Abstract
Inversion transfer (IT) is a well-established technique with multiple attractive features for analysis of kinetics. However, its application in measurement of ATP synthesis rate in vivo has lagged behind the more common ST techniques. One well-recognized issue with IT is the complexity of data analysis in comparison to much simpler analysis by ST. This complexity arises, in part, because the γ-ATP spin is involved in multiple chemical reactions and magnetization exchanges, whereas Pi is involved in a single reaction, Pi → γ-ATP. By considering the reactions involving γ-ATP only as a lumped constant, the rate constant for the reaction of physiological interest, kPi→γATP, can be determined. Here, we present a new IT data analysis method to evaluate kPi→γATP using data collected from resting human skeletal muscle at 7T. The method is based on the basic Bloch-McConnell equation, which relates kPi→γATP with ṁPi, the rate of Pi magnetization change. The kPi→γATP value is accessed from ṁPi data by more familiar linear correlation approaches. For a group of human subjects (n = 15), the kPi→γATP value derived for resting calf muscle was 0.066 ± 0.017 s−1, in agreement with literature reported values. In this study we also explored possible time-saving strategies to speed up data acquisition for kPi→γATP evaluation using simulations. The analysis indicates that it is feasible to carry out a 31P inversion transfer experiment in ~10 minutes or shorter at 7T with reasonable outcome in kPi→γATP variance for measurement of ATP synthesis in resting human skeletal muscle. We believe that this new IT data analysis approach will facilitate the wide acceptance of IT to evaluate ATP synthesis rate in vivo.
Keywords: skeletal muscle, magnetization transfer, ATP, chemical exchange, T1 relaxation time, 7T, inversion transfer, energy metabolism
INTRODUCTION
Saturation transfer (ST) and inversion transfer (IT) are two techniques to probe the kinetics of magnetization exchange (ME) between spins at different resonance frequency (1–6). 31P NMR and ST have been the preferred techniques for noninvasive analysis of high energy phosphate metabolism for the last three decades (7–20). Extension of ST to high magnetic fields should be advantageous because of improved sensitivity, but implementation of prolonged saturating pulses in human subjects may be challenging because of hardware and SAR limitations, and additional data must be acquired to correct for off-resonance saturation, the so-called "spillover" effect (21,22), in spite of increased chemical shift dispersion (23). Inversion transfer may be technically simpler to implement at high fields, and it offers the advantages of low SAR, an inherently large initial labeling effect (Mz → -Mz versus Mz → 0), and insensitivity to “spillover” side effect.
Despite these potential advantages and applications in a number of kinetic studies (3–6,24–27), the inversion transfer technique is rarely used to measure the rate constant (kPi→ATP) for ATP synthesis in vivo while ST has been the method of choice (7–20,28–34). One reason that ST is so popular is that the prolonged saturation (5 – 9 seconds) at γ-ATP allows magnetization transfer effects to build up in Pi despite the intrinsically slow Pi ↔ γ-ATP exchange rate. Frequency-selective inversion at γ-ATP is limited by the relatively fast relaxation of the γ-ATP spin (apparent T1 relaxation ~ 1 – 2 sec) and consequently the duration of any magnetization transfer effect is brief. At higher magnetic fields, the increased effect of chemical shift anisotropy further accelerates T1 relaxation of ATP 31P spins. Our recent work has shown that the problem of fast relaxing γ-ATP can be overcome by using a band-selective inversion of multiple 31P resonances at 7T (35). Co-inversion of phosphocreatine (PCr) and all exchanging ATP spins results in a longer apparent T1 relaxation of γ-ATP because the inverted magnetization is stored in the longer T1 PCr pool (~ 5 – 6 sec). This approach leads to an increase in the magnetization transfer effect at Pi, making it suitable for quantitative evaluation of kPi→ATP at 7T.
Analysis of inversion transfer data, however, is complex. The high-energy phosphate exchanges in vivo are described by a five-spin network including 31P in Pi, PCr, α-, β- and γ-ATP. In addition to the well-known chemical exchanges, intramolecular NOE effects have also been identified between γ-ATP ↔ β-ATP and between γ-ATP ↔ α-ATP (6,34,36). For such a complex system a full analytical solution is difficult to achieve. Instead, a numerical solution, although not intuitive, is more practical (6). In contrast to analysis of IT data, the complexity of the 31P spin exchange system in vivo does not affect ST data analysis because upon saturation of γ-ATP, the magnetization of Pi is uncoupled from the rest of the exchange network, resulting in a simple analytical solution to relate the Pi signal reduction and T1 relaxation time to kPi→ATP (7,10,23).
In this study, we outline a new IT data analysis approach to obtain kPi→ATP based on a single Bloch-McConnell differential equation defined for Pi. By direct access to the rate of Pi magnetization change, it eliminates the need to solve the Bloch-McConnell differential equations for other exchange spins (PCr, α-, β- and γ-ATP) (Figure 1), thereby permitting an evaluation of kPi→ATP graphically by simple linear regression. The validity of this data analysis approach is demonstrated using datasets acquired from resting human calf muscle using EBIT, a band-selective inversion technique (35). We believe that this new IT data analysis approach will facilitate the wide acceptance of IT to evaluate ATP synthesis rate in vivo, a rate of fundamental importance in physiology (9–20).
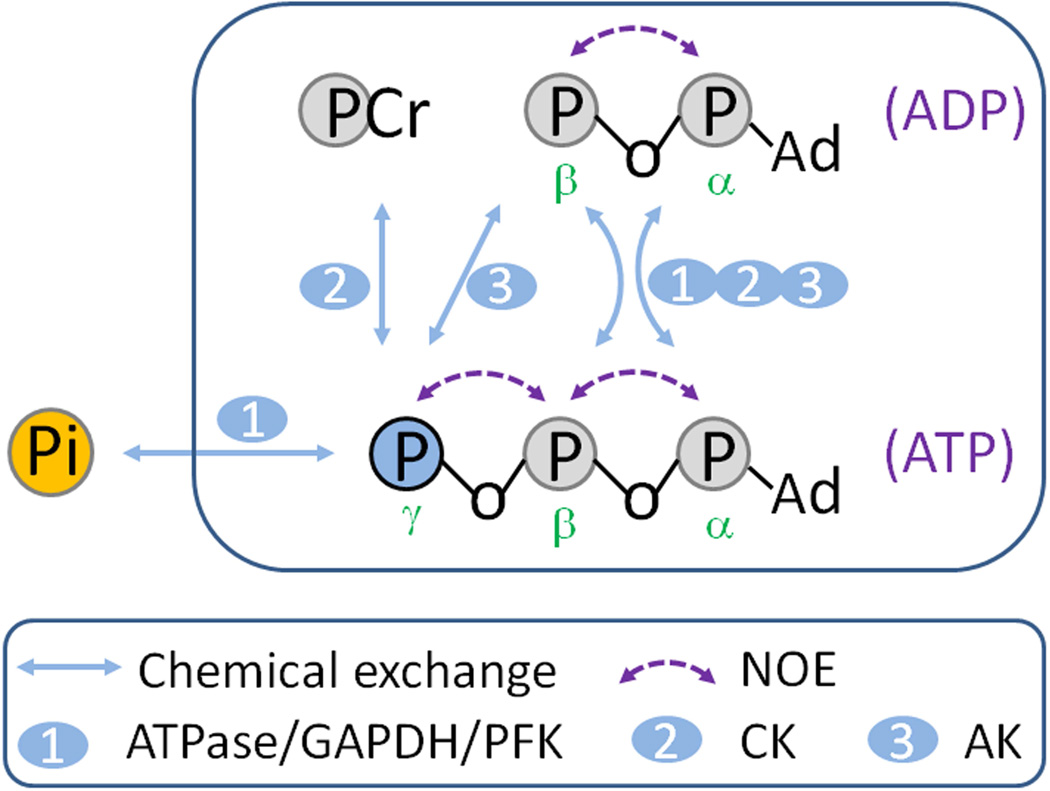
FIG. 1.
