Abstract
Objectives:
To evaluate the efficacy of orally-administered alendronate compared with intravenously-administered zoledronate.
Methods:
This prospective study was carried out at Barts Health HNS Trust between April 2010 and March 2012. This study compares changes in bone mineral density (BMD) in 234 patients treated with 2 bisphosphonates: alendronate taken orally, and zoledronate administered intravenously. One hundred and eighteen patients received alendronate at 70 mg/week, while 116 patients received zoledronate once annually. Dual energy x-ray absorptiometry was used to measure BMD of the left hip and anterior-posterior spine (lumbar L1-L4) skeletal sites at baseline, and at one-, and 2-years post-treatment.
Results:
This study provides evidence that lumbar spine BMD increased by 3.6% in patients receiving alendronate, and 5.7% in patients receiving zoledronate after 2 years compared with baseline values (p=0.0001 for both). Total hip BMD decreased in patients treated with alendronate by 0.4% but increased in patients receiving zoledronate by 0.8% (p=0.0001).
Conclusion:
This study provides evidence that zoledronate is more effective than alendronate in treating patients with osteoporosis and with no gastrointestinal (GI) serious side effects. Furthermore, zoledronate appears to have the added advantage of a better safety profile in patients suffering from GI intolerance of oral bisphosphonates.
Osteoporosis is a disease characterized by low bone mass and micro-architectural deterioration of associated tissue.1,2 It is estimated that over 200 million people worldwide have the disease,3 and suffer clinical consequences, including increased incidence of bone fractures, morbidity, and premature mortality.2,3 Approximately 30% of all post-menopausal women suffer from osteoporosis.3 The importance of developing treatment strategies that reduce the risk of fracture is therefore, evident both from individual and societal perspectives. Treatment with nitrogen-containing bisphosphonates, a class of potent therapeutic agents that inhibit osteoclast-mediated bone resorption, is one of the major pharmacological interventions for osteoporosis.4-8 The primary therapeutic goal of treatment with bisphosphonates is to reduce fracture risks.9 In placebo-controlled clinical trials, the efficacy of bisphosphonates in improving bone mineral density (BMD) at key skeletal sites, particularly at the lumbar spine has been demonstrated.10-12 When compared with placebo, all bisphosphonate formula at different dose levels, routes, and frequency of administration significantly increased BMD. In 2012, the Agency for Healthcare Research and Quality (AHRQ) published an update following a systematic review of the comparative effectiveness of treatments for osteoporosis.13 The AHRQ report provided evidence for decreased risk of hip and other non-vertebral fractures in alendronate-, risedronate- and zoledronate-treated patients even in high-risk post-menopausal women.13 As pharmaceutical agents, however, bisphosphonates can cause a number of adverse events, including some that are attributed to mode of administration. For example, gastrointestinal (GI) complaints, such as dysphagia, esophagitis, and esophageal and gastric ulcers are seen predominantly with orally-administered bisphosphonates, such as alendronate, risedronate, and ibandronate.7,8,14-18 In randomized clinical trials of alendronate involving large cohorts of patients, high incidences of upper GI complaints, such as dyspepsia were reported.19-21 However, prospective studies involving untreated osteoporosis patients to compare therapeutic efficacy and side effects of different bisphosphonates are limited. Intravenous bisphosphonates may be followed by an acute-phase reaction within one to 3 days of infusion, characterized by low-grade temperature, and muscle and joint pain. The current study was carried out to evaluate the efficacy of orally-administered alendronate compared with intravenously-administered zoledronate. Our starting hypothesis was that zoledronate would be as effective as alendronate but with no, or fewer, GI-associated adverse events. Changes in lumbar spine and hip BMD in patients with osteoporosis receiving either a single intravenous (i.v.) dose of zoledronate at 5 mg/year, or oral alendronate at 70 mg/week in a clinical setting were compared.
