SUMMARY
Background
Current guidelines do not recommend screening for nonalcoholic fatty liver disease (NAFLD) or advanced fibrosis. Patients with type 2 diabetes mellitus (T2DM) are known to be at increased risk for NAFLD and advanced fibrosis.
Aim
We aimed to assess the feasibility in diabetics in a primary care setting of screening for NAFLD and advanced fibrosis by using non-invasive magnetic resonance imaging (MRI) to estimate the hepatic proton density fat fraction (MRI-PDFF) and magnetic resonance elastography (MRE) to estimate hepatic stiffness.
Methods
We performed a cross-sectional analysis of a prospective study that included 100 (53% men) consecutively enrolled diabetics who did not have any other etiology of liver disease. All patients underwent a standardized research visit, laboratory tests, MRI-PDFF, and MRE.
Results
Mean (± SD) age and BMI was 59.7 (±11.2) years and 30.8 (±6.5) kg/m2, respectively. The prevalence of NAFLD (defined as MRI-PDFF ≥ 5%) and advanced fibrosis (defined as MRE ≥ 3.6 kPa) was 65% and 7.1%, respectively. One patient with advanced fibrosis had definite hepatocellular carcinoma. When compared to those without NAFLD, patients with NAFLD were younger (p=0.028) and had higher mean BMI (p=0.0008), waist circumference (p<0.0001) and prevalence of metabolic syndrome (84.6% vs. 40.0%, p<0.0001). Only 26% of those with NAFLD had elevated ALT.
Conclusions
This proof-of-concept study demonstrates that T2DM has significant rates of both NAFLD and advanced fibrosis. Concomitant screening for NAFLD and advanced fibrosis by using MRI-PDFF and MRE in T2DM is feasible and may be considered after validation in a larger cohort.
Keywords: non-invasive, biomarker, nonalcoholic fatty liver disease (NAFLD), fibrosis, liver fat, type 2 diabetes
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the Western world with an estimated prevalence in the United States ranging between 19–46% depending on the study population characteristics and diagnostic modality.1, 2 NAFLD is a frequent finding in patients with type 2 diabetes mellitus (T2DM) due to their common underlying pathogenic mechanism of insulin resistance.3, 4 Previous studies on the prevalence of NAFLD in T2DM were based on abdominal ultrasound evaluation,5, 6 magnetic resonance spectroscopy (MRS)7, 8 or small samples of patients with liver biopsies.2, 9 Due to the differences in the study methodologies and diagnostic techniques, the reported prevalence of NAFLD in T2DM patients ranges broadly between 43% and 94%.2, 5–9 It is also known that the presence of T2DM is an independent predictor of advanced fibrosis in NAFLD,10 with a greater prevalence of cirrhosis in diabetic compared to non-diabetic patients.11 However, the exact prevalence of NAFLD in T2DM as well as the utility of screening for NAFLD in T2DM remain uncertain.
With the steadily increasing prevalence of obesity and T2DM, the health burden of NAFLD in diabetes raises an important concern and imposes the need for early identification of patients who are at an increased risk of progressive liver disease and cirrhosis. Currently, routine screening of NAFLD in either diabetics (as a high-risk group for progression of liver disease) or in the general population is not recommended due to controversies regarding the lack of accurate non-invasive diagnosis of NAFLD and advanced fibrosis and limited therapeutic options.12
Serum aminotransferase elevation is an inaccurate marker of NAFLD as normal alanine aminotransferase (ALT) has been noted in up to 86% of patients with NAFLD.5 Abdominal ultrasound lacks sensitivity for mild steatosis (< 30%) and has high inter- and intra-observer variability.13 Computed tomography (CT) has limited sensitivity for mild disease and is associated with radiation exposure. Therefore, liver biopsy remains the gold standard for the diagnosis and staging of NAFLD and assessment of fibrosis.12 However, liver biopsy is an invasive procedure, which is limited due to its associated cost and risk of complications, including pain, infection, bleeding, and very rarely even death. Furthermore, liver biopsy has significant sampling variability, and inter-observer variability, and it is impractical tool for screening of patients who are at increased risk for presence of NAFLD and advanced fibrosis.14
Therefore, we aimed to investigate the feasibility of screening for NAFLD in T2DM using magnetic resonance imaging-estimated proton density fat fraction (MRI-PDFF), a novel, recently validated imaging-based biomarker which allows for the quantification of liver fat content in the entire liver that correlates strongly with MR spectroscopy – measured liver fat15–17 and liver histology-determined steatosis grade.18–20 We also aimed to assess the usefulness of magnetic resonance elastography (MRE), a noninvasive imaging modality shown to differentiate advanced from non-advanced fibrosis,21, 22 to screen our cohort for advanced fibrosis. Our hypotheses were that the feasibility of using MRI-PDFF and MRE to screen for NAFLD and advanced fibrosis would be excellent with no or low failure rates, and that asymptomatic diabetics in primary care setting would have high prevalence of NAFLD and advanced fibrosis. We followed STROBE guidelines for the reporting of prospectively conducted cross-sectional studies.23
METHODS
Study design and setting
This is a cross-sectional analysis of a prospective study involving 100 consecutive eligible adult patients with T2DM recruited via newspaper advertisement and from primary care practices in the greater San Diego area. The patients underwent a standardized research visit including history, physical exam, anthropometric measurements, and biochemical and serological laboratory tests at the Clinical and Translational Research Institute, University of California, San Diego (UCSD). Within 30 days of the research visit, patients were scanned at the UCSD MR3T Research Laboratory and underwent same day MRI and MRE to estimate hepatic PDFF and hepatic stiffness, respectively. The study was approved by the UCSD Institutional Review Board. All enrolled patients provided written informed consent before data collection.
Derivation of cohort
Between March 2013 and September 2014, one hundred ninety-five subjects were screened. Ninety-five were excluded and 100 subjects were enrolled in the study. See Supplementary Figure 1 for details of cohort derivation.
Patient Population
We included patients with T2DM, age 21 years or older, diagnosed with T2DM according to the American Diabetes Association (ADA) clinical practice recommendations-201224: hemoglobin A1c ≥ 6.5% or fasting plasma glucose ≥ 126 mg/dL or 2-hour plasma glucose ≥ 200mg/dL during an oral glucose tolerance test (OGTT) or patient with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose ≥ 200mg/dL. All enrolled patients were asymptomatic with respect to liver disease and did not have any history of known or diagnosed liver disease.
Exclusion criteria: Subjects were excluded if they had evidence of any other chronic liver disease shown by the presence of hepatitis B surface antigen (HBsAg) or hepatitis C antibody (anti-HCV Ab), documented history of any other liver disease (such as alpha-1 antitrypsin deficiency, autoimmune hepatitis, drug-induced liver injury, primary biliary cirrhosis, primary sclerosing cholangitis or any other liver disease), or cirrhosis, bile duct obstruction on prior radiology tests, use of medications associated with secondary NAFLD (corticosteroids, tamoxifen, amiodarone, methotrexate), major diabetic end-organ damage (advanced cardiovascular disease requiring coronary artery bypass graft surgery or congestive heart failure; chronic kidney disease, stage ≥ 3), alcohol consumption of more than 30 grams per day in the previous 10 years or greater than 10 grams per day in the previous year assessed with the Alcohol Use Disorders Identification Test (AUDIT) questionnaire25, positive HIV test, pregnancy or contraindications to MRI.
