Abstract
Ror proteins are a conserved family of tyrosine kinase receptors that function in developmental processes, including skeletal and neuronal development, cell movement, and cell polarity. While Ror (receptor tyrosine kinase-like orphan receptor) proteins were originally named because the associated ligand and signaling pathway were unknown, recent studies in multiple species now establish that Ror proteins are Wnt receptors. Depending on the cellular context, Ror proteins can either activate or repress transcription of Wnt target genes and can modulate Wnt signaling by sequestering Wnt ligands. New evidence implicates Ror proteins in planar cell polarity (PCP), an alternative Wnt pathway. Here, we review the progress made in understanding these mysterious proteins and in particular we focus on their function as Wnt receptors.
Introduction
Receptor tyrosine kinases (RTKs) play crucial roles in many cellular processes including differentiation, proliferation, migration, angiogenesis and survival. Therefore, it is not surprising that dysfunctional RTKs cause severe developmental defects and diseases such as cancer. Ror proteins are no exception and disruptions of human Ror proteins are associated with skeletal deformities and with leukemia. RTKs normally enable communication between a cell and its environment by binding to an extracellular ligand and initiating an intracellular signaling cascade. For a long time, the Ror family of RTKs was one of the few types of RTK whose ligand and signaling pathway remained elusive, giving rise to their ‘orphan’ nomenclature; however, recent work has greatly advanced our understanding of Ror function. In particular, Ror proteins have emerged as central regulators of Wnt signaling, an important developmental signaling pathway.
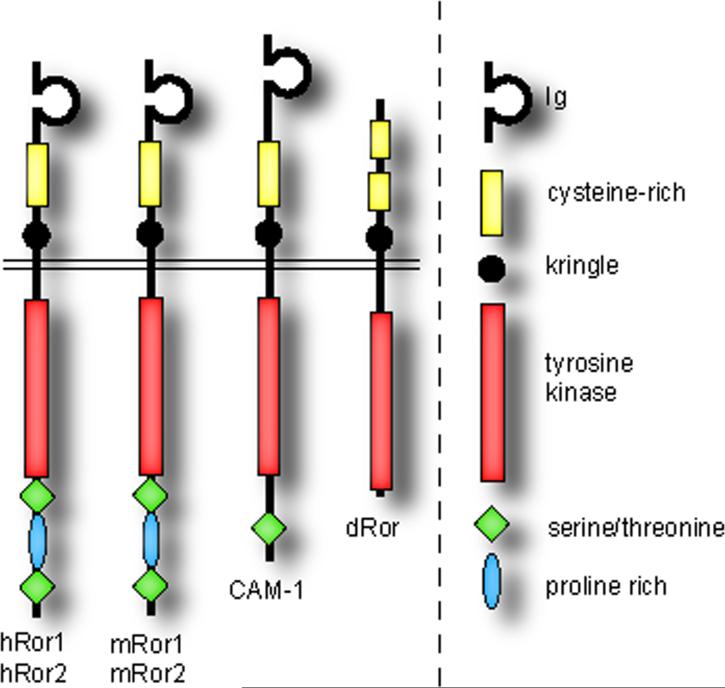
Ror proteins are type I transmembrane receptor tyrosine kinases (Figure 1). Like other RTKs, they are predominantly located in the plasma membrane [1]. The extracellular region of vertebrate Ror proteins contains an immunoglobulin (Ig) domain, a cysteine-rich domain (CRD), also called a Frizzled domain, and a Kringle (Kr) domain. Intracellularly, Ror proteins possess a tyrosine kinase (TK) domain, and a proline-rich domain straddled by two serine-threonine-rich (S/T1 and S/T2) domains [2].
Figure 1. Structure of Ror RTKs in different species.
Structure of Ror receptor tyrosine kinases (RTKs) in different species. Domain organization (approximately to scale) of Ror proteins in human (hROR1, hROR2), mouse (mRor1, mRor2), C. elegans (CAM-1) and Drosophila (dROR). The N-terminal extracellular domain (ECD) is above and the intracellular domain (ICD) is below the double line representing the plasma membrane (image adapted from Refs [68, 69]).
Vertebrates have two ROR family members encoded by ROR1 and ROR2 (formerly known as NTRKR1 and NTRKR2, respectively), first identified in a human neuroblastoma cell line by a PCR-based search for tyrosine kinases similar to Trk neurotrophic receptors [2]. Splice variants of ROR1 encoding truncated proteins lacking either the extracellular domains [3] or the transmembrane and intracellular domains (Genbank locus NM_001083592) have been described. Since the former, called truncated ROR1 (t-ROR1), might be artefactual [4] and the latter has not been analyzed in detail, in this review we will consider only full-length ROR proteins. Despite their lack of several amino acids highly conserved in protein tyrosine kinases, ROR1 and ROR2 each have kinase activity in vitro [2, 5]. ROR orthologs have been identified in fruit flies (Drosophila melanogaster; dROR) [6], roundworms (Caenorhabditis elegans; cam-1) [7, 8], sea slugs (Aplysia californica; Apror) [9], zebrafish (Danio rerio; Ror2 and Ror2) [10], chickens (Gallus gallus; cRor1 and cRor2) [11, 12], frogs (Xenopus laevis; XRor1 and XRor2) [13] and mice (Mus musculus; mRor1 and mRor2) [5]. Drosophila Dnrk [14] has been excluded here as it is now thought of as a MuSK ortholog [15] (see Box 1). While the CRD, Kringle and TK domains are characteristic of all ROR proteins, the architecture of the other domains varies between species (Figure 1).
Box 1. Evolution of Ror.
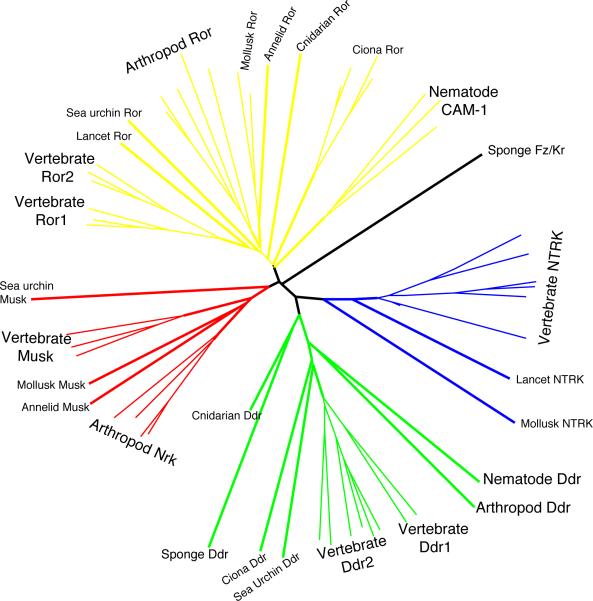
The Ror family is part of the NTRK superfamily of receptor tyrosine kinases, which also includes the MuSK (muscle-specific kinase) and Nrk (neuro-specific receptor kinase) family, the Ddr (Discoidin domain receptors) family, and the Ntrk (neurotrophic tyrosine kinase) family, also known as the Trk (tropomyosin-related kinase) family (Box Figure 1). The Ddr family appears to have split from the ancestral family first, being present as a separate family in the sponges (Porifera). The sponge Frizzled-Kringle protein might be representative of an ancestral family of proteins present in early metazoa, before the Ror, MuSK, and Ntrk split. The Ntrk proteins appear to have branched off near the same time as the MuSK and Ror split, although Ror and Ddr are the only families present in sea anemone (Cnidaria) and demonstrably basal to bilateria [15]. All four families must have been present in early bilateria, though different families have been lost in divergent modern phyla. While vertebrates retain all four families, molluscs appear to have lost the Ddr family, insects have lost the Ntrk family (Dnrk is now viewed as a representative of the MuSK and Nrk family owing to domain and sequence similarity), and nematodes have lost both the Ntrk and the MuSK families [15]. Certain species have significantly more divergent representatives of a family than would be expected for their phyla (such as the echinoderm Ror proteins that appear basal to those of vertebrates, molluscs and arthropods), indicating rapid evolution of various domains, especially the kinase domain. C. elegans CAM-1, whose conserved kinase, kringle and Ig domains clearly place it in the Ror family, has a cysteine-rich (Frizzled) domain, whose amino acid sequence resembles that of a MuSK as much as that of a Ror [8, 15]. As nematodes lack MuSK, the cam-1 frizzled domain could be converging with the MuSK frizzled domains of other organisms. The propensity for continued duplication and mutation of this family is reflected in the more recent divergence of Ror1 and Ror2 and Ddr1 and Ddr2 in early vertebrates.