Methods
A cohort of 234 patients attending the osteoporosis clinics at the Royal London and St Bartholomew’s Hospitals, Barts Health HNS Trust between April 2010 and March 2012 was recruited for the study. For recruitment, patients had to have a T score of at least minus 2.0 (-2.0) at the lumbar spine, or the total hip as measured by dual energy x-ray absorptiometry (DEXA) scan. Patients who were bedridden, had diabetes mellitus, renal disease, cancer, or were receiving steroids were excluded from the study. One hundred and eighteen of the 234 recruited patients received alendronate (70 mg/week) taken orally, while 116 patients received 5 mg/year zoledronate once a year intravenously. Patients were prescribed zoledronate if they had a history of gastrointestinal (GI) problems, or developed GI complications within one month of starting treatment with alendronate. Apart from the latter patients, none of the patients was previously treated with bisphosphonates. Patients with poor or no compliance were excluded from the analysis and no data from such patients is, consequently, included in the analyses.
This study was a random prospective audit of patients receiving alendronate or zoledronate for osteoporosis. Patients were given the choice of either oral alendronate, or i.v. zoledronate by the treating physician based on guidelines by the National Institute of Clinical Excellence (NICE) in UK, and also on their history of GI problems. Standard Arthritis Research patient leaflets on alendronate and zoledronate were given to the patients to aid in the decision-making on drug choice. All recruited patients were referred to the Radiology Department of the Royal London or St. Bartholomew’s Hospital for DEXA scans (Hologic Discovery QDR series, Waltham, MA, USA). The study was authorized by the Clinical Audit Office of Barts Health HNS Trust and was carried out according to principles of the Helsinki Declaration. All patients signed consent forms before treatment, and their DEXA assessment to permit the release of de-identified demographic information.
Sites assessed by DEXA were total left hip and anterior-posterior spine (lumbar L1-L4) skeletal sites. The BMD at both sites was measured simultaneously at baseline, and then after one year, and then 2 years after starting treatment to obtain BMD and their T-scores. The BMD data on 116 of the 118 patients treated with alendronate was available after one year, and on all 118 patients after 2 years of the study. For patients treated with zoledronate, BMD data was available on 113 patients at one year, and on all 116 patients at 2 years of the study. All patients received oral daily calcium (≤1 g) and vitamin D (≤10 µg). To ensure reproducibility of measurements and consistency of comparisons, short- and long-term precisions were measured on a daily basis. Short-term precision was assessed by scanning an anthromorphic spine phantom 20 times per day. The phantom contains a human-like spine segment made of calcium hydroxyapatite, enclosed in a block of water-simulant epoxy. This phantom has a BMD equal to 0.995 g/cm2. The phantom was used so that errors due to patient positioning did not affect measurements, and to evaluate performance of the scanner over a range of bone density values, and avoid operator and patient sources of error. The long-term precision of the scanner was determined on a daily basis using BMD measurements of the spine phantom as a routine part of quality control. Serum levels of calcium, phosphate, and alkaline phosphatase were routinely measured for the patients. All patients had values within the normal range for these tests. Demographic, clinical and lifestyle data were collected by interviewer-administered questionnaire at baseline, and after one-, and 2-years during DEXA scan appointments. Data collected consisted of age, lifestyle (smoking, alcohol consumption of more than 2 servings a day, and physical exercise), personal and family history of fractures, ethnic origin, current medications, and history of prednisone use.
Statistical analysis
Pearson’s chi-square test for dichotomous variables was used to compare the distribution of risk factors for osteoporosis between groups. Student’s t-test was applied to continuous variables. Changes in BMD between the 2 groups were compared using Student’s t-tests with the Statistical Package for Social Sciences version 19 software (IBM Corp., Armonk, NY, USA). P<0.05 values were considered statistically significant.
