Abstract
Study question What is the extent and effect of excessive testing for glycated hemoglobin (HbA1c) among adults with controlled type 2 diabetes?
Methods A retrospective analysis of data from a national administrative claims database included commercially insured individuals in the USA, 2001-13. Study patients were aged 18 years or older, had type 2 diabetes with stable glycemic control (two consecutive tests showing HbA1c<7.0% within 24 months), did not use insulin, had no history of severe hypoglycemia or hyperglycemia, and were not pregnant. HbA1c testing frequency was measured within 24 months after the second (index) HbA1c test, and classified as guideline recommended (≤2 times/year), frequent (3-4 times/year), and excessive (≥5 times/year). Changes in treatment regimen were ascertained within three months of the index test.
Study answer and limitations Of 31 545 patients in the study cohort (mean age 58 years; mean index HbA1c 6.2%), HbA1c testing frequency was excessive in 6% and frequent in 55%. Despite good glycemic control at baseline, treatment was further intensified by addition of glucose lowering drugs or insulin in 8.4% of patients (comprising 13%, 9%, and 7% of those tested excessively, frequently, and per guidelines, respectively; P<0.001). Compared with guideline recommended testing, excessive testing was associated with treatment intensification (odds ratio 1.35 (95% confidence interval 1.22 to 1.50)). Excessive testing rates remained unchanged in 2001-08, but fell significantly after 2009. The odds of excessive testing was 46% lower in 2011 than in 2001-02. The study population is not representative of all US patients with type 2 diabetes because it was restricted to commercially insured adults with stable and controlled diabetes not receiving insulin treatment. The study design did not capture the underuse of HbA1c testing.
What this study adds In this US cohort of adults with stable and controlled type 2 diabetes, more than 60% received too many HbA1c tests, a practice associated with potential overtreatment with hypoglycemic drugs. Excessive testing contributes to the growing problem of waste in healthcare and increased patient burden in diabetes management.
Funding, competing interests, data sharing NDS and RGM are funded partly by the Agency for Healthcare Research and Quality (R18HS18339) and AcademyHealth Delivery System Science Fellowship (2013), respectively. No competing interests declared. Additional data are available from mccoy.rozalina@mayo.edu.
Introduction
Regulatory bodies, both public and private, use metrics on glycated hemoglobin (HbA1c) to guide pay-for-performance reimbursement and public reporting in the care of patients with type 2 diabetes.1 2 3 Current clinical practice guidelines recommend HbA1c of less than 6.5%, 7.0%, or 8.0%, depending on the agency and the individual patient’s goals and clinical complexity.4 5 6 7 8 9 10 Frequent HbA1c monitoring is often needed for patients with variable glycemic control, receiving intensive insulin treatment, or needing tightly regulated control (such as pregnant women). However, for patients who are meeting treatment goals, have stable glycemic control, and have had no complications of glucose lowering treatment such as hypoglycemia, current guidelines agree that HbA1c should be tested less frequently, once or twice annually.4 5 6 7 8 9 Currently, guidelines do not prescribe minimum testing frequencies and implications of exceeding recommended frequencies are not discussed, probably because of a lack of evidence that excessive testing causes patient harm. Yet, among patients with stable and controlled disease, more frequent HbA1c testing might not benefit the patient, but would contribute to their treatment burden, resource use, and healthcare costs. Potential effects on overtreatment and resultant hypoglycemia, however, are not known.
Several studies raised concerns about HbA1c testing frequency among patients with diabetes. A study of patients with newly diagnosed diabetes in the Veterans Affairs Administration (VA) found that during the year following a new diabetes diagnosis, 38% of patients were tested at least three times and 4.2% were tested at least five times.11 Several other studies conducted in the United States, Europe, and Turkey similarly suggested a high prevalence of redundant HbA1c testing, but—like the VA study—did not differentiate across patients needing different degrees of glycemic monitoring.12 13 14 Although most agree that testing five or more times per year is redundant (considering that HbA1c is a measure of average glycemia over three months), quarterly monitoring might be indicated in patients with newly diagnosed or pregnancy associated diabetes, rapidly changing HbA1c levels, treatment changes, insulin use, or history of hypoglycemia.11 To strictly evaluate the magnitude of unequivocally inappropriate testing in the general US population, patients at low risk for deterioration of glycemic control and no indication for intensive monitoring should therefore be examined separately.
Moreover, excessive testing could prompt providers to overtreat, either by intensifying treatment despite glycemic targets that are still at goal, or by failure to de-escalate treatment with stable, albeit low HbA1c levels. Such overtreatment could lead to patient harm through hypoglycemia, increased cost of care, and other treatment side effects. While identifying these adverse health and economic outcomes was beyond the scope of this study, research into this important area is ongoing. In this work, we present a population level estimate of HbA1c testing frequencies and treatment consequences among commercially insured adults and Medicare Advantage beneficiaries with type 2 diabetes across the USA who have achieved and maintained glycemic control without use of insulin.
Methods
Dataset
We conducted a retrospective analysis of data from the Optum Labs Data Warehouse (OLDW), an administrative claims database of more than 86 million commercially insured and Medicare Advantage enrollees throughout the USA between 2001 and 2014.15 OLDW includes individuals of all ages and races, and in all 50 US states, with greatest representation in the midwest and south US census regions.16 The plan provides fully insured coverage for professional, facility, and outpatient prescription medication services. Medical claims include ICD-9-CM (international classification of diseases, 9th revision, clinical modification) diagnosis and procedure codes, Current Procedural Terminology (CPT) version 4 procedure codes, Healthcare Common Procedure Coding System procedure codes, site of service codes, and provider specialty codes. Laboratory data, available for a subset of individuals based on data sharing agreements, includes test names, logical observation identifiers names and codes (LOINC), and test results.17
Study data were statistically deidentified and accessed according to the Health Insurance Portability and Accountability Act (HIPAA) 164.514 privacy rule. The Mayo Clinic Institutional Review Board exempted this study from approval as it represents research on pre-existing, deidentified data.
Patient population
We identified all patients with diabetes mellitus, aged at least 18 years, who had an HbA1c laboratory test performed between 1 January 2001 and 31 December 2011. Patients with diabetes were identified on the basis of criteria from the Healthcare Effectiveness Data and Information Set.3 We excluded patients with type 1 diabetes (ICD-9-CM codes 250.x1 and 250.x3), gestational diabetes (ICD-9-CM codes 648.0x, 648.8x), non-clinical diabetes (ICD-9-CM code 790.29), and secondary diabetes (ICD-9-CM codes 249.x). These conditions have different pathophysiologies and natural histories, and therefore different monitoring and treatment goals. ICD-9-CM codes from the top three diagnoses of any evaluation and management (E/M) encounter were queried to identify these criteria. Eligible patients had at least 24 months of continuous enrolment before and after the index HbA1c test.
