Natural isothiocyanate sulforaphane targets multiple inflammasomes to inhibit caspase-1 activation, IL-1β maturation, and secretion in in vivo inflammatory responses.
Keywords: caspase-1, IL-1, NLRP1, NLRP3, inflammation
Abstract
The inflammasomes are intracellular complexes that have an important role in cytosolic innate immune sensing and pathogen defense. Inflammasome sensors detect