Abstract
Background
Knock-in mice provide useful models of congenital and age-related cataracts caused by α-crystallin mutations. R49C αA-crystallin and R120G αB-crystallin mutations are linked with hereditary cataracts. Knock-in αA-R49C+/− heterozygotes develop cataracts by 1–2 months, whereas homozygote mice have cataracts at birth. The R49C mutation drastically reduces lens protein water solubility and causes cell death in knock-in mouse lenses. Mutant crystallin cannot function as a chaperone, which leads to protein aggregation and lens opacity. Protein aggregation disrupts the lens fiber cell structure and normal development and causes cell death in epithelial and fiber cells. We determined what aspects of the wild-type phenotype are agedependently altered in the mutant lens.
Methods
Wild-type, heterozygote (αA-R49C+/−), and homozygote (αA-R49C+/+) mouse lenses were assessed pre- and postnatally for lens morphology (electron microscopy, immunohistochemistry), and autophagy or unfolded protein response markers (immunoblotting).
Results
Morphology was altered by embryonic day 17 in R49C+/+ lenses; R49C+/− lens morphology was unaffected at this stage. Active autophagy in the lens epithelium of mutant lenses was indicated by the presence of autophagosomes using electron microscopy. Protein p62 levels, which are degraded specifically by autophagy, increased in αA-R49C mutant versus wild-type lenses, suggesting autophagy inhibition in the mutant lenses. The unfolded protein response marker XBP-1 was upregulated in adult lenses of αB-R120G+/+ mice, suggesting its role in lens opacification.
Conclusions
Mutated crystallins alter lens morphology, autophagy, and stress responses.
General Significance
Therapeutic modulation of autophagic pathways may improve protein degradation in cataractous lenses and reduce lens opacity.
Keywords: cataract, crystallin, mutation, knock-in mouse, unfolded protein response, autophagy
1. Introduction
The crystallin protein family accounts for 90% of lens proteins and plays a key role in lens transparency [1,2]. Point mutations in the genes encoding α-, β-, and γ-crystallins cause hereditary human cataract formation at birth or at an early age [3–5]. Alphacrystallin is an oligomer of two polypeptides, αA- and αB-crystallin, that are expressed in lens epithelial and fiber cells. Human patients harboring single point mutations in αA- and αB-crystallin genes develop hereditary cataracts [6–8]. The distribution of α- crystallin in human lenses changes with aging [9,10]. There is strong correlation between loss of α-crystallin from the lens soluble protein fraction and increased light scattering and lens opacification in human cataracts [9]. Genetically defined cataracts are attractive model systems for studying the mechanisms of cataract formation. Age-related cataracts are very heterogeneous and multifactorial in origin. Cataracts caused by specific mutations in crystallin genes occur earlier in life and can be studied in animal models. Cataract-causing mutations identified in human αA-crystallin include the R49C [11], R116C [8], R116H [12], G98R [13], R54L [14], R21Q [15], and W9X mutations [16]. The αA-R49C mutation causes protein aggregation, cell death, and mislocalization of the mutant protein to the cellular nucleus. The mutant protein also forms crosslinks with other crystallins in vitro. Mutations in the αB-crystallin gene that cause human cataracts include the R120G, R11H, and P20S mutations [7,17,18]. The αB-R120G mutant protein also forms high-molecular weight aggregates, and knock-in mice have been generated to understand the mechanism of cataract formation by this mutant [19].
Studies in the past two decades have demonstrated the importance of genetic factors in the etiology of age-related cataract [20,21]. Functional studies of hereditary cataract formation in animal models can improve our understanding of the etiology of age-related cataracts [22,23]. To understand disease etiology in hereditary cataracts, we have used embryonic stem cell-based technologies to generate knock-in mice expressing α-crystallin proteins containing either the αA-R49C or αB-R120G mutation. These two mutations are associated with human autosomal dominant hereditary cataracts. Point mutation gene knock-in mice have a single-nucleotide mutation in a gene that does not ablate the gene but merely changes its function. Knock-in mice offer the advantage of comparing wild type, heterozygous, and homozygous mice, and permit studying the effects of gene dosing in vivo. Knock-in mice have been used to study the role of specific mutations in connexin and αA- and αB-crystallin genes that cause cataract pathology [19,24,25].
2. Materials and Methods
Experiments using mice were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research. All animal procedures were performed by the Washington University Mouse Genetics Core, following protocols approved by the Washington University Animals Studies Committee. Mice were euthanized via CO2 asphyxiation followed by cervical dislocation. For neonatal mice, decapitation was used. Eyes were enucleated and lenses were extracted on ice in Dulbecco’s phosphate-buffered saline (PBS) containing protease inhibitors (1:1000 vol/vol of protease inhibitor cocktail; Sigma-Aldrich, St. Louis, MO). Lenses were manually homogenized to extract water-soluble proteins, and homogenates were centrifuged at 20,800 × g for 30 min to separate soluble and insoluble fractions. After withdrawing the soluble supernatant fraction, the remaining protein pellet was dissolved in 1x PBS containing 8M urea. Bradford assays provided sample protein concentrations for uniform gel loading. Western blotting used primary antibodies against β-actin (Sigma A1978), p62 (Sigma P0067), and XBP-1 (Santa Cruz Biotech), at dilutions of 1:500–1:1000 in either Odyssey Blocking Buffer (LI-COR, Inc., Lincoln, NE) or 5% non-fat dried milk in Tris-buffered saline. After washing, IRDye secondary antibodies (LI-COR) were used to image the blots on an Odyssey SA Infrared Imaging System (LI-COR); band intensities were quantified using Odyssey 3.0 software. Histological and immunocytochemical analysis was performed on lens sections according to methods described previously [26,27]. Transmission electron microscopy was performed by our Molecular Microbiology Imaging Core facility [27].