Clinical assessment and formulas
All patients were evaluated at the UCSD NAFLD Clinical and Translational Research Institute. A detailed history was obtained from all patients. A physical exam including vital signs, height, weight, and anthropometric measurements was performed by a trained investigator. Alcohol consumption was assessed by using the AUDIT questionnaire, a validated tool used to screen for heavy drinking and/or active alcohol abuse or dependence.25 All patients had blood drawn in a fasting state for laboratory analysis.
Body mass index (BMI) was calculated by dividing body weight (in kilograms) by the square of the height (in meters). Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated in a fasting state based on the formula: HOMA-IR = [glucose (mg/dL) x insulin (mIU/mL)]/405. Adipose tissue IR (Adipo-IR) was calculated in a fasting state using the formula: Adipo-IR = free fatty acids (mmol/L) x insulin (mIU/mL).
Covariates
The following demographic and anthropometric characteristics were collected: age, gender, race, BMI and waist circumference. Clinical characteristics included: presence of hypertension (defined as blood pressure ≥ 130/85 mmHg or use of antihypertensive medication), metabolic syndrome based on the American Heart Association and the National Heart, Lung, and Blood Institute criteria26 (3 out of 5 conditions present: hypertension as defined above; fasting glucose ≥ 100 mg/dL (5.6 mmol/L) or receiving therapy for hyperglycemia; hypertriglyceridemia ≥ 150 mg/dL (1.7 mmol/L); high-density lipoprotein cholesterol (HDL-C) < 50 mg/dL (1.29 mmol/L) in women and < 40 mg/dL (1.03 mmol/L) in men; waist circumference ≥ 102 cm in men or ≥ 88 cm in women, or ≥ 90 cm in Asian men and ≥ 80 cm in Asian women), duration of diabetes, diabetic complications (diabetic retinopathy, neuropathy or nephropathy), current treatment of diabetes, antilipemic therapy, and current therapy with vitamin E.
Primary and secondary outcomes
The primary outcome of this study was the assessment of the feasibility of screening for NAFLD in T2DM in primary care setting by using MRI to estimate PDFF. Our secondary outcome was the evaluation of the feasibility of screening for advanced fibrosis in T2DM in primary care setting by using MRE.
MR Examinations
MR examinations were performed using a 3T research scanner (GE Signa EXCITE HDxt, GE Healthcare, Waukesha, WI) at the UCSD MR3T Research Laboratory. The MR examinations were performed by experienced MR technologists and analyzed by a single image analyst who was blinded to clinical and biochemical data. Patients were scanned in the supine position with a torso phased-array coil placed over the abdomen. To reduce potential physiologic confounding factors, patients were instructed to fast for a minimum of four hours before the exam. Two MR techniques were performed: MRI to estimate hepatic PDFF for NAFLD screening and MRE to estimate hepatic stiffness for advanced fibrosis screening.
For screening of NAFLD: MRI-PDFF protocol for assessment of liver fat content
The MRI-PDFF protocol has been previously described in details.19, 27 For full protocol, see supplementary materials.
Rationale for using MRI-PDFF for NAFLD screening
MRI-PDFF was chosen to screen for NAFLD, as this is an accurate and robust quantitative imaging-based biomarker that allows for precise fat quantification in all nine segments of the liver.20, 28, 29 It has been proven to correlate well with MRS15–17, 20 and histology-proven steatosis grade.18–20, 30 This technique is accurate, reproducible,31 has high inter- and intra-examination repeatability,15, 28 and can serve as a marker for longitudinal assessment.
For screening of advanced fibrosis: MRE protocol for assessment of advanced fibrosis
The MRE protocol has been previously described in detail.21 For full protocol, see supplementary materials.
Rationale for using MRE for screening of advanced fibrosis
MRE is an accurate, safe, non-invasive imaging with excellent diagnostic accuracy in differentiating between normal liver and mild fibrosis (stage 0–2) and between non-advanced fibrosis and advanced fibrosis (stage 3–4).22, 32 A recent prospective study has confirmed this finding with the diagnostic accuracy of AUROC 0.92 (p-value < 0.0001). The most optimal threshold for the diagnosis of advanced fibrosis on MRE was 3.6 kPa with a negative predictive value of 0.97 (95% CI, 0.91–0.99).21
Statistical analysis
Patients’ demographic, anthropometric, clinical and biochemical characteristics were summarized. Categorical variables were shown as counts and percentages and associations were tested using a Chi-square test (χ2) or Fisher’s exact test. Normally distributed continuous variables were shown as mean (+/− standard deviation) and differences between groups were analyzed using a two-independent- samples t-test. Values that were not normally distributed were reported as medians and interquartile ranges (IQR) with differences assessed using the Wilcoxon-Mann-Whitney test. A two-tailed p-value of <0.05 was considered statistically significant for all analyses. Statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
Baseline characteristics
Mean (±SD) age and BMI was 59.7 (±11.2) years and 30.8 (±6.5) kg/m2, respectively. 53% were men and the mean duration of T2DM was 8.5 years. 69% had metabolic syndrome and 66% had hypertension. Detailed description of anti-diabetic and antilipemic therapies is listed in Supplementary Table 1. Elevated ALT (>40 U/L) was noted only in 18% with median ALT of 24 U/L.
Primary Outcome
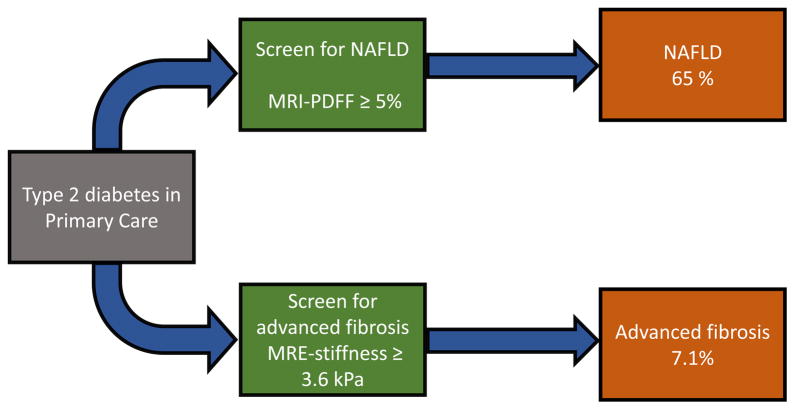
All 100 patients successfully underwent MRI to estimate PDFF. NAFLD as assessed by MRI-PDFF ≥ 5% was present in 65% of the patients (Figure 1). There were no technical failures.
Figure 1. Prevalence of NAFLD and advanced fibrosis among patients with type 2 diabetes in primary care.
Patients with type 2 diabetes in the primary care setting were screened for NAFLD with magnetic resonance imaging-estimated proton density fat fraction (MRI-PDFF). NAFLD was defined by the presence of hepatic steatosis ≥ 5% on MRI-PDFF. Screening for advanced fibrosis was performed using magnetic resonance elastography (MRE) with a threshold of 3.6 kPa to identify those with advanced fibrosis.
