Abstract
The exposure of insects to xenobiotics, such as insecticides, triggers a complex defence response necessary for survival. This response includes the induction of genes that encode key Cytochrome P450 monooxygenase detoxification enzymes. Drosophila melanogaster Malpighian (renal) tubules are critical organs in the detoxification and elimination of these foreign compounds, so the tubule response induced by dietary exposure to the insecticide permethrin was examined. We found that expression of the gene encoding Cytochrome P450-4e3 (Cyp4e3) is significantly up-regulated by Drosophila fed on permethrin and that manipulation of Cyp4e3 levels, specifically in the principal cells of the Malpighian tubules, impacts significantly on the survival of permethrin-fed flies. Both dietary exposure to permethrin and Cyp4e3 knockdown cause a significant elevation of oxidative stress-associated markers in the tubules, including H2O2 and lipid peroxidation byproduct, HNE (4-hydroxynonenal). Thus, Cyp4e3 may play an important role in regulating H2O2 levels in the endoplasmic reticulum (ER) where it resides, and its absence triggers a JAK/STAT and NF-κB-mediated stress response, similar to that observed in cells under ER stress. This work increases our understanding of the molecular mechanisms of insecticide detoxification and provides further evidence of the oxidative stress responses induced by permethrin metabolism.
Keywords: Cytochrome P450-4e3, Pyrethroid, Insecticide detoxification, Drosophila melanogaster, Malpighian tubule, Oxidative stress
Graphical abstract
Highlights
-
•
We examined the Malpighian tubule response induced by exposure to the insecticide permethrin.
-
•
Cyp4e3 expression is induced in Drosophila fed on oxidants and permethrin.
-
•
Manipulation of Cyp4e3 levels impact significantly on the survival of the whole fly.
-
•
Endoplasmic reticulum stress responses are induced by permethrin metabolism.
-
•
We provide better understanding of the molecular mechanisms of insecticide detoxification.
1. Introduction
The evolution of insecticide resistance is a continuing problem in the control of insect pest species. One major mechanism of insecticide resistance involves transcriptional induction of detoxification enzymes that are capable of metabolizing insecticides, i.e., the P450 monoxygenases (P450s), the glutathione-S-transferases (GSTs) and the carboxylesterases (Feyereisen, 1995, Daborn et al., 2002, Enayati et al., 2005, Heidari et al., 2005). Increased activity of members from all these families has been associated with pyrethroid resistance in different insect species (Jamroz et al., 2000, Kasai and Scott, 2000, Vontas et al., 2001).
The synthetic pyrethroids are among the most commonly used pesticides for controlling agricultural and insect pests. Distinct classes of pyrethroids have been identified, based on different behavioural, neuropsychological and biochemical properties (Soderlund and Bloomquist, 1989, Shafer et al., 2005, Breckenridge et al., 2009). In addition to its functions as a neurotoxin, the induction of oxidative stress conditions in different tissues following pyrethroid exposure has been proposed as the major cause of cytotoxicity and ecotoxicity (Hill, 1989, Banerjee et al., 1999).
The cytochrome P450s (monooxygenases) compose a large enzyme superfamily involved in the detoxification of many xenobiotics (Feyereisen, 1999) and several studies have shown that increased cytochrome P450s catalysed metabolism is an important mechanism of pyrethroid resistance in many insect species (Tomita et al., 1995, Maitra et al., 1996, Pittendrigh et al., 1997). Drosophila Malpighian (renal) tubules are highly enriched for many P450 enzymes, GSTs, esterases and multidrug resistance-associated proteins (MRPs) (Dow and Davies, 2006, Chintapalli et al., 2007, Chahine and O'Donnell, 2009), and the importance of the tubules in xenobiotic metabolism has previously been demonstrated, by manipulation of a single P450 gene (Cyp6g1) in the tubules, which altered the survival of the whole fly following exposure to dichlorodiphenyltrichloroethane (DDT) (Yang et al., 2007).
Recent studies have shown increased reactive oxygen species (ROS) production upon permethrin (PM) exposure in rat adrenal pheochromocytoma cells or cardiac myocytes (Hu et al., 2010, Vadhana et al., 2011). Thus, we examined if the oxidative effects induced by PM metabolism are recapitulated in an organotypic context, the Malpighian (renal) tubule, an important epithelial tissue for xenobiotic handling. In the present study, Drosophila melanogaster was used as a simple genetic model to investigate the role of Cytochrome P450-4e3 (Cyp4e3) in oxidative stress responses. Here, we show that manipulation of Cyp4e3 in the Malpighian tubule results in a continuous production of ROS, ultimately causing lipid peroxidation, ER stress, and death upon PM exposure.
2. Materials and methods
2.1. Drosophila stocks
All lines were maintained on a standard Drosophila diet at 22 °C, 55% humidity with a 12:12 h light:dark photoperiod. Wild-type Canton-S (CS), ‘cantonised’ white1118 and the UAS-Catalase RNAi (TRIP line) were obtained from the Bloomington Stock Center (Bloomington, IN). The UAS-cyto-roGFP2-Orp1, a cytosolic hydrogen peroxide reporter line was obtained from the German Cancer Research Centre, Heidelberg, Germany (Albrecht et al., 2011). The capaR-GAL4 driver, which drives expression in adult tubule principal cells, was previously generated in-house (Terhzaz et al., 2012). The c564-GAL4 driver specific to the fat body (Takehana et al., 2004) was a kind gift of Prof. Shoichiro Kurata, Tohoku University, Japan.
2.2. Generation of UAS-Cyp4e3 RNAi and UAS-Cyp4e3-eYFP(Venus) transgenic lines
To generate the construct for heritable RNAi of the Cyp4e3 gene, an inverted repeat of a 270-base pair fragment of Cyp4e3 was generated by PCR using the primers described in Table S1 and cloned in opposite orientations separated by a ftz intron into the P-element vector pRISE (Kondo et al., 2006). To generate the construct for overexpression of the Cyp4e3 gene, the coding sequence of Drosophila Cyp4e3 (CG4105) was amplified by PCR using DreamTaq green PCR master mix (Thermo Scientific) with the primer pairs listed in Table S1. The product was cloned into a pENTR donor vector (Invitrogen) and transferred to the pTWV destination vector (DGRC) using Gateway LR Clonase II Enzyme mix according to the manufacturer's instructions. The sequence integrity for each construct was confirmed by GATC Biotech, and transgenic lines were generated using standard methods for P-element-mediated germline transformation (BestGene Inc, USA).
2.3. Quantitative RT-PCR
For validation of whole fly or tubule mRNA expression, qRT-PCR was performed using an Opticon DNA engine 4 (Bio-Rad Technologies UK) with Brilliant III Ultra-Fast SYBR Green QPCR master mix (Agilent, UK) and primer pairs described in Table S1. Data was normalized against the rp49 standard and expressed as fold change compared to controls ± SEM (n = 3).
2.4. Immunofluorescence
Adult Malpighian tubules were dissected in Schneider's medium (Invitrogen, UK) and fixed with 4% (wt/vol) paraformaldehyde (PFA) for 20 min at room temperature. Mouse anti-GFP primary antibody (1:500, Zymed) and the rabbit anti-PDI (1:1000, Abcam) were used. Incubations in the primary antibodies were performed overnight. FITC or Texas red-conjugated affinity-purified goat anti-mouse or anti-rabbit antibodies (Jackson Immunologicals) were used in a dilution of 1:1000 for visualization of the primary antiserum. Samples were mounted on poly-l-Lysine (0.1% w/vol in H2O, Sigma–Aldrich) covered 35 mm glass bottom dishes (MatTek Corporation, MA, USA) in Vectashield (Vector Laboratories, Burlingame, CA). Confocal images were taken by using a Zeiss LSM META 510 microscope and processed with LSM 510 image examiner and Adobe Photoshop CS 5.1.
2.5. Measurement of hydrogen peroxide and peroxidase activity
For tubule quantification of hydrogen peroxide (H2O2) levels or peroxidase activity, 20 pairs of rapidly dissected tubules from D. melanogaster wild-type male flies fed on PM or Cyp4e3 transgenic flies were added to 60 μl of 5 mM sodium phosphate (pH 7.4, Invitrogen), ruptured by brief sonication to release cellular contents, and spun down briefly at 5000 g at 4 °C; 50 μl of the supernatant was used immediately to measure either H2O2 levels or peroxidase activity using an Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (Invitrogen) as previously described (Terhzaz et al., 2010). Total H2O2 levels and peroxidase activity were normalized to protein amounts in each homogenate using a Bradford assay (Bio-Rad).