To restrict the population to patients with controlled and stable disease, only patients with two consecutive HbA1c test readings below 7.0% within a 24 month period were included. The index test was the second HbA1c test; the first HbA1c test was required to establish stability of glycemic control. Thus, all patients included in the study had at least two HbA1c tests within 24 months. We excluded patients who were not subject to usual care and monitoring frequency.4 5 6 7 8 9 10 Excluded individuals were those treated with insulin within 120 days before the index date, who were pregnant, or with a history of hypoglycemic and hyperglycemic acute diabetes complications as one of three diagnoses during an E/M encounter within 12 months before the index date. Such complications included diabetic ketoacidosis (ICD-9-CM codes 250.1x), hyperglycemic hyperosmolar state (250.2x), unspecified diabetic coma (250.3x), hypoglycemia (251.x and 250.8 according to the Ginde algorithm),18 or poisoning by insulin or antidiabetic drugs (962.3). Patients entered the study cohort once, the first time they became eligible by having a second sequential HbA1c result less than 7.0%, and were followed prospectively for 24 months. All patients therefore had at least four years of uninterrupted enrollment, two years before and two years after the index test date.
We identified HbA1c studies using CPT-4 codes 83036 and 83037 in the claims files and LOINC codes 4548-4, 4549-2, 17856-6, 59261-8, 62388-4, and 4547-6 in the laboratory results file (web appendix 1]). Patients were stratified by baseline HbA1c measurements (≤5.6%; 5.7-6.4%; and 6.5-6.9%). These HbA1c thresholds were chosen to differentiate between very tight (≤5.6%), tight (5.7-6.4%), and guideline recommended (6.5-6.9%) glycemic control.
Patient involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing up of results. There are no plans to disseminate the results of the research to study participants or the relevant patient community.
Primary outcome
The primary outcome was HbA1c testing frequency, measured as the mean number of tests per year obtained during the first 24 months following the index HbA1c test. We categorized testing frequency according to the most recent clinical practice guidelines (web appendix 2):
Guideline recommended: up to two tests per year, or tests obtained six or more months apart
Frequent: three to four tests per year, or tests obtained at least three but less than six months apart
Excessive: at least five tests per year, or tests obtained less than three months apart.
Secondary outcome
We assessed changes to diabetes treatment regimen by comparing pharmacy claims 120 days after the index date with claims 120 days before the index date. Treatment intensification was defined as the increase in the number of glucose lowering drugs or addition of insulin, while treatment deintensification was defined as the discontinuation of at least one drug; none of the patients was using insulin at baseline. Drug changes that maintained the same number of substances used were classified as class switches, and assumed to be neither intensification nor deintensification.
Independent variables
To assess the influence of disease burden and clinical complexity, the Charlson comorbidity index for one calendar year before the index test date was derived from ICD-9-CM diagnoses included in administrative claims. The Charlson index is a widely used measure that weighs comorbid conditions by the strength of their association with one year mortality;19 it has been previously validated for use in diabetes.20 21 Year of the index HbA1c (2001-11) was recorded and presented as two year increments except for 2011. Demographics included age, sex, race or ethnic origin, and census region. Age and sex were obtained directly from enrolment data. We obtained information on race or ethnic origin by linking administrative claims to a third party dataset of demographic characteristics.
Baseline treatment regimens were identified from pharmacy claims issued within 120 days preceding the index test date. We grouped drugs into nine classes (web appendix 3). Drug combinations were considered as belonging to both classes. We used E/M CPT codes to determine the number of face to face clinical encounters. The number and specialties of unique providers seen were measured for the 12 month period following the index HbA1c test. We ascertained provider specialty on the basis of the provider specialty code. Cholesterol, creatinine, and HbA1c tests obtained on the same day (with or without fasting plasma glucose or urine microalbumin) were categorized as a bundled test. Web appendix 1 presents the CPT codes used to identify these studies.
Statistical analysis
We calculated frequencies and means for baseline characteristics and diabetes management stratified by testing frequency or baseline HbA1c. Comparisons were tested by χ2 test for categorical variables and t test for continuous variable. Time to repeat HbA1c testing was calculated, and the distribution was graphed, stratified by testing frequency.
We computed odds ratios and 95% confidence intervals by modeling logistic regression with the dependent variable as the odds of testing excessively and frequently (versus testing according to the guidelines). We also computed odds ratios and 95% confidence intervals for the odds of treatment intensification and deintensification (versus no change or drug class change only) of the two categories indicative of too many tests compared with guideline recommended frequency. Odds of treatment intensification were assessed among all patients in the cohort, while odds of treatment deintensification were assessed only among those patients receiving at least one diabetes drug at baseline. For all analyses, two sided P values less than 0.05 were considered significant. We used SAS software version 9.3 (SAS Institute) for all analyses.
Results
Study population
Table 1 describes the baseline characteristics of 31 545 patients included in the study. The cohort had a mean age of 58 years (standard deviation 11), and a mean index HbA1c of 6.2% (0.4); 61.5% of patients had low disease burden as judged by the Charlson index; and 83.7% received care from both primary care providers and medical subspecialists. Distribution of HbA1c results at the time of index testing showed that 10.4% of patients had HbA1c levels of at least 5.6%; 57.1% had HbA1c between 5.7% and 6.4%; and 32.5% had HbA1c between 6.5% and 6.9%. Furthermore, at the time of the index HbA1c test, 32.9% of patients did not receive any glucose lowering drugs, 37.7% were treated with one drug, 21.3% were treated with two drugs, and 8.2% were treated with three or more drugs.
Table 1 .
