Abstract
Objective
To assess the impact of sepsis classification and multidrug resistance status on outcome in patients receiving appropriate initial antibiotic therapy.
Design
A retrospective cohort study.
Setting
Barnes-Jewish Hospital, a 1250-bed teaching hospital.
Patients
Individuals with Enterobacteriaceae sepsis, severe sepsis, and septic shock that received appropriate initial antimicrobial therapy between June 2009 and December 2013.
Interventions
Clinical outcomes were compared according to multidrug resistance status, sepsis classification, demographics, severity of illness, comorbidities, and antimicrobial treatment.
Measurements and Main Results
We identified 510 patients with Enterobacteriaceae bacteremia and sepsis, severe sepsis, or septic shock. Sixty-seven patients (13.1%) were non-survivors. Mortality increased significantly with increasing severity of sepsis (3.5%, 9.9%, and 28.6%, for sepsis, severe sepsis, and septic shock, respectively, p<0.05). Time to antimicrobial therapy was not significantly associated with outcome. APACHE II was more predictive of mortality than age-adjusted Charlson comorbidity index. Multidrug resistance status did not result in excess mortality. Length of intensive care unit and hospital stay increased with more severe sepsis. In multivariate logistic regression analysis, African-American race, sepsis severity, APACHE II score, solid organ cancer, cirrhosis, and transfer from an outside hospital were all predictors of mortality.
Conclusions
Our results support sepsis severity, but not multidrug resistance status as being an important predictor of death when all patients receive appropriate initial antibiotic therapy. Future sepsis trials should attempt to provide appropriate antimicrobial therapy and take sepsis severity into careful account when determining outcomes.
Keywords: Sepsis mortality, multi-drug resistance
INTRODUCTION
Inappropriate initial antimicrobial therapy leads to higher mortality in patients with severe sepsis or septic shock [1–5]. Multidrug resistant pathogens are prone to treatment with initial inappropriate antimicrobial therapy, but whether drug resistance alone increases mortality in the setting of appropriate therapy is unclear [6–10]. The presence of severe sepsis with organ failure and shock requiring vasopressor support are predictors of greater mortality in populations with variable rates of appropriate antimicrobial therapy [1, 11–16]. Neither the presence of severe sepsis or shock nor multidrug resistance as predictors of mortality has been studied in a cohort of septic patients that all received appropriate initial antimicrobial therapy. Our primary goal was to compare thirty-day all-cause mortality among patients with sepsis, severe sepsis, and septic shock treated with appropriate initial antimicrobial therapy to more directly assess the impact of sepsis severity on outcome. Our secondary objective was to examine the impact of multidrug resistance on mortality in the same cohort. We selected only patients with Enterobacteriaceae bacteremia for two reasons: 1) the incidence of multidrug resistant Enterobacteriaceae infections is increasing worldwide [17–21] and 2) for a homogeneous population in order to minimize pathogen related confounders.
MATERIALS AND METHODS
Study Location and Patient Population
This study was conducted at Barnes-Jewish Hospital, a 1250 bed academic medical center located in St. Louis, MO. The study period was June 1, 2009 through December 31, 2013, corresponding to the length of time for which an electronic medical record was available that could verify time of antibiotic administration. All consecutive hospitalized patients with sepsis, severe sepsis, or septic shock and a positive blood culture for an organism in the Enterobacteriaceae family during the study period were analyzed for eligibility. This study was approved by the Washington University School of Medicine Human Studies Committee.
Study Design and Data Collection
Utilizing a retrospective cohort study design, all patients age ≥ 18 with sepsis, severe sepsis, or septic shock were identified by the presence of a positive blood culture for an organism in the Enterobacteriaceae family. Patients were included only if they had positive blood cultures with a single organism from the Enterobacteriaceae family; patients with polymicrobial blood cultures were excluded from the study. ICD-9 codes indicative of acute organ dysfunction or the need for vasopressors were used to classify patients as having severe sepsis or septic shock, respectively. The primary endpoint was all-cause 30-day mortality, calculated from the time that a positive blood culture was drawn. Secondary endpoints included length of hospital stay (LOS), length of intensive care unit (ICU) stay (ICU LOS), and the number of procedures performed. Only the first episode of sepsis, severe sepsis, or septic shock was evaluated. Baseline characteristics, including age, gender, race, place of origin, healthcare exposure, receipt of antibiotics within 30 days of positive culture, presence of immunosuppression, Acute Physiology and Chronic Health Evaluation (APACHE) II [22] scores (calculated based on clinical data present during the 24 hours after positive blood cultures were drawn), Charlson Comorbidity Index, and medical comorbidities were obtained.