For organotypic imaging, live and PFA fixed intact transgenic tubules expressing roGFP2 probes were allowed to adhere to glass-bottomed dishes coated with poly-l-lysine. To conserve the redox state of the roGFP2-based probes and prevent post-dissection oxidation, 20 mM of the thiol-alkylating agent N-Ethylmaleimide (NEM, Sigma–Aldrich) was added to Schneider's medium and fixation solution, with the exception for the live imaging experiments. The cytosolic hydrogen peroxide sensing reporter was assessed by treatment with H2O2 (25 μM, Sigma–Aldrich) or dithiothreitol (DTT, 10 mM, Invitrogen) by imaging on a Zeiss 510 meta confocal system coupled to an inverted Zeiss microscope. The reporter was excited at 405 nm and 488 nm, and the emission was filtered through a 505–530 nm band pass filter. Real time images of tubule-expressed roGFP2 were captured, and average fluorescence values for specified regions of interest were calculated for each frame. Images were saved as 16-bit tiff files and processed using ImageJ. Background was subtracted using the function “disable smoothing”. Images were then converted to a 32-bit format and displayed in false colours using the lookup table “fire”.
2.6. Measurement of oxidative damage to lipids
For each sample, 20 pairs of tubules from male wild-type flies fed on PM or Cyp4e3 transgenic flies were added to 60 μl of 1X phosphate buffered saline (pH 7.4, Gibco), ruptured by brief sonication to release cellular contents, and spun down briefly at 5000 g at 4 °C. Protein amounts in each homogenate were quantified using a Bradford assay (Bio-Rad) and 10 μg/ml input protein were used. Triplicate samples were generated for each genotype. The lipid peroxidation byproduct, HNE (4-hydroxynonenal)-Histidine protein adduct, was measured using the OxiSelect HNE-His adduct ELISA Kit (Cell Biolabs) according to the manufacturer's protocol. The signals generated by the colorimetric assays were detected at 450 nm on a Mithras LB 940 plate reader luminometer (Berthold technologies).
2.7. Xenobiotic survival assays
For feeding assays, D. melanogaster wild-type or Cyp4e3 transgenic 7-day-old flies were briefly anesthetized on CO2, starved for 4–6 h and then placed in 30-ml cellulose-capped vials containing 5 ml of 1% aqueous agarose/1% sucrose medium (control) or fed with either 1% H2O2 or specified concentration of permethrin or fenvalerate (all Sigma–Aldrich). The vials were placed in an incubator at 22 °C, 55% humidity with 12:12 h light:dark photoperiod and checked for dead flies every 3–6 h until no living flies were left. All experiments were run in triplicate with at least 30 flies in each run of a specified genotype. For topical application, D. melanogaster wild-type or transgenic 7-day-old male flies were briefly immobilised on CO2, and a 69 nl volume of permethrin (0, 2, 5, 6, 8, 10, 12.5, 15, 17.5 and 20 ng/fly doses which were then converted to ng/mg by dividing the dose by the average weight, n = 25 flies with at least 3 biological replicates) was applied to the thoracic notum using a microinjector (NanojectII, Drummond Scientific Company). Treated flies were placed in an inverted food vial to prevent mortality through adherence to the food and vials were placed in the same incubator at 22 °C (55% humidity with 12:12 light:dark h photoperiod) because pyrethroids have a temperature dependent toxicity. Mortality data was recorded 24 h after application.
2.8. Data analysis
A two-tailed Student's t-test (for two independent groups: non treated vs treated), or one-way ANOVA (for three independent groups: capaR-Gal4/ + vs Cyp4e3 RNAi/ + or Cyp4e3/ + vs capaR-Gal4>Cyp4e3 RNAi or capaR-Gal4>Cyp4e3) was used to compare whole fly or tubule mRNA expression, H2O2 levels, peroxidase activity and lipid peroxidation in wild-type Canton-S and transgenic flies. For ANOVAs, P-values were adjusted with the Sidak multiple comparisons test. For insecticide feeding assays, significance was assessed by the LogRank (Mantel–Cox) test. Data was plotted using GraphPad Prism 6.0 software (GraphPad Software Inc., USA). For topical application assays, data was corrected using Microsoft Excel for control mortality using Abbott's formula before using Probit analysis to linearize the cumulative Gaussian distribution. Probits were plotted against the log of the dose per mg body weight using Graphpad Prism 6.0 software. A linear regression analysis was performed and the 50% lethal dose (LD50) determined. Statistical significance between LD50s of different genotypes was determined using the Litchfield & Wilcoxon method.
3. Results
3.1. Cyp4e genes expression are enriched in Malpighian tubules
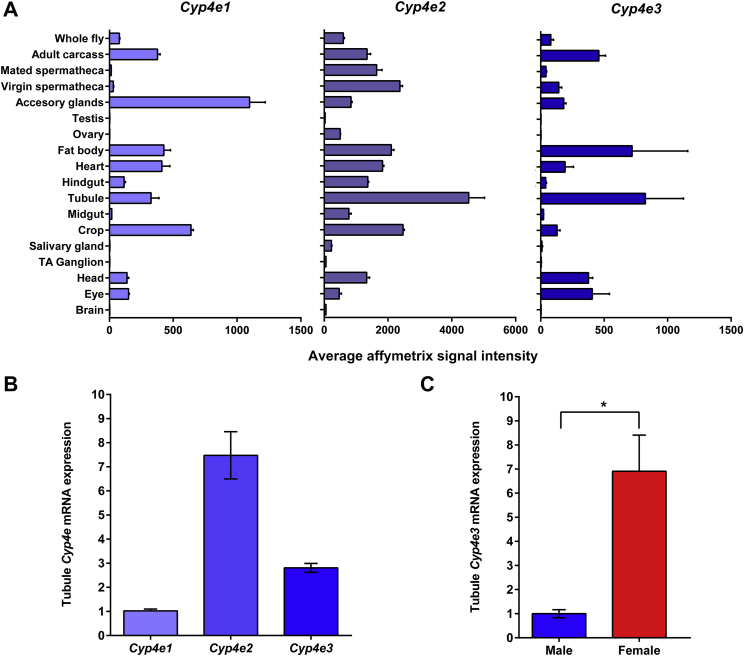
All three Cyp4e genes encoded by the D. melanogaster genome show highly abundant expression in adult Malpighian tubules and fat body (Chintapalli et al., 2007) (Supplementary Fig. S1A and B), which are tissues acknowledged to have functions analogous to the human kidney and liver respectively. All three Cyp4e genes encode monooxygenases which catalyse many reactions involved in xenobiotic metabolism. The genes Cyp4e1 (CG2062) and Cyp4e2 (CG2060) are very similar as they share 79% identity at the AA sequence level, and their close proximity at the genomic level could imply that these genes may have been derived from a DNA-based tandem duplication. As well as being expressed in the canonical detoxification tissues, Cyp4e1 also has strong expression in accessory glands, and could potentially metabolize endogenous compounds such as hormones, fatty acids, and steroids. Based on the tissue distributions, high expression levels and gene function, we focused our attention on the functional role of the Cyp4e3 (CG4105) gene in Malpighian tubules.
Recently, a Drosophila Malpighian tubule microarray study showed the gender-specific expression of several defence genes in tubules (Chintapalli et al., 2012). For example, males preferentially express a series of cytochrome P450s, including the cardinal insecticide resistance locus, Cyp6g1 (2.5x enrichment). In contrast, female-enriched genes include the detoxification enzyme cytochrome P450 Cyp4e3 (6.5x enrichment). This finding was confirmed by qRT-PCR, where wild-type female tubule Cyp4e3 mRNA levels were 7-fold higher compared to males (P = 0.0016, Supplementary Fig. S1C). The gender-specific expression of several defence genes in tubules implies that males and females meet differing defence challenges.