Results
Demographic data on the patient cohort, their medical history and information on their lifestyle are summarized in Table 1. All recruited patients were interviewed by at least 2 members of the study team, and information recorded based on face-to-face interviews. There were no dropouts from the 234 patients whose data is included in this study, and all 118 patients treated with alendronate confirmed compliance with the treatment at interviews. However, it was not possible to verify patient statements on compliance by studying prescription refills. None of the patients treated with zoledronate had previously taken oral bisphosphonates for more than one month. Patients were excluded from treatment with alendronate only if they had a history of GI problem, or when prescribed alendronate show such problems within the first month of treatment. After the first month of starting treatment, none of the patients whose data are included in this study discontinued alendronate treatment to be treated with zoledronate. There were no statistically significant differences in perceived risk factors between the treatment groups (Table 1). The mean age of all patients was 68.5 ± 12.5 years (range: 46.5-72.6 years). For patients treated with alendronate, the mean ± SEM ages was 67.5 ± 12.5, and for zoledronate the mean ± SEM ages was 69.6 ± 11.1. The proportion of post-menopausal women in each group was also not statistically different (Table 1). However, the number of patients with fracture history was higher in the zoledronate-treated group compared with the alendronate group. This could suggest an element of unintended selection bias based on the higher fracture risk in the patients concerned. Nevertheless, this does not appear to have adversely influenced the analysis, and indeed, highlights the superior response in the zoledronate-treated group. At the end of the study, the mean duration of bisphosphonate treatment was 23.8 ± 1.3 months. Baseline BMD in both treatment groups at the lumbar spine and total hip sites were comparable at the start of treatment (Table 2 & Figure 1). Improvement in BMD in patients from the 2 treatment groups after 2 years was however, different. The BMD of the total hip decreased after 2 years in patients receiving alendronate by 0.4% from the baseline, whereas in the zoledronate-treated group, it increased by 0.8% (Figure 1A). The BMD at the lumbar spine increased after 2 years in patients receiving alendronate by 3.6%, and zoledronate by 5.7% (Figure 1B). The increase in BMD at both sites was significantly greater in patients receiving zoledronate compared with patients receiving alendronate (p=0.001).
Table 1.
Demographic, clinical, and lifestyle data on the patient cohort studied for treatment efficacy of alendronate and zoledronate.
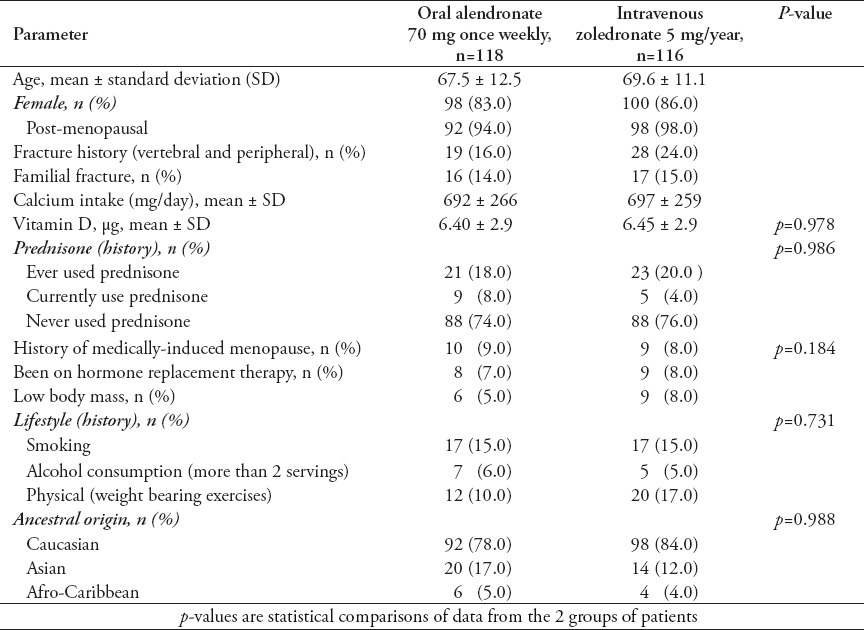
Table 2.
Mean bone mineral density (BMD) and T-score changes in the alendronate and zoledronate treated groups for 2 years.