Possible 31P magnetization exchange pathways in skeletal muscle. Abbreviations: CK, creatine kinase; AK adenylate kinase; PFK, phosphofructokinase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NOE, nuclear Overhauser effect.
METHODS
Theory
For the chemical exchange reaction Pi ↔ γ − ATP, after applying an inversion pulse at γ − ATP either frequency-selective or band-selective, the rate of change in Pi Z-magnetization Mz (for clarity, the subscript “z” is omitted hereafter) in the inversion delay time t, can be described by the Bloch-McConnell equation as follows,
| [1] |
where k denotes pseudo first-order kinetic rate constant, with the forward and reverse rate constants related by
| [2] |
in which and denote the Z-magnetization at Boltzmann thermal equilibrium for Pi and γ-ATP, respectively. Substituting kγATP→Pi in Eq [1] by Eq [2] gives:
| [3] |
This expression can be rearranged into two different linear equations:
| [3a] |
and
| [3b] |
where m is the normalized Z-magnetization defined by:
| [4] |
Eq [3] indicates that two separate terms contribute to ṁPi: the first is the T1 relaxation term, expressed as the product of 1/T1,Pi by (1 − mPi), and the second is the chemical exchange term, expressed as the product of kPi→γATP by (mPi − mγATP). Note that the quantity (1 − mPi) represents the exchange effect observed at Pi and that the quantity (mPi − mγATP) represents the net difference in normalized magnetization between Pi and γ-ATP. Since both (1 − mPi) and (mPi − mγATP) are experimentally measurable, those two unknowns T1,Pi and kPi→γATP in Eq [3] thus can be solved by a simple linear fitting to ṁPi. Alternatively, the parameters kPi→γATP and 1/T1,Pi can be evaluated graphically from the intercept or slope of the linear relationship versus , or versus , as defined by Equations [3a] and [3b].
It is clear from Eqs [3, 3a and 3b] that, in order to obtain kPi→γATP and 1/T1,Pi, the value of ṁPi must be known. A way to obtain ṁPi is first to construct the mathematical equation for the function mPi(t), from which ṁPi(t) values can be obtained. With the boundary conditions mPi|t = 0 = 1 and mPi|t→∞ = 1, the time-dependent function of mPi can be described by
| [5] |
where a, λ1 and λ2 are three constants, which can be accessed by numerical fitting the mPi ~ t dataset. With these three constants, it is straightforward to derive the ṁPi values using the following differential equation (by applying ∂/∂t operation on Eq [5]),
| [6] |
Based on Eq [3], one may also estimate the parameters kPi→γATP and T1,Pi from the experimental mPi ~ t curve at a few special time points.
One example occurs when ṁPi(t)=0 the transition point at which the curve mPi ~ t reaches a minimum and the effect of chemical exchange reaches the maximum.
or
| [7] |
This particular time point is denoted by tmin. Equation [7] is equivalent to the formula given by Kuchel (1).
A second example is at t = 0, immediately after the inversion of γ-ATP, at which mPi = 1 but ṁPi ≠ 0. At this initial time point, the T1 relaxation term in Eq [3] becomes zero, and ṁPi is given by:
| [8] |
Equation [8] is equivalent to the formula given by Malloy et al (5).
For description of γ-ATP signal, the Bloch-McConnell-Solomon formula has to be used which contains more exchange terms contributed from five pools (Figure 1):
| [9] |
where σ denotes the rate constant for the cross-relaxation between γ-ATP and β-ATP (NOE) and ρ denotes the rate constant for the chemical exchange between γ-ATP and β-ATP mediated by AK. If all chemical exchange and cross-relaxation effects among PCr, γ-, β-, and α-ATP are lumped in one single pool represented by γ-ATP, then a two-pool model will apply. Under the condition of band inversion with co-inversion of PCr and all ATP spins, this simplified two-pool exchange model yields:
| [10] |
where T1app,γATP denotes the apparent T1 relaxation time of γ-ATP, which lumps T1 relaxation and all exchange effects together except the exchange pathway involving Pi (Figure 1). In other words, the interaction of γ-ATP with other 31P spins in PCr, ADP and ATP is treated as part of molecular “lattice” and is incorporated into T1app,γATP (35). Using this approximation, the kPi→γATP and T1,Pi values can be evaluated numerically by the following matrix equation:
| [11] |
in which ; M(0) = [MPi(0); MγATP(0)], which is the initial Z-magnetization immediately after band inversion; and A is the exchange matrix
| [12] |
Note that, unlike Eqs [3,3a,3b], Equation [11] contains an additional unknown parameter T1app,γATP through the matrix A.
Human Subjects
The protocol was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center. Prior to the MRS study, informed written consent was obtained from all participants. Fifteen subjects (9 male and 6 female), aged 27 ± 4 yr and BMI 24 ± 2, participated in the study. Spectra from 10 subjects reported elsewhere (35) were reanalyzed using the new methods, and new data from 5 subjects were acquired and analyzed. All subjects were in good general health with no history of peripheral vascular, systemic or myopathic diseases. To avoid possible exercise-associated physiological variations among subjects, all subjects were asked to refrain from any physical activity of moderate or high intensity for 24 hours prior to the study, and subjects sat at rest for 20 minutes prior to the scan. Heart rate and blood oxygen saturation level were monitored during the scan. The study was well-tolerated by all subjects.
MRS Protocol
The general MRS acquisition protocol has been described previously (35). Briefly, FID-based 31P spectra were acquired from calf muscle for each subject using a band-selective inversion sequence (180° – t – 90° – τ), which was comprised of an adiabatic inversion pulse, followed by a varying inversion delay time t, a hard 90° readout pulse, and a recovery period τ. The inversion time was varied from 30 to 10000 ms with 12 data points spaced logarithmically (t = 30, 626, 1253, 1923, 2641, 3414, 4252, 5167, 6174, 7294, 8556 and 10000 msec). The inversion pulse is a trapezoid-shaped adiabatic pulse with 42 ms duration and an excitation frequency band of 2500 Hz, centered at 150 Hz upfield from α-ATP, which is set to co-invert PCr and ATP spins but not Pi and PDE. Other NMR parameters were TR 30 sec, NA 4 averages, 4 K sampling points zero-filled to 8 K. The chemical shift of the 31P MR spectra was referenced to PCr at 0 ppm. Each 31P NMR spectrum was Fourier transformed, processed with Gaussian apodization (6 Hz) using the scanner software (SpectroView, Philips Healthcare), and further analyzed using Matlab (Mathworks) routines to evaluate the normalized magnetization for Pi, PCr, α-, β- and γ-ATP, based on the magnitude of the observed 31P peaks. The mPi data were fitted by Equation [5] to obtain the fitting parameters a, λ1 and λ2.
To evaluate the exchange rate constant kPi→γ-ATP and T1 value for Pi, the ṁPi values were first obtained by Eq [6]. Then these ṁPi data were fit by Eq [3] against the quantities (1 − mPi) and (mPi − mγATP). Alternatively, simpler linear relationship can be obtained to evaluate kPi→γ-ATP and T1,Pi by plotting versus , or versus , as defined by Eqs [3a and 3b] respectively. Note that, since the quantity (1 − mPi) appears as denominator in Eq [3a], we chose to exclude the first data point with (1 − mPi) ~ 0 from data analysis. For comparison, the kPi→γ-ATP and T1,Pi values were also obtained by solving Eq [11] numerically.
