Abstract
The global figure of 14 million new cancer cases in 2012 is projected to rise to almost 22 million by 2030, with the burden in low- and middle-income countries (LMICs) shifting from 59% to 65% of all cancer cases worldwide over this time. While the overheads of cancer care are set to rapidly increase in all countries worldwide irrespective of income, the limited resources to treat and manage the growing number of cancer patients in LMICs threaten national economic development. Current data collated in the recent second edition of The Cancer Atlas by the American Cancer Society and International Agency for Research on Cancer show that a substantial proportion of cancers are preventable and that prevention is cost-effective. Therefore, cancer control strategies within countries must prioritize primary and secondary prevention, alongside cancer management and palliative care and integrate these measures into existing health care plans. There are many examples of the effectiveness of prevention in terms of declining cancer rates and major risk factors, including an 80% decrease in liver cancer incidence rates among children and young adults following universal infant hepatitis B vaccination in Taiwan and a 46% reduction in smoking prevalence in Brazil after the implementation of a more aggressive tobacco control program beginning in 1989. Prevention can bring rich dividends in net savings but actions must be promoted and implemented. The successful approaches to combatting certain infectious diseases provide a model for implementing cancer prevention, particularly in LMICs, via the utilization of existing infrastructures for multiple purposes.
Cancer is one of the leading causes of death worldwide and it is a major component of the noncommunicable disease (NCD) burden. Its societal and economic impact will certainly rise as morbidity and mortality continue to increase and the demands on cancer services escalate. Over 14 million new cancer cases occur every year worldwide, but they are projected to reach almost 22 million by 2030 (1). During the corresponding period, the proportion of newly diagnosed cases occurring in low- and middle-income countries (LMICs) is projected to increase from 59% to almost 65% because of the aging and growth of the population but also because of marked increases in the prevalence of known or putative risk factors for cancer associated with westernization (2).
The predicted increase in the cancer burden can only be reduced if primary and secondary prevention strategies are prioritized. The management of cancer is often extremely expensive because of the chronic nature of the disease and the high cost of therapies. LMICs can ill afford the diagnostic and therapeutic work-up required by the new wave of cancers linked with westernization that are now becoming evident, in addition to those already established. Only rapid acceleration in the implementation of cancer control programs at the national and/or regional levels, set up for surveillance, primary prevention, early detection, and treatment and integrated into NCD plans, is likely to have a major impact in reducing the projected burden.
Current data, comprehensively collated for the recent second edition of The Cancer Atlas released by the American Cancer Society and the International Agency for Research on Cancer (1), argues that many cancers are largely preventable and prevention is cost-effective. The prevention approach also substantively reduces the number of cancer patients in forthcoming generations requiring diagnosis, treatment, and follow-up. Prevention is much less expensive than implementing all the required health infrastructures currently lacking in LMICs to manage and treat cancer patients. However, as we argue later, prevention is better achieved by leveraging upon health care infrastructures, which means that prevention and care must go hand in hand, and at a minimum resource-dependent treatment and palliative care services are needed in every LMIC. This paper provides the rationale for the feasibility, effectiveness, and cost-effectiveness of primary prevention of cancer in economically developing countries.
The Rapidly Changing Global Landscape of Cancer
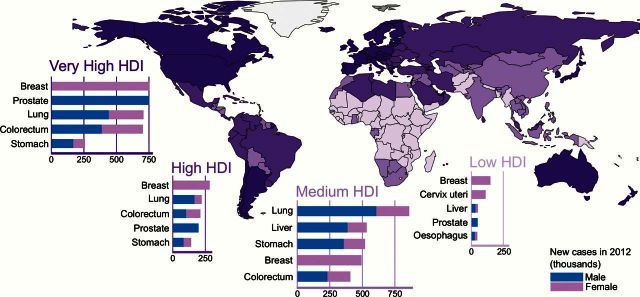
If we look at the global burden of cancer, regional diversity is one of the most striking aspects. There are two main features that differentiate LMICs from high-income countries (HICs). First, LMICs still have a high burden of infection-related cancers, such as cervix and other human papillomavirus (HPV)–related cancers, stomach cancer, and liver cancer. Infection-related cancers account for 33% of the total cancer cases in sub-Saharan regions compared with only 3.3% in Australia/New Zealand and 4% in North America (1). Second, LMICs are facing a rapid epidemiologic transition specific to the major NCDs, which are the predominant sources of morbidity and mortality in affluent societies. Similar to the increase in the risk of cardiovascular disease, chronic obstructive pulmonary disease (COPD), and diabetes in many transitioning countries, certain forms of cancer such as lung, colon, and breast cancer are also rapidly rising in these countries (Figure 1).
