Abstract
Skeletal Muscle Ultrastructure and Function in Statin-Tolerant Individuals:
Introduction
Statins have well-known benefits on cardiovascular mortality, though up to 15% of patients experience side effects. With guidelines from the American Heart Association, American College of Cardiology, and American Diabetics Association expected to double the number of statin users, the overall incidence of myalgia and myopathy will increase.
Methods
We evaluated skeletal muscle structure and contractile function at the molecular, cellular, and whole tissue levels in 12 statin tolerant and 12 control subjects.
Results
Myosin isoform expression, fiber type distributions, single fiber maximal Ca2+-activated tension, and whole muscle contractile force were similar between groups. No differences were observed in myosin-actin cross-bridge kinetics in myosin heavy chain (MHC) I or IIA fibers.
Discussion
We found no evidence for statin-induced changes in muscle morphology at the molecular, cellular, or whole tissue levels. Collectively, our data show that chronic statin therapy in healthy asymptomatic individuals does not promote deleterious myofilament structural or functional adaptations.
Keywords: myalgia, myopathy, statin, skeletal muscle, ultrastructure
Introduction
HMG Co-Reductase inhibitors (statins), have well-known health benefits, the most prominent being the reduction of low-density lipoprotein cholesterol and cardiovascular and all-cause mortality1, 2. Additionally, they have been shown to reduce inflammation3, decrease the hypertensive effects of the angiotensin system4, and improve endothelial function5 and arterial compliance6. According to the National Center for Health Statistics, 25% of Americans aged 45 or older were prescribed statin therapy from 2005-2008. With updated guidelines released by the American Heart Association and American College of Cardiology7 and the American Diabetes Association8, the number of potential statin users may double. However, despite documented reductions in mortality and additional pleiotropic benefits, statin use also has potential side effects. These include myalgia9, myopathy10, rhabdomyolysis11, insulin resistance12, and hepatotoxicity13. Furthermore, statins may be associated with increased fatigue with exertion14, greater susceptibility to muscle damage with vigorous exercise15, and mitochondrial dysfunction16,17. Attenuated cardiorespiratory benefits with exercise training18 and poor tolerance by elite athletes19 have been reported.
The incidence of documented myopathy ranges from 1-5% in randomized clinical trials, though actual cases may be up to 10% in the clinical setting10,20. Furthermore, observational reports indicate myalgia rates of 10-15%10. Despite numerous reports of muscle complaints, the mechanism of statin-induced myopathy is not completely understood and may result from a combination of mitochondrial dysfunction17, dysregulation of cellular apoptosis and protein degradation21, depletion of the mevalonate pathway22, drug interactions23, genetic predisposition24, and immune-mediated myopathy25,26. Additionally, there is the potential for sub-clinical myopathies that impair skeletal muscle contractile or energetic function, as evidenced by reports of increased production of reactive oxygen species15, diminished mitochondrial capacity, and reduced biogenesis15,18,27. These adaptations could affect the fundamental contractile capacity of skeletal muscle28. If present, the resultant reduction in physiological capacity could actually cause patients to become less physically active, which could produce a counterbalancing effect of increasing the likelihood of inactivity-related co-morbidities29-31.
In a prospective comparative study, 15% of reported adverse reactions were associated with objective muscle weakness in statin users32. As statin therapy may have myotoxic effects33 and has been shown to decrease both twitch and tetanic peak force and duration in rat models34, alterations to skeletal muscle contractility could be expected. Additionally, animal models have shown that statins potentiate muscle fiber atrophy35, which would decrease muscle strength and performance. Despite the evidence for myopathic effects, no studies have rigorously explored the effects of statins on skeletal muscle fiber size or function in humans. Although some studies have been conducted at the whole muscle level36,37, these indices are influenced by myriad physiological systems and volitional effort, which can confound detection of statin effects. Assessment of muscle structure and function at the cellular, sub-cellular, and molecular levels would provide a more accurate picture of the effects of statins on intrinsic skeletal muscle size and function, as well as determine if sub-clinical myopathy is present with chronic therapy. If present in otherwise asymptomatic individuals, it would provide insight into potential mechanisms in which statin-intolerance induces muscle dysfunction. Therefore, we sought to determine whether statin use affects muscle size, composition, and contractility via measurements at the whole muscle, cellular, sub-cellular, and molecular levels in subjects on stable statin therapy without complaints of myalgia or elevated creatine kinase (CK) levels.
METHODS
Subjects
This retrospective study utilized a cohort of 24 (10 men, 14 women) healthy older volunteers who were recruited for past and on-going studies in our laboratory and who differed clinically by their use of statins. Data from volunteers participating in 2 separate studies were combined for this analysis. In the first study38, we used data from 8 physically inactive subjects including 4 (3 women, 1 man) on a stable regimen of statins and 4 (3 women, 1 man) not taking statins (ie, non-statin controls). Data from a second study28, including both healthy active subjects and patients with advanced-stage knee osteoarthritis, were used with 8 taking statins (4 women, 4 men; 4 healthy, 4 knee OA) and 8 non-statin controls (4 women, 4 men; 4 healthy, 4 knee OA). In statin tolerant subjects, the type and dose varied (see Table 1 for details), as did the length of therapy, from 4 months to 10 years (4 ± 1 year). Based on a large review of side-effects attributed to statins, we pooled various statin medications, as all were found to promote myopathy at reasonably similar rates10. Volunteers were considered healthy based on medical screening and routine clinical/laboratory tests. Participants had no prior history of cancer (within past 10 yrs, excluding non-melanoma skin cancer), chronic lung or cardiovascular disease or neurologic disease, and all were non-smokers. None were taking any medications, aside from statins, which are known to influence skeletal muscle. None of the statin users reported muscle pain or weakness. Written informed consent was obtained from all volunteers prior to their participation, and protocols for controls and statin subjects were approved by the Committees on Human Research at the University of Vermont.
Table 1.
Physical and clinical characteristics.
| Control | Statin | P-value | ||
|---|---|---|---|---|
| 12 (5/7) | 12 (5/7) | |||
| n (M/W) | ||||
| Age (yr) | 70 ± 2 | 70 ± 1 | 0.78 | |
| Body mass (kg) | 71.1 ± 3.6 | 80.3 ± 3.6 | 0.08 | |
| Height (m) | 1.67 ± 0.03 | 1.64 ± 0.03 | 0.44 | |
| Body mass index (kg/m2) | 25.4 ± 0.8 | 29.9 ± 0.9 | 0.001 | |
| Fat mass (kg) | 24.1 ± 2.6 | 31.6 ± 1.6 | 0.02 | |
| Fat-free mass (kg) | 44.2 ± 2.9 | 46.2 ± 2.9 | 0.64 | |
| Appendicular skeletal muscle mass (kg) |
25.6 ±1.8 | 26.6 ± 2.1 | 0.73 | |
| Activity level (kcal/d) | 283 ± 38 | 360 ± 47 | 0.21 | |
| Creatine kinase (U/L) | 89 ± 22 | 125 ± 15 | 0.20 | |
|
Statin Medication
(Type/Dose) |
Mean
Duration of Use (yr) |
|||
| Simvastatin | 20 mg | 0 | 4 | 4.8 |
| Atorvastatin | 20 mg | 0 | 3 | 1.4 |
| 10 mg | 1 | 0.3 | ||
| Lovastatin | 40 mg | 0 | 2 | 6 |
| Pravastatin | 20 mg | 0 | 1 | 10 |
| 10 mg | 1 | 2 | ||
Data are mean ± SE, except statin medication information, which reflects number of patients taking each medication and mean duration of use for each medication. Activity level reflects weight-bearing activity measured via accelerometry. Sample sizes were 12 per group, with the exception of creatine kinase (11/12 statin).