Definitions
Patients were considered to have a bloodstream infection due to Enterobacteriaceae if any blood culture obtained within 48 hours of developing sepsis, severe sepsis, or septic shock were positive for Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Klebsiella granulomatis, Proteus mirabilis, Proteus vulgaris, Enterobacter aerogenes, Enterobacter cloacae, Enterobacter sakasakii, Serratia marcescens, Citrobacter freundii, Citrobacter koseri, Citrobacter amalonaticus, Edwardsiella tarda, Hafnia alvei, Morganella morganii, Pantoea agglomerans, Plesiomonoas shigelloides, Providencia stuartii, Providencia rettgeri, Salmonella enterica, Shigella dysenterii, Shigella flexneri, Shigella sonnei, Shigella boydii, Yersinia enterocolitica, Yersinia pestis, Yersinia pseudotuberculosis, Ewingella americana, or Kluyvera spp.
Patients were required to have at least one of the following ICD-9 codes 995.91 (sepsis), 995.92 (severe sepsis), 038 (septicemia), 790.7 (bacteremia NOS), or 785.52 (septic shock). For patients to be included in the septic shock group, they had to receive blood pressure support with any of the following medications within 24 hours of positive blood culture: norepinephrine, phenylephrine, epinephrine, dopamine, dobutamine, or vasopressin. All patients had to receive appropriate initial antibiotic therapy, defined as antibiotics that had in vitro activity against the cultured organism (and were not single-agent aminoglycosides), that was administered within 12 hours of when a positive blood culture was drawn and continued for at least 24 hours. For extended-spectrum β-lactamase producing organisms, initial use of a carbapenem was required to be classified as appropriate treatment. Antimicrobial susceptibilities were determined using disc diffusion methodology. Multidrug resistance was defined as nonsusceptibility to at least one antimicrobial agent from at least three different antimicrobial classes [23]. Appropriate antibiotics administered ≤ 12 hours before positive blood cultures were drawn were considered to have a time of administration of 0 minutes. Patients with pathogens resistant to ampicillin were not considered to have received appropriate therapy if they received ampicillin/sulbactam.
Only the first episode of bacteremia during a hospitalization was considered. Patients who had an episode of bacteremia during their hospitalization prior to Enterobacteriaceae bacteremia were excluded (only two cases, one with Staphylococcus epidermidis, one with Enterococcus). The following organisms were considered contaminants if not recultured within 72 hours: coagulase-negative Staphylococci, Corynebacterium, Propionibacterium acnes, or Viridans group Streptococcus. Patients were excluded if they were under 18 years of age or if they had a blood culture positive for more than one organism. All patients who did not receive antibiotics within 12 hours of when positive blood cultures were drawn were excluded. Discharge on hospice was considered a mortality equivalent. All patients discharged on hospice were considered to expire at the time of hospital discharge. In the number of procedures analysis, codes for vasopressors and mechanical ventilation were not included in the final tally. Blood product administration was considered a procedure, but only one instance was counted. Otherwise, all ICD9 procedure codes were considered in the number of procedures analysis. Length of hospital stay was calculated from time a positive blood culture was drawn. Patients that never required ICU admission were considered to have an ICU stay length of 0 days. Healthcare exposure was defined as chemotherapy within the prior 30 days, residence in a nursing home or other long-term care facility, hospitalization in an acute care hospital for two or more days within the prior 90 days, or attendance at a hospital or hemodialysis clinic within the prior 30 days.
Thirty-day mortality was assessed using the BJC Healthcare informatics database. Barnes-Jewish Hospital serves as the main teaching institution for BJC Healthcare, a large integrated healthcare system of both inpatient and outpatient care. The system includes a total of thirteen hospitals in a compact geographic region surrounding and including St Louis, Missouri. Persons treated within this healthcare system are, in nearly all cases, readmitted to one of the system’s participating hospitals or evaluated in a BJC Healthcare outpatient practice. If a patient who receives healthcare in the system presents to a non-system hospital, he/she is often transferred back into the integrated system because of issues of insurance coverage. Death certificate records and autopsy reports are included in the informatics database. All data was derived from the informatics database provided by the Center for Clinical Excellence, BJC HealthCare.
