Summary
Background
The American Academy of Pediatrics recommends a permissive hypoxaemic target for an oxygen saturation of 90% for children with bronchiolitis, which is consistent with the WHO recommendations for targets in children with lower respiratory tract infections. No evidence exists to support this threshold. We aimed to assess whether the 90% or higher target for management of oxygen supplementation was equivalent to a normoxic 94% or higher target for infants admitted to hospital with viral bronchiolitis.
Methods
We did a parallel-group, randomised, controlled, equivalence trial of infants aged 6 weeks to 12 months of age with physician-diagnosed bronchiolitis newly admitted into eight paediatric hospital units in the UK (the Bronchiolitis of Infancy Discharge Study [BIDS]). A central computer randomly allocated (1:1) infants, in varying length blocks of four and six and without stratification, to be clipped to standard oximeters (patients treated with oxygen if pulse oxygen saturation [SpO2] <94%) or modified oximeters (displayed a measured value of 90% as 94%, therefore oxygen not given until SpO2 <90%). All parents, clinical staff, and outcome assessors were masked to allocation. The primary outcome was time to resolution of cough (prespecified equivalence limits of plus or minus 2 days) in the intention-to-treat population. This trial is registered with ISRCTN, number ISRCTN28405428.
Findings
Between Oct 3, and March 30, 2012, and Oct 1, and March 29, 2013, we randomly assigned 308 infants to standard oximeters and 307 infants to modified oximeters. Cough resolved by 15·0 days (median) in both groups (95% CI for difference −1 to 2) and so oxygen thresholds were equivalent. We recorded 35 serious adverse events in 32 infants in the standard care group and 25 serious adverse events in 24 infants in the modified care group. In the standard care group, eight infants transferred to a high-dependency unit, 23 were readmitted, and one had a prolonged hospital stay. In the modified care group, 12 infants were transferred to a high-dependency unit and 12 were readmitted to hospital. Recorded adverse events did not differ significantly.
Interpretation
Management of infants with bronchiolitis to an oxygen saturation target of 90% or higher is as safe and clinically effective as one of 94% or higher. Future research should assess the benefits and risks of different oxygen saturation targets in acute respiratory infection in older children, particularly in developing nations where resources are scarce.
Funding
National Institute for Health Research, Health Technology Assessment programme.
Introduction
Oxygen saturation targets standardise clinical care in patients with acute disease, aiming to maintain tissue oxygenation and improve clinical outcomes. No randomised clinical trial has assessed oxygen saturation targets in management of patients with acute lower respiratory infection (LRTI).1 The most common acute LRTI in early life is acute viral bronchiolitis, which causes about 128 000 hospital admissions each year in the USA at an estimated cost of US$1·73 billion.2 Applying standard unit cost estimates to annual rates in the UK reveals that the annual cost of paediatric bronchiolitis to NHS England would be about £83·8 million. This figure is based on 37 200 cases of paediatric bronchiolitis per year3 with an average cost per case of £2254.4 In 2006, two evidence-based guidelines for the management of bronchiolitis included contrasting recommendations for oxygen saturation targets. The UK SIGN guideline (SIGN 915) recommended a normoxic pulse oxygen saturation (SpO2) target (≥94%), whereas the American Academy of Pediatrics (AAP)6 recommended a permissive hypoxaemic SpO2 target (≥90%; reiterated 20147). The AAP's advice is consistent with WHO's recommended SpO2 target of 90% and higher in infants with LRTI. However, WHO's recommendation is considered pragmatic advice for best use of scarce oxygen availability in low-resource settings,8 and neither AAP or WHO targets have been tested in a randomised controlled trial.
Despite AAP's guidance, recommended SpO2 targets for infants with bronchiolitis continue to vary between national guideline portals for the USA,9 Europe,10 and Australasia. This inconsistency might show clinical hesitance to adopt a lower SpO2 target for infants than is currently recommended for older children with pneumonia (≤92%)11 and asthma (<94%),12 or a reticence to use hypoxaemic targets provided by expert opinion when similar recommendations have been associated with unanticipated adverse events once tested in a randomised controlled trial.13 We aimed to assess whether a target oxygen saturation of 90% or higher would be equivalent to 94% or higher for resolution of illness in acute viral bronchiolitis. To do this we assessed time to resolution of cough, which is associated with airway inflammation and might be influenced by hypoxia.14, 15 Cough is a ubiquitous symptom in bronchiolitis consistently identified by parents and has a duration well documented in many trials.16
Research in context.
Evidence before this study
We searched PubMed and Scopus for clinical trials published in English with the terms “oxygen”, “oximetry”, “saturation”, “bronchiolitis”, “pneumonia”, and “lower respiratory tract infection” in humans (all ages) up to Oct 26, 2014. We could identify no randomised controlled trials. A Cochrane systematic review of oxygen therapy for lower respiratory tract infection in children aged 3 months to 15 years identified no randomised controlled trials up to April 30, 2008. An observational study in Papua New Guinea (Duke et al, 2008) identified that deaths from lower respiratory tract infection were reduced in children provided with supplemental oxygen for SpO2 lower than 90% when compared with historical controls.
Added value of this study
To our knowledge, this is the first randomised controlled trial of oxygen saturation targets of children with bronchiolitis or any lower respiratory tract infection. Providing supplemental oxygen to a saturation target of 90% in infants with bronchiolitis was safe and clinically effective. There was some evidence of better outcomes in infants managed to a 90% SpO2 target.