3. Results and Discussion
3.1 αA-crystallin mutation affects embryonic histology at embryonic day 17
Human patients heterozygous for the αA-R49C mutation in αA-crystallin develop cataracts at birth or during infancy [11]. The αA-R49C heterozygous mice that mimic the heterozygosity of human patients have one mutant allele and one wild-type allele. Knock-in heterozygote mice exhibit minor lens defects by 2 months of age, including reduced protein solubility and increased lens opacification. Homozygous αA-R49C knock-in mice that express only mutant αA-crystallin have more serious lens defects that are present at birth. Comparison of wild-type, heterozygous, and homozygous lenses allow us to study gene-dosage effects. We initially focused on αA-R49C homozygous mice, where the changes are expected to be more readily observable [26,28,29].
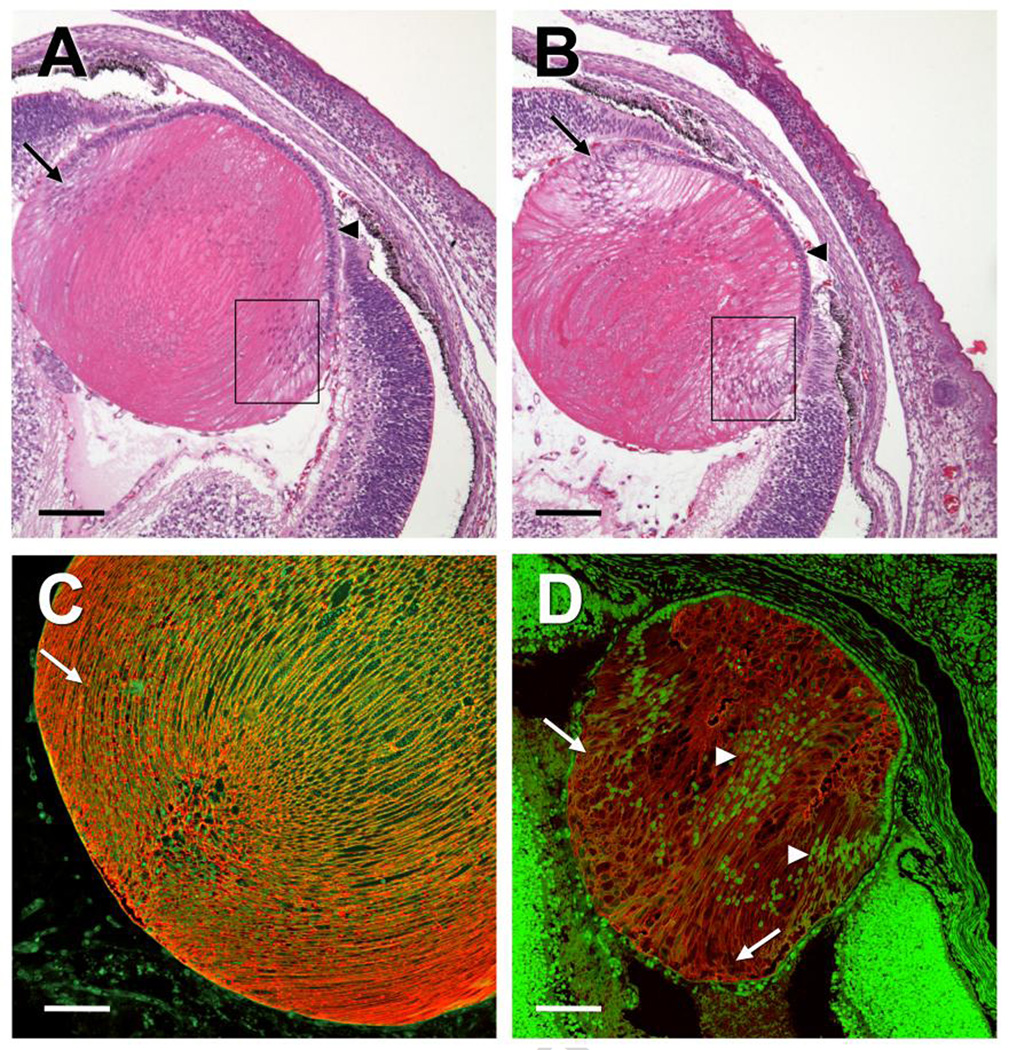
To understand the mechanism of cataract formation, it is necessary to investigate the earliest changes that occur during lens development in the presence of the mutant αA-R49C protein. Mice underwent timed pregnancy, with embryos extracted at different times after conception. The αB-crystallin gene is expressed very early during mouse development, with transcripts present in the lens placode at embryonic day (E) 9.5; αA-crystallin transcripts appear at E10.5 [30]. We determined what aspects of the wild-type phenotype are lost in the mutant lens, and tested whether these changes occur soon after mutant αA-crystallin protein is expressed in the embryonic mouse lens. Thus, we hypothesized that cataracts will develop on, or after, E11.5. αA-crystallin transcripts first appear at E10.5 in the mouse lens, and αA-crystallin is strongly expressed at E 12.5 [30,31]. Figure 1 shows the histology of wild-type and αA-R49C knock-in homozygous mutant mouse lenses at E17. Increased vacuole formation in the anterior region and loss of regular fiber cell morphology was first evident in homozygote mutants at E17. By birth, the morphology was significantly disrupted in anterior, posterior, and equatorial regions of the lens. The histology of heterozygous knock-in mutant mice appeared normal in embryonic development (not shown). Taken together, our findings indicate that mutated αA-crystallin affects lens development and homeostasis a short time after the mutant protein is expressed in vivo.
Figure 1.
