Abstract
Green tea, particularly epigallocatechin-3-gallate (EGCG), may affect body weight and composition, possibly by enhancing fat oxidation. The aim of this double-blind, randomized placebo-controlled cross-over study was to investigate whether 3-day supplementation with EGCG (282mg/day) stimulates fat oxidation and lipolysis in 24 overweight subjects (age = 30 ± 2yrs, BMI = 27.7 ± 0.3 kg/m2). Energy expenditure, substrate metabolism and circulating metabolites were determined during fasting and postprandial conditions. After 6 h, a fat biopsy was collected to examine gene expression. In 12 subjects, skeletal muscle glycerol, glucose and lactate concentrations were determined using microdialysis. EGCG-supplementation did not alter energy expenditure and substrate oxidation compared to placebo. Although EGCG reduced postprandial circulating glycerol concentrations (P = 0.015), no difference in skeletal muscle lipolysis was observed. Fasting (P = 0.001) and postprandial (P = 0.003) skeletal muscle lactate concentrations were reduced after EGCG-supplementation compared to placebo, despite similar tissue blood flow. Adipose tissue leptin (P = 0.05) and FAT/CD36 expression (P = 0.08) were increased after EGCG compared to placebo. In conclusion, 3-day EGCG-supplementation decreased postprandial plasma glycerol concentrations, but had no significant effects on skeletal muscle lipolysis and whole-body fat oxidation in overweight individuals. Furthermore, EGCG decreased skeletal muscle lactate concentrations, which suggest a shift towards a more oxidative muscle phenotype.
The prevalence of obesity has become an epidemic problem during the last few decades1,2. Being overweight or obese is considered to be the most important risk factor for the onset of type 2 diabetes mellitus3.
In recent years there has been an increased interest in the health benefits of polyphenols in the prevention of obesity and type 2 diabetes mellitus. Green tea is rich in polyphenols, especially catechins. These catechins are comprised primarily of epigallocatechin -3- gallate (EGCG), epigallocatechin (EGC), and epicatechin (EC)4. Many of the beneficial health effects of green tea have been attributed to the most abundant catechin EGCG and were initially mainly related to their anti-oxidant activity5,6,7,8. More recently, interest has increased in the anti-obesity effect of green tea9. Consumption of green tea extracts (270 mg EGCG) in combination with caffeine supplementation (150 mg caffeine) has been shown to increase fat oxidation10,11 and energy expenditure10 in an acute manner in lean subjects, and to reduce body weight in overweight subjects12,13. However, studies are not consistent yet, which may relate to different doses of EGCG and caffeine, subjects’ BMI and genetic predisposition11,14,15,16,17. Preliminary data suggest that short-term EGCG supplementation may stimulate fat oxidation during postprandial conditions in overweight subjects18. Moreover, we recently showed that EGCG, in combination with resveratrol, increased fasting and postprandial energy expenditure, associated with an increased metabolic flexibility19, which may be associated with an increased insulin sensitivity20. Although supplementation of a green tea supplement for 24 hours in healthy men increased the Matsuda index21, reflecting a higher insulin sensitivity based on glucose and insulin concentrations after an oral glucose tolerance test22, most studies found no short-term effect on glucose homeostasis in humans16,23,24.
The underlying mechanisms behind the possible effect of green tea components on energy expenditure and lipid metabolism are not well studied in humans. One of the putative mechanisms is that EGCG may modulate energy expenditure by inhibiting catechol-o-methyltransferase (COMT)10,11, an enzyme involved in the degradation of norepinephrine25. As a consequence there is a prolonged stimulation of the adrenergic receptors, thereby increasing lipolysis and fat oxidation. Another mechanism may involve activation of sirtuins, especially sirtuin1 (SIRT1) and its transcriptional co-factor peroxisome proliferator-activated receptor-γ coactivator 1 alpha (PGC-1α), although few data of human studies are available26.
Watanabe et al.27 have shown that EGCG inhibited acetyl CoA-carboxylase (ACC) in 3T3-L1 cells suggesting that EGCG could alter the partitioning of lipids from storage towards oxidation. In addition, chronic feeding of green tea extract to mice has been shown to elevate skeletal muscle gene expression of factors involved in lipid transport and oxidation, such as FAT/CD36, medium-chain acyl-CoA dehydrogenase (MCAD) and uncoupling protein 3 (UCP3)28,29. Moreover, green tea extract reduced malonyl-CoA in skeletal muscle, which is an inhibitor of carnitine palmitoyl transferase (CPT), an enzyme involved in fatty acid transportation into the mitochondria30. The latter studies in mice, as well as our recent human study showing that combined EGCG and resveratrol supplementation affects flexibility of postprandial substrate oxidation without changes in systemic lipolysis19 may suggest that skeletal muscle is a major target tissue for the EGCG-induced metabolic effects. Nevertheless, whether the effects of EGCG and/or green tea extract in humans are mediated through direct effects on muscle metabolism, or secondary to a transient increase in free fatty acids (FFA) related to changes in adipose tissue metabolism or a regulatory action on gene expression remains to be established.
Therefore, we aimed to investigate whether a 3-day supplementation of 282 mg/day EGCG would increase fasting and postprandial fat oxidation, lipolysis (as indicated by circulating FFA and glycerol) and alter adipose tissue gene expression. Furthermore, in a subset of subjects (n = 12), local skeletal muscle metabolism was also assessed using microdialysis.
Results
Bioavailability
Plasma EGCG reached maximum concentration (394 ± 73 ng/mL (0.86 ± 0.16 μmol/L)) one hour after intake of EGCG (t = 0) and gradually declined below the detection limit (20 ng/mL) at the end of the meal-test (t = 360, Fig. 1). Relative bioavailability (AUCplasma/dose) was 19 ± 2%.
Figure 1. Time-course of plasma Epigallocatechin-gallate after oral intake of 300 mg/day or placebo.