Implications of all the available evidence
Further trials are warranted of older children with lower respiratory infection, and our results suggest that the current 90% SpO2 threshold might be open to investigation, especially in low-resource settings with little availability of supplemental oxygen.
Methods
Study design and participants
We did a parallel-group, randomised, controlled, equivalence trial at eight paediatric hospitals in the UK during two 6-month winter bronchiolitis seasons (five centres in season one with three additional centres added in season two; appendix p 2). The protocol is available online. A health economic cost-effectiveness analysis was also done and is reported separately.
Infants aged between 6 weeks and 12 months of age (corrected for prematurity) who presented to a participating hospital emergency department or acute assessment area, either by general practice referral or spontaneous attendance, were assessed by acute care medical staff for potential trial recruitment. To be eligible for recruitment, infants had to have a clinical diagnosis of bronchiolitis (consistent with SIGN 91 Bronchiolitis5) made by receiving acute medical staff and required admission to hospital for supportive care. The clinical decision to admit an infant with bronchiolitis prompted randomisation to the study. We excluded infants who: were preterm (<37 weeks' gestation) and had received oxygen therapy in the past 4 weeks; had cyanotic or haemodynamically significant heart disease; had cystic fibrosis or interstitial lung disease; had documented immune function deficit; were directly admitted to a high dependency or intensive care area; or were previously randomised.
South East Scotland Research Ethics Committee 3 provided multicentre UK ethical approval. Written, informed consent was obtained from the parent or legal guardian.
Randomisation and masking
We randomly allocated (1:1) infants to a standard pulse saturation oximeter or a modified pulse saturation oximeter. Randomisation was by a central internet-based secure password-protected computer. The random allocation sequence was generated by computer programme at the Edinburgh Clinical Trials Unit, UK. Randomisation was by blocks of varying length (four and six) without stratification. Infants were enrolled by clinicians and research or specialist nurses in the hospital emergency department or acute assessment area, and group allocation was concealed until definite enrolment. Clinicians (mostly nursing staff) attached either a standard or modified pulse oximeter according to the computer-generated randomisation code. Oximeters were attached with an interval of at least 1 min after removal of the hospital oximeter and within 4 h of arrival at hospital, and most typically at the point of preparing to transfer from hospital emergency department or acute assessment area to a hospital ward. Monitors were identical in appearance (except for study number). The average difference between displayed standard and modified oximeter values was 2% SpO2 (appendix p 3). To reduce risk of unmasking, oximeters had new study numbers allocated for season two. All study staff involved in day-to-day running of the trial, hospital staff, and parents were masked to study intervention, as were those assessing outcomes. The mask was not broken for any infant during the study.
Procedures
Standard pulse oximeters (Rad 8 with LNC 10 patient cable, Masimo, CA, USA) measured and displayed oxygen saturation in the typical way. Identical-looking modified oximeters measured arterial oxygen saturation as per standard oximeters, but manufacturer-altered internal algorithms provided a non-standard display—for the range 85–100% the algorithm skewed the displayed values so that at a measured value of 90% the monitor would display 94%, with smoothed displayed values above and below this point (appendix). Staff were instructed to provide supplemental oxygen to infants with an SpO2 of lower than 94%. Therefore, infants with modified oximeters would not be given supplemental oxygen at a displayed 94% oxygen saturation, but actual 90% oxygen saturation. Pilot work for the trial showed very high variability of SpO2 improvement in bronchiolitis during recovery17 and this information was provided to staff during study education.
Study staff followed up participants and parents or carers during the hospital stay and also by structured telephone questionnaire at 7, 14, and 28 days, and 6 months after randomisation to assess outcomes. To maximise accurate recall, we provided parents with study cards at discharge to remind them of outcomes that would be assessed at each follow-up. Every effort was made to minimise dropout and non-compliance. We measured parental anxiety with the anxiety subscale of The Hospital Anxiety and Depression Score (HADS).18 Viral identification was done with routine PCR or near patient testing of nasal secretions.
Discharge from hospital was at the discretion of the paediatrician responsible for care; however, we asked that study infants be considered eligible and fit for discharge from hospital once they had attained stable SpO2 of 94% or higher continuously for 4 h (including a period of sleep) and were feeding orally at 75% or more of their expected intake of milk daily. In all other aspects, management was according to local paediatric care.
Outcomes
The primary outcome for equivalence was time to resolution of cough. Evidence of limits of equivalence could not be determined from published data and so we used expert opinion from a survey of ten general paediatricians from Royal Hospital for Sick Children, Edinburgh, UK, as plus or minus 2 days (appendix). Cough resolution as determined by parents was noted during the routine telephone contacts. Parents were reminded at each contact of the information they would be asked to provide at the next call and requested to note the date of cough resolution on their study card. Subgroup analyses were prespecified for length of illness before randomisation (0–3 days vs 4 or more), use of antibiotics (any vs none), and household smoking (any vs none).
Further prespecified outcomes assessed for equivalence were time to feed adequately (≥75% usual) with limits of equivalence of plus or minus 4 h; time to a parental perception of back to normal with the limits of equivalence plus or minus 2 days; SpO2 measured at 28 days with limits of equivalence of plus or minus 1%. Subgroup analyses were prespecified for time to fit for discharge and actual discharge by in-hospital oxygen requirement. After a review by the data monitoring committee of data from season one for SpO2 at 28 days, equivalence was identified with no safety concerns and further measurement was stopped to help with study logistics.
