Abstract
Background
Patients hospitalized with an exacerbation of inflammatory bowel disease (IBD) often receive antibiotics in addition to intravenous steroids. However, their efficacy in this setting is unclear.
Aim
To ascertain if the addition of antibiotics to intravenous steroids modifies short and long-term clinical outcomes.
Methods
Our study included IBD patients hospitalized between 2009 – 2014 who received intravenous (IV) steroids with or without adjuvant antibiotics. Outcomes of interest included length of stay, need for medical and surgical rescue therapy during the hospitalization, and at 90 and 365 days. A meta-analysis of previously published randomized trials was additionally performed.
Results
A total of 354 patients were included (145 ulcerative colitis (UC); 209 Crohn’s disease (CD)). In CD, combination of IV steroids and antibiotics did not change need for in-hospital medical rescue therapy, surgery or hospitalizations at 1 year but was associated with greater LOS (6.1 vs. 4.6 days, p=0.02). In UC, patients receiving antibiotics were less likely to require in-hospital medical rescue therapy (Odds ratio (OR) 0.42, 95% confidence interval (CI) 0.19 – 0.93) but experienced no statistically significant differences in LOS, in-hospital surgery, re-hospitalizations or surgery by 1 year. A meta-analysis of 3 relevant randomized trials demonstrated no difference in clinical improvement with antibiotics over placebo (OR 1.08, 95% CI 0.50 – 2.32).
Conclusions
The addition of antibiotics to intravenous steroids for treatment of IBD exacerbations was associated with a reduced need for in-hospital medical rescue therapy in UC without significant long-term benefit and did not affect short- or long-term outcomes in CD.
Keywords: Crohn’s disease, ulcerative colitis, antibiotics, hospitalization, surgery, steroids
INTRODUCTION
Inflammatory bowel diseases (IBD) (Crohn’s disease [CD] or ulcerative colitis [UC]) are chronic immunologically mediated diseases of the gastrointestinal tract that are rising in incidence worldwide and are associated with significant morbidity 1, 2. Up to two-thirds of patients with CD and 1 in 10 patients with UC will require at least one surgery for management of their refractory disease or related complications3–5. A larger proportion of patients will require at least one hospitalization for management of their disease and represent a subgroup at a particularly high risk of adverse outcomes3, 6. Advances in IBD therapies aimed toward immunologic modulation have improved our ability to achieve remission and reduce surgical procedures and hospitalizations7. However, patients continue to be hospitalized at an increasing rate 8, 9. Furthermore, temporal declines in the need for definitive surgical management of patients with CD or UC appear to have been achieved primarily in patients with moderate severity of disease 10. Those with the greatest severity requiring hospitalization have experienced more modest or no reduction in the need for surgical intervention despite the growing availability of biologic agents, emphasizing the importance of optimizing treatment protocols for management of such patients 11.
The cornerstone of treatment of hospitalized patients with severe UC or CD involves administration of intravenous steroids11, 12. Responders are transitioned to oral steroids with optimization of their maintenance regimen. Non-responders often require initiation of rescue medications, either anti-tumor necrosis factor (anti-TNF) biologics or cyclosporine, or undergo definitive surgery for refractory disease12. Antibiotics that target gastrointestinal flora, typically ciprofloxacin and metronidazole, are often given concurrently with steroids to further facilitate symptom resolution 13. However, the evidence behind this practice is limited. Several trials and recent meta-analyses of patients with both CD and UC showed a modest benefit for antibiotics 14–16. However, these studies almost exclusively included patients treated in the outpatient setting with variable use of steroids. The few randomized controlled trials performed in hospitalized inpatients suggested no benefit but were limited by a sample size of fewer than twenty-five patients in each arm 17–19. On the other hand, inappropriate use of antibiotics may increase risk for outcomes such as Clostridium difficile (C. difficile) infection. Consequently, there is a need to examine the potential benefit (or lack thereof) of antibiotics in the management of hospitalized IBD patients.
We performed this retrospective observational study of IBD exacerbations requiring hospitalization to (1) compare in-hospital outcomes including need for rescue therapy or surgery between patients receiving both intravenous steroids and antibiotics compared to those receiving steroids alone; (2) compare short and long-term outcomes at 90 days and 1 year between the two groups; and (3) perform a meta-analysis of existing randomized controlled trial (RCT) data on the role of adjuvant antibiotics in the hospitalized IBD patient.