To examine the reliability of these different evaluation approaches, the kPi→γ-ATP and T1 values for Pi were also analyzed from the simulated mPi and mγATP datasets generated using a set of known parameters (T1,P = 6.5 sec, T1app,γATP = 4.0 sec, kPi→γ-ATP = 0.065 sec−1, and Moγ-ATP / MoPi = 2.6) at varying delay time (30 – 10000 msec, 12 data points) based on 2-site chemical exchange model. To imitate the effect of spectral background noise on small Pi signal, the mPi dataset was modulated by pseudo-random noise with a Gaussian distribution variance of 2% (σ ranged from −1% to 1%) generated by Box Muller transformation. A total of 1000 noise-mixed mPi datasets were analyzed using a self-written Matlab routine for evaluation of the variance in kPi→γ-ATP and T1,Pi values.
An interesting feature embedded in Eqs [3, 3a and 3b] is the absence of T1app,γATP in ṁPi. To test this feature, simulated mPi and mγATP datasets were generated at varying T1app,γATP values (1, 2, 4, and 6 sec) to examine its effect on the outcome of kPi→γ-ATP and T1,pi.
Our in vivo 31P study takes a total scan time of 24 minutes for collecting a series of 12 spectra at varying inversion delay times with constant TR of 30 s and 4 repeated acquisitions. Determination of kPi→γ-ATP and T1,Pi as rapidly as possible is an important experimental goal, and for this reason, the following scenarios were examined by simulations. In simulation #1 (denoted as S1), the noise level was doubled (σ ranged from −2% to 2% in Gaussian distribution), corresponding to one single data acquisition for each inversion time data point, or a simulated total scan time of 6 minutes for the whole series with 12 delay times. In simulation #2 (denoted as S2), only the first 6 data points in the inversion delay series were sampled (inversion delay time = 30, 626, 1253, 1923, 2641, and 3414 msec), corresponding to a scan time of 12 minutes. In simulation #3 (denoted as S3), 6 data points were acquired at large intervals (inversion delay time = 30, 1253, 2641, 4252, 6174, and 8556 msec), which also corresponds to a reduced scan time of 12 minutes.
Statistical Analysis
All data are reported as mean ± standard deviation, calculated using standard functions in Matlab.
RESULTS
Observation of magnetization exchange between Pi and γ-ATP
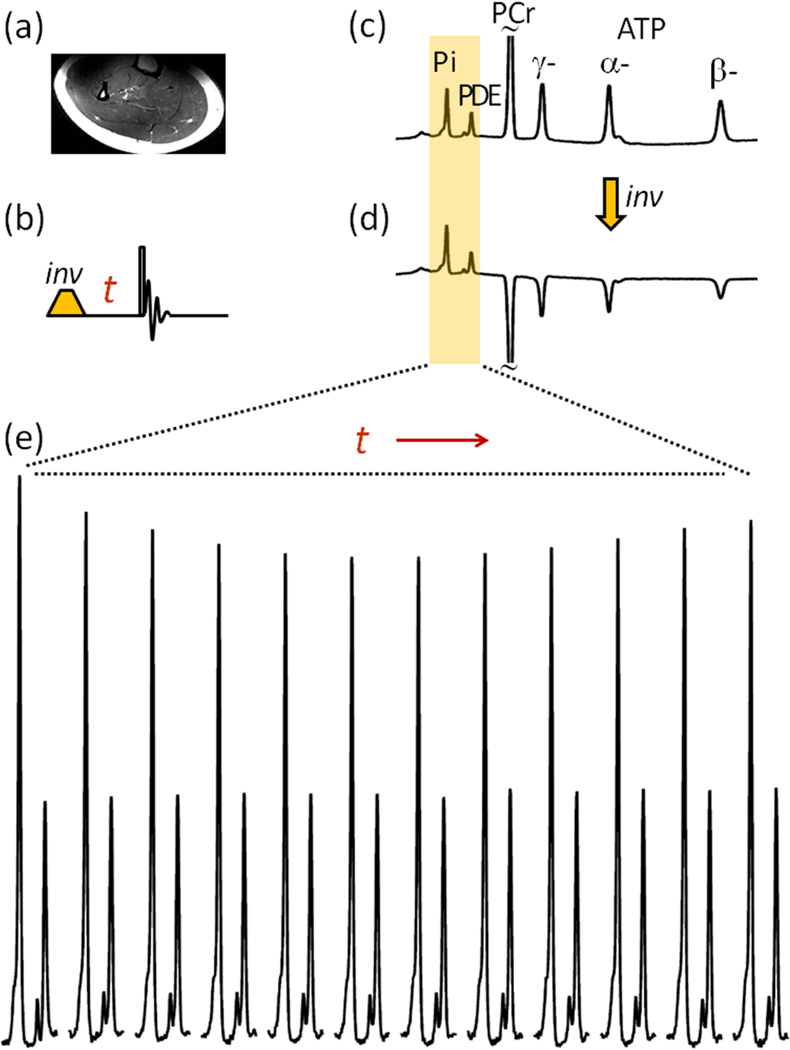
As illustrated in Figure 2, the increased chemical shift dispersion at ultra-high field 7T facilitates band-selective inversion. Upon co-inversion of ATP and PCr signals, the intensity of Pi signal in calf muscle is significantly reduced due to Pi ↔ γ-ATP exchange as shown (Figure 2e, n = 15 subjects). In contrast, there is no change in signal intensity of PDE (Figure 2e), a relatively inert metabolite not actively involved in exchange with other high energy phosphates in vivo. The absence of effect of the inversion pulse on the PDE signal illustrates the limited spillover to the Pi signal. As anticipated, the Pi signal continues to decrease as the inversion delay time t increases. After reaching a minimum in intensity, the Pi signal gradually recovers back to the equilibrium state with a further increase in t (Figure 2e). Such a biphasic feature of Pi signal change was clearly observed in the 31P spectra of all subjects studied (n = 15); the maximal reduction of Pi signal ranged from 13 to 21 % and typically occurred between t ~ 3.4 – 4.5 sec (Figure 3).
FIG. 2.
Image and 31P NMR of Human Calf Muscle. (a) T2w MR image. (b) Sequence of the band-selective inversion pulse used in the experiment. (c) and (d) 31P NMR spectra acquired from resting muscle before (c) and after (d) applying the band inversion sequence. (e) Evolution of the Pi signal with the inversion delay time t. The spectral data represents averages for n = 15 subjects.