A predetermined statistical analysis plan included an assessment of differences between the groups for the time from randomisation until fit for discharge and actual discharge for all infants admitted with bronchiolitis; proportion of infants with health-care reattendance (primary care, emergency department, readmission; parental phone calls); child well enough to attend to childcare (parental phone calls) for days 0–7, 7–14, and 14–28; and difference in parental anxiety score at 7, 14, and 28 days and 6 month follow-up. A subgroup analysis was made of time from randomisation to fit for discharge and actual discharge from hospital in those infants with an oxygen requirement during their stay. We did a full economic analysis alongside the trial, to calculate the incremental cost and effectiveness of the 90% compared with the 94% oxygen saturation discharge procedure in terms of health, social care, and societal costs, as well as the clinical and quality-of-life outcome measures. The analysis was undertaken from the UK NHS perspective, with a sensitivity analysis considering societal costs from the parents' perspective.
Statistical analysis
Sample size was established based on the primary outcome of time to resolution of cough after randomisation. An estimate of 544 participants needed was made by assumption that there would be no difference between the treatment groups, with a common SD of 8·3 days from Plint and colleagues19 The SD of 8·3 was calculated by dividing the IQR by 1·35.19, 20 This used a two-sided test (overall α of 0·05), with power of 80%, and limits of equivalence of 2 days (ie, the difference between the two groups could be up to 2 days in either direction). To allow for skewness in the outcome measure, drop-outs, and non-compliance, we aimed to recruit 600 infants. There were no preplanned stopping rules.
The primary analysis was done by intention to treat, in which we analysed all randomly assigned infants by allocated treatment, irrespective of treatment received. However, for equivalence outcomes, we also did per-protocol analyses of infants who received a pulse oximeter analysed by treatment received; firm conclusions were only declared when intention-to-treat and per-protocol analyses agreed. Safety analyses were done by intention to treat. The primary analysis was unadjusted. All statistical tests were two-sided with 5% significance. For all outcomes, we used a complete case analysis, but for some equivalence analyses (time to cough resolution and time back to normal), imputation was used for the primary analysis, as follows. If a date of event was known, it was used. If it was known that the event occurred, but the precise date was not known, a value was chosen between the date that the event was last known to not be present and the date of the follow-up when it was present. Then a value was chosen at random from patients in the same treatment group whose cough stopped in a similar timeframe. If it was known that the event had not occurred by 6 months, then the event date was taken at random from a uniform distribution capped at 200 days.21 If it was known that the event had not occurred by the last follow-up done, but the patient was not followed to 6 months, then a random value was chosen from a uniform distribution starting at the last known follow-up point until 200 days. This process was repeated 100 times, and the analysis done on each dataset. The mean values for the estimate of the median, and the estimates of the CI limits were used.
For equivalence outcomes, the difference in medians (and 95% CIs) between treatments was estimated using a non-parametric method.22 The treatments were considered equivalent if the 95% CI lay within prespecified equivalence limits. For time to re-establish adequate feeding, no imputations were necessary. For oxygen saturation, we estimated the difference in mean oxygen saturation by an independent sample t test.
We used logistic regression for binary outcomes, poisson regression for counts, normal linear models for continuous outcomes, and ANCOVA, adjusting for baseline score where relevant data were recorded.