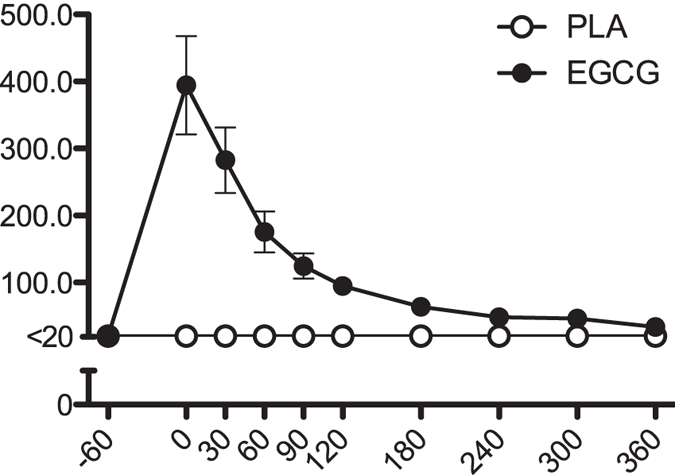
Results represent mean ± SEM; n = 24.
Energy expenditure and substrate oxidation
Supplementation of EGCG did not change fasting or postprandial fat and carbohydrate oxidation (Fig. 2A,B). Similarly, there were no differences in energy expenditure between conditions (Fig. 2C).
Figure 2. Time-course of substrate oxidation and energy expenditure after intake of 300 mg/day EGCG or placebo.
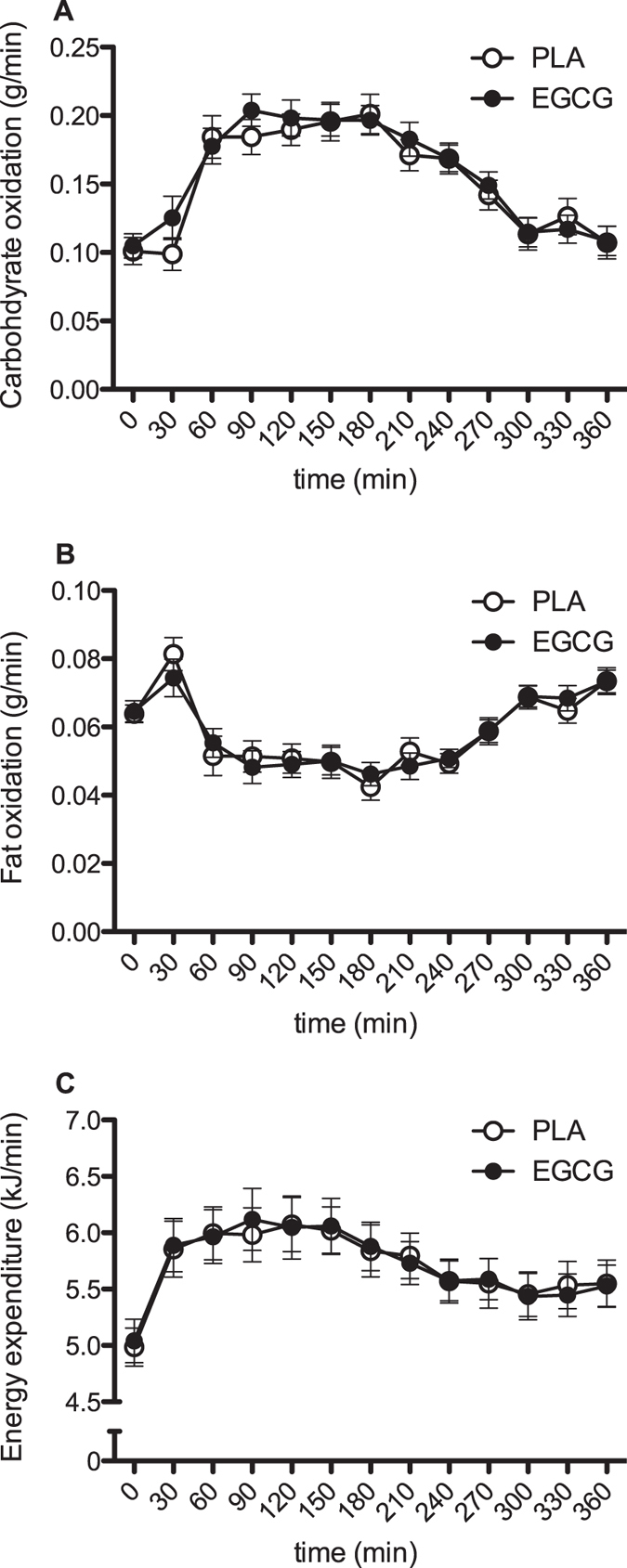
Results represent mean ± SEM; n = 24. Carbohydrate oxidation (A), fat oxidation (B) and energy expenditure (C) before and after a mixed meal.
Circulating metabolites
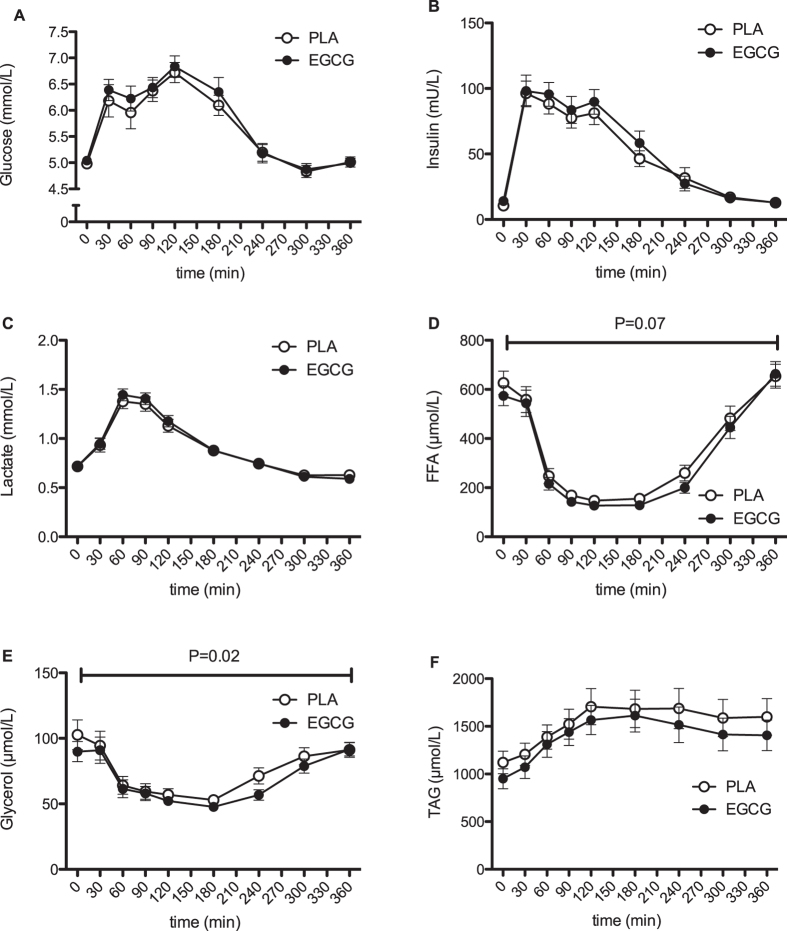
EGCG supplementation showed no differences in plasma glucose, insulin and lactate concentration compared to placebo (Fig. 3A–C). Plasma free fatty acids (FFA) concentrations tended to be reduced after EGCG supplementation (AUC, P = 0.07, Fig. 3D). Furthermore, plasma glycerol concentrations were significantly reduced after EGCG supplementation as compared with placebo (AUC, P = 0.02, Fig. 3E), which was most pronounced in the late postprandial period (240–360 min). Plasma concentration responses to the meal, expressed as incremental area under the curve, were not affected by EGCG (p = N.S.). EGCG supplementation did not induce differences in plasma TAG concentrations, although fasting TAG concentrations tended to be reduced after EGCG (P = 0.07, Fig. 3F).