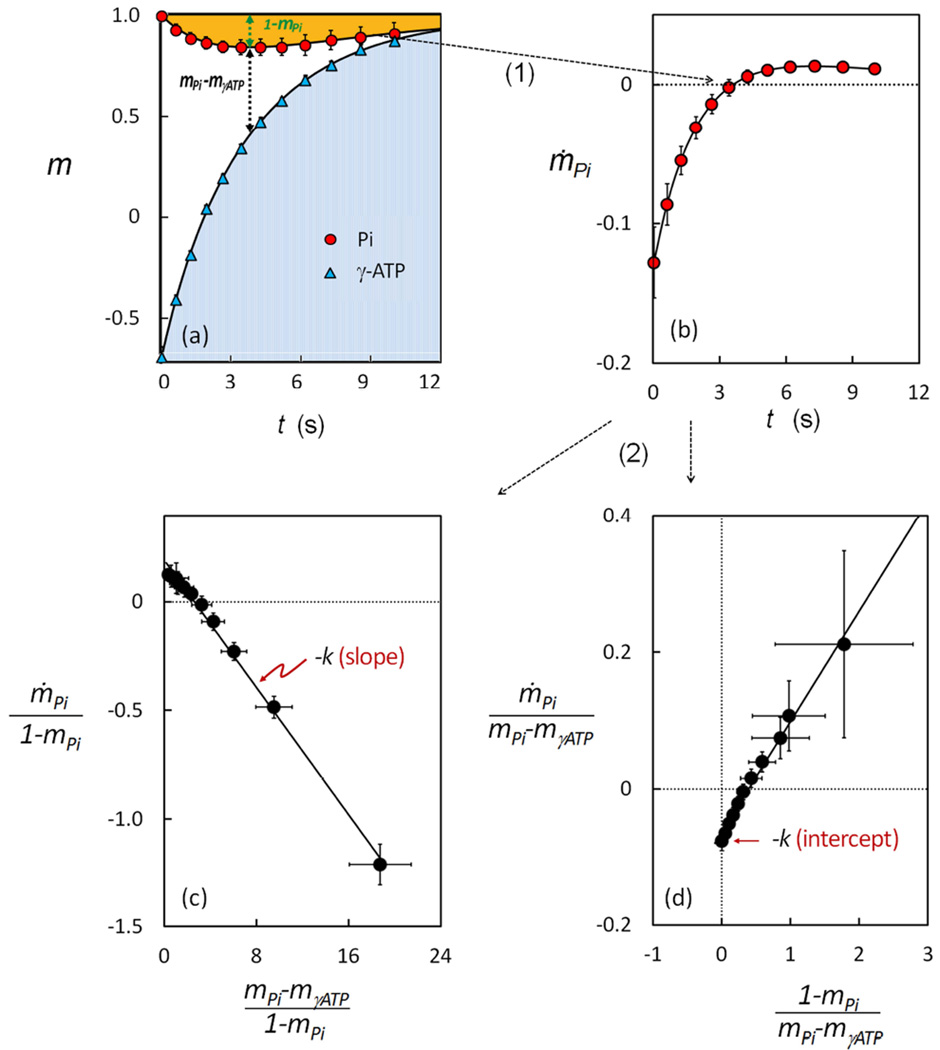
FIG. 3.
Alternative Analyses of Band Inversion-Transfer Data Sets (n = 15 subjects). (a) The m-plot, showing dependence of Pi and γ-ATP magnetization (mPi and mγATP) on inversion delay time t. (b) The ṁ–plot, showing the rate of change of Pi signal with inversion delay time t. (c) The linear relationship between and , with a slope defined by - kPi→γATP and an intercept defined by 1/T1,Pi. (d) The linear relationship between and , with a slope defined by 1/T1,Pi and an intercept defined by - kPi→γATP.
Unlike the non-inverted 31P signals (Pi and PDE), all the inverted signals (PCr, α-, β- and γ-ATP) recovered exponentially with t (Figure 3 and Supplementary Figure 1S), but at varying recovery rates (PCr < γ-ATP < β-ATP < α-ATP). Notably, the recovery of γ-ATP becomes much slower than that of α- and β-ATP, and more similar to the trend observed for PCr. This yields a long period of delay time with (mPi − mγATP) > 0, the driving factor for producing the ME effect at Pi (Figure 3a). As will be clear later, to be able to contribute to the net buildup of the ME effect at Pi, the exchange term kPi→γATP (mPi − mγATP) has to be sufficiently large to overcome the draining effect of the T1 relaxation term (1 − mPi)/T1,Pi, which occurs only in the period t = 0 ~ 4 sec during which the ME effect (1 − mPi) is in a trend of increase (Figure 3a).
Evaluation of ṁPi from mPi
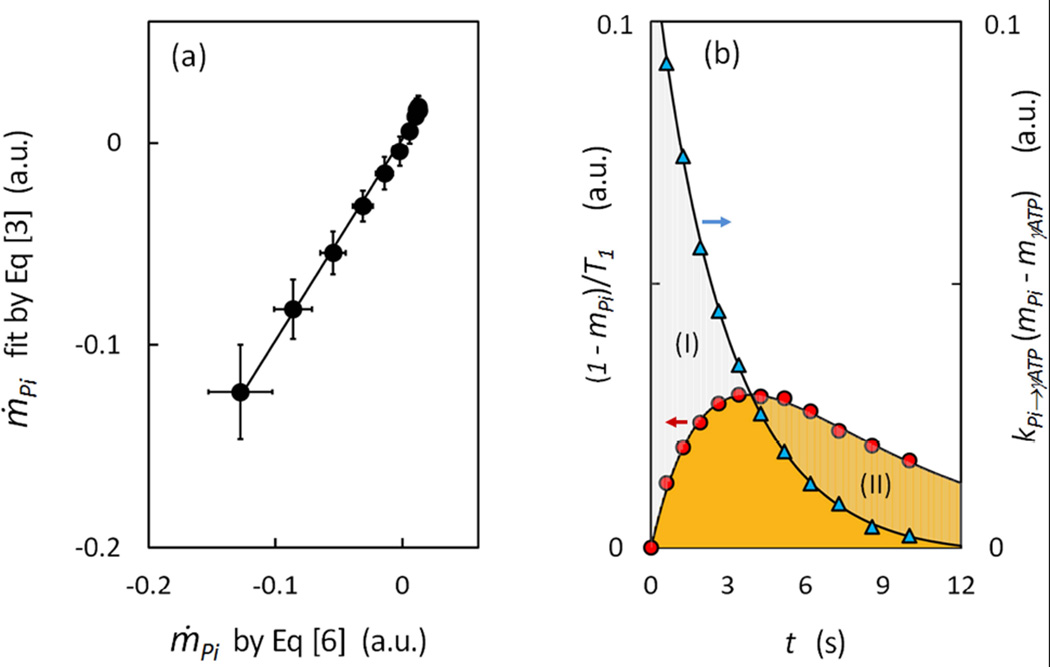
To take advantage of the simple relationship between ṁPi and kPi→γ-ATP, as described by Bloch-McConell equation [3] or its two simpler linear forms (Eqs [3a] and [3b]), for calculation of kPi→γ-ATP, the first step is to evaluate the ṁPi values. These values were derived by Eq [6] using the fitting constants (a, λ1 and λ2) obtained by fitting the experimental mPi(t) data (Figure 3a) according to Eq [5]. As shown in Figure 3b (n = 15 subjects), with increase in inversion delay time, the ṁPi values is changed from negative to positive, with transition occurring at tmin ~ 4 sec. Within t = 0 – 4 sec, the ṁPi magnitude decreases with t (Figure 3b). However, a further increase in inversion delay time (t > 4 sec) leads to a rise and then fall in the ṁPi magnitude, with a maximum occurring at t ~ 7.3 (Figure 3b). This feature is not visually identifiable in the curve mPi ~ t (Figure 3a), but becomes obvious when the ṁPi value is separated into the relaxation and exchange term by Eq [3] (Figure 4b).
FIG. 4.
Analysis of Relaxation and Exchange Components in Rate of Change of Pi Signal. (a) Linear correlation of ṁPi data derived by Eq [6] and those by fitting to Eq [3] (n = 15 subjects). The resultant fitting parameters kPi→γATP and T1,Pi are presented in (b) with a multiplication factor (mPi − mγATP) and (1 − mPi) to separate the relaxation term from the exchange term in ṁPi as defined by Eq [3]. The region marked by (I) indicates the dominance of the exchange term over the relaxation term, and the opposite for the region marked by (II).