Statistical Analysis
Thirty day all-cause mortality was compared between the sepsis, severe sepsis, and septic shock groups. Univariate analysis was performed by chi-square or Fischer’s exact test where appropriate for categorical values. Student’s t-test or Mann-Whitney U test was used where appropriate for continuous variables. Continuous variables were reported as means with standard deviations. Categorical data were expressed as frequencies. A p-value of <0.05 was considered significant. Multivariate analysis comparing survivors and non-survivors was used to determine risk factors for mortality. Factors associated with mortality in univariate analysis (p < 0.20) were entered into a multivariate logistic regression analysis to determine odds ratios for mortality. All variables entered into the model were assessed for co-linearity, and interaction terms were tested. Goodness-of-fit was assessed via the Hosmer-Lemeshow c-statistic. All tests were two-tailed. All analysis was done using SPSS v22.
RESULTS
Five-hundred ten patients with sepsis, severe sepsis, or septic shock due to Enterobacteriaceae met the inclusion criteria. There were no cases with multiple episodes of Enterobacteriaceae bacteremia identified. Baseline characteristics of the patients are listed in Table 1. Patients with septic shock had the greatest APACHE II scores and need for mechanical ventilation. Time to appropriate initial antibiotic therapy was shortest for patients with septic shock. The distribution of pathogens is shown in Table 2. There was no significant difference in pathogen distribution according to sepsis classification. The most common organism was Escherichia coli. There were no significant differences in the proportion of individual pathogens between survivors and non-survivors (data not shown). Among the 510 cases, 99 (19.4%) met MDR criteria. As the severity of sepsis increased, so did the LOS, ICU LOS, number of procedures, and mortality (Table 3). Kaplan-Meier curves confirmed that increasing sepsis severity was associated with greater 30-day mortality (Figure 1). Total LOS, prevalence of MDR pathogens, and number of procedures performed were not significantly different between the survivors and non-survivors. Non-survivors had significantly longer ICU LOS (Table 3).
Table 1.
Patient Characteristics According to Sepsis Severity and Survival Status
| Characteristics | Sepsis (n=172) | Severe Sepsis (n=191) | Septic Shock (n=147) | Survivors (n=443) | Non-survivors (n=67) | P value (survivors vs. non-survivors) |
|---|---|---|---|---|---|---|
| Age, yrs | 58.4 ± 15.9 | 61.9 ± 16.4* | 59.6 ± 14.4 | 59.2 ± 16.0 | 65.2 ± 12.3 | 0.003 |
| Male, % (#) | 49.4 (85) | 52.9 (101) | 55.1 (81) | 51.8 (229) | 56.7 (38) | 0.382 |
| African-American, % (n) | 25.6 (44) | 37.2 (71)* | 30.6 (45) | 29.6 (131) | 43.3 (29) | 0.024 |
| Mechanical ventilation, % (n) | 0 (0) | 16.2 (31)* | 48.3 (71) *@ | 16.0 (71) | 46.3 (31) | <0.001 |
| Bone marrow transplant, % (n) | 2.9 (5) | 6.3 (12) | 4.1 (6) | 4.5 (20) | 4.5 (3) | 1 |
| Solid organ transplant, % (n) | 3.5 (6) | 5.2 (10) | 3.4 (5) | 4.5 (20) | 1.5 (1) | 0.338 |
| CHF, % (n) | 9.9 (17) | 14.1 (27) | 21.8 (32)* | 13.5 (60) | 23.9 (16) | 0.027 |