METHODS
Study Population
This was a retrospective observational cohort study of patients hospitalized for a diagnosis of UC or CD at a single tertiary referral center, Massachusetts General Hospital (MGH), serving over 3 million patients in the Greater Boston metropolitan area. Eligible patients were identified using the Partners Research Patient Data Registry (RPDR)20. As previously described, this is a comprehensive data warehouse of all patients receiving inpatient or outpatient care at MGH, or any of the other Partners healthcare-affiliated hospitals, and is continually populated with information from administrative sources including billing, radiology, endoscopy, inpatient stays and procedures21, 22. For the purpose of this study, a preliminary screen identified all patients admitted between 2009 and 2014 with a diagnosis of UC (International Classification of Diseases, 9th Edition, clinical modification (ICD-9-CM) 556.x) or CD (ICD-9-CM 555.x). Manual chart review of all patients by two study authors (V.G. and R.R.) was performed to identify hospitalizations related to acute exacerbations of CD or UC who received intravenous steroids. This group was further stratified into those receiving antibiotics (defined as receiving antibiotics for at least 24 hours in conjunction with intravenous steroids) or those receiving steroids alone. Patients were excluded if they were found to have (i) Clostridium difficile infection within 48hrs of admission, (ii) other documented sources of infection such as pneumonia, urinary tract infection, or bacteremia for which antibiotics were started, (iii) high fevers (defined as a temperature greater than 101.0F) and (iv) suspicion for sepsis (no other documented source or localizing symptom with concern for microbial translocation across inflamed bowel wall) (iv) no use of intravenous steroids or hospitalization for an alternate indication; (v) CD with imaging showing intra-abdominal abscess, phlegmon, or fluid collection; or (vi) active perianal CD.
Variables and outcomes
The electronic medical records of included patients underwent further review for data acquisition. Covariates extracted included age, gender, IBD type, disease extent (in UC) and disease location and behavior (CD) according to the Montreal classification. Upper gastrointestinal (GI) tract or perianal involvement in CD was noted, as well. Information was also obtained about prior medical and surgical treatments for their IBD including use of immunomodulators or biologics at the time of admission. As a measure of severity of the episode, data was extracted about baseline laboratories values (within 24 hours of admission) of hemoglobin, albumin, white blood cell count, platelet count, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) where available.
The primary outcomes of interest were need for in-hospital rescue therapy (new initiation of infliximab, adalimumab, certolizumab pegol, or cyclosporine), in-hospital surgery, and length of stay. Secondary outcomes included re-hospitalization at 90 days and 1 year, IBD-related surgery at 90 days and 1 year, and therapy escalation (immunomodulator or new biologic) at 180 days.
Statistical Analysis
All data was analyzed using Stata 13.2 (Stata Corp, College Station, TX). Continuous variables were summarized using means and standard deviations while categorical variables were expressed as proportions and compared using the chi-square test (with Fisher’s exact test applied where appropriate). To reduce the bias introduced by repeat hospitalizations for the same patient during the study period, for our primary analysis, only index hospitalizations were considered. However, in a sensitivity analysis, we included all hospitalizations irrespective of re-admissions. Univariate logistic (or linear for outcome of LOS) regression was performed to identify predictors of each of our primary outcomes; variables significant at p < 0.2 in this analysis were carried forward into a multivariable regression model where a two-sided p-value < 0.05 indicated independent statistical significance.
As the decision to initiate antibiotics was non-random and could be skewed by severity, we performed a sensitivity analysis introducing a propensity score. This score estimated the likelihood of a patient receiving antibiotics based on previous disease characteristics including use of immunomodulators or biologics, and admission laboratory values. This score was then introduced as a covariate in the multivariable model to determine if there was an independent effect of adjuvant antibiotics. Institutional Review Board approval was obtained from the Partners Healthcare Human Subjects Research Committee.
Meta-analysis
A systematic search of eligible randomized controlled trials (RCT) examining the effect of antibiotics in conjunction with intravenous corticosteroids in IBD was performed on MEDLINE from inception to June 2015 with the search terms “ulcerative colitis” or “Crohn’s disease” or “inflammatory bowel disease” AND “corticosteroids” AND “antibiotics” or “ciprofloxacin” or “metronidazole”. Review of all such identified abstracts was performed independently by two of the study authors (A.N.A and V.G) and eligible articles were identified for full text review. In addition, references of review articles, position statements, and guidelines were scrutinized for additional eligible studies. From eligible RCTs thus identified, we extracted information on number of patients in each arm (steroids+antibiotics vs. steroids alone), and the proportion responding to therapy or requiring surgical or medical rescue therapy. A fixed effects model (due to no significant heterogeneity between studies) was used to determine pooled effect size and 95% confidence intervals (CI) according to the methods of Der-Simonian and Laird23.
RESULTS
Study Population
An initial search during the 6-year period encompassing 2009 to 2014 resulted in 929 unique patients who had an ICD-9-CM diagnosis code for IBD and had received IV steroids. Applying inclusion and exclusion criteria to these patients resulted in 354 unique patients, spanning 552 different admissions. Of these, 145 patients had a diagnosis of UC and 209 with CD. A smaller proportion of UC patients received adjuvant antibiotics in combination with steroids (39%) when compared to CD (63%, p < 0.01). Nearly all the patients (97%) received intravenous methylprednisolone (typically 40–60mg daily) with the rest receiving intravenous hydrocortisone. The most frequently used antibiotics were ciprofloxacin, metronidazole, or both in combination in 90% of CD patients and 97% of UC patients in the antibiotic arm. Fewer than 5% of patients received ampicillin, gentamycin, oral vancomycin, or other antibiotics.