Figure 1.
The cancer burden varies by Human Development Index level (from Cancer Atlas, American Cancer Society, Atlanta, 2014). HDI = Human Development Index.
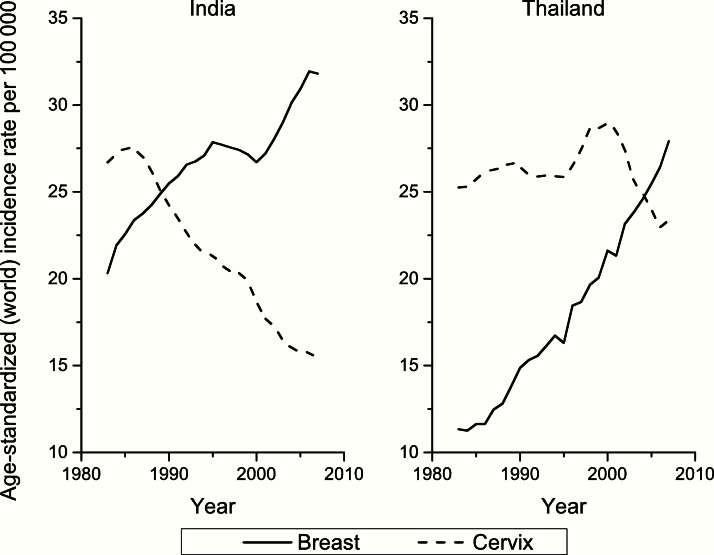
For example, Figure 2 shows the crossing of the curves for breast and cervical cancer incidence over time, a typical indicator of transition of cancer patterns in LMICs towards those of HICs. Along with NCDs generally, transitions in cancer profiles are largely driven by changes in reproductive patterns as well as lifestyle factors that include tobacco consumption, unhealthy diet, and obesity; these are often exacerbated by factors linked to the built environment (encouraging sedentary lifestyles) and national policy, including the extent to which multinational tobacco companies are able to target an economically developing country to expand market share and maximize profit (3,4).
Figure 2.
The crossing of the curves for breast and cervical cancers is a typical indicator of transition of low-income countries to a pattern typical of high-income countries (from Cancer Atlas, American Cancer Society, Atlanta, 2014).
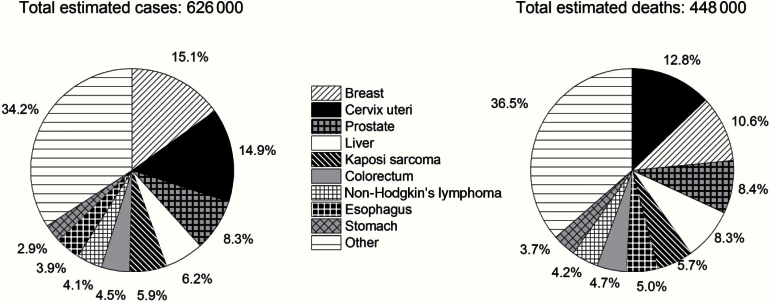
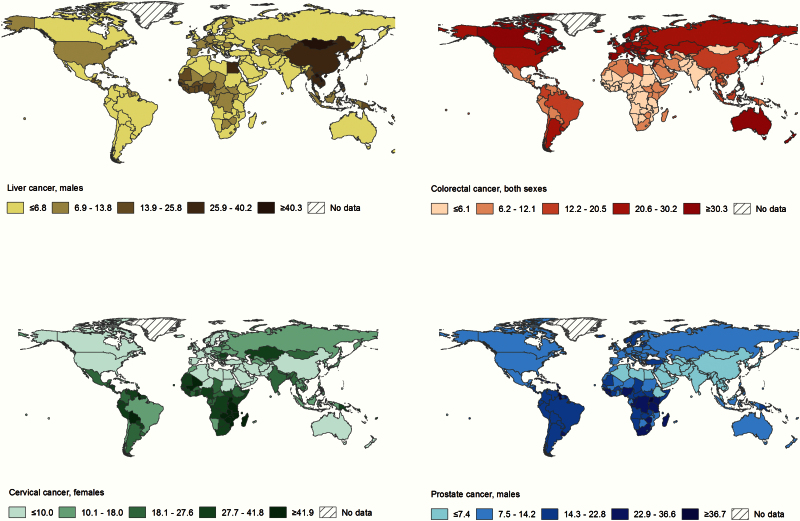
The socioeconomic transitions are far from being uniform, however, and the most striking observations of regional diversity come from the comparison of incidence rates (newly occurring cases per unit population and time period) and mortality rates in many LMICs. In sub-Saharan Africa, mortality to incidence ratios, a proxy of case fatality, tend to be higher than in other world regions. In 2012, the ratio for sub-Saharan Africa was 0.7 (448 000/626 000), compared with 0.4 (692 000/1 786 000) in North America (US and Canada) (Figure 3). Poverty and infection-related cancers are among a number of prominent barriers that preclude higher life expectancies in some countries in Africa and other parts of the world (5). Clearly the prevalence of risk factors (known or otherwise) and the availability of diagnostics and/or treatment largely determine the magnitude of rates across countries for a specific cancer, for example, cancers of the liver, cervix, colorectum, and prostate (Figure 4).