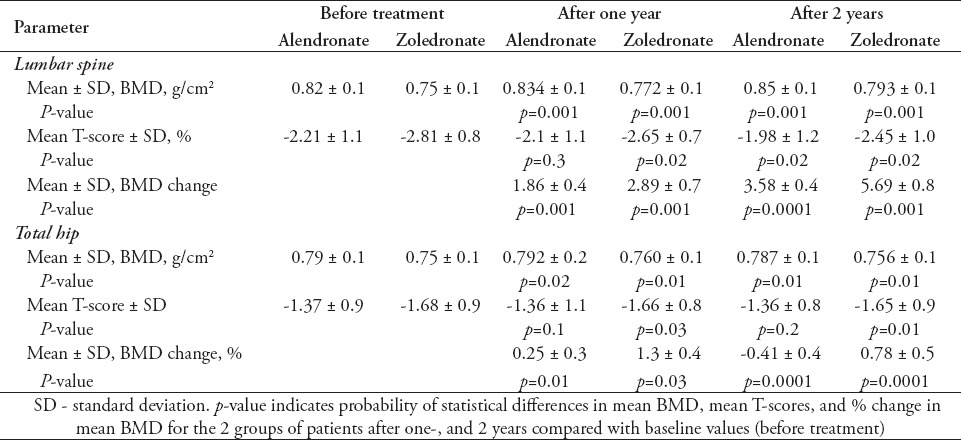
Figure 1.
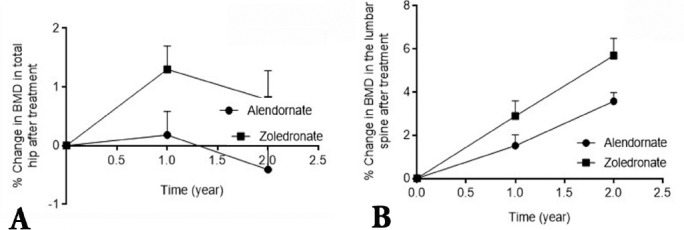
Change in bone mineral density (BMD) from baseline to one and 2 years after treatment at: A) the hip; and B) the lumbar spine sites in patients with osteoporosis receiving oral alendronate, or zoledro≠nate. The BMD of the hip and lumbar spine were determined by dual energy x-ray absorptiometry scan at baseline (time 0), and at one, and 2 years post treatment initiation. Percent changes in BMD values are presented as the mean for each group with their corresponding standard deviations. p=0.0001 in change in BMD from baseline to 2 years between patients treated with zoledronate compared with patients treated with alendronate in both sites after 2 years.
Discussion
This study demonstrates that treatment of osteoporosis patients with zoledronate once a year by i.v. injection is more effective than oral alendronate in increasing BMD at the hip and lumbar spine sites. Improvement in BMD in response to treatment with both agents was seen in both sets of patients after one year. However, BMD increased by a significantly greater magnitude in patients treated with zoledronate than patients treated with alendronate, suggesting superiority of the medication in improving BMD. The overall lower increase in BMD in the hip region compared with the lumbar spine is likely to be due to higher bone remodelling processes that are especially prominent in cancellous bone abundant in the lumbar spine.22 Differences in BMD between the 2 treatment groups, however, were not due to differences in baseline demographics of the patients, such as age, gender, or menopausal status since these were not significantly different between both groups.
The observations made in the study could be of clinical relevance with the small caveat that the findings could have been marginally influenced by selection bias, such as selecting patients with a higher fracture history, and also patients with GI intolerance to receive zoledronate rather than alendronate. However, positive effects on total hip BMD were observed after 2 years of treatment only in patients treated with zoledronate indicating that this agent is an effective alternative to alendronate in treating osteoporosis patients. Although the data clearly shows a superior response of osteoporosis patients to treatment with zoledronate as compared with alendronate, we consider that treatment compliance of patients with alendronate have to also be taken into consideration although significant efforts were made in the current study to corroborate patient compliance. Thus, even mild adverse effects, such as GI intolerance and concerns regarding such side effects could have led to lack of adherence to, or persistence with treatment by some patients as observed by other investigators.5 This is important considering that there is some evidence to suggest that treatment compliance and persistence increases with reduced dosing and frequency of administration.23,24 Treatment with zoledronate presents the opportunity for once-yearly i.v. administration, and is reported to increase treatment-adherence, especially in patients with polypharmacy.25 Furthermore, improvement in adherence has been associated with a reduction in fracture risk.26 For these reasons, zoledronate has become often the preferred treatment of choice for those with GI intolerance to other treatments in the same class.24 However, patients with confirmed, or suspected poor, or no compliance were excluded from the analysis in our study when the information was available. Our results, therefore, provide good evidence that zoledronate has a notable superior effect on improving BMD at both assessed sites.