Demographic, clinical, biochemical and imaging characteristics in diabetics with NAFLD compared to those without NAFLD
Table 1 presents a detailed description of patients with and without NAFLD. In comparison to those without NAFLD, patients with NAFLD were younger (p = 0.028), and had higher mean BMI (32.5 vs. 27.6, p = 0.0008), mean waist circumference (106.6 cm vs. 95.1 cm, p <0.0001), and prevalence of metabolic syndrome (84.6% vs. 40%, p <0.0001). Patients with NAFLD were more likely to have elevated aspartate aminotransferase (AST) and ALT although only 26.2% had elevated ALT. Average ALT and gamma-glutamyl transpeptidase (GGT) were higher in those with NAFLD but these were within normal reference limits. Lower HDL-C and higher triglyceride levels characterized lipid profile of NAFLD patients when compared to those without NAFLD. The duration of diabetes and control of diabetes as assessed by hemoglobin A1c did not differ between the NAFLD versus the non-NAFLD group. However, as expected, compared to non-NAFLD group, participants in the NAFLD group had higher fasting glucose (p = 0.0366), fasting insulin (p = 0.0151), HOMA-IR (p = 0.0007), and adipo-IR (p = 0.0033).
Table 1.
Baseline population characteristics and comparison between diabetics with and without NAFLD
| N | NAFLD (MRI-PDFF ≥ 5%) (N=65) | No NAFLD (MRI-PDFF <5%) (N=35) | p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Male, n (%) | 100 | 33 (50.8%) | 20 (57.1%) | 0.5425 |
| Age (yrs), mean ± SD | 100 | 57.9 ± 11.3 | 63.1 ± 10.5 | 0.0280 |
| Race | 100 | 0.1125 | ||
| Am Indian/Alaskan Native, n (%) | 1 (1.5%) | 0 | ||
| Asian, n (%) | 9 (13.8%) | 2 (5.7%) | ||
| Black, n (%) | 5 (7.7%) | 12 (12%) | ||
| White, n (%) | 34 (52.3%) | 22 (62.9%) | ||
| Hispanic, n (%) | 16 (24.6%) | 4 (11.4%) | ||
| Anthropometric | ||||
| BMI (kg/m2), mean ± SD | 100 | 32.5 ± 5.4 | 27.6 ± 7.2 | 0.0008 |
| Waist (cm), mean ± SD | 100 | 106.6 ± 12.6 | 95.1 ± 12.9 | <0.0001 |
| Clinical | ||||
| Hypertension, n (%) | 100 | 42 (64.6%) | 24 (68.6%) | 0.6904 |
| Metabolic syndrome, n (%) | 100 | 55 (84.6%) | 14 (40.0%) | <0.0001 |
| Duration of DM (years) | 84 | 7.0 (8.0) | 11.0 (11.0) | 0.1869 |
| Oral hypoglycemic therapy, n (%) | 100 | 55 (84.6%) | 26 (74.3%) | 0.2091 |
| Metformin, n (%) | 100 | 49 (75.4%) | 25 (71.4%) | 0.6671 |
| Thazolidinediones, n (%) | 100 | 2 (3.1%) | 0 | 0.5404 |
| Antilipemic therapy, n (%) | 100 | 37 (56.9%) | 19 (54.3%) | 0.7999 |
| Insulin therapy, n (%) | 100 | 13 (20.0%) | 10 (28.6%) | 0.3313 |
| Vitamin E, n (%) | 100 | 1 (1.5%) | 1 (2.9%) | 1.0000 |
| Biochemical | ||||
| AST (U/L), median (IQR) | 100 | 22.0 (12.0) | 20.0 (10.0) | 0.1965 |
| Elevated AST (>40 U/L), n (%) | 100 | 8 (12.3%) | 0 | 0.0477 |
| ALT (IU/L), median (IQR) | 100 | 29.0 (23.0) | 19.0 (12.0) | 0.0003 |
| Elevated ALT (>40 U/L), n (%) | 100 | 17 (26.2%) | 1 (2.9%) | 0.0027 |
| GGT (U/L), median (IQR) | 99 | 27.5 (19.0) | 20.0 (11.0) | 0.0119 |
| Alkaline phosphatase (U/L), median (IQR) | 100 | 72.0 (20.0) | 77.0 (44.0) | 0.6078 |
| HDL-C (mmol/L), median (IQR) | 100 | 1.27 (0.49) | 1.58 (0.85) | 0.0360 |
| LDL-C (mmol/L), median (IQR) | 100 | 2.28 (1.14) | 2.28 (1.27) | 0.7124 |
| Triglycerides (mmol/L), median (IQR) | 100 | 1.83 (0.85) | 1.04 (0.65) | <0.0001 |
| Platelet (109/L), mean ± SD | 100 | 261.8 ± 73.0 | 247.7±66.4 | 0.3447 |
| HbA1C (%), median (IQR) | 100 | 7.1 (1.6) | 7.0 (1.3) | 0.2592 |
| Ferritin (pmol/L), median (IQR) | 98 | 200.0 (382.0) | 155.0 (283.1) | 0.2062 |
| Fasting glucose (mmol/L), median (IQR) | 98 | 7.33 (3.44) | 5.89 (3.00) | 0.0366 |
| Fasting insulin (mIU/mL/ pmol/L), median (IQR) | 94 | 17.5/121.5 (20.0/138.9) | 11.0/76.4 (14.0/97.2) | 0.0151 |
| HOMA-IR, median (IQR) | 92 | 5.8 (5.3) | 3.0 (3.7) | 0.0007 |
| FFA, (mmol/L), mean ± SD | 94 | 0.55 ± 0.21 | 0.52 ± 0.27 | 0.5181 |
| Adipo –IR, median (IQR) | 93 | 9.1 (10.9) | 5.1 (8.4) | 0.0033 |
| Imaging | ||||
| MRI-PDFF (%), median (IQR) | 100 | 12.3 (9.2) | 2.7 (1.9) | <0.0001 |
| NAFLD (MRI-PDFF ≥ 5%), n (%) | 100 | |||
| MRE (kPa), median (IQR) | 98 | 2.6 (0.7) | 2.4 (0.5) | 0.1127 |
| Advanced Fibrosis (MRE ≥ 3.6 kPa), n (%) | 98 | 5 (7.8%) | 2 (5.9%) | 1.0000 |
p-values: n (%) → chi-square test or Fisher’s exact test, mean ± SD → two independent samples t-test, median (IQR) → Wilcoxon-Mann-Whitney test
Abbreviations: Adipo-IR: adipose tissue insulin resistance, ALT: alanine aminotransferase, AST: aspartate aminotransferase, BMI: body mass index, DM: diabetes mellitus, FFA, free fatty acids, GGT: gamma-glutamyl transpeptidase, HbA1C: hemoglobin A1c, HDL-C: high-density lipoprotein cholesterol, HOMA-IR: Homeostatic Model Assessment of Insulin Resistance, NAFLD: nonalcoholic fatty liver disease; MRE: magnetic resonance elastography, MRI-PDFF: magnetic resonance imaging-estimated proton density fat fraction.