Figure 3.
Time-course of systemic metabolite and hormone concentrations response after intake of 282 mg/day EGCG or placebo. Results represent mean ± SEM; n = 24. Plasma glucose (A), insulin (B), lactate (C), FFA (D), glycerol (E) and TAG (F) concentrations before and after a mixed meal. P: paired Student’s t-test for AUC-values.
Skeletal muscle lipolysis
Skeletal muscle interstitial glycerol, glucose concentrations, as well as ethanol in/out ratio were not different between EGCG supplementation and placebo (Fig. 4A,C, glucose data not shown). However, interstitial lactate was reduced with EGCG both for fasting levels (P<0.001) as well during the postprandial period (P = 0.003, Fig. 4B). Moreover, the postprandial increase of interstitial lactate concentrations tended to be attenuated by EGCG (iAUC, PLA: 15.2 ± 2.1 mmol/(L*360 min), EGCG: 9.8 ± 1.5 mmol/(L*360 min), P = 0.06).
Figure 4. Time-course of interstitial metabolite concentrations in skeletal muscle after intake of 282 mg/day EGCG or placebo.
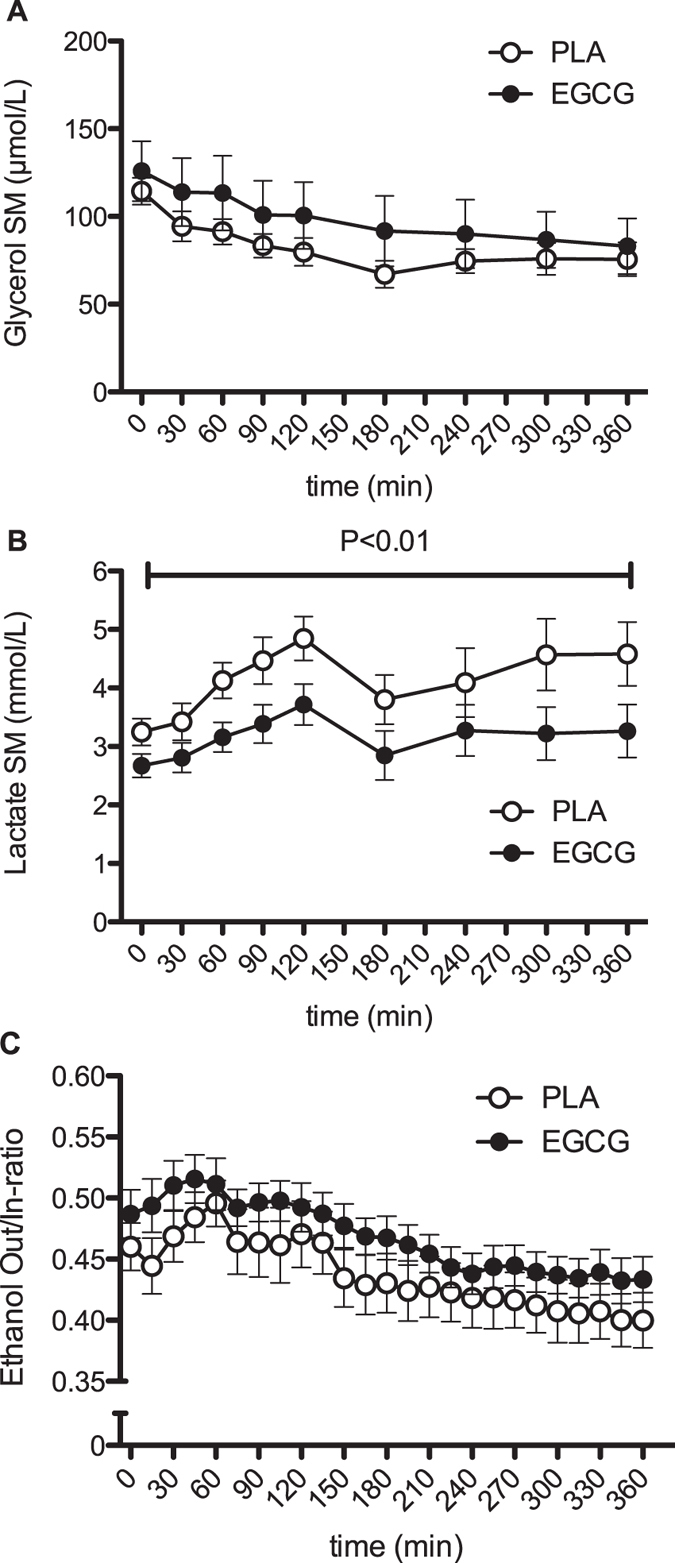
Results represent mean ± SEM; n = 12. Skeletal muscle interstitial glycerol (A) and lactate (B) concentrations and ethanol Out/In-ratio (C) before and after a mixed meal. P: paired Student’s t-test for AUC-values.