Evaluation of kPi→γ-ATP from ṁPi
The simplest way to assess kPi→γ-ATP is to divide ṁPi value by (mPi − mγATP) at t = 0 (Eq [8]). This yielded 0.076 ± 0.014 s−1 using the measurements at the first data point (t = 0.03 sec) as an approximation. The value of kPi→γ-ATP can also be estimated, if T1,Pi is known, from the ratio (1 − mPi) / (mPi − mγATP) at ṁPi = 0 when t = tmin (Eq. [7]). This yielded a kPi→γ-ATP value in the range 0.048 – 0.096 s−1 assuming a possible T1,Pi ranging from 3.5 sec (37) to 7.0 sec (6).
To evaluate kPi→γ-ATP by linear graphic approach, we plotted the data against , (Figure 3c) and against (Figure 3d), using the above-derived ṁPi values (n = 15 subjects). Both datasets yielded a remarkably good linear relationship (y = −0.069 x + 0.181 and R2 = 0.996 in Figure 3c, and y = 0.159 x − 0.061 and R2 = 0.997), as anticipated from Eqs [3a] and [3b], respectively. More importantly, the resultant kPi→γ-ATP values from two different linear datasets agreed reasonably well, as summarized in Table 1. In addition, a direct ṁPi fitting by Eq [3] also yielded an excellent linear correlation (y = 0.996x + 0.002 and R2 = 0.996, Figure 4a), with kPi→γ-ATP result (Table 1) comparable to those by other two linear methods (Eqs [3a] and [3b]) and by the numeric matrix approach (Eq [11]). On average, these three different linear data analysis methods yielded a rate constant kPi→γ-ATP of 0.066 ± 0.017 sec−1 and T1,Pi value of 5.75 ± 1.91 sec for the group of the subjects studied (n = 15). An apparent T1 value of 4.02 sec was also obtained by the numeric matrix approach for γ-ATP. Additionally, the fitting procedure by Eq [3] also provides a quantitative separation of ṁPi into two parts distributable to relaxation- and exchange-related terms, respectively, which clearly illustrates the evolution of these two terms over the inversion delay time (Figure 4b).
Table 1.
Unidirectional rate constant for the ATP synthesis reaction Pi → γ-ATP in resting human skeletal muscle (n = 15)
The Pi → ATP flux in resting skeletal muscle was 8.47 ± 2.18 mmol/kg wet weight, calculated by the product of the above-derived kPi→γ-ATP value, and the Pi concentration of 2.14 mmol/kg wet weight measured from the integral of Pi signal at 4.83 ppm in reference to that of γ-ATP (5.5 mmol/kg wet weight) in fully relaxed 31P MR spectrum.
Data simulation and analysis
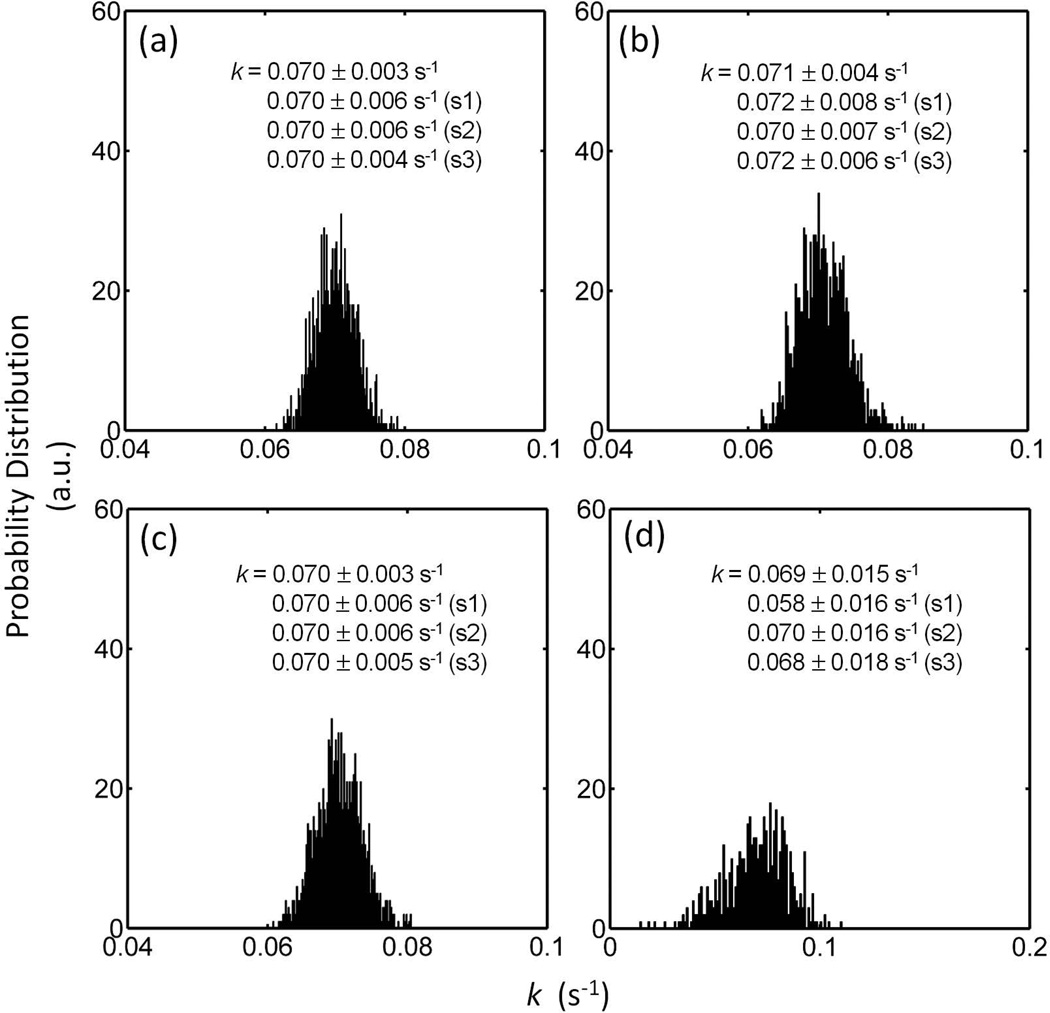
Figures 5 shows the analysis results for kPi→γ-ATP using the mPi and mγATP datasets generated at given kPi→γ-ATP and T1,Pi values and mixed with random noises. For a large number of executions (N = 1000), the mean values obtained for kPi→γ-ATP by these four different data analysis approaches are very similar to the input value (kPi→γ-ATP = 0.07 sec−1), but each approach shows a different dispersion pattern, as indicated by the data histograms (Figure 5). A significant large variance in kPi→γ-ATP is noticed in the histogram by Eq [3b] approach, as compared to other three methods defined by Eqs [3], [3a] and [11], suggesting it may be a less favorable approach. Among the three remaining approaches with smaller variances, Eqs [3] and [11] yielded slightly better outcomes in kPi→γ-ATP in terms of mean and standard deviation (Figure 5). Similar features also appear in the histograms for T1,Pi analysis results (Supplementary Figure S2).
FIG. 5.
Effects of Noise on Evaluation of kPi→γATP. The histograms represent the results of 1000 datasets in simulation of a scan with 12 data points at noise level ± 1%, using analysis approaches defined by Eqs [11] (a), [3] (b), [3a] (c) and [3b] (d). The derived kPi→γATP results are compared with those obtained for simulation at a higher noise level of ± 2% (S1), simulation of a scan collecting only the first 6 data points (S2), and simulation of a scan with 6 data points at large intervals in a wide range of delay times (S3).