Baseline distribution of HbA1c testing frequencies in patients with stable and controlled type 2 diabetes. Data are no (%) of patients per testing frequency category
| HbA1c testing frequency | P | |||
|---|---|---|---|---|
| Recommended (≤2 per year) | Frequent (3-4 per year) | Excessive (≥5 per year) | ||
| Patients in each testing category (n=31 545; 100%) | 12 535 (39.7) | 17 182 (54.5) | 1828 (5.8) | — |
| Patient age (years) | ||||
| 18-44 (n=3561; 11.3%) | 1711(48.1) | 1684 (47.3) | 166 (4.7) | <0.001 |
| 45-54 (n=8130; 25.8%) | 3404 (41.9) | 4314 (53.1) | 412 (5.1) | |
| 55-64 (n=11 781; 37.4%) | 4502 (38.2) | 6586 (55.9) | 693 (5.9) | |
| 65-74 (n=4643; 14.7%) | 1660 (35.8) | 2666 (57.4) | 317 (6.8) | |
| ≥75 (n=3430; 10.9%) | 1258 (36.7) | 1932 (56.3) | 240 (7.0) | |
| Sex | ||||
| Male (n=16 060; 50.9%) | 6448 (40.2) | 8664 (53.9) | 948 (5.9) | 0.16 |
| Female (n=15 485; 49.1%) | 6087 (39.3) | 8518 (55.0) | 880 (5.7) | |
| Race or ethnic origin | ||||
| White (n=18 190; 57.7%) | 7242 (39.8) | 9919 (54.5) | 1029 (5.7) | <0.001 |
| Black (n=4757; 15.1%) | 2042 (42.9) | 2481 (52.2) | 234 (4.9) | |
| Hispanic (n=3482; 11.0%) | 1442 (41.4) | 1852 (53.2) | 188 (5.4) | |
| Asian (n=2243; 7.1%) | 834 (37.2) | 1280 (57.1) | 129 (5.8) | |
| Unknown (n=2873; 9.1%) | 975 (33.9) | 1650 (57.4) | 248 (8.6) | |
| US census region | ||||
| Midwest (n=3675; 11.7%) | 1545 (42.0) | 1985 (54.0) | 145 (4.0) | <0.001 |
| Northeast (n=7954; 25.2%) | 2782 (35.0) | 4467 (56.2) | 705 (8.9) | |
| South (n=17 481; 55.4%) | 7113 (40.7) | 9512 (54.4) | 856 (4.9) | |
| West (n=2433; 7.7%) | 1095 (45.0) | 1217 (50.0) | 121 (5.0) | |
| Unknown (n=2; 0.0%) | 0 | 1 (50.0) | 1 (50.0) | |
| Comorbidities | ||||
| Myocardial infarction (n=383; 1.2%) | 125 (32.6) | 227 (59.3) | 31 (8.1) | 0.01 |
| Heart failure (n=720; 2.3%) | 251 (34.9) | 403 (56.0) | 66 (9.2) | <0.001 |
| Kidney disease (n=655; 2.1%) | 211 (32.2) | 372 (56.8) | 72 (11.0) | <0.001 |
| Cancer (n=2073; 6.6%) | 728 (35.1) | 1176 (56.7) | 169 (8.2) | <0.001 |
| Charlson comorbidity index | ||||
| 0-1 (n=19 411; 61.5%) | 8316 (42.8) | 10225 (52.7) | 870 (4.5) | <0.001 |
| 2 (n=4814; 15.3%) | 1836 (38.1) | 2646 (55.0) | 332 (6.9) | |
| 3 (n=4316; 13.7%) | 1468 (34.0) | 2515 (58.3) | 333 (7.7) | |
| ≥4 (n=3004; 9.5%) | 915 (30.5) | 1796 (59.8) | 293 (9.8) | |
| Index HbA1c | ||||
| ≤5.6% (n=3283; 10.4%) | 1599 (48.7) | 1498 (45.6) | 186 (5.7) | <0.001 |
| 5.7-6.4% (n=18 016; 57.1%) | 7445 (41.3) | 9601 (53.3) | 970 (5.4) | |
| 6.5-6.9% (n=10 246; 32.5%) | 3491 (34.1) | 6083 (59.4) | 672 (6.6) | |
| Baseline treatment | ||||
| Lifestyle (n=10 370; 32.9%) | 4798 (46.3) | 5069 (48.9) | 503 (4.9) | <0.001 |
| 1 drug (n=11 883; 37.7%) | 4617 (38.9) | 6572 (55.3) | 694 (5.8) | |
| 2 drugs (n=6711; 21.3%) | 2383 (35.5) | 3920 (58.4) | 408 (6.1) | |
| ≥3 drugs (n=2581; 8.2%) | 737 (28.6) | 1621 (62.8) | 223 (8.6) | |
| Treatment change | ||||
| No change before or after; lifestyle (n=8976; 28.5%) | 4245 (47.3) | 4328 (48.2) | 403 (4.5) | <0.001 |
| No change before or after; treated with at least one drug (n=16 746; 53.1%) | 6246 (37.3) | 9546 (57.0) | 954 (5.7) | |
| Drug class change only (n=247; 0.8%) | 59 (23.9) | 158 (64.0) | 30 (12.2) | |
| Intensification (n=2644; 8.4%) | 900 (34.0) | 1515 (57.3) | 229 (8.7) | |
| Deintensification (n=2932; 9.3%) | 1085 (37.0) | 1635 (55.8) | 212 (7.2) | |
| Specialties seen* | ||||
| Primary care (n=30 331; 96.2%) | 12 062 (39.8) | 16 554 (54.6) | 1715 (5.7) | <0.001 |
| Endocrinology (n=4225; 13.4%) | 1044 (24.7) | 2626 (62.2) | 555 (13.1) | <0.001 |
| Cardiology (n=8237; 26.1%) | 2828 (34.3) | 4755 (57.7) | 654 (7.9) | <0.001 |
| Ophthalmology (n=5772; 18.3%) | 1967 (34.1) | 3396 (58.8) | 409 (7.1) | <0.001 |
| Gynecology (n=6601; 20.9%) | 2462 (37.3) | 3717 (56.3) | 422 (6.4) | <0.001 |
| Nephrology (n=1433; 4.5%) | 419 (29.2) | 838 (58.5) | 176 (12.3) | <0.001 |
| Laboratory studies obtained on the same day | ||||
| HbA1c with or without glucose (n=16 149; 51.2%) | 5866 (36.3) | 9115 (56.4) | 1168 (7.2) | <0.001 |
| Bundled tests (n=15 396; 48.8%)† | 6669 (43.3) | 8067 (52.4) | 660 (4.3) | |
| Coordination of care | ||||
| Primary care only (n=3927; 12.5%) | 1906 (48.5) | 1909 (48.6) | 112 (2.9) | <0.001 |
| Primary care and specialist care (n=26 404; 83.7%) | 10 156 (38.5) | 14 645 (55.5) | 1603 (6.1) | |
| Specialist care only (n=1109; 3.5%) | 436 (39.3) | 576 (51.9) | 97 (8.8) | |
| None (n=105; 0.3%) | 37 (35.2) | 52 (49.5) | 16 (15.2) | |
| Encounters per year | ||||
| Median (interquartile range) | 5.5 (3.5-9.0) | 7.5 (5.0-11.5) | 11.0 (7.5-16.0) | <0.001 |
| Range | 0.0-76.5 | 0.0-73.0 | 0.0-124.0 | |
| Year of index HbA1c test | ||||
| 2001-02 (n=776; 2.5%) | 220 (28.4) | 485 (62.5) | 71 (9.5) | <0.001 |
| 2003-04 (n=1827; 5.8%) | 750 (41.1) | 978 (53.5) | 99 (5.5) | |
| 2005-06 (n=2922; 9.3%) | 1258 (43.1) | 1498 (51.3) | 166 (5.7) | |
| 2007-08 (n=9203; 29.2%) | 3240 (35.2) | 5345 (58.1) | 618 (6.8) | |
| 2009-10 (n=12 648; 40.1%) | 5261 (41.6) | 6697 (53.0) | 690 (5.5) | |
| 2011 (n=4169; 13.2%) | 1806 (43.3) | 2179 (52.3) | 184 (4.4) | |
*Each patient could see different specialties of healthcare providers, and could be included in one or more categories; percentages therefore add up to more than 100%.
†Bundled tests include lipid profile (n=10 668; 33.8%) and serum creatinine with or without urine microalbumin (n=4728; 15.0%).