Morphology of lenses from embryonic and newborn mice. Wild type and αA-R49C knock-in mutant mouse eyes were analyzed by hematoxylin/eosin staining (A, B) and aquaporin-0 immunofluorescence (C, D). Eyes from embryonic day 17 (E17) mice showed normal lens morphology (A), whereas those from αA-R49C homozygous mutants (B) have an aberrant morphology and uneven staining of cortical fiber cells (box), and increased vacuole formation in the fiber cells of the equatorial (arrows) and anterior region (arrowheads) of the lens. C) Immunofluorescence of aquaporin-0 expression in lens fiber cells of Day 0 (P0) mouse lenses. Note the regular morphology of fiber cells in the nuclear and posterior regions. (D) αA-R49C homozygous knock-in mutant mouse lenses showed irregularly shaped fiber cell morphology and a disrupted alignment of lens fiber cells. The nuclei (green) appear to accumulate in the posterior and nuclear regions in the homozygous mutant lenses (arrowheads). Red = Aquaporin-0 immunofluorescence. Green = DRAQ5. Scale bar = 150 µm (A, B, D), 100 µm (C).
3.2 Autophagy in knock-in αA-crystallin mutant mouse lenses
Autophagy is a result of signaling pathways in which protein substrates targeted for degradation are packaged into inclusion bodies and engulfed by double-membrane organelles called autophagosomes [32–34]. Autophagosomes fuse with lysosomes and damaged proteins are then degraded by lysosomal proteases. We previously detected autophagosomes in lens epithelial cells of αB-R120G knock-in mutant mice [27]. Our data further demonstrated an increase in the size of the punctate bodies containing the protein p62, a specific marker for autophagy, suggesting that the mutation affects protein degradation [27]. These findings strongly suggest inhibition of autophagic degradation in the αB-R120G mutant [23]. Similar dysfunctional autophagy has been linked to increased accumulation of aggregated αB-R120G protein in cardiomyocytes [35,36].
The role of autophagy in lens function was suggested by the discovery of human cataracts associated with a mutation in the gene encoding FYVE and coiled-coil domain containing 1 (FYCO1), a protein involved in autophagosome transport [37]. Expression of autophagy genes in human lens epithelial and fiber cells was previously reported [38], and we have demonstrated that autophagy is inhibited in αB-R120G knock-in mice [27]. The autophagosome markers LC3B and FYCO1 are detected throughout the lens fibers in newborn mutant mice [39]. These proteins are also detected in microdissected human lens fibers, and the number of LC3B-positive puncta is increased in cultured lens epithelial cells upon serum starvation, suggesting that autophagy is induced in lens cells under stress [38]. Decreased autophagy can result in the accumulation of aggregation-prone proteins, cellular degeneration, and cell death and is usually accompanied by the accumulation of p62 in large perinuclear aggregates [40–42]. p62 is a ubiquitin- and LC3-binding scaffold protein that co-localizes with ubiquitinated protein aggregates in many protein aggregation diseases associated with neurodegeneration, myofibrillar myopathies, and cataracts [43,44]. Because p62 is specifically degraded by autophagy it accumulates in cells and tissues of autophagy-deficient mice, and p62 accumulation is used as a marker for autophagy inhibition [42]. p62 accumulates in the lens of a mouse model for autophagy inhibition with lens-specific deletion of the ATG5 gene [45]. We hypothesize that autophagy is essential for normal lens homeostasis and that inhibited autophagy is associated with increased risk of cataract formation.
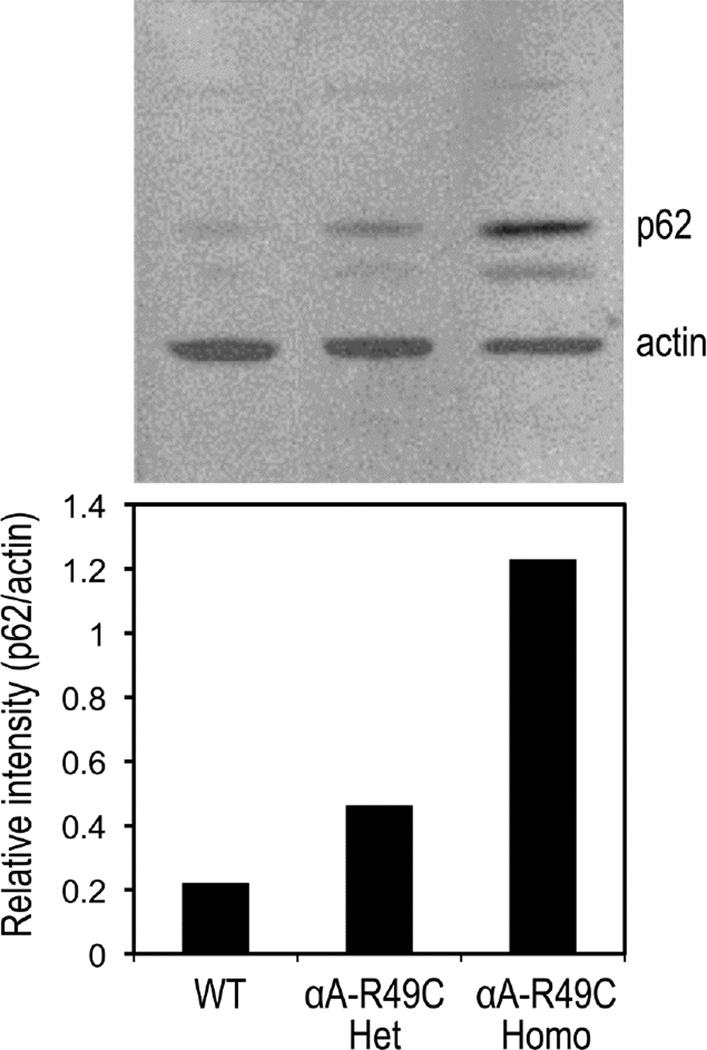
We examined p62 levels in wild-type vs αA-R49C knock-in mutant mouse lenses at an early postnatal age by immunoblotting and autophagosome size by electron microscopy to determine whether autophagy is altered in the αA-R49C mutant lens. Figure 2 shows expression of the autophagy marker p62 in lenses of wild-type and αA-R49C knock-in mice. Western blotting for p62, a protein that is specifically degraded by autophagy and is thus a marker for autophagy, indicated impaired autophagy in mice containing one or two copies of the R49C mutation. p62 levels in the αA-R49C knock-in lenses were increased. Elevated p62 accumulation in the mutants is evidence of a partial shutdown of autophagy pathways.