For the three different time-saving acquisition strategies examined by simulation, it is found that a 100% increase in noise level (simulation S1), equivalent to a 4-fold decrease in NA and scan time, led to a 100% increase in variance in kPi→γ-ATP (Figures 5), as evaluated by Eqs [3], [3a] and [11]. A comparison between the two scenarios with 50% reduced data points (S2 and S3) indicates that the strategy with larger sampling intervals (S3) appears to be a better choice for kPi→γ-ATP determination. The T1,Pi analysis on the same simulation datasets showed the similar findings (Supplementary Figure S2).
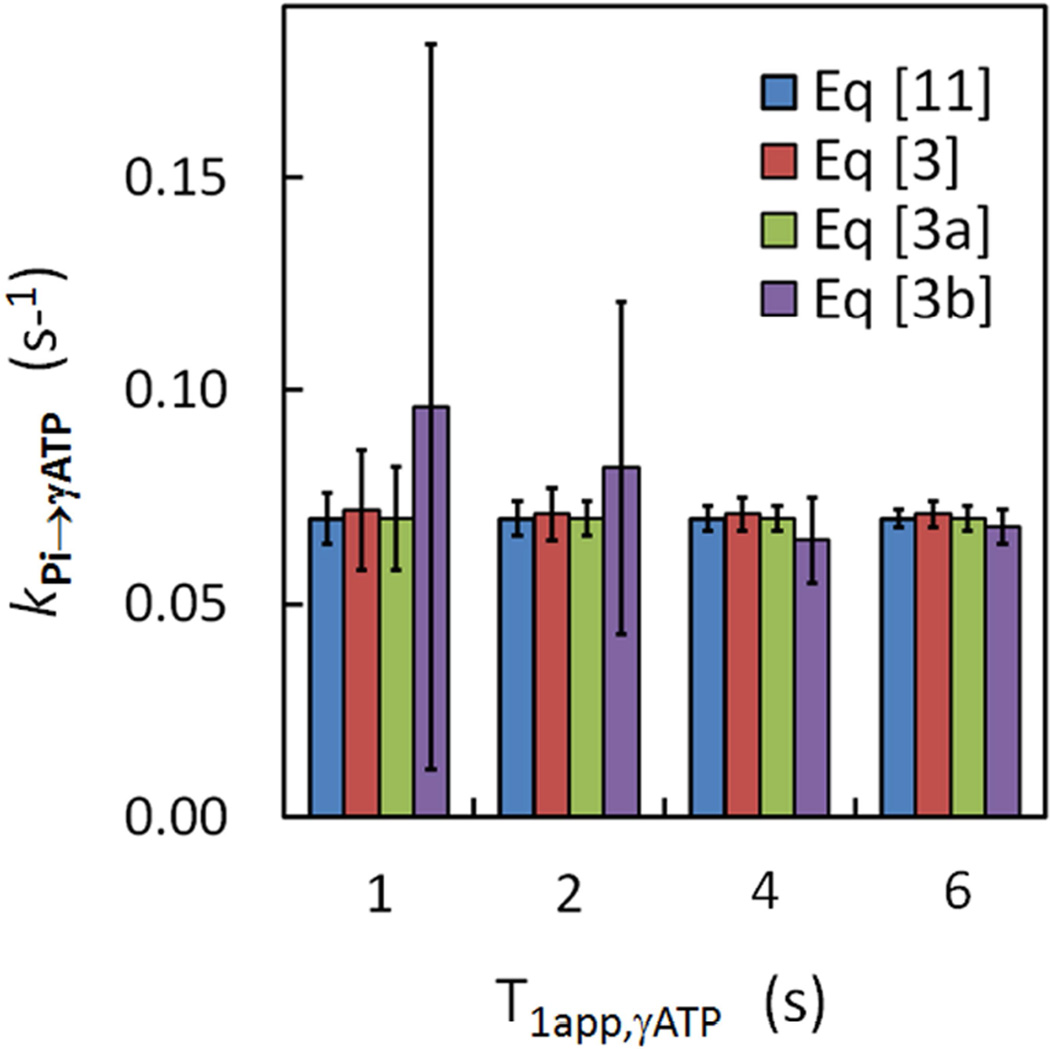
For those three linear equations (Eqs [3], [3a] and [3b]), an obvious feature is the absence of T1app,γ-ATP, in contrast to Eq [11] in which T1app,γ-ATP is an unknown that has to be solved in order to get kPi→γ-ATP. Simulations were performed to test whether a change in T1app,γATP would affect kPi→γ-ATP determination. For the two linear approaches defined by Eqs. [3 and 3a], simulation results (Figure 6) indicate that there was no significant difference in mean kPi→γ-ATP value when T1app,γATP was varied from 1 to 6 sec; the obtained kPi→γ-ATP values were comparable to that by the matrix approach defined by Eq [11] and consistent with the input value. However, the difference in kPi→γ-ATP between the linear approach by Eq [3b] and other approaches is noticeably large (Figure 6). Especially, the variance in kPi→γ-ATP by Eq [3b] was increased dramatically when T1app,γATP was decreased from 4 to 1 sec, suggesting that Eq [3b] approach may not be reliably applicable for kPi→γ-ATP determination when γ-ATP relaxation is fast. At T1app,γATP = 1 sec, which is equivalent to narrow band selective inversion, the matrix approach defined by Eq [11] yielded the best performance (Figure 6), with a variance ~2 – 3 fold smaller than those by Eqs [3] and [3a]. Similar performance was also found in T1,Pi analysis (Supplementary Figure S3) in which a large variance in T1,Pi was given by Eq [3b] approach, as compared to other approaches.
FIG. 6.
Effects of T1app,γATP on analysis of kPi→γATP. Comparison of different fitting approaches on analysis of simulation data at different apparent T1 values for γ-ATP.
DISCUSSION
Although the ability to probe exchange kinetics between metabolites using IT techniques has long been recognized, its use has behind alternative ST techniques for measurement of ATP synthesis rates (commentary (17) and the references therein). The added chemical shift dispersion and sensitivity of high field research scanners provide the motivation to reexamine the possible benefits of measuring such exchanges in vivo using IT methods (6). The preference of ST over IT may also have much to do with the ease of data analysis in ST, which permits the evaluation of kPi→ATP by a simple expression, rather than a non-intuitive way as in IT, numerically solving two or more simultaneous equations with multiple unknown parameters. Certain strategies toward simplifying IT data analysis were suggested in early kinetic studies. For example, early studies (4,5) showed that the rate constant for a two-spin system can be obtained from the initial slope of the curve Mz(t) ~ t by a formula equivalent to Eq [8]. derived directly from the basic Bloch-McConnell equation. In 1990 Kuchel (1) also proposed a simple formula, equivalent to Eq [7], to calculate the rate constant based on the inverted and non-inverted magnetizations at the time point with ṁ = 0 (for non-inverted spin).
In the current study, we suggest a method to directly utilize ṁPi, as defined by the original Bloch-McConnell equation for analysis of kPi→γ-ATP. The ṁPi data can be easily derived from the experimental mPi data (Eqs [5] and [6], and Figures 3a and 3b). Unlike mPi, which has a complex relationship with k (5), ṁPi is linearly correlated to k (Eq [3]), with a simple proportional coefficient (mPi − mγATP). As illustrated in Figure 3b, the trend in ṁPi ~ t is governed by two counteractive terms, the exchange term kPi→γATP (mPi − mγATP), which measures the magnitude of the net ME flux, and the relaxation term (1 − mPi)/T1,Pi, which measure the magnitude of T1 relaxation effect at Pi.