The extracellular CRD of Ror is similar to the Wnt-binding domain found in Frizzled receptors [16-20], suggesting that Ror proteins also bind to Wnt ligands. This was later shown (see below). Wnt proteins are a family of secreted glycoproteins that play crucial roles in development and disease (reviewed by [21]). In the classic model of Wnt signaling, a Wnt ligand binds to a Frizzled (Fzd) receptor and to the Lrp5/6 co-receptor. This interaction results in the stabilization of cytoplasmic β-catenin, allowing it to accumulate, translocate to the nucleus, and act as a transcriptional co-activator with TCF, a DNA-binding protein. Other mechanisms of Wnt signaling include Wnt–calcium signaling, Wnt–JNK signaling and the planar cell polarity (PCP) pathway (reviewed by [22]). The degree to which these pathways overlap is presently unclear.
Several studies report diverse, and sometimes conflicting, interactions of Ror with Wnt signaling. It is likely that the discrepancies reflect the diversity of systems tested, with Ror proteins in fact having multiple functions depending on the cellular context (the coexistence of other Wnt pathway components operating in a given cell). In this review, we have clustered compatible observations into a handful of signaling mechanisms, discussed in detail below, following a brief account of Ror function during development. We conclude with a description of other Ror interactions not yet associated with Wnt signaling.
Ror function during development
In humans, Ror protein functions are known primarily in skeletal development. hROR2 mutations cause well-characterized skeletal defects: dominant brachydactyly type B (BDB), a condition of shortened or missing digits [23, 24], and recessive Robinow syndrome (RRS), a form of short-limbed dwarfism [25, 26]. Ror2 polymorphisms are also associated with variations in human bone length and mineral density [27]. In mouse and chick, Ror genes play a partially redundant role in skeletal development and are also required for development of the cardiac and respiratory systems [1, 4, 5, 28-32]. Notably, the skeletal defects of mRor2 mutant mice, dwarfism, shortened limbs and facial abnormalities, resemble the deformities of the human disease RRS. While mutations in hROR1 have not been linked to any human disease, hROR1 is overexpressed in chronic lymphocytic leukemia (CLL) and confers a survival advantage to these cells in vitro [33, 34]. Consistent with there being role for hROR1 in cancer, ROR1 was identified as a potent survival kinase in HeLa cervical carcinoma cells [35]. The signaling events downstream of Ror receptors, which are still largely unknown, will need to be deciphered in order to treat Ror-based diseases and malignancies. Table 1 presents a summary of the biological functions and expression patterns of Ror proteins.
Table 1.
Summary of Ror function and expression in various species
| Receptor | Species | Developmental process (disease) | Expression pattern* | References |
|---|---|---|---|---|
| hRORl | human | cancer (chronic lymphocytic leukemia) | See note† | [3, 4, 33, 34] |
| hROR2 | human | skeletal development (Recessive Robinow Syndrome, Brachydactyly Type B) | ND | [23-27] |
| mRorl | mouse | skeletal , respiratory, and cardiac development | Craniofacial region, cardiovascular system, respiratory system, nervous system, digestive system, thymus, developing limb | [1, 4, 5, 28] |
| mRor2 | mouse | skeletal, respiratory, and cardiac development, planar cell polarity | Developing nervous, cardiovascular, respiratory, urogenital, skeletal and digestive systems, developing limb, craniofacial region, inner ear. | [1, 4, 5, 30-32, 49, 51] |
| cRorl | chick | ND | Developing limb | [11] |
| cRor2 | chick | skeletal development | Developing limb, nervous system, skeletal system, digestive system, mesonephros, heart, muscles, liver, lung | [12] |
| XRor2 | Xenopus | convergent extension | Dorsal mesoderm, posterior neuroectoderm, neural crest, pharyngeal arches | [13, 47, 49] |
| ApRor | Aplysia californica | ND | Nervous system | [9] |
| CAM-1 | C. elegans | cell migration, neuronal development, vulva development, dauer larva formation, locomotion, asymmetric cell division | Neurons, muscle, intestine, gonad, pharynx, vulva | [7, 8, 39, 40, 45, 52] |
| dROR | Drosophila | ND | Nervous system | [6] |
Expression has been detected in the tissues listed. In many species, expression has not been comprehensively examined.
One study reports that a truncated version of hRORl, “t-RORl,” is expressed in the central nervous system and in cancer tissue [3]; however, these results are contested [4] The same study also reports hRORl expression in fetal and adult heart, lung and kidney. In contrast, two recent studies report that hRORl is not expressed in these or other normal adult tissues [33, 34]. Fetal hRORl expression was not reexamined.
Although Ror proteins are strongly expressed in the developing nervous systems of many species (Table 1), the role of Ror proteins in neuronal development remains unclear. The mutant phenotype of dROR, which is expressed exclusively in the developing nervous system of Drosophila [6] has not been described. In mice, although the largely non-overlapping expression patterns of mROR1 and mROR2 in the developing nervous system makes redundancy unlikely, mROR2 knockout mice do not display obvious neurological defects [4, 5]; however, it is possible that a subtle phenotype is masked by the early lethality of these mice.
Despite the apparent lack of a vertebrate neuronal phenotype, strong neuronal expression and structural similarity to MuSK (muscle-specific kinase) protein, which is an RTK required for synapse formation [36], suggest that Ror proteins are involved in neuronal development. Evidence supporting this comes from C. elegans, where CAM-1 regulates neuronal migration, axon outgrowth and axon guidance [8, 37-39]. CAM-1 also regulates the localization of acetylcholine receptors at the neuromuscular synapse [40], a function performed by the MuSK receptor in mammals [36]. Interestingly, although CAM-1's closest homolog is Ror, CAM-1 turns up as the closest homolog for both Ror and MuSK in C. elegans (see Box 1), raising the possibility that CAM-1 fulfills the roles of both Ror and MuSK. While it is unknown whether Ror proteins perform neuronal functions in species that have a distinct MuSK protein, Ror proteins display a localization pattern in cultured mammalian neurons consistent with functions in neurite extension and the organization of neuronal subdomains [9, 41-43].