Knee extensor muscle function
Knee extensor isometric torque production was measured at 30°, 70°, and 90° (0°=full extension) and isokinetic torque production at rates of 60°/second and 180°/second, as described39.
Total and regional body composition
Body mass was measured on a digital scale (ScaleTronix, Wheaton, IL). Total and regional fat mass, fat-free mass, and bone mass were measured by dual energy x-ray absorptiometry as described.39,40
Muscle tissue processing
Percutaneous muscle biopsy of the vastus lateralis was performed, as described41. Muscle tissue was partitioned for mechanical, morphological, and biochemical analysis. For single fiber mechanical assessments, tissue was placed immediately into cold (4°C) dissecting solution (all solutions discussed herein were previously described).42 Thereafter, muscle fiber bundles were dissected and processed for single fiber measurements, as described42. Muscle tissue used for morphological analysis was processed as follows. Immediately after obtaining the biopsy, a small bundle of muscle fibers (~100 fibers) was dissected and tied to glass rods at a slightly stretched length and placed into 2.5%/1% glutaradlehyde/paraformaldehyde (sodium cacodylate) fixative for electron microscopy (EM) studies and then processed for EM measurements, as described42. The remaining tissue not aliquoted for mechanical or morphological analysis was immediately frozen in liquid nitrogen and stored at −80°C until analysis.
Myosin heavy chain (MHC) and actin protein content and MHC isoform distribution
Myosin and actin protein content were evaluated via gel electrophoresis in tissue homogenates (~10 mg), as was MHC isoform distribution, as described42.
Single muscle fiber morphology
Single fiber cross-sectional area (CSA) was measured along the length (every 250 μm) of segments of chemically-skinned muscle fibers (average = 2937 ± 24 μm), as described previously42, which accounts for possible longitudinal inhomogeneity in fiber size43.
Ultrastructural measurements
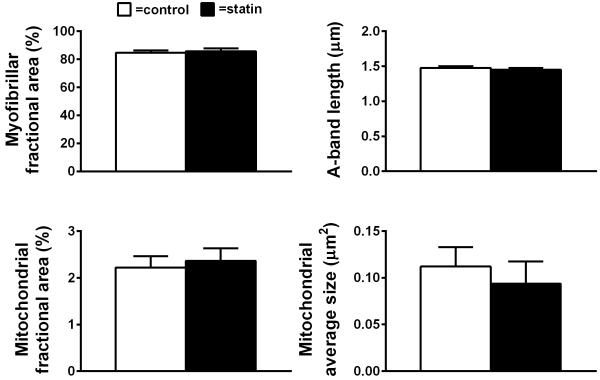
EM measurements were conducted on intact (ie, unskinned) skeletal muscle fiber bundles to assess myofibrillar area fraction, A-band length, intermyofibrillar mitochondrial area fraction, and average area, as described.41,42
Single muscle fiber mechanical measurements
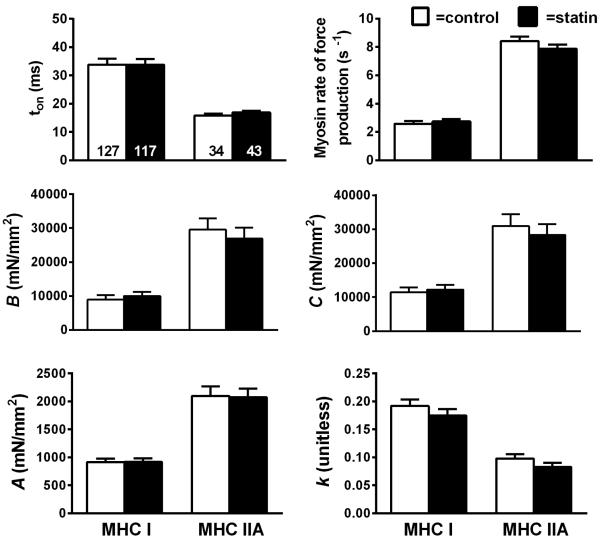
Segments (~2.5 mm) of chemically-skinned single fibers were isolated and processed for mechanical measurements on controls (n=127 MHC I fibers and n=34 MHC IIA fibers) and statin subjects (n=117 MHC I fibers and n=43 MHC IIA fibers), as described in detail.42,44 Sinusoidal analysis was performed under maximal Ca2+-activated conditions (pCa 4.5; 25°C and 5 mM Pi) to estimate myosin-actin cross-bridge mechanics and kinetics, as described44, with single muscle fiber tension (force/CSA) derived from the plateau of tension following maximal Ca2+ activation prior to beginning sinusoidal length oscillations
Following mechanical assessments, single fibers were placed in gel loading buffer, heated for 2 min at 65°C and stored at −80°C until determination of MHC isoform composition by SDS-PAGE as described42. We restricted this study to MHC I and IIA fibers, as the numbers of IIX and other hybrid (IIA/X, I/IIA, I/IIA/IIX) fibers were too few to permit analysis.
To characterize myosin-actin cross-bridge mechanics and kinetics using sinusoidal analysis, complex modulus data (elastic and viscous moduli, derived as described previously)44 at peak calcium activation were fitted mathematically, as described44 to derive 3 characteristic processes, A, B, and C, which relate to various mechanical (A, B, C, and k) and kinetic [2πb and (2πc)−1] properties of the cross-bridge cycle, as described44 in detail. Briefly, the frequency portion of the B-process, 2πb, is interpreted as the rate of myosin transition between the weakly- and strongly-bound states45,46. The inverse of the frequency portion of the C-process, or (2πc)−1, represents the average myosin attachment time (ton) to actin47. The magnitudes of the B- and C-processes (parameters B and C) are proportional to the number of myosin heads strongly bound to actin and the cross-bridge stiffness45. Finally, as the A-process has no kinetic or enzymatic dependence48, it represents the viscoelastic properties of the non-enzymatic, passive elements of the myofilaments, which under Ca2+-activated conditions represents the underlying stiffness of the lattice structure and the attached myosin heads in series48. The parameter A indicates the magnitude of the viscoelastic modulus and k the angle at which the A-process lies relative to the x-axis, which reflects the viscous to elastic modulus relationship of the A-process (k=0 purely elastic vs. k=1 purely viscous).