Figure 2.
Immunoblot analysis of the autophagy-specific protein p62 in in wild-type, heterozygous αA-R49C (R49C Het), and homozygous αA-R49C (R49C Homo) lenses. Four-week-old lenses were analyzed using antibodies against p62 and actin. Band densities at 62 kDa (p62) and 43 kDa (actin) were quantified and presented as relative ratios. Note the mutant gene-dosage-dependent increase in p62 expression in the heterozygous and homozygous knock-in mutant lens protein as compared with wild-type controls. The results are representative of three independent experiments with a standard deviation of 10%.
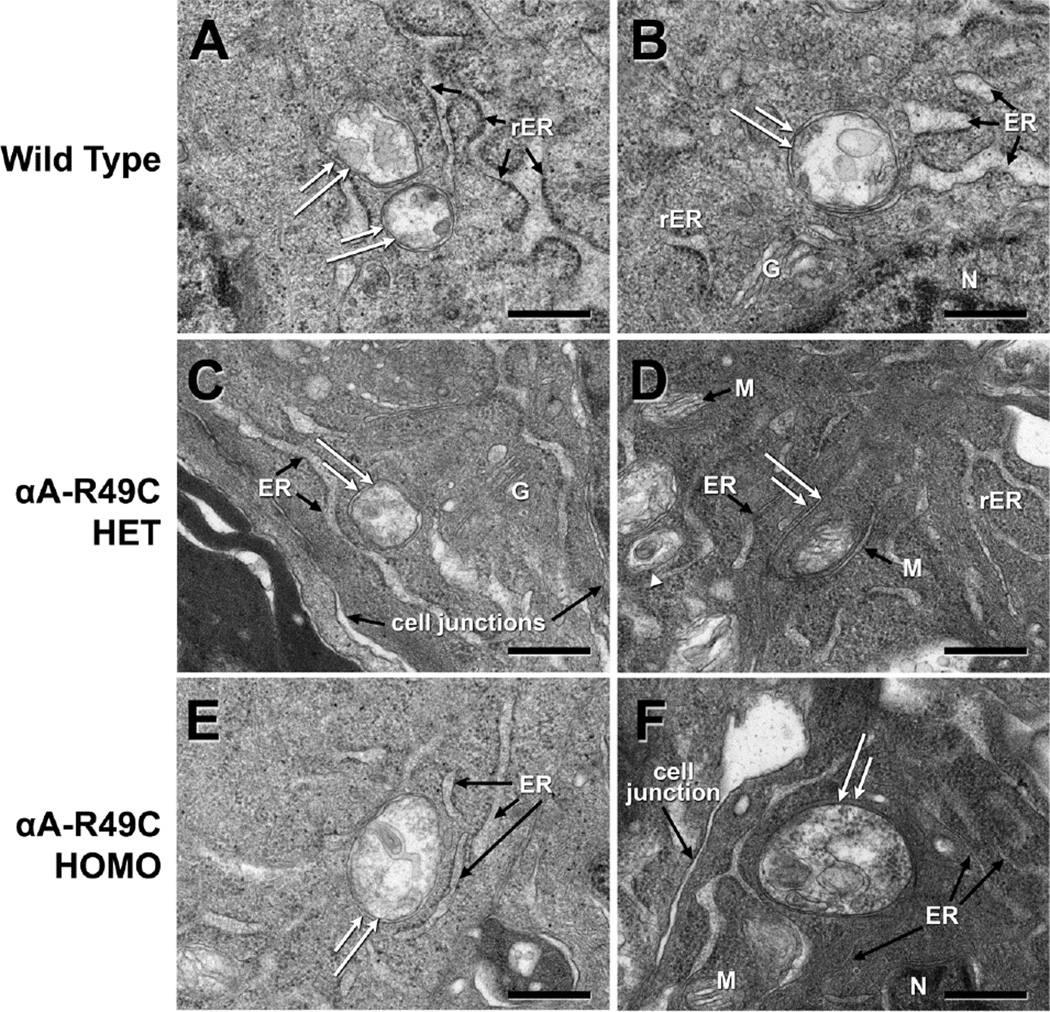
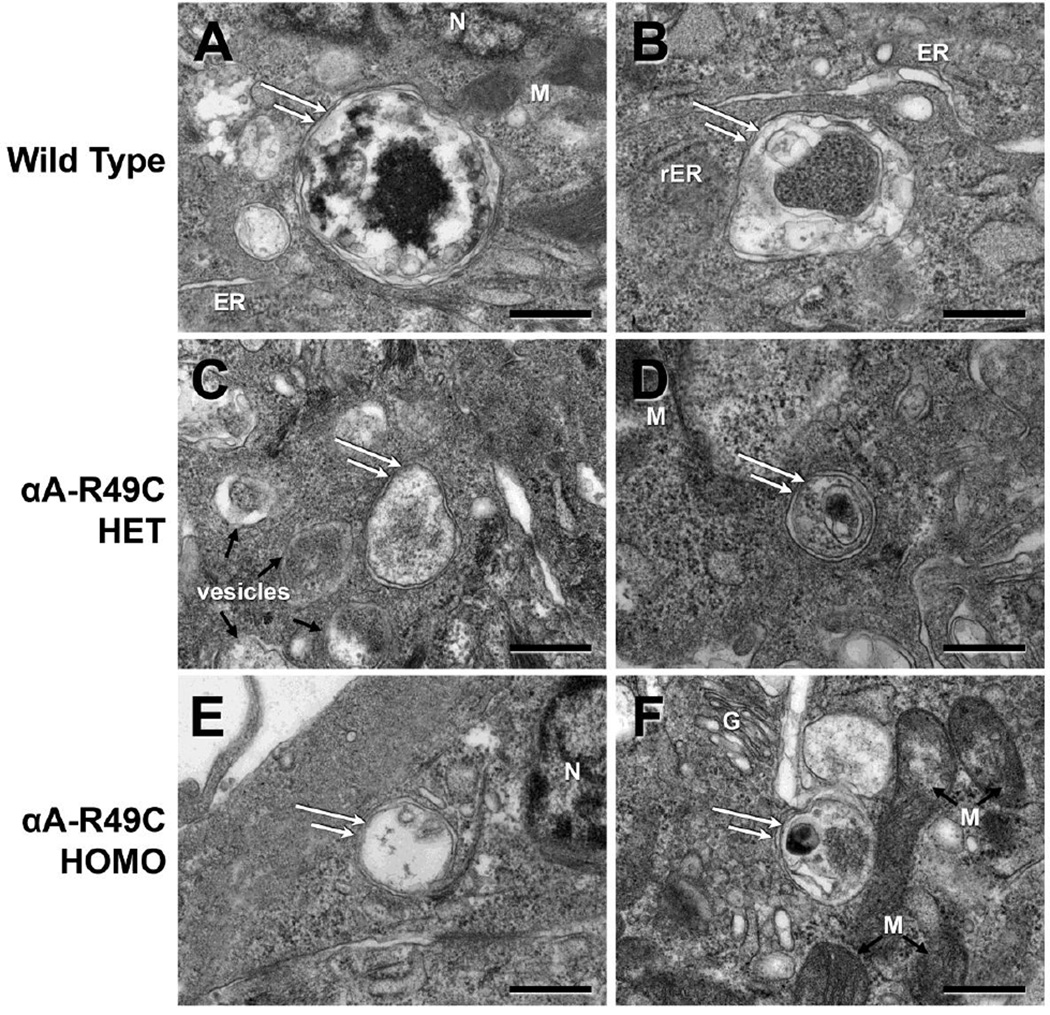
Autophagosomes were evident in the central and equatorial regions of the lens epithelium of wild-type and αA-R49C mutant lenses. To further examine the effect of the mutation on autophagy, we examined autophagosomes in 2-week-old wild-type, heterozygous, and homozygous mutant lenses by electron microscopy. Figure 3 shows the autophagosomes detected in the central epithelial region of these mouse lenses. Autophagosome size per unit cytoplasmic area was two-fold smaller in the αA-R49C+/− lenses in the central epithelium, but was not significantly affected in αA-R49C+/+ lenses. However, autophagosomes were also two-fold smaller in the equatorial epithelium of the αA-R49C+/− and αA-R49C+/+ knock-in lenses (Fig. 4). The area of autophagosomes per unit cellular area was 85.54 ± 31.68 for wild-type (n = 7), 40.72 ± 23.16 for αA-R49C +/− (n = 7) and 32.015 ± 3.00 for αA-R49C +/+ mutant lenses (n = 4), p < 0.01 wild-type vs. mutant. These observations suggest that the αA-R49C mutation inhibits autophagosome development in this model.
Figure 3.