Testing frequency
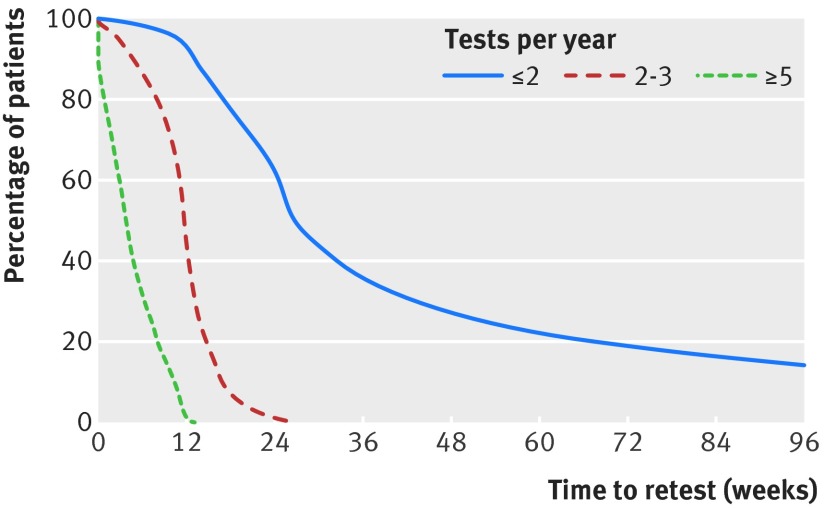
Most patients received frequent (54.5%) or excessive (5.8%) HbA1c testing (table 1). The figure shows the median time to retesting following the index HbA1c test in each group. These times were four weeks, 12 weeks, and 27 weeks for patients receiving excessive, frequent, and guideline recommended testing, respectively. Patients receiving too many tests (for example, excessive and frequent testing frequencies) were older, had more comorbid conditions, were taking more diabetes drugs, and had higher index HbA1c (all P<0.001; table 1). There was a small amount of variability in testing frequency as a function of race, with a lower proportion of black (4.9%) and Hispanic (5.4%) patients undergoing excessive testing than white (5.7%) and Asian (5.8%) patients (P<0.001). There was significant geographical variability in excessive testing, with the highest prevalence in the northeast US census region (8.9%) and the lowest prevalence in the midwest region (4.0%).
Median times to HbA1c retesting in study population following an index test that was at glycemic goal (that is, HbA1c<7.0%)
In the multivariable analysis, the odds of frequent and excessive testing as compared with guideline recommended testing increased with patient complexity. This increase was reflected by the Charlson comorbidity index (≥4), treatment (≥three diabetes drugs), index HbA1c (6.5-6.9%), and involvement of an endocrinologist or nephrologist in the patient’s care (table 2). There was also direct correlation between the number of discrete healthcare providers seen by the patient annually and the likelihood of excessive and frequent testing; each additional provider increased the odds of being tested (odds ratio 1.14 (95% confidence interval 1.10 to 1.18) and 1.05 (1.04 to 1.07)) for receiving excessive and frequent testing compared with guidance recommended testing. Patients living in the northeast US census region had significantly higher odds of undergoing excessive testing than those living in the south region (1.60 (1.44 to 1.78)), while patients living in the midwest region were significantly less likely (0.67 (0.58 to 0.79)). Finally, use of bundled testing decreased the odds of excessive testing compared with use of glycemic tests alone (0.82 (0.77 to 0.88)).
Table 2 .
Correlates of increased frequency of HbA1c testing
| Excessive HbA1c testing | Frequent HbA1c testing | ||||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | ||
| Patient age (years) | |||||
| 18-44 | Ref | Ref | Ref | Ref | |
| 45-54 | 0.94 (0.85 to 1.05) | 0.29 | 0.97 (0.93 to 1.02) | 0.23 | |
| 55-64 | 1.08 (0.98, 1.19) | 0.10 | 1.07 (1.03 to 1.12) | <0.001 | |
| 65-74 | 1.17 (1.03 to 1.32) | 0.01 | 1.12 (1.06 to 1.19) | <0.001 | |
| ≥75 | 1.10 (0.95 to 1.27) | 0.21 | 1.07 (1.00 to 1.15) | 0.05 | |
| Sex | |||||
| Male | Ref | Ref | Ref | Ref | |
| Female | 0.95 (0.89 to 1.02) | 0.13 | 0.98 (0.96 to 1.01) | 0.22 | |
| Race | |||||
| White | Ref | Ref | Ref | Ref | |
| Non-white | 0.99 (0.93 to 1.04) | 0.64 | 0.99 (0.97 to 1.01) | 0.40 | |
| US census region | |||||
| South | Ref | Ref | Ref | Ref | |
| Midwest | 0.67 (0.58 to 0.79) | <0.001 | 0.95 (0.90 to 1.01) | 0.12 | |
| Northeast | 1.60 (1.44 to 1.78) | <0.001 | 1.14 (1.08 to 1.19) | <0.001 | |
| West | 1.01 (0.86 to 1.18) | 0.93 | 0.90 (0.84 to 0.97) | 0.004 | |
| Year of index HbA1c test | |||||
| 2001-02 | Ref | Ref | Ref | Ref | |
| 2003-04 | 0.95 (0.78 to 1.16) | 0.61 | 0.93 (0.85 to 1.01) | 0.09 | |
| 2005-06 | 0.97 (0.83 to 1.14) | 0.73 | 0.86 (0.80 to 0.92) | <0.001 | |
| 2007-08 | 1.11 (0.99 to 1.24) | 0.08 | 1.10 (1.05 to 1.16) | <0.001 | |
| 2009-10 | 0.74 (0.67 to 0.83) | <0.001 | 0.85 (0.80 to 0.89) | <0.001 | |
| 2011 | 0.54 (0.46 to 0.64) | <0.001 | 0.77 (0.72 to 0.83) | <0.001 | |
| Index HbA1c | |||||
| ≤5.6% | Ref | Ref | Ref | Ref | |
| 5.7-6.4% | 0.95 (0.88 to 1.03) | 0.19 | 1.02 (0.99 to 1.06) | 0.22 | |
| 6.5-6.9% | 1.29 (1.18 to 1.41) | <0.001 | 1.31 (1.26 to 1.37) | <0.001 | |
| Charlson comorbidity index | |||||
| 0-1 | Ref | Ref | Ref | Ref | |
| 2 | 0.97 (0.87 to 1.09) | 0.65 | 0.98 (0.93 to 1.03) | 0.43 | |
| 3 | 0.99 (0.88 to 1.10) | 0.82 | 1.03 (0.98 to 1.09) | 0.23 | |
| ≥4 | 1.44 (1.29 to 1.61) | <0.001 | 1.15 (1.08 to 1.22) | <0.001 | |
| Laboratory studies obtained on the same day | |||||
| HbA1c with or without glucose | Ref | Ref | Ref | Ref | |
| Bundled tests | 0.82 (0.77 to 0.88) | <0.001 | 0.93 (0.90 to 0.95) | <0.001 | |
| Baseline treatment | |||||
| Lifestyle | Ref | Ref | Ref | Ref | |
| 1 drug | 0.95 (0.87 to 1.03) | 0.21 | 0.95 (0.92 to 0.99) | 0.02 | |
| 2 drugs | 1.05 (0.95 to 1.16) | 0.38 | 1.08 (1.03 to 1.14) | <0.001 | |
| ≥3 drugs | 1.61 (1.41 to 1.85) | <0.001 | 1.39 (1.29 to 1.49) | <0.001 | |
| Treatment change | |||||
| No change | Ref | Ref | Ref | Ref | |
| Deintensification | 0.78 (0.65 to 0.93) | 0.004 | 0.80 (0.73 to 0.88) | <0.001 | |
| Intensification | 1.24 (1.03 to 1.49) | 0.02 | 1.09 (0.98 to 1.20) | 0.12 | |
| Drug class change only | 1.53 (1.04 to 2.25) | 0.03 | 1.32 (1.05 to 1.67) | 0.02 | |
| No of healthcare providers per year | 1.14 (1.10 to 1.18) | <0.001 | 1.05 (1.04 to 1.07) | <0.001 | |
| Coordination of care | |||||
| Primary care only | Ref | Ref | Ref | Ref | |
| Primary care and specialist care | 0.73 (0.60 to 0.87) | <0.001 | 1.02 (0.91 to 1.15) | 0.72 | |
| Specialist care only | 0.70 (0.55 to 0.90) | 0.006 | 0.77 (0.67 to 0.90) | <0.001 | |
| None | 3.24 (2.01 to 5.24) | <0.001 | 1.31 (0.94 to 1.81) | 0.11 | |