3.2. Permethrin toxicity in D. melanogaster and Cyp4e3 response
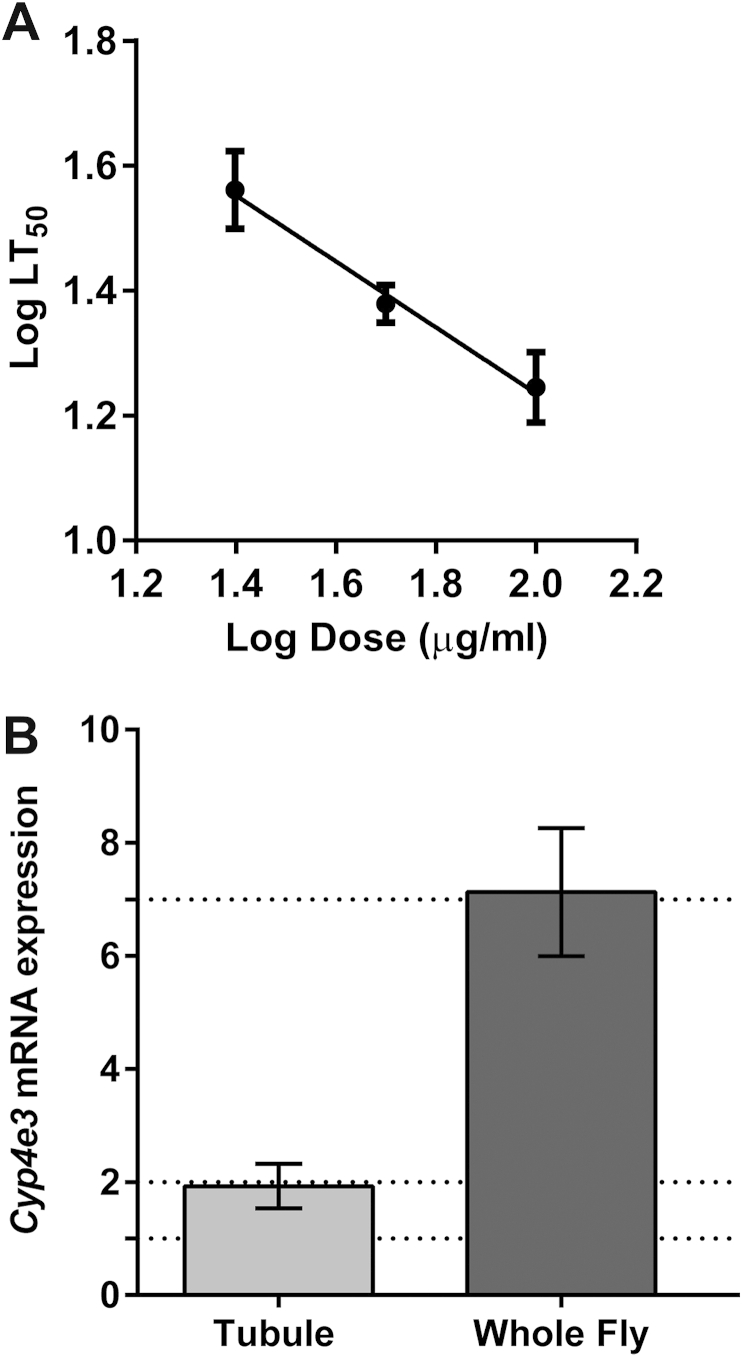
We first tested PM toxicity using feeding survival assays. Wild-type male flies were fed on increasing concentrations of PM and the LD50 after 24 h exposure was established at a concentration of approximately 75 μg/ml (Fig. 1A).
Fig. 1.
PM toxicity assay and up-regulation of Cyp4e3 mRNA levels. (A) Wild-type Canton-S male flies were exposed to increasing concentrations of PM, and survival recorded until 100% mortality was reached. Log of the median lethal time (LT50) was plotted against the log of the dose (μg/ml), and error bars show 95% confidence intervals (n = 120–130 male adult wild-type flies for each concentration tested). (B) Relative tubule and whole animal Cyp4e3 mRNA expression after 24 h feeding with either sucrose alone (control) or sucrose supplemented with 75 μg/ml PM. Data are expressed as fold change compared to non-treated control ± SEM (n = 3).
Exposure of insects to insecticides triggers a defence response, inducing genes that encode key detoxification enzymes. We therefore determined if dietary exposure to PM could induce changes in Cyp4e3 mRNA levels. Wild-type male flies were treated for 24 h with either sucrose alone or sucrose supplemented with 75 μg/ml PM. Whole fly and tubule RNA was extracted from these animals and Cyp4e3 mRNA expression determined by qRT-PCR. Dietary exposure to PM elicited significant increases in Cyp4e3 mRNA levels (2- and 7-fold in tubule and whole fly, respectively) compared to untreated controls (Fig. 1B). This suggests that Cyp4e3 enrichment in adult tubules, fat body, carcass by 10.2, 8.9 and 5.7-fold respectively compared to the rest of the fly (Flyatlas.org) may explain the increases of Cyp4e3 mRNA levels in the whole fly compared to that observed in tubules. Although only a small subset of tissues have been tested, these data suggest that Cyp4e3 is an insecticide-responsive gene and that several detoxification tissues, including tubule, fat body and carcass, might also be involved in the PM response.
3.3. Manipulation of Cyp4e3 expression alters survival to permethrin
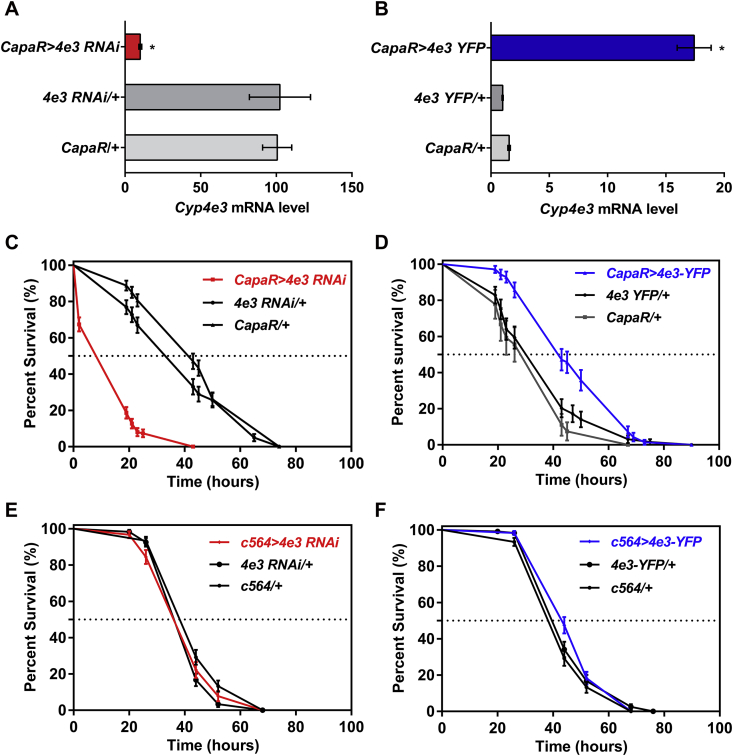
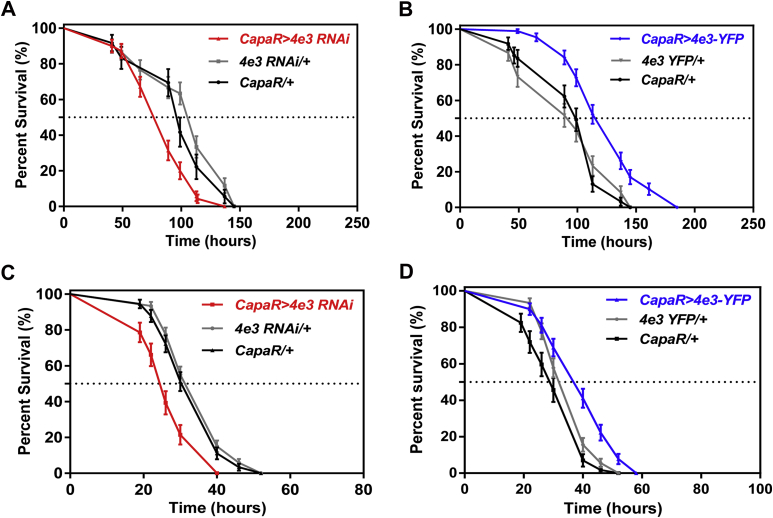
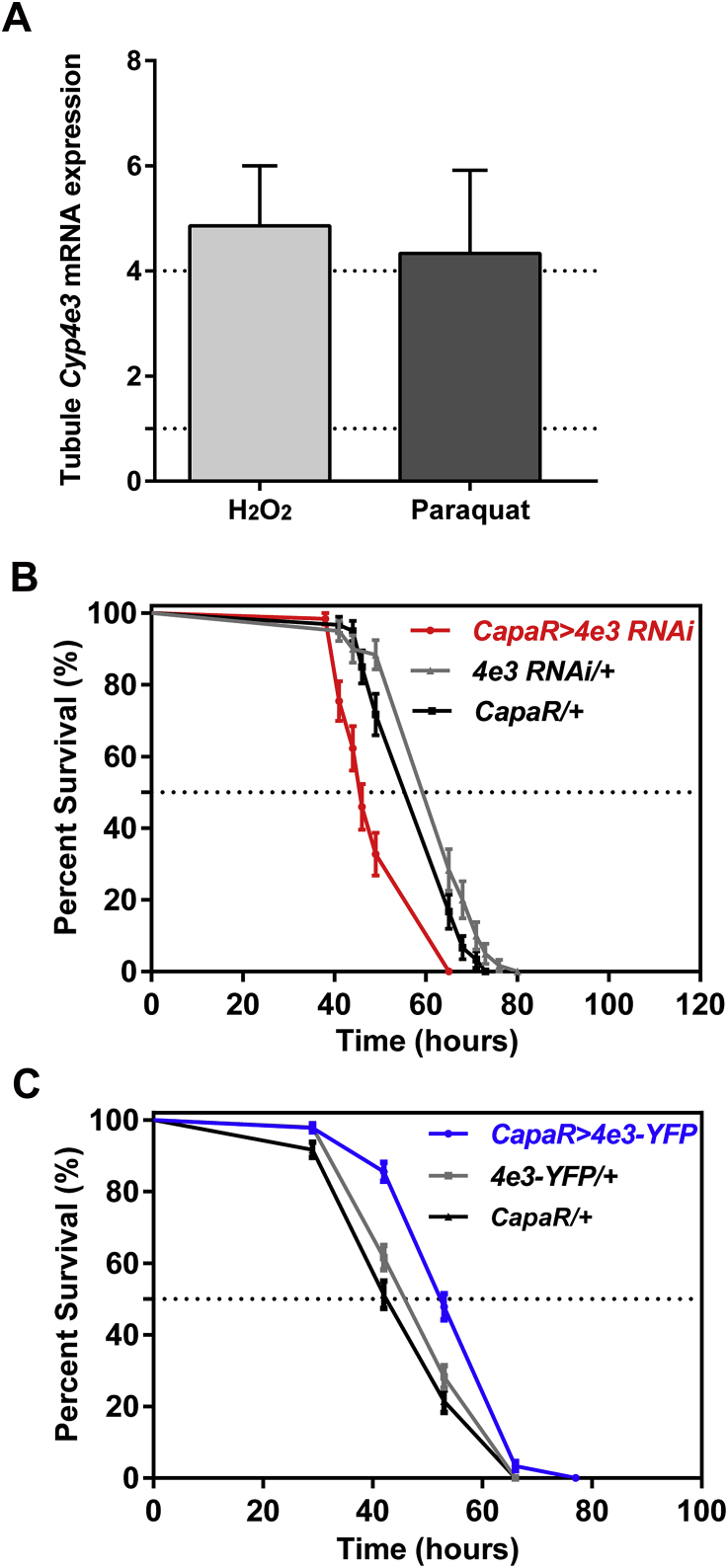
The GAL4/UAS binary expression system allows the knockdown or overexpression of any genes in specific cells within an otherwise normal organism, under control of the appropriate GAL4 driver lines. We therefore generated UAS-dsRNA- and eYFP-tagged- Cyp4e3 transgenic fly lines and drove expression of both constructs in tubule principal cells using the tubule principal cell specific capaR-Gal4 line. The UAS-Cyp4e3 RNAi line produced a strong knockdown (∼90%) while the UAS-Cyp4e3-eYFP showed an ∼17-fold increase in overall tubule expression of Cyp4e3 mRNA when driven in principal cells compared to parental control lines (Fig. 2A,B).