Incidences of serious adverse events were not recorded for either treatment-group in the current study. However, there is published evidence to indicate that orally-administered bisphosphonates are more likely to cause GI side effects than intravenous ones. Moreover, it can be inferred from the dose-route and regime used that patients are more likely to comply, and persist with zoledronate than with alendronate treatment. Nevertheless, incidences of acute-phase responses (APR) have been reported in patients treated with zoledronate.27 However, all APR components had their peak onset within one day, the median duration for 3 days, and severity rated as mild, or moderate in 90% of patients. Vitamin D supplementation was reported to reduce the musculoskeletal pain element of APR, and deficiencies must, therefore, be corrected prior to administration of zoledronate.27,28 Calcium supplementation is also prescribed to counter the calcium-lowering effects of zoledronate through its effect on reducing bone turnover. It is also important to mention that renal deterioration progressing to renal failure has been observed by other investigators after an average of 56 days of zoledronate administration in some patients.29 Zoledronate is, therefore, contraindicated in patients with renal impairment, and all patients administered with zoledronate should be monitored for renal function regularly as a precautionary measure.
One limitation of the current study to be taken into consideration when treating patients is that assessment of the effects of alendronate and zoledronate involved measuring BMD, which is a surrogate measure of osteoporosis progression. However, the primary goal of treatment remains fracture reduction although low BMD remains the most recognized risk factor for predicting fractures.30 Nevertheless, extrapolation from these results to the wider clinical application of bisphosphonate should be undertaken with caution, since the data were collected over 2 years only. Studies for longer periods will be needed to assess the long-term efficacy and side effects of zoledronate. In addition, the use of biochemical bone turnover markers could be helpful in confirming our data, and in addition, could also help gain further insights into the mechanism of action of these therapeutic agents.
In summary, the current study confirms that treating osteoporosis patients with oral alendronate or with i.v. zoledronate is therapeutically beneficial for patients with osteoporosis. However, treatment with zoledronate offers therapeutic advantages. Therefore, zoledronate appears to be an acceptable alternative to oral bisphosphonates in the treatment of osteoporosis patients in general, and especially those who cannot tolerate oral bisphosphonates.
Acknowledgment
The authors gratefully acknowledge all staff at the Departments of Rheumatology and Radiology, The Royal London Hospital, Mile End Road, UK for their support with the study.
Footnotes
Statistics.
Excerpts from the Uniform Requirements for Manuscripts Submitted to Biomedical Journals updated November 2003.
Available from www.icmje.org
Describe statistical methods with enough detail to enable a knowledgeable reader with access to the original data to verify the reported results. When possible, quantify findings and present them with appropriate indicators of measurement error or uncertainty (such as confidence intervals). Avoid relying solely on statistical hypothesis testing, such as the use of P values, which fails to convey important information about effect size. References for the design of the study and statistical methods should be to standard works when possible (with pages stated). Define statistical terms, abbreviations, and most symbols. Specify the computer software used.