We used SAS version 9.3 for Windows for all analyses. The data monitoring committee were all based in the UK. They worked to a prespecified data monitoring committee charter and convened a meeting on three occasions to review unblinded data. A data monitoring committee met at the end of season one and recommended the study continue as planned. This trial is registered with ISRCTN, number ISRCTN28405428.
Role of the funding source
The funder was not involved in the collection, analysis, interpretation of data, writing of the report or the decision to publish. Masimo provided altered algorithms for study oximeters, but was not involved in study design or collection, analysis, interpretation of data, writing of the report, or the decision to publish. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
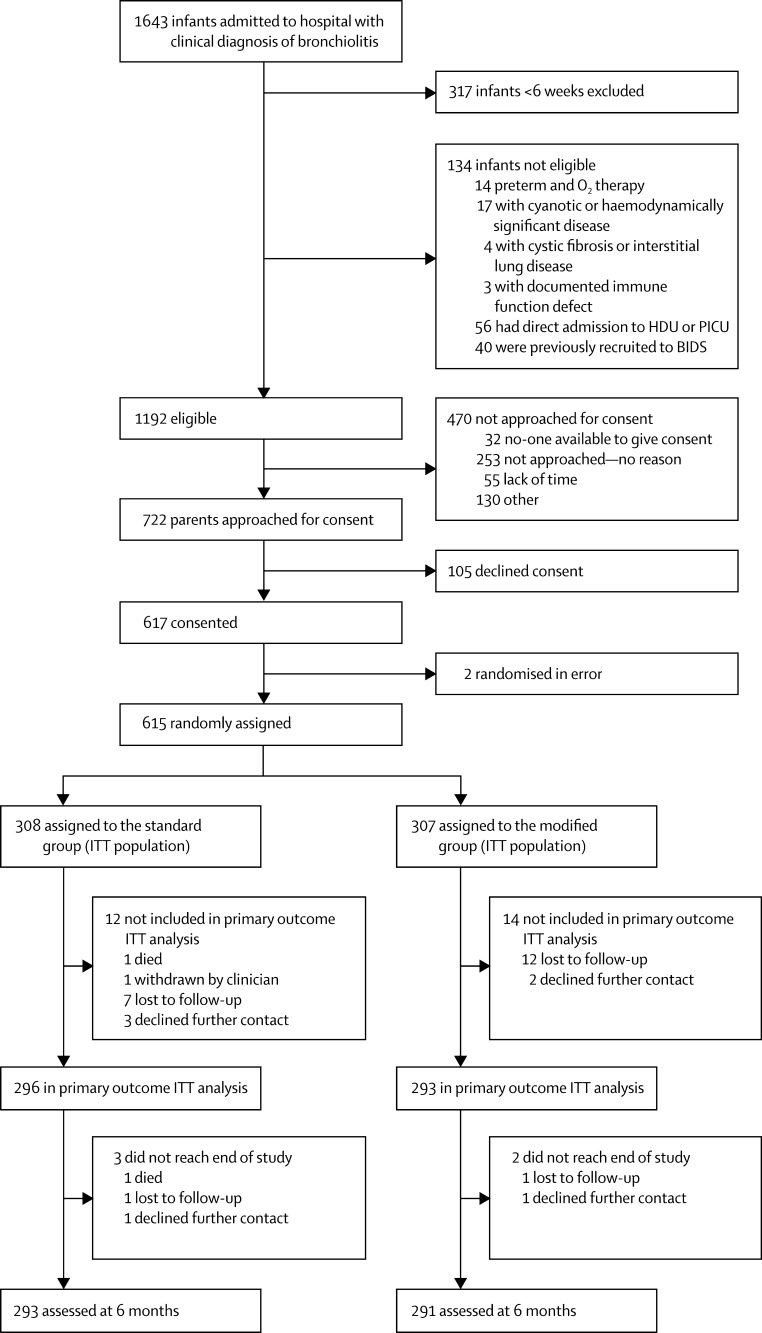
Between Oct 3, and March 30, 2012, and Oct 1, and March 29, 2013, we recruited 615 infants of median age 21·3 weeks (IQR 11·7–31·6). We randomly assigned 308 infants to be monitored with standard oximeter (standard group) and 307 to be monitored with the modified oximeters that displayed SpO2 90% as SpO2 94% (modified group; figure 1). The trial steering committee decided to continue to the end of season two, which is why the sample size is 615 people instead of 600 as planned.
Figure 1.
Study profile
HDU=high-dependency unit. PICU=paediatric intensive care unit. BIDS=Bronchiolitis of Infancy Discharge Study (BIDS). ITT=intention to treat.
There were protocol deviations relating to the pulse oximeters (attached late, removed early, or never attached) on 26 occasions in 26 infants in the standard group and 31 occasions in 30 infants in the modified group. Seven infants were incorrectly attached to a standard oximeter when randomised to modified care, and one given a modified oximeter when randomised to standard care. These infants were analysed in the group to which they were allocated in the ITT analysis, but to that received in the per protocol analysis. Six standard and nine modified pulse oximeters were never attached.
Baseline characteristics were similar between the two groups, apart from the modified group had fewer boys and more infants were preterm (table 1). 194 (70%) of 278 infants in the standard group and 213 (76%) of 279 in the modified group tested positive for respiratory syncytial virus by PCR or near patient testing.
Table 1.
Baseline clinical data
| Standard group (n=308) | Modified group (n=307) | |
|---|---|---|
| Age (weeks) | 21·3 (12·6–31·1) | 21·1 (11·1–32·0) |
| Boys | 186 (60%) | 166 (54%) |
| Preterm (<37 weeks') | 28/278 (10%) | 45/279 (16%) |
| Eczema | 51/305 (17%) | 44/303 (15%) |
| Food allergy | 8/305 (3%) | 11/302 (4%) |
| Household smoking | 133/303 (44%) | 130/304 (43%) |
| 1 or more siblings | 221/304 (73%) | 211/304 (69%) |
| Primary care visits in previous 4 weeks | 1 (1–2) | 1 (0–2) |
| Heart rate on arrival (bpm) | 159 (146–173) | 158 (148–172) |
| Respiratory rate on arrival (breaths per min) | 50 (44–58) | 49 (42–58) |
| Antibiotics on arrival | 24/305 (8%) | 23/304 (8%) |
| Bronchodilator on arrival | 17/305 (6%) | 16/304 (5%) |
| Length of Illness (days) on arrival | 4 (3–5) | 4 (3–5) |
| Apnoea on arrival | 3/303 (1%) | 3/304 (1%) |
| SpO2 on arrival (%) | 95% (93–97) | 95% (93–97) |
| SpO2 on arrival ≤94% | 121/304 (40%) | 119/303 (39%) |
Data are n (%), n/N (%), or median (IQR). On arrival relates to arrival at the emergency department. Data were missing for the following numbers of patients (standard group, modified): primary care attendances (7, 4), heart rate (3, 4), respiratory rate (9, 5), length of illness (3, 5), and SpO2 (4, 4). SpO2=pulse oxygen saturation.
Median time to cough resolution was 15·0 days in both standard and modified groups with a median difference of 1·0 day (95% CI −1 to 2; table 2). This interval and 95% CI falls within the prespecified equivalence limits of plus and minus 2 days. Both the prespecified complete-case analysis for the ITT population and per-protocol analysis (n=297 for standard group; n=279 for modified group) had a median difference of 0 days (95% CI −1 to 2; table 2, appendix p 4). Because all intervals fell within the prespecified equivalence limits, we consider the groups equivalent for the primary outcome. No differences of note were found in the prespecified subgroup analyses for time to cough resolution (appendix p4).