Fig. 2.
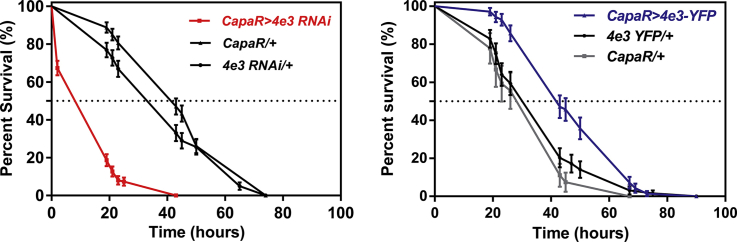
Consequence of loss- and gain-of-function of the Cyp4e3 gene upon PM exposure. (A–B) Down- and up-regulation of Cyp4e3 in principal cells compared to both Cyp4e3 RNAi/ + and capaR/ + parental lines (*P < 0.05). The observed difference in sample quantity corresponds to (A) an approximately 90% decrease in Cyp4e3 mRNA levels and (B) an approximately 17-fold increase of Cyp4e3 mRNA expression. Data are expressed as (A) percentage and (B) fold change compared to parental controls ± SEM (n = 3). Effect of Cyp4e3 manipulation has on PM dietary exposure (75 μg/ml). (C–D) Survival was significantly reduced after knockdown of Cyp4e3 in just the principal cells of Malpighian tubules (C) while over-expression of Cyp4e3 significantly increased survival compared to parental controls (D). In C and D, P < 0.001 was used as the significance level; Log-rank test; n = 100–130 male flies for the different genotypes. (E–F) Selective knockdown (E) or overexpression (F) of Cyp4e3 in fat body using the c564-GAL4 driver does not change sensitivity to PM (n > 210 male flies for the different genotypes).
Given the induction of Cyp4e3 mRNA levels by PM exposure, we investigated the role of the Cyp4e3 gene in organismal insecticide survival. Targeted knockdown of Cyp4e3 specifically to tubule principal cells in males resulted in significantly reduced survival under PM dietary exposure (median life span of 19 h, as compared with the median control life span of 43 h) (Fig. 2C). Conversely, male flies with increased Cyp4e3 levels exhibited significantly extended survival compared to parental controls (Fig. 2D) (in C and D, P < 0.001; Logrank test, Mantel–Cox). These results clearly show that the whole insect tolerance to insecticide is related to the expression level of Cyp4e3 in the metabolically active tubule principal cells. A similar pattern in PM survival was observed in females (Supplementary Fig. S2A and B). The enrichment of Cyp4e3 mRNA in the fat body prompted us to assess if the manipulation of Cyp4e3 levels, specifically in this tissue, impacted on the survival of PM-fed flies. We show that selective knockdown or overexpression of Cyp4e3 in the fat body (Supplementary Fig. S3) does not change sensitivity to PM (Fig. 2E and F). These data are consistent with those obtained for the DDT insecticide resistance gene, Cyp6g1 (Yang et al., 2007), and suggest that the Malpighian tubules constitute the key tissue for insecticide metabolism.
We next examined if the survival phenotype observed in the Cyp4e3 knockdown flies could be recapitulated using a member of the type II synthetic pyrethroid insecticide family. Fenvalerate, characterised by the presence of an alpha-cyano group, is known to be more resistant to degradation and thus more potent than the type I PM. We therefore tested if manipulation of Cyp4e3 expression alters survival to dietary fenvalerate at a concentration ∼15-fold lower than PM. As expected, manipulation of Cyp4e3 expression, specifically in the principal cells of the Malpighian tubule, impacts significantly on fly survival upon fenvalerate exposure (Supplementary Fig. S2C and D).
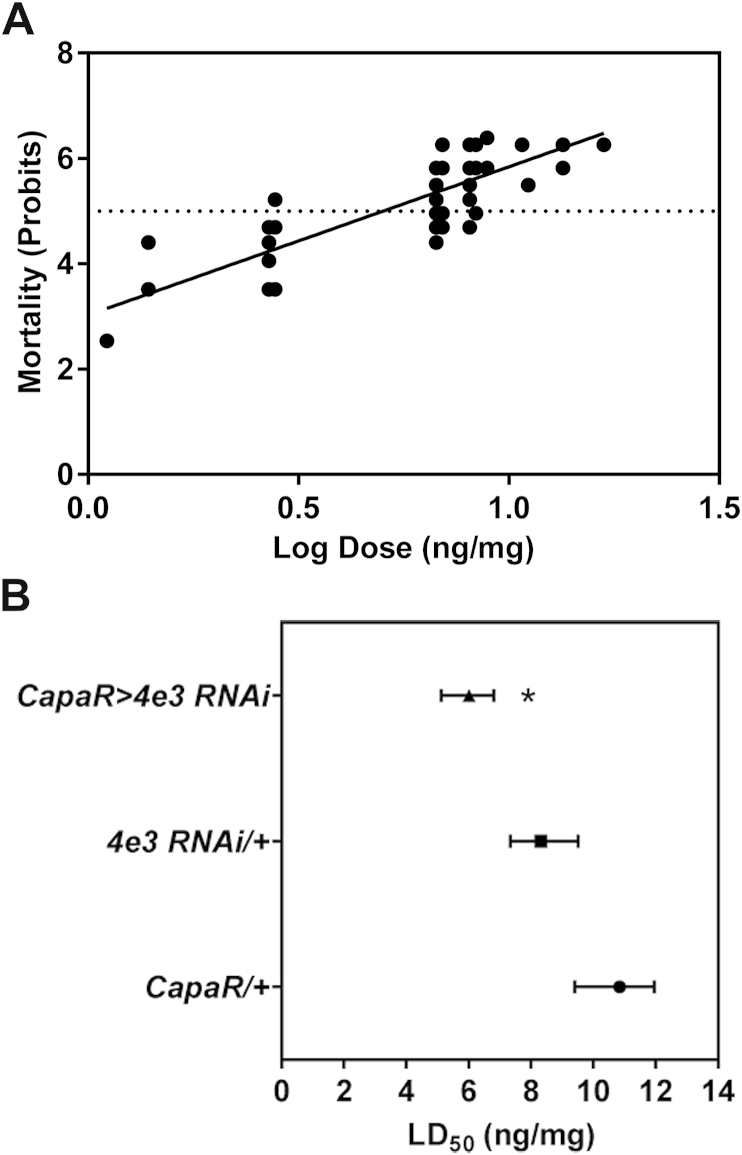
In addition to the “kidney” tissue, the carcass expresses high Cyp4e3 mRNA levels, so we performed PM topical application survival assays. Wild-type Canton-S male flies were treated with different PM doses and the mortality after 24 h exposure was determined (Fig. 3A). Although the carcass and fat body tissue can also impact on the topical application of insecticides, the tubules are more likely to handle topically applied agents that appear in the hemocoel. Selective knockdown of Cyp4e3 in the principal cells of the Malpighian tubule significantly increases PM susceptibility of the whole organism (P < 0.001 against both parental controls) and demonstrate a major role of the tubule in insecticide metabolism (Fig. 3B).