References
- 1.Sandhu SK, Hampson G. The pathogenesis, diagnosis, investigation and management of osteoporosis. J Clin Pathol. 2011;64:1042–1050. doi: 10.1136/jcp.2010.077842. [DOI] [PubMed] [Google Scholar]
- 2.Harvey N, Dennison E, Cooper C. Osteoporosis: a lifecourse approach. J Bone Miner Res. 2014;29:1917–1925. doi: 10.1002/jbmr.2286. [DOI] [PubMed] [Google Scholar]
- 3.Cauley JA, Chalhoub D, Kassem AM, Fuleihan Gel-H. Geographic and ethnic disparities in osteoporotic fractures. Nat Rev Endocrinol. 2014;10:338–351. doi: 10.1038/nrendo.2014.51. [DOI] [PubMed] [Google Scholar]
- 4.Giger EV, Castagner B, Leroux JC. Biomedical applications of bisphosphonates. J Control Release. 2013;167:175–188. doi: 10.1016/j.jconrel.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 5.Russell RG. Bisphosphonates: the first 40 years. Bone. 2011;49:2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Watts NB, Diab DL. Long-Term Use of Bisphosphonates in Osteoporosis. J Clin Endocrinol Metab. 2010;95:1555–1565. doi: 10.1210/jc.2009-1947. [DOI] [PubMed] [Google Scholar]
- 7.Kanis JA, Reginster JY, Kaufman JM, Ringe JD, Adachi JD, Hiligsmann M, et al. A reappraisal of generic bisphosphonates in osteoporosis. Osteoporos Int. 2012;23:213–221. doi: 10.1007/s00198-011-1796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee S, Glendenning P, Inderjeeth CA. Efficacy, side effects and route of administration are more important than frequency of dosing of anti-osteoporosis treatments in determining patient adherence: a critical review of published articles from 1970 to 2009. Osteoporos Int. 2011;22:741–753. doi: 10.1007/s00198-010-1335-x. [DOI] [PubMed] [Google Scholar]
- 9.Bauer DC, Schwartz A, Palermo L, Cauley J, Hochberg M, Santora A, et al. Fracture prediction after discontinuation of 4 to 5 years of alendronate therapy: the FLEX study. JAMA Intern Med. 2014;174:1126–1134. doi: 10.1001/jamainternmed.2014.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boonen S, Reginster JY, Kaufman JM, Lippuner K, Zanchetta J, Langdahl B, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2012;367:1714–1723. doi: 10.1056/NEJMoa1204061. [DOI] [PubMed] [Google Scholar]
- 11.Miller PD, Recker RR, Harris S, Silverman S, Felsenberg D, Reginster J, et al. Long-term fracture rates seen with continued ibandronate treatment: pooled analysis of DIVA and MOBILE long-term extension studies. Osteoporos Int. 2014;25:349–357. doi: 10.1007/s00198-013-2518-z. [DOI] [PubMed] [Google Scholar]
- 12.Donaldson MG, Palermo L, Ensrud KE, Hochberg MC, Schousboe JT, Cummings SR. Effect of alendronate for reducing fracture by FRAX score and femoral neck bone mineral density: the Fracture Intervention Trial. J Bone Miner Res. 2012;27:1804–1810. doi: 10.1002/jbmr.1625. [DOI] [PubMed] [Google Scholar]
- 13.Levis S, Theodore G. Summary of AHRQ’s comparative effectiveness review of treatment to prevent fractures in men and women with low bone density or osteoporosis: update of the 2007 report. J Manag Care Pharm. 2012;18(4 Suppl B):S1–S15. doi: 10.18553/jmcp.2012.18.s4-b.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClung MR, Zanchetta JR, Racewicz A, Roux C, Benhamou CL, Man Z, et al. Efficacy and safety of risedronate 150-mg once a month in the treatment of postmenopausal osteoporosis: 2-year data. Osteoporos Int. 2013;24:293–299. doi: 10.1007/s00198-012-2056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merck Sharp and Dohme Limited. Fosamax: Summary of Product Characteristics. 2010. [cited 22 October 2012]. Available from: http://www.medicines.org.uk/emc/medicine/1175/SPC/Fosamax/
- 16.Bianchi G, Czerwinski E, Kenwright A, Burdeska A, Recker RR, Felsenberg D. Long-term administration of quarterly IV ibandronate is effective and well tolerated in postmenopausal osteoporosis: 5-year data from the DIVA study long-term extension. Osteoporos Int. 2012;23:1769–1778. doi: 10.1007/s00198-011-1793-9. [DOI] [PubMed] [Google Scholar]