Statistics
Differences between statin subjects and controls were determined using analysis of variance or analysis of covariance in the case of knee extensor muscle function (SPSS version 19; IBM SPSS Statistics, Armonk, NY). For the variables in which multiple observations were performed within the same individual (eg, single fiber ultrastructural, morphological, and mechanical indices), a linear mixed model (SAS Version 9.3; SAS Institute, Cary, NC) was used, with group assignment being the between-subject factor (ie, non-statin versus statin for most comparisons). In this model, a random effect is used to account for the clustering of observations within individuals. Inclusion of this effect is necessary because the standard general linear model assumes that each measurement is independent, which is not the case for fibers evaluated from different volunteers (ie, fibers from the same subject are related). In other words, each fiber cannot be considered a single observation. Additionally, as we have found that sex influences adaptations in myofilament function with aging and muscle disuse28,49 we also included sex in the aforementioned models to explore whether any effects of statins may differ in men and women. All data are reported as mean ± SEM.
RESULTS
Physical characteristics
Body composition and physical characteristics of statin subjects and controls are shown in Table 1. No differences in age, height, body mass, fat-free mass, appendicular skeletal muscle mass, or physical activity were found between groups. Additionally, CK levels were similar between statin subjects and controls. Individuals on statins had greater adiposity, using either body mass index (P=0.001) or absolute fat mass measurement (P=0.02), compared to controls.
Whole muscle function
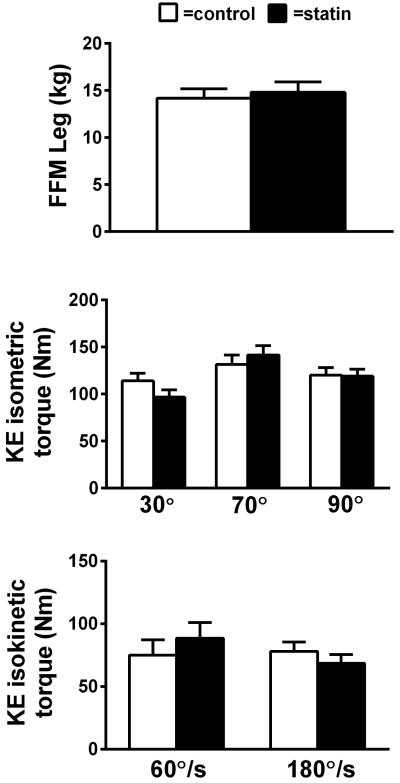
Leg fat-free mass and knee extensor contractile function are shown in Figure 1. No differences in leg fat-free mass or knee extensor isometric or isokinetic torque were found between individuals on statins and controls. When adjusted for leg fat-free mass, contractile function remained similar between groups across all knee angles and contraction velocities.
Figure 1.
Knee extensor isometric and isokinetic torque in controls and subjects taking statins. For group comparisons, data were adjusted for leg fat-free mass. Sample sizes were 12 per group (statin/control), with the exception of 30° and 90° isometric (11/12 statin, 10/12 control) and 180°/s isokinetic (10/12 control). Data are mean ± SE. (P=NS)
Single muscle fiber size and distribution
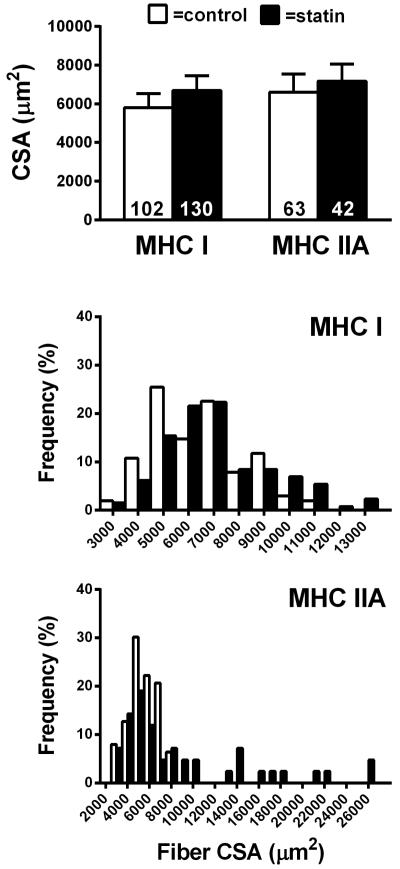
Single fiber CSA in MHC I and IIA fibers was not significantly different between groups, and no group by sex interaction was noted (P=0.41 and P=0.66; Figure 2, upper panel). Frequency distributions of individual fiber size were generally similar for MHC I and IIA fibers, though individuals on statins had greater proportions of large fibers. MHC IIA/X hybrid fibers were not found in all subjects and were therefore not included in the figure (n=16/24 subjects). Subsequent analysis revealed no difference in CSA in this limited sample (P=0.09) (number of fibers analyzed; control n=37, statin n=39). Of note, although this analysis shows a trend towards a difference, it favored larger muscle fiber size in statin users.
Figure 2.
Skeletal muscle fiber morphology in controls and subjects taking statins. Data are shown for muscle fiber cross-sectional area average size and relative frequency for MHC I and IIA fibers. Data are mean ± SE. (P=NS)
Myosin isoform expression and fiber type distributions
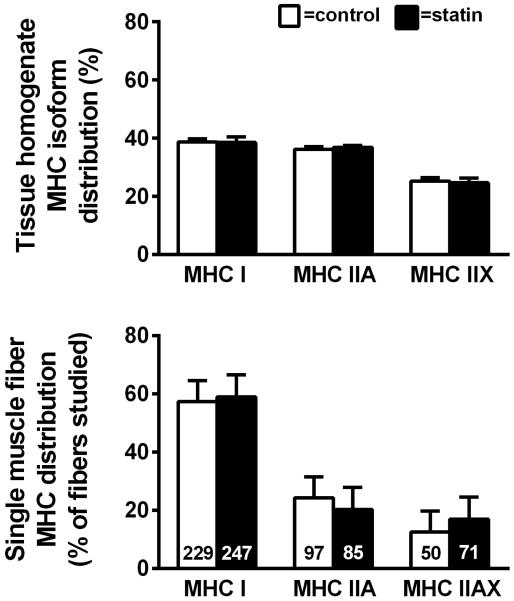
The relative expression of myosin isoforms measured in tissue homogenates by gel electrophoresis and the relative composition of single fibers analyzed for morphological and mechanical outcome are shown in Figure 3. No differences in myosin protein content were found between statin subjects and controls, arguing against an effect of statins on muscle fiber type distribution. The isoform expression in single muscle fibers was measured in those fibers studied for morphological (Figure 2) and mechanical (Figure 4) outcomes (n=34 ± 1 fibers/volunteer) by SDS-PAGE. When all of the fibers are considered together, the relative proportion of MHC I vs. IIA vs. IIAX fiber types did not differ between individuals on statin therapy and controls (lower panel). Of note, there was no effect of sex to modify these parameters, and the number of other fiber types (MHC IIX) and hybrid fibers (MHC I/IIA and I/IIA/IIX) were too few to permit analysis.