Secondary outcome
Ninety-eight patients (98%) underwent MRE. Two patients did not have MRE due to scheduling conflict. There were no MRE technical failures. The prevalence of advanced fibrosis (defined as MRE ≥ 3.6 kPa) was 7.1% (7 out of 98 patients who underwent MRE) (Figure 2A).
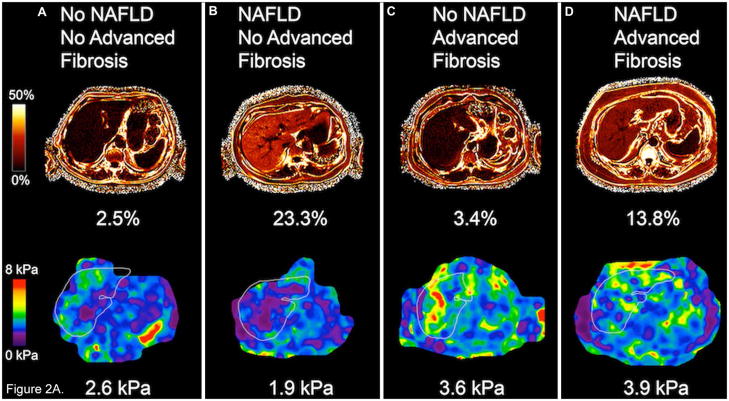
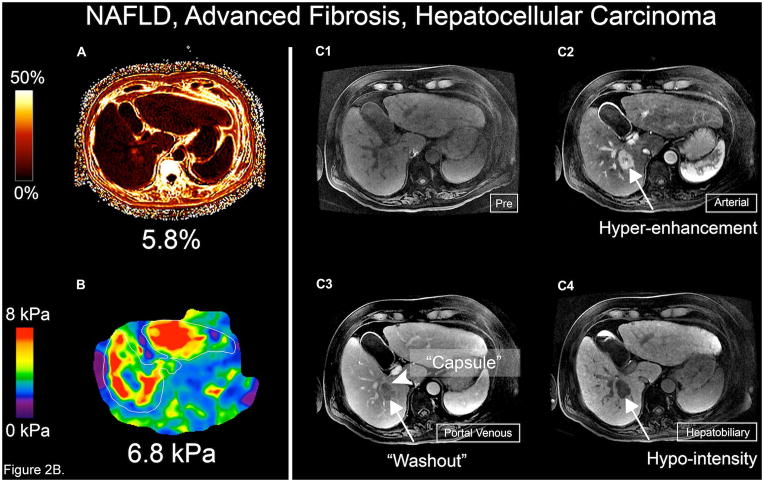
Figure 2.
Figure 2A. Hepatic steatosis by MRI-PDFF (upper row) and liver stiffness by MRE (lower row) in 4 different patients. (A) Example of a patient with no NAFLD and no advanced fibrosis, (B) patient with NAFLD, but no advanced fibrosis, (C) patient without NAFLD, but with advanced fibrosis, and (D) patient with both NAFLD and advanced fibrosis. Fat fraction on MRI-PDFF was expressed in percentages and liver stiffness in kPa, shown underneath each image.
Figure 2B. MRI-PDFF, MRE, and Gadoxetic acid-enhanced 3T MRI of the patient with hepatocellular carcinoma. (A) MRI-PDFF shows 5.8 % hepatic steatosis, (B) MRE shows significant liver stiffness of 6.8 kPa, (C1) Pre-contrast 3D MRI image, (C2) Late arterial phase image shows 3-cm hyperenhancing lesion (arrow) in segment VI of the liver, (C3) Portal venous phase with definite “washout” (arrow) and “capsule” (arrowhead) of the lesion, typical features confirming the diagnosis of HCC; (C4) Hepatobiliary phase reveals markedly hypointense lesion (arrow)
Other outcomes
One patient was noted to have definite hepatocellular carcinoma (HCC) (1% of the entire population) (Figure 2B).
Demographic, clinical, biochemical and imaging characteristics in diabetics with and without advanced fibrosis
Table 2 provides detailed comparison between groups stratified by presence or absence of advanced fibrosis. Two patients with advanced fibrosis had MRI-PDFF < 5%, likely reflecting more advanced liver disease. There was no difference between the two groups in age, gender, ethnicity, metabolic syndrome or BMI. As expected, the advanced fibrosis group had higher AST and lower low-density lipoprotein cholesterol (LDL-C), both suggestive of advanced liver disease. Similar to the NAFLD group, advanced fibrosis group was also characterized by significantly elevated fasting serum insulin (p = 0.0196), higher IR as assessed by HOMA (p = 0.0452) and adipo-IR (p = 0.0062).
Table 2.
Comparison between diabetics with and without advanced fibrosis
| Advanced Fibrosis (MRE ≥ 3.6kPa) (N=7) | No Advanced Fibrosis (MRE < 3.6kPa) (N=91) | p-value | |
|---|---|---|---|
| Demographics | |||
| Male, n (%) | 5 (71.4%) | 47 (51.7%) | 0.4423 |
| Age (yrs), mean ± SD | 67.0 ± 8.9 | 59.2 ± 11.3 | 0.0779 |
| Race | 0.9397 | ||
| Am Indian/Alaskan Native, n (%) | 0 | 1 (1.1%) | |
| Asian, n (%) | 0 | 11 (12.1%) | |
| Black, n (%) | 1 (14.3%) | 11 (12.1%) | |
| White, n (%) | 5 (71.4%) | 49 (53.9%) | |
| Hispanic, n (%) | 1 (14.3%) | 19 (20.9%) | |
| Anthropometric (mean ± SD) | |||
| BMI (kg/m2), mean ± SD | 31.0 ± 8.3 | 30.8 ± 6.4 | 0.9396 |
| Waist (cm), mean ± SD | 104.0 ± 22.9 | 102.3 ± 13.1 | 0.8522 |
| Clinical | |||
| Hypertension, n (%) | 7 (100.0%) | 57 (62.6%) | 0.0921 |
| Metabolic syndrome, n (%) | 5 (71.4%) | 63 (69.2%) | 1.0000 |
| Duration of DM (years) | 10.0 (3.0) | 8.0 (8.0) | 0.8459 |
| Oral hypoglycemic therapy, n (%) | 6 (85.7%) | 74 (81.3%) | 1.0000 |
| - Metformin, n (%) | 6 (85.7%) | 67 (73.6%) | 0.6743 |
| - Thiazolidinediones, n (%) | 0 | 2 (2.2%) | 1.0000 |
| Antilipemic therapy, n (%) | 6 (85.7%) | 48 (52.8%) | 0.1253 |
| Insulin therapy, n (%) | 2 (28.6%) | 19 (20.9%) | 0.6401 |
| Vitamin E, n (%) | 0 | 2 (2.2%) | 1.0000 |
| Biochemical | |||
| AST (U/L), median (IQR) | 28.0 (19.0) | 20.0 (10.0) | 0.0154 |
| Elevated AST (>40 U/L), n (%) | 3 (42.9%) | 5 (5.5%) | 0.0110 |
| ALT (IU/L), median (IQR) | 28.0 (22.0) | 23.0 (16.0) | 0.2878 |
| Elevated ALT (>40 U/L), n (%) | 2 (28.6%) | 16 (17.6%) | 0.6090 |
| GGT (U/L), median (IQR) | 38.0 (36.0) | 25.0 (16.0) | 0.2163 |
| Alkaline phosphatase (ALP) (U/L), median (IQR) | 73.0 (29.0) | 73.0 (31.0) | 1.0000 |
| HDL-C (mmol/L), median (IQR) | 1.45 (0.49) | 1.34 (0.72) | 0.7825 |
| LDL-C (mmol/L), median (IQR) | 1.78 (0.85) | 2.33 (1.11) | 0.0372 |
| Triglycerides (mmol/L), median (IQR) | 1.38 (0.75) | 1.58 (1.00) | 0.5302 |
| Platelet (109/L), mean ± SD | 217.0 ± 115.1 | 259.8 ± 67.0 | 0.3668 |
| HbA1C (%), median (IQR) | 6.7 (1.5) | 7.1 (1.6) | 0.8684 |