Functions of Ror as a Wnt receptor
ROR proteins sequester Wnt ligands
A series of studies led to the discovery that CAM-1, the C. elegans Ror protein, inhibits the function of a C. elegans Wnt ligand, EGL-20 [8, 39, 44]. It was first shown that cam-1 mutations cause defects in the migration of several neurons along the anterior–posterior axis [8]. This migration phenotype was later determined to be reciprocal to the phenotype caused by loss of the Wnt ligand egl-20 [39]. Further investigation revealed that cam-1 overexpression mimics the egl-20 mutant phenotype and that egl-20 overexpression mimics the cam-1 mutant phenotype. Thus, cam-1 and Wnt/egl-20 appeared to have an antagonistic relationship. Experiments using engineered cam-1 deletions showed that the membrane-anchored CAM-1 CRD was sufficient to rescue cell migration in cam-1 mutants, suggesting that CAM-1 might function to regulate the spatial distribution of Wnt/EGL-20 (Table 2A) [44]. The hypothesis that CAM-1 can sequester Wnt proteins was recently confirmed by a study of cam-1 function in C. elegans vulva development. During vulva development, cam-1 mutations result in elevated Wnt pathway activity in the vulval precursor cells (VPCs), and overexpression of cam-1 between the source of Wnt expression and the VPCs acts as a barrier to reduce Wnt pathway activity in the VPCs [45]. Also, this study showed that the membrane-anchored CAM-1 CRD is sufficient to bind Wnt ligands in vitro and non-autonomously inhibit their activity in vivo.
Table 2.
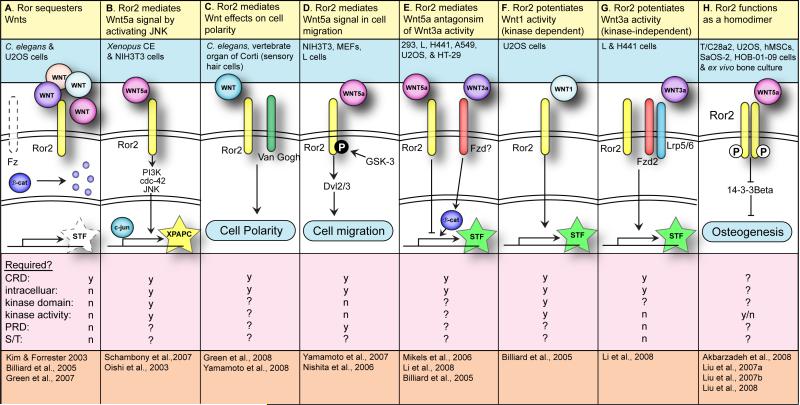
Ror proteins as Wnt receptors
| 1. Ror function | A. Ror sequesters Wnts | B. Ror2 mediates Wnt5a signal by activating JNK | C. Ror2 mediates Wnt effects on cell polarity | D. Ror2 mediates Wnt5a signal in cell migration | E. Ror2 mediates Wnt5a antagonism of Wnt3a activity | F. Ror2 potentiates Wntl activity (kinase-dependent) | G. Ror2 potentiates Wnt3a activity (kinase-independent) | H. Ror2 functions as a homodimer |
| 2. System(s) | C. elegans & U2OS cells | Xenopus CE & NIH3T3 cells | C. elegans, vertebrate organ of Corti (sensory hair cells) | NIH3T3, MEFs, A7, L cells | 293, L, H441, A549, U2OS & HT-29 cells | U2OS cells | L & H441 cells | T/C28a2, U2OS, hMSCs, SaOS-2, HOB-01-09 cells & ex vivo bone culture |
| 3. Molecular interactions | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 |
| 4. Domains: | ||||||||
| CRD | y | y | y | y | y | y | y | ? |
| Intracellular | n | y | y | y | y | y | y | ? |
| Kinase domain | n | y | ? | n | ? | y | ? | ? |
| Kinase activity | n | y | ? | n | ? | y | n | y/n |
| PRD | n | ? | ? | ? | ? | ? | n | ? |
| S/T1 | n | ? | ? | ? | ? | ? | n | ? |
| S/T2 | n | ? | ? | ? | ? | ? | n | ? |
| 5. References | [44-46] | [47, 49] | [51, 52] | [53-55] | [46, 59, 60] | [46] | [59] | [56, 61-63] |
Columns A-G include a description of Ror function (row 1), the system in which the function was observed (row 2), a diagram of Ror activity (row 3), the domains of Ror required (row 4) and the references that describe the function (row 5). Domain abbreviations: CRD (cysteine-rich domain), PRD (proline-rich domain), S/T1 (serine-threonine-rich domain 1) and S/T2 (serine-threonine-rich domain 2). We have taken some liberty in grouping observations from different systems into similar Ror functions, and each reference listed might not describe all of the interactions depicted. Within the diagrams, the extracellular space is at the top, cytoplasmic is in the middle and nuclear is at the bottom, separated by double lines. Stars represent transcriptional readouts: STF (SuperTOPFLASH) and XPAPC (paraxial protocadherin). Dotted lines represent inactivity. In (C), (D) and (H), where transcriptional activity has not been described, the phenomenological assay is indicated in a blue oval. A white 'P' in a black circle indicates phosphorylation on Ser/Thr residues. A black 'P' in a white circle indicates phosphorylation on Tyr residues.
The function of Ror proteins in other systems is also consistent with Wnt sequestration. For example, in U2OS human osteosarcoma cells, Ror2 binds to Wnt1 and Wnt3 and antagonizes Wnt1- and Wnt3-mediated stabilization of cytosolic β-catenin by a mechanism that does not require the Ror2 kinase domain [46]. However, there are also many examples where the influence of Ror proteins on Wnt signaling cannot be explained by simple sequestration of Wnt proteins, indicating that Ror functions include additional mechanisms, as explained below.
Wnt5a, Ror2 and JNK act in a distinct pathway
Studies of Xenopus convergent extension (CE), a polarized morphogenetic movement in which lengthening and narrowing of a field of cells occurs in embryogenesis, showed that XRor2 binds to Wnt5a [13] and transmits a Wnt5a signal via the Ser/Thr kinase JNK (c-Jun N-terminal kinase) [47]. XWnt5a and XRor2 regulate constriction by activating a JNK pathway, which upregulates expression of the paraxial protocadherin XPAPC (Table 2B). XPAPC loss-of-function causes constriction defects in Keller explants, which are sections of dorsal mesoderm and ectoderm from Xenopus embryos that undergo CE in culture. Knockdown of XWnt5a, XRor2 or XJNK phenocopies the constriction defect caused by XPAPC loss-of-function. Conversely, XWnt5a overexpression upregulates XPAPC expression and this activity requires the kinase domain of XRor2. Activated XJNK similarly upregulates XPAPC expression and XJNK activity is stimulated by XWnt5a overexpression and is reduced by XWnt5a depletion. Therefore, Wnt5a, Ror2 and JNK probably constitute a distinct functional pathway in vivo.