Once ṁPi is derived, one can easily determine kPi→γATP and T1,Pi by Eq [3, 3a or 3b], each having certain advantages and limitations. Eqs [3a and 3b] both are visually intuitive, with kPi→γATP and 1/T1,Pi shown as the slope and intercept (or vice versa) of a visible line while Eq [3] provides a direct quantitative separation of relaxation and exchange terms (Figure 4b), from which one can easily understand how these two terms evolve with delay time and contribute to the observed ME effect.
Despite the difference in mathematical expression, for resting human calf muscle, the data analysis using Eqs [3, 3a and 3b] yielded comparable kPi→γATP results, as that by the numerical matrix approach defined by Eq [11] (Table 1). These results are also in agreement with the corresponding kPi→γATP value (0.050 s−1) by an integrated numerical analysis method on data acquired using EKIT sequence (6). They are also consistent with the literature reported kPi→γATP values ranging from 0.04 to 0.12 s−1 using ST approach (review (9) and the references therein). The T1,Pi relaxation times obtained by these linear approaches are also in reasonably agreement with the literature values (6,37) at 7T.
It should be noted that, although the IT technique is not able to decouple Pi from the rest of the 31P exchange network in vivo as ST does through prolonged irradiation at γ-ATP, the IT data analysis method presented here requires no detailed knowledge about the various exchange reaction that the inverted γ-ATP is involved for evaluation of kPi→γATP. This means that, selection of IT or ST techniques depends on practical considerations such as available sequences in the MRI scanner and the required experimental time. Since IT technique does not require additional mirror irradiation to control the “spillover” effect, it is an advantage over progressive ST on this respect.
A careful analysis of the simulated datasets indicates that it is possible to get reliable rate constant kPi→γATP by using fewer data points or fewer acquisition averages to save scan time. One most attractive strategy is to acquire data at relatively large intervals to cover a wide range of delay times (simulation S3). It is possible that one can complete the data acquisition with ~10 minutes and still able to obtain reliable kPi→γATP values. While all linear approaches presented here are mathematically simpler than the numerical matrix approach, the linear data analysis approach defined by Eq [3a] offers less variance values and larger suitability range (Figures 5–6).
In summary, we demonstrated that, using simple linear fitting procedure, IT data acquired by band inversion technique can be used to derive the kPi→γATP parameter for evaluation of the physiologically important Pi → ATP flux. Band inversion transfer is one alternative to ST for the measurement of ATP synthesis in vivo.
Supplementary Material
FIG. S1. Evolution of PCr, α- and β-ATP signals with the inversion delay time t.
FIG. S2. Effects of Noise on Evaluation of T1,Pi. The histograms represent the results of 1000 datasets in simulation of a scan with 12 data points at noise level ± 1%, using analysis approaches defined by Eqs [11] (a), [3] (b), [3a] (c) and [3b] (d). The derived T1,Pi results are compared with those obtained for simulation at a higher noise level of ± 2% (S1), simulation of a scan collecting only the first 6 data points (S2), and simulation of a scan with 6 data points at large intervals in a wide range of delay times(S3).
FIG. S3. Effects of T1app,γ ATP on analysis of T1,Pi. Comparison of different fitting approaches on analysis of simulation data at different apparent T1 values for γ-ATP.
ACKNOWLEDGEMENTS
The authors are grateful for the technical support from Drs. Baolian Yang and Ivan Dimitrov (Philips Medical Systems), Salvador Pena for operational assistance. Jeannie Davis and Janet Jerrow recruited and managed the human subjects. This project was supported by the National Center for Research Resources and the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health through P41EB015908, DK081186, R37HL034557, P01DK058398 and R01AR050597, Department of Defense Grant W81XWH-06-2-0046.
Abbreviation used
- IT
inversion transfer
- ST
saturation transfer
- NOE
nuclear Overhauser effect
- ME
magnetization exchange
- MT
magnetization transfer
- SAR
specific absorption ratio
- EKIT
exchange kinetics by inversion transfer
- EBIT
exchange kinetics by band inversion transfer
- Eq
Equation
- Pi
inorganic phosphate
- ATP
adenosine triphosphate
- ADP
adenosine diphosphate
- PCr
phosphocreatine
- PDE
phosphodiesters
- CK
creatine kinase
- AK
adenylate kinase
- PFK
phosphofructokinase
- GAPDH
glyceraldehydes-3-phosphate dehydrogenase
REFERENCES
- 1.Kuchel PW. Spin-exchange NMR spectroscopy in studies of the kinetics of enzymes and membrane transport. NMR Biomed. 1990;3(3):102–119. doi: 10.1002/nbm.1940030303. [DOI] [PubMed] [Google Scholar]
- 2.Noggle JH, Schirmer RE. The nuclear overhauser effect - chemical applications. New York and London: Acedemic press; 1971. [Google Scholar]
- 3.Meyer RA, Kuchmerick MJ, Brown TR. Application of 31P-NMR spectroscopy to the study of striated muscle metabolism. Am J Physiol. 1982;242(1):C1–C11. doi: 10.1152/ajpcell.1982.242.1.C1. [DOI] [PubMed] [Google Scholar]
- 4.Alger JR, Prestegard JH. Investigation of peptide bond isomerization by magnetization transfer NMR. J Magn Reson. 1977;27(1):137–141. [Google Scholar]
- 5.Malloy CR, Sherry AD, Nunnally RL. Carbon 13 NMR measurement of flux through alanine animotransferase by inversion and saturation transfer methods. J Mag Res. 1985;64:243–254. [Google Scholar]
- 6.Ren J, Yang B, Sherry AD, Malloy CR. Exchange kinetics by inversion transfer: Integrated analysis of the phosphorous metabolite kinetics exchanges in resting human skeletal muscle at 7T. Magn Reson Med. 2014 doi: 10.1002/mrm.25256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown TR, Ugurbil K, Shulman RG. 31P nuclear magnetic resonance measurements of ATPase kinetics in aerobic Escherichia coli cells. Proc Natl Acad Sci USA. 1977;74(12):5551–5553. doi: 10.1073/pnas.74.12.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kingsley-Hickman P, Sako EY, Andreone PA, St Cyr JA, Michurski S, Foker JE, From AH, Petein M, Ugurbil K. 31P NMR measurement of ATP synthesis rate in perfused intact rat hearts. FEBS Lett. 1986;198(1):159–163. doi: 10.1016/0014-5793(86)81204-2. [DOI] [PubMed] [Google Scholar]