| Ferritin (pmol/L), median (IQR) | 184.3 (343.8) | 186.5 (283.1) | 0.6828 |
| Fasting glucose (mmol/L), median (IQR) | 6.97 (5.55) | 6.89 (2.72) | 0.7969 |
| Fasting insulin (mIU/mL / pmol/L), median (IQR) | 45.0/312.5 (114.0/791.7) | 15.0/104.2 (16.0/111.1) | 0.0196 |
| HOMA-IR, median (IQR) | 9.1 (14.7) | 5.0 (5.4) | 0.0452 |
| FFA, (mmol/L), mean ± SD | 0.65 ± 0.28 | 0.53 ± 0.23 | 0.2418 |
| Adipo –IR, median (IQR) | 22.6 (28.1) | 7.4 (8.3) | 0.0062 |
| Imaging | |||
| MRI-PDFF (%), median (IQR) | 6.4 (10.6) | 8.2 (9.7) | 0.7932 |
| MRE (kPa), median (IQR) | 4.2 (2.0) | 2.5 (0.6) | <0.0001 |
| NAFLD (MRI-PDFF ≥5%), n (%) | 5 (71.4%) | 59 (64.8%) | 1.0000 |
p-values: n (%) → chi-square test or Fisher’s exact test, mean ± SD → two independent samples t-test, median (IQR) → Wilcoxon-Mann-Whitney test
Abbreviations: Adipo-IR: adipose tissue insulin resistance, ALT: alanine aminotransferase, AST: aspartate aminotransferase, BMI: body mass index, DM: diabetes mellitus, FFA, free fatty acids, GGT: gamma-glutamyl transpeptidase, HbA1C: hemoglobin A1c, HDL-C: high-density lipoprotein cholesterol, HOMA-IR: Homeostatic Model Assessment of Insulin Resistance, NAFLD: nonalcoholic fatty liver disease; MRE: magnetic resonance elastography, MRI-PDFF: magnetic resonance imaging-estimated proton density fat fraction.
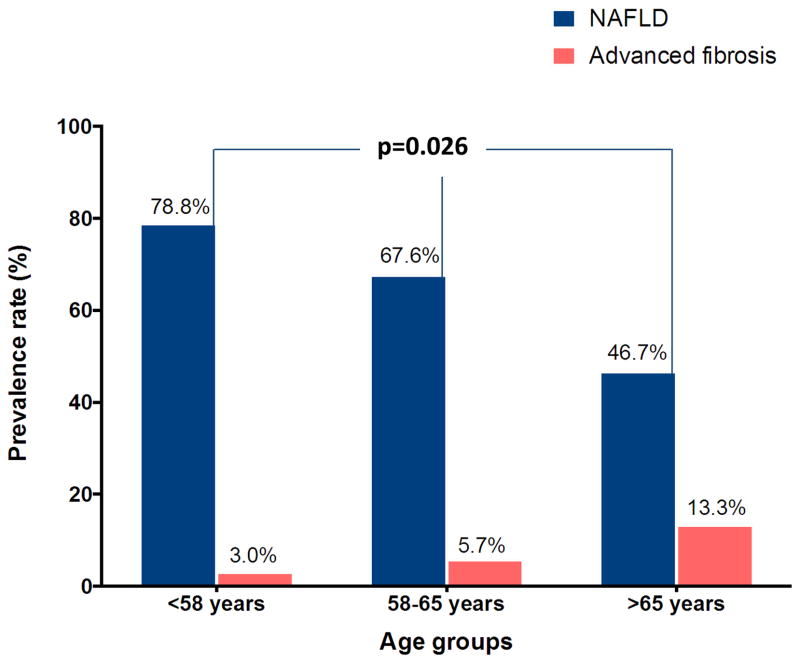
When divided into different age groups, younger patients had higher prevalence of NAFLD and lower prevalence of advanced fibrosis with p = 0.026 for the association between age and NAFLD (Figure 3).
Figure 3.
Prevalence of NAFLD and advanced fibrosis in different age groups.
DISCUSSION
Main findings
This is the first prospective study that assessed the feasibility of screening for both NAFLD and advanced fibrosis in type 2 diabetic patients in a primary care setting by using two accurate, precise, and validated non-invasive image-based biomarkers: MRI-PDFF and MRE-stiffness. The study cohort included a diverse population of patients with T2DM that has been managed and followed by primary care providers, and was conducted in the population likely to benefit from such screening program. All patients underwent MRI to estimate hepatic PDFF (100%) and 98% had MRE to estimate hepatic stiffness. We found a 65% prevalence of NAFLD and 7.1% prevalence of advanced fibrosis. One patient (1%) was noted to have definite HCC.
In context with published literature
Previous studies that aimed to screen for NAFLD in T2DM were mainly based on ultrasound imaging.5, 6 Targher and colleagues5 conducted a cross-sectional study enrolling 2839 diabetic patients in Italy and found a 71.1% age-adjusted prevalence of NAFLD in men and 68% in women based on ultrasound evaluation. Similarly, 939 randomly selected participants in the Edinburgh Type 2 Diabetes Study (ET2DS) were screened for NAFLD using ultrasound, but the prevalence of NAFLD was only 42.6%.6 The cohort of ET2DS was further screened for advanced fibrosis by using elevated hyaluronic acid level,33 a test with high negative predictive value (96%), but moderate positive predictive value (51%).34 Based on this test, 5.7% had evidence of hepatic fibrosis.33 Two patients (0.2%) in that cohort were diagnosed with HCC.
Only small population samples of diabetics have been assessed with liver biopsies.2, 9, 35, 36 Williams and colleagues2 analyzed 54 patients with T2DM and ultrasound-based diagnosis of NAFLD who underwent liver biopsies. NAFLD was confirmed in 74% and 22% had NASH in comparison to 11% in nondiabetics (p = 0.03). Furthermore, studies from Brazil and India have shown even higher histologic prevalence of NASH in diabetics ranging between 63 and 78%.9, 36 However, these studies also initially screened diabetics with ultrasound, and biopsies were performed only in those patients with positive imaging findings. Two studies have used MRS to assess NAFLD prevalence:7, 8 Petit and colleagues8 found NAFLD prevalence of 60.3% in 101 outpatient diabetics based on MRS > 5.5%. The other recent study of Portillo Sanchez and colleagues7 enrolled 103 mainly obese male diabetics with normal aminotransferases. The NAFLD prevalence was 76% based on MRS > 5.5% while NASH was noted in 56% based on liver biopsies of 37 patients. The prevalence of NAFLD in our study was based on an accurate and reproducible image-based biomarker, MRI-PDFF, and is in accordance with the previously reported prevalences.