Additional evidence linking Ror2 and Wnt5a comes from mice, where the mWnt5a expression pattern is highly similar to that of mRor2 in the developing embryo. The gross morphological phenotypes of mRor2 and mWnt5a mutants are also similar; both display dwarfism, facial abnormalities and shortened limbs [30, 48, 49]. Subsequent analysis revealed a functional relationship between the two gene products. mRor2 physically interacts with Wnt5a, but not Wnt3a, in vitro and mRor2 and Wnt5a synergistically activate JNK in NIH3T3 cells, supporting the existence of a distinct Wnt5a–Ror2–JNK pathway (Table 2B) [49].
Ror and planar cell polarity
PCP is a process wherein cells, or groups of cells, are polarized along the plane of the epithelium, perpendicular to the apical–basal axis (reviewed by [50]). PCP is regulated by the PCP pathway, which includes the conserved core components Fzd, Dishevelled (Dvl), Van Gogh, Prickle and Flamingo. Although the PCP pathway is considered an alternative type of Wnt signaling, the contribution of Wnt proteins to PCP is not well understood. Ror2 was previously suspected to act in the PCP pathway during Xenopus CE; however, recent evidence suggests that XRor2 function during this process occurs by a different mechanism, the Wnt5a–Ror2–JNK pathway (see above and Table 2B) [47]. Nevertheless, new studies in vertebrates and C. elegans reveal a connection between Ror proteins and PCP signaling after all, as described below.
An established model for the study of PCP in vertebrates is the organ of Corti in the mammalian inner ear, in which PCP defects manifest as misoriented sensory hair cells. mRor2 is strongly expressed in the organ of Corti during embryogenesis and the hair cells of mRor2 mutant mice display characteristic PCP abnormalities [51].
CAM-1 regulates the polarity of the C. elegans VPCs [52]. The VPCs are epithelial cells that divide asymmetrically along the anterior–posterior axis of the nematode. VPC orientation resembles PCP in that the cells are polarized along the plane of the vulval epithelium. Several Wnt proteins, including Wnt/EGL-20, determine the orientation of VPC division. During VPC orientation, CAM-1 mediates EGL-20 activity by a JNK-independent mechanism that requires the CAM-1 intracellular domain (Table 2C). In this process, CAM-1 imparts directional information from EGL-20 to the VPCs. Van Gogh, a core component of the PCP pathway acts in the same pathway as CAM-1 and EGL-20, suggesting that Ror proteins interact with the PCP pathway. It is important to distinguish the function of CAM-1 in VPC orientation, which requires the intracellular domain and is thus probably cell-autonomous, from the non-autonomous inhibition of Wnt signaling in the VPCs when CAM-1 is expressed in other tissues (see above). CAM-1 also regulates neuronal polarity and the asymmetric division of several neurons [8]; however, whether CAM-1 interacts with Wnt proteins in these processes is unknown.
Ror and cell migration
Another context where the Wnt ligand Wnt5a and Ror2 appear to act together is during cell migration. Wnt5a-induced migration of mouse embryonic fibroblasts (MEFs) requires the CRD and C-terminal domain of Ror2 (Table 2D) [53]. Treatment of mouse NIH3T3 cells with Wnt5a causes glycogen synthase kinase-3 (GSK-3)-mediated phosphorylation of Ror2 on serine/threonine residues. GSK-3 is required for Wnt5a to mobilize these cells, suggesting that phosphorylation of Ror2 by GSK-3 might be required for Ror2 function in cell migration [54]. JNK, PKCζ and the actin-binding protein filamin A are also required for Wnt5a-induced polarization and cell migration in NIH3T3 cells [55]. Besides the migration described above, Ror2 can influence the cytoskeleton independently of Wnt5a, as measured by the formation of actin-rich structures known as filopodia. Since these cytoplasmic extensions are probably associated with Wnt- and Ror2-mediated cell migration, we will discuss them now.
Ror2 overexpression induces filopodia formation in MEFs and this effect is independent of the Ror2 CRD and of Wnt5a [53]. Notably, Ror2-induced formation of filopodia is not sufficient to stimulate migration; however, it is possible that Ror2 mobilizes the cytoskelton allowing MEFs to respond to the Wnt5a migratory cue, when present. In MEF filopodia, Ror2 colocalizes with actin and the Ror2 cytoplasmic domain associates with filamin A. The C-terminal portion of Ror2 containing the PRD and S/T2 domains is required for association of Ror2 with filamin A. Interestingly, CAM-1, which regulates cell-polarization and cell motility in C. elegans, lacks a PRD and has only a single S/T domain (Figure 1). It is unknown whether the single S/T domain of CAM-1 can recapitulate the Ror2-filamin A interaction.
While Ror2 appears to positively regulate filopodia in MEFs, it has a different effect on filopodia in Keller open-face explants, in which knockdown of XRor2 or XWnt5a results in an increase in transient filopodia [47]. One explanation that would reconcile these observations is that Ror2 functions to stabilize filopodia. In this case, Ror2 overexpression might cause increased filopodia, as seen in MEFs, and Ror2 knockdown might cause more transient filopodia, as seen in the Keller open-face explants. Ror proteins also influence the cytoskeleton in several other cell types. For example, transfection of Ror2 in MCF7 human breast cancer cells, T/C28a2 human chondrocytes, mouse B16BL6 melanoma cells, and mouse L cells causes extensive formation of filopodia [53, 56]. Also, in cultured hippocampal neurons, Ror proteins mobilize the cytoskeleton to regulate neurite and axon extension and branching [42].
Ror2 inhibits expression of Wnt target genes
Experiments in cell culture demonstrate that Ror2 modulates the expression of Wnt target genes independently of the sequestration mechanism described above for CAM-1. Wnt–β-catenin pathway activity is commonly measured by a reporter called TOPFLASH that has multiple TCF binding sites driving expression of the gene encoding luciferase [57, 58]. SUPERTOPFLASH (STF) is similar to TOPFLASH, but has a greater number of TCF sites. Ror2 inhibits classic Wnt–β-catenin signaling in mouse L cells and the A549 and H441 human lung carcinoma cell lines, in which Wnt5a antagonizes Wnt3a-induced STF expression in the presence of Ror2 [59] (Table 2E). In human embryonic kidney 293 cells, Wnt5a is reported to inhibit Wnt3a-induced STF expression, not by influencing β-catenin levels, but by reducing gene expression downstream of β-catenin [60]. This Wnt5a signal is mediated by Ror2 and does not involve Ca2+ signaling. Overexpression of Ror2 enhances the ability of Wnt5a to block Wnt3a activation of STF and the Ror2 intracellular domain is required for this activity, arguing against a sequestration function of Ror2 in this context. Contrary to U2OS human osteosarcoma cells where Ror2 binds to Wnt3a, [46], Ror2 does not bind to Wnt3a in 293 cells (see specificity section below).
