Frailty represents one of the most critical issues facing health care due to its inherent relationship with poor health care outcomes. Frailty is present in 10% to 20% of individuals 65 years and older1,2 and increases with advancing age. Currently, 15% of the United States population is 65 years and older; a number that is forecast to increase to 21% by the year 2030.3
Older adults make up a large portion of surgical practice in the United States. In 2010, 37% of all inpatient operations performed in the United States were in patients 65 years and older,4 and this percentage will rise in the coming decades.5 Given the inevitable rise of the aging population, it is vital that surgeons understand the concept of frailty and how it may affect surgical decisions and outcomes. To address this gap in knowledge, the National Institute on Aging and the American Geriatrics Society sponsored a 2-day conference held March 2 and 3, 2015, specifically addressing the topic of frailty for specialists. Global leaders in frailty management and research served as faculty. The purpose of this manuscript is to summarize the key points regarding frailty and perioperative management in a clinically relevant context.
FRAILTY AND SURGICAL OUTCOMES
Frailty in older adults is closely associated with adverse surgical outcomes. Baseline preoperative frailty forecasts adverse postoperative events (Table 1). Multiple studies across a range of surgical disciplines consistently relate baseline preoperative frailty to poor surgical outcomes including serious complications,6–9 prolonged length of stay,8 need for discharge to an institutional care facility,7,8,10–12 hospital readmission, 30-day mortality,12 and long-term mortality.10 Frailty has only recently been recognized in the surgical literature; a statement evidenced by the fact that the term frailty did not appear as a title word in a major surgical journal until a letter to the editor reported in 2005,13 and the first scientific publication was in 2009.10
Table 1.
Why Frailty Matters: Frail Older Adults Are at Highest Risk
| Risks |
| Falls |
| Disability |
| Comorbid disease states |
| Delirium |
| Cognitive decline |
| Iatrogenic complications |
| Social withdrawal |
| Death |
Multiple examples exist in the peer-reviewed literature to support the close association between frailty and poor surgical outcomes. The following 3 studies represent the initial publications relating frailty to surgical outcomes. Dasgupta and colleagues7 measured frailty preoperatively with the Edmonton Frail Scale and found that high frailty scores were associated with increased postoperative complications (odds ratio [OR] 5.02; 95% CI 1.55 to 16.25) and a lower chance of being discharged home (40%; p = 0.02). Robinson and colleagues10 found that accumulation of a high number of frailty characteristics was related to an increased risk of 6-month mortality, with 81% sensitivity and 86% specificity. Makary and colleagues8 measured preoperative phenotypic frailty and found an association between frailty and increased postoperative complications (OR 2.54; 95% CI 1.12 to 5.77), length of stay (incidence rate ratio 1.69; 95% CI 1.28 to 2.23), and discharge to an institutional care facility (OR 20.48; 95% CI 5.54 to 75.68).
DEFINING FRAILTY
Frailty can be conceptualized as an age-related, multi-dimensional state of decreased physiologic reserves that results in diminished resiliency, loss of adaptive capacity, and increased vulnerability to stressors.14–18 By definition, frailty is a state with high vulnerability to adverse health care outcomes including hospitalization, functional dependence, disability, falls, need for institutionalization, and mortality.19 Frailty has been conceptualized as a pre-disability state,20 but has also been described as co-existing with disability.21 The frail state has been described as an accelerated accumulation of health deficits, making individuals more vulnerable to adverse health outcomes at the same age.
Commonalities for conceptualizing frailty include age-related vulnerability, a decline in multiple physiologic systems, an age-related condition that often co-exists with disability and chronic disease but can be independent of these conditions, and deficit accumulation. There is broad acceptance of this conceptual definition of frailty.21,22 The biologic hallmarks of aging, which clinically express themselves as frailty, are listed in Table 2.23
Table 2.
Proposed Biologic Underpinnings of Aging23
| Genomic instability |
| Telomere attrition |
| Epigenetic alterations |
| Loss of proteostasis |
| Deregulated nutrient sensing |
| Mitochondrial dysfunction |
| Cellular senescence |
| Stem cell exhaustion |
| Altered intercellular communication |
Despite broad agreement on the definitions and conceptualizations of frailty, no consensus exists on which tool is best to use to assess frailty.21 Indeed, using the literature to identify an appropriate tool to measure frailty can be difficult because of the enormous heterogeneity in how frailty is measured. Part of this confusion around choosing a tool rests in the fact that multiple tools have been developed and that there are at least 2 major schools of thought regarding how to operationalize the measurement of frailty. To knowledgeably read the literature and understand how the tools can best be used, the clinician must understand the differing approaches to operationalizing frailty.
CONCEPTUALIZING AND MEASURING FRAILTY
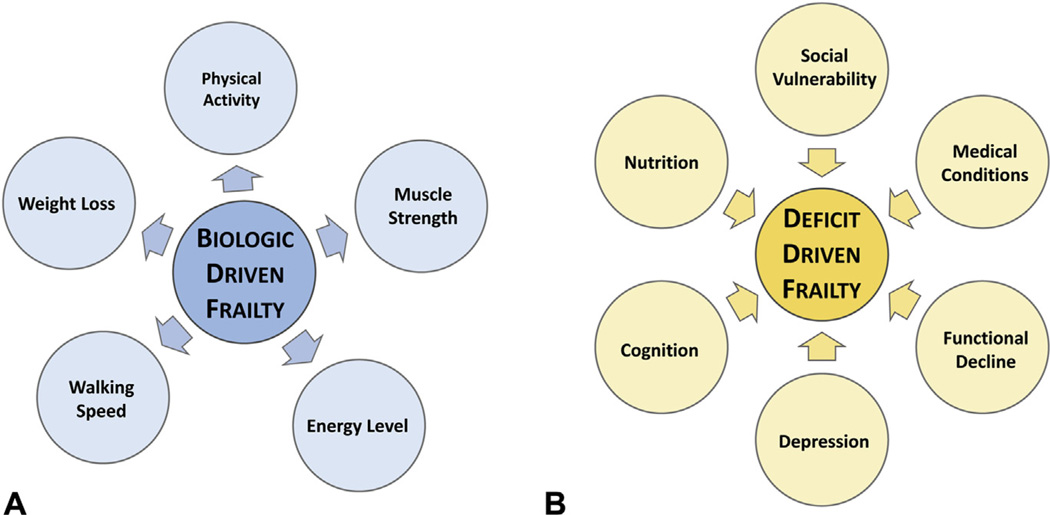
Two of the tools most commonly cited in the literature include the phenotypic definition of frailty15 and the accumulation of deficits definition of frailty24,25 (Fig. 1).