Figure 3.
Skeletal muscle tissue homogenate and single fiber isoform distribution in controls and subjects taking statins. Data are mean ± SE. (P=NS)
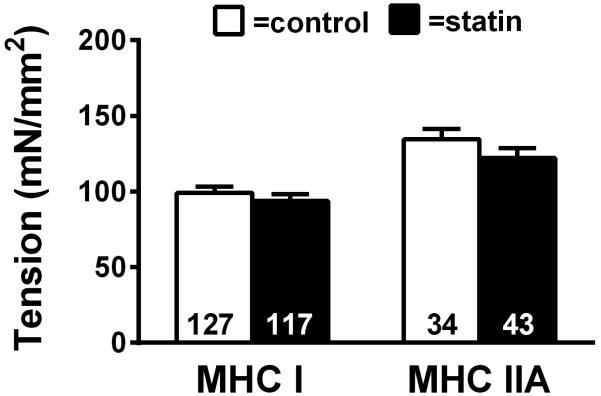
Figure 4.
Single skeletal muscle fiber maximal Ca2+-activated (pCa 4.5) isometric tension in MHC I and MHC IIA fibers (25°C) in controls and subjects taking statins. The number of fibers evaluated is at the base of each bar. Data are mean ± SE. (P=NS)
Single fiber contractile function
Single fiber maximal Ca2+-activated tension data for MHC I and IIA fibers are shown in Figure 4. Single fiber tension was similar between statin subjects and controls for both MHC I and IIA fibers and sex did not alter the results. MHC IIA/X hybrid fibers were excluded from the figure due to the restricted sample size (n=13/24 subjects), though similar single fiber tension was found between groups (P=0.43) (number of fibers analyzed: control n=13, statin n=32).
Myosin-actin cross-bridge mechanics and kinetics
To further explore the effects of statin use on muscle function at the molecular level, we employed sinusoidal analysis to estimate myosin-actin cross-bridge mechanics and kinetics (Figure 5). In both MHC I and IIA fibers, there were no differences in cross-bridge mechanics or kinetics and no effect by sex to modify any of these parameters, as evidenced by similar myosin attachment times (ton), rate of myosin force production, parameters reflecting the number of strongly-bound cross-bridges and cross-bridge stiffness (B and C), and viscoelastic properties (A and k) between statin subjects and controls. MHC IIA/X hybrid fibers were not found in all subjects and therefore not included in the figure (n=13/24 subjects). Subsequent analysis revealed no differences across all measures of cross-bridge mechanics and kinetics in the limited sample (ton, P=0.92; rate of myosin force production, P=0.91; B, P=0.60; C, P=0.61; A, P=0.29; k, P=0.66) (number of fibers analyzed; control n=13, statin n=32).
Figure 5.
Sinusoidal analysis model parameters for maximal Ca2+-activated (pCa 4.5) MHC I and IIA fibers in controls and subjects taking statins (n = 8). The number of fibers studied is shown at the base of each bar on the panel showing myosin attachment time. Definitions for each variable are provided in Methods. (P=NS)
Myofibrillar and mitochondrial structure
We further evaluated the effect of statins on skeletal muscle fiber ultrastructure by evaluating myofilament fractional content and filament length. Moreover, as statin treatment has been associated with altered mitochondrial protein content and dysfunction16-18, we also measured mitochondrial content and average area (Figure 6) to determine if either of these phenotypes is accompanied by structural alterations in mitochondria. Neither myofibrillar fractional area nor A-band length differed between groups. Moreover, no differences in mitochondrial fractional area or average mitochondrial size were observed, and no groups by sex interactions were noted for any parameter.
Figure 6.
Skeletal muscle myofilament ultrastructure and mitochondrial density in controls and subjects taking statins. Average data are shown for myofibrillar fractional area, A-band length, mitochondrial fractional area, and mitochondrial average size. Data are mean ± SE. (P=NS)
Discussion
The prevalence of statin use is likely to increase in the future, with recent recommendations calling for their broader application to individuals at risk for coronary artery disease7 and new indications for their use are growing rapidly50. Although incidences of statin-induced myalgia and myopathy are low (1-5%myalgia, 1-10%myopathy)10,20, higher rates of adverse effects have been found by some studies, suggesting that statins may promote generalized muscle weakness/dysfunction32,51. Although our cohort consisted of statin tolerant individuals and was nonrandomized, we pursued our analysis, since there is evidence of varying levels of statin-induced CK elevation without muscle complaints9. While not statistically significant, CK levels in our volunteers taking a statin were mildly elevated to an extent (~20 U/L) similar to those in a randomized clinical trial investigating the effects of statin therapy on muscle function9, suggesting that sub-clinical myopathies associated with statin use might be observed in our cohort.
The mechanism(s) underlying statin-induced myopathy, myalgia, or modest reductions in muscle strength are not completely understood. To better understand the potential effects of statins on muscle function, we performed measurements of muscle size and function across the anatomic spectrum, from the whole muscle to the molecular level. Our data show no evidence for an effect of chronic statin use on skeletal muscle size, myofilament protein content/isoform expression, structure, or function at the cellular, sub-cellular, or molecular levels in subjects without myalgia or elevated CK levels, which argues against a generalized subclinical, statin-induced myopathy associated with myofilament proteins. We discuss these findings on a descending anatomic scale, starting with the whole muscle (ie, tissue) level and progressing to the cellular and sub-cellular/molecular levels.
Whole muscle strength and size
Statin therapy is linked to decreases in whole muscle strength, though reports have been conflicting. El-Salem et al. found that 15% of patients with adverse symptoms displayed objective weakness, and only 2 of their patients had elevated CK levels32, suggesting the possibility of reduced muscle strength with statin use even in the absence of elevated CK. Additionally, in older adults statin therapy has been associated with reductions in leg strength and increased fall risk scores even while the percentage of appendicular lean mass increased52. However, the STOMP trial presented no difference in muscle function following the use of 80 mg of atorvastatin for 6 months. Although the investigators found moderate decreases in arm and leg strength in a subset of patients presenting with myalgia, the observed weakness occurred in both statin and placebo groups, suggesting that any weakness was the result of underlying muscle dysfunction and not statin therapy9. Furthermore, other studies have shown no effect on measures of muscle strength53,54 while another found improvements based on better performance for sit-to-stand chair tests55. In a small review based in part on these studies, Krishnan and Thompson found insufficient data to determine whether statins affect muscle strength and performance36. Our data would argue against any effect of statins on muscle strength (isometric or isokinetic), and we found no differences in leg fat-free mass. Whole muscle assessments of contractile function, however, are complicated by the fact that they are determined by so many physiological systems/parameters (eg, neurological, skeletal, biomechanical, antagonist muscle co-activation, volitional effort, etc), and variation in any or several of these parameters could affect strength. Similarly, assessments of whole muscle size with imaging techniques carry some uncertainty56. To provide a clearer delineation of the effect of statins on muscle structure and function, we have assessed these parameters at the cellular and sub-cellular level, where these confounding factors and methodological limitations are eliminated.