| COPD, % (n) | 10.5 (18) | 15.2 (29) | 20.4 (30)* | 15.8 (70) | 10.4 (7) | 0.254 |
| Diabetes mellitus, type 2, % (n) | 25.0 (43) | 30.4 (58) | 32.0 (47) | 28.9 (128) | 29.9 (20) | 0.872 |
| CKD, % (n) | 5.2 (9) | 22.0 (42)* | 12.2 (18)*@ | 12.4 (55) | 20.9 (14) | 0.059 |
| RRT, % (n) | 1.2 (2) | 2.6 (5) | 6.1 (9)* | 2.9 (13) | 4.5 (3) | 0.454 |
| Solid organ malignancy, % (n) | 26.7 (46) | 27.7 (53) | 30.6 (45) | 25.3 (112) | 47.8 (32) | <0.001 |
| Leukemia, % (n) | 19.2 (33) | 20.4 (39) | 15.6 (23) | 20.1 (89) | 9.0 (6) | 0.029 |
| Lymphoma, % (n) | 5.8 (10) | 6.3 (12) | 5.4 (8) | 5.6 (25) | 7.5 (5) | 0.555 |
| Cirrhosis, % (n) | 1.7 (3) | 4.2 (8) | 12.2 (18)*@ | 3.6 (16) | 19.4 (13) | <0.001 |
| Antibiotics within 30 days, % (n) | 39.5 (68) | 38.7 (74) | 36.1 (53) | 37.9 (168) | 40.3 (27) | 0.709 |
| Healthcare exposure, % (n) | 70.3 (121) | 66.5 (127) | 72.1 (106) | 67.9 (301) | 79.1 (53) | 0.065 |
| MDR, % (n) | 18.0 (31) | 17.8 (34) | 23.1 (34) | 19.2 (85) | 20.9 (14) | 0.742 |
| Time to appropriate antibiotics (hours) | 3.6 ± 2.9 | 3.6 ± 3.2 | 3.0 ± 2.8* | 3.4 ± 3.0 | 3.8 ± 3.2 | 0.314 |
| Immunosuppressed, % (n) | 37.2 (64) | 38.7 (74) | 33.3 (49) | 36.8 (163) | 35.8 (24) | 0.877 |
| Charlson Comorbidity Score | 1.5 ± 1.2 | 1.8 ± 1.3* | 1.5 ± 1.2 | 1.6 ± 1.3 | 2.0 ± 1.2 | 0.003 |
| APACHE II score | 10.7 ± 3.6 | 12.5 ± 4.7* | 17.6 ± 5.4*@ | 12.8 ± 5.0 | 17.5 ± 6.2 | <0.001 |
| Presence of multiple other pathogens, % (n) | 5.2 (9) | 11.0 (21)* | 13.6 (20)* | 9.5 (42) | 11.9 (8) | 0.528 |
| Patient origin, % (n) | ||||||
| Nursing home, SNF, or LTACH | 3.5 (6) | 11.0 (21)* | 11.6 (17)* | 9.0 (40) | 6.0 (4) | 0.492 |
| Community | 67.4 (116) | 49.7 (95)* | 45.6 (67)* | 55.5 (246) | 47.8 (32) | 0.233 |
| OSH | 9.9 (17) | 9.4 (18) | 11.6 (17) | 8.6 (38) | 20.9 (14) | 0.019 |
| In hospital | 19.2 (33) | 29.8 (57)* | 31.3 (46)* | 26.9 (119) | 25.4 (17) | 0.797 |
| Infection source, % (n) | ||||||
| Central venous catheter | 9.3 (16) | 9.4 (18) | 8.8 (13) | 10.2 (45) | 3.0 (2) | 0.068 |
| Genitourinary | 40.7 (70) | 43.4 (83) | 42.2 (62) | 42.4 (188) | 40.3 (27) | 0.741 |
| Pulmonary | 3.5 (6) | 5.2 (10) | 8.8 (13)* | 4.7 (21) | 11.9 (8) | 0.177 |
| Gastrointestinal | 18.6 (32) | 13.6 (26) | 17.0 (25) | 15.6 (69) | 20.9 (14) | 0.271 |
| CNS | 0 (0) | 0.5 (1) | 0.7 (1) | 0.2 (1) | 1.5 (1) | 0.245 |
| Skin/soft tissue | 0.6 (1) | 1.0 (2) | 0.7 (1) | 0.7 (3) | 1.5 (1) | 0.431 |
| Unknown | 25.6 (44) | 25.6 (49) | 21.1 (31) | 24.8 (110) | 20.9 (14) | 0.484 |
| Infected surgical vascular graft | 0 (0) | 1.0 (2) | 0 (0) | 0.5 (2) | 0 | 1 |
| Muscle | 0.6 (1) | 0 (0) | 0 (0) | 0.2 (1) | 0 | 1 |
| Joint | 0 (0) | 0 (0) | 0.7 (1) | 0.2 (1) | 0 | 1 |
| Osteomyelitis | 0.6 (1) | 0 (0) | 0 (0) | 0.2 (1) | 0 | 1 |
| Gynecologic | 0.6 (1) | 0 (0) | 0 (0) | 0.2 (1) | 0 | 1 |
p<0.05 compared to sepsis group.
p<0.05 compared to severe sepsis group.
Values are reported as percentages (number) or mean value ± standard deviation.
CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease; RRT: renal replacement therapy; MDR: multi-drug resistance; APACHE II: Acute physiology and chronic health evaluation II; SNF: skilled nursing facility; LTACH: long-term acute care hospital; OSH: outside hospital; CNS: central nervous system.
Table 2.