Ror2 promotes expression of Wnt target genes
While the above studies indicate that Ror2 can antagonize Wnt–β-catenin signaling, other studies indicate that Ror2 potentiates Wnt–β-catenin signaling in multiple cell types. In U2OS osteosarcoma cells, Ror2 potentiates Wnt1-induced TOPFLASH expression by a mechanism requiring the Ror2 kinase domain [46]. That Ror2 antagonizes Wnt1-mediated stabilization of β-catenin (described above, see sequestration section), yet potentiates the transcriptional response to Wnt1 in the same cell line is an enigma and presents a challenge to current views of Wnt signaling. One possibility is that the interactions between Ror2 and Wnt1 reflect two distinct Ror2 functions. Perhaps Ror2 antagonizes Wnt1-mediated stabilization of β-catenin by sequestering Wnt1 (Table 2A) and Ror2 potentiates Wnt1-induced reporter activation by a signaling mechanism involving the Ror2 kinase domain (Table 2F). In H441 lung carcinoma cells, Ror2 cooperates with the receptor Fzd2 to activate STF in response to Wnt3a [59]. In this context, Ror2 function requires the Ror2 intracellular domain, but not Ror2 kinase activity (Table 2G). Thus, because Ror2 can both increase and decrease expression of Wnt reporters, caution should be used when classifying Ror proteins as activators or inhibitors of Wnt signaling.
Specificity
Wnt3a binds to Ror2 in U2OS osteosarcoma cells [46], but not in 293 cells [60]. One explanation for the difference in binding specificity between the cell types is that 293 cells do not express a cofactor necessary for binding. As previously reported [60], a recent study similarly showed that Ror2 does not bind to Wnt3a in 293 cells; however, this study showed that Ror2 does bind to Wnt3a in 293 cells in the presence of collagen triple helix repeat-containing protein 1 (Cthrc1), a secreted glycoprotein that stabilizes Wnt ligand–receptor interactions [51]. Cthrc1 binds to Wnt proteins and to Ror2 (independently of the Ror2 CRD), and Cthrc1 enhances binding of both Wnt3a and Wnt5a to Ror2. The Fzd receptor is another good candidate for a binding cofactor as Ror2 associates with Wnt3a in 293 cells when Fzd6 is co-expressed [51]. Although the Ror2 CRD physically interacts with several Fzd receptors, the biological significance of Fzd–Ror interactions has not been tested [49, 59].
Ror2 homodimerization
Like many other RTKs, Ror proteins form homodimers (Table 2H). Homodimerization of Ror2, which occurs upon overexpression in U2OS cells, can be enhanced by treatment with a bivalent antibody against Ror2 [61] or by fusion to the dimeric Fc portion of human Ig [56]. Ror2 homodimerization results in tyrosine phosphorylation of the receptor [56, 61, 62], and, in this regard, Ror2 appears to function as a typical RTK. In U2OS osteosarcoma cells, in which both Wnt3a and Wnt5a bind to Ror2 [46], only Wnt5a promotes Ror2 homodimerization and tyrosine phosphorylation. [62]. In NIH3T3 cells, by contrast, Wnt5a does not induce tyrosine phosphorylation of Ror2 (homodimerization was not examined) [54]. Again, these differences could be due to the varying cellular contexts.
Downstream of Wnt5a and Ror
Forced dimerization of Ror2 or treatment with Wnt5a activates Ror2 signaling, as evidenced by increased bone formation in organ culture and increased osteogenesis in human mesenchymal stem cells (hMSCs) [61-63]. These effects could be mediated by inhibition of the cytoplasmic14-3-3-β scaffold protein, which antagonizes osteogenesis. Immunoprecipitation followed by mass-spectrometric analysis of FLAG-tagged Ror2 revealed that the 14-3-3-β scaffold protein is a Ror2 binding partner (Table 2H). In U2OS osteosarcoma cells, the Ror2 intracellular domain directly interacts with and phosphorylates 14-3-3-β, and treatment with Wnt5a also promotes phosphorylation of 14-3-3-β. Interestingly, 14-3-3-β exhibits stronger binding to kinase-inactive Ror2 (Ror2-KD) than to wild-type Ror2, suggesting that 14-3-3-β might be released by Ror2 phosphorylation.
Another candidate signal transducer is Src protein-tyrosine kinase, which is activated when Ror2-expressing T/C28a2 human chondrocytes are treated with Wnt5a [56]. In these cells, activation by Wnt5a causes robust tyrosine phosphorylation of Ror2 followed by rapid internalization of Ror2. While most functional studies of mammalian Ror proteins have focused on Ror2 because of its disease association, Wnt5a also binds to Ror1, and co-transfection of these two proteins in 293 cells causes activation of the pleiotropic transcription factor NF-κB [34]. Thus, NF-κB is another potential downstream effector of Wnt5a–Ror signaling.
While the signaling cascade downstream of activated Ror is poorly understood, some of the components that interact with the Ror intracellular domain are beginning to be elucidated. The interactions below are not apparently related to Wnt signaling, but a connection to Wnt signaling might be exposed upon further investigation.
Potentially Wnt-independent Ror functions
A yeast-two-hybrid (Y2H) protein–protein interaction screen using the mRor1 and mRor2 C-termini as bait identified Dlxin-1 as a protein that interacts with Ror2 but not with Ror1 (Table 3A) [64]. Dlxin-1 is a melanoma-associated-antigen (MAGE) family member and was confirmed to bind to Ror2, but not to Ror1, by co-immunoprecipitation. Ror2 kinase activity is not required for this association. Ror2, by means of its C-terminal proline-rich or S/T2 domains, recruits Dlxin-1 from the cytoplasm to the plasma membrane, and Dlxin-1 is localized to the nucleus in the absence of Ror2. Through this subcellular localization, Ror2 indirectly affects the transcriptional activity of the Dlxin-1 interactor Msx2. Consistent with an interaction between Ror2 and Dlxin-1, their spatiotemporal expression patterns significantly overlap in the developing mouse face and lung. As with the interaction of Ror2 with filamin A during cell migration (see above), it will be interesting to see whether the single S/T domain in the CAM-1 C-terminus is sufficient to recapitulate the Ror2–Dlixin-1 interaction.
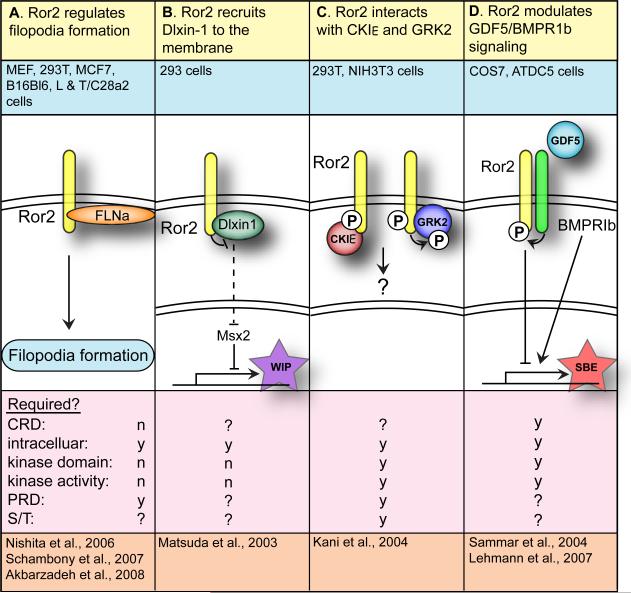
Table 3.