Figure 3.
Comparison of incidence rates (newly occurring cases per unit population) and mortality rates in sub-Saharan Africa, showing that incidence and mortality are very close to each other. In 2012, the ratio for sub-Saharan Africa was 0.7 (448,000/626,000), compared with 0.4 (692,000/1,786,000) in North America (US and Canada) (from Cancer Atlas, American Cancer Society, Atlanta, 2014).
Figure 4.
Cancer incidence reflects the distribution of risk factors and availability of diagnostic services. Estimated cancer incidence rates per 100 000, age-standardized rate (world), 2012 (from Cancer Atlas, American Cancer Society, Atlanta, 2014).
Based on the observations above, global inequalities are a major source of avoidable cancer deaths. In particular, cervical cancer incidence rates in many LMIC countries (including sub-Saharan and some Latin-American countries) remain high, and there is also a residual but high burden of HIV/AIDS-related cancers in sub-Saharan Africa. This adds to other cancers that are not preventable based on current knowledge, for example, the cluster of unexplained esophageal cancer in Uganda, Zimbabwe, Malawi, and other Eastern African countries.
Diversity is not only related to income, and simple epidemiological transition models often fail to grasp the geographical and temporal heterogeneity of cancer in global terms. While the declines in noncardia gastric cancer can be considered part of an “unplanned triumph” of primary prevention, rates are still very high in parts of Asia including Japan (eg, Miyagi prefecture) and South Korea (Ulsan), China (Cixian), and India (Mizoram). The risk of death from cervical cancer is unacceptably high among young women in middle- and high-income countries of the former Soviet Union (6). This is because of an absence of effective screening (eg, in Russia, Belarus, and the Ukraine) in an era of changing sexual behaviors and thus a higher risk of cervical cancer among recent generations. Further, the increasing burden of cancer associated with unhealthy diet and obesity involves both LMIC and high-income countries. For example, colorectal cancer incidence rate in Japan has increased rapidly over the past few decades, so much so that it has now exceeded the rates in historically high-incidence countries, including the United States and Australia (7). According to a recent study, high body mass index accounted for 3% to 6% of cancer cases in low–human development index (HDI) countries and 16% of the cases in very high-HDI countries (8), with the burden expected to continue increasing in the future as the obesity prevalence rises and the associated cancer epidemic unfolds (8). Also, alcohol intake is an increasingly important cause of noncommunicable diseases, including cancer of the liver, upper aero-digestive tract, breast, and colorectum (9). It is slightly increasing worldwide, though there are strong geographic differences, with increases since the mid-1980s in Russia and more recently since the mid-2000s in China and India and large decreases over the past several decades in Spain, France, and Italy (10).
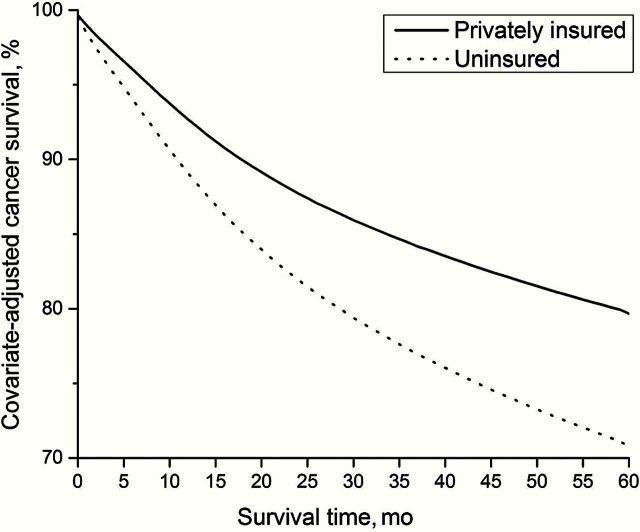
In addition to between-country differences, there are large variations within countries linked to socioeconomic position or race/ethnicity. In the United States, survival after a cancer diagnosis is much lower in the uninsured than privately insured persons (Figure 5) and among blacks compared with whites. In Australia, a recent study revealed that Indigenous Australians were less likely to receive cancer treatment, had more comorbidities, and had more advanced cancer stage at diagnosis than nonindigenous cancer patients, factors that contribute to their poorer cancer survival (11).