Figure 1.
Two conceptualizations of frailty. (A) Phenotypic frailty.15 Phenotypic frailty is conceptualized as a clinical syndrome driven by age-related biologic changes that drive physical characteristics of frailty and eventually, adverse outcomes. (B) Deficit accumulation frailty.18 The deficit model of frailty proposes that frailty is driven by the accumulation of medical, functional, and social deficits, and that a high accumulation of deficits represents accelerated aging. An important distinction between these 2 conceptualizations of frailty is that biologic driven frailty causes the physical characteristics of frailty (arrows pointed outward). In contrast, deficit accumulation frailty is caused by accumulated abnormal clinical characteristics (arrows pointed inward). Table 4 describes measuring phenotypic frailty. Table 5 describes measuring deficit accumulation frailty.
Phenotypic frailty views frailty as a biologic syndrome of decreased reserve resulting from cumulative declines across multiple biologic systems.15 This definition of frailty is often referred to as physical frailty. This methodology has an underlying hypothesized cycle with domains of decline related to skeletal muscle, nutrition, and energy metabolism, which results in the development of a frail phenotype. This hypothesized phenotype was operationalized into a screening tool that includes measures of unintentional weight loss, grip strength weakness, slow walking speed, self-reported exhaustion, and low activity levels.15 Those considered frail met 3 to 5 of the cutpoints; those with 1 or 2 were considered pre-frail, and those with none were considered not frail or robust. The phenotypic frailty measurement was initially validated by demonstrating strong relationships between frailty and a range of adverse health outcomes related to disability, falls, and mortality by using data from the Cardiovascular Health Study.15
The accumulation of deficits definition of frailty, often termed the frailty index, proposes that frailty is a nonspecific age-associated vulnerability that is reflected in an accumulation of medical, social, and functional deficits and that can be measured by counting an individual’s health problems or deficits.25 A wide range of deficits can be measured, including patients’ symptoms, medical diagnoses, lab abnormalities, and disabilities,26 though deficits must be acquired and associated with increasing age and adverse health outcomes.27 The greater the number of deficits one has, the higher the likelihood of frailty and the greater the likelihood of adverse health outcomes, including disability, institutionalization, additional deficit accumulation, and death.25 This definition is operationalized by generating an index score, which divides the number of deficits present in an individual by the number of deficits measured. Among community dwelling individuals, deficits are accumulated at rate of 3% per year28 and the frailty index has been associated with increasing age and mortality.28–30
TOOLBOX OF FRAIL MEASURES
Despite the lack of agreement on choosing a single measurement tool to assess frailty, a recent consensus conference on frailty suggested that those over age 70 should be screened for physical frailty, in part because physical frailty can be potentially treated or prevented with specific modalities, and by extension, the adverse outcomes associated with frailty ameliorated.31 Currently, more than 70 tools exist to measure frailty, which can be bewildering to those attempting to integrate frailty measurement into clinical practice. Many of these tools have not been extensively validated and/or reproduced in more than 1 population or study, and very few have been compared against each other in their capacity to predict adverse outcomes or utility in prevention or intervention development strategies, making it difficult to set clear recommendation standards. Frailty tools can range from measuring 1 item to more than 90 items. The reason for the proliferation of frailty tools is likely multifactorial and represents a need for differing assessment strategies based on clinical need, use of measures already collected rather than de novo targeted measurements, and lack of feasibility for developing tools retrospectively based on available clinical information. Frailty screening and frailty assessment practically may have different purposes. For example, a brief screening tool may be appropriate for risk stratification; a more formal frailty assessment may be required to define preoperative interventions to modify surgical outcomes. An independent assessment of disability may also be of clinical importance in surgical preoperative assessment because of the clinical importance of impaired function, which may need to be addressed by the health care professional.
Given the wide array of tools and the wide variety of populations in which the tools may need to be implemented, the choice of which to use must be tailored to a clinical situation and clinical need. In addition, choosing tools that have been previously used in a variety of populations and have demonstrated predictive validity in several settings should also influence the choice of tools. Given the short time available for preoperative assessments, another major factor to be considered when choosing a frailty measurement tool is the time required to complete the test. If time or resources impede wide-spread implementation, a good rule of thumb is to start by preoperatively screening individuals older than 70 years with weight loss. Tools used for frailty assessment are listed in order of shortest time to complete to longest time to complete and include the following.
Single item surrogate assessments
For feasibility, single item measurement tools have been proposed to quantify surgical risk as a surrogate for a more formal frailty measurement. Gait speed is recognized as a highly reliable single measurement tool.32 Slower gait speed (measured while a patient walked a 5-meter distance) is an incremental predictor of higher mortality and major morbidity after cardiac operations.33 A timed up-and-go score (the time it takes to rise from a chair, walk 10 feet, turn around, and return to sitting in the chair) ≥15 seconds is closely related to both postoperative complications and 1-year mortality.34 The timed up-and-go has been found to be both sensitive and specific for identifying frailty.35 Some of these single measures are components of both the frailty index and frailty phenotype approaches, and although they can be easy to use and predictive of certain outcomes, they can lack sensitivity and specificity of the full frailty assessment tools.