Adipose tissue gene and protein expression
The mRNA expression of CPT-1, ACC-1, adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) were similar after supplementation with EGCG or placebo capsules. EGCG increased leptin (P = 0.05) and fatty acid translocase (FAT/CD36) (P = 0.08) mRNA expression compared to placebo (Table 1).
Table 1. Normalized mRNA expression in adipose tissue after EGCG or placebo.
| EGCG | Placebo | P-value | |
|---|---|---|---|
| CPT-1 mRNA | 1,06·10−5 ± 1,0·10−6 | 9,3·10−6 ± 7,4·10−7 | 0.31 |
| ATGL mRNA | 3,51·10−4 ± 3,7·10−5 | 3,7·10−4 ± 3,0·10−5 | 0.12 |
| HSL mRNA | 5,38·10−4 ± 4,7·10−5 | 5,19·10−4 ± 4,1·10−5 | 0.73 |
| FAT/CD36 mRNA | 1,73·10−3 ± 1,2·10−4 | 1,52·10−3 ± 9,1·10−5 | 0.08 |
| ACC-1 mRNA | 2,16·10−4 ± 2,1·10−5 | 2,18·10−4 ± 2,6·10−5 | 0.81 |
| Leptin mRNA | 5,2·10−4 ± 5,9·10−5 | 4,3·10−4 ± 5,9·10−5 | 0.05 |
CPT-1, carnitine-palmitoyl-transferase-1; ATGL, adipose triglyceride lipase; HSL, hormone-sensitive lipase; FAT/CD36, fatty acid-translocase/cluster of differentiation; ACC-1, acetyl-Coenzyme-A-Carboxylase.
Discussion
This study was designed to study the acute effects of EGCG supplementation (282 mg/day), the main catechin of green tea, on whole-body and skeletal muscle lipolysis and whole-body fat oxidation in overweight subjects. Supplementation of 282 mg/day EGCG for 3 days decreased circulating glycerol and tended to reduce FFA concentrations but did not alter local muscle lipolysis, substrate oxidation and energy expenditure compared to placebo. Skeletal muscle lactate concentrations were significantly reduced by EGCG compared to placebo, whilst skeletal muscle glucose concentrations were comparable, suggesting an ECCG-induced shift towards a more oxidative skeletal muscle phenotype. Bioavailability was in line with previously published data31.
It has been suggested that EGCG stimulates fat oxidation by augmenting sympathic nervous stimulation10,32, however human studies have shown conflicting results19,33. In the present study, no significant effect on postprandial fat oxidation and energy expenditure was found, which is consistent with findings of Lonac et al.16 (945 mg EGCG in 48 h) and Gregersen et al.17 (494–684 mg/day catechins and 150 mg caffeine) after 2 days EGCG, respectively acute EGCG-enriched catechin supplementation, in healthy adults. In contrast to our results, Thielecke et al.24 observed an increased fat oxidation in the first 2 h of the postprandial period following 3-day supplementation of EGCG. This may be explained by an extra dose of 150 mg EGCG that was ingested 1 h before meal intake, additional to the daily dose ingested in the morning. In addition, Dulloo et al.10 showed an increase in fat oxidation and energy expenditure following a single dose of catechins (375 mg catechins, 270 mg EGCG) + 150 mg caffeine in healthy young men. Since caffeine has been shown to independently stimulate energy expenditure in a dose-dependent manner, with doses as low as 100 mg showing effects34, synergistic effects of EGCG and caffeine could possibly explain the discrepancy between the study of Dulloo et al.10 and our present findings. Of note, in the study of Gregersen et al.17, lower doses of combined EGCG and caffeine (600 mg, respectively 150 mg, 6 small doses over 11 h) did not induce significant increases in fat oxidation and energy expenditure. The synergistic action of different supplements may explain the increased energy expenditure that we reported in overweight men and women after combined EGCG and RSV supplementation19.
Although there was no significant effect on postprandial fat oxidation in the present study, skeletal muscle lactate concentrations were reduced after EGCG supplementation both in the fasted state as well as during the postprandial period. These data might indicate a shift towards a less glycolytic and/or more oxidative muscle phenotype after EGCG. It is increasingly recognized that skeletal muscle lipolysis may play an important role in the regulation of mitochondrial function in skeletal muscle by activation of PPARs35,36. Since we found no significant differences in muscle lipolysis, this has not driven the shift in oxidative potential in muscle after EGCG supplementation. Also, we found no differences in adipose tissue lipase expression, and even reduced systemic FFA concentrations, indicating that the shift towards a more oxidative phenotype in skeletal muscle was not related to differences in the supply of exogenous fatty acids, but may possibly be due to a direct effect on mitochondrial function which has been reported earlier26,37. However, the reduction of interstitial lactate, indicative for a more oxidative phenotype, does not translate into significant alterations in substrate oxidation or glucose disposal. Still, even though the acute effects of EGCG on fat oxidation were not significantly different from placebo treatment, this does not rule out the possibility that green tea can have beneficial effects on fat oxidation38,39, body composition12,13,38,40 and insulin sensitivity41 over longer time periods. Therefore, the duration of supplementation, addition of caffeine or other polyphenols and combination with exercise are factors that have to be taken into account for future studies.
Systemic glycerol concentrations were significantly reduced in the postprandial period with EGCG, but not during fasting conditions. This decrease may indicate a slightly improved insulin-mediated suppression of adipose tissue lipolysis after EGCG treatment. Further studies are necessary to examine this mechanism or possible others, including glycerol clearance. Additionally, EGCG supplementation resulted in an upregulation of leptin mRNA expression in adipose tissue, whilst mRNA expression of the fatty acid transporter FAT/CD36 tended to be higher after EGCG supplementation compared to placebo. Similarly, we have previously found increased fasting concentrations of the adipose tissue derived satiety hormone leptin after short-term supplementation with EGCG and resveratrol19. In line, Josic et al.42 suggested that green tea might increase satiety although the data should be confirmed in a large clinical trial with overweight and obese subjects. Altogether, these data indicate slight effects on adipose tissue metabolism and function; more pronounced effects might require a longer period of supplementation and/or higher dosage of EGCG.