| Specialties seen | |||||
| Endocrinology | 1.87 (1.75 to 2.00) | <0.001 | 1.34 (1.29 to 1.40) | <0.001 | |
| Cardiology | 1.04 (0.98 to 1.11) | 0.19 | 1.03 (1.00 to 1.06) | 0.06 | |
| Ophthalmology | 1.06 (0.99 to 1.14) | 0.09 | 1.07 (1.03 to 1.10) | <0.001 | |
| Gynecology | 1.03 (0.95 to 1.12) | 0.41 | 1.05 (1.02 to 1.09) | 0.006 | |
| Nephrology | 1.36 (1.23 to 1.52) | <0.001 | 1.09 (1.02 to 1.16) | 0.008 | |
Data are odds ratios and 95% confidence intervals (CI) of excessive and frequent testing, versus guideline recommended testing.
Ref=reference group.
Treatment changes in response to testing
Most patients (81.6%) in the study did not have their treatment altered after the index HbA1c test (table 1). However, despite meeting recommended targets for glycemic control as per study inclusion criteria, treatment was further intensified by addition of glucose lowering drugs or insulin in 13% (n=229), 9% (n=1515), and 7% (n=900) of patients tested excessively, frequently, and per the guidelines, respectively (P<0.001; table 1).
We specifically examined the rates of excessive testing among patients as a function of treatment change at the time of index HbA1c testing (table 1). Of the 31 545 patients included in the study, 8976 (28.5%) did not receive any glucose lowering drugs before or after the index HbA1c test. Yet of these patients, 403 (4.5%) were still tested excessively in the following two years. An additional 16 746 (53.1%) patients of the study population were treated with the same non-insulin drugs before and after testing; of these patients, 954 (5.7%) continued to be tested excessively. Of 2644 (8.4%) patients whose regimens intensified after the index test, 229 (8.7%) were tested excessively. Finally, of 2932 (9.3%) patients whose treatments deintensified, 212 (7.2%) were tested excessively.
Compared with testing rates among patients who remained on the same treatment regimen after the index HbA1c test, excessive testing was significantly less likely among patients whose treatments deintensified (odds ratio 0.78 (95% confidence interval 0.65 to 0.93)). On the other hand, excessive testing was significantly more likely among patients who switched drug classes (1.53 (1.04 to 2.25)) or whose treatments intensified (1.24 (1.03 to 1.49); table 2).
Table 3 presents the correlates of treatment intensification and deintensification. Patients who were tested excessively were significantly more likely to have treatment intensified after the index HbA1c test (odds ratio 1.35 (95% confidence interval 1.22 to 1.50)) but not deintensified (1.08 (0.97 to 1.20)). Older age was associated with decreased odds of both treatment intensification and deintensification, while comorbidity burden was not associated with either. Additional predictors of treatment intensification included care by an endocrinologist (1.27 (1.20 to 1.34)), and HbA1c levels of 6.5-6.9% versus 5.6% or under (1.72 (1.61 to 1.85)).
Table 3 .
Correlates of treatment regimen change after index HbA1c testing
| Treatment intensification | Treatment deintensification | ||||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | ||
| Patient age (years) | |||||
| 18-44 | Ref | Ref | Ref | Ref | |
| 45-54 | 1.13 (1.04 to 1.23) | 0.003 | 1.08 (1.00 to 1.17) | 0.04 | |
| 55-64 | 1.06 (0.99 to 1.14) | 0.10 | 0.93 (0.87 to 1.00) | 0.04 | |
| 65-74 | 0.92 (0.83 to 1.02) | 0.10 | 0.92 (0.84 to 1.02) | 0.11 | |
| ≥75 | 0.70 (0.62 to 0.80) | <0.001 | 0.84 (0.74 to 0.95) | 0.005 | |
| Sex | |||||
| Male | Ref | Ref | Ref | Ref | |
| Female | 1.06 (1.01 to 1.11) | 0.03 | 1.06 (1.01 to 1.11) | 0.02 | |
| Race | |||||
| White | Ref | Ref | Ref | Ref | |
| Non-white | 1.00 (0.95 to 1.04) | 0.82 | 1.07 (1.03 to 1.11) | 0.002 | |
| US census region | |||||
| South | Ref | Ref | Ref | Ref | |
| Midwest | 0.79 (0.70 to 0.88) | <0.001 | 0.93 (0.83 to 1.04) | 0.18 | |
| Northeast | 0.90 (0.83 to 0.98) | 0.01 | 0.96 (0.88 to 1.04) | 0.34 | |
| West | 1.28 (1.14 to 1.44) | <0.001 | 1.09 (0.97 to 1.21) | 0.18 | |
| Year of index HbA1c test | |||||
| 2001-02 | Ref | Ref | Ref | Ref | |
| 2003-04 | 0.90 (0.77 to 1.05) | 0.18 | 0.89 (0.75 to 1.05) | 0.15 | |
| 2005-06 | 1.07 (0.95 to 1.20) | 0.28 | 1.02 (0.89 to 1.17) | 0.75 | |
| 2007-08 | 0.97 (0.89 to 1.05) | 0.40 | 1.04 (0.94 to 1.15) | 0.45 | |
| 2009-10 | 0.80 (0.74 to 0.87) | <0.001 | 1.15 (1.04 to 1.27) | 0.007 | |
| 2011 | 0.79 (0.70 to 0.89) | <0.001 | 1.26 (1.11 to 1.42) | <0.001 | |
| Index HbA1c | |||||
| ≤5.6% | Ref | Ref | Ref | Ref | |
| 5.7-6.4% | 0.88 (0.82 to 0.93) | <0.001 | 0.96 (0.91 to 1.02) | 0.21 | |
| 6.5-6.9% | 1.72 (1.61 to 1.85) | <0.001 | 0.79 (0.74 to 0.84) | <0.001 | |
| Charlson comorbidity index | |||||
| 0-1 | Ref | Ref | Ref | Ref | |
| 2 | 1.02 (0.94 to 1.12) | 0.59 | 0.97 (0.89 to 1.07) | 0.56 | |
| 3 | 1.01 (0.92 to 1.11) | 0.85 | 1.00 (0.91 to 1.09) | 0.92 | |
| ≥4 | 1.04 (0.94 to 1.15) | 0.42 | 1.07 (0.97 to 1.18) | 0.15 | |
| Frequency of HbA1c testing | |||||
| Recommended (≤2 tests/year) | Ref | Ref | Ref | Ref | |
| Frequent (3-4 tests/year) | 0.96 (0.90 to 1.03) | 0.23 | 0.92 (0.86 to 0.98) | 0.009 | |
| Excessive (≥5 tests/year) | 1.35 (1.22 to 1.50) | <0.001 | 1.08 (0.97 to 1.20) | 0.18 | |
| No of healthcare providers per year | 1.01 (0.98 to 1.04) | 0.51 | 1.05 (1.02 to 1.09) | <0.001 | |
| Baseline treatment | |||||
| Lifestyle | Ref | Ref | — | — | |
| 1 drug | 0.80 (0.74 to 0.86) | <0.001 | Ref | Ref | |
| 2 drugs | 0.79 (0.72 to 0.87) | <0.001 | 0.84 (0.79 to 0.89) | <0.001 | |