Table 1 compares the baseline characteristics of patients who received antibiotics and steroids compared to those who received steroids alone. Among those with CD, there was a trend towards younger age and more women in the steroids-antibiotics group. One in five patients in each group had isolated colonic CD. A larger proportion in the steroids-antibiotics group had inflammatory phenotype (48% vs. 28%, p=0.02) and nearly half the patients in both groups had previously received biologics. Among patients with UC, there was no difference in age or gender between the two groups, and the majority of patients in the steroids or combined steroids-antibiotics group had pancolitis (53% vs. 63%, p=0.4). Approximately one-third of UC patients in each group had previously received biologic therapy at the time of admission. There was no statistically significant difference in the baseline laboratory values between the two groups for either disease except for a lower platelet count in UC patients receiving steroids alone compared to steroids-antibiotics (407,300 vs. 513,800/mm3, p < 0.01) (Table 2).
Table 1.
Characteristics of included patients, hospitalized for exacerbations of Crohn’s disease or ulcerative colitis, receiving intravenous corticosteroids
| Characteristic | Steroids Alone | Steroids + Antibiotics | P-value |
|---|---|---|---|
| Ulcerative Colitis | |||
| Patients, n | 88 | 57 | |
| Mean Age (in years) | 35.0 | 35.6 | 0.87 |
| Female, % | 55.7 | 59.7 | 0.64 |
| Extent, % | 0.45 | ||
| Limited colitis | 46.6 | 36.8 | |
| Pancolitis | 53.4 | 63.2 | |
| Previous Therapy, % | |||
| 5-ASA | 90.9 | 84.2 | 0.22 |
| Biologics | 37.5 | 29.8 | 0.34 |
| Immunomodulators | 45.5 | 36.8 | 0.31 |
| Current Therapy % | |||
| 5-ASA | 54.5 | 49.1 | 0.48 |
| Biologics | 21.6 | 17.5 | 0.56 |
| Immunomodulators | 18.1 | 15.9 | 0.76 |
| Crohn’s Disease | |||
| Patients, n | 78 | 131 | |
| Mean age (in years) | 35.1 | 30.2 | 0.06 |
| Female, % | 43.6 | 56.5 | 0.07 |
| Location, % | 0.61 | ||
| Ileocolic | 51.3 | 55.7 | |
| Ileal | 30.8 | 24.4 | |
| Colonic | 18.0 | 19.9 | |
| Upper GI, % | 12.8 | 9.9 | 0.52 |
| Perianal, % | 28.2 | 24.4 | 0.55 |
| Behavior, % | 0.02 | ||
| Inflammatory | 28.1 | 48.1 | |
| Stricturing | 37.2 | 27.5 | |
| Penetrating | 34.6 | 24.4 | |
| Previous Surgeries, % | 39.7 | 29.8 | 0.14 |
| Previous Therapy, % | |||
| 5-ASA | 75.6 | 69.5 | 0.34 |
| Biologics | 47.4 | 47.3 | 0.99 |
| Immunomodulators | 68.0 | 58.0 | 0.15 |
| Current Therapy % | |||
| 5-ASA | 23.1 | 26.0 | 0.63 |
| Biologics | 28.2 | 26.7 | 0.88 |
| Immunomodulators | 29.5 | 18.3 | 0.07 |
Table 2.
Admission laboratory characteristics of included patients hospitalized for Crohn’s disease or ulcerative colitis exacerbations
| Laboratory test |
Ulcerative Colitis | Crohn’s Disease | ||||
|---|---|---|---|---|---|---|
| Steroids Alone |
Steroids + Antibiotics |
P-value | Steroids Alone |
Steroids + Antibiotics |
P-value | |
| Hemoglobin (in g/dL) | 11.5 | 11.4 | 0.85 | 12.6 | 12.2 | 0.28 |
| WBC Count (/mm3) | 11.3 | 12.5 | 0.15 | 9.7 | 10.6 | 0.09 |
| Platelets (in thousands/mm3) | 407.3* | 513.8* | 0.001 | 396.2 | 413.3 | 0.47 |
| Albumin (in g/dL) | 4.0 | 3.4 | 0.30 | 3.9 | 3.7 | 0.09 |
| ESR (in mm) | 36.9 | 39.3 | 0.62 | 31.9 | 35.2 | 0.45 |
| CRP (in mg/L) | 46.2 | 63.5 | 0.12 | 47.2 | 58.8 | 0.20 |
P-value <0.05
Outcomes – Crohn’s disease
There was no difference in need for in-hospital rescue medical therapy (15% vs. 14%, p=0.82) between the groups receiving or not receiving adjuvant antibiotics. Though numerically greater, there was no statistically significant difference in the rate of in-hospital surgery between patients receiving steroids-antibiotics (6%) compared to those receiving steroids alone (3%, p=0.25) (Table 3). In contrast, the antibiotic group had longer hospital stay (6.1 days) when compared to those receiving steroids alone (4.6 days, p=0.02). There was also no difference in need for re-hospitalization or surgery at 90 days or 1 year between the two groups (Table 3).