In our cohort of diabetic patients, we found a 7.1% prevalence of advanced fibrosis using the precise method of MRE and based on a recently determined threshold.21 This finding emphasizes the potential importance of initiating concomitant NAFLD and advanced fibrosis screening in diabetics early, perhaps at the time of diagnosis of diabetes, in order to prevent further damage. Previous studies have demonstrated more aggressive course of NAFLD in diabetes and higher risk of cirrhosis in patients with T2DM and NAFLD.37, 38 The risk of advanced liver disease was present even when newly diagnosed diabetes patients were evaluated in a large population-based study.39 Moreover, patients with T2DM and NAFLD were noted to have a 2.2-fold increased risk of overall mortality compared to those without NAFLD, which was partially attributed to liver-related death.40 Population-based studies and meta-analysis also indicate that T2DM is associated with 2–3-fold increase in the risk of HCC41, 42 and HCC-related death (HR 3.38, CI 2.35–4.86)43, which further strengthens the importance for surveillance in this population. We also identified one patient with HCC although our study was not designed for HCC screening.
Two patients with advanced fibrosis in our study did not meet criteria for NAFLD. This finding is in accordance with recent meta-analysis that showed fibrosis progression over time in patients with NAFLD.44 Moreover, it again confirms the previously shown observation that liver fat declines in patients with advanced fibrosis (burnt-out NASH)45 and can be missed if diabetics are screened for NAFLD only.
Cost and limited availability of MRI-PDFF and MRE remain a concern. Furthermore, several serum biomarkers as well as ultrasound-based imaging biomarkers such as transient elastography (TE) have been studied in the non-invasive assessment of steatosis and fibrosis in patients with NAFLD.46–49 Although the available clinical prediction rules and TE are less expensive and more widely available than MRI, these tests have several limitations, especially in patients with NAFLD.46 TE is a reproducible, easy to perform, non-invasive method measuring both fibrosis and steatosis. However, it carries several disadvantages. Controlled Attenuation Parameter (CAP) for detection of steatosis can be used only with the conventional M probe, but not with the XL probe that has been designed to be used in overweight and obese patients. A large prospective study showed an overall CAP failure rate of 7.7%, with failure of 19.4% and 58.4% in those with BMI ≥ 30 kg/m2 and >40 kg/m2, respectively.50 CAP failure was independently associated with female gender, age >55 years, BMI ≥ 30 kg/m2, and metabolic syndrome. Similarly, liver stiffness measurement by TE fails to determine hepatic fibrosis in 3–10%, while unreliable examinations range between 15 and 26% when using the M probe.51, 52 A large study that evaluated performance of XL probe in biopsy-proven NAFLD found an overall reliable measurement by XL probe of 75% and of only 65% in patients with BMI ≥ 30 kg/m2.52 Moreover, when compared to histology, the results by XL probe showed discordance of at least two stages in 9% with single risk factor for discordance being BMI ≥ 35 kg/m2. All these disadvantages make TE a suboptimal technique for routine screening of steatosis and advanced fibrosis in diabetics as obesity is very common in patients with NAFLD.
MRE is thought to have higher accuracy, sensitivity and specificity for the diagnosis of advanced fibrosis than TE.21, 22, 53 Furthermore, MRE is not limited by obesity or presence of cirrhosis while TE has a high failure rate in these patients.54 In future, we expect that it may be feasible to stratify patients based upon their BMI and utilize MRE in those who are obese versus performing ultrasound-based tests such as TE or acoustic radiation force impulse (ARFI) in those who are not obese. Further studies are needed to address these issues.
Several clinical models for prediction of steatosis, steatohepatitis, and fibrosis have been studied and proposed in NAFLD. However, although they are fast, inexpensive, and widely available, these tests lack accuracy and are unable to appropriately classify patients into distinct categories.47 A large study evaluated the utility of 5 clinical biomarkers for prediction of steatosis in biopsy-proven NAFLD and noted that the biomarkers were able to detect the presence of steatosis, but unable to accurately quantify hepatic steatosis and more importantly, fibrosis and inflammation were significant confounders.48 Cui et al recently demonstrated in a prospective study that two-dimensional MRE had significantly higher accuracy for diagnosis of advanced fibrosis than 8 commonly used prediction rules.55 These data suggest that the best approach in screening diabetics for NAFLD would be a sequential algorithm using both clinical models and imaging modalities.56 Future studies should evaluate the best and most cost-effective modalities for screening of advanced fibrosis in diabetics with NAFLD.
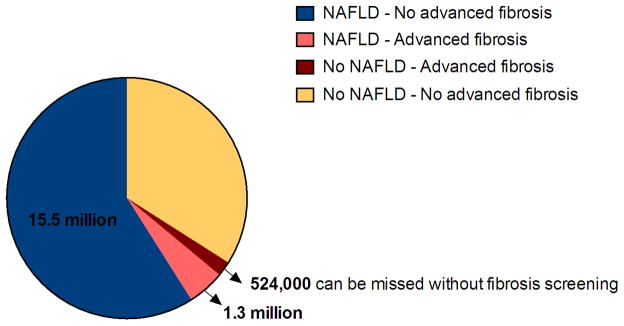
Recent analyses of prevalence and incidence of diabetes in the U.S. population showed doubling of both parameters between 1990 and 2008 and plateauing during 2008–2012.57 However, currently 29.1 million Americans - 9.3 % of the U.S. population - have diabetes.58 Of those, 90–95% are type 2 diabetics. Extrapolating the prevalences observed in our study (59% with NAFLD only, 5 % with NAFLD and advanced fibrosis, and 2% with advanced fibrosis, but no NAFLD) in asymptomatic type 2 diabetics to the entire population of diabetics, we estimate that there are about 15.5 million diabetic persons with NAFLD in the U.S.; approximately 1.3 million may have NAFLD and advanced fibrosis, and more than half million may have advanced fibrosis alone and could be missed if only NAFLD screening were performed (Figure 4). Compounding the problem, patients with NAFLD, and especially those with metabolic disturbances, have low awareness of NAFLD and its complications and management.59 Furthermore, a recent study assessed NAFLD management in primary care settings and noted that the magnitude and proportion of ALT elevation was the only predictor of NAFLD care and only 3% of those with high risk of fibrosis were referred to a specialist.60 All these findings imply that prompt changes are needed in surveillance strategy, management, and education of both patients and primary care providers taking care of patients at high risk of NAFLD, especially those with T2DM. We found that concomitant use of MRI to estimate PDFF and MRE to estimate stiffness are feasible for NAFLD and advanced fibrosis screening and can be used to identify patients at risk.
Figure 4.