Cellular level: Muscle fiber cross-sectional area and fiber type
Animal models have shown that statins can promote muscle atrophy34,35. The effect of chronic statin use on skeletal muscle fiber size in humans, however, has not been defined clearly. We found no significant differences in CSA in both MHC I and IIA fibers, in line with our assessments of whole muscle size discussed above. Additionally, distributions of individual fiber size for MHC I and IIA fibers and relative proportions of myosin isoforms expressed in tissue homogenates, 2 indices that reflect the distribution of fiber types within the muscle, were similar between groups. As the relative distribution of fiber types affects both oxidative and contractile properties of muscle57-59, these results collectively argue against any effect of statins on muscle function through modifications in muscle fiber size or relative fiber distribution in asymptomatic subjects.
Sub-cellular structure: Myofilament ultrastructure and mitochondrial content/morphology
Effects of statins to cause alterations in muscle function may relate to modifications in myofilament structure, without gross alterations in fiber size. Indeed, statin usage has been shown to shorten sarcomeres in zebrafish, likely through damage to thick filaments; this is preventable by circumventing HMG Co-A reductase inhibition via administration of mevalonate60. However, we found no evidence for alterations in the fractional content of myofilament proteins in muscle fibers, suggesting that any variation in muscle function per unit fiber cannot be explained by modulation of myofilament fractional content by statins. Moreover, there were no differences in A band length. The A band length primary reflects the length of the myosin-containing thick filament. It has a clear effect on muscle function, as the length of the thick filament can influence the degree of filament overlap and, in turn, force production. Collectively, our results argue against any effect of chronic statin use on myofilament ultrastructure in asymptomatic subjects.
Statin therapy has been associated with altered mitochondrial protein content and dysfunction. In 1 study, patients presenting with myopathy displayed increased production of reactive oxidative species and decreased mitochondrial biogenesis in skeletal muscle15. Furthermore, recent studies suggest that statin use is associated with an attenuated increase in cardiorespiratory fitness and mitochondrial content in response to training18. In contrast to these results, we found no effect of chronic statin use on mitochondrial fractional area or average mitochondria size, indicating statin use does not alter mitochondrial content or structure. Additionally, we have recently reported that chronic statin use does not attenuate improvements in peak oxygen consumption in response to cardiac rehabilitation in patients with coronary heart disease61. Considering that increases in peak oxygen consumption in these patients are primarily explained by improvements in muscle mitochondrial function62, these findings further argue against an effect of statin use on the adaptability of muscle oxidative capacity to training61. Furthermore, statins have been shown to have protective effects by reducing production of reactive oxygen species in cardiac muscle63. Longitudinal evaluation of adaptations in indices of mitochondrial function in humans is needed to comprehensively define the effects of statins on mitochondrial biology. Nonetheless, our results argue against an effect of statins on sub-cellular structure/mitochondrial content in ways that could impact skeletal muscle contractility or oxidative function.
Cellular and molecular muscle function
Despite the fact that muscle weakness and pain is a reasonably common side effect of statin use, little is known about effects on the contractile properties of skeletal muscle. Studies in rodents and humans have shown decreases in contractility in skeletal muscle with statin treatment34,64,65. Such contractile deficits have generally been attributed to impaired excitation-contraction coupling/Ca2+ homeostasis, as statins may disrupt sarcoplasmic reticulum integrity and Ca2+ levels66, 67. Indeed, this is thought to be a mechanism whereby statins may induce myopathy via activation of intracellular Ca2+-activated proteases and degradation of muscle fibers, with type II glycolytic fibers being most susceptible37,65,68,69. Because adaptations in Ca2+ regulatory systems are apparent in statin treated patients with no evidence of myopathy67, it is tenable to hypothesize that such disruptions may affect muscle function secondary to degradation of myofilaments by Ca2+-activated proteases. To investigate the possibility of statins affecting the intrinsic contractility of skeletal muscle myofilament proteins, we evaluated both cellular and molecular muscle function in chemically-skinned single muscle fiber segments. We found no evidence for differences between groups in single fiber tension or myosin-actin cross-bridge mechanics and kinetics regardless of fiber type. The fact that we did not find evidence for statin modulation of sensitive indices of molecular muscle function at the level of the myosin-actin cross-bridge interaction strongly argues against an effect on muscle dysfunction through alterations in myofilament function.
Several caveats to our study should be acknowledged. First, none of our subjects were symptomatic. Additionally, they varied by type and dosage of statin, and therefore results can only be generalized to statins as a class. While limited to subjects on stable statin regimens, we were able to investigate adaptations in myofilament function, muscle fiber size, and other parameters that may have been present but masked by compensatory adaptations in other systems. That is, because many of the parameters studied are first derivatives of muscle function (eg, molecular function, myofilament ultrastructure, muscle fiber size), adaptations in other regulatory systems (eg, excitation-contraction coupling, neural activation, etc) could occur to maintain whole muscle performance. While appearing asymptomatic, individuals would still have impaired myocellular structure/function and conceivably less physiological reserve in these other regulatory systems to buffer deficits in physically disabling stimuli that occur frequently in elderly humans. Nevertheless, we were able to address a novel aspect of statin therapy by assessing measurements of muscle structure and function at the cellular, sub-cellular, and molecular levels, assessments that do not suffer from the many confounding factors that influence whole muscle measurements. Moreover, we found no evidence for myopathy extending down to the molecular level, suggesting that asymptomatic individuals without elevated CK levels, who represent the vast majority of those who take statins, do not display any signs of a sub-clinical myopathy. Documented myopathy and myalgia in patients may be a result of potential risk factors such as age, duration of use, diabetes mellitus, stroke, and low BMI32. Therefore, alterations in skeletal muscle may result from a combination of statin use and individual disease characteristics. However, from a clinical perspective, our results suggest that this is not likely a concern in otherwise healthy individuals. To remove potential confounding variables, we matched subjects by age and sex, and subjects in the statin group had been prescribed consistent therapy in most cases for multiple years. Although the purpose of this study was to address potential structural changes in the absence of symptoms, it remains possible that patients with statin-intolerance or objective muscle weakness present with cellular and molecular changes in muscle structure and function. Therefore, further research in statin-intolerant patients is of great importance in determining whether elevated CK levels or symptomatic myalgia results in alterations to muscle myofilament structure and function.