Lastly, the interaction between polyphenols and the gut microbiota may modulate the effect of EGCG supplementation43. Previous research suggests pre- and antibiotic properties of EGCG, which may influence peripheral metabolism through changes in microbiota composition and microbial products like short-chain fatty acids44. Moreover, it should be considered that microbial polyphenolic metabolites may have distinct effects on host metabolism45.
In conclusion, EGCG supplementation for 3 days decreased postprandial plasma glycerol concentrations, but had no significant effects on skeletal muscle lipolysis and whole-body fat oxidation in overweight individuals. Interestingly, EGCG decreased skeletal muscle lactate concentrations, suggesting a shift towards a more oxidative muscle phenotype. It can be speculated that the shift towards a more oxidative phenotype may be beneficial over a longer period in the prevention of obesity and related complications20.
Methods
Ethics Statement
The study was reviewed and approved by the Medical Ethical Committee of the Maastricht University Medical Centre+ and all subjects gave written informed consent before participation. All procedures were carried out in accordance with the approved guidelines.
Subjects
Twenty-four overweight men (n = 9) and women (n = 15) with a low habitual caffeine intake (<300 mg/day) were recruited for this study. Sample size is calculated to detect a physiological relevant change in fat oxidation of 20% with a power of 90%, assuming a significance level of α = 0.05 and a drop-out of 20%, based on pilot data by Boschmann et al.18. Subjects‘ characteristics are presented in table 2. Subjects with type 2 diabetes and/or overt cardiovascular complications, and those using medication for digestive disorders were excluded from the participation. All subjects tolerated the EGCG capsules well and no adverse effects were observed on liver enzymes ALAT and ASAT.
Table 2. Subjects’ characteristic’s.
| Mean ± SEM (N = 24, 9M/15F) | |
|---|---|
| Age (years) | 30 ± 2 |
| BMI (kg/m2) | 27.7 ± 0.3 |
| Waist circumference (cm) | 89 ± 1.7 |
| Hip circumference (cm) | 99 ± 1.1 |
| WHR | 0.90 ± 0.01 |
| Fat mass (%) | 28.8 ± 1.9 |
| Fat free mass (%) | 71.2 ± 1.9 |
| Glucose (mmol/L) | 5.20 ± 0.06 |
| Insulin (μU/mL) | 12.5 ± 0.7 |
| HOMA-IR | 2.9 ± 0.2 |
| Systolic blood pressure (mmHg) | 114 ± 2 |
| Diastolic blood pressure (mmHg) | 75 ± 1 |
BMI, Body mass index; WHR, Waist-to-hip ratio; HOMA-IR, Homeostatic assessment model for insulin resistance.
Study design
The effects of EGCG and placebo on postprandial fat oxidation were studied in a double blind, randomised cross-over design, with a washout-period of at least 7 days between both treatments. Subjects consumed the capsules during 2 days with breakfast and dinner (at both occasions 1 capsule of 141 mg). At day 3, subjects came to the university (Maastricht University Medical Centre+) for a test (test day). At this day the 2 capsules were ingested simultaneously, 1 h before the ingestion of a liquid mixed meal.
Test product
The test product EGCG (Teavigo TG Lot: UT05080001) was provided by DSM Nutritional Products Ltd to Temmler Werke GmbH (Munchen, Germany). All capsules supplied by Temmler Werke GmbH were manufactured, tested and released according to Good Manufacturing Practice (GMP) guidelines. TEAVIGO TG™ contains >90% EGCG on a dry weight basis. The placebo capsules were filled with partially hydrolysed cellulose. The capsules were of identical appearance containing either 141 mg EGCG or placebo. 282 mg EGCG per day has been shown to be safe and well tolerated in single doses as well as repeated dosing46.
Protocol Test day
All subjects were asked to refrain from drinking alcohol, smoking and doing strenuous exercise for a period of 24 h before the test day. Subjects came to the laboratory by car or bus in the morning after an overnight fast. At the beginning of the test day (day 3), a cannula was inserted into an antecubital vein for blood sampling. The EGCG or placebo capsules were consumed 1 h before metabolic testing and a fasting blood sample was drawn to determine baseline values of EGCG (t = −60 min). A liquid test meal was consumed 60 min after ingestion of EGCG/placebo capsules. Primary outcomes, energy expenditure and substrate utilization, were measured before and for 6 h after ingestion of the liquid test meal, using a ventilated hood system (Omnical, Maastricht University, The Netherlands)47. Gas analyses, which occurred every minute, are performed by dual paramagnetic O2 analysers and dual infrared CO2 analysers (type 1156, 1507, 1520; Servomex, Cowborough, Sussex, UK), similar to the analysis system described by Schoffelen et al.48. Blood samples were taken before ingestion of the liquid meal (t = 0 min) and for 6 h after meal ingestion at t = 30, 60, 90, 120, 180, 240, 300 and 360 min after EGCG/placebo ingestion to determine circulating metabolites and hormone concentrations.
The liquid meal had a total energy content equivalent of 40% of calculated 24 h resting energy expenditure based upon the formula of Harris and Benedict49. The energy content of the test meal was accounted for 49En% CHO, 35En% FAT and 16En% protein and was consumed within 20 minutes. A fat biopsy was taken at the end of the 6 h postprandial period in each condition (t = 360 min). Blood samples and fat biopsy were snap frozen in liquid nitrogen and stored at −80 °C until analysis.
Fat biopsy
A small amount (about 1 g) of abdominal subcutaneous adipose tissue was collected under local anesthesia using a needle biopsy technique and snap frozen in liquid nitrogen. Total RNA was isolated, using the total RNA stabilization and purification kit for human samples Qiagen (Qiagen, Hombrechtikon, Switzerland). Gene expression of HSL, ATGL, CPT-1, ACC-1, FAT/CD36 and leptin was measured by the Taqman multiplex method using the ABI 7900 quantitative real-time RT-PCR instrument (Applied Biosystems, Rotkreuz, Switzerland) as described by Heim50. All probe and primer sets were designed with the Primer Express program version 1.0 (Applied Biosystems) and initially tested to have comparable (>90%) efficiency in multiplex assays using 18S rRNA as an internal control. An overview of the primers and probes is listed in Supplementary Table S1.