Fig. 3.
PM topical application and the effect on Cyp4e3 knockdown flies. (A) Wild-type Canton-S male flies were exposed to PM, and mortality measured at 24 h. Probits were plotted against the Log of the dose per mg body weight (n = 150 male adult wild-type flies for each concentrations tested). (B) For the different genotypes, a linear regression analysis was performed to determine the dose at which 50% mortality was observed (LD50). Significant change in LD50 was tested using the Litchfield & Wilcoxon method. Knockdown of Cyp4e3 in principal cells of Malpighian tubules significantly reduced the PM LD50 dose compared to both parental controls (*P < 0.001; n > 380 male flies for the different genotypes).
3.4. Cyp4e3 knockdown flies display increased tubule oxidative stress
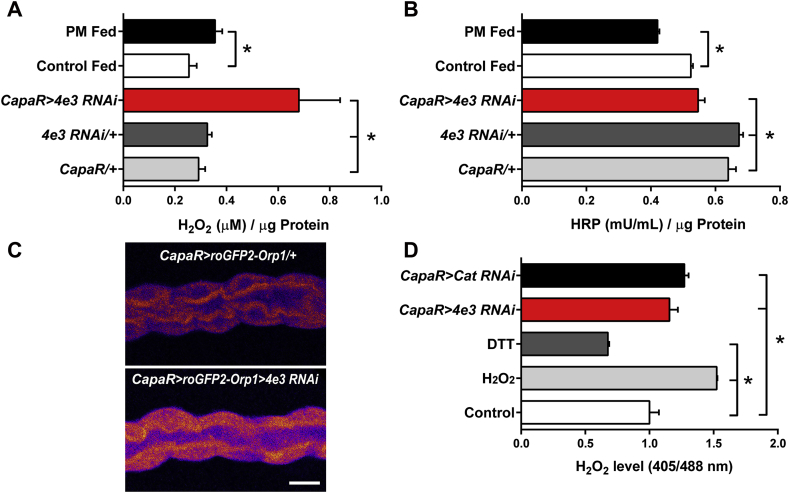
Which mechanisms contribute to the deleterious effects on survival observed in the Cyp4e3 knockdown flies on PM diet? Previous studies have shown an increase in ROS production upon PM exposure (Hu et al., 2010, Vadhana et al., 2011). To determine if the oxidative effects induced by the insecticide metabolism is recapitulated in wild-type flies fed with PM, we measured tubule H2O2 levels. Dietary exposure to PM was associated with a significant increase in H2O2 levels in the Malpighian tubules in comparison with tubules from flies reared on the control diet (Fig. 4A). We next investigated if Cyp4e3 knockdown flies displayed a significant elevation in oxidative stress, leading to reduced survival on PM exposure. Data shown in Fig. 4A revealed that H2O2 production in isolated Cyp4e3 knockdown tubules is significantly increased compared to either parental line, suggesting that Cyp4e3 knockdown mediates increased H2O2 production in tubules principal cells. Therefore, Cyp4e3 and PM each directly modulate tubule H2O2 production and these effects could be additive. This increased H2O2 production in tubules, presumably by the mitochondria, can either result from Superoxide Dismutase actions on superoxide anions (Miwa et al., 2003, Murphy, 2009) or could be the consequence of decreased tubule peroxidase activity. Data shown in Fig. 4B revealed that, indeed, flies fed on PM or cyp4e3 knockdown flies had significantly decreased peroxidase activity compared to normally-fed flies and parental lines respectively. These data suggest that PM exposure or the absence of Cyp4e3 plays an important role in regulating tubule antioxidant function leading to oxidative stress. Interestingly, we found that tubules dissected from flies fed with H2O2 or the superoxide generator paraquat elicited significant increases in Cyp4e3 mRNA levels (more than fourfold compared to controls) (Supplementary Fig. S4A), and manipulation of Cyp4e3 in the tubules alters the survival of the whole fly during exposure to H2O2 (Supplementary Fig. S4B and C), suggesting that Drosophila Cyp4e3 may play a role in the regulation of cellular redox balance.
Fig. 4.
PM fed and Cyp4e3 knockdown flies display increased levels of H2O2 and decreased peroxidase activity. Tubules from wild-type Canton-S male flies fed for 24 h with either sucrose alone (control) or sucrose supplemented with 75 μg/ml PM and from Cyp4e3 knockdown or parental controls were collected and homogenates assayed for (A) H2O2 levels and (B) peroxidase activity. Error bars represent SEM, n ≥ 3, independent samples of 20 pairs of tubules of male flies each in triplicate; *P < 0.05. (C) Ex vivo imaging of the cytosolic roGFP2-Orp1 probe targeted to Malpighian tubule principal cells. Silencing of Cyp4e3 leads to increased cytosolic H2O2 levels as evidenced by the higher oxidation of the specific H2O2 probe. Scale bar = 25 μm. (D) Redox state of the cytosolic roGFP2-Orp1 probe targeted to Malpighian tubule principal cells in control (Schneider's medium), challenged with 25 mM H2O2, 10 mM DTT, or targeted Cyp4e3 and Catalase knockdowns. Data from different tubule principal cells are expressed as the relative H2O2 level (405/488 nm) ± SEM (n ≥ 6), where *P < 0.05.
To further validate these results, we performed in vivo imaging using redox sensitive GFPs (roGFPs) (Dooley et al., 2004) that have been converted into specific probes for H2O2 by coupling them to the microbial H2O2 sensor oxidant receptor peroxidase 1 (Orp1) (Gutscher et al., 2009). The cytosolic roGFP2-Orp1 probe was targeted to Malpighian tubules principal cells and the fluorescence of the reporter imaged in intact live tubules to determine H2O2 levels. Intact tubules exhibited higher levels of H2O2 compared to other tissues as evidenced by the stronger level of oxidation of the H2O2 probe, confirming the intense metabolic activity occurring within the tubule principal cells. Next, we tested the H2O2 probe for in situ responsiveness, and Supplementary Fig. S5A and B show that, as expected, exogenous application of DTT and H2O2 elicited complete probe reduction and oxidation respectively, which permitted calibration of the probe and therefore the quantification of H2O2 levels.
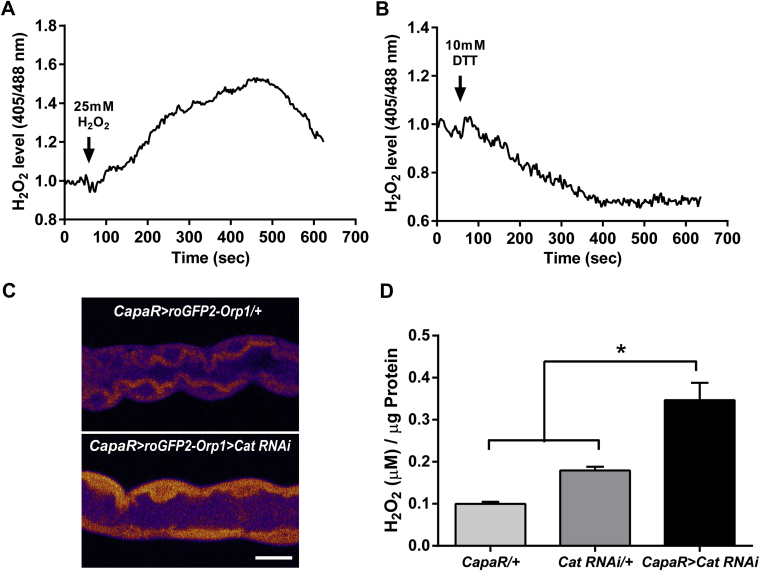
We also explored the possibilities of combining redox imaging with reverse genetics. For this purpose, we created a fly line expressing the H2O2 probe stably and specifically in the tubule principal cells that was used together with dsRNA constructs for antioxidant genes. The Malpighian tubules constitute a major sources of ROS in the organism due to the high rates of metabolic activity, and play a significant role in the organismal response to oxidative stress (Terhzaz et al., 2010). Interestingly, the tubules have specific adaptations to counter the high production of ROS as the expression of genes encoding antioxidant enzymes are enriched in tubules compared to the rest of the fly. For example, the Catalase (Cat) gene, which is an important H2O2 scavenger, by catalysing the decomposition of hydrogen peroxide to water and oxygen, is enriched in tubules by 4.7-fold compared to the rest of the fly (Flyatlas.org). As expected, gene silencing of Cat led to significantly increased H2O2 levels in the cytosol compared to parental control lines (Supplementary Fig. S5C and D). Moreover, when Cyp4e3 was silenced, we also observed a cytosolic increase in H2O2 levels in the Malpighian tubules (Fig. 4C,D). This suggests that silencing of Cyp4e3 increases steady-state H2O2 levels in tubule principal cells and that Cyp4e3 mediates production of H2O2 by potentially regulating enzyme activity, i.e. peroxidases.