Collectively, our data show that when tolerated, chronic statin therapy in otherwise healthy asymptomatic individuals does not lead to myopathic effects on muscle structure or function at the cellular, subcellular, or molecular levels. Thus, our results suggest expansion of statin use to additional at-risk statin tolerant populations would not likely impair physical functional capacity via alterations in skeletal muscle fiber size or myofilament protein ultrastructure or function, and tolerance would be expected long-term. However, further examinations of muscle size and function at the cellular and molecular levels prior to and following statin administration will be important for resolving whether statin therapy leads to sub-clinical myopathies that might impact physical functionality and muscle size long-term.
Acknowledgments
Funding for this study was provided in part by:
NIH through the national institute on aging: R01-AG033547 (Dr. Toth)
NIH through NHLBI: HL-007647 (Dr. Callahan)
National Institutes of Health Center of Biomedical Research Excellence award from the National Institute of General Medical Sciences: P20GM103644 (Dr. Ades)
ABBREVIATIONS
- CK
Creatine kinase
- CSA
Cross-sectional area
- EM
Electron microscopy
- statin
HMG Co-Reductase inhibitors
- MHC
Myosin heavy chain
Footnotes
Disclosure: This manuscript is not under consideration for publication elsewhere and none of the contents have previously been published. All authors have read and approved the manuscript. There are no relationships with industry to report.
REFERENCES
- 1.Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32(14):1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 2.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2012;33(13):1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 3.Jain MK, Ridker PM. Anti-Inflammatory Effects of Statins: Clinical Evidence and Basic Mechanisms. Nat Rev Drug Discov. 2005;4(12):977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 4.Nickenig G, Baumer AT, Temur Y, Kebben D, Jockenhovel F, Bohm M. Statin-sensitive dysregulated AT1 receptor function and density in hypercholesterolemic men. Circulation. 1999;100(21):2131–2134. doi: 10.1161/01.cir.100.21.2131. [DOI] [PubMed] [Google Scholar]
- 5.Wolfrum S, Jensen KS, Liao JK. Endothelium-dependent effects of statins. Arterioscler Thromb Vasc Biol. 2003;23(5):729–736. doi: 10.1161/01.ATV.0000063385.12476.A7. [DOI] [PubMed] [Google Scholar]
- 6.Ferrier KE, Muhlmann MH, Baguet JP, Cameron JD, Jennings GL, Dart AM, et al. Intensive cholesterol reduction lowers blood pressure and large artery stiffness in isolated systolic hypertension. J Am Coll Cardiol. 2002;39(6):1020–1025. doi: 10.1016/s0735-1097(02)01717-5. [DOI] [PubMed] [Google Scholar]
- 7.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. 25 Pt B. [DOI] [PubMed] [Google Scholar]
- 8.Standards of medical care in diabetes-2015: summary of revisions. Diabetes Care. 2015;(38 Suppl):S4. doi: 10.2337/dc15-S003. [DOI] [PubMed] [Google Scholar]
- 9.Parker BA, Capizzi JA, Grimaldi AS, Clarkson PM, Cole SM, Keadle J, et al. Effect of statins on skeletal muscle function. Circulation. 2013;127(1):96–103. doi: 10.1161/CIRCULATIONAHA.112.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auer J, Sinzinger H, Franklin B, Berent R. Muscle- and skeletal-related side-effects of statins: tip of the iceberg? Eur J Prev Cardiol. doi: 10.1177/2047487314550804. Published online Septermber 17, 2014. DOI: 10.1177/2047487314550804. [DOI] [PubMed] [Google Scholar]
- 11.Graham DJ, Staffa JA, Shatin D, Andrade SE, Schech SD, La Grenade L, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. Jama. 2004;292(21):2585–2590. doi: 10.1001/jama.292.21.2585. [DOI] [PubMed] [Google Scholar]
- 12.Henriksbo BD, Lau TC, Cavallari JF, Denou E, Chi W, Lally JS, et al. Fluvastatin causes NLRP3 inflammasome-mediated adipose insulin resistance. Diabetes. 2014;63(11):3742–3747. doi: 10.2337/db13-1398. [DOI] [PubMed] [Google Scholar]
- 13.Chang CH, Chang YC, Lee YC, Liu YC, Chuang LM, Lin JW. Severe hepatic injury associated with different statins in patients with chronic liver disease: A nationwide population-based cohort study. J Gastroenterol Hepatol. 2015;30(1):155–162. doi: 10.1111/jgh.12657. [DOI] [PubMed] [Google Scholar]
- 14.Golomb BA, Evans MA, Dimsdale JE, White HL. Effects of statins on energy and fatigue with exertion: results from a randomized controlled trial. Arch Intern Med. 2012;172(15):1180–1182. doi: 10.1001/archinternmed.2012.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouitbir J, Charles AL, Rasseneur L, Dufour S, Piquard F, Geny B, et al. Atorvastatin treatment reduces exercise capacities in rats: involvement of mitochondrial impairments and oxidative stress. J Appl Physiol. 2011;111(5):1477–1483. doi: 10.1152/japplphysiol.00107.2011. [DOI] [PubMed] [Google Scholar]
- 16.Wagner BK, Kitami T, Gilbert TJ, Peck D, Ramanathan A, Schreiber SL, et al. Large-scale chemical dissection of mitochondrial function. Nat Biotechnol. 2008;26(3):343–351. doi: 10.1038/nbt1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galtier F, Mura T, Raynaud de Mauverger E, Chevassus H, Farret A, Gagnol JP, et al. Effect of a high dose of simvastatin on muscle mitochondrial metabolism and calcium signaling in healthy volunteers. Toxicol Appl Pharmacol. 2012;263(3):281–286. doi: 10.1016/j.taap.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Mikus CR, Boyle LJ, Borengasser SJ, Oberlin DJ, Naples SP, Fletcher J, et al. Simvastatin impairs exercise training adaptations. J Am Coll Cardiol. 2013;62(8):709–714. doi: 10.1016/j.jacc.2013.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinzinger H, O'Grady J. Professional athletes suffering from familial hypercholesterolaemia rarely tolerate statin treatment because of muscular problems. Br J Clin Pharmacol. 2004;57(4):525–528. doi: 10.1111/j.1365-2125.2004.02044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez G, Spatz ES, Jablecki C, Phillips PS. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med. 2011;78(6):393–403. doi: 10.3949/ccjm.78a.10073. [DOI] [PubMed] [Google Scholar]