Table 2.
Clinical outcomes
| Standard group (n=308) | Modified group (n=307) | Median difference* | HR estimate† | p value | |
|---|---|---|---|---|---|
| Time to resolution cough (days)‡ | 15·0 (10·0 to 42·5); n=296 | 15·0 (10·0 to 41·0); n=293 | 1·00 (−1·0 to 2·0) | .. | .. |
| Time feeding returned to ≥75% normal (h)§ | 24·1 (6·5 to 62·1); n=304 | 19·5 (6·3 to 47·2); n=296 | 2·7 (−0·3 to 7·3) | .. | .. |
| Time back to normal (days)¶ | 12·0 (7·0 to 25·0); n=296 | 11·0 (6·0 to 20·0); n=293 | 1·0 (0 to 3·0) | .. | .. |
| Time to fit to discharge (h) | 44·2 (18·6 to 87·5); n=283 | 30·2 (15·6 to 59·7); n=276 | .. | 1·46 (1·23 to 1·73) | <0·0001 |
| Time to actual discharge (h) | 50·9 (23·1 to 93·4); n=303 | 40·9 (21·8 to 67·3); n=301 | .. | 1·28 (1·09 to 1·50) | 0·003 |
| Time to no further supplemental oxygen (h) | 27·6 (0 to 68·1); n=305 | 5·7 (0 to 32·4); n=304 | .. | 1·37 (1·12 to 1·68) | 0·0021 |
Data are median (IQR); n or estimate of difference (95% CI), unless otherwise stated.
Median difference is standard–modified (<0 indicates benefit to standard practice).
HR is standard/modified (<1 indicates benefit to standard practice).
Equivalence defined as plus or minus 2 days.
Equivalence defined as plus or minus 4 h.
Equivalence defined as plus or minus 2 days.
Infants returned to adequate feeding 2·7 h sooner (median) in the modified group, with the 95% CI (–0·3 to 7) falling outside the prespecified limits of plus or minus 4 h. Similarly, infants in the modified group were considered back to normal 1 day sooner by parents (table 2) with the 95% CI of 0 to 3 also falling outside prespecified limits (plus and minus 2 days). These findings were not anticipated.
We did a post-hoc analysis for the difference between time-to-event outcomes using Cox proportional hazards regression to estimate the treatment effect (HRs given for modified vs standard). Time to return to adequate feeding gave a HR of 1·22 (95% CI 1·04–1·44, p=0·015), and for time back to normal a HR of 1·19 (95% CI 1·00–1·41, p=0·043). Thus, we have not proved equivalence for these outcomes and suggest that the modified group might have benefited more.
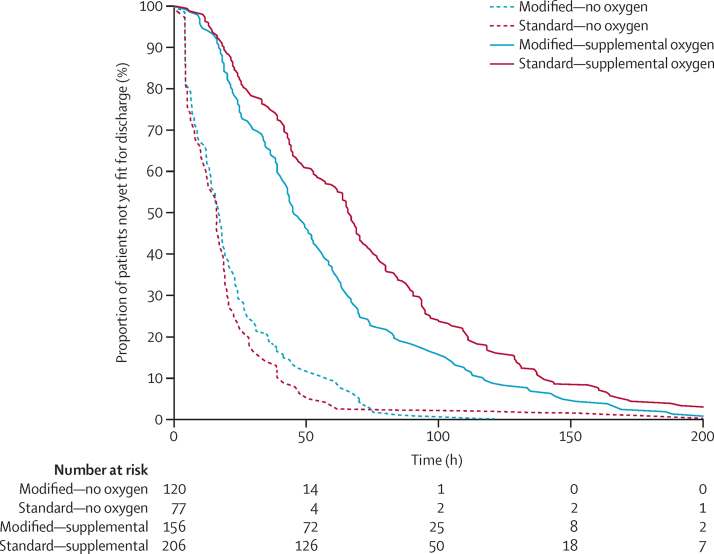
At day 28, SpO2 values (median 99%; IQR 97–100) did not significantly differ between the groups, with a mean difference of 0·111 (95% CI −0·35 to 0·57, p=0·64; prespecified limits of agreement plus or minus 1%). Supplemental oxygen was provided to 223 (73%) of 305 of infants in the standard group and 169 (56%) of 304 in the modified group. Oxygen was provided for significantly longer in the standard group than the modified group (table 2). Infants were also fit for discharge and were discharged significantly sooner in the modified group (table 2), with benefit to those who required supplemental oxygen (figure 2).
Figure 2.
Time to fit to discharge by oxygen requirement and group allocation
Serious adverse events and adverse events were recorded up to 28 days after randomisation. We recorded 35 serious adverse events in 32 infants in the standard care group and 25 serious adverse events in 24 infants in the modified care group. In the 28 days after randomisation 173 adverse events were recorded in 144 infants: 89 adverse events in 75 infants in the standard care group and 84 adverse events in 69 infants in the modified care group. No important differences were noted between group for type of event (respiratory, gastrointestinal) or severity (appendix p 5).
The standard care group had eight episodes of high-dependency unit admission (in eight infants), 26 readmissions to hospital (in 23 infants), and one prolonged hospital stay. The modified care group had 13 episodes of high-dependency unit admission (in 12 infants) and 12 readmissions to hospital (in 12 infants). Time to readmission to hospital did not significantly differ between groups; median difference was 0·9 days (95% CI 0·4–2·0, p=0·8410). The number of health-care contacts (primary, emergency, or hospital) up to 28 days was similar (table 3).