- 9.Kemp GJ, Brindle KM. What do magnetic resonance-based measurements of Pi→ATP flux tell us about skeletal muscle metabolism? Diabetes. 2012;61(8):1927–1934. doi: 10.2337/db11-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Befroy DE, Rothman DL, Petersen KF, Shulman GI. 31P-magnetization transfer magnetic resonance spectroscopy measurements of in vivo metabolism. Diabetes. 2012;61(11):2669–2678. doi: 10.2337/db12-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, et al. Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen KF, Dufour S, Shulman GI. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med. 2005;2(9):e233. doi: 10.1371/journal.pmed.0020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 14.Szendroedi J, Schmid AI, Chmelik M, Toth C, Brehm A, et al. Muscle Mitochondrial ATP Synthesis and Glucose Transport/Phosphorylation in Type 2 Diabetes. PLoS Med. 2007;4(5):e154. doi: 10.1371/journal.pmed.0040154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebon V, Dufour S, Petersen KF, Ren J, Jucker BM, et al. Effect of triiodothyronine on mitochondrial energy coupling in human skeletal muscle. J Clin Invest. 2001;108:733–737. doi: 10.1172/JCI11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jucker BM, Dufour S, Ren J, Cao X, Previs SF, Underhill B, Cadman KS, Shulman GI. Assessment of mitochondrial energy coupling in vivo by 13C/31P NMR. Proc Natl Acad Sci U S A. 2000;97(12):6880–6884. doi: 10.1073/pnas.120131997. Erratum in: Proc Natl Acad Sci U S A 2001; 98(6):3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koretsky AP, Basus VJ, James TL, Klein MP, Weiner MW. Detection of exchange reactions involving small metabolite pools using NMR magnetization transfer techniques: relevance to subcellular compartmentation of creatine kinase. Magn Reson Med. 1985;2(6):586–594. doi: 10.1002/mrm.1910020610. [DOI] [PubMed] [Google Scholar]
- 19.Balaban RS, Koretsky AP. Interpretation of 31P NMR saturation transfer experiments: what you can't see might confuse you. Focus on "Standard magnetic resonance-based measurements of the Pi→ATP rate do not index the rate of oxidative phosphorylation in cardiac and skeletal muscles". Am J Physiol Cell Physiol. 2011;301(1):C12–C15. doi: 10.1152/ajpcell.00100.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nabuurs CI, Hilbers CW, Wieringa B, Heerschap A. Letter to the editor: "Interpretation of 31P NMR saturation transfer experiments: do not forget the spin relaxation properties". Am J Physiol Cell Physiol. 2012;302(10):C1566–C1567. doi: 10.1152/ajpcell.00409.2011. [DOI] [PubMed] [Google Scholar]
- 21.Kingsley PB, Monahan WG. Corrections for off-resonance effects and incomplete saturation in conventional (two-site) saturation-transfer kinetic measurements. Magn Reson Med. 2000;43(6):810–819. doi: 10.1002/1522-2594(200006)43:6<810::aid-mrm6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 22.Baguet E, Roby C. Off-Resonance Irradiation Effect in Steady-State NMR Saturation Transfer. J Magn Reson. 1997;128(2):149–160. doi: 10.1006/jmre.1997.1230. [DOI] [PubMed] [Google Scholar]
- 23.Valkovič L, Bogner W, Gajdošík M, Považan M, Kukurová IJ, Krššák M, Gruber S, Frollo I, Trattnig S, Chmelík M. One-dimensional image-selected in vivo spectroscopy localized phosphorus saturation transfer at 7T. Magn Reson Med. 2014 doi: 10.1002/mrm.25058. In press. [DOI] [PubMed] [Google Scholar]
- 24.Degani H, Laughlin M, Campbell S, Shulman RG. Kinetics of creatine kinase in heart: a 31P NMR saturation- and inversion-transfer study. Biochemistry. 1985;24(20):5510–5516. doi: 10.1021/bi00341a035. [DOI] [PubMed] [Google Scholar]
- 25.Brown TR, Ogawa S. 31P nuclear magnetic resonance kinetic measurements on Adenylate kinase. Proc Natl Acad Sci. 1972;74:3627–3631. doi: 10.1073/pnas.74.9.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brindle KM. NMR methods for measuring enzyme kinetics in vivo. Progress in NMR Spectroscopy. 1988;20:257–293. [Google Scholar]
- 27.Morris GA, Freeman R. Selective excitation in Fourier transform nuclear magnetic resonance. J Magn Reson. 1978;29:433–462. doi: 10.1016/j.jmr.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 28.Schmid AI, Schrauwen-Hinderling VB, Andreas M, Wolzt M, Moser E, Roden M. Comparison of measuring energy metabolism by different (31) P-magnetic resonance spectroscopy techniques in resting, ischemic, and exercising muscle. Magn Reson Med. 2012;67(4):898–905. doi: 10.1002/mrm.23095. [DOI] [PubMed] [Google Scholar]
- 29.Valkovič L, Chmelík M, Just Kukurova I, Krššák M, Gruber S, Frollo I, Trattnig S, Bogner W. Time-resolved phosphorous magnetization transfer of the human calf muscle at 3 T and 7 T: a feasibility study. Eur J Radiol. 2013;82(5):745–751. doi: 10.1016/j.ejrad.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 30.Brindle KM, Blackledge MJ, Challiss RA, Radda GK. 31P NMR magnetization-transfer measurements of ATP turnover during steady-state isometric muscle contraction in the rat hind limb in vivo. Biochemistry. 1989;28(11):4887–4893. doi: 10.1021/bi00437a054. [DOI] [PubMed] [Google Scholar]
- 31.Shoubridge EA, Briggs RW, Radda GK. 31P NMR saturation transfer measurements of the steady state rates of creatine kinase and ATP synthetase in the rat brain. FEBS Lett. 1982;140(2):289–292. doi: 10.1016/0014-5793(82)80916-2. [DOI] [PubMed] [Google Scholar]
- 32.Lei H, Ugurbil K, Chen W. Measurement of unidirectional Pi to ATP flux in human visual cortex at 7 T by using in vivo 31P magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 2003;100(24):14409–14414. doi: 10.1073/pnas.2332656100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du F, Zhu XH, Qiao H, Zhang X, Chen W. Efficient in vivo 31P magnetization transfer approach for noninvasively determining multiple kinetic parameters and metabolic fluxes of ATP metabolism in the human brain. Magn Reson Med. 2007;57(1):103–114. doi: 10.1002/mrm.21107. [DOI] [PubMed] [Google Scholar]
- 34.Nabuurs C, Huijbregts B, Wieringa B, Hilbers CW, Heerschap A. 31P saturation transfer spectroscopy predicts differential intracellular macromolecular association of ATP and ADP in skeletal muscle. J Biol Chem. 2010;285(51):39588–39596. doi: 10.1074/jbc.M110.164665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren J, Sherry AD, Malloy CR. Amplification of the effects of magnetization exchange by band inversion for measuring adenosine triphosphate synthesis rates in human skeletal muscle. Magn Reson Med. 2014 doi: 10.1002/mrm.25514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong Q, Du F, Zhu X, Zhang P, Suntharalingam P, Ippolito J, Kamdar FD, Chen W, Zhang J. ATP production rate via creatine kinase or ATP synthase in vivo: a novel superfast magnetization saturation transfer method. Circ Res. 2011;108(6):653–663. doi: 10.1161/CIRCRESAHA.110.231456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bogner W, Chmelik M, Schmid AI, Moser E, Trattnig S, Gruber S. Assessment of (31)P relaxation times in the human calf muscle: a comparison between 3 T and 7 T in vivo. Magn Reson Med. 2009;62(3):574–582. doi: 10.1002/mrm.22057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIG. S1. Evolution of PCr, α- and β-ATP signals with the inversion delay time t.
FIG. S2. Effects of Noise on Evaluation of T1,Pi. The histograms represent the results of 1000 datasets in simulation of a scan with 12 data points at noise level ± 1%, using analysis approaches defined by Eqs [11] (a), [3] (b), [3a] (c) and [3b] (d). The derived T1,Pi results are compared with those obtained for simulation at a higher noise level of ± 2% (S1), simulation of a scan collecting only the first 6 data points (S2), and simulation of a scan with 6 data points at large intervals in a wide range of delay times(S3).
FIG. S3. Effects of T1app,γ ATP on analysis of T1,Pi. Comparison of different fitting approaches on analysis of simulation data at different apparent T1 values for γ-ATP.