Frail Scale (<5 minutes)
The Frail Scale was developed as a screening tool.20 The Geriatric Advisory Panel of the International Academy of Nutrition and Aging developed this approach to define frailty as a case-finding tool.32 This brief tool simply requires asking 5 straightforward questions (Table 3).
Table 3.
| F | Fatigue (Are you fatigued?) |
| R | Resistance (Can you climb 1 flight of stairs?) |
| A | Ambulation (Can you walk 1 block?) |
| I | Illnesses (greater than 5) |
| L | Loss of weight (greater than 5%) |
Scoring: 0 = robust; 1 – 2 = pre-frail; ≥ 3 frail.
Phenotypic frailty (10 to 15 minutes)
Phenotypic frailty is the most widely used measurement tool used by frailty researchers. This frailty evaluation was 1 of 2 strategies recognized by the American College of Surgeons/American Geriatric Society’s optimal preoperative assessment of the older adult.36 The tool requires a questionnaire, a hand-held dynamometer, and a stopwatch (Table 4).
Table 4.
| Criteria and scoring | Measurement |
|---|---|
| Frailty criteria | |
| Shrinking (weight loss) | ≥10-pound weight loss in past year |
| Weakness | Grip strength in lowest 20% based on sex and BMI |
| Exhaustion | Self-reported exhaustion |
| Slowness | Walking speed over 15 feet in lowest 20% |
| Low activity | Kilocalories per week expended in lowest 20% |
| Scoring for surgical patients8 | |
| Robust | 0 or 1 abnormalities |
| Intermediate or pre-frail | 2 or 3 abnormalities |
| Frail | 4 or 5 abnormalities |
Deficit accumulation index
The most widely recognized deficit accumulation method to measure frailty was developed from the Canadian Health and Aging Study.18 Between 21 and 70 deficits are suggested to be measured. Although considerable time may be needed to gather information in the initial developmental stages of individualized frailty indices; data may be quickly accessible if they are already available in the electronic medical record. The frailty index score is calculated as the number of characteristics that are abnormal (or “deficits”) divided by the total number of characteristics measured (Table 5).
Table 5.
Deficit Accumulation Frailty Index – Range Of Time
| Frailty characteristic | Measurement |
|---|---|
| Mobility | Walking speed |
| Number of falls in past six months | |
| Cognition | History of dementia |
| Mini-Cog Test or Mini-Mental Status Exam | |
| Function | Dependence in activities of daily living |
| Dependence in instrumental activities of daily living | |
| Exhaustion | Energy level |
| Tiredness | |
| Burden of chronic disease | Charlson Index |
| > 5 chronic medications | |
| Nutritional status | >10 lbs weight loss in past 6 months |
| Low albumin | |
| Poor appetite | |
| Mood | Depression |
| Sadness | |
| Anxiety | |
| Social vulnerability | Presence of social support |
| Lack of interactions with other people |
Common strategies to score an accumulation of deficits frail score are twofold: (1) Sum the number of abnormal characteristics and reference to published “cutoff” score; and (2) calculate the ratio of abnormal frail characteristics and divide by the number of total assessed characteristics.
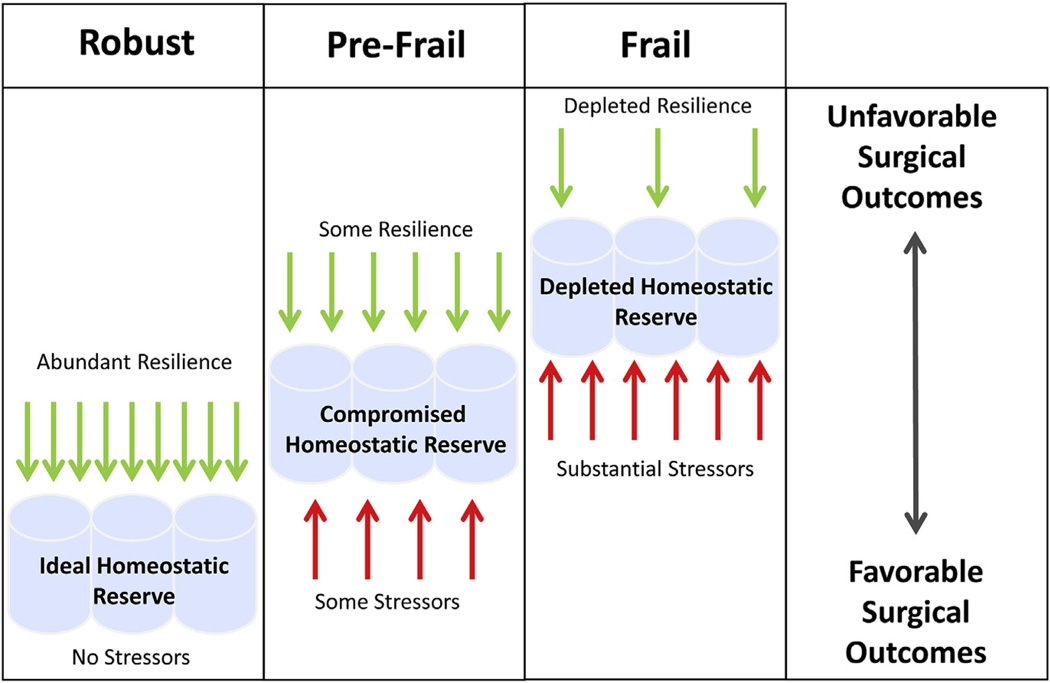
RESILIENCE AND FRAILTY
Vulnerability to stressors can cause an imbalance between physiologic demands and the body’s ability to cope with these demands, resulting in the dysregulation of multiple physiologic systems, physical disability, and adverse health outcomes.15,29,37 Resilience is defined as the positive capacity of an organism to deal with stress and other detrimental challenges.38 An alternative definition might be the ability to respond appropriately to a stressor because not all stress is detrimental. In other words, the amount of resilience represents how competent an organism is to respond to stress. Resilience and frailty are inter-related but are not opposites. An equally powerful, yet relatively underexplored, research discipline would be to define why some individuals maintain resiliency (Fig. 2). The mechanisms that promote frailty and resiliency are likely intertwined and might include environment, genetic, demographic, social, humoral, functional, and inflammatory processes.23 The concept of frailty to quantify physiologic compromise as a tool for surgical decision making is accepted and commonplace. In contrast, measuring resiliency as a measure of positive biologic reserve has not been explored as a strategy to aid in surgical decision making.38
Figure 2.