A gallery of electron micrographs of autophagosomes in the central lens epithelial cells of mouse lenses. Double-membrane structures (white double arrows) consistent with autophagosomes were detected in each genotype. (A, B), Wild-type; (C, D), αA-R49C heterozygous mutant; (E, F), αA-R49C homozygous mutant. The structures contained cytoplasmic material and/or organelles and were surrounded by cytoplasm. Note the proximity of the autophagosomes to the ER and Golgi. The size of autophagosomes per unit cellular area was smaller in the αA-R49C heterozygous mutant than in the wild-type. ER, endoplasmic reticulum; rER, rough endoplasmic reticulum; G, Golgi; M, mitochondria; N, nucleus. Scale bar = 500 nm.
Figure 4.
Electron micrographs of autophagosomes in the equatorial epithelial cell region of mouse lenses. Sections of lens epithelial cells, approximately 20 cells before differentiation and cell elongation starts, were analyzed. (A, B), Wild-type; (C, D), αAR49C heterozygous mutant; (E, F), αA-R49C homozygous mutant. The area of the autophagosomes per unit cellular area was smaller in the αA-R49C mutant lenses than in the wild-type. ER, endoplasmic reticulum; rER, rough endoplasmic reticulum; G, Golgi; M, mitochondria; N, nucleus. Scale bar = 500 nm.
3.3 Activation of unfolded protein response (UPR) in knock-in mutant mouse lenses
Human patients heterozygous for the αB-R120G mutation in αB-crystallin develop desmin-related myopathy and cataracts at an early age [7]. The αB-R120G knock-in mice develop lens opacity, appearing even in 3-week-old mice, which increase in severity with age [19]. The molecular weight of the α-crystallin fraction increased from 1130 to 2014 kDa and 2855 kDa in lenses from 4-month-old αB-R120G wild-type mice, heterozygote mutants, and homozygote mutants, respectively [19]. This increased molecular weight, together with the increased protein content in the water-insoluble fraction, suggests accumulation of aggregated proteins in the mutant lenses. It is not yet known how high-molecular weight protein aggregates are degraded.