3.5. Cyp4e3 knockdown flies display ER stress
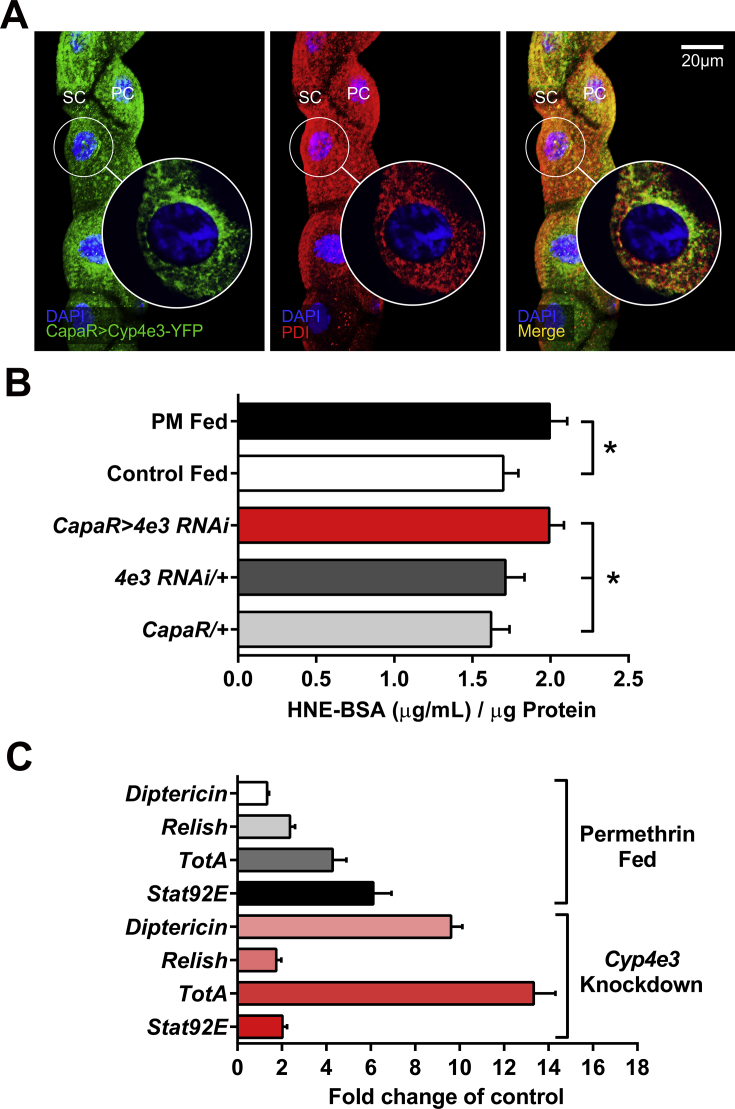
Cytochrome P450s are membrane-bound enzymes commonly located in the endoplasmic reticulum (ER), the inner mitochondrial membrane and peroxisomes. Targeted expression of the eYFP-tagged Cyp4e3 construct in the tubule principal cells enabled determination of the subcellular localization of the CYP4E3 protein. The eYFP fluorescence was seen throughout the cytosol with a clear reticular pattern (Fig. 5A), characteristic of ER, as demonstrated by co-localization with the antibody for the ER protein disulfide isomerase (PDI). As endogenous CYP4E3 is localized to the ER, and may play an important role in regulating H2O2 levels in the ER, we investigated the possible effect of targeted knockdown of Cyp4e3 or PM dietary exposure on ER stress. ER redox status has to be maintained as high levels of H2O2 may affect protein-folding capacity and lipid peroxidation which is the formation of unsaturated lipid oxidation products that occur as a result of oxidative damage. Samples prepared from flies fed on PM and Cyp4e3 knockdown flies were immunoassayed for the detection and quantification of HNE-His adduct. HNE (4-Hydroxynonenal), a natural catabolite of lipid peroxidation, is capable of covalently binding to proteins and forming stable adducts, causing both structural and functional changes. Data shown in Fig. 5B revealed that the production of HNE-His adduct in isolated Cyp4e3 knockdown tubules or PM fed tubules is significantly increased compared to either parental line and normally-fed flies, suggesting that Cyp4e3 knockdown and PM dietary exposure mediates increased H2O2 and HNE production in tubule principal cells.
Fig. 5.
PM fed and Cyp4e3 knockdown flies display ER stress and increased immune and stress response genes. (A) Endoplasmic reticulum localization of CYP4E3. Tubule principal cells with eYFP fluorescence (green, left) and the antibody for the ER protein disulfide isomerase (PDI, red, middle) shows co-localization (yellow, merge, right). Maximum projection of confocal z-series with inserts showing single focal plane images of selected regions. Scale bar = 20 μm. (B) Tubules from wild-type Canton-S male flies fed for 24 h with 75 μg/ml PM and Cyp4e3 knockdown flies and respective controls were collected and homogenates assayed for HNE-His adduct levels. Error bars represent SEM, n = 3, independent samples of 20 pairs of tubules each in triplicate; *P < 0.05. (C) Immune and stress genes were up-regulated in adult wild-type males fed for 24 h with 75 μg/ml PM and Cyp4e3 knockdown flies. Data are expressed as fold change compared to controls ± SEM (n = 3). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
A number of conditions affecting ER function have been observed to cause the activation of the NF-κB pathways and JAK/STAT stress response pathways (Pahl and Baeuerle, 1997). This prompted an assessment of both immune response (Imd) and stress response (JAK/STAT) pathways in the Cyp4e3 knockdown or PM fed flies to detect any disruption in homeostasis as revealed by the expression of “readout” genes that are specific for each pathway. The D. melanogaster orthologue of NF-κB transcription factor, Relish (Stoven et al., 2003), is associated with the expression of anti-microbial peptide (AMP) genes like Diptericin (Lemaitre et al., 1997). Interestingly, Cyp4e3 knockdown flies exhibited a several fold elevation in the expression of Diptericin (Fig. 5C), clearly indicating the activation of the NF-κB pathway. We found that the expression of a downstream target of the JAK/STAT pathway, the cytokine-like protein Turandot A (TotA) (Ekengren and Hultmark, 2001), was also induced in these flies as well as in PM fed flies (Fig. 5C). The JAK/STAT signaling leads to activation of the transcription factor Stat92E, which then translocates to the nucleus and triggers the expression of TotA. Interestingly, it has been shown that Relish can also regulate the expression of TotA (Agaisse et al., 2003), so the high levels of TotA expression may be the result of both a JAK/STAT and NF-κB-mediated response to stress.
4. Discussion
An important mechanism of pyrethroid resistance involves increased expression of enzymes involved in insecticide metabolism. Insect Cytochrome P450 genes compose a large superfamily with approximately 90 members in the D. melanogaster genome. Deciphering the individual enzymes responsible for insecticide metabolism is therefore a major challenge. Although several P450 genes and multiple tissues might impinge on insecticide detoxification, we have shown that targeted, cell specific manipulation of a single gene, Cyp4e3, in the Malpighian tubule principal cells of adult Drosophila alters the survival of the insect following pyrethroid feeding or topical application. A previous study has demonstrated that Cyp4e3 is significantly up-regulated in a Drosophila strain resistant to the neonicotinoid insecticide Imidacloprid (Kalajdzic et al., 2012), and here we show that Cyp4e3 is a pyrethroid insecticide-responsive gene.
D. melanogaster is a useful model for the functional validation of genes involved in insecticide resistance. We have shown that gain- and loss-of-function of Cyp4e3 significantly impacts on fly survival when fed on PM. The overexpression of Cyp4e3 in tubules conferred an increased survival, while Cyp4e3 knockdown a dramatically reduced survival. We found that both dietary exposure to PM and Cyp4e3 knockdown causes a significant elevation in oxidative stress with increased H2O2 levels and lipid peroxidation in tubules. These findings corroborate a recent study describing the effect of pyrethroids on the oxidative stress response of Helicoverpa armigera larvae, along with their neurotoxic effects (Akbar et al., 2012) and further confirms that oxidative stress is one of the main mechanisms of pyrethroid action. Given the induction of Cyp4e3 mRNA levels by the in vivo ROS generators paraquat, H2O2 and by PM dietary exposure, Drosophila Cyp4e3 is likely to play a role in ROS metabolism. Silencing Cyp4e3 in the tubule may result in a continuous production of ROS causing lipid peroxidation and reduced survival, while overexpression of Cyp4e3 demonstrated a protective function on exposure to oxidants and PM.