Microbiology of Enterobacteriaceae Sepsis, Severe Sepsis and Septic Shock
| Pathogen | Sepsis % (n) | Severe Sepsis % (n) | Septic Shock % (n) |
|---|---|---|---|
| Escherichia coli | 57.5 (99) | 55.5 (106) | 52.4 (77) |
| Klebsiella pneumoniae | 31.9 (55) | 27.2 (52) | 35.4 (52) |
| Klebsiella oxytoca | 3.5 (6) | 5.2 (10) | 4.1 (6) |
| Enterobacter aerogenes | 0 (0) | 1.0 (2) | 0.7 (1) |
| Enterobacter cloacae | 2.9 (5) | 4.2 (8) | 2.0 (3) |
| Citrobacter freundii | 0 (0) | 0.5 (1) | 0 (0) |
| Citrobacter koseri | 0 (0) | 0.5 (1) | 0.7 (1) |
| Morganella morganii | 0.6 (1) | 0.5 (1) | 0 (0) |
| Pantoea agglomerans | 0.6 (1) | 0 (0) | 0 (0) |
| Hafnia alvei | 0.6 (1) | 0 (0) | 0 (0) |
| Proteus mirabilis | 2.3 (4) | 0.5 (1) | 3.4 (5) |
| Providencia stuartii | 0 (0) | 0 (0) | 0.7 (1) |
| Serratia marcescens | 0 (0) | 0.5 (1) | 0.7 (1) |
Table 3.
Clinical Outcomes According to Sepsis Severity and Survival Status
| Outcome | Sepsis | Severe Sepsis | Septic Shock | Survivors | Non-survivors |
|---|---|---|---|---|---|
| Thirty day mortality, % (n) | 3.5 (6) | 9.9 (19)* | 28.6 (42)*@ | – | – |
| Length of stay, days | 9.2 ± 9.4 | 15.3 ± 14.7* | 20.8 ± 22.6*@ | 15.1 ± 17.2 | 13.1 ± 12.5 |
| Length of ICU stay, days | 2.5 ± 7.6 | 4.8 ± 10.9* | 9.8 ± 13.2*@ | 5.4 ± 11.4 | 6.1 ± 8.5ϕ |
| Number of procedures | 2.2 ± 2.1 | 3.2 ± 3.3* | 5.5 ± 3.9*@ | 3.4 ±3.5 | 3.9 ± 3.3 |
| MDR, % (n) | 18.0 (31) | 17.8 (34) | 23.1 (34) | 19.2 (85) | 20.9 (14) |
p<0.05 compared to sepsis group.
p<0.05 compared to severe sepsis group.
p<0.05 compared to survivors.
MDR = multidrug resistance.
Values are reported as percentages (number) or mean value ± standard deviation.
Figure 1.
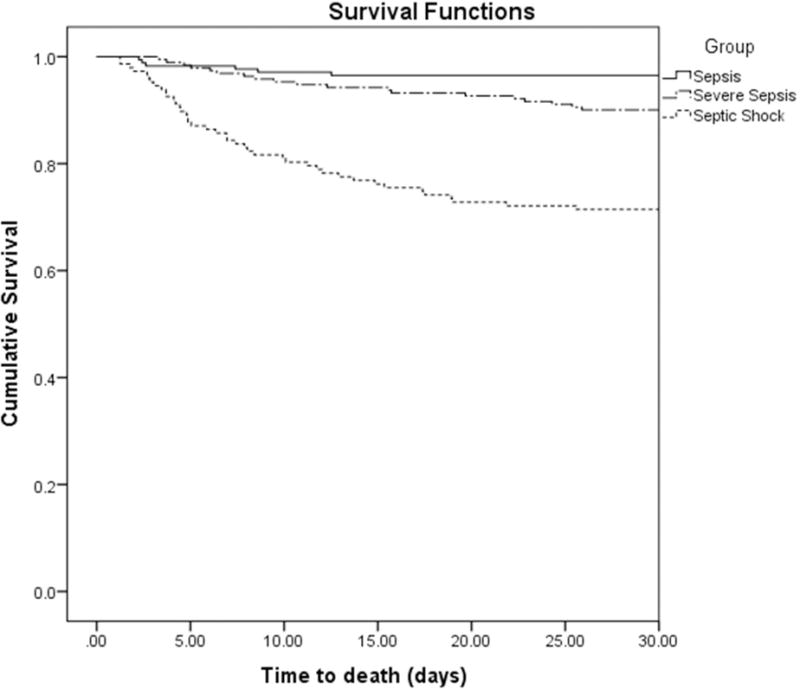
Kaplan-Meier curve for thirty-day survival according to sepsis classification. Thirty-day survival was significantly lower for patients with septic shock (p < 0.001; Log-Rank Test).