The concept of resilience. Resilience (a health-based, rather than disease-based, model) implies that disease is the consequence of inadequate reserve in the face of overwhelming stressors, which predispose to unstable and adverse health outcomes. Stressors may differ in number and magnitude, such that a few severe stressors may exert similar effects as many mild stressors.
INTERSECTION OF MULTI-MORBIDITY AND FRAILTY
Multi-morbidity is the co-occurrence of 2 or more chronic diseases in the same individual.39 The surgical literature has examples in which unfavorable outcomes in older adults are associated with a higher number of chronic diseases, and the resultant score is labeled as “frailty.”40,41 This is probably a misnomer due to the fact that frailty and multi-morbidity are thought to be distinct syndromes19 even though they share the attribute of elevated surgical risk. This biologic-based distinction between frailty and multi-morbidity is challenged by large dataset findings demonstrating that the majority of Medicare beneficiaries in whom geriatric syndromes of falls, functional dependence, and incontinence are common have 3 or more chronic conditions.42,43
The clinically relevant point for surgeons is that addressing multi-morbidity in the frail older adult needs to be managed from a patient-centric standpoint. High surgical risk due to multi-morbidity is commonly addressed by medical optimization of individual chronic diseases (eg, improved glucose control for diabetes). The tricky part for frail individuals is that treatment of 1 condition can exacerbate other conditions that do not lead to net health improvements (eg, improved glucose control leads to hypoglycemia, resulting in falls). The suggested management strategy is to identify and treat clinically dominant conditions that eclipse other less important conditions, which may be better left alone.44,45
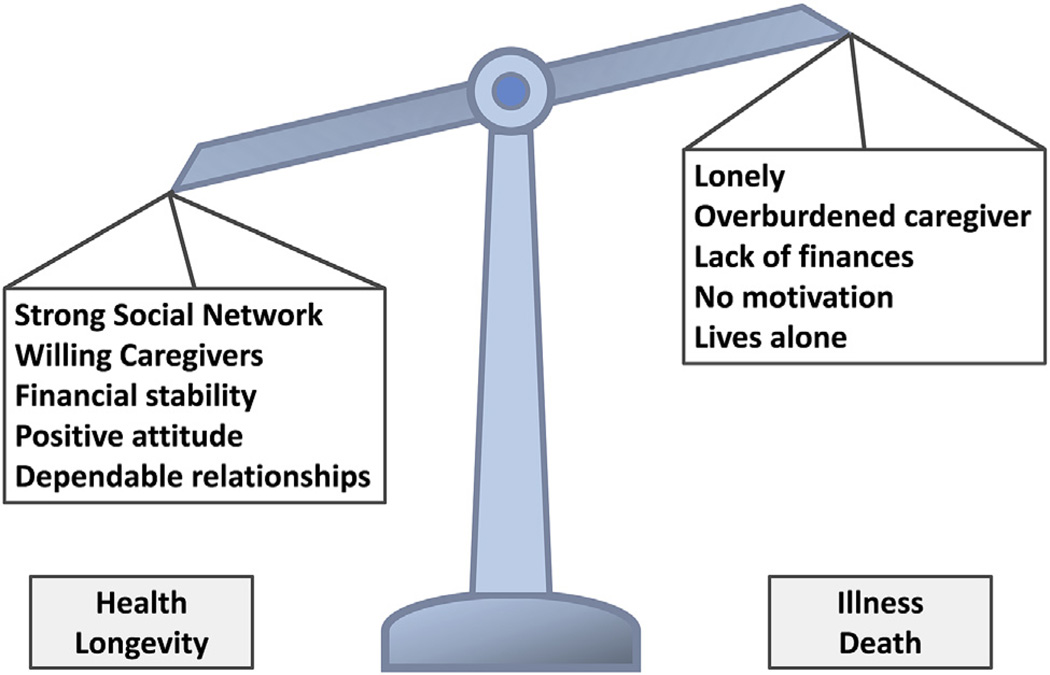
FRAILTY AND SOCIAL VULNERABILITY
To project an older adult’s health risk requires looking beyond intrinsic biologic and physiologic parameters and, incorporating the extrinsic social context in which they live.46 The tactic of looking beyond intrinsic physiology to extrinsic factors is relatively novel for surgical risk assessment and is part of how frail individuals are defined through the accumulation of deficits strategy. Social vulnerability is a unifying concept of an individual’s social situation and has been found to be reproducibly related to frailty and fitness, yet these constructs are distinct.47 A Social Vulnerability Index, which includes factors such as living alone, social engagement, empowerment/life-control, and socioeconomic status, is associated with an older adult’s risk of mortality47 (Fig. 3).
Figure 3.
The impact on health of social vulnerability.