Table 3.
Outcomes of Crohn’s disease patients receiving intravenous corticosteroids with or without adjuvant antibiotics
| Outcome | Steroids Alone (%) |
Steroids + Antibiotics (%) |
P-value | Multivariate adjusted odds ratio (95% CI)‡ |
|---|---|---|---|---|
| Length of stay (in days) | 4.6* | 6.1* | 0.02 | 1.27 (0.11 – 2.44) ║ # |
| In-hospital medical rescue therapy† | 14.1 | 15.3 | 0.82 | 1.04 (0.42 – 2.60) # |
| In Hospital Surgery | 2.6 | 6.1 | 0.25 | 2.55 (0.44 – 14.77) # |
| 90 Day Surgery | 23.1 | 13.0 | 0.06 | 0.60 (0.27 – 1.29) |
| 365 Day Surgery | 24.4 | 21.4 | 0.62 | 1.13 (0.55 – 2.31) |
| 90 Day Re-hospitalization | 15.4 | 14.5 | 0.86 | 0.91 (0.40 – 2.08) |
| 365 Day Re-hospitalization | 21.8 | 34.4 | 0.06 | 1.92 (0.96 – 3.82) |
| 6 Month New Biologic | 35.9 | 40.9 | 0.51 | 1.24 (0.67 – 2.29) |
| 6 Month New Immunomodulator | 15.4 | 20.8 | 0.34 | 1.38 (0.63 – 3.00) |
P-value < 0.05
Adjusted for age, gender, Crohn’s disease behavior, and need for prior surgeries
Regression co-efficient and 95% confidence intervals
in-hospital medical rescue included infliximab, adalimumab, or cyclosporine
Additionally adjusted for prior biologic therapy, admission hemoglobin, albumin, C-reactive protein, and platelet count
On multivariate analysis, adjusting for relevant confounders, adjuvant antibiotic use was not associated with need for in-hospital rescue therapy (odds ratio (OR) 1.04, 95% confidence interval (CI) 0.42 – 2.60), in-hospital surgery (OR 2.55, 95% CI 0.44 – 14.77) or IBD-related surgery at 90 days (OR 0.59, 95% CI 0.27 – 1.29) but was associated with a modest increase in length of stay (co-efficient 1.27 days, 95% CI 0.11 – 2.44 days). Receiving antibiotics was also not associated with need for new immunomodulators (OR 1.38, 95% CI 0.63 – 3.00) or biologic treatment (OR 1.24, 95% CI 0.67 – 2.29) by 6 months. There was no difference in the rate of C. difficile infections between the two groups at 1 year (0% vs. 2.3%).
Outcomes – Ulcerative Colitis
Among hospitalized UC patients, we found that patients in the steroids-antibiotic group had significantly lower utilization of in-hospital medical rescue therapies when compared to those receiving steroids alone (21% vs. 37.5%, p=0.04; adjusted OR 0.42, 95% CI 0.19 – 0.93), (Table 4). However, there was no difference in the need for in-hospital surgery (5.3% vs. 10.2%, adjusted OR 0.31, 95% CI 0.05 – 1.97), or length of stay. There was also no difference in surgery at 90 days (14% vs. 24%, adjusted OR 0.57, 95% CI 0.22 – 1.50) or 1 year (19.3% vs. 29.6%, adjusted OR 0.61, 95% CI 0.24 to 1.54) or in escalation of biologic (39% vs. 42%, adjusted OR 0.85, 95% CI 0.42 – 1.74) or immunomodulator therapies at 6 months (Table 4). There was no difference in the rate of C difficile infections at 1 year (3.5% vs. 2.2%).
Table 4.