Age, Charlson Comorbidity Index, APACHE II, leukemia, cirrhosis, solid organ malignancy, origin from an outside hospital, pulmonary source of infection, and CHF were significantly different between survivors and non-survivors in univariate analysis (Table 1). Time to antibiotic therapy after a positive blood culture was drawn did not differ between survivors and nonsurvivors. In multivariate analysis, patients who died were more likely to be African-American, have greater sepsis severity, have higher APACHE II scores, have solid organ cancer, cirrhosis, and be transferred from an outside hospital (Table 4).
Table 4.
Factors associated with mortality in multivariate logistic regression analysis
| Factor | Odds ratio (95% confidence intervals) |
|---|---|
| Sepsis severity | 2.07ϕ(1.62 – 2.65) |
| African American race | 2.65 (1.93 – 3.66) |
| APACHE II (1-point increments) | 1.12 (1.09 – 1.15) |
| Solid organ cancer | 2.92 (2.14 – 3.97) |
| Cirrhosis | 6.17 (3.82 – 9.97) |
| Patient origin from OSH | 3.37 (2.21 – 5.12) |
= Represents the odds ratio for death between two groups (sepsis and severe sepsis, severe sepsis and septic shock). When comparing sepsis and septic shock, the odds ratio is multiplied by two for each degree of separation.
APACHE II: Acute physiology and chronic health evaluation II; OSH: outside hospital.
Hosmer-Lemeshow c-statistic = 0.457.
DISCUSSION
We found that sepsis severity predicted mortality among patients receiving appropriate initial antimicrobial therapy. The presence of multidrug resistance did not appear to influence outcome when the initial therapy was appropriate for the causative pathogen. Interestingly, patients with septic shock had a significantly shorter time to appropriate antibiotic administration than patients with sepsis alone, but still had significantly higher mortality. These results suggest that appropriate antimicrobials are insufficient to completely overcome the systemic calamity associated with septic shock. However, appropriate therapy is still crucial, as suggested by the low overall mortality in our cohort (13.1%) compared to studies with varying levels of appropriate empiric antimicrobial therapy [24]. In the future, clinical trials should strive to provide appropriate antimicrobial therapy and take sepsis severity into careful account when determining outcomes. Unsurprisingly, as sepsis severity increased, LOS, ICU LOS, and number of procedures significantly increased. Length of stay was not statistically different between survivors and non-survivors, but this is likely a result of hospital stays truncated by death.
Multidrug resistance was not statistically different when comparing sepsis severity groups nor between survivors and non-survivors. Past studies have posited that the presence of multidrug resistance leads to worse outcomes and possibly even conveys increased virulence [10, 25]. Prior studies have shown that multidrug resistance is a risk factor for mortality in the setting of inappropriate initial antimicrobial therapy [26]. Our study population included only patients who received appropriate antimicrobial therapy; we found no evidence of increased mortality due to multidrug resistance. Our data demonstrate that this increased mortality is negated with appropriate and timely antimicrobial therapy. The current paucity of agents to treat multidrug resistant pathogens limits our ability to administer appropriate initial therapy for many drug resistant bacteria. Fortunately, there are quality improvement measures, improving molecular technologies, and new antimicrobials in the pipeline that provide hope to stay in step with increasing drug resistance [27–31].
African-American race as a risk factor for mortality is likely an unfortunate marker for racial disparities in healthcare rather than a genetic predisposition. Transfer from an OSH may increase mortality because of the high level of acuity of patients transferred from institutions with limited resources. Increasing APACHE II scores, underlying malignancy, and cirrhosis have previously been shown to be independent risk factors for mortality in sepsis and reflect acute and chronic illness severity [1, 8, 26, 32].
Our study is limited in several ways. The retrospective nature of the study makes it difficult to elucidate possible confounders that could have biased the outcome measures. This was a single-center study and results may not be generalizable to other centers. However, the lack of increased mortality with multidrug resistant pathogens should be applicable to other cohorts that have received appropriate therapy. We did not study outcomes in patients with Gram-positive infections or non-Enterobacteriaceae Gram-negative infections. It is possible that there would be different results in these populations and this is an area ripe for future studies. Another limitation is the method of determining 30-day mortality. It is possible that some patients died outside of the BJC Healthcare network and that we were unable to capture their mortality status. However, it is unlikely that this would have influenced our results given the clear signal observed between sepsis severity and outcome. We are also limited by a lack of antimicrobial minimum inhibitory concentration (MIC) data to determine if the administered antibiotics were therapeutic at a given MIC. However, our microbiology lab uses well-validated disc diffusion methodology to determine antimicrobial susceptibilities. Utilizing susceptibility data, our pharmacy uses an antimicrobial control program and reviews all antimicrobial orders as previously described [8], which minimizes inadequate therapy.