Estimated population with NAFLD and advanced fibrosis in type 2 diabetes based on diabetes prevalence in 2012* and results of our study#
Strengths and limitations
The key strengths of our study are that this was a prospective study, which included asymptomatic patients in different clinical stages of T2DM. Patients were referred for the study by their primary care providers and constitute a representative sample of diabetics. Advanced MR imaging techniques were used for concomitant screening of NAFLD (MRI-PDFF) and advanced fibrosis (MRE-stiffness). Both imaging techniques are safe, accurate and reproducible and have been validated to correlate well with histologic changes in liver fat and fibrosis.20, 21 However, we would like to acknowledge some limitations of our study. This is a single-center study conducted at a specialized center for both clinical and MRI research in NAFLD. Although validated, MRI-PDFF and MRE-stiffness have not been approved for screening of NAFLD and advanced fibrosis and currently are available mainly in specialized tertiary centers.
Implications for future research and patient care
Patients with T2DM have a high prevalence of NAFLD and advanced fibrosis. This frequently remains unrecognized and overlooked in the primary care setting. This is the first proof-of-concept study that used advanced MR methods to accurately screen for both NAFLD and advanced fibrosis. Future studies should include larger populations of diabetic patients and utilize different non-invasive clinical prediction models and imaging techniques, (including advanced and emerging ultrasound-based methods)53, 61 to better assess the most accurate and cost-effective modality, the best time to start screening, and the optimal screening algorithm in this population in order to prevent advanced liver damage and reduce liver-related complications and mortality in diabetics.
Supplementary Material
Acknowledgments
Declaration of Funding interests: The study was conducted at the Clinical and Translational Research Institute, University of California at San Diego. RL is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award; Funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Association of Specialty Professors, and the American Gastroenterological Association and grant K23-DK090303. JC is supported by NIH T32 training grant 5TL1TR000098-05.
Role of study sponsor: The study sponsor(s) had no role in the study design, collection, analysis, interpretation of the data, and/or drafting of the manuscript.
Abbreviations
- Adipo-IR
adipose tissue insulin resistance
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUDIT
Alcohol Use Disorders Identification Test
- BMI
body mass index
- CT
computed tomography
- ET2DS
Edinburgh Type 2 Diabetes Study
- GGT
gamma-glutamyl transpeptidase
- HDL-C
high-density lipoprotein cholesterol
- HCC
hepatocellular carcinoma
- HOMA-IR
Homeostatic Model Assessment of Insulin Resistance
- LDL-C
low-density lipoprotein cholesterol
- MRE
magnetic resonance elastography
- MRI-PDFF
magnetic resonance imaging-estimated proton density fat fraction
- MRS
magnetic resonance spectroscopy
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- TE
transient elastography
- T2DM
type 2 diabetes mellitus
- UCSD
University of California, San Diego
Footnotes
The prevalence of type 2 diabetes in the U.S. in 2012 was 29.1 million. For the purpose of this figure we accepted that 90% of those have type 2 diabetes, which equals 26.2 million
15.5 million (59%) would have NAFLD only; 1.3 million (5%) would have both NAFLD and advanced fibrosis. Approximately 524,000 of all diabetics would have only advanced fibrosis and no NAFLD. These patients can be missed if only screening for NAFLD is performed.
All authors report no conflicts of interest.
AUTHORSHIP
Guarantor of the article: Rohit Loomba
Author contributions: Iliana Doycheva - study concept and design, interpretation of data, drafting of the manuscript, critical revision of the manuscript, approved final submission. Jeffrey Cui – statistical analysis, data analysis, critical revision of the manuscript, approved final submission. Phirum Nguyen - patient visits, acquisition of data, critical revision of the manuscript, approved final submission. Eduardo A. C. Costa – image analysis, critical revision of the manuscript, approved final submission. Jonathan Hooker - image analysis, critical revision of the manuscript, approved final submission. Sharon S. Brouha - critical revision of the manuscript, approved final submission. Heather Hofflich - critical revision of the manuscript, approved final submission. Ricki Bettencourt - statistical analysis, critical revision of the manuscript, approved final submission. Claude Sirlin - analysis and interpretation of images and data, critical revision of the manuscript, approved final submission. Rohit Loomba - study concept and design, obtained funding, study supervision, critical revision of the manuscript, approved final submission. All authors approved the final version of the manuscript.
Additional supporting information may be found in the online version of this article:
Methods: Detailed description of MRI-PDFF and MRE protocols
Table S1. Oral hypoglycemic and antilipemic therapy.
Figure S1. Derivation of patient cohort.
References
- 1.Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2013;178:38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 3.Cusi K. Role of insulin resistance and lipotoxicity in non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:545–63. doi: 10.1016/j.cld.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Zarrinpar A, Loomba R. Review article: the emerging interplay among the gastrointestinal tract, bile acids and incretins in the pathogenesis of diabetes and non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2012;36:909–21. doi: 10.1111/apt.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212–8. doi: 10.2337/dc06-2247. [DOI] [PubMed] [Google Scholar]
- 6.Williamson RM, Price JF, Glancy S, et al. Prevalence of and risk factors for hepatic steatosis and nonalcoholic Fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care. 2011;34:1139–44. doi: 10.2337/dc10-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Portillo Sanchez P, Bril F, Maximos M, et al. High Prevalence of Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J Clin Endocrinol Metab. 2014:jc20142739. doi: 10.1210/jc.2014-2739. [DOI] [PubMed] [Google Scholar]
- 8.Petit JM, Guiu B, Terriat B, et al. Nonalcoholic fatty liver is not associated with carotid intima-media thickness in type 2 diabetic patients. J Clin Endocrinol Metab. 2009;94:4103–6. doi: 10.1210/jc.2009-0541. [DOI] [PubMed] [Google Scholar]
- 9.Leite NC, Villela-Nogueira CA, Pannain VL, et al. Histopathological stages of nonalcoholic fatty liver disease in type 2 diabetes: prevalences and correlated factors. Liver Int. 2011;31:700–6. doi: 10.1111/j.1478-3231.2011.02482.x. [DOI] [PubMed] [Google Scholar]