The UPR is an intracellular signaling mechanism by which cells respond to the presence of misfolded proteins [46,47]. The UPR has been implicated in the pathogenesis of protein aggregation diseases including Alzheimer’s, Parkinson’s, and cataracts [48,49]. Recent studies on the nervous system suggest a cross-talk between autophagy and UPR, wherein deficiency of a key UPR transcription factor leads to increased autophagy [50,51]. Lens cells in diabetic cataracts and in lenses expressing mutant collagen 4a or the αA-crystallin G98R mutant experience endoplasmic reticulum (ER) stress and activate the UPR [49,52,53]. When UPR is activated, the protein-folding capacity of the cell increases. This includes upregulating synthesis of chaperonin proteins, decreasing general transcription, and enhancing ER-associated degradation [47]. If these steps do not restore cellular integrity, then apoptosis pathways are activated. In non-stressed conditions the ER chaperone binding immunoglobulin protein (BiP), a sensor of altered ER homeostasis, binds to three juxtaposed stress transducers in the ER membrane, i.e., inositol-requiring enzyme 1 (IRE1), activating transcription factor 6 (ATF6), and protein kinase RNA-like endoplasmic reticulum kinase (PERK). Accumulation of misfolded proteins causes IRE1 dissociation from regulatory proteins, oligomerization, and activation to increase expression of X-box binding protein 1 (XBP-1), which is a potent transcriptional activator. ATF6 released from BiP activates UPR-responsive genes. PERK released from BiP increases transcription of stress-responsive genes with apoptotic functions such as CCAAT-enhancer-binding protein homologous protein (CHOP). We previously demonstrated enhanced expression of ATF4 and CHOP, proteins normally linked with the activation of the PERK pathway and subsequent cell death in αA-R49C knock-in homozygous mutant lenses [54]. Furthermore, XBP-1 and ATF6 were downregulated in the αA-R49C mutant lenses, suggesting downregulation of the IRE1 and ATF6 branches of the UPR that elicit cytoprotective responses to restore cellular homeostasis [46–48].
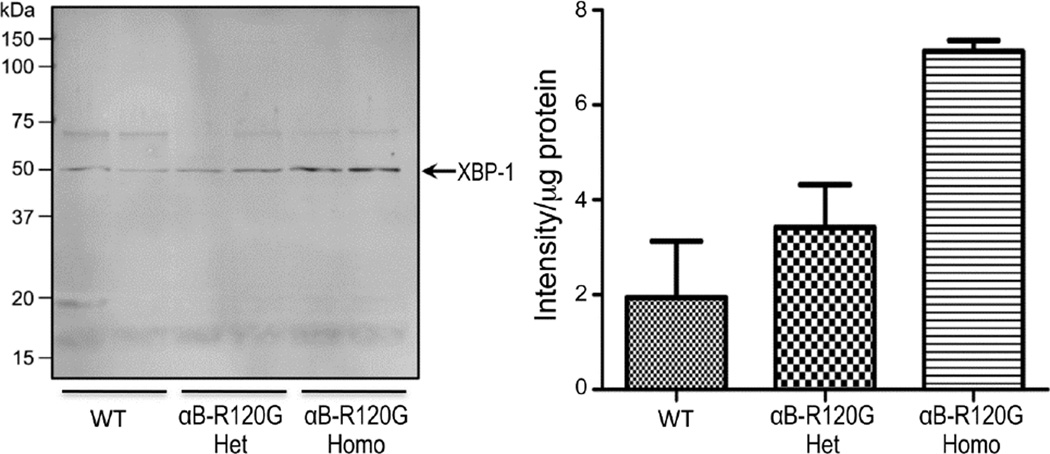
Analysis of the UPR in the αB-R120G knock-in mouse model demonstrated that the PERK and ATF6 branches of the UPR were unaffected in these lenses, and there were no significant changes in the levels of CHOP, ATF6, or ATF4 (not shown), but the levels of XBP-1 increased, suggesting an activation of the IRE1 branch of the UPR (Fig. 5). These observations are consistent with the absence of cell death observed in the αB-R120G knock-in mutant mouse lenses. Thus the two knock-in mouse models investigated in the current work demonstrate distinct mechanisms of pathway activation leading to cell homeostasis in the lens.
Figure 5.
Immunoblot analysis of the UPR-associated protein XBP-1 in wild-type, heterozygous αB-R120G (R120G Het), and homozygous αB-R120G (R120G Homo) lenses. Forty-µg protein/sample was analyzed and the relative intensity of XBP-1 band per µg protein was determined by image analysis. Note the increase in XBP-1 in αB-R120G mutant lenses as compared with wild-type. The two wells are replicate aliquots of single samples. The error bars reflect standard deviations. The data are representative of three independent experiments.
4. Conclusions
We are using an integrated program aimed at elucidating the function of mutant α-crystallin in vivo. This approach will increase our understanding of the mechanisms underlying congenital cataracts caused by αA-crystallin mutations and will provide crucial information concerning the mechanisms of age-related cataract. Our studies demonstrate different mechanisms of protein homeostasis in two mouse models with either αA-crystallin or αB-crystallin gene mutations. The αA-R49C homozygous mouse lenses display upregulation of the PERK UPR pathway, which leads to lens cell death, whereas the αB-R120G mutation activates the IRE pathway in lenses, a cytoprotective response. Autophagy appears to be inhibited in the αB-R120G model of cataract, and the current work suggests that this also occurs in the αA-R49C knock-in lenses. Both models demonstrate increased lens accumulation of p62 protein, which is specifically degraded by autophagy, suggesting autophagy inhibition during cataract formation. We propose that autophagy is essential for normal lens homeostasis and that the inhibition of autophagy is associated with increased risk of cataract formation.
Highlights.
Knock-in mice provide useful models of congenital and age-related cataracts caused by α-crystallin mutations.
R49C αA-crystallin and R120G αB-crystallin mutations are linked with hereditary cataracts.
Mutant crystallin cannot function as a chaperone, which leads to protein aggregation and lens opacity
Mutated crystallins alter lens morphology, autophagy, and stress responses.
Therapeutic modulation of autophagic pathways may improve protein degradation in cataractous lenses and reduce lens opacity.