Outcomes of Ulcerative Colitis patients receiving intravenous corticosteroids with or without adjuvant antibiotics
| Outcome | Steroids Alone (%) |
Steroids + Antibiotics (%) |
P-value | Multivariate adjusted odds ratio (95% CI) |
|---|---|---|---|---|
| Length of stay (in days) | 8.8 | 7.6 | 0.25 | −0.22 (−2.01 – 1.57) ║ # |
| In-hospital medical rescue therapy† | 37.5* | 21.0* | 0.04 | 0.42 (0.19 – 0.93) # |
| In Hospital Surgery | 10.2 | 5.3 | 0.29 | 0.31 (0.05 – 1.97) # |
| 90 Day Surgery | 23.9 | 14.0 | 0.15 | 0.57 (0.21 – 1.50) |
| 365 Day Surgery | 29.6 | 19.3 | 0.17 | 0.61 (0.24 – 1.54) |
| 90 Day Re-hospitalization | 15.9 | 8.8 | 0.21 | 0.55 (0.17 – 1.75) |
| 365 Day Re-hospitalization | 23.9 | 17.5 | 0.37 | 0.72 (0.30 – 1.76) |
| 6 Month New Biologic | 42.1 | 38.6 | 0.68 | 0.85 (0.42 – 1.74) |
| 6 Month New Immunomodulator | 33.0 | 26.3 | 0.40 | 0.73 (0.34 – 1.54) |
P-value < 0.05
Adjusted for age, gender, UC extent and prior biologic and immunomodulator therapies
Regression co-efficient and 95% confidence intervals
in-hospital medical rescue included infliximab, adalimumab, or cyclosporine
Additionally adjusted for admission hemoglobin, albumin, C-reactive protein, and platelet count
Sensitivity Analyses
Adjustment for likelihood of receiving antibiotics based on clinical characteristics using a propensity score was performed. The greater length of stay in the antibiotic-steroid group compared to steroids alone in CD (1.29 days, 95% CI 0.04 – 2.54) as well as the lower need for in-hospital rescue in UC patients in the steroids-antibiotic group (OR 0.38, 95% CI 0.15 – 0.99) remained significant. Additionally, administration of antibiotics along with steroids was associated with a slightly lower 90 day (OR 0.37, 95% CI 0.14 – 0.97) but not 1 year surgery rate (OR 0.83, 95% CI 0.35 – 1.96) in CD. As well, an analysis including all 552 hospitalizations irrespective of re-hospitalizations yielded similar results (data not shown). Given the different spectrums of activity of the included antibiotics, we repeated the analysis comparing those who received both ciprofloxacin and metronidazole to those who received neither. The need for in-hospital medical rescue therapy (OR 1.01, 95% CI 0.33 – 3.11) or surgery (OR 3.84, 95% CI 0.61 – 24.21) remained unchanged in CD. The association with in-hospital medical rescue therapy (OR 0.24, 95% CI 0.07 – 0.77) remained significant in UC while there was no association with need for surgery (OR 0.24, 95% CI 0.02 – 3.09).
Meta-analysis of RCTs Examining Benefit of Adjuvant Antibiotics
To further determine if adjuvant antibiotics improved outcomes for IBD patients hospitalized for an exacerbation, we performed a search of literature for RCTs in which both control and intervention groups received IV steroids. Our search strategy yielded 3 randomized controlled trials, totaling 66 patients receiving intravenous steroids alone and 67 receiving antibiotics and steroids17–19 (Supplemental Table 1). The antibiotics studied were metronidazole (2 studies)17, 18, ciprofloxacin (1 study)19, and tobramycin18 (1 study, in combination with metronidazole). The examined outcome in each trial was the need for surgery after failure of inpatient medical therapy. A pooled fixed effect meta-analysis demonstrated no benefit to adjuvant antibiotics in UC patients receiving intravenous corticosteroids (OR 1.08, 95% CI 0.50 – 2.32) with no heterogeneity between the studies (i2 = 0; p=0.958) (Figure 1).
Figure 1.
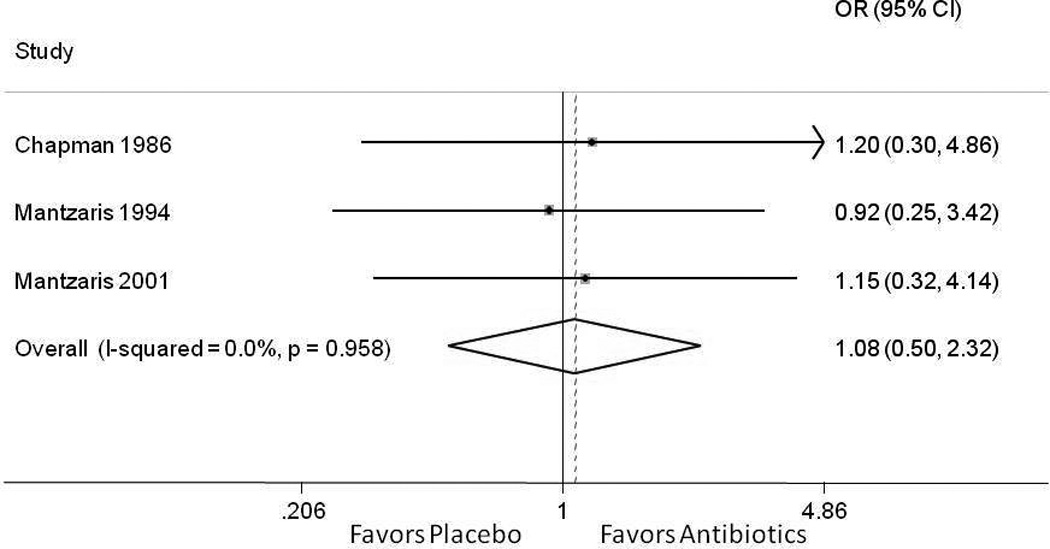
Meta-analysis of clinical response to antibiotics compared to placebo among hospitalized patients with ulcerative colitis receiving intravenous corticosteroids
DISCUSSION
Both Crohn’s disease and ulcerative colitis are prone to exacerbations that require hospitalization for treatment with intravenous corticosteroids. Adjuvant antibiotics are frequently used to treat such inflammatory exacerbations despite guidelines and expert opinions recommending the contrary12. In a large, retrospective observational cohort, we demonstrate that while in UC, adjuvant antibiotics were associated with a lower rate of in-hospital medical rescue therapy, for both diseases, use of antibiotics did not significantly modify length of stay, need for surgery or hospitalizations in the short-term or at 1 year following hospitalizations, suggesting limited role for antibiotics as an add-on therapy to intravenous corticosteroids. Complementing the results of the observational arm of our study was the meta-analysis of three previously published randomized controlled trials. Similarly, this demonstrated no benefit to antibiotics when used in conjunction with intravenous corticosteroids in acute exacerbations of UC requiring hospitalization.