- 21.Sacher J, Weigl L, Werner M, Szegedi C, Hohenegger M. Delineation of myotoxicity induced by 3-hydroxy-3-methylglutaryl CoA reductase inhibitors in human skeletal muscle cells. J Pharmacol Exp Ther. 2005;314(3):1032–1041. doi: 10.1124/jpet.105.086462. [DOI] [PubMed] [Google Scholar]
- 22.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. Jama. 2003;289(13):1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 23.Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80(6):565–581. doi: 10.1016/j.clpt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, et al. SLCO1B1 variants and statin-induced myopathy--a genomewide study. N Engl J Med. 2008;359(8):789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 25.Needham M, Fabian V, Knezevic W, Panegyres P, Zilko P, Mastaglia FL. Progressive myopathy with up-regulation of MHC-I associated with statin therapy. Neuromuscul Disord. 2007;17(2):194–200. doi: 10.1016/j.nmd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve. 2010;41(2):185–190. doi: 10.1002/mus.21486. [DOI] [PubMed] [Google Scholar]
- 27.Kwak HB, Thalacker-Mercer A, Anderson EJ, Lin CT, Kane DA, Lee NS, et al. Simvastatin impairs ADP-stimulated respiration and increases mitochondrial oxidative stress in primary human skeletal myotubes. Free Radic Biol Med. 2012;52(1):198–207. doi: 10.1016/j.freeradbiomed.2011.10.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Callahan DM, Miller MS, Sweeny AP, Tourville TW, Slauterbeck JR, Savage PD, et al. Muscle disuse alters skeletal muscle contractile function at the molecular and cellular levels in older adult humans in a sex-specific manner. J Physiol. 2014;592:4555–4573. doi: 10.1113/jphysiol.2014.279034. Pt 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116(19):2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fogelholm M. Physical activity, fitness and fatness: relations to mortality, morbidity and disease risk factors. A systematic review. Obes Rev. 2010;11(3):202–221. doi: 10.1111/j.1467-789X.2009.00653.x. [DOI] [PubMed] [Google Scholar]
- 31.Sieverdes JC, Sui X, Lee DC, Church TS, McClain A, Hand GA, et al. Physical activity, cardiorespiratory fitness and the incidence of type 2 diabetes in a prospective study of men. Br J Sports Med. 2010;44(4):238–244. doi: 10.1136/bjsm.2009.062117. [DOI] [PubMed] [Google Scholar]
- 32.El-Salem K, Ababneh B, Rudnicki S, Malkawi A, Alrefai A, Khader Y, et al. Prevalence and risk factors of muscle complications secondary to statins. Muscle Nerve. 2011;44(6):877–881. doi: 10.1002/mus.22205. [DOI] [PubMed] [Google Scholar]
- 33.Echaniz-Laguna A, Mohr M, Tranchant C. Neuromuscular symptoms and elevated creatine kinase after statin withdrawal. N Engl J Med. 2010;362(6):564–565. doi: 10.1056/NEJMc0908215. [DOI] [PubMed] [Google Scholar]
- 34.Fuzi M, Palicz Z, Vincze J, Cseri J, Szombathy Z, Kovacs I, et al. Fluvastatin-induced alterations of skeletal muscle function in hypercholesterolaemic rats. J Muscle Res Cell Motil. 2012;32(6):391–401. doi: 10.1007/s10974-011-9272-7. [DOI] [PubMed] [Google Scholar]
- 35.Hanai J, Cao P, Tanksale P, Imamura S, Koshimizu E, Zhao J, et al. The muscle-specific ubiquitin ligase atrogin-1/MAFbx mediates statin-induced muscle toxicity. J Clin Invest. 2007;117(12):3940–3951. doi: 10.1172/JCI32741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krishnan GM, Thompson PD. The effects of statins on skeletal muscle strength and exercise performance. Curr Opin Lipidol. 2010;21(4):324–328. doi: 10.1097/MOL.0b013e32833c1edf. [DOI] [PubMed] [Google Scholar]
- 37.Parker BA, Thompson PD. Effect of statins on skeletal muscle: exercise, myopathy, and muscle outcomes. Exerc Sport Sci Rev. 2012;40(4):188–194. doi: 10.1097/JES.0b013e31826c169e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toth MJ, Miller MS, VanBuren P, Bedrin NG, LeWinter MM, Ades PA, et al. Resistance training alters skeletal muscle structure and function in human heart failure: effects at the tissue, cellular and molecular levels. J Physiol. 2012;590:1243–1259. doi: 10.1113/jphysiol.2011.219659. Pt 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toth MJ, Shaw AO, Miller MS, VanBuren P, LeWinter MM, Maughan DW, et al. Reduced knee extensor function in heart failure is not explained by inactivity. Int J Cardiol. 2010;143(3):276–282. doi: 10.1016/j.ijcard.2009.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, et al. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr. 1990;52(2):214–218. doi: 10.1093/ajcn/52.2.214. [DOI] [PubMed] [Google Scholar]
- 41.Toth MJ, Miller MS, Ward KA, Ades PA. Skeletal muscle mitochondrial density, gene expression, and enzyme activities in human heart failure: minimal effects of the disease and resistance training. J Appl Physiol. 2012;112(11):1864–1874. doi: 10.1152/japplphysiol.01591.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller MS, Vanburen P, Lewinter MM, Lecker SH, Selby DE, Palmer BM, et al. Mechanisms underlying skeletal muscle weakness in human heart failure: alterations in single fiber myosin protein content and function. Circ Heart Fail. 2009;2(6):700–706. doi: 10.1161/CIRCHEARTFAILURE.109.876433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bua EA, McKiernan SH, Wanagat J, McKenzie D, Aiken JM. Mitochondrial abnormalities are more frequent in muscles undergoing sarcopenia. J Appl Physiol. 2002;92(6):2617–2624. doi: 10.1152/japplphysiol.01102.2001. [DOI] [PubMed] [Google Scholar]
- 44.Miller MS, VanBuren P, LeWinter MM, Braddock JM, Ades PA, Maughan DW, et al. Chronic heart failure decreases cross-bridge kinetics in single skeletal muscle fibres from humans. J Physiol. 2010;588:4039–4053. doi: 10.1113/jphysiol.2010.191957. Pt 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawai M, Saeki Y, Zhao Y. Crossbridge scheme and the kinetic constants of elementary steps deduced from chemically skinned papillary and trabecular muscles of the ferret. Circ Res. 1993;73(1):35–50. doi: 10.1161/01.res.73.1.35. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y, Kawai M. The effect of the lattice spacing change on cross-bridge kinetics in chemically skinned rabbit psoas muscle fibers. II. Elementary steps affected by the spacing change. Biophys J. 1993;64(1):197–210. doi: 10.1016/S0006-3495(93)81357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palmer BM, Suzuki T, Wang Y, Barnes WD, Miller MS, Maughan DW. Two-state model of acto-myosin attachment-detachment predicts C-process of sinusoidal analysis. Biophys J. 2007;93(3):760–769. doi: 10.1529/biophysj.106.101626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mulieri LA, Barnes W, Leavitt BJ, Ittleman FP, LeWinter MM, Alpert NR, et al. Alterations of myocardial dynamic stiffness implicating abnormal crossbridge function in human mitral regurgitation heart failure. Circ Res. 2002;90(1):66–72. doi: 10.1161/hh0102.103221. [DOI] [PubMed] [Google Scholar]