Figure 5.
In the United States, cancer survival is lower for those without insurance compared with those with private insurance (from Cancer Atlas, American Cancer Society, Atlanta, GA, 2014)
The Potential of Prevention
Prevention has many advantages over cure. There are several examples of the effectiveness of prevention, both primary and secondary, in terms of declining cancer rates. Evidently, the greatest success in public health in recent decades has been the decrease in lung cancer mortality in men in most HICs. For example, 40% of the overall decrease in cancer mortality rate between 1991 and 2003 in men in the United States has been because of a reduction in cigarette smoking (12). In other cases, a decline in mortality has been achieved by other means, including screening. Cervical cancer incidence rates in many western countries such as Finland and Sweden have decreased by more than 70%, largely because of organized screening (13,14). In the case of breast cancer, mortality in Europe is decreasing in spite of an increasing incidence; this is interpreted as the effect of earlier diagnosis and more effective therapies at earlier stages (15,16). In fact, screening has played a role in decreasing mortality, but reasons for the decline go beyond screening per se and include earlier diagnosis due to greater awareness and enhancements in breast cancer management and therapy. Curative treatment for prostate cancer in the last 20 years has been a major driver of mortality reduction in men in some HICs, while corresponding colorectal cancer mortality trends show declines for similar reasons including an impact of screening in the United States (1). However, colorectal cancer incidence rates continue to increase in young adults in the United States (<50 years of age) and in other HICs (7,17), as they are in many LMICs rapidly transiting towards higher levels of development (7).
Three Examples of the Preventative Approach in Practice
Taiwan and Hepatitis B Virus Vaccination
Very good evidence of the effectiveness of hepatitis B virus (HBV) vaccination is available from Taiwan, where vaccination started in 1984. Since then, liver cancer in children and young adults has decreased by as much as 80% (18). As with other preventive programs, the campaign had a number of positive outcomes: It has successfully lowered the prevalence of chronic HBV carriers, mortality from infant fulminant hepatitis, and chronic liver disease in vaccinated birth cohorts.
Rwanda and the HPV Vaccine
Rwanda, in spite of its limited resources, is at the avant-garde of HPV vaccination, with a stunning 93% coverage of the target population in 2011 (19). This was made possible through school-based vaccination and community involvement, a public-private partnership between Merck and the Ministry of Health in developing the program and providing Gardasil free of cost, and a nationwide sensitization campaign that preceded delivery of the first dose (19). This compares with the complete lack of vaccination in the vast majority of African and Asian States.
Tobacco Policy in Brazil and Thailand
Tax and prices of tobacco are among the most effective policies on the side of demand. There is strong evidence from Thailand and Brazil that increases in taxes and prices had a large and durable impact on the decline in tobacco use (20,21). In Brazil, smoking prevalence declined by 46% between 1989 and 2008, with 48% and 14% of the relative decline attributed to taxes and advertisement bans, respectively (20). Similarly, increased taxes and advertisement bans, respectively, accounted for 61% and 22% of the relative decrease in smoking prevalence between 1991 and 2006 in Thailand (21). Other examples of the effectiveness of the increasing price of cigarettes on reducing cigarette consumption come from South Africa and France (22). The Framework Convention on Tobacco Control (FCTC), the world’s first global health treaty, entered into force in 2005 and continues to provide an impetus for further tobacco control in countries around the world (23).
The different timing in the perception of benefits of prevention has to be considered because politics often suffers from “short-termism.” For certain risk factors the impact of prevention can be demonstrated soon after implementation. Reducing smoking leads to a substantial reduction in risk of lung cancer and cardiovascular disease a few years after cessation (24). However, eliminating smoking uptake is the optimal policy, as risk among former smokers does not return to the levels of never-smokers for several decades (depending on the age at cessation) (25). As discussed above, certain countries have been particularly effective in reducing smoking prevalence through taxation and advertisement bans. The chemical arsenic is another exposure where elimination rapidly reduces cancer risk (26). Millions of people are exposed to arsenic poisoning in drinking water in China, Bangladesh, Chile, Taiwan (China), and other countries, resulting in an increased burden of skin and bladder cancers (27).