Microdialysis
In a subset of 12 subjects (6 men, 6 women, representative for the whole-study group with respect to subjects characteristics), the lipolytic effects of EGCG in skeletal muscle were determined by the microdialysis technique. On arrival, two microdialysis probes (CMA 60, CMA microdialysis AB, Stockholm, Sweden) were inserted in the medial portion of the m. gastrocnemius of both legs after anesthesia (xylocaine 2% without adrenalin, Astra Zeneca). Thereafter, 90 min was allowed for recovery of muscle from insertion trauma.
One probe was perfused with Ringer’s solution (147 mM sodium, 4 mM potassium, 2.25 mM calcium and 156 mM chloride, Baxter BV, Utrecht, The Netherlands) at a rate of 0.3 μL/min to obtain a near 100% recovery. Microdialysate was collected from these probes in 30 min fractions during the baseline period and during the early postprandial period (0–120 min) and at 60 min fractions during the last 4 h postprandially (120–360 min), to determine glycerol, glucose and lactate concentrations. Baseline concentrations of glycerol, lactate and glucose were determined by the average of three baseline samples. The second microdialysis probe was used for determining tissue blood flow using the ethanol dilution technique51,52. For this, the probes were perfused with Ringer’s solution supplemented with 50 mM ethanol, at a flow rate of 5 μL/min (Harvard microinfusion pump, Plato BV, Diemen, The Netherlands).
Ethanol concentrations were determined both in the ingoing and outgoing perfusion solvent to assess the ethanol inflow/outflow ratio as an indicator for local nutritive blood flow. Ethanol concentrations were determined at the same day, whereas microdialysate samples for measurement of glycerol, glucose and lactate concentrations were immediately frozen and stored at −80 °C until analysis.
Biochemical analyses
At all time points, 8 mL blood was collected in pre-chilled tubes with 200 μL of 0.2 M EDTA (Sigma, Dorset, UK). After collection, blood samples were centrifuged immediately at 4 °C for 10 min at 1000 g and frozen at −80 °C until analysis. Additionally, 500 μL of the cell free plasma supernatant was combined with exactly 500 μL stabilization buffer at ambient temperature for the determination of EGCG concentration by HPLC. Plasma glucose (Uni Kit III, Roche, Basel, Switzerland), lactate, FFA (NEFA-C, Wako Chemicals, Neuss, Germany), TAG and free glycerol (148270, Roche Diagnostics, Indianapolis, IN, USA) concentrations were analyzed with a COBAS FARA semi-automatic analyzer (Roche). Insulin was analyzed by radioimmunoassay (Human Insulin RIA Kit, LINCO Research Inc, St. Charles, MO).
Glycerol, glucose and lactate concentrations from the microdialysates were measured by bioluminescence after enzymatic oxidation of L-Lactate53. Ethanol concentrations were measured spectrophotometrically using a standard enzymatic technique (R-Biopharm AG, Darmstadt, Germany).
Calculations
Substrate oxidation was calculated from VO2 (L/min) and VCO2 (L/min) according to the equations of Frayn54. Nitrogen excretion was calculated based on the assumption that protein oxidation represents ~15% of total energy expenditure. Energy expenditure was calculated using the formula of Weir55. For each time point, the average of 20 minutes was used for calculation.
Carbohydrate oxidation (CHO) (g/min) = (4.55*VCO2)–(3.21*VO2)–(2.87*N)
FAT oxidation (g/min) = (1.67*VO2)–(1.67*VCO2)–(1.92*N)
N (g/min) = ((0.15* EE)/17)/6.25
Statistics
All data are expressed as means ± SEM. The total response of parameters after ingestion EGCG or placebo was expressed as the total area under the curve (AUC) and calculated by the trapezoid method. Differences between placebo and EGCG were analyzed by means of student’s paired t-test. Normal distribution was tested by the Kolmogorov-Smirnov test. If parameters were not normally distributed they were ln transformed. Plasma lactate, TAG, glycerol and the genes CPT-1, ACC-1, HSL and leptin data were ln transformed. SPSS 15 for Windows was used to perform all calculation. The level of statistical significance was set at P ≤ 0.05.
Additional Information
How to cite this article: Most, J. et al. A 3-day EGCG-supplementation reduces interstitial lactate concentration in skeletal muscle of overweight subjects. Sci. Rep. 5, 17896; doi: 10.1038/srep17896 (2015).
Supplementary Material
Acknowledgments
This study was funded by DSM Nutritional Products, Switzerland. The authors thank Jos Stegen for expert technical assistance. They also thank Pascale Fuchs and Petra Buchwald Hunziker for the mRNA measurement.