Other Ror interactions
| 1. Ror function | A. Ror2 recruits Dlixin-1 to the membrane | B. Ror2 interacts with CKIε and GRK2 | C. Ror2 modulates GDF5/BMPR1b signaling |
| 2. System(s) | C. elegans & U2OS cells | 293, NIH3T3 cells | COS7, ATDC5 cells |
| 3. Molecular interactions | T9 | T10 | T11 |
| 4. Domains: | |||
| CRD | y | y | y |
| Intracellular | n | y | y |
| Kinase domain | n | y | ? |
| Kinase activity | n | y | ? |
| PRD | n | ? | ? |
| S/T1 | n | ? | ? |
| S/T2 | n | ? | ? |
| 5. References | [64] | [65] | [66, 67] |
This Y2H screen also identified casein kinase I epsilon (CKIε) as a Ror2 binding partner. Endogenous Ror2 and CKIε co-immunoprecipitated from NIH3T3 cells and also coimmunoprecipitated when overexpressed in 293 cells (Table 3B) [65]. CKIε phosphorylates Ser/Thr residues in the S/T2 domain of Ror2 and induces autophosphorylation of tyrosine residues in the Ror2 proline-rich domain. After activation by CKIε, Ror2 associates with and tyrosine phosphorylates G-protein-coupled receptor kinase 2 (GRK2/beta-adrenergic receptor kinase 1). The expression pattern of GRK2 is similar to that of Ror2 and Dlxin-1 in mice [65]. Association of Ror2 with CKIε may be specific to vertebrates, as invertebrate Ror proteins do not have the proline-rich domain (Figure 1). Neither the Ror2-Dlixin-1 nor the Ror2- CKIε interactions have thus far been associated with any biological function.
Another study, using a candidate-driven approach, identified growth/differentiation factor 5 (GDF5) and bone morphogenetic protein receptor type-1B (BMPR1b) as Ror-interacting proteins [66]. This study was based on the premise that the molecular mechanisms responsible for BDB, caused by Ror2 mutation, might be shared among other forms of brachydactyly. Brachdactyly type C (BDC) and brachydactyly type A2 (BDA2) are caused by mutation in the gene encoding GDF5, which is a TGF-β/BMP family member, and its receptor, BMPR1b, respectively. A genetic and physical interaction between Ror2, BMPR1b and GDF5 was described, in which BMPR1b binds to and phosphorylates Ror2, which inhibits the GDF5–BMPR1b pathway (Table 3C). A relationship between Ror2 and BMP signaling was reproduced in a second study where mutations were detected in the gene encoding the GDF5 antagonist NOGGIN, in patients with BDB without Ror2 mutations [67]. The signaling events that take place following these interactions and the mechanism by which they cause the brachydactyly phenotype are presently unclear. It remains to be shown whether GDF5 directly binds to Ror2 and if so, which domains are involved.
Remaining questions and future directions
Ror proteins are involved in a multitude of cellular processes and signaling events. A striking theme, however, is that Ror proteins function as Wnt receptors. In particular, there is general consensus that Wnt5a binds to and activates Ror2; therefore, Wnt5a can be considered a bona fide Ror2 ligand. While much progress has been made in understanding Ror2 function as a Wnt receptor, several key questions remain unanswered. What is the connection between Ror and the PCP pathway? What is the mechanism by which Ror2 either activates or inhibits Wnt targets? What determines whether Ror2 will transduce a Wnt signal versus sequester the Wnt? Are these exclusive functions or do both occur at once? Future work should address these issues.
While there is abundant evidence in many systems that Ror proteins act as Wnt receptors, most of these functions depend on the CRD. To date, there is no knowledge of the function of the other extracellular domains – Kringle and Ig. These domains could be required to interact with a co-receptor, or be involved in receptor localization, or they could be required for binding to unidentified non-Wnt ligands. Similarly, the function of the intracellular domain remains ambiguous. Studies involving Y2H assays and mass-spectrometry, as well as candidate-driven approaches, have identified multiple potential binding partners; however the biological significance of many of these interactions remains to be determined.
Ror2 has been the focus of many studies because of its involvement in human disease; by contrast, Ror1 function has been studied less rigorously. In light of its recent implication in human cancer, it will be important also to decipher the signaling properties of Ror1. The unique domain architecture of these RTKs and the variety of processes in which they are involved hold promise for exciting revelations about Ror signaling. While great progress has been made in placing these orphan receptors into the cellular signaling network, Ror signaling remains rich with mystery.
Figure 2.
Ror proteins as Wnt receptors
Figure 3.
Other Ror functions
Box Figure 1. The different families within the NTRK superfamily of tyrosine kinases.
The tree, which has no root, represents an approximation of the evolutionary divergence, as different domains within the proteins have evolved at different rates in different species and have experienced independent introductions of the Ig domain. Highlighted are the NTRK (blue), Ddr (green), MuSK/Nrk (red), and Ror (yellow) families; each line represents a single protein from a species in the labelled clade (i.e. the three lines for nematode CAM-1 represent CAM-1 of C. elegans, Brugia malayi, and Pristionchus pacificus). The sponge Frizzled-Kringle protein (black) does not fit into any family. Sequences are from GenBank (http://www.ncbi.nlm.nih.gov/sites/entrez), Ensembl (http://www.ensembl.org), UCSC Genome Browser (http://genome.ucsc.edu), and JGI (http://genome.jgi-psf.org). Tree generated by ClustalX (v1.83.1; [71]) and Phylodendron (v0.8d; http://iubio.bio.indiana.edu/treeapp/treeprint-form.html).
Acknowledgements
P.W.S. is an investigator with the HHMI. J.L.G. was supported by the Thomas Hunt Morgan Fellowship and S.G.K. was supported by the NIH training grant for graduate study toward the Doctor of Philosophy degree in Biology at the California Institute of Technology.