Table 3.
Adverse events (number of patients) and safety outcomes
| Standard group (n=308) | Modified group (n=307) | Mean difference (95% CI) | Odds ratio (95% CI) | p | Reduction in events per 1000 treated | ||
|---|---|---|---|---|---|---|---|
| Adverse events* | |||||||
| Any adverse event | 75 (24%) | 69 (22%) | .. | .. | .. | .. | |
| Respiratory adverse event | 49 (16%) | 50 (16%) | .. | .. | .. | .. | |
| Gastrointestinal adverse event | 7 (2%) | 12 (4%) | .. | .. | .. | .. | |
| Other adverse event | 27 (9%) | 14 (5%) | .. | .. | .. | .. | |
| Safety outcomes | |||||||
| Deaths | 2 (1%) | 0 | .. | .. | .. | .. | |
| Episodes of high dependency care | 8 (3%) | 13 (4%) | .. | .. | .. | .. | |
| Heart rate at discharge (bpm) | 133·8 (16·2)† | 135·0 (15·7)† | −1·16 (−3·70 to 1·37) | .. | 0·37 | .. | |
| Respiratory rate at discharge (breaths per min) | 38·0 (7·9) | 38·0 (6·4) | 0·09 (−1·05 to 1·23) | .. | 0·88 | .. | |
| Re-admission to hospital within 7 days (episodes; infants [%]) | 8 (6; 2%) | 5 (5; 2%) | .. | .. | .. | .. | |
| Re-admission to hospital within 28 days (episodes; infants [%]) | 26 (23; 7%) | 12 (12; 4%) | .. | .. | .. | .. | |
| Re-attendance health care within 7 days | 39/270 (14%) | 34/267 (13%) | .. | 0·98 (0·65 to 1·49) | 0·94 | 2 (−56 to 45) | |
| Re-attendance health care within 14 days | 76/267 (29%) | 70/258 (27%) | .. | 1·07 (0·73 to 1·57) | 0·73 | −14 (−88 to 79) | |
| Re-attendance health care after 28 days | 127/274 (46%) | 128/262 (49%) | .. | 0·90 (0·64 to 1·27) | 0·56 | 26 (−99 to 104) | |
| Antibiotics after discharge | 24/305 (8%) | 10/304 (3%) | .. | .. | .. | .. | |
Data are n (%), mean (SD), n/N (%), or difference (95% CI), unless otherwise specified. bpm=beats per min.
See appendix p 5 for more information about adverse events.
n=304.
Resource use of therapies in hospital other than oxygen was similar in the two groups. A cost analysis from a UK National Health Service (NHS) perspective showed a $496 (£290) mean cost saving per patient associated with the modified oximeters over the study period (appendix). This was a statistically insignificant result; however, exploring the individual components of the total NHS cost, the hospital inpatient stay variable was significantly higher in the standard group (£1055 compared to £886, p<0·05).
Early discharge did not result in parents in the modified group having greater levels of anxiety at 7, 14, or 28 days (appendix). Lead carers discharged home sooner in the modified group actually lost fewer hours to usual activities than did those in the standard group discharged later, with a median gain of 13·5 h by 7 days, 17·3 h by 14 days and 16·1 h by 28 days (appendix p 7). Only few children were attending child-care at the time of admission, with similar times to return to child-care from randomisation to 28 days (appendix p 7).
Discussion
In children with acute viral bronchiolitis, the time taken for symptoms to resolve was the same whether they were managed to a target oxygen saturation of 90% or 94%. As might be expected, when managed to a 90% SpO2 target, fewer infants needed oxygen, those that did needed it for a shorter duration, and the infants were discharged home sooner. Somewhat unexpectedly, we found that infants managed to the lower target of 90% might regain satisfactory feeding and be back to normal sooner and have fewer readmissions to hospital.
The return to adequate oral feeding a median difference of 2·7 h sooner in the modified group might simply show a more assertive approach to oral feeding by staff when infants were considered to have oxygen saturation in the normal range. However, feeding difficulties could also be prolonged in infants (obligate nasal breathers) by the drying of nasal secretions by oxygen gases and the increased resistance to nasal airflow created by a nasal cannula to deliver oxygen. Parents of infants in the modified group considered them to return to normal health 1 day sooner than did parents of infants in the standard group. Although this finding might represent a time from discharge effect, it could also be consistent with a previous report of better outcomes in children with bronchiolitis when exposed to fewer interventions.23 Infants were considered by parents to be back to normal health sooner than parents reported complete cough resolution, presumably denoting that resolving cough is one component of being back to normal health but does not define it. We did not anticipate that fewer readmissions would occur in the modified group because we considered these infants would be discharged sooner in the course of their illness and therefore more likely to be readmitted. The 5% absolute reduction in readmissions for infants at day 28 in the modified group (14 fewer admissions) could be considered a clinically important difference. The reasons for differential readmission rates can only be speculative, but those with a longer stay in hospital might have been at greater risk of nosocomial infection or possible oxygen toxicity leading to readmission.