- 10.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–62. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 11.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 12.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 13.Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011;21:87–97. doi: 10.1007/s00330-010-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pais R, Charlotte F, Fedchuk L, et al. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol. 2013;59:550–6. doi: 10.1016/j.jhep.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Yokoo T, Shiehmorteza M, Hamilton G, et al. Estimation of hepatic proton-density fat fraction by using MR imaging at 3. 0 T. Radiology. 2011;258:749–59. doi: 10.1148/radiol.10100659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoo T, Bydder M, Hamilton G, et al. Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1. 5 T. Radiology. 2009;251:67–76. doi: 10.1148/radiol.2511080666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meisamy S, Hines CD, Hamilton G, et al. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology. 2011;258:767–75. doi: 10.1148/radiol.10100708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang A, Desai A, Hamilton G, et al. Accuracy of MR Imaging-estimated Proton Density Fat Fraction for Classification of Dichotomized Histologic Steatosis Grades in Nonalcoholic Fatty Liver Disease. Radiology. 2014:140754. doi: 10.1148/radiol.14140754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang A, Tan J, Sun M, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267:422–31. doi: 10.1148/radiol.12120896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noureddin M, Lam J, Peterson MR, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930–40. doi: 10.1002/hep.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loomba R, Wolfson T, Ang B, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology. 2014;60:1920–8. doi: 10.1002/hep.27362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim D, Kim WR, Talwalkar JA, Kim HJ, Ehman RL. Advanced fibrosis in nonalcoholic fatty liver disease: noninvasive assessment with MR elastography. Radiology. 2013;268:411–9. doi: 10.1148/radiol.13121193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes A. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35 (Suppl 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 26.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 27.Le TA, Chen J, Changchien C, et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology. 2012;56:922–32. doi: 10.1002/hep.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Negrete LM, Middleton MS, Clark L, et al. Inter-examination precision of magnitude-based MRI for estimation of segmental hepatic proton density fat fraction in obese subjects. J Magn Reson Imaging. 2014;39:1265–71. doi: 10.1002/jmri.24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonekamp S, Tang A, Mashhood A, et al. Spatial distribution of MRI-Determined hepatic proton density fat fraction in adults with nonalcoholic fatty liver disease. J Magn Reson Imaging. 2014;39:1525–32. doi: 10.1002/jmri.24321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Permutt Z, Le TA, Peterson MR, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36:22–9. doi: 10.1111/j.1365-2036.2012.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang GH, Cruite I, Shiehmorteza M, et al. Reproducibility of MRI-determined proton density fat fraction across two different MR scanner platforms. J Magn Reson Imaging. 2011;34:928–34. doi: 10.1002/jmri.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin M, Talwalkar JA, Glaser KJ, et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5:1207–1213. e2. doi: 10.1016/j.cgh.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williamson RM, Price JF, Hayes PC, et al. Prevalence and markers of advanced liver disease in type 2 diabetes. QJM. 2012;105:425–32. doi: 10.1093/qjmed/hcr233. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki A, Angulo P, Lymp J, Li D, Satomura S, Lindor K. Hyaluronic acid, an accurate serum marker for severe hepatic fibrosis in patients with non-alcoholic fatty liver disease. Liver Int. 2005;25:779–86. doi: 10.1111/j.1478-3231.2005.01064.x. [DOI] [PubMed] [Google Scholar]
- 35.Gupte P, Amarapurkar D, Agal S, et al. Non-alcoholic steatohepatitis in type 2 diabetes mellitus. J Gastroenterol Hepatol. 2004;19:854–8. doi: 10.1111/j.1440-1746.2004.03312.x. [DOI] [PubMed] [Google Scholar]
- 36.Prashanth M, Ganesh HK, Vima MV, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. J Assoc Physicians India. 2009;57:205–10. [PubMed] [Google Scholar]
- 37.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–8. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004;2:262–5. doi: 10.1016/s1542-3565(04)00014-x. [DOI] [PubMed] [Google Scholar]
- 39.Porepa L, Ray JG, Sanchez-Romeu P, Booth GL. Newly diagnosed diabetes mellitus as a risk factor for serious liver disease. CMAJ. 2010;182:E526–31. doi: 10.1503/cmaj.092144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105:1567–73. doi: 10.1038/ajg.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533–9. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28:109–22. doi: 10.1002/dmrr.1291. [DOI] [PubMed] [Google Scholar]
- 43.Chiang CH, Lee LT, Hung SH, et al. Opposite association between diabetes, dyslipidemia, and hepatocellular carcinoma mortality in the middle-aged and elderly. Hepatology. 2014;59:2207–15. doi: 10.1002/hep.27014. [DOI] [PubMed] [Google Scholar]
- 44.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis Progression in Nonalcoholic Fatty Liver vs Nonalcoholic Steatohepatitis: A Systematic Review and Meta-analysis of Paired-Biopsy Studies. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Poorten D, Samer CF, Ramezani-Moghadam M, et al. Hepatic fat loss in advanced nonalcoholic steatohepatitis: are alterations in serum adiponectin the cause? Hepatology. 2013;57:2180–8. doi: 10.1002/hep.26072. [DOI] [PubMed] [Google Scholar]
- 46.Kwok R, Tse YK, Wong GL, et al. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease--the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharmacol Ther. 2014;39:254–69. doi: 10.1111/apt.12569. [DOI] [PubMed] [Google Scholar]
- 47.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–49. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 48.Fedchuk L, Nascimbeni F, Pais R, et al. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014;40:1209–22. doi: 10.1111/apt.12963. [DOI] [PubMed] [Google Scholar]
- 49.Verna EC, Patel J, Bettencourt R, et al. Novel association between serum pentraxin-2 levels and advanced fibrosis in well-characterised patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2015;42:582–90. doi: 10.1111/apt.13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Ledinghen V, Vergniol J, Capdepont M, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014;60:1026–31. doi: 10.1016/j.jhep.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 51.Castera L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828–35. doi: 10.1002/hep.23425. [DOI] [PubMed] [Google Scholar]
- 52.Wong VW, Vergniol J, Wong GL, et al. Liver stiffness measurement using X probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2012;107:1862–71. doi: 10.1038/ajg.2012.331. [DOI] [PubMed] [Google Scholar]
- 53.Loomba R, Sirlin CB, Ang B, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial) Hepatology. 2015;61:1239–50. doi: 10.1002/hep.27647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castera L, Vilgrain V, Angulo P. Noninvasive evaluation of NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:666–75. doi: 10.1038/nrgastro.2013.175. [DOI] [PubMed] [Google Scholar]
- 55.Cui J, Ang B, Haufe W, et al. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: a prospective study. Aliment Pharmacol Ther. 2015;41:1271–80. doi: 10.1111/apt.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bazick J, Donithan M, Neuschwander-Tetri BA, et al. Clinical Model for NASH and Advanced Fibrosis in Adult Patients With Diabetes and NAFLD: Guidelines for Referral in NAFLD. Diabetes Care. 2015;38:1347–55. doi: 10.2337/dc14-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geiss LS, Wang J, Cheng YJ, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312:1218–26. doi: 10.1001/jama.2014.11494. [DOI] [PubMed] [Google Scholar]
- 58.CDC. National Diabetes Statistics Report. 2014. [Google Scholar]
- 59.Wieland AC, Mettler P, McDermott MT, Crane LA, Cicutto LC, Bambha KM. Low awareness of nonalcoholic Fatty liver disease among patients at high metabolic risk. J Clin Gastroenterol. 2015;49:e6–e10. doi: 10.1097/MCG.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 60.Blais P, Husain N, Kramer JR, Kowalkowski M, El-Serag H, Kanwal F. Nonalcoholic Fatty Liver Disease is Underrecognized in the Primary Care Setting. Am J Gastroenterol. 2015;110:10–4. doi: 10.1038/ajg.2014.134. [DOI] [PubMed] [Google Scholar]
- 61.Lin SC, Heba E, Wolfson T, et al. Noninvasive Diagnosis of Nonalcoholic Fatty Liver Disease and Quantification of Liver Fat Using a New Quantitative Ultrasound Technique. Clin Gastroenterol Hepatol. 2015;13:1337–1345. e6. doi: 10.1016/j.cgh.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.