Footnotes
Author Contributions J.M., J.v.C., E.E.B. and J.J.H. were involved in the protocol design, J.M., J.v.C., J.W.D. and G.G. conducted the study, J.J. and I.B. performed part of the analysis and all authors were involved in writing and reviewing the manuscript
References
- IASO. Overweight & Obesity in the EU27, <http://www.worldobesity.org/site_media/uploads/v2PDFforwebsiteEU27.pdf> (2008) (Date of access: 28/09/2015). [Google Scholar]
- Organization, W. H. Obesity and overweight - Fact sheet N˚311, <http://www.who.int/mediacentre/factsheets/fs311/en/> (2015) (Date of access: 28/09/2015). [Google Scholar]
- Kahn S. E., Hull R. L. & Utzschneider K. M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846, doi: 10.1038/nature05482 (2006). [DOI] [PubMed] [Google Scholar]
- Sano M. et al. Simultaneous determination of twelve tea catechins by high-performance liquid chromatography with electrochemical detection. Analyst 126, 816–820 (2001). [DOI] [PubMed] [Google Scholar]
- Jung Y. D. & Ellis L. M. Inhibition of tumour invasion and angiogenesis by epigallocatechin gallate (EGCG), a major component of green tea. Int J Exp Pathol 82, 309–316 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartippour M. R. et al. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J Nutr 132, 2307–2311 (2002). [DOI] [PubMed] [Google Scholar]
- Kavanagh K. T. et al. Green tea extracts decrease carcinogen-induced mammary tumor burden in rats and rate of breast cancer cell proliferation in culture. J Cell Biochem 82, 387–398 (2001). [DOI] [PubMed] [Google Scholar]
- Osada K. et al. Tea catechins inhibit cholesterol oxidation accompanying oxidation of low density lipoprotein in vitro. Comp Biochem Physiol C Toxicol Pharmacol 128, 153–164 (2001). [DOI] [PubMed] [Google Scholar]
- Diepvens K., Westerterp K. R. & Westerterp-Plantenga M. S. Obesity and thermogenesis related to the consumption of caffeine, ephedrine, capsaicin, and green tea. Am J Physiol Regul Integr Comp Physiol 292, R77–85, doi: 10.1152/ajpregu.00832.2005 (2007). [DOI] [PubMed] [Google Scholar]
- Dulloo A. G. et al. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr 70, 1040–1045 (1999). [DOI] [PubMed] [Google Scholar]
- Hursel R., Janssens P. L., Bouwman F. G., Mariman E. C. & Westerterp-Plantenga M. S. The role of catechol-O-methyl transferase Val(108/158)Met polymorphism (rs4680) in the effect of green tea on resting energy expenditure and fat oxidation: a pilot study. PLoS One 9, e106220, doi: 10.1371/journal.pone.0106220 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A. et al. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J Am Coll Nutr 29, 31–40 (2010). [DOI] [PubMed] [Google Scholar]
- Brown A. L. et al. Health effects of green tea catechins in overweight and obese men: a randomised controlled cross-over trial. Br J Nutr 106, 1880–1889, doi: 10.1017/S0007114511002376 (2011). [DOI] [PubMed] [Google Scholar]
- Berube-Parent S., Pelletier C., Dore J. & Tremblay A. Effects of encapsulated green tea and Guarana extracts containing a mixture of epigallocatechin-3-gallate and caffeine on 24 h energy expenditure and fat oxidation in men. Br J Nutr 94, 432–436 (2005). [DOI] [PubMed] [Google Scholar]
- Gosselin C. & Haman F. Effects of green tea extracts on non-shivering thermogenesis during mild cold exposure in young men. Br J Nutr 110, 282–288, doi: 10.1017/S0007114512005089 (2013). [DOI] [PubMed] [Google Scholar]
- Lonac M. C., Richards J. C., Schweder M. M., Johnson T. K. & Bell C. Influence of short-term consumption of the caffeine-free, epigallocatechin-3-gallate supplement, Teavigo, on resting metabolism and the thermic effect of feeding. Obesity (Silver Spring) 19, 298–304, doi: 10.1038/oby.2010.181 (2011). [DOI] [PubMed] [Google Scholar]
- Gregersen N. T. et al. Effect of moderate intakes of different tea catechins and caffeine on acute measures of energy metabolism under sedentary conditions. Br J Nutr 102, 1187–1194, doi: 10.1017/S0007114509371779 (2009). [DOI] [PubMed] [Google Scholar]
- Boschmann M. & Thielecke F. The effects of epigallocatechin-3-gallate on thermogenesis and fat oxidation in obese men: a pilot study. J Am Coll Nutr 26, 389S–395S (2007). [DOI] [PubMed] [Google Scholar]
- Most J., Goossens G. H., Jocken J. W. & Blaak E. E. Short-term supplementation with a specific combination of dietary polyphenols increases energy expenditure and alters substrate metabolism in overweight subjects. Int J Obes (Lond) 38, 698–706, doi: 10.1038/ijo.2013.231 (2014). [DOI] [PubMed] [Google Scholar]
- Corpeleijn E., Saris W. H. & Blaak E. E. Metabolic flexibility in the development of insulin resistance and type 2 diabetes: effects of lifestyle. Obes Rev 10, 178–193, doi: 10.1111/j.1467-789X.2008.00544.x (2009). [DOI] [PubMed] [Google Scholar]
- Venables M. C., Hulston C. J., Cox H. R. & Jeukendrup A. E. Green tea extract ingestion, fat oxidation, and glucose tolerance in healthy humans. Am J Clin Nutr 87, 778–784 (2008). [DOI] [PubMed] [Google Scholar]
- Matsuda M. & DeFronzo R. A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22, 1462–1470 (1999). [DOI] [PubMed] [Google Scholar]
- Stote K. S. et al. Effect of cocoa and green tea on biomarkers of glucose regulation, oxidative stress, inflammation and hemostasis in obese adults at risk for insulin resistance. Eur J Clin Nutr 66, 1153–1159, doi: 10.1038/ejcn.2012.101 (2012). [DOI] [PubMed] [Google Scholar]
- Thielecke F. et al. Epigallocatechin-3-gallate and postprandial fat oxidation in overweight/obese male volunteers: a pilot study. Eur J Clin Nutr 64, 704–713, doi: 10.1038/ejcn.2010.47 (2010). [DOI] [PubMed] [Google Scholar]
- Borchardt R. T. & Huber J. A. Catechol O-methyltransferase. 5. Structure-activity relationships for inhibition by flavonoids. J Med Chem 18, 120–122 (1975). [DOI] [PubMed] [Google Scholar]
- Valenti D. et al. Epigallocatechin-3-gallate prevents oxidative phosphorylation deficit and promotes mitochondrial biogenesis in human cells from subjects with Down's syndrome. Biochim Biophys Acta 1832, 542–552, doi: 10.1016/j.bbadis.2012.12.011 (2013). [DOI] [PubMed] [Google Scholar]
- Watanabe J., Kawabata J. & Niki R. Isolation and identification of acetyl-CoA carboxylase inhibitors from green tea (Camellia sinensis). Biosci Biotechnol Biochem 62, 532–534 (1998). [DOI] [PubMed] [Google Scholar]