There is growing recognition of the role of the microbiome in the pathogenesis of IBD. In addition, several of the genetic risk loci identified in large IBD cohorts appear to influence pathogen recognition, and the innate and adaptive immune responses to such microbiota24. This has led to continued interest in microbiome directed therapeutics in the management of both diseases, including a potential role for antibiotics. Inconsistent evidence tying mycobacterial infections (Mycobacterium paratuberculosis) to CD pathogenesis led to clinical trials of antimycobacterial therapies in the management of CD, demonstrating equivocal findings25, 26. Several recent meta-analyses examined the existing literature on the role of antibiotics in the management of UC and CD14, 15, 27–29. In one that included 1,160 patients with CD and 662 with active UC, Khan et al. concluded that antibiotics were associated with a statistically significant, albeit modest, increase in likelihood of achieving remission for both diseases14. However, there was considerable heterogeneity across the included studies in terms of patient population, concomitant therapies, and importantly, antibiotic regimens used. Also, the studies included in the Khan et al. meta-analysis were predominantly comprised of IBD exacerbations managed in the ambulatory setting and results may not be applicable to severe exacerbations requiring hospitalizations. Indeed, as shown in our study, in contrast to the nearly 2,000 patients included in the above study by Khan et al.14, only three prior RCTs have examined the role of adjuvant antibiotics in acute severe colitis, each comprising fewer than 25 patients in each arm (133 patients in total)17–19. Thus, there is a substantial gap in the literature robustly examining the role of antibiotics in hospitalized IBD patients, perhaps contributing to the wide variation in practice, highlighted by the large proportion of patients receiving antibiotics with both diseases in our study.
The main positive finding of our study was that adjuvant antibiotics were associated with a lower rate of in-hospital medical rescue therapy in UC, suggesting a possible short-term effect. Indeed, while the odds ratios numerically favored antibiotic use in in-hospital and 90-day surgery, this difference was not statistically significant. The potential difference in these short-term outcomes could have a few explanations. One, our findings may imply the existence of a subgroup of patients within UC who may benefit from antibiotic co-administration along with intravenous steroids. Secondly, medical rescue therapy initiation may also be more susceptible to provider-level variation than need for surgery in refractory colitis, correlating with antibiotic use. While the numerically superior odds ratios do warrant further consideration, given the lack of a robust, statistically significant effect on other important outcomes (length of stay, colectomy), and lack of effect in our meta-analysis, one cannot make absolute conclusions about the short-term benefits of routine antibiotic co-administration. However, some of our findings are intriguing enough to trigger further, larger studies before modifying clinical practice.
With the exception of this short-term finding in UC, there did not appear to be any significant long-term benefits of adjuvant antibiotics in UC and CD for likelihood of re-hospitalization, therapy escalation, and surgery by 1 year, suggesting a lack of durable benefit. These results are also not explained by differing severity of disease in the two groups as it remained without statistical significance on adjustment for such covariates as well as in our propensity-score adjusted models. The reason for the discrepancy between our results observed in the hospitalized setting (no effect) to that previously observed in ambulatory IBD (mild benefit) could be several. First, the severity of disease and resultant circulating inflammatory cytokine burden in hospitalized patients may overwhelm the potential benefit of modulating the microbiome through antibiotics in this setting. Second, a majority of inpatients received ciprofloxacin or metronidazole while there was variation in the antibiotics used in prior studies of ambulatory IBD patients suggesting that perhaps different antibiotics (rifamycins, antimycobacterials) may have a role. Whether antibiotics are associated with improvements in symptom scores, quality of life, or fecal inflammatory markers may be examined in future studies. However, altering the natural history of disease, such as the need for surgery, is an important threshold for any intervention to meet in order to alter practice.