FRAILTY ACCELERATORS BEYOND AGE
The acceleration of aging is influenced by both an intrinsic rate of background insults as well as an environmental rate of insults, which is balanced against the ability of the organism’s repair mechanisms.48 The biologic syndrome of frailty due to advanced chronologic age has less hope as a target to reverse the clinical detriments of frailty. In contrast, frailty due to a specific underlying cause that can be reversed appears to be a more fruitful target for interventions. Uncompensated single-end organ dysfunction, chronic infection, and malignancy are all recognized to promote a frail state. These causes of frailty are not clinically distinguishable from frailty due to advanced age alone; however, these “age-accelerating” causes of frailty may represent reversible causes of frailty. Two examples of age accelerating causes of frailty which may be responsive to and reversible by a surgical intervention follow.
Congestive heart failure
Older adults account for the majority of patients with heart failure.49 Patients with heart failure have physical dysfunction not only from heart failure, but also from aging and multi-morbidity. Heart failure exacerbations are proposed to create a cycle of immobility, loss of skeletal muscle, followed by delayed, incomplete recovery. This cycle promotes muscle abnormalities in skeletal muscle that include mitochondrial dysfunction and excess adipose deposition.50 Heart failure is a systemic syndrome that involves inflammatory, humoral, and other systemic factors that may be closely related to the synchronous development of frailty. Phenotypic frailty in a patient with heart failure is associated with a comorbidity-adjusted increase of 92% for emergency room visits and a 65% increased risk of hospitalization.51 Surgical interventions to treat heart failure (eg, left ventricular assist devices or percutaneous aortic valve placement to relieve aortic stenosis) may represent treatment strategies that reverse the frail state associated with congestive heart failure.
End-stage renal disease
Physical frailty is present at a 5- to 7-times higher rate in dialysis-dependent patients in comparison with community dwelling older adults.52 The relationship of frailty to chronic kidney disease is likely multifactorial and due to the systemic effects of end-stage renal disease, including protein wasting, anemia, inflammation, acidosis, and hormonal changes.53 Frail dialysis patients are at a 2.6-times higher risk of death (95% CI 1.04 to 6.49) compared with a comorbidity-adjusted group of non-frail dialysis patients.52 Frailty due to end-stage renal disease is potentially reversible by the surgical intervention of renal transplantation.
Congestive heart failure and end-stage renal disease both represent accelerators of clinical frailty that have the potential to be reversible. The reversibility of these end-organ, disease-driven frail states contrast with frailty associated solely with advanced chronologic age. These differences suggest that the measurement of frailty may be too blunt to differentiate between underlying drivers of the frail state. Other recognized accelerators of frailty include end-stage liver disease54 and HIV infection.55
UTILITY OF FRAILTY FOR SURGEONS
Quantifying frailty in older adults undergoing surgical therapy should be performed in the context of modifying patient care based on the frailty assessment findings. The goals of interventions for frail older adults are to improve quality of life, prevent worsening chronic disease, reduce the risk of catastrophic outcomes, and provide risk assessment to guide therapeutic decisions. Strategies for incorporating frailty assessment and intervention into the perioperative care of older adults are listed in Table 6.
Table 6.
The Utility of Frailty in the Care of Older Adults Undergoing Surgery
| Preoperative risk assessment |
| Trauma triage |
| Prehabilitation to modify risk |
| Tailored anesthesia approach |
| Implementation of team-based care pathways |
| Delirium prevention |
| Palliative care approaches |
Preoperative risk assessment
Preoperative frailty assessment is well established to forecast surgical outcomes including complications, length of stay, need for discharge to a skilled nursing facility or nursing home, and death.7,8,10–12 Quantification of frailty that forecasts elevated surgical risk can modify care in 2 ways. First, the aggressiveness of surgery can be modified. Frailty assessment allows tailoring of surgical recommendations to the real physiologic capacity of the patient. For example, endovascular valve replacement might be recommended over open surgery, or endoscopic stenting of an obstructing colon lesion might be recommended rather than resection. Research is needed to define the efficacy of outcomes based on frailty assessment risk and tailoring of the surgical recommendation. Second, appropriate counseling of anticipated outcomes can be provided. Knowledge of need for post-discharge nursing home stays and increased risk of complications prepares patients and families for their postoperative course. Recognizing and addressing co-dependence of the frail older adults’ partner before a major hospitalization relieves family worry and burden.
Trauma triage
Geriatric trauma presentations are increasingly common, particularly with the fall from standing height mechanism. A trauma-specific frailty assessment performed on initial presentation has been developed to aid trauma clinicians in their decision-making.56 This baseline frailty assessment on admission is able to forecast which patients require discharge to an institutionalization care setting.57,58
Prehabilitation to modify risk
Intervening before an operation to increase a frail patient’s physiologic reserve to withstand an operative stress is feasible. Exercise and nutrition are 2 common modalities of prehabilitation. Preoperative physical therapy improves pulmonary complications and decreases length of stay after cardiac operations.59 Multi-modal preoperative strategies including physical therapy, nutritional supplements, and anxiety reduction are also being studied to better prepare patients, with the goal of improving surgical outcomes.60
Tailor anesthesia regimen
Anesthesia choice for frail older adults includes optimizing regional anesthetics and minimizing sedation. An optimal anesthesia regimen for frail older adults has not been established. Regional anesthetics to control pain in the perioperative setting are proven to minimize postoperative delirium.61,62 Early evidence suggests that light sedation may reduce postoperative delirium in older adults undergoing hip fracture surgery under spinal anesthetic.63
Implement team-based care pathways
Team-based care approaches have proven beneficial for frail older adults in both the inpatient and outpatient settings. For inpatients, the Acute Care for Elders (ACE) model has proven to help maintain frail older adults’ functional status during acute hospital stays.64 For outpatients, the Program of All-inclusive Care for the Elderly (PACE) provides community-dwelling older adults with integrated health, social, recreational, and nutritional support, and preserves older adults’ function.65
Delirium prevention
Baseline physical frailty is associated with the development of postoperative delirium.66 Delirium is a critically relevant outcome for older adults due to its close relationship to adverse postoperative outcomes including increased complications, prolonged length of stay, increased discharge to a skilled nursing facility or nursing home, and death. Delirium is preventable in up to 40% of cases.67 Establishing baseline frailty can trigger immediate ordering of evidence-based postoperative multicomponent delirium prevention programs.61,62
Palliative care approaches
Palliative care is not synonymous with withdrawal of care. Frail patients require palliative care input preoperatively to help define their care goals.68 To determine if surgery is necessary, assessing the “need value” of an operation for a frail patient is vital. Value decisions are grounded in patient-oriented expectations and outcomes and should be discussed before any major operative intervention.