| ≥3 drugs | 0.80 (0.70 to 0.92) | 0.001 | 2.30 (2.15 to 2.45) | <0.001 | |
| Specialties involved | |||||
| Endocrinology | 1.27 (1.20 to 1.34) | <0.001 | 1.05 (0.99 to 1.11) | 0.09 | |
| Cardiology | 1.01 (0.96 to 1.06) | 0.68 | 0.96 (0.91 to 1.01) | 0.11 | |
| Ophthalmology | 1.01 (0.95 to 1.06) | 0.80 | 0.95 (0.90 to 1.00) | 0.07 | |
| Gynecology | 0.94 (0.89 to 1.00) | 0.06 | 1.01 (0.95 to 1.08) | 0.68 | |
| Nephrology | 1.09 (0.99 to 1.20) | 0.09 | 1.10 (1.01 to 1.20) | 0.04 | |
Odds ratio compares the odds of treatment intensification or deintensification after index HbA1c test with either no treatment change or drug class change only. The intensification group includes all patients, while the deintensification group includes only those patients receiving at least one glucose lowering drug at baseline.
CI=confidence interval; Ref=reference group.
Temporal trends
Testing frequency varied over the course of the study, and the prevalence of both excessive and frequent testing decreased significantly after 2009 (tables 1 and 2). Excessive testing rates were unchanged in 2003-08 compared with 2001-02 (all P>0.05), but excessive testing was less likely in 2009-10 (odds ratio 0.74 (95% confidence interval 0.67 to 0.83)) and 2011 (0.54 (0.46 to 0.63); table 2). Trends for treatment intensification were similar, with no significant change in 2003-08 compared with 2001-02, but showed a reduction after 2009. Compared with 2001-02, the odds of treatment intensification were 0.80 (95% confidence interval 0.74 to 0.87) in 2009-10 and 0.79 (0.70 to 0.89) in 2011 (table 3). We saw no significant change over time in the likelihood of treatment deintensification.
Discussion
Principal findings
In this study, we looked at a national cohort of 31 545 adults with type 2 diabetes in the USA who had achieved and maintained stable glycemic control with HbA1c less than 7.0%, had no use of insulin, and had no apparent indications for intensive monitoring or treatment. In this cohort, more than 60% of patients received too many HbA1c tests despite current guidance recommendations of one or two tests per year.4 5 6 7 8 9 10 In total, we found that 5.8% of patients had five or more tests over one year, and 54.5% had three or four tests over one year. We also identified a direct association between excessive testing and likelihood of treatment intensification that, in the context of HbA1c being already less than 7%, is concerning for overtreatment. In 2008, the US National Quality Forum designated unnecessary laboratory tests to be one of nine areas of wasteful or inappropriate care,22 potentially explaining the recent improvements in excessive testing rates in our patient population seen after 2009.
Strengths and limitations of the study
To our knowledge, this is the largest national study of glycemic overtesting among clinically stable and controlled people with diabetes, and includes commercially insured adults of all ages from across the USA. While the study population is not representative of all patients with diabetes, it was specifically chosen to reflect clinical situations where the frequency of guideline recommended HbA1c testing should suffice. It is likely that the prevalence of overtesting would have been even greater had all patients with diabetes been included, as in previous studies. Finally, the study population is representative of commercially insured adults in the USA, but not of the entire US population, because it does not include those insured by government payers.
This study focused on patients who had at least one HbA1c test performed during the study period. Although we could assess clinical overuse as a result, we could not capture the prevalence of underuse. Study design was also based on the assumption that frequent monitoring was not needed for patients with non-insulin treated type 2 diabetes who have stable HbA1c within the target range, are not pregnant, and have no documented hypoglycemia or hyperglycemia. It is possible that such patients experienced deterioration of glycemic control during the study, warranting an increase in testing frequency. However, testing frequency in routine clinical practice is determined prospectively, using population level data to infer the likelihood of glycemic deterioration or new instances of hypoglycemic and hyperglycemic acute diabetes complications. Our study was restricted to patients whose histories did not warrant frequent testing, so that their repeat HbA1c test should have been scheduled six to 12 months after the index test, rather than one or three months after—as we observed for patients in the excessive and frequent testing groups, respectively. Finally, self-reported hypoglycemia—which is known to impair quality of life23 and increase risk of mortality,24 or glycemic variability on glucose self-monitoring—could have prompted glycemic testing but remain unseen in our data sources. However, because self-reporting of hypoglycemia24 and glycemic variability do not always correlate with HbA1c, additional testing in these situations might not be helpful.
In view of these limitations, this study is the largest and (by virtue of strict inclusion and exclusion criteria to only include individuals at the lowest risk) most rigorous of glycemic overtesting among US patients so far. This was made possible by the large number of patients and clinical data available in the OLDW dataset, and is therefore generalizable to other commercially insured adults in the USA.