The strength of this study is that we provide evidence-based target oxygen saturation for the management of acute bronchiolitis. Guideline-recommended expert opinion for permissive hypoxaemic targets has previously been associated with poor outcomes when tested in randomised trials, prompting the recommended saturation target to be raised.13
However, some limitations should be noted. We did no assessment of neurocognitive outcomes. Concerns were raised after following the AAP recommendation for permissive hypoxemia that the practice might affect neurocognition.24 However mild hypoxaemia seems to have no significant neurocognitive effect in children with mild or moderate obstructive sleep apnoea older than 6 months.25 Infants recovering from bronchiolitis supplemented to a stable SpO2 of 90% would have less hypoxia over a shorter timescale than that typically seen in those with obstructive sleep apnoea, and so the risk to infants in our study seem very low or absent. Another limitation is that we did not assess safe oxygen saturation for the management of children in a primary care or emergency department setting; however, the protocol did incorporate a pragmatic brief observation period (4 h) with the purpose that this trial could provide supportive evidence for a future study of safe targets for SpO2 in bronchiolitis in hospital emergency department or acute assessment area and primary care. Recent studies suggest that a more flexible approach to SpO2 in the assessment of bronchiolitis in emergency departments should be pursued.26 Furthermore, in designing a trial to study the effects of oxygen saturation targets in emergency admissions to hospital wards, we had to meet the challenges of high levels of recruitment in an acute setting and satisfactory masking. Our pilot work27 revealed that 48% of infants admitted with bronchiolitis have satisfactory oxygen saturation on arrival at the emergency department with desaturation after admission. The logistics of recruitment, randomisation, and maintained masking of infants who were admitted and subsequently desaturated persuaded us that observation of the whole population admitted to hospital would provide the best opportunity to study this proposed policy change. The trial design reported here therefore has the effect that the intervention is limited to those infants in both groups of the trial who were admitted to hospital and desaturated below 94% with provision of supplemental oxygen (73% standard group). The effect of the inclusion of infants who were not affected by the study intervention is difficult to estimate, but on a population basis the effect was equivalent.
Because children in the modified group managed to a target of 90% had the same or better outcomes than did those managed to a 94% target, the WHO oxygen saturation target of 90% might not be the lower limit of tolerance for oxygen saturation in acute respiratory disease in children. Particularly in low-resource settings, the risk and benefit balance of managing acute respiratory infection at a target lower than the current WHO recommended SpO2 target could be considered. However, with increased risk of death shown for children with pneumonia and preterm infants with chronic lung disease managed at target oxygen saturation of lower than 90%,28, 29, 30 the ethical boundaries to such a study would need careful consideration.31, 32
Acknowledgments
Acknowledgments
We were funded by the UK National Institute for Health Research (NIHR), Health Technology Assessment (HTA) programme (project number 09/91/16) and will be published in full in Health Technology Assessment. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the HTA programme, NIHR, NHS, or the UK Department of Health. The BIDS study team acknowledges the financial support of NHS Research Scotland (NRS), through the Edinburgh Clinical Research Facility. We also received non-financial support from the Scottish Children's Research Network (ScotCRN) and the NIHR Clinical Research Network. Masimo, CA, USA, produced oxygen saturation monitors with standard and altered algorithm to the specification required for the study, which we then bought from Masimo. We thank Michael Shields (Queen's University Belfast), Colin Powell (University of Cardiff), and Clare Murray (University of Manchester) for serving as the trial steering committee, and to Sheila McKenzie (NHS Highland), Mike McKean (Newcastle University), and Carrol Gamble (University of Liverpool) for serving as the data monitoring committee.
Contributors
SC devised the study concept, was chief investigator, and wrote the first version of the manuscript, and with SL and EM devised the study protocol. SC, SCL, and EM, together with MM and FW, provided trial management. AR, IB, and SCL did the statistical analysis. EM and KAB did the economic analysis. TA, BE, JYP, KR, HT, SWT, CW, and JM were involved in design for study logistics and data collection. All authors contributed towards and approved the final version of the manuscript.
Declaration of interests
SC is Chair of the UK National Institute for Health and Care Excellence (NICE) Bronchiolitis Guideline Group, has received consultancy fees (paid to NHS Lothian) by Ablynx Pharmaceuticals (Belgium) related to role as international coordinating investigator for ALX-0171 phase 1 and 2 trials in bronchiolitis, and is Principal Investigator for Alios Pharmaceuticals (San Francisco, USA) for phase 1 and 2 trials of ALS-8176 in bronchiolitis. All other authors declare no competing interests.
Supplementary Material
References
- 1.Rojas MX, Granados Rugeles C, Charry-Anzola LP. Oxygen therapy for lower respiratory tract infections in children between 3 months and 15 years of age. Cochrane Database Syst Rev. 2009;1:CD005975. doi: 10.1002/14651858.CD005975.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Hasegawa K, Tsugawa Y, Brown DFM, Mansbach JM, Camargo CA., Jr Trends in bronchiolitis hospitalizations in the United States, 2000–2009. Pediatrics. 2013;132:28–36. doi: 10.1542/peds.2012-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence Costing Statement: Bronchiolitis in children 2015; implementing the NICE guideline on bronchiolitis in children (NG9) June 2015. https://www.nice.org.uk/guidance/ng9/resources/costing-statement-62382925 (accessed Aug 24, 2015). [PubMed]
- 4.UK Department of Health. National Schedule of Reference Costs 2013–14 https://www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014 (accessed Aug 24, 2015).
- 5.Network SIG. Bronchiolitis in Children (SIGN 91). NHS Quality Improvement Scotland; 2006.