Endogenous CYP4E3 localizes to the ER, and so may impact on the ER redox state by regulation of H2O2 levels causing a JAK/STAT and NF-κB-mediated stress response, similar to that observed in cells under ER stress (Radyuk et al., 2013). In agreement with regulation by the transcription factors Sta92E and Relish, cytokine-like protein TotA was induced in the Cyp4e3 knockdown flies as well as in PM fed flies. The Turandot gene is induced in response to a variety of stressors, including heat, mechanical stress, ROS, and bacteria (Ekengren and Hultmark, 2001) and although TotA is known to be mainly expressed in the fat body, our data suggest that TotA plays a novel role in mediating stress responses in the Malpighian (renal) tubule. Furthermore, ER stress is known to cause an induction of immunity-related genes from the NF-κB pathway that is regulated through activation of the NF-κB transcription factor Relish. The up-regulation of this pathway in Cyp4e3 knockdown flies is confirmed by the observed increase in tubule levels of diptericin. Ultimately, it would be interesting to manipulate both NF-κB and JAK/STAT stress response pathways in the whole fly during survival under PM exposure.
The main function of the monoxygenase system is the oxygenation of exogenous compounds, and xenobiotics undergo deactivation by Cytochrome P450 enzyme, either directly or by facilitated excretion from the body. Therefore, one might expect that the Cyp4e3 gene would participate in the metabolism and excretion of PM by the tubules and thus, the effects on PM insecticide tolerance could be explained by variation in the effective dose experienced under each of the Cyp4e3 transgenic manipulations. Further insight in to this possible mechanism could be obtained by assessment of either levels of PM in the fed flies or examining metabolite profiles of PM in the Cyp4e3 transgenic flies. Now that an important Cytochrome P450 enzyme (Cyp4e3) involved in PM insecticide metabolism has been identified, determining if the D. melanogaster CYP4E3 protein is capable of catalysing the detoxification of pyrethroid insecticides might help in designing new strategies for the control of insect pest species.
Author contributions
S.T., J.A.T.D. and S.-A.D. designed the research. S.T., P.C., R.A.B. and K.A.H. performed the experiments. S.T., P.C., R.A.B., K.A.H. and S.-A.D analysed and interpreted the data, S.T. wrote the manuscript, and all authors revised the manuscript.
Acknowledgements
We thank Ruma G. Singh, Shaun K. Bremner and Ravi Sabherval for technical assistance. This work was funded by grants from the UK Biotechnology and Biological Sciences Research Council (BB/G020620 and BB/L002647/1) (to S.-A.D., J.A.T.D., and S.T.). K.A.H was funded by an individual postdoctoral fellowship from the Danish Council for Independent Research | Natural Sciences (Grant no. 0602-02523B).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ibmb.2015.06.002.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Fig. S1.
Cytochrome P450-4e family expression profiles in Drosophila melanogaster. (A) X-axis shows mean affymetrix microarray signal for each tissue along the y-axis. FlyAtlas data identified three Cyp4e genes, all three show abundant expression in adult Malpighian tubule. (B) D. melanogaster male tubule Cyp4e mRNA expression. Expression levels of Cyp4e mRNA were normalized against the housekeeping reference rp49 and values expressed as fold change relative ± SEM (n = 3). (C) D. melanogaster male and female Cyp4e3 mRNA expression. Female tubule Cyp4e3 mRNA levels were 7-fold higher compared to males. Bars indicated with an asterisk were significantly different (*P = 0.0016 as determined by Student's t-test).
Fig. S2.
Effect of manipulation of Cyp4e3 levels on (A-B) permethrin (75 μg/ml) and on (C-D) fenvalerate (5 μg/ml) dietary exposure. (A) Cyp4e3 knockdown female flies display significantly reduced survival and (B) conversely display significantly increased survival after over-expression of Cyp4e3 compared to parental controls. The same phenotype was seen in Cyp4e3 transgenic males with fenvalerate survival experiments. In all survival experiments, P < 0.001 against both controls; Log-rank test; n > 90 flies for each genotypes.
Fig. S3.
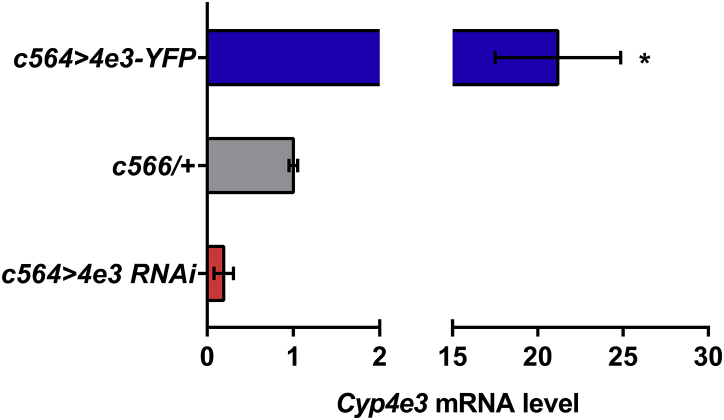
Down- and up-regulation of Cyp4e3 in fat body tissue. The observed difference in sample quantity corresponds to an approximately 80% decrease in Cyp4e3 mRNA levels in the Cyp4e3 knockdown flies and an approximately 21-fold increase of Cyp4e3 mRNA expression in the Cyp4e3 overexpressor flies. Data are expressed as fold change compared to c564/ + parental control ± SEM (n = 3).
Fig. S4.
Up-regulation of Cyp4e3 mRNA levels and Cyp4e3 transgenic fly survival under exposure to H2O2. (A) Wild-type (Canton-S) flies were fed on normal food and on 1% H2O2 or 20 mM paraquat-containing food for 24 h, tubules were removed, and assessed for Cyp4e3 expression levels, data expressed as fold change relative ± SEM (n = 3). (B) Reduced Cyp4e3 levels or (C) increased Cyp4e3 levels in tubule principal cells significantly alters survival of flies fed on 1% H2O2 (P < 0.001 against both controls; log-rank test; n = 100–120 male flies for the different genotypes).
Fig. S5.
Single cell imaging for H2O2 level measurements. Intact live tubules were dissected in Schneider's medium in the presence of 20 mM of NEM and were challenged with (A) 25 mM H2O2 and (B) 10 mM DTT. The effect was imaged over 10 min and the resulting in situ redox state of the probe was determined by microscopy. (C) In vivo confocal imaging of the cytosolic roGFP2-Orp1 probe targeted to Malpighian tubule principal cells. Silencing of Catalase leads to increased cytosolic H2O2 levels as shown by the higher oxidation of the specific H2O2 probe. Scale bar = 25 μm. (D) Hydrogen peroxide (H2O2) production in Malpighian tubules. Tubules from Catalase knockdown flies show increased H2O2 production compared to parental controls. Data is shown as mean μM H2O2 ± SEM (n ≥ 3), where *P < 0.05.