Comparison with other studies
Our findings confirm observations from previous studies that detected redundant HbA1c testing; however, none of these studies explicitly differentiated between patients who needed close monitoring and those who did not.5 11 25 26 27 The geographical variability of excessive HbA1c testing also parallels other areas of healthcare overuse,28 suggesting non-clinical and patient unrelated factors driving such laboratory overuse. This observation warrants closer examination of institutional and individual clinical practices that might promote frequent testing.29 Further investigation should also look at any similar overtesting trends in other disciplines and clinical circumstances, reinforcing the culture of test reliance and high healthcare use.
Conclusions and policy implications
Excessive HbA1c testing is ineffective and inefficient, and could contribute to the growing problem of waste in healthcare and increasing patient burden in the management of type 2 diabetes.25 30 Our study did not directly quantify the excess costs incurred by overtesting, which would include the direct cost of HbA1c tests as well as the indirect costs of phlebotomy and laboratory services, patient time, and burden of completing testing (especially when bundled with a fasting lipid panel). Because HbA1c is a measure of average glycemia over three months, more frequent monitoring is also not representative of steady state glycemic control and is generally not clinically informative.
There are several potential reasons for frequent testing. These include clinical uncertainty; misunderstanding of the nature of the test (for example, the fact that HbA1c represents three months of glycemic control, on average); desire for diagnostic and management thoroughness; fragmentation of care; and the need to fulfill patient, institution, and regulatory demands.31 32 Inefficiency can result from inadequate care coordination between different healthcare providers involved in a patient’s care.17 This inefficiency was evident in our study, because the odds of redundant testing increased with the number of doctors seen per year. Some specialists, particularly endocrinologists, could be accustomed to seeing high risk patients who need frequent monitoring, and extend those practices to patients who might be at lower risk. Public reporting of diabetes performance measures and performance based reimbursement could also drive repeat testing, although this was less likely to influence testing frequency in our study where all patients had already satisfied the reporting criterion of HbA1c levels being less than 7.0%.
Clinically unnecessary testing can have detrimental effects for both the patient and the healthcare system. Excessive tests can cause unnecessary patient discomfort and anxiety, and because of the potential for false positive results caused by expected short term biological and analytical variability of the HbA1c test,33 they can increase the risk of further needless testing, specialist referral, and treatment change.34 Availability of a laboratory result could compel a doctor and patient to act, even if not clinically indicated or beneficial. We saw this in our study population, in which excessive testing was associated with an increased likelihood of treatment intensification despite normal levels of HbA1c.
We found that patients over age 65 years and those with high underlying disease burden were more likely to have excessive HbA1c testing. Healthcare providers could have decided that such patients needed to be monitored more closely, yet all these patients had stable HbA1c levels of less than 7.0%, with no insulin use or documented hypoglycemia or hyperglycemia. Moreover, current guidelines recommend that patients with significant comorbidities should in fact be treated less intensely and have relaxed HbA1c targets above 7.0%,4 5 6 7 8 9 10 making frequent testing in this population even less useful. We were reassured to find that patients aged 75 years and older were less likely to have intensified treatment after the index HbA1c test than those younger than 45 years. But these older patients were also less likely to have deintensified treatment after the index HbA1c test, suggesting a propensity to maintain the status quo (for example, clinical inertia) in this tightly controlled population.
The association between overtesting and potential overtreatment is concerning, particularly considering clinical trial data linking intensive glucose lowing treatment to adverse health outcomes, including hypoglycemia, cardiovascular events, and mortality.35 36 37 38 39 Because all study patients had index HbA1c levels lower than 7.0% at study entry, none was eligible for treatment intensification, and some were eligible for treatment deintensification (particularly if they are overwhelmed by treatment burden or hypoglycemia). Thus, all treatment intensification observed during our study was not warranted and is, in essence, overtreatment. The implications of such overtreatment needs further work to identify whether it results in direct patient harm, specifically hypoglycemia and other adverse drug reactions, in addition to burden of polypharmacy and increased healthcare use. The reasons for overtreatment, particularly treatment intensification, should be further investigated through qualitative analysis of patient, provider, and system factors leading to overtesting, overtreatment, and increased healthcare use.
In 2012, the American Board of Internal Medicine Foundation launched the Choosing Wisely campaign, aimed at reducing healthcare waste from low value, unnecessary, or redundant tests and procedures.40 Although HbA1c testing was not included among the “five things physicians and patients should question,” doctors were cautioned against excessive frequency of self-glucose monitoring in adults with stable type 2 diabetes.40 We argue that the same caution should be applied to HbA1c testing and other routine chronic disease management tests. Ultimately, patients and doctors should question the value of routine tests and test bundles, increasingly built as defaults within protocols and algorithms to improve compliance with quality metrics and performance in those metrics. Doing so could reduce the waste associated with marginally informative and otherwise excessive testing, and might also mitigate the practice of responding to small variations in test results with equally unwarranted and excessive treatment changes. Unnecessary testing is not only wasteful, but also potentially harmful to patients.
What is already known on this topic
Professional societies and regulatory bodies recommend that glycated hemoglobin (HbA1c) is checked once or twice a year in non-pregnant adults with type 2 diabetes who have achieved and maintained glycemic control without use of insulin and without recent acute diabetes complications
Several studies have suggested a high prevalence of repeat HbA1c testing, contributing to redundancy and waste in healthcare
These studies were relatively small, did not differentiate among patients needing different degrees of glycemic monitoring and control, and did not assess the effect of excessive testing on treatment
What this study adds
In a national US cohort of 31 545 non-pregnant adults with type 2 diabetes, who did not use insulin and who achieved and maintained HbA1c levels of less than 7.0%, 6% of patients had five or more HbA1c tests over one year, and 55% had three or four tests over one year
Excessive testing increased the odds of diabetes treatment intensification despite normal HbA1c levels (odds ratio 1.35 (95% confidence interval 1.22 to 1.50)), compared with guideline recommended testing
Excessive testing is ineffective and inefficient, contributing to the growing problem of waste in healthcare and increasing patient burden in the management of type 2 diabetes
Contributors: RGM and NDS conceived and designed the study. HKVH carried out data collection and statistical analysis, with input from RGM and NDS. RGM wrote the manuscript, and all authors (NDS, HKVH, JSR, and VMM) contributed to manuscript critical appraisal and review. RGM and NDS are the guarantors of this study. All authors had full access to all of the data, including statistical reports and tables, and take responsibility for the integrity of the data and the accuracy of data analysis. All authors reviewed and agreed on the final version of the manuscript.
Funding: NDS is funded partly by the Agency for Healthcare Research and Quality (R18HS18339). RGM was supported partly by the AcademyHealth Delivery System Science Fellowship (2013). The funders had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the Agency for Healthcare Research and Quality and AcademyHealth for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Study data were statistically deidentified and accessed according to the Health Insurance Portability and Accountability Act 164.514 privacy rule. The Mayo Clinic Institutional Review Board exempted this study from approval as it represents research on pre-existing, deidentified data. Participant consent was not obtained because all presented data were anonymized prior to dataset creation and risk of identification is low.