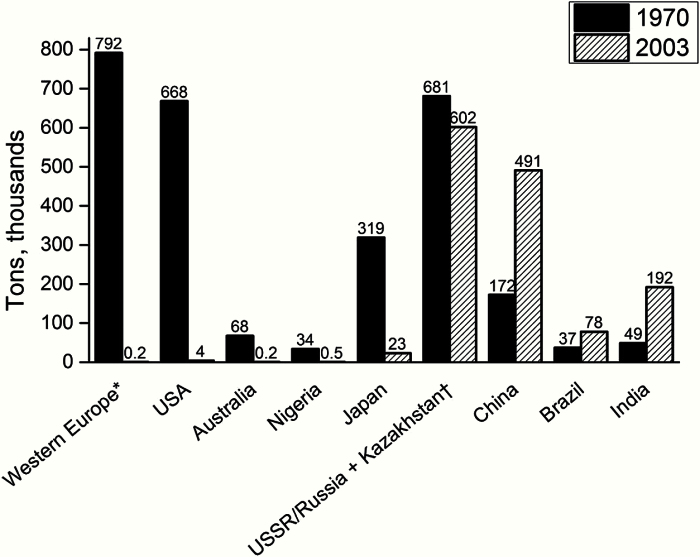
However, sometimes the benefits of cancer prevention can only be observed many years after the intervention. Asbestos is a striking example of a potent carcinogen that still acts for several decades after the removal of exposure, with the persistent increases in asbestos-related mesotheliomas in the UK projected to peak circa 2025, decades after the drastic reduction in exposure (28). Carcinogens like asbestos that, for biological reasons, take decades to be cleared from the body, require focused attention. The use of asbestos is declining in many countries of the world, but its use is being transferred from HICs to LMICs (Figure 6). The public health benefit of reduced exposure in avoidance of mesothelioma deaths (in terms of numbers) becomes evident only when cohorts reach the ages at which the risk of cancer is sufficiently high.
Figure 6.
The use of asbestos is declining in many countries of the world, but is being transferred from high- to low-income counties and emerging economies (from Cancer Atlas, American Cancer Society, Atlanta, GA, 2014; adapted from: Virta RL. Worldwide asbestos supply and consumption trends from 1900 through 2003: U.S. Geological Survey Circular 1298. Reston, VA; United States Geological Survey; 2006. Available from http://pubs.usgs.gov/circ/2006/1298/c1298.pdf , accessed July 25, 2014.). * The top seven Western European consumers in 1970: UK, Italy, W. Germany, E. Germany, France, Spain, and Belgium/Luxembourg. †USSR for 1970; Russia and Kazakhstan for 2003.
Cancer Prevention as an Economic Investment
The costs of cancer diagnosis and therapy are extremely high, while prevention leads to net savings. The combined costs of cancer diagnosis and treatment, loss of productivity because of morbidity and premature death, and informal care costs have been estimated at €126 billion in the EU in 2009, more than the entire EU budget (€112 billion) (29). The direct medical costs (total all healthcare expenditures) in the United States in 2011 were estimated to be around $88.7 billion per year (30). There are no similar data on cost of cancer from LMICs. However, costs are likely to increase, both in HICs because of expanding costs of therapies and in LMICs because of the increasing burden of cancer.
In the absence of the implementation of prevention, LMICs will not have the resources to diagnose and treat all new cancer patients, and the economic burden will soon become unsustainable. Such a scenario can only amplify socio-economic differentials, making effective therapies a preserve of the richest in most societies. There are many examples of a complete lack of essential infrastructure to presently tackle cancer in LMICs. Ethiopia, the second most populous country in Africa with a population of over 80 million, is presently served with only two functioning radiotherapy machines (31). Africa and other less developed regions in general are very poorly served when it comes to radiotherapy availability (1, 31). Despite 60% of the cancer burden falling on LMICs, only 32% of the radiotherapy machines available worldwide are operating on the continent. (1) This is the case with other indicators of very limited health infrastructure: For example, with the exceptions of South Africa and Botswana, countries in sub-Saharan Africa average less than one pathologist per 500 000 people (32).