For ease of data collection and interpretation, we focused on culture-positive patients with sepsis. We recognize that historically, culture-negative patients fare better than their culture-positive counterparts. However, assessing antimicrobial appropriateness according to our definition was impractical without culture data. With improving molecular technologies, the number of culture-negative patients will likely decrease, thereby increasing the spectrum of patients that can be assessed for antimicrobial appropriateness. We are also limited in the assessment of differential outcomes based on specific pathogens due to the low frequency of infection with pathogens other than E. coli and K. pneumoniae.
In conclusion, severity of sepsis is an important predictor of mortality, even in patients that all receive appropriate initial antimicrobial therapy. In patients that receive appropriate initial therapy, we found no difference in outcome based on time to appropriate therapy, as long as it was administered within 12 hours after a positive culture was drawn. Our results will assist in the interpretation of outcomes in sepsis clinical trials. Additionally, physicians will now be better able to prognosticate for patient families with evidence to support the claim that the presence of shock quadruples the risk of death as compared to its absence, even in the setting of appropriate antimicrobials. Our findings suggest that appropriate initial antimicrobial therapy eliminates the impact of multidrug resistance on mortality, providing another impetus for improved diagnostics and antimicrobials for drug-resistant pathogens. Future studies can assess similar outcomes in patients with Gram-positive or Gram-negative non-Enterobacteraiaceae sepsis.
Footnotes
Work was performed at Barnes-Jewish Hospital, St. Louis, MO.
- Dr. Burnham has no conflicts of interest to report.
- Dr. Kollef’s effort was supported by the Barnes-Jewish Hospital Foundation.
- Dr. Lane has received career development support from the Goldfarb Patient Safety & Quality Fellowship program and the Barnes-Jewish Hospital Foundation. Dr. Lane was also supported by the Washington University Institute of Clinical and Translational Sciences grants UL1 TR000448 and KL2 TR000450 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
No reprints will be ordered.
References
- 1.Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115:462–474. doi: 10.1378/chest.115.2.462. [DOI] [PubMed] [Google Scholar]
- 2.Kollef MH. Broad-spectrum antimicrobials and the treatment of serious bacterial infections: getting it right up front. Clin Infect Dis. 2008;47:S3–13. doi: 10.1086/590061. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118:146–155. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 5.Shorr AF, Micek ST, Welch EC, Doherty JA, Reichley RM, Kollef MH. Inappropriate antibiotic therapy in Gram-negative sepsis increases hospital length of stay. Crit Care Med. 2011;39:46–51. doi: 10.1097/CCM.0b013e3181fa41a7. [DOI] [PubMed] [Google Scholar]
- 6.Blot S, Vandewoude K, De Bacquer D, Colardyn F. Nosocomial bacteremia caused by antibiotic-resistant gram-negative bacteria in critically ill patients: clinical outcome and length of hospitalization. Clin Infect Dis. 2002;34:1600–1606. doi: 10.1086/340616. [DOI] [PubMed] [Google Scholar]
- 7.Figueiredo CS. Impact of antimicrobial resistance on the treatment and outcome of patients with sepsis. Shock. 2008;30:23–29. doi: 10.1097/SHK.0b013e3181818990. [DOI] [PubMed] [Google Scholar]
- 8.Labelle A, Juang P, Reichley R, et al. The determinants of hospital mortality among patients with septic shock receiving appropriate initial antibiotic treatment. Crit Care Med. 2012;40:2016–2021. doi: 10.1097/CCM.0b013e318250aa72. [DOI] [PubMed] [Google Scholar]
- 9.Paterson DL, Ko WC, Von Gottberg A, et al. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clin Infect Dis. 2004;39:31–37. doi: 10.1086/420816. [DOI] [PubMed] [Google Scholar]
- 10.Schwaber MJ, Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother. 2007;60:913–920. doi: 10.1093/jac/dkm318. [DOI] [PubMed] [Google Scholar]
- 11.Micek ST, Isakow W, Shannon W, Kollef MH. Predictors of hospital mortality for patients with severe sepsis treated with Drotrecogin alfa (activated) Pharmacotherapy. 2005;25:26–34. doi: 10.1592/phco.25.1.26.55615. [DOI] [PubMed] [Google Scholar]