SUMMARY/FUTURE DIRECTIONS
Developing frailty tools for surgical needs may require more than 1 instrument. For example, the best frailty measurement to assess postoperative fall risk, delirium, or complications will likely differ from a tool aimed to define frailty biology. A surgical frail score probably should be tailored to define frail characteristics that are modifiable, with the goal of improving surgical outcomes.
Incorporation of frailty assessments into the preoperative flow will have additional downstream effects than simple risk assessment because it will open a window into patient-centric care. The current culture of being “too busy” to pursue clinical questions that uncover patient-centric expectations and goals will need to be challenged with the ultimate goal of practically fitting this line of clinical inquiry into the routine preoperative clinical evaluation. Frailty evaluation can catalyze this necessary change. Additionally, understanding frailty will facilitate new treatment strategies, and the implications of a positive frailty score on perioperative care will allow surgeons to improve the surgical care of older adults.
Acknowledgments
Disclosures outside the scope of this work: Dr Hurria is paid as a consultant for GTx, Inc and Boehringer Ingelheim Pharma; he is employed by On Q Health; and he received a grant from GSK Celgene.
Support: This conference was supported by U13 AG048721 (Dr Hurria was the principal investigator): Physician Scientists in Aging: Developing and Activating Specialty Investigators, and the John A Hartford Foundation, Inc. Dr Walston’s efforts are supported by Johns Hopkins University Claude D Pepper Older Americans Independence Center, National Institute on Aging, NIA P30AG021334. Dr Brummel is supported by the National Institute on Aging under R03AG040549 and the National Center for Advancing Translational Sciences KL2TR000446. He also received support for travel from the American Geriatrics Society. Dr Brown’s efforts are supported by the Research Career Development Core of the Johns Hopkins Pepper Older Americans Independence Center, NIA P30AG02133.
APPENDIX 1. NATIONAL INSTITUTE ON AGING FRAILTY FOR SPECIALISTS PLANNING COMMITTEE AND SPEAKERS
Principal Investigators: Arti Hurria, MD, City of Hope National Medical Center, Duarte, CA; Chris Carpenter, MD, MSc, Washington University School of Medicine, Saint Louis, MO.
Co-Chairs: Tom Robinson, MD, University of Colorado, Aurora, CO; Jeremy Walston, MD, Johns Hopkins University, Baltimore, MD.
Planning Committee Members: Susan Zieman, MD, PhD, National Institute on Aging, National Institutes of Health, Bethesda, MD; Basil Eldadah, MD, PhD, National Institute on Aging, National Institutes of Health, Bethesda, MD; Nancy Lundebjerg, MPA, American Geriatrics Society, New York, NY.
Speakers: Keri Althoff, PhD, Johns Hopkins University, Baltimore, MD; Caroline Blaum, MD, MS, New York University School of Medicine, New York, NY; Patrick Brown, PhD, Columbia University, New York, NY; Deborah Culley, MD, Harvard Medical School, Boston, MA; Wesley Ely, MD, MPH, Vanderbilt University, Nashville, TN; Luigi Ferrucci, MD, PhD, National Institute on Aging, National Institutes of Health, Bethesda, MD; Bill Hazzard, MD, Wake Forest University, Winston Salem, NC; Kevin High, MD, Wake Forest University, Winston Salem, NC; Dalane Kitzman, MD, Wake Forest University, Winston Salem, NC; Stephen Kritchevsky, PhD, Wake Forest University, Winston Salem, NC; Ken Rockwood, MD, FRCPC, FRCP, Dalhousie University, Halifax, Nova Scotia; Kenneth Schmader, MD, Duke University, Durham, NC; Dorry Segev, MD, PhD, Johns Hopkins University, Baltimore, MD; Felipe Sierra, PhD, National Institute on Aging, National Institutes of Health, Bethesda, MD; Stephanie Studenski, MD, MPH, National Institute on Aging, National Institutes of Health, Bethesda, MD; Ravi Varadhan, PhD, Johns Hopkins University, Baltimore, MD.
American Geriatrics Society Staff: Erin Obrusniak, Marianna Drootin, MPA, Kelly Middleton, American Geriatrics Society, New York, NY.
Footnotes
Disclosure Information: Nothing to disclose.
Author Contributions
Study conception and design: Robinson, Walston, Hurria
Acquisition of data: Robinson, Walston, Brummel, Deiner, Brown, Kennedy
Analysis and interpretation of data: Robinson, Walston, Brummel, Deiner, Brown, Kennedy, Hurria
Drafting of manuscript: Robinson, Brummel, Deiner, Brown, Kennedy
Critical revision: Robinson, Walston, Brummel, Deiner, Brown, Kennedy, Hurria
REFERENCES
- 1.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 2.Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58:681–687. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- 3.United States Census Bureau. 2014 National Population Projections. [Accessed September 22, 2015]; Available at: https://www.census.gov/population/projections/data/national/2014.html.