Acknowledgments
Electron microscopy was performed by Dr. Wandy Beatty in the Molecular Microbiology Imaging Core at Washington University School of Medicine. The authors thank Brittney McGlasson and Jonathan Wignes for technical assistance. This work was supported by NIH grants R01-EY05681 (UPA), Core grant EY02687, and RPB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, et al. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Slingsby C, Wistow GJ. Functions of crystallins in and out of lens: roles in elongated and post-mitotic cells. Prog Biophys Mol Biol. 2014;115:52–67. doi: 10.1016/j.pbiomolbio.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiels A, Hejtmancik JF. Genetic origins of cataract. Archives of Ophthalmology. 2007;125:165–173. doi: 10.1001/archopht.125.2.165. [DOI] [PubMed] [Google Scholar]
- 4.Andley UP. Crystallins and hereditary cataracts: molecular mechanisms and potential for therapy. Expert Rev Mol Med. 2006;8:1–19. doi: 10.1017/S1462399406000111. [DOI] [PubMed] [Google Scholar]
- 5.Graw J. Genetics of crystallins: cataract and beyond. Exp Eye Res. 2009;88:173–189. doi: 10.1016/j.exer.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Mackay DS, Andley UP, Shiels A. Cell death triggered by a novel mutation in the alphaA-crystallin gene underlies autosomal dominant cataract linked to chromosome 21q. Eur J Hum Genet. 2003;11:784–793. doi: 10.1038/sj.ejhg.5201046. [DOI] [PubMed] [Google Scholar]
- 7.Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, et al. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20:92–95. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- 8.Litt M, Kramer P, LaMorticella DM, Murphey W, Lovrien EW, et al. Autosomal dominant congenital cataract associated with a missense mutation in the human alpha crystallin gene CRYAA. Hum Mol Genet. 1998;7:471–474. doi: 10.1093/hmg/7.3.471. [DOI] [PubMed] [Google Scholar]
- 9.Datiles MB, 3rd, Ansari RR, Suh KI, Vitale S, Reed GF, et al. Clinical detection of precataractous lens protein changes using dynamic light scattering. Arch Ophthalmol. 2008;126:1687–1693. doi: 10.1001/archophthalmol.2008.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy D, Spector A. Absence of low-molecular-weight alpha crystallin in nuclear region of old human lenses. Proc Natl Acad Sci U S A. 1976;73:3484–3487. doi: 10.1073/pnas.73.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackay DS, Andley UP, Shiels A. Cell death triggered by a novel mutation in the alphaA-crystallin gene underlies autosomal dominant cataract linked to chromosome 21q. Eur J Hum Genet. 2003;11:784–793. doi: 10.1038/sj.ejhg.5201046. [DOI] [PubMed] [Google Scholar]
- 12.Richter L, Flodman P, Barria von-Bischhoffshausen F, Burch D, Brown S, et al. Clinical variability of autosomal dominant cataract, microcornea and corneal opacity and novel mutation in the alpha A crystallin gene (CRYAA) Am J Med Genet A. 2008;146:833–842. doi: 10.1002/ajmg.a.32236. [DOI] [PubMed] [Google Scholar]
- 13.Santhiya ST, Soker T, Klopp N, Illig T, Prakash MV, et al. Identification of a novel, putative cataract-causing allele in CRYAA (G98R) in an Indian family. Mol Vis. 2006;12:768–773. [PubMed] [Google Scholar]
- 14.Yang Z, Su D, Li Q, Ma Z, Yang F, et al. A R54L mutation of CRYAA associated with autosomal dominant nuclear cataracts in a Chinese family. Curr Eye Res. 2013;38:1221–1228. doi: 10.3109/02713683.2013.811260. [DOI] [PubMed] [Google Scholar]
- 15.Laurie KJ, Dave A, Straga T, Souzeau E, Chataway T, et al. Identification of a novel oligomerization disrupting mutation in CRYAlphaA associated with congenital cataract in a South Australian family. Hum Mutat. 2013;34:435–438. doi: 10.1002/humu.22260. [DOI] [PubMed] [Google Scholar]
- 16.Pras E, Frydman M, Levy-Nissenbaum E, Bakhan T, Raz J, et al. A nonsense mutation (W9X) in CRYAA causes autosomal recessive cataract in an inbred Jewish Persian family. Invest Ophthalmol Vis Sci. 2000;41:3511–3515. [PubMed] [Google Scholar]
- 17.Sacconi S, Feasson L, Antoine JC, Pecheux C, Bernard R, et al. A novel CRYAB mutation resulting in multisystemic disease. Neuromuscul Disord. 2012;22:66–72. doi: 10.1016/j.nmd.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Berry V, Francis P, Reddy MA, Collyer D, Vithana E, et al. Alpha-B crystallin gene (CRYAB) mutation causes dominant congenital posterior polar cataract in humans. Am J Hum Genet. 2001;69:1141–1145. doi: 10.1086/324158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andley UP, Hamilton PD, Ravi N, Weihl CC. A knock-in mouse model for the R120G mutation of alphaB-crystallin recapitulates human hereditary myopathy and cataracts. PLoS One. 2011;6:e17671. doi: 10.1371/journal.pone.0017671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heiba IM, Elston RC, Klein BE, Klein R. Evidence for a major gene for cortical cataract. Invest Ophthalmol Vis Sci. 1995;36:227–235. [PubMed] [Google Scholar]
- 21.Hammond CJ, Snieder H, Spector TD, Gilbert CE. Genetic and environmental factors in age-related nuclear cataracts in monozygotic and dizygotic twins. N Engl J Med. 2000;342:1786–1790. doi: 10.1056/NEJM200006153422404. [DOI] [PubMed] [Google Scholar]
- 22.Moore AT. Understanding the molecular genetics of congenital cataract may have wider implications for age related cataract. Br J Ophthalmol. 2004;88:2–3. doi: 10.1136/bjo.88.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francis PJ, Moore AT. Genetics of childhood cataract. Curr Opin Ophthalmol. 2004;15:10–15. doi: 10.1097/00055735-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Andley UP, Hamilton PD, Ravi N. Mechanism of insolubilization by a single-point mutation in alphaA-crystallin linked with hereditary human cataracts. Biochemistry. 2008;47:9697–9706. doi: 10.1021/bi800594t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White TW. Unique and redundant connexin contributions to lens development. Science. 2002;295:319–320. doi: 10.1126/science.1067582. [DOI] [PubMed] [Google Scholar]