- Murase T., Haramizu S., Shimotoyodome A., Nagasawa A. & Tokimitsu I. Green tea extract improves endurance capacity and increases muscle lipid oxidation in mice. Am J Physiol Regul Integr Comp Physiol 288, R708–715, doi: 10.1152/ajpregu.00693.2004 (2005). [DOI] [PubMed] [Google Scholar]
- Sae-Tan S., Grove K. A., Kennett M. J. & Lambert J. D. (-)-Epigallocatechin-3-gallate increases the expression of genes related to fat oxidation in the skeletal muscle of high fat-fed mice. Food Funct 2, 111–116, doi: 10.1039/c0fo00155d (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase T., Haramizu S., Shimotoyodome A., Tokimitsu I. & Hase T. Green tea extract improves running endurance in mice by stimulating lipid utilization during exercise. Am J Physiol Regul Integr Comp Physiol 290, R1550–1556, doi: 10.1152/ajpregu.00752.2005 (2006). [DOI] [PubMed] [Google Scholar]
- Henning S. M. et al. Bioavailability and antioxidant effect of epigallocatechin gallate administered in purified form versus as green tea extract in healthy individuals. J Nutr Biochem 16, 610–616, doi: 10.1016/j.jnutbio.2005.03.003 (2005). [DOI] [PubMed] [Google Scholar]
- Klaus S., Pultz S., Thone-Reineke C. & Wolfram S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int J Obes (Lond) 29, 615–623, doi: 10.1038/sj.ijo.0802926 (2005). [DOI] [PubMed] [Google Scholar]
- Lorenz M. et al. The activity of catechol-O-methyltransferase (COMT) is not impaired by high doses of epigallocatechin-3-gallate (EGCG) in vivo. Eur J Pharmacol 740, 645–651, doi: 10.1016/j.ejphar.2014.06.014 (2014). [DOI] [PubMed] [Google Scholar]
- Astrup A. et al. Caffeine: a double-blind, placebo-controlled study of its thermogenic, metabolic, and cardiovascular effects in healthy volunteers. Am J Clin Nutr 51, 759–767 (1990). [DOI] [PubMed] [Google Scholar]
- Haemmerle G. et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med 17, 1076–1085, doi: 10.1038/nm.2439 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meex R. C. et al. ATGL-mediated triglyceride turnover and the regulation of mitochondrial capacity in skeletal muscle. Am J Physiol Endocrinol Metab 308, E960–970, doi: 10.1152/ajpendo.00598.2014 (2015). [DOI] [PubMed] [Google Scholar]
- Liu J. et al. (-)-Epigallocatechin-3-gallate attenuated myocardial mitochondrial dysfunction and autophagy in diabetic Goto-Kakizaki rats. Free Radic Res 48, 898–906, doi: 10.3109/10715762.2014.920955 (2014). [DOI] [PubMed] [Google Scholar]
- Roberts J. D., Roberts M. G., Tarpey M. D., Weekes J. C. & Thomas C. H. The effect of a decaffeinated green tea extract formula on fat oxidation, body composition and exercise performance. J Int Soc Sports Nutr 12, 1, doi: 10.1186/s12970-014-0062-7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler A. et al. Metabolic response to epigallocatechin-3-gallate in relapsing-remitting multiple sclerosis: a randomized clinical trial. Am J Clin Nutr 101, 487–495, doi: 10.3945/ajcn.113.075309 (2015). [DOI] [PubMed] [Google Scholar]
- Suliburska J. et al. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol Trace Elem Res 149, 315–322, doi: 10.1007/s12011-012-9448-z (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanski P. et al. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res 32, 421–427, doi: 10.1016/j.nutres.2012.05.007 (2012). [DOI] [PubMed] [Google Scholar]
- Josic J., Olsson A. T., Wickeberg J., Lindstedt S. & Hlebowicz J. Does green tea affect postprandial glucose, insulin and satiety in healthy subjects: a randomized controlled trial. Nutr J 9, 63, doi: 10.1186/1475-2891-9-63 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin L., Miguelez E. M., Villar C. J. & Lombo F. Bioavailability of dietary polyphenols and gut microbiota metabolism: antimicrobial properties. Biomed Res Int 2015, 905215, doi: 10.1155/2015/905215 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfora E. E., Jocken J. W. & Blaak E. E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol, doi: 10.1038/nrendo.2015.128 (2015). [DOI] [PubMed] [Google Scholar]
- Lambert J. D., Sang S. & Yang C. S. Biotransformation of green tea polyphenols and the biological activities of those metabolites. Mol Pharm 4, 819–825, doi: 10.1021/mp700075m (2007). [DOI] [PubMed] [Google Scholar]
- Ullmann U. et al. Plasma-kinetic characteristics of purified and isolated green tea catechin epigallocatechin gallate (EGCG) after 10 days repeated dosing in healthy volunteers. Int J Vitam Nutr Res 74, 269–278 (2004). [DOI] [PubMed] [Google Scholar]
- Adriaens M. P., Schoffelen P. F. & Westerterp K. R. Intra-individual variation of basal metabolic rate and the influence of daily habitual physical activity before testing. Br J Nutr 90, 419–423 (2003). [DOI] [PubMed] [Google Scholar]
- Schoffelen P. F., Westerterp K. R., Saris W. H. & Ten Hoor F. A dual-respiration chamber system with automated calibration. J Appl Physiol 83, 2064–2072 (1997). [DOI] [PubMed] [Google Scholar]
- Harris J. A. & Benedict F. G. A Biometric Study of Human Basal Metabolism. Proc Natl Acad Sci USA 4, 370–373 (1918). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim M. et al. The phytoestrogen genistein enhances osteogenesis and represses adipogenic differentiation of human primary bone marrow stromal cells. Endocrinology 145, 848–859, doi: 10.1210/en.2003-1014 (2004). [DOI] [PubMed] [Google Scholar]
- Arner P. & Bulow J. Assessment of adipose tissue metabolism in man: comparison of Fick and microdialysis techniques. Clin Sci (Lond) 85, 247–256 (1993). [DOI] [PubMed] [Google Scholar]
- Hickner R. C. et al. Ethanol may be used with the microdialysis technique to monitor blood flow changes in skeletal muscle: dialysate glucose concentration is blood-flow-dependent. Acta Physiol Scand 143, 355–356 (1991). [DOI] [PubMed] [Google Scholar]
- Arner P. Techniques for the measurement of white adipose tissue metabolism: a practical guide. Int J Obes Relat Metab Disord 19, 435–442 (1995). [PubMed] [Google Scholar]
- Frayn K. N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55, 628–634 (1983). [DOI] [PubMed] [Google Scholar]
- Weir J. B. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109, 1–9 (1949). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.