References
- 1.Matsuda T, et al. Expression of the receptor tyrosine kinase genes, Ror1 and Ror2, during mouse development. Mech Dev. 2001;105(1-2):153–6. doi: 10.1016/s0925-4773(01)00383-5. [DOI] [PubMed] [Google Scholar]
- 2.Masiakowski P, Carroll RD. A novel family of cell surface receptors with tyrosine kinase-like domain. J Biol Chem. 1992;267(36):26181–90. [PubMed] [Google Scholar]
- 3.Reddy UR, Phatak S, Pleasure D. Human neural tissues express a truncated Ror1 receptor tyrosine kinase, lacking both extracellular and transmembrane domains. Oncogene. 1996;13(7):1555–9. [PubMed] [Google Scholar]
- 4.Al-Shawi R, et al. Expression of the Ror1 and Ror2 receptor tyrosine kinase genes during mouse development. Dev Genes Evol. 2001;211(4):161–71. doi: 10.1007/s004270100140. [DOI] [PubMed] [Google Scholar]
- 5.Oishi I, et al. Spatio-temporally regulated expression of receptor tyrosine kinases, mRor1, mRor2, during mouse development: implications in development and function of the nervous system. Genes Cells. 1999;4(1):41–56. doi: 10.1046/j.1365-2443.1999.00234.x. [DOI] [PubMed] [Google Scholar]
- 6.Wilson C, Goberdhan DC, Steller H. Dror, a potential neurotrophic receptor gene, encodes a Drosophila homolog of the vertebrate Ror family of Trk-related receptor tyrosine kinases. Proc Natl Acad Sci U S A. 1993;90(15):7109–13. doi: 10.1073/pnas.90.15.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koga M, et al. Control of DAF-7 TGF-(alpha) expression and neuronal process development by a receptor tyrosine kinase KIN-8 in Caenorhabditis elegans. Development. 1999;126(23):5387–98. doi: 10.1242/dev.126.23.5387. [DOI] [PubMed] [Google Scholar]
- 8.Forrester WC, et al. A C. elegans Ror receptor tyrosine kinase regulates cell motility and asymmetric cell division. Nature. 1999;400(6747):881–5. doi: 10.1038/23722. [DOI] [PubMed] [Google Scholar]
- 9.McKay SE, et al. Aplysia ror forms clusters on the surface of identified neuroendocrine cells. Mol Cell Neurosci. 2001;17(5):821–41. doi: 10.1006/mcne.2001.0977. [DOI] [PubMed] [Google Scholar]
- 10.Katoh M, Katoh M. Comparative genomics on ROR1 and ROR2 orthologs. Oncol Rep. 2005;14(5):1381–4. doi: 10.3892/or.14.5.1381. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Niedenfuhr M, Prols F, Christ B. Expression and regulation of ROR-1 during early avian limb development. Anat Embryol (Berl) 2004;207(6):495–502. doi: 10.1007/s00429-004-0381-6. [DOI] [PubMed] [Google Scholar]
- 12.Stricker S, et al. Cloning and expression pattern of chicken Ror2 and functional characterization of truncating mutations in Brachydactyly type B and Robinow syndrome. Dev Dyn. 2006;235(12):3456–65. doi: 10.1002/dvdy.20993. [DOI] [PubMed] [Google Scholar]
- 13.Hikasa H, et al. The Xenopus receptor tyrosine kinase Xror2 modulates morphogenetic movements of the axial mesoderm and neuroectoderm via Wnt signaling. Development. 2002;129(22):5227–39. doi: 10.1242/dev.129.22.5227. [DOI] [PubMed] [Google Scholar]
- 14.Oishi I, et al. A novel Drosophila receptor tyrosine kinase expressed specifically in the nervous system. Unique structural features and implication in developmental signaling. J Biol Chem. 1997;272(18):11916–23. doi: 10.1074/jbc.272.18.11916. [DOI] [PubMed] [Google Scholar]
- 15.Sossin WS. Tracing the evolution and function of the Trk superfamily of receptor tyrosine kinases. Brain Behav Evol. 2006;68(3):145–56. doi: 10.1159/000094084. [DOI] [PubMed] [Google Scholar]
- 16.Saldanha J, Singh J, Mahadevan D. Identification of a Frizzled-like cysteine rich domain in the extracellular region of developmental receptor tyrosine kinases. Protein Sci. 1998;7(8):1632–5. [PMC free article] [PubMed] [Google Scholar]
- 17.Roszmusz E, et al. Localization of disulfide bonds in the frizzled module of Ror1 receptor tyrosine kinase. J Biol Chem. 2001;276(21):18485–90. doi: 10.1074/jbc.M100100200. [DOI] [PubMed] [Google Scholar]
- 18.Xu YK, Nusse R. The Frizzled CRD domain is conserved in diverse proteins including several receptor tyrosine kinases. Curr Biol. 1998;8(12):R405–6. doi: 10.1016/s0960-9822(98)70262-3. [DOI] [PubMed] [Google Scholar]
- 19.Masiakowski P, Yancopoulos GD. The Wnt receptor CRD domain is also found in MuSK and related orphan receptor tyrosine kinases. Curr Biol. 1998;8(12):R407. doi: 10.1016/s0960-9822(98)70263-5. [DOI] [PubMed] [Google Scholar]
- 20.Rehn M, et al. The frizzled motif: in how many different protein families does it occur? Trends Biochem Sci. 1998;23(11):415–7. doi: 10.1016/s0968-0004(98)01290-0. [DOI] [PubMed] [Google Scholar]
- 21.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 22.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5(3):367–77. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 23.Oldridge M, et al. Dominant mutations in ROR2, encoding an orphan receptor tyrosine kinase, cause brachydactyly type B. Nat Genet. 2000;24(3):275–8. doi: 10.1038/73495. [DOI] [PubMed] [Google Scholar]
- 24.Schwabe GC, et al. Distinct mutations in the receptor tyrosine kinase gene ROR2 cause brachydactyly type B. Am J Hum Genet. 2000;67(4):822–31. doi: 10.1086/303084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Bokhoven H, et al. Mutation of the gene encoding the ROR2 tyrosine kinase causes autosomal recessive Robinow syndrome. Nat Genet. 2000;25(4):423–6. doi: 10.1038/78113. [DOI] [PubMed] [Google Scholar]
- 26.Afzal AR, et al. Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nat Genet. 2000;25(4):419–22. doi: 10.1038/78107. [DOI] [PubMed] [Google Scholar]
- 27.Ermakov S, et al. Family-based association study of ROR2 polymorphisms with an array of radiographic hand bone strength phenotypes. Osteoporos Int. 2007 doi: 10.1007/s00198-007-0401-5. [DOI] [PubMed] [Google Scholar]
- 28.Nomi M, et al. Loss of mRor1 enhances the heart and skeletal abnormalities in mRor2-deficient mice: redundant and pleiotropic functions of mRor1 and mRor2 receptor tyrosine kinases. Mol Cell Biol. 2001;21(24):8329–35. doi: 10.1128/MCB.21.24.8329-8335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeChiara TM, et al. Ror2, encoding a receptor-like tyrosine kinase, is required for cartilage and growth plate development. Nat Genet. 2000;24(3):271–4. doi: 10.1038/73488. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi S, et al. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes Cells. 2000;5(1):71–8. doi: 10.1046/j.1365-2443.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- 31.Schwabe GC, et al. Ror2 knockout mouse as a model for the developmental pathology of autosomal recessive Robinow syndrome. Dev Dyn. 2004;229(2):400–10. doi: 10.1002/dvdy.10466. [DOI] [PubMed] [Google Scholar]
- 32.Raz R, et al. The mutation ROR2W749X, linked to human BDB, is a recessive mutation in the mouse, causing brachydactyly, mediating patterning of joints and modeling recessive Robinow syndrome. Development. 2008 doi: 10.1242/dev.015149. [DOI] [PubMed] [Google Scholar]
- 33.Baskar S, et al. Unique Cell Surface Expression of Receptor Tyrosine Kinase ROR1 in Human B-Cell Chronic Lymphocytic Leukemia. Clin Cancer Res. 2008;14(2):396–404. doi: 10.1158/1078-0432.CCR-07-1823. [DOI] [PubMed] [Google Scholar]
