Figure 2. Hypothetic scheme of Fus1 functioning in mitochondria.
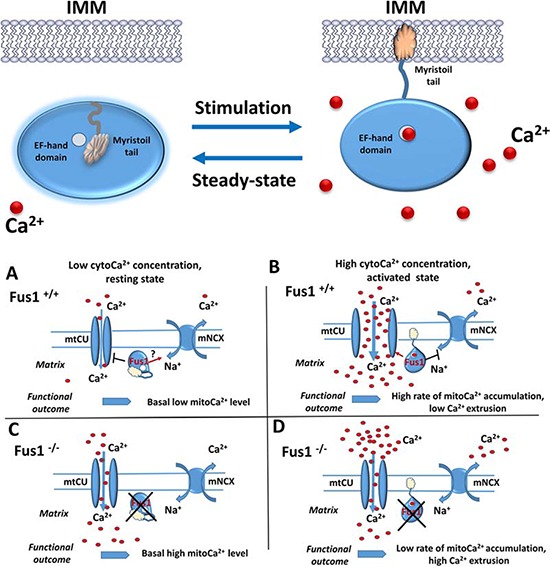
Upper panel. Putative mechanism of molecular organization and activity of Fus1. At Ca2+- free or low Ca2+ conditions (steady-state), EF-hand domain (open circle) of Fus1 protein is not occupied by Ca2+ ions while its myristoyl tail is folded inside the hydrophobic pocket of the protein preventing Fus1 from anchoring the membranes (left). Upon stimulation, elevation of Ca2+ in cytosol (red circles) results in Ca2+ ion binding to the EF-hand domain, release of myristoyl tail and anchoring of Fus1 protein to IMM. Lower panel. A. At Ca2+-free or low Ca2+ conditions, Fus1 may inhibit mtCU while simultaneously maintain the mNCX active state thus preventing accumulating Ca2+ in mitochondria. B. Elevation of Ca2+ leads to Fus1 activation and anchoring in IMM. Membrane tethering allows Fus1 to activate mtCU and inhibit mNCX that leads to retention of Ca2+ in mitochondrial matrix. C. When Fus1-mediated gatekeeping function is lost at a steady state, the mtCU channel is not tightly closed allowing cytosolic Ca2+ uptake while mNCX does not return Ca2+ ions back to cytosol. Thus, Ca2+ concentration in mitochondria of Fus1-deficient cells is higher. D. When Fus1-mediated gatekeeping function is absent during activation, the mtCU channel does not open to a full extent leading to insufficient buffering of cytosolic Ca2+ signals. Additionally, mNCX is over-activated resulting in extrusion of Ca2+ from mitochondria and an overall slow rate of mitochondrial Ca2+ accumulation.