- 26.Andley UP, Reilly MA. In vivo lens deficiency of the R49C alphaA-crystallin mutant. Experimental Eye Research. 2010;90:699–702. doi: 10.1016/j.exer.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wignes JA, Goldman JW, Weihl CC, Bartley MG, Andley UP. p62 expression and autophagy in alphaB-crystallin R120G mutant knock-in mouse model of hereditary cataract. Experimental Eye Research. 2013;115:263–273. doi: 10.1016/j.exer.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reilly MA, Andley UP. Quantitative biometric phenotype analysis in mouse lenses. Mol Vis. 2010;16:1041–1046. [PMC free article] [PubMed] [Google Scholar]
- 29.Xi JH, Bai F, Gross J, Townsend RR, Menko AS, et al. Mechanism of small heat shock protein function in vivo: a knock-in mouse model demonstrates that the R49C mutation in alpha A-crystallin enhances protein insolubility and cell death. J Biol Chem. 2008;283:5801–5814. doi: 10.1074/jbc.M708704200. [DOI] [PubMed] [Google Scholar]
- 30.Robinson ML, Overbeek PA. Differential expression of alpha A- and alpha B-crystallin during murine ocular development. Invest Ophthalmol Vis Sci. 1996;37:2276–2284. [PubMed] [Google Scholar]
- 31.Shaham O, Smith AN, Robinson ML, Taketo MM, Lang RA, et al. Pax6 is essential for lens fiber cell differentiation. Development. 2009;136:2567–2578. doi: 10.1242/dev.032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao Y, Klionsky DJ. New insights into autophagy using a multiple knockout strain. Autophagy. 2008;4:1073–1075. doi: 10.4161/auto.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider JL, Cuervo AM. Autophagy and human disease: emerging themes. Curr Opin Genet Dev. 2014;26:16–23. doi: 10.1016/j.gde.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Q, Su H, Ranek MJ, Wang X. Autophagy and p62 in cardiac proteinopathy. Circ Res. 2011;109:296–308. doi: 10.1161/CIRCRESAHA.111.244707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tannous P, Zhu H, Johnstone JL, Shelton JM, Rajasekaran NS, et al. Autophagy is an adaptive response in desmin-related cardiomyopathy. Proc Natl Acad Sci U S A. 2008;105:9745–9750. doi: 10.1073/pnas.0706802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, Ma Z, Jiao X, Fariss R, Kantorow WL, et al. Mutations in FYCO1 cause autosomal-recessive congenital cataracts. Am J Hum Genet. 2011;88:827–838. doi: 10.1016/j.ajhg.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brennan LA, Kantorow WL, Chauss D, McGreal R, He S, et al. Spatial expression patterns of autophagy genes in the eye lens and induction of autophagy in lens cells. Mol Vis. 2012;18:1773–1786. [PMC free article] [PubMed] [Google Scholar]
- 39.Costello MJ, Brennan LA, Basu S, Chauss D, Mohamed A, et al. Autophagy and mitophagy participate in ocular lens organelle degradation. Experimental Eye Research. 2013;116C:141–150. doi: 10.1016/j.exer.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- 41.Williams A, Jahreiss L, Sarkar S, Saiki S, Menzies FM, et al. Aggregate-prone proteins are cleared from the cytosol by autophagy: therapeutic implications. Current Topics in Developmental Biology. 2006;76:89–101. doi: 10.1016/S0070-2153(06)76003-3. [DOI] [PubMed] [Google Scholar]
- 42.Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. Journal of Biological Chemistry. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 44.Kuusisto E, Salminen A, Alafuzoff I. Ubiquitin-binding protein p62 is present in neuronal and glial inclusions in human tauopathies and synucleinopathies. Neuroreport. 2001;12:2085–2090. doi: 10.1097/00001756-200107200-00009. [DOI] [PubMed] [Google Scholar]
- 45.Morishita H, Eguchi S, Kimura H, Sasaki J, Sakamaki Y, et al. Deletion of autophagy-related 5 (Atg5) and Pik3c3 genes in the lens causes cataract independent of programmed organelle degradation. Journal of Biological Chemistry. 2013;288:11436–11447. doi: 10.1074/jbc.M112.437103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 47.Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mulhern ML, Madson CJ, Danford A, Ikesugi K, Kador PF, et al. The unfolded protein response in lens epithelial cells from galactosemic rat lenses. Invest Ophthalmol Vis Sci. 2006;47:3951–3959. doi: 10.1167/iovs.06-0193. [DOI] [PubMed] [Google Scholar]
- 50.Hetz C, Thielen P, Matus S, Nassif M, Court F, et al. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 2009;23:2294–2306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vidal RL, Figueroa A, Court FA, Thielen P, Molina C, et al. Targeting the UPR transcription factor XBP1 protects against Huntington's disease through the regulation of FoxO1 and autophagy. Hum Mol Genet. 2012;21:2245–2262. doi: 10.1093/hmg/dds040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Firtina Z, Danysh BP, Bai X, Gould DB, Kobayashi T, et al. Abnormal expression of collagen IV in lens activates unfolded protein response resulting in cataract. J Biol Chem. 2009;284:35872–35884. doi: 10.1074/jbc.M109.060384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gong B, Zhang LY, Pang CP, Lam DS, Yam GH. Trimethylamine N-oxide alleviates the severe aggregation and ER stress caused by G98R alphaA-crystallin. Mol Vis. 2009;15:2829–2840. [PMC free article] [PubMed] [Google Scholar]
- 54.Watson GW, Andley UP. Activation of the unfolded protein response by a cataract-associated alphaA-crystallin mutation. Biochemical and Biophysical Research Communications. 2010;401:192–196. doi: 10.1016/j.bbrc.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]