- 34.Fukuda T, et al. Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0712148105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacKeigan JP, Murphy LO, Blenis J. Sensitized RNAi screen of human kinases and phosphatases identifies new regulators of apoptosis and chemoresistance. Nat Cell Biol. 2005;7(6):591–600. doi: 10.1038/ncb1258. [DOI] [PubMed] [Google Scholar]
- 36.DeChiara TM, et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85(4):501–12. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- 37.Zinovyeva AY, et al. Complex Network of Wnt Signaling Regulates Neuronal Migrations During Caenorhabditis elegans Development. Genetics. 2008;179(3):1357–71. doi: 10.1534/genetics.108.090290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forrester WC, Garriga G. Genes necessary for C. elegans cell and growth cone migrations. Development. 1997;124(9):1831–43. doi: 10.1242/dev.124.9.1831. [DOI] [PubMed] [Google Scholar]
- 39.Forrester WC, Kim C, Garriga G. The Caenorhabditis elegans Ror RTK CAM-1 inhibits EGL-20/Wnt signaling in cell migration. Genetics. 2004;168(4):1951–62. doi: 10.1534/genetics.104.031781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Francis MM, et al. The Ror receptor tyrosine kinase CAM-1 is required for ACR-16- mediated synaptic transmission at the C. elegans neuromuscular junction. Neuron. 2005;46(4):581–94. doi: 10.1016/j.neuron.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 41.Paganoni S, Ferreira A. Expression and subcellular localization of Ror tyrosine kinase receptors are developmentally regulated in cultured hippocampal neurons. J Neurosci Res. 2003;73(4):429–40. doi: 10.1002/jnr.10674. [DOI] [PubMed] [Google Scholar]
- 42.Paganoni S, Ferreira A. Neurite extension in central neurons: a novel role for the receptor tyrosine kinases Ror1 and Ror2. J Cell Sci. 2005;118(Pt 2):433–46. doi: 10.1242/jcs.01622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paganoni S, Anderson KL, Ferreira A. Differential subcellular localization of Ror tyrosine kinase receptors in cultured astrocytes. Glia. 2004;46(4):456–66. doi: 10.1002/glia.20023. [DOI] [PubMed] [Google Scholar]
- 44.Kim C, Forrester WC. Functional analysis of the domains of the C elegans Ror receptor tyrosine kinase CAM-1. Dev Biol. 2003;264(2):376–90. doi: 10.1016/j.ydbio.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Green JL, Inoue T, Sternberg PW. The C. elegans ROR receptor tyrosine kinase, CAM-1, non-autonomously inhibits the Wnt pathway. Development. 2007;134(22):4053–62. doi: 10.1242/dev.005363. [DOI] [PubMed] [Google Scholar]
- 46.Billiard J, et al. The orphan receptor tyrosine kinase Ror2 modulates canonical Wnt signaling in osteoblastic cells. Mol Endocrinol. 2005;19(1):90–101. doi: 10.1210/me.2004-0153. [DOI] [PubMed] [Google Scholar]
- 47.Schambony A, Wedlich D. Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell. 2007;12(5):779–92. doi: 10.1016/j.devcel.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 48.Yamaguchi TP, et al. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126(6):1211–23. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- 49.Oishi I, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8(7):645–54. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 50.Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8(2):126–38. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto S, et al. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell. 2008;15(1):23–36. doi: 10.1016/j.devcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Green JL, Inoue T, Sternberg PW. Opposing Wnt pathways orient cell polarity during organogenesis. Cell. 2008 doi: 10.1016/j.cell.2008.06.026. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishita M, et al. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol. 2006;175(4):555–62. doi: 10.1083/jcb.200607127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamamoto H, et al. Wnt5a modulates glycogen synthase kinase 3 to induce phosphorylation of receptor tyrosine kinase Ror2. Genes Cells. 2007;12(11):1215–23. doi: 10.1111/j.1365-2443.2007.01128.x. [DOI] [PubMed] [Google Scholar]
- 55.Nomachi A, et al. Receptor tyrosine kinase Ror2 mediates Wnt5a-induced polarized cell migration by activating c-Jun N-terminal kinase via actin-binding protein filamin A. J Biol Chem. 2008 doi: 10.1074/jbc.M802325200. [DOI] [PubMed] [Google Scholar]
- 56.Akbarzadeh S, et al. The deleted in brachydactyly B domain of ROR2 is required for receptor activation by recruitment of Src. PLoS ONE. 2008;3(3):e1873. doi: 10.1371/journal.pone.0001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Molenaar M, et al. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86(3):391–9. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 58.van de Wetering M, et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88(6):789–99. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- 59.Li C, et al. Ror2 modulates the canonical Wnt signaling in lung epithelial cells through cooperation with Fzd2. BMC Mol Biol. 2008;9:11. doi: 10.1186/1471-2199-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4(4):e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y, et al. Homo-dimerization of Ror2 Tyrosine Kinase Receptor Induces 14 3-3{beta} Phosphorylation and Promotes Osteoblast Differentiation and Bone Formation. Mol Endocrinol. 2007 doi: 10.1210/me.2007-0323. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y, et al. Wnt5a induces homodimerization and activation of Ror2 receptor tyrosine kinase. J Cell Biochem. 2008 doi: 10.1002/jcb.21848. [DOI] [PubMed] [Google Scholar]
- 63.Liu Y, et al. The orphan receptor tyrosine kinase Ror2 promotes osteoblast differentiation and enhances ex vivo bone formation. Mol Endocrinol. 2007;21(2):376–87. doi: 10.1210/me.2006-0342. [DOI] [PubMed] [Google Scholar]
- 64.Matsuda T, et al. The receptor tyrosine kinase Ror2 associates with the melanoma-associated antigen (MAGE) family protein Dlxin-1 and regulates its intracellular distribution. J Biol Chem. 2003;278(31):29057–64. doi: 10.1074/jbc.M302199200. [DOI] [PubMed] [Google Scholar]
- 65.Kani S, et al. The receptor tyrosine kinase Ror2 associates with and is activated by casein kinase Iepsilon. J Biol Chem. 2004;279(48):50102–9. doi: 10.1074/jbc.M409039200. [DOI] [PubMed] [Google Scholar]
- 66.Sammar M, et al. Modulation of GDF5/BRI-b signalling through interaction with the tyrosine kinase receptor Ror2. Genes Cells. 2004;9(12):1227–38. doi: 10.1111/j.1365-2443.2004.00799.x. [DOI] [PubMed] [Google Scholar]
- 67.Lehmann K, et al. A new subtype of brachydactyly type B caused by point mutations in the bone morphogenetic protein antagonist NOGGIN. Am J Hum Genet. 2007;81(2):388–96. doi: 10.1086/519697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hubbard SR, Till JH. Protein tyrosine kinase structure and function. Annu Rev Biochem. 2000;69:373–98. doi: 10.1146/annurev.biochem.69.1.373. [DOI] [PubMed] [Google Scholar]
- 69.Forrester WC. The Ror receptor tyrosine kinase family. Cell Mol Life Sci. 2002;59(1):83–96. doi: 10.1007/s00018-002-8407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang H, et al. Heterodimerization of Msx and Dlx homeoproteins results in functional antagonism. Mol Cell Biol. 1997;17(5):2920–32. doi: 10.1128/mcb.17.5.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thompson JD, et al. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25(24):4876–82. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]