There are a few implications from our results. First, our findings demonstrate that there appears to be no durable benefit to routine use of antibiotics in combination with intravenous steroids in hospitalized patients with IBD exacerbations. However, our findings remain applicable only to those with luminal inflammatory disease and do not extend to those with findings such as an inflammatory phlegmon or draining fistulae in CD. As well, our findings do not extend to those with severe systemic signs including fevers or peritoneal findings on exam. While our study did not identify any harm to the use of antibiotics in terms of increased risk for C difficile infection, larger cohorts have clearly established an association between antibiotic use and such complications30.
We readily acknowledge several limitations in our study. Ours was a retrospective observational study where use of antibiotics in conjunction with steroids was non-random at the discretion of the treating gastroenterologist or medical team. Consequently, unmeasured confounders could have influenced our findings. However, the lack of significant effect in our propensity score adjusted model lends confidence to the robustness of our findings. Second, the number of patients meeting eligibility criteria for our study may have limited statistical power for identification of an effect; though, the total number of hospitalizations included in our study is several-fold greater than prior data in the literature. Similarly, there may be specific subgroups of patients who may benefit from antibiotics; this requires larger cohorts to identify a treatment effect. Finally, we did not have information on change in disease activity indices or inflammatory markers as these were not systematically noted in all patients.
In conclusion, our retrospective observational cohort study and meta-analysis of randomized controlled trials do not support the routine use of adjuvant antibiotics to steroids in the treatment of UC or CD exacerbations that require hospitalization. However, the reduction in need for medical rescue therapy in UC associated with antibiotic use merits further study and there is a need for larger rigorous prospective studies and randomized trials to firmly guide clinical practice.
Supplementary Material
Acknowledgments
Conflicts of Interest: Ananthakrishnan has served on the scientific advisory boards for Abbvie, Exact Sciences, and Cubist pharmaceuticals and has received grant support from Cubist and Amgen. Khalili has received consultant fee from Abbvie. Yajnik has received consulting fees from NPS, Janssen Pharmaceuticals, and UCB.
Source of funding: Ananthakrishnan is supported in part by a grant from the National Institutes of Health (K23 DK097142). Khalili is supported by a career development award from the American Gastroenterological Association (AGA) and by National Institute of Diabetes and Digestive and Kidney Diseases (K23 DK099681). This work is also supported by the National Institutes of Health (NIH) (P30 DK043351) to the Center for Study of Inflammatory Bowel Diseases.
Footnotes
| Vikas Gupta | – no conflict of interest exists |
| Rodrigo Rodrigues | – no conflict of interest exists |
| Deanna D Nguyen | – no conflict of interest exists |
| Jenny Sauk | – no conflict of interest exists |
| Hamed Khalili | - Consulting fee from Abbvie |
| Vijay Yajnik | - Consulting fee from NPS, Janssen pharmaceuticals, and UCB |
| Ashwin Ananthakrishnan | - Scientific advisory board for Cubist pharmaceuticals, Abbvie, Exact Sciences |
| V Gupta | study design, data collection, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content |
| R Rodrigues | data collection, analysis and interpretation of data, critical revision of the manuscript for important intellectual content |
| D Nguyen | data collection, critical revision of the manuscript for important intellectual content. |
| J Sauk | data collection, critical revision of the manuscript for important intellectual content. |
| V Yajnik | data collection, critical revision of the manuscript for important intellectual content. |
| H Khalili | data collection, critical revision of the manuscript for important intellectual content. |
| A Ananthakrishnan | study design, data collection, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, supervision for the study |
REFERENCES
- 1.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin. Gastroenterol. Hepatol. 2007;5:1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46.e42-–54.e42-. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein CN, Loftus EV, Jr, Ng SC, et al. Hospitalisations and surgery in Crohn's disease. Gut. 2012;61:622–629. doi: 10.1136/gutjnl-2011-301397. [DOI] [PubMed] [Google Scholar]
- 4.Frolkis AD, Dykeman J, Negron ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996–1006. doi: 10.1053/j.gastro.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan GG, Seow CH, Ghosh S, et al. Decreasing colectomy rates for ulcerative colitis: a population-based time trend study. Am J Gastroenterol. 2012;107:1879–1887. doi: 10.1038/ajg.2012.333. [DOI] [PubMed] [Google Scholar]