References
- Agaisse H., Petersen U.M., Boutros M., Mathey-Prevot B., Perrimon N. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev. Cell. 2003;5:441–450. doi: 10.1016/s1534-5807(03)00244-2. [DOI] [PubMed] [Google Scholar]
- Akbar S.M.D., Sharma H.C., Jayalakshmi S.K., Sreeramulu K. Effect of pyrethroids, permethrin and fenvalarate, on the oxidative stress of Helicoverpa armigera. World J. Sci. Technol. 2012;2:01–05. [Google Scholar]
- Albrecht S.C., Barata A.G., Grosshans J., Teleman A.A., Dick T.P. In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell. Metab. 2011;14:819–829. doi: 10.1016/j.cmet.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Banerjee B.D., Seth V., Bhattacharya A., Pasha S.T., Chakraborty A.K. Biochemical effects of some pesticides on lipid peroxidation and free-radical scavengers. Toxicol. Lett. 1999;107:33–47. doi: 10.1016/s0378-4274(99)00029-6. [DOI] [PubMed] [Google Scholar]
- Breckenridge C.B., Holden L., Sturgess N., Weiner M., Sheets L., Sargent D., Soderlund D.M., Choi J.S., Symington S., Clark J.M. Evidence for a separate mechanism of toxicity for the type I and the type II pyrethroid insecticides. Neurotoxicology. 2009;30(Suppl. 1):S17–S31. doi: 10.1016/j.neuro.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Chahine S., O'Donnell M.J. Physiological and molecular characterization of methotrexate transport by Malpighian tubules of adult Drosophila melanogaster. J. Insect Physiol. 2009;55:927–935. doi: 10.1016/j.jinsphys.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Chintapalli V.R., Terhzaz S., Wang J., Al Bratty M., Watson D.G., Herzyk P., Davies S.A., Dow J.A. Functional correlates of positional and gender-specific renal asymmetry in Drosophila. PloS One. 2012;7:e32577. doi: 10.1371/journal.pone.0032577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli V.R., Wang J., Dow J.A. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- Daborn P.J., Yen J.L., Bogwitz M.R., Le Goff G., Feil E., Jeffers S., Tijet N., Perry T., Heckel D., Batterham P. A single p450 allele associated with insecticide resistance in Drosophila. Science. 2002;297:2253–2256. doi: 10.1126/science.1074170. [DOI] [PubMed] [Google Scholar]
- Dooley C.T., Dore T.M., Hanson G.T., Jackson W.C., Remington S.J., Tsien R.Y. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J. Biol. Chem. 2004;279:22284–22293. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- Dow J.A., Davies S.A. The Malpighian tubule: rapid insights from post-genomic biology. J. Insect Physiol. 2006;52:365–378. doi: 10.1016/j.jinsphys.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Ekengren S., Hultmark D. A family of turandot-related genes in the humoral stress response of Drosophila. Biochem. Biophys. Res. Commun. 2001;284:998–1003. doi: 10.1006/bbrc.2001.5067. [DOI] [PubMed] [Google Scholar]
- Enayati A.A., Ranson H., Hemingway J. Insect glutathione transferases and insecticide resistance. Insect Mol. Biol. 2005;14:3–8. doi: 10.1111/j.1365-2583.2004.00529.x. [DOI] [PubMed] [Google Scholar]
- Feyereisen R. Molecular biology of insecticide resistance. Toxicol. Lett. 1995;82–83:83–90. doi: 10.1016/0378-4274(95)03470-6. [DOI] [PubMed] [Google Scholar]
- Feyereisen R. Insect P450 enzymes. Annu. Rev. Entomol. 1999;44:507–533. doi: 10.1146/annurev.ento.44.1.507. [DOI] [PubMed] [Google Scholar]
- Gutscher M., Sobotta M.C., Wabnitz G.H., Ballikaya S., Meyer A.J., Samstag Y., Dick T.P. Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J. Biol. Chem. 2009;284:31532–31540. doi: 10.1074/jbc.M109.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidari R., Devonshire A.L., Campbell B.E., Dorrian S.J., Oakeshott J.G., Russell R.J. Hydrolysis of pyrethroids by carboxylesterases from Lucilia cuprina and Drosophila melanogaster with active sites modified by in vitro mutagenesis. Insect Biochem. Mol. Biol. 2005;35:597–609. doi: 10.1016/j.ibmb.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Hill I.R. Aquatic organisms and pyrethroids. Pestic. Sci. 1989;27:429–465. [Google Scholar]
- Hu F., Li L., Wang C., Zhang Q., Zhang X., Zhao M. Enantioselective induction of oxidative stress by permethrin in rat adrenal pheochromocytoma (PC12) cells. Environ. Toxicol. Chem. SETAC. 2010;29:683–690. doi: 10.1002/etc.73. [DOI] [PubMed] [Google Scholar]
- Jamroz R.C., Guerrero F.D., Pruett J.H., Oehler D.D., Miller R.J. Molecular and biochemical survey of acaricide resistance mechanisms in larvae from Mexican strains of the southern cattle tick, Boophilus microplus. J. Insect Physiol. 2000;46:685–695. doi: 10.1016/s0022-1910(99)00157-2. [DOI] [PubMed] [Google Scholar]
- Kalajdzic P., Oehler S., Reczko M., Pavlidi N., Vontas J., Hatzigeorgiou A.G., Savakis C. Use of mutagenesis, genetic mapping and next generation transcriptomics to investigate insecticide resistance mechanisms. PloS One. 2012;7:e40296. doi: 10.1371/journal.pone.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai S., Scott J.G. Overexpression of cytochrome P450CYP6D1 is associated with monooxygenase-mediated pyrethroid resistance in house flies from Georgia. Pestic. Biochem. Physiol. 2000;68:34–41. [Google Scholar]
- Kondo T., Inagaki S., Yasuda K., Kageyama Y. Rapid construction of Drosophila RNAi transgenes using pRISE, a P-element-mediated transformation vector exploiting an in vitro recombination system. Genes Genet. Syst. 2006;81:129–134. doi: 10.1266/ggs.81.129. [DOI] [PubMed] [Google Scholar]
- Lemaitre B., Reichhart J.M., Hoffmann J.A. Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc. Natl. Acad. Sci. U. S. A. 1997;94:14614–14619. doi: 10.1073/pnas.94.26.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra S., Dombrowski S.M., Waters L.C., Ganguly R. Three second chromosome-linked clustered Cyp6 genes show differential constitutive and barbital-induced expression in DDT-resistant and susceptible strains of Drosophila melanogaster. Gene. 1996;180:165–171. doi: 10.1016/s0378-1119(96)00446-5. [DOI] [PubMed] [Google Scholar]
- Miwa S., St-Pierre J., Partridge L., Brand M.D. Superoxide and hydrogen peroxide production by Drosophila mitochondria. Free Radic. Biol. Med. 2003;35:938–948. doi: 10.1016/s0891-5849(03)00464-7. [DOI] [PubMed] [Google Scholar]
- Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl H.L., Baeuerle P.A. The ER-overload response: activation of NF-kappa B. Trends Biochem. Sci. 1997;22:63–67. doi: 10.1016/s0968-0004(96)10073-6. [DOI] [PubMed] [Google Scholar]
- Pittendrigh B., Aronstein K., Zinkovsky E., Andreev O., Campbell B., Daly J., Trowell S., Ffrench-Constant R.H. Cytochrome P450 genes from Helicoverpa armigera: expression in a pyrethroid-susceptible and -resistant strain. Insect Biochem. Mol. Biol. 1997;27:507–512. doi: 10.1016/s0965-1748(97)00025-8. [DOI] [PubMed] [Google Scholar]
- Radyuk S.N., Klichko V.I., Michalak K., Orr W.C. The effect of peroxiredoxin 4 on fly physiology is a complex interplay of antioxidant and signaling functions. FASEB J. 2013;27:1426–1438. doi: 10.1096/fj.12-214106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer T.J., Meyer D.A., Crofton K.M. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ. Health Perspect. 2005;113:123–136. doi: 10.1289/ehp.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund D.M., Bloomquist J.R. Neurotoxic actions of pyrethroid insecticides. Annu. Rev. Entomol. 1989;34:77–96. doi: 10.1146/annurev.en.34.010189.000453. [DOI] [PubMed] [Google Scholar]
- Stoven S., Silverman N., Junell A., Hedengren-Olcott M., Erturk D., Engstrom Y., Maniatis T., Hultmark D. Caspase-mediated processing of the Drosophila NF-kappaB factor Relish. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5991–5996. doi: 10.1073/pnas.1035902100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehana A., Yano T., Mita S., Kotani A., Oshima Y., Kurata S. Peptidoglycan recognition protein (PGRP)-LE and PGRP-LC act synergistically in Drosophila immunity. EMBO J. 2004;23:4690–4700. doi: 10.1038/sj.emboj.7600466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhzaz S., Cabrero P., Robben J.H., Radford J.C., Hudson B.D., Milligan G., Dow J.A., Davies S.A. Mechanism and function of Drosophila capa GPCR: a desiccation stress-responsive receptor with functional homology to human neuromedinU receptor. PloS One. 2012;7:e29897. doi: 10.1371/journal.pone.0029897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhzaz S., Finlayson A.J., Stirrat L., Yang J., Tricoire H., Woods D.J., Dow J.A., Davies S.A. Cell-specific inositol 1,4,5 trisphosphate 3-kinase mediates epithelial cell apoptosis in response to oxidative stress in Drosophila. Cell. Signal. 2010;22:737–748. doi: 10.1016/j.cellsig.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Tomita T., Liu N., Smith F.F., Sridhar P., Scott J.G. Molecular mechanisms involved in increased expression of a cytochrome P450 responsible for pyrethroid resistance in the housefly, Musca domestica. Insect Mol. Biol. 1995;4:135–140. doi: 10.1111/j.1365-2583.1995.tb00018.x. [DOI] [PubMed] [Google Scholar]
- Vadhana D., Carloni M., Fedeli D., Nasuti C., Gabbianelli R. Perturbation of rat heart plasma membrane fluidity due to metabolites of permethrin insecticide. Cardiovasc. Toxicol. 2011;11:226–234. doi: 10.1007/s12012-011-9116-0. [DOI] [PubMed] [Google Scholar]
- Vontas J.G., Small G.J., Hemingway J. Glutathione S-transferases as antioxidant defence agents confer pyrethroid resistance in Nilaparvata lugens. Biochem. J. 2001;357:65–72. doi: 10.1042/0264-6021:3570065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., mccart C., Woods D.J., Terhzaz S., Greenwood K.G., ffrench-Constant R.H., Dow J.A. A Drosophila systems approach to xenobiotic metabolism. Physiol. Genom. 2007;30:223–231. doi: 10.1152/physiolgenomics.00018.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.