- 4.Centers for Disease Control and Prevention. National Hospital Discharge Summary (2010) [Accessed September 22, 2015]; Available at: http://www.cdc.gov/nchs/nhds.htm.
- 5.Etzioni DA, Liu JH, Maggard MA, Ko CY. The aging population and its impact on the surgery workforce. Ann Surg. 2003;238:170–177. doi: 10.1097/01.SLA.0000081085.98792.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson TN, Wu DS, Pointer L, et al. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg. 2013;206:544–550. doi: 10.1016/j.amjsurg.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dasgupta M, Rolfson DB, Stolee P, et al. Frailty is associated with postoperative complications in older adults with medical problems. Arch Gerontol Geriatr. 2009;48:78–83. doi: 10.1016/j.archger.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 9.Kristjansson SR, Nesbakken A, Jordhoy MS, et al. Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: a prospective observational cohort study. Crit Rev Oncol Hematol. 2010;76:208–217. doi: 10.1016/j.critrevonc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Robinson TN, Eiseman B, Wallace JI, et al. Redefining geriatric preoperative assessment using frailty, disability and comorbidity. Ann Surg. 2009;250:449–455. doi: 10.1097/SLA.0b013e3181b45598. [DOI] [PubMed] [Google Scholar]
- 11.Robinson TN, Wallace JI, Wu DS, et al. Accumulated frailty characteristics predict postoperative discharge institutionalization in the geriatric patient. J Am Coll Surg. 2011;213:37–42. doi: 10.1016/j.jamcollsurg.2011.01.056. discussion 42–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DH, Buth KJ, Martin BJ, et al. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010;121:973–978. doi: 10.1161/CIRCULATIONAHA.108.841437. [DOI] [PubMed] [Google Scholar]
- 13.Ferrucci L, Maggio M, Ceda GP, et al. Acute postoperative frailty. J Am Coll Surg. 2006;203:134–135. doi: 10.1016/j.jamcollsurg.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bortz WM., 2nd A conceptual framework of frailty: a review. J Gerontol A Biol Sci Med Sci. 2002;57:M283–M288. doi: 10.1093/gerona/57.5.m283. [DOI] [PubMed] [Google Scholar]
- 15.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 16.Lipsitz LA. Dynamics of stability: the physiologic basis of functional health and frailty. J Gerontol A Biol Sci Med Sci. 2002;57:B115–B125. doi: 10.1093/gerona/57.3.b115. [DOI] [PubMed] [Google Scholar]
- 17.Lipsitz LA, Goldberger AL. Loss of ‘complexity’ and aging. Potential applications of fractals and chaos theory to senescence. JAMA. 1992;267:1806–1809. [PubMed] [Google Scholar]
- 18.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 20.Abellan van Kan G, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc. 2008;9:71–72. doi: 10.1016/j.jamda.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Manas L, Feart C, Mann G, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68:62–67. doi: 10.1093/gerona/gls119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen RR, Lagoo-Deenadayalan SA, Heflin MT, et al. Exploring predictors of complication in older surgical patients: a deficit accumulation index and the Braden Scale. J Am Geriatr Soc. 2012;60:1609–1615. doi: 10.1111/j.1532-5415.2012.04109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-Otin C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Scientific World J. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 26.de Vries NM, Staal JB, van Ravensberg CD, et al. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10:104–114. doi: 10.1016/j.arr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27:17–26. doi: 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–2189. doi: 10.1111/j.1532-5415.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 29.Kulminski AM, Ukraintseva SV, Culminskaya IV, et al. Cumulative deficits and physiological indices as predictors of mortality and long life. J Gerontol A Biol Sci Med Sci. 2008;63:1053–1059. doi: 10.1093/gerona/63.10.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulminski AM, Ukraintseva SV, Kulminskaya IV, et al. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56:898–903. doi: 10.1111/j.1532-5415.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abellan van Kan G, Rolland Y, Bergman H, et al. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12:29–37. doi: 10.1007/BF02982161. [DOI] [PubMed] [Google Scholar]
- 33.Afilalo J, Eisenberg MJ, Morin JF, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–1676. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 34.Robinson TN, Wu DS, Sauaia A, et al. Slower walking speed forecasts increased postoperative morbidity and 1-year mortality across surgical specialties. Ann Surg. 2013;258:582–588. doi: 10.1097/SLA.0b013e3182a4e96c. discussion 588–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savva GM, Donoghue OA, Horgan F, et al. Using timed up-and-go to identify frail members of the older population. J Gerontol A Biol Sci Med Sci. 2013;68:441–446. doi: 10.1093/gerona/gls190. [DOI] [PubMed] [Google Scholar]
- 36.Chow WB, Rosenthal RA, Merkow RP, et al. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg. 2012;215:453–466. doi: 10.1016/j.jamcollsurg.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 37.Buchner DM, Wagner EH. Preventing frail health. Clin Geriatr Med. 1992;8:1–17. [PubMed] [Google Scholar]
- 38.De Alfieri W, Borgogni T. Through the looking glass and what frailty found there: looking for resilience in older adults. J Am Geriatr Soc. 2010;58:602–603. doi: 10.1111/j.1532-5415.2010.02754.x. [DOI] [PubMed] [Google Scholar]
- 39.Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 40.Obeid NM, Azuh O, Reddy S, et al. Predictors of critical care-related complications in colectomy patients using the National Surgical Quality Improvement Program: exploring frailty and aggressive laparoscopic approaches. J Trauma Acute Care Surg. 2012;72:878–883. doi: 10.1097/TA.0b013e31824d0f70. [DOI] [PubMed] [Google Scholar]