While introducing screening for colorectal and breast cancers is not only cost prohibitive but may not be supported by existing healthcare infrastructure in most LMICs, most preventive activities are relatively cheap and feasible to implement, including tobacco control as described in the FCTC’s MPOWER measures (higher tobacco taxes, dissemination campaigns on health risks of smoking, restrictions on smoking in public places) (4), dietary advice, and a policy on prices that promotes healthy diet (33). Comprehensive cervical cancer prevention programs are feasible and cost as little as $0.20 per capita in the ten countries that currently have the highest mortality rates in the world (Mali, Guinea, Burundi, Zambia, Zimbabwe, Tanzania, Comoros, Mozambique, Malawi, and Swaziland) (34). Equally, there would be enormous dividends in LMICs following complete coverage of HPV vaccination: The cost to avert one disability-adjusted life-year is less than gross domestic product per capita, which makes it a very cost-effective intervention by World Health Organization standards (costs at the price negotiated by the Global Alliance for Vaccine and Immunization (GAVI)) (35).
Often, risk factors are shared among many different diseases. For example, smoking, obesity, and poor diet are risk factors for several major cancers but are also determinants of cardiovascular disease, diabetes, and some neurological diseases. Thus prevention has an impact on multiple NCDs. There are several examples of how preventive activities might lead to net savings. The smoking cessation program in Taipei (involving counseling and nicotine replacement) led to 215 million USD savings in 15 years (36). According to a simulation model, an intensive six-month mass media antismoking campaign in Australia will lead to an estimated $912 million savings over the lifetime of 190 000 quitters (37), which is equivalent to Australia’s government investment in early childhood education. Dietary advice to obese and overweight people in the Netherlands has been estimated to save up to $2.5 billion over five years (38).
Implementing Prevention Using Existing Infrastructures
What is the best strategy to implement prevention in the most effective way, particularly in LMICs? Noncommunicable diseases, including cancer, need to be prioritized, and prevention is a good investment. The promotion of national, universal healthcare systems would be a leap forward for cancer control in every country, if coupled with sound public health initiatives. Prevention implementation must therefore be integrated with cancer diagnosis and care for two reasons. One is that a minimum level of good quality disease management (including palliative care) is needed in LMICs. Secondly, leveraging on existing health care infrastructures has been an effective way to promote prevention for infectious diseases. The way forward has been shown by the successful approaches adopted to tackle infectious diseases such as HIV/AIDS, using the existing infrastructures for multiple purposes (though the role of highly active antiretroviral therapy for AIDS has been crucial, while a similar life-saving treatment is currently unavailable for most cancers, an additional reason to promote prevention). The issues involved in improving the responsiveness of health systems to NCDs have been reviewed by Atun et al. (39). They note that management of NCD patients, sometimes with multiple conditions, is particularly challenging in low- and middle-income countries with weak health systems, characterized by fragmented health care services. A key lesson from HIV/AIDS is the broad-based governance in identification of problems, needs, and responses, involving the engagement of civil society, affected communities, and the private sector. Atun and colleagues have shown the efficacy of an integrated approach for infectious diseases and a possible pathway for combatting NCDs. The first steps in this direction may be seen, for example, with implementation of HBV and HPV immunization, particularly in GAVI-eligible countries; however, one must also have actions focussed on supporting the implementation of the HPV vaccine nationally in noneligible LMICs (39).
Leveraging existing public health infrastructure is an important strategy for cancer control in LMICs, where resources to address the burden of chronic disease are limited. The United States Centers for Disease Control and Prevention (CDC) works with Ministries of Health and other partners to establish sustainable Field Epidemiology Training Programs (FETPs: http://www.cdc.gov/globalhealth/FETP/default.htm), which help to build and strengthen workforce capacity for disease detection, laboratory services, and outbreak response. Since 1980, 50 of these programs have produced more than 2800 graduates in 69 countries, with more than 80% of graduates serving as public health leaders in their home countries. There is great potential to leverage FETP infrastructure and expertise to build capacity and leadership for the prevention and control of cancer and other chronic diseases; the program has already begun to reach into NCDs through five initial NCD focus countries (China, Colombia, Jordan, Tanzania, and Thailand). The Pink Ribbon Red Ribbon (PRRR: http://pinkribbonredribbon.org/) initiative is an innovative public-private partnership that uses evidence-based approaches to deliver health care services for women’s cancers in sub-Saharan Africa. PRRR-supported programs increase access to cervical cancer screening and treatment, HPV vaccine, and breast and cervical cancer education for underserved women in this region. Similarly, GAVI (http://www.gavi.org/pledging2015/) has worked with the World Health Organization Expanded Program on Immunization to prevent cervical cancer in low income countries by increasing access to HPV and HBV vaccines.