- 17.Hongo M, Miyakoshi N, Kasukawa Y, Ishikawa Y, Shimada Y. Additive effect of elcatonin to risedronate for chronic back pain and quality of life in postmenopausal women with osteoporosis: a randomized controlled trial. J Bone Miner Metab. 2015;33:432–439. doi: 10.1007/s00774-014-0603-9. [DOI] [PubMed] [Google Scholar]
- 18.Brown JP, Roux C, Ho PR, Bolognese MA, Hall J, Bone HG, et al. Denosumab significantly increases bone mineral density and reduces bone turnover compared with monthly oral ibandronate and risedronate in postmenopausal women who remained at higher risk for fracture despite previous suboptimal treatment with an oral bisphosphonate. Osteoporos Int. 2014;25:1953–1961. doi: 10.1007/s00198-014-2692-7. [DOI] [PubMed] [Google Scholar]
- 19.Modi A, Siris ES, Steve Fan CP, Sajjan S. Gastrointestinal events among patients initiating osteoporosis therapy: a retrospective administrative claims database analysis. Clin Ther. 2015;37:1228–1234. doi: 10.1016/j.clinthera.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 20.McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412–420. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 21.Hadji P, Gamerdinger D, Spieler W, Kann PH, Loeffler H, Articus K, et al. Rapid Onset and Sustained Efficacy (ROSE) study: results of a randomised, multicentre trial comparing the effect of zoledronic acid or alendronate on bone metabolism in postmenopausal women with low bone mass. Osteoporos Int. 2012;23:625–633. doi: 10.1007/s00198-011-1583-4. [DOI] [PubMed] [Google Scholar]
- 22.Jiang E, Wang Z, Meng Q, Li S, Wang F, Shao G, et al. Study on bone density at various skeletal sites for the diagnosis of primary osteoporosis. Cell Biochem Biophys. 2012;64:1–3. doi: 10.1007/s12013-012-9361-2. [DOI] [PubMed] [Google Scholar]
- 23.Hadji P, Claus V, Ziller V, Intorcia M, Kostev K, Steinle T. GRAND: the German retrospective cohort analysis on compliance and persistence and the associated risk of fractures in osteoporotic women treated with oral bisphosphonates. Osteoporos Int. 2012;23:223–231. doi: 10.1007/s00198-011-1535-z. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Roddam A, Gitlin M, Taylor A, Shepherd S, Shearer A, et al. Persistence with osteoporosis medications among postmenopausal women in the UK General Practice Research Database. Menopause. 2012;19:33–40. doi: 10.1097/gme.0b013e318221bacd. [DOI] [PubMed] [Google Scholar]
- 25.Pereira RM, Carvalho JF, Paula AP, Zerbini C, Domiciano DS, Gonçalves H, et al. Guidelines for the prevention and treatment of glucocorticoid-induced osteoporosis. Rev Bras Reumatol. 2012;52:580–593. English, Portuguese. [PubMed] [Google Scholar]
- 26.Rietbrock S, Olson M, van Staa TP. The potential effects on fracture outcomes of improvements in persistence and compliance with bisphosphonates. QJM. 2009;102:35–42. doi: 10.1093/qjmed/hcn130. [DOI] [PubMed] [Google Scholar]
- 27.Reid R, Gamble GD, Mesenbrink P, Lakatos P, Black DM. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab. 2010;95:4380–4387. doi: 10.1210/jc.2010-0597. [DOI] [PubMed] [Google Scholar]
- 28.Catalano A, Morabito N, Atteritano M, Basile G, Cucinotta D, Lasco A. Vitamin D Reduces musculoskeletal pain after infusion of zoledronic acid for postmenopausal osteoporosis. Calcif Tissue Int. 2012;90:279–285. doi: 10.1007/s00223-012-9577-6. [DOI] [PubMed] [Google Scholar]
- 29.Diel IJ, Weide R, Köppler H, Antràs L, Smith M, Green J, et al. Risk of renal impairment after treatment with ibandronate versus zoledronic acid: a retrospective medical records review. Support Care Cancer. 2009;17:719–725. doi: 10.1007/s00520-008-0553-7. [DOI] [PubMed] [Google Scholar]
- 30.Unnanuntana A, Gladnick BP, Donnelly E, Lane JM. The assessment of fracture risk. J Bone Joint Surg Am. 2010;92:743–753. doi: 10.2106/JBJS.I.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]