- 41.Keller DS, Bankwitz B, Nobel T, Delaney CP. Using frailty to predict who will fail early discharge after laparoscopic colorectal surgery with an established recovery pathway. Dis Colon Rectum. 2014;57:337–342. doi: 10.1097/01.dcr.0000442661.76345.f5. [DOI] [PubMed] [Google Scholar]
- 42.Centers for Medicare and Medicaid Services. Chronic Conditions among Medicare Beneficiaries Chartbook: 2012 edition. Baltimore, MD: 2012. [Accessed September 22, 2015]. Available at https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Chronic-Conditions/Downloads/2012Chartbook.pdf. [Google Scholar]
- 43.Cigolle CT, Lee PG, Langa KM, et al. Geriatric conditions develop in middle-aged adults with diabetes. J Gen Intern Med. 2011;26:272–279. doi: 10.1007/s11606-010-1510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29:725–731. doi: 10.2337/diacare.29.03.06.dc05-2078. [DOI] [PubMed] [Google Scholar]
- 45.Reuben DB, Tinetti ME. Goal-oriented patient care–an alternative health outcomes paradigm. N Engl J Med. 2012;366:777–779. doi: 10.1056/NEJMp1113631. [DOI] [PubMed] [Google Scholar]
- 46.Andrew MK. Social vulnerability in old age. In: Fillit HM, Rockwood K, Woodhous K, editors. Brocklehurst’s Textbook of Geriatric Medicine and Gerontology. Philadelphia: Saunders; 2010. [Google Scholar]
- 47.Andrew MK, Mitnitski AB, Rockwood K. Social vulnerability, frailty and mortality in elderly people. PLoS One. 2008;3:e2232. doi: 10.1371/journal.pone.0002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brothers TD, Rockwood K. Biologic aging, frailty, and age-related disease in chronic HIV infection. Curr Opin HIV AIDS. 2014;9:412–418. doi: 10.1097/COH.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 49.Hall MJ, DeFrances CJ, Williams SN, et al. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report. 2010:1–20. 24. [PubMed] [Google Scholar]
- 50.Haykowsky MJ, Kouba EJ, Brubaker PH, et al. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211–1216. doi: 10.1016/j.amjcard.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McNallan SM, Singh M, Chamberlain AM, et al. Frailty and healthcare utilization among patients with heart failure in the community. JACC Heart Fail. 2013;1:135–141. doi: 10.1016/j.jchf.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61:896–901. doi: 10.1111/jgs.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim JC, Kalantar-Zadeh K, Kopple JD. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J Am Soc Nephrol. 2013;24:337–351. doi: 10.1681/ASN.2012010047. [DOI] [PubMed] [Google Scholar]
- 54.Lai JC, Feng S, Terrault NA, et al. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14:1870–1879. doi: 10.1111/ajt.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erlandson KM, Schrack JA, Jankowski CM, et al. Functional impairment, disability, and frailty in adults aging with HIV-infection. Curr HIV/AIDS Rep. 2014;11:279–290. doi: 10.1007/s11904-014-0215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joseph B, Pandit V, Zangbar B, et al. Superiority of frailty over age in predicting outcomes among geriatric trauma patients: a prospective analysis. JAMA Surg. 2014;149:766–772. doi: 10.1001/jamasurg.2014.296. [DOI] [PubMed] [Google Scholar]
- 57.Joseph B, Pandit V, Zangbar B, et al. Validating trauma-specific frailty index for geriatric trauma patients: a prospective analysis. J Am Coll Surg. 2014;219:10.e1–17.e1. doi: 10.1016/j.jamcollsurg.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 58.Joseph B, Pandit V, Khalil M, et al. Managing older adults with ground-level falls admitted to a trauma service: the effect of frailty. J Am Geriatr Soc. 2015;63:745–749. doi: 10.1111/jgs.13338. [DOI] [PubMed] [Google Scholar]
- 59.Hulzebos EH, Smit Y, Helders PP, van Meeteren NL. Preoperative physical therapy for elective cardiac surgery patients. Cochrane Database Syst Rev. 2012;11:CD010118. doi: 10.1002/14651858.CD010118.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li C, Carli F, Lee L, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc. 2013;27:1072–1082. doi: 10.1007/s00464-012-2560-5. [DOI] [PubMed] [Google Scholar]
- 61.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg. 2015;220:136.e1–148.e1. doi: 10.1016/j.jamcollsurg.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 62.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. 2015;63:142–150. doi: 10.1111/jgs.13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sieber FE, Zakriya KJ, Gottschalk A, et al. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010;85:18–26. doi: 10.4065/mcp.2009.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Landefeld CS, Palmer RM, Kresevic DM, et al. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332:1338–1344. doi: 10.1056/NEJM199505183322006. [DOI] [PubMed] [Google Scholar]
- 65.Eng C, Pedulla J, Eleazer GP, et al. Program of All-inclusive Care for the Elderly (PACE): an innovative model of integrated geriatric care and financing. J Am Geriatr Soc. 1997;45:223–232. doi: 10.1111/j.1532-5415.1997.tb04513.x. [DOI] [PubMed] [Google Scholar]
- 66.Leung JM, Tsai TL, Sands LP. Brief report: preoperative frailty in older surgical patients is associated with early postoperative delirium. Anesth Analg. 2011;112:1199–1201. doi: 10.1213/ANE.0b013e31820c7c06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 68.Statement of Principles of Palliative Care. Chicago: American College of Surgeons; 2005. American College of Surgeons Task Force on Surgical Palliative Care and the Committee on Ethics. [PubMed] [Google Scholar]
- 69.Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012;16:601–608. doi: 10.1007/s12603-012-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woo J, Yu R, Wong M, et al. Frailty screening in the community using the FRAIL scale. J Am Med Dir Assoc. 2015;16:412–419. doi: 10.1016/j.jamda.2015.01.087. [DOI] [PubMed] [Google Scholar]