Another example is the IARC-coordinated Global Initiative for Cancer Registry Development (GICR; http://gicr.iarc.fr), which has brought together a group of major international and national agencies to work collaboratively to redefine the level of cancer surveillance worldwide for cancer control action. Advocating the central role of population-based cancer registries in national planning, reference centers (IARC Regional Hubs) are becoming operational in six world regions to provide targeted support for cancer control implementation, monitoring, and evaluation. The World Health Organization has endorsed the GICR as a tool to support Member States in addressing the related indicator (cancer incidence by type per 100 000) within the NCD Global Monitoring Framework.
Conclusions
In the absence of prevention, the cancer burden will soon become overwhelming in many lower-income countries in sub-Saharan Africa, Asia, Latin America, the Caribbean, and the Pacific Islands because of the aging of the population, tobacco and other risk factors, and a chronic lack of adequate medical and public health infrastructure. This is one of the central arguments to accelerate the implementation of primary prevention while the opportunity still exists.
Funding
This commentary was written by staff of the American Cancer Society (A. Jemal and L. Torre), the International Agency for Research on Cancer (F. Bray and D. Forman), and Imperial College London (P. Vineis). These authors were responsible for the commentary, including interpretation, writing, and presenting the manuscript.
The authors wish to thank Mr. Mathieu Laversanne, International Agency for Research on Cancer, for graphics development and Ms. Kimberly D. Miller, American Cancer Society, for graphics and content development.
References
- 1. Jemal A, Vineis P, Bray F, et al. The Cancer Atlas 2nd ed. Atlanta, GA: American Cancer Society; 2014. [Google Scholar]
- 2. Bray F, Soerjomataran I. The changing global burden of cancer: transitions in human development and implications for cancer prevention and control. In: Gelband H, Jha P, Sankaranarayanan R, Horton S, editors. Disease Control Priorities, 3rd ed Ed: Cancer; 2015. [PubMed] [Google Scholar]
- 3. Stuckler D, McKee M, Ebrahim S, et al. Manufacturing epidemics: the role of global producers in increased consumption of unhealthy commodities including processed foods, alcohol, and tobacco. PLoS Med. 2012;9(6):e1001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. WHO Report on the Global Tobacco Epidemic, 2013. Geneva: 2013. [Google Scholar]
- 5. Bray F, Jemal A, Grey N, et al. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012;13(8):790–801. [DOI] [PubMed] [Google Scholar]
- 6. Bray F, Lortet-Tieulent J, Znaor A, et al. Patterns and trends in human papillomavirus-related diseases in Central and Eastern Europe and Central Asia. Vaccine. 2013;31 Suppl 7:H32–H45. [DOI] [PubMed] [Google Scholar]
- 7. Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1688–1694. [DOI] [PubMed] [Google Scholar]
- 8. Arnold M, Pandeya N, Byrnes G, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Cancer Research Fund / American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, D.C: AICR; 2007. [Google Scholar]
- 10. World Health Organization. Global Status Report on Alcohol and Health 2014. Geneva: WHO Press; 2014. [Google Scholar]
- 11. Moore SP, Green AC, Bray F, et al. Survival disparities in Australia: an analysis of patterns of care and comorbidities among indigenous and non-indigenous cancer patients. BMC Cancer. 2014;14:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thun MJ, Jemal A. How much of the decrease in cancer death rates in the United States is attributable to reductions in tobacco smoking? Tob Control. 2006;15(5):345–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. International Agency for Research on Cancer. Cervix Cancer Screening. Lyon: IARC Press; 2004. [Google Scholar]
- 14. Vaccarella S, Franceschi S, Engholm G, et al. 50 years of screening in the Nordic countries: quantifying the effects on cervical cancer incidence. Br J Cancer. 2014;111(5):965–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. [DOI] [PubMed] [Google Scholar]
- 16. Kalager M, Zelen M, Langmark F, et al. Effect of screening mammography on breast-cancer mortality in Norway. N Engl J Med. 2010;363(13):1203–1210. [DOI] [PubMed] [Google Scholar]