- 6.Ananthakrishnan AN, Issa M, Beaulieu DB, et al. History of medical hospitalization predicts future need for colectomy in patients with ulcerative colitis. Inflamm Bowel Dis. 2009;15:176–181. doi: 10.1002/ibd.20639. [DOI] [PubMed] [Google Scholar]
- 7.D'Haens GR, Panaccione R, Higgins PD, et al. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn's and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol. 2011;106:199–212. doi: 10.1038/ajg.2010.392. quiz 213. [DOI] [PubMed] [Google Scholar]
- 8.Bewtra M, Su C, Lewis JD. Trends in hospitalization rates for inflammatory bowel disease in the United States. Clin. Gastroenterol. Hepatol. 2007;5:597–601. doi: 10.1016/j.cgh.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen GC, Tuskey A, Dassopoulos T, et al. Rising hospitalization rates for inflammatory bowel disease in the United States between 1998 and 2004. Inflammatory Bowel Diseases. 2007;13:1529–1535. doi: 10.1002/ibd.20250. [DOI] [PubMed] [Google Scholar]
- 10.Ananthakrishnan AN, McGinley EL, Binion DG, et al. A nationwide analysis of changes in severity and outcomes of inflammatory bowel disease hospitalizations. J Gastrointest Surg. 2011;15:267–276. doi: 10.1007/s11605-010-1396-3. [DOI] [PubMed] [Google Scholar]
- 11.Turner D, Walsh CM, Steinhart AH, et al. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007;5:103–110. doi: 10.1016/j.cgh.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 12.Pola S, Patel D, Ramamoorthy S, et al. Strategies for the care of adults hospitalized for active ulcerative colitis. Clin Gastroenterol Hepatol. 2012;10:1315–1325. e4. doi: 10.1016/j.cgh.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. YGAST. 2004;126:1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Khan KJ, Ullman TA, Ford AC, et al. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am. J. Gastroenterol. 2011;106:661–673. doi: 10.1038/ajg.2011.72. [DOI] [PubMed] [Google Scholar]
- 15.Rahimi R, Nikfar S, Rezaie A, et al. A meta-analysis of antibiotic therapy for active ulcerative colitis. Dig. Dis. Sci. 2007;52:2920–2925. doi: 10.1007/s10620-007-9760-1. [DOI] [PubMed] [Google Scholar]
- 16.Wang S-L, Wang Z-R, Yang C-Q. Meta-analysis of broad-spectrum antibiotic therapy in patients with active inflammatory bowel disease. Exp Ther Med. 2012;4:1051–1056. doi: 10.3892/etm.2012.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman RW, Selby WS, Jewell DP. Controlled trial of intravenous metronidazole as an adjunct to corticosteroids in severe ulcerative colitis. Gut. 1986;27:1210–1212. doi: 10.1136/gut.27.10.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantzaris GJ, Hatzis A, Kontogiannis P, et al. Intravenous Tobramycin and Metronidazole as a Adjunct to Corticosteroids in Acute, Severe Ulcerative Colitis. Am. J. Gastroenterol. 1994;89:43–46. [PubMed] [Google Scholar]
- 19.Mantzaris GJ, Petraki K, Archavlis E, et al. A Prospective Randomized Controlled Trial of Intravenous Ciprofloxacin as an Adjunct to Corticosteroids in Acute, Severe Ulcerative Colitis. Scand J Gastroenterol. 2001;36:971–974. doi: 10.1080/003655201750305503. [DOI] [PubMed] [Google Scholar]
- 20.Nalichowski R, Keogh D, Chueh HC, et al. Calculating the benefits of a Research Patient Data Repository. AMIA Annu Symp Proc. 2006:1044. [PMC free article] [PubMed] [Google Scholar]
- 21.Desai A, Zator ZA, de Silva P, et al. Older age is associated with higher rate of discontinuation of anti-TNF therapy in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:309–315. doi: 10.1002/ibd.23026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shelton E, Chaudrey K, Sauk J, et al. New onset idiosyncratic liver enzyme elevations with biological therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:972–979. doi: 10.1111/apt.13159. [DOI] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bach H. What Role Does Mycobacterium avium subsp. paratuberculosis Play in Crohn's Disease? Curr Infect Dis Rep. 2015;17:463. doi: 10.1007/s11908-015-0463-z. [DOI] [PubMed] [Google Scholar]
- 26.Selby W, Pavli P, Crotty B, et al. Two-Year Combination Antibiotic Therapy With Clarithromycin, Rifabutin, and Clofazimine for Crohn’s Disease. Gastroenterology. 2007;132:2313–2319. doi: 10.1053/j.gastro.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 27.Rahimi R, Nikfar S, Rezaie A, et al. A meta-analysis of broad-spectrum antibiotic therapy in patients with active Crohn's disease. Clin Ther. 2006;28:1983–1988. doi: 10.1016/j.clinthera.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Su JW, Ma JJ, Zhang HJ. Use of antibiotics in patients with Crohn's disease: a systematic review and meta-analysis. J Dig Dis. 2015;16:58–66. doi: 10.1111/1751-2980.12216. [DOI] [PubMed] [Google Scholar]
- 29.Wang SL, Wang ZR, Yang CQ. Meta-analysis of broad-spectrum antibiotic therapy in patients with active inflammatory bowel disease. Exp Ther Med. 2012;4:1051–1056. doi: 10.3892/etm.2012.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ananthakrishnan AN, Issa M, Binion DG. Clostridium difficile and inflammatory bowel disease. Med Clin North Am. 2010;94:135–153. doi: 10.1016/j.mcna.2009.08.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