- 12.Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother. 2005;49:1306–1311. doi: 10.1128/AAC.49.4.1306-1311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ha YE, Kang CI, Cha MK, et al. clinical outcomes of bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in patients with cancer. Int J Antimicrob Agents. 2013;42:403–409. doi: 10.1016/j.ijantimicag.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Lin YC, Chen TL, Ju HL, et al. risk factors for attributable mortality in Enterobacter cloacae bacteremia. J Microbiol Immunol Infect. 2006;39:67–72. [PubMed] [Google Scholar]
- 15.Legrand M, Max A, Peigne V, et al. Survival in neutropenic patients with severe sepsis or septic shock. Crit Care Med. 2012;40:43–49. doi: 10.1097/CCM.0b013e31822b50c2. [DOI] [PubMed] [Google Scholar]
- 16.Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother. 2010;54:4851–4863. doi: 10.1128/AAC.00627-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben-Ami R, Schwaber MJ, Navon-Venezia S, et al. Influx of extended-spectrum beta-lactamase-producing enterobacteriaceae into the hospital. Clin Infect Dis. 2006;42:925–934. doi: 10.1086/500936. [DOI] [PubMed] [Google Scholar]
- 18.Deshpande LM, Jones RN, Fritsche TR, Sader HS. Occurrence and characterization of carbapenemase-producing Enterobacteriaceae: report from the SENTRY Antimicrobial Surveillance Program (2000–2004) Microb Drug Resist. 2006;12:223–230. doi: 10.1089/mdr.2006.12.223. [DOI] [PubMed] [Google Scholar]
- 19.Guzman-Blanco M, Labarca JA, Villegas MV, Gotuzzo E. Extended spectrum beta-lactamase producers among nosocomial Enterobacteriaceae in Latin America. Braz J Infect Dis. 2014;18:421–433. doi: 10.1016/j.bjid.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livermore DM. Current epidemiology and growing resistance of gram-negative pathogens. Korean J Intern Med. 2012;27:128–142. doi: 10.3904/kjim.2012.27.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Nosocomial Infections Surveillance (NNIS) System Report, Data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 22.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 23.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 24.Leligdowicz A, Dodek PM, Norena M, et al. Association between source of infection and hospital mortality in patients who have septic shock. Am J Respir Crit Care Med. 2014;189:1204–1213. doi: 10.1164/rccm.201310-1875OC. [DOI] [PubMed] [Google Scholar]
- 25.Schwaber MJ, Navon-Venezia S, Kaye KS, Ben-Ami R, Schwartz D, Carmeli Y. Clinical and economic impact of bacteremia with extended- spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2006;50:1257–1262. doi: 10.1128/AAC.50.4.1257-1262.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zilberberg MD, Shorr AF, Micek ST, Vazquez-Guillamet C, Kollef MH. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: a retrospective cohort study. Crit Care. 2014;18:596. doi: 10.1186/s13054-014-0596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Micek ST, Heard KM, Gowan M, Kollef MH. Identifying critically ill patients at risk for inappropriate antibiotic therapy: a pilot study of a point-of-care decision support alert. Crit Care Med. 2014;42:1832–1838. doi: 10.1097/CCM.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 28.Awad SS, Rodriguez AH, Chuang YC, et al. A Phase 3 Randomized Double-Blind Comparison of Ceftobiprole Medocaril Versus Ceftazidime Plus Linezolid for the Treatment of Hospital-Acquired Pneumonia. Clin Infect Dis. 2014 doi: 10.1093/cid/ciu219. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucasti C, Hershberger E, Miller B, et al. Multicenter, double-blind, randomized, phase II trial to assess the safety and efficacy of ceftolozane-tazobactam plus metronidazole compared with meropenem in adult patients with complicated intra-abdominal infections. Antimicrob Agents Chemother. 2014;58:5350–5357. doi: 10.1128/AAC.00049-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doern CD. Integration of technology into clinical practice. Clin Lab Med. 2013;33:705–729. doi: 10.1016/j.cll.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Poulakou G, Bassetti M, Righi E, Dimopoulos G. Current and future treatment options for infections caused by multidrug-resistant Gram-negative pathogens. Future Microbiol. 2014;9:1053–1069. doi: 10.2217/fmb.14.58. [DOI] [PubMed] [Google Scholar]
- 32.Iregui M, Ward S, Sherman G, Fraser VJ, Kollef MH. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest. 2002;122:262–268. doi: 10.1378/chest.122.1.262. [DOI] [PubMed] [Google Scholar]