- 49.Miller MS, Bedrin NG, Callahan DM, Previs MJ, Jennings ME, 2nd, Ades PA, et al. Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans. J Appl Physiol. 2013;115(7):1004–1014. doi: 10.1152/japplphysiol.00563.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Q, Liao JK. Pleiotropic effects of statins. - Basic research and clinical perspectives. Circ J. 2010;74(5):818–826. doi: 10.1253/circj.cj-10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phillips PS, Haas RH, Bannykh S, Hathaway S, Gray NL, Kimura BJ, et al. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137(7):581–585. doi: 10.7326/0003-4819-137-7-200210010-00009. [DOI] [PubMed] [Google Scholar]
- 52.Scott D, Blizzard L, Fell J, Jones G. Statin therapy, muscle function and falls risk in community-dwelling older adults. Qjm. 2009;102(9):625–633. doi: 10.1093/qjmed/hcp093. [DOI] [PubMed] [Google Scholar]
- 53.Traustadottir T, Stock AA, Harman SM. High-dose statin use does not impair aerobic capacity or skeletal muscle function in older adults. Age (Dordr) 2008;30(4):283–291. doi: 10.1007/s11357-008-9070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ashfield TA, Syddall HE, Martin HJ, Dennison EM, Cooper C, Aihie Sayer A. Grip strength and cardiovascular drug use in older people: findings from the Hertfordshire Cohort Study. Age Ageing. 2010;39(2):185–191. doi: 10.1093/ageing/afp203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agostini JV, Tinetti ME, Han L, McAvay G, Foody JM, Concato J. Effects of statin use on muscle strength, cognition, and depressive symptoms in older adults. J Am Geriatr Soc. 2007;55(3):420–425. doi: 10.1111/j.1532-5415.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- 56.Maden-Wilkinson TM, Degens H, Jones DA, McPhee JS. Comparison of MRI and DXA to measure muscle size and age-related atrophy in thigh muscles. J Musculoskelet Neuronal Interact. 2013;13(3):320–328. [PubMed] [Google Scholar]
- 57.Ivy JL, Costill DL, Maxwell BD. Skeletal muscle determinants of maximum aerobic power in man. Eur J Appl Physiol Occup Physiol. 1980;44(1):1–8. doi: 10.1007/BF00421757. [DOI] [PubMed] [Google Scholar]
- 58.Ivy JL, Withers RT, Brose G, Maxwell BD, Costill DL. Isokinetic contractile properties of the quadriceps with relation to fiber type. Eur J Appl Physiol Occup Physiol. 1981;47(3):247–255. doi: 10.1007/BF00422470. [DOI] [PubMed] [Google Scholar]
- 59.Gur H, Gransberg L, vanDyke D, Knutsson E, Larsson L. Relationship between in vivo muscle force at different speeds of isokinetic movements and myosin isoform expression in men and women. Eur J Appl Physiol. 2003;88(6):487–496. doi: 10.1007/s00421-002-0760-8. [DOI] [PubMed] [Google Scholar]
- 60.Huang SH, Hsiao CD, Lin DS, Chow CY, Chang CJ, Liau I. Imaging of zebrafish in vivo with second-harmonic generation reveals shortened sarcomeres associated with myopathy induced by statin. PLoS One. 2011;6(9):e24764. doi: 10.1371/journal.pone.0024764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rengo JL, Savage PD, Toth MJ, Ades PA. Statin Therapy Does Not Attenuate Exercise Training Response in Cardiac Rehabilitation. Journal of the American College of Cardiology. 2014;63(19):2050–2051. doi: 10.1016/j.jacc.2014.02.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ades PA, Savage PD, Brawner CA, Lyon CE, Ehrman JK, Bunn JY, et al. Aerobic capacity in patients entering cardiac rehabilitation. Circulation. 2006;113(23):2706–2712. doi: 10.1161/CIRCULATIONAHA.105.606624. [DOI] [PubMed] [Google Scholar]
- 63.Antoniades C, Demosthenous M, Reilly S, Margaritis M, Zhang MH, Antonopoulos A, et al. Myocardial redox state predicts in-hospital clinical outcome after cardiac surgery effects of short-term pre-operative statin treatment. J Am Coll Cardiol. 2012;59(1):60–70. doi: 10.1016/j.jacc.2011.08.062. [DOI] [PubMed] [Google Scholar]
- 64.Guis S, Figarella-Branger D, Mattei JP, Nicoli F, Le Fur Y, Kozak-Ribbens G, et al. In vivo and in vitro characterization of skeletal muscle metabolism in patients with statin-induced adverse effects. Arthritis Rheum. 2006;55(4):551–557. doi: 10.1002/art.22100. [DOI] [PubMed] [Google Scholar]
- 65.Simsek Ozek N, Bal IB, Sara Y, Onur R, Severcan F. Structural and functional characterization of simvastatin-induced myotoxicity in different skeletal muscles. Biochim Biophys Acta. 2014;1840(1):406–415. doi: 10.1016/j.bbagen.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 66.Draeger A, Monastyrskaya K, Mohaupt M, Hoppeler H, Savolainen H, Allemann C, et al. Statin therapy induces ultrastructural damage in skeletal muscle in patients without myalgia. J Pathol. 2006;210(1):94–102. doi: 10.1002/path.2018. [DOI] [PubMed] [Google Scholar]
- 67.Draeger A, Sanchez-Freire V, Monastyrskaya K, Hoppeler H, Mueller M, Breil F, et al. Statin therapy and the expression of genes that regulate calcium homeostasis and membrane repair in skeletal muscle. Am J Pathol. 2010;177(1):291–299. doi: 10.2353/ajpath.2010.091140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Westwood FR, Bigley A, Randall K, Marsden AM, Scott RC. Statin-induced muscle necrosis in the rat: distribution, development, and fibre selectivity. Toxicol Pathol. 2005;33(2):246–257. doi: 10.1080/01926230590908213. [DOI] [PubMed] [Google Scholar]
- 69.Liantonio A, Giannuzzi V, Cippone V, Camerino GM, Pierno S, Camerino DC. Fluvastatin and atorvastatin affect calcium homeostasis of rat skeletal muscle fibers in vivo and in vitro by impairing the sarcoplasmic reticulum/mitochondria Ca2+-release system. J Pharmacol Exp Ther. 2007;321(2):626–634. doi: 10.1124/jpet.106.118331. [DOI] [PubMed] [Google Scholar]