Data sharing statement: Technical appendix, statistical code, and dataset are available from the corresponding author at mccoy.rozalina@mayo.edu.
The lead author and the manuscript’s guarantor (RGM) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Cite this as: BMJ 2015;351:h6138
Web Extra. Extra material supplied by the author
Web appendix: Supplemental materials
References
- 1.Song Z, Safran DG, Landon BE, et al. Health care spending and quality in year 1 of the alternative quality contract. N Engl J Med 2011;365:909-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Medicare and Medicaid Services Physician Quality Reporting System: Registry Reporting. Registry reporting. 2015. www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS/Registry-Reporting.html.
- 3.National Committee for Quality Assurance. HEDIS 2009 volume 2 technical update. www.ncqa.org/portals/0/PolicyUpdates/HEDIS%20Technical%20Updates/09_CDC_Spec.pdf.
- 4.International Diabetes Federation clinical guidelines task force. Global guidelines for type 2 diabetes, 2012. https://www.idf.org/sites/default/files/IDF%20T2DM%20Guideline.pdf.
- 5.Reithof M, Flavin PL, Lindvall B, et al. Institute for Clinical Systems Improvement. Diagnosis and management of type 2 diabetes mellitus in adults. 2012. http://bit.ly/Diabetes0412.
- 6.American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care 2015;38(suppl 1). [PubMed]
- 7.Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE/ACE comprehensive diabetes management algorithm 2015. Endocr Pract 2015;21:438-47. [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Health and Care Excellence. Type 2 diabetes: the management of type 2 diabetes. 2009. https://www.nice.org.uk/guidance/cg87/chapter/guidance.
- 9.US Department of Veteran Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of diabetes mellitus. Department of Veteran Affairs, Department of Defense, 2010. www.healthquality.va.gov/guidelines/CD/diabetes/DM2010_FUL-v4e.pdf.
- 10.Berard LD, Blumer I, Houlden R, Miller D, Woo V. Monitoring glycemic control. Can J Diabetes 2013;37 (suppl 1):S35-9. [DOI] [PubMed]
- 11.Laxmisan A, Vaughan-Sarrazin M, Cram P. Repeated hemoglobin A1C ordering in the VA health system. Am J Med 2011;124:342-9. [DOI] [PubMed] [Google Scholar]
- 12.Pivovarov R, Albers DJ, Hripcsak G, Sepulveda JL, Elhadad N. Temporal trends of hemoglobin A1c testing. J Am Med Inform Assoc 2014;21:1038-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Driskell OJ, Holland D, Hanna FW, et al. Inappropriate requesting of glycated hemoglobin (Hb A1c) is widespread: assessment of prevalence, impact of national guidance, and practice-to-practice variability. Clin Chem 2012;58:906-15. [DOI] [PubMed] [Google Scholar]
- 14.Akan P, Cimrin D, Ormen M, et al. The inappropriate use of HbA1c testing to monitor glycemia: is there evidence in laboratory data? J Eval Clin Pract 2007;13:21-4. [DOI] [PubMed] [Google Scholar]
- 15.Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH. Optum Labs: building a novel node in the learning health care system. Health Aff (Millwood) 2014;33:1187-94. [DOI] [PubMed] [Google Scholar]
- 16.Optum. Optum research data assets. 2014. https://www.optum.com/content/dam/optum/resources/productSheets/5302_Data_Assets_Chart_Sheet_ISPOR.pdf.
- 17.Romano MJ, Segal JB, Pollack CE. The association between continuity of care and the overuse of medical procedures. JAMA Intern Med 2015;175:1148-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ginde AA, Blanc PG, Lieberman RM, Camargo CA Jr. Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord 2008;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245-51. [DOI] [PubMed] [Google Scholar]
- 20.McEwen LN, Kim C, Karter AJ, et al. Risk factors for mortality among patients with diabetes: the Translating Research Into Action for Diabetes (TRIAD) Study. Diabetes Care 2007;30:1736-41. [DOI] [PubMed] [Google Scholar]
- 21.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-9. [DOI] [PubMed] [Google Scholar]
- 22.National Priorities Partnership. National Priorities and Goals: aligning our efforts to transform America’s healthcare. 2008. https://www.qualityforum.org/Setting_Priorities/NPP/National_Priorities_Partnership_Goals.aspx.
- 23.McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Self-report of hypoglycemia and health-related quality of life in patients with type 1 and type 2 diabetes. Endocr Pract 2013:1-28. [DOI] [PubMed]
- 24.McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 2012;35:1897-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Institute of Medicine, Committee on Quality of Health Care in America. Crossing the quality chasm: a new health system for the 21st century. National Academy Press, 2001.
- 26.Lewis S, Foreman J. Low-cost diagnostic technologies and clinical outcomes. The impact of inappropriate utilization. Int J Technol Assess Health Care 1997;13:501-11. [DOI] [PubMed] [Google Scholar]
- 27.Janssens PM. Managing the demand for laboratory testing: options and opportunities. Clin Chim Acta 2010;411:1596-602. [DOI] [PubMed] [Google Scholar]
- 28.Yasaitis LC, Bubolz T, Skinner JS, Chandra A. Local population characteristics and hemoglobin a1c testing rates among diabetic Medicare beneficiaries. PLoS One 2014;9:e111119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colla CH, Sequist TD, Rosenthal MB, Schpero WL, Gottlieb DJ, Morden NE. Use of non-indicated cardiac testing in low-risk patients: choosing wisely. BMJ Qual Saf 2014;24:149-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bentley TG, Effros RM, Palar K, Keeler EB. Waste in the U.S. Health care system: a conceptual framework. Milbank Q 2008;86:629-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Axt-Adam P, van der Wouden JC, van der Does E. Influencing behavior of physicians ordering laboratory tests: a literature study. Medical care 1993;31:784-94. [DOI] [PubMed] [Google Scholar]
- 32.Alonso-Cerezo MC, Martin JS, Garcia Montes MA, de la Iglesia VM. Appropriate utilization of clinical laboratory tests. Clin Chem Lab Med 2009;47:1461-5. [DOI] [PubMed] [Google Scholar]
- 33.Glasziou PP, Irwig L, Heritier S, Simes RJ, Tonkin A. Monitoring cholesterol levels: measurement error or true change? Ann Intern Med 2008;148:656-61. [DOI] [PubMed] [Google Scholar]
- 34.Rang M. The Ulysses syndrome. Can Med Assoc J 1972;106:122-3. [PMC free article] [PubMed] [Google Scholar]
- 35.Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376:419-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52:2288-98. [DOI] [PubMed] [Google Scholar]
- 37.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010;340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129-39. [DOI] [PubMed] [Google Scholar]
- 39.Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410-8. [DOI] [PubMed] [Google Scholar]
- 40.Choosing wisely: an initiative of the ABIM Foundation. 2014. www.choosingwisely.org/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplemental materials