- 17. Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1695–1698. [DOI] [PubMed] [Google Scholar]
- 18. Chiang CJ, Yang YW, You SL, et al. Thirty-year outcomes of the national hepatitis B immunization program in Taiwan. JAMA. 2013;310(9):974–976. [DOI] [PubMed] [Google Scholar]
- 19. Binagwaho A, Wagner CM, Gatera M, et al. Achieving high coverage in Rwanda’s national human papillomavirus vaccination programme. Bull World Health Organ. 2012;90(8):623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levy D, de Almeida LM, Szklo A. The Brazil SimSmoke policy simulation model: the effect of strong tobacco control policies on smoking prevalence and smoking-attributable deaths in a middle income nation. PLoS Med. 2012;9(11):e1001336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levy DT, Benjakul S, Ross H, et al. The role of tobacco control policies in reducing smoking and deaths in a middle income nation: results from the Thailand SimSmoke simulation model. Tob Control. 2008;17(1):53–59. [DOI] [PubMed] [Google Scholar]
- 22. Jha P. Avoidable global cancer deaths and total deaths from smoking. Nat Rev Cancer. 2009;9(9):655–664. [DOI] [PubMed] [Google Scholar]
- 23. World Health Organization. WHO Framework Convention on Tobacco Control Overview. http://www.who.int/fctc/text_download/en/index.html. Last acce ssed March 2, 2015. [Google Scholar]
- 24. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking- 50 Years of Progress: A Report of the Surgeon General. In. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014. [Google Scholar]
- 25. Centers for Disease Control and Prevention. Best Practices for Comprehensive Tobacco Control Programs- 2007. Atlanta, GA: October 2007. [Google Scholar]
- 26. Su CC, Lu JL, Tsai KY, et al. Reduction in arsenic intake from water has different impacts on lung cancer and bladder cancer in an arseniasis endemic area in Taiwan. Cancer Causes Control. 2011;22(1):101–108. [DOI] [PubMed] [Google Scholar]
- 27. Chen Y, Ahsan H. Cancer burden from arsenic in drinking water in Bangladesh. Am J Public Health. 2004;94(5):741–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hodgson JT, McElvenny DM, Darnton AJ, et al. The expected burden of mesothelioma mortality in Great Britain from 2002 to 2050. Br J Cancer. 2005;92(3):587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luengo-Fernandez R, Leal J, Gray A, et al. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 2013;14(12):1165–1174. [DOI] [PubMed] [Google Scholar]
- 30. Agency for Healthcare Research and Quality Medical Expenditure Panel Survey. Total Expenses and Percent Distribution for Selected Conditions by type of Service: United States, 2012. http://meps.ahrq.gov/mepsweb/data_stats/tables_compendia_hh_interactive.jsp?_SERVICE=MEPSSocket0&_PROGRAM=MEPSPGM.TC.SAS&File=HCFY2012&Table=HCFY2012_CNDXP_C&_Debug. Last accessed March 2, 2015. [Google Scholar]
- 31. Abdel-Wahab M, Bourque JM, Pynda Y, et al. Status of radiotherapy resources in Africa: an International Atomic Energy Agency analysis. Lancet Oncol. 2013;14(4):e168–e175. [DOI] [PubMed] [Google Scholar]
- 32. Adesina A, Chumba D, Nelson AM, et al. Improvement of pathology in sub-Saharan Africa. Lancet Oncol. 2013;14(4):e152–e157. [DOI] [PubMed] [Google Scholar]
- 33. World Health Organization. Global status report on noncommunicable diseases, 2014. 2014. [Google Scholar]
- 34. World Health Organization. Scaling up action against noncommunicable diseases: how much will it cost? France: 2011. [Google Scholar]
- 35. Goldie SJ, O’Shea M, Campos NG, et al. Health and economic outcomes of HPV 16,18 vaccination in 72 GAVI-eligible countries. Vaccine. 2008;26(32):4080–4093. [DOI] [PubMed] [Google Scholar]
- 36. Chen PC, Lee YC, Tsai ST, et al. A cost-benefit analysis of the outpatient smoking cessation services in Taiwan from a societal viewpoint. Nicotine Tob Res. 2012;14(5):522–530. [DOI] [PubMed] [Google Scholar]
- 37. Hurley SF, Matthews JP. Cost-effectiveness of the Australian National Tobacco Campaign. Tob Control. 2008;17(6):379–384. [DOI] [PubMed] [Google Scholar]
- 38. SEO Economic Research. Cost-benefit analysis of dietary treatment. https://www.bda.uk.com/improvinghealth/healthprofessionals/cost_benefit_of_dietitians. Last accessed March 2, 2015. [Google Scholar]
- 39. Atun R, Jaffar S, Nishtar S, et al. Improving responsiveness of health systems to non-communicable diseases. Lancet. 2013;381(9867):690–697. [DOI] [PubMed] [Google Scholar]