- 6.American Academy of Pediatrics Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–1793. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- 7.Ralston SL, Lieberthal AS, Meissner HC. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474–e1502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 8.WHO . Pocket book of hospital care for children. 2nd edn. WHO Publications; Geneva: 2013. [Google Scholar]
- 9.National Guideline Clearinghouse (NGC) Guideline synthesis: prevention, diagnosis and treatment of pediatric bronchiolitis. http://www.guideline.gov/syntheses/synthesis.aspx?id=48172&search=bronchiolitis (accessed Aug 21, 2015).
- 10.Working Group of the Clinical Practice Guideline on Acute Bronchiolitis; Sant Joan de Déu Foundation Fundació Sant Joan de Déu, coordinator; Clinical Practice Guideline on Acute Bronchiolitis; Quality Plan for the Spanish National Healthcare System of the Spanish Ministry for Health and Social Policy; Catalan Agency for Health Technology Assessment, 2010; Clinical Practice Guidelines in the Spanish National Healthcare System: CAHTA no. 2007/05.
- 11.Harris M, Clark J, Coote N. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(suppl 2):ii1–ii23. doi: 10.1136/thoraxjnl-2011-200598. [DOI] [PubMed] [Google Scholar]
- 12.British Thoracic Society Scottish Intercollegiate Guidelines N British guideline on the management of asthma. Thorax. 2014;69(suppl 1):i1–i192. [PubMed] [Google Scholar]
- 13.Hudson KL, Guttmacher AE, Collins FS. In support of SUPPORT—a view from the NIH. N Engl J Med. 2013;368:2349–2351. doi: 10.1056/NEJMp1306986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarze J, Mackenzie KJ. Novel insights into immune and inflammatory responses to respiratory viruses. Thorax. 2013;68:108–110. doi: 10.1136/thoraxjnl-2012-202291. [DOI] [PubMed] [Google Scholar]
- 15.Ali S, Hirschfeld AF, Mayer ML. Functional genetic variation in NFKBIA and susceptibility to childhood asthma, bronchiolitis, and bronchopulmonary dysplasia. J Immunol. 2013;190:3949–3958. doi: 10.4049/jimmunol.1201015. [DOI] [PubMed] [Google Scholar]
- 16.Thompson M, Vodicka TA, Blair PS. Duration of symptoms of respiratory tract infections in children: systematic review. BMJ. 2013;347:f7027. doi: 10.1136/bmj.f7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham S, McMurray A. Observational study of two oxygen saturation targets for discharge in bronchiolitis. Arch Dis Child. 2012;97:361–363. doi: 10.1136/adc.2010.205211. [DOI] [PubMed] [Google Scholar]
- 18.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 19.Plint AC, Johnson DW, Patel H. Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med. 2009;360:2079–2089. doi: 10.1056/NEJMoa0900544. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions, version 5, 2011. http://handbook.cochrane.org/ (accessed Aug 24, 2015).
- 21.Shields MD, Thavagnanam S. The difficult coughing child: prolonged acute cough in children. Cough. 2013;9:11. doi: 10.1186/1745-9974-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altman DG, Machin D, Bryant TN, Gardner MJ. Statistics with confidence. 2nd edn. BMJ Books; London: 1989. p. 19. [Google Scholar]
- 23.Skjerven HO, Hunderi JO, Brugmann-Pieper SK. Racemic adrenaline and inhalation strategies in acute bronchiolitis. N Engl J Med. 2013;368:2286–2293. doi: 10.1056/NEJMoa1301839. [DOI] [PubMed] [Google Scholar]
- 24.Bass JL, Gozal D. Oxygen therapy for bronchiolitis. Pediatrics. 2007;119:611. doi: 10.1542/peds.2006-3002. [DOI] [PubMed] [Google Scholar]
- 25.Marcus CL, Moore RH, Rosen CL. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368:2366–2376. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuh S, Freedman S, Coates A. Effect of oximetry on hospitalization in bronchiolitis: a randomized clinical trial. JAMA. 2014;312:712–778. doi: 10.1001/jama.2014.8637. [DOI] [PubMed] [Google Scholar]
- 27.Unger S, Cunningham S. Effect of oxygen supplementation on length of stay for infants hospitalized with acute viral bronchiolitis. Pediatrics. 2008;121:470–475. doi: 10.1542/peds.2007-1135. [DOI] [PubMed] [Google Scholar]
- 28.Duke T, Wandi F, Jonathan M. Improved oxygen systems for childhood pneumonia: a multihospital effectiveness study in Papua New Guinea. Lancet. 2008;372:1328–1333. doi: 10.1016/S0140-6736(08)61164-2. [DOI] [PubMed] [Google Scholar]
- 29.Stenson BJ, Tarnow-Mordi WO, Darlow BA. Oxygen saturation and outcomes in preterm infants. N Engl J Med. 2013;368:2094–2104. doi: 10.1056/NEJMoa1302298. [DOI] [PubMed] [Google Scholar]
- 30.Carlo WA, Finer NN, Walsh MC. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362:1959–1969. doi: 10.1056/NEJMoa0911781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drazen JM, Solomon CG, Greene MF. Informed consent and SUPPORT. N Engl J Med. 2013;368:1929–1931. doi: 10.1056/NEJMe1304996. [DOI] [PubMed] [Google Scholar]
- 32.Macklin R, Shepherd L, Dreger A. The OHRP and SUPPORT–another view. N Engl J Med. 2013;369:e3. doi: 10.1056/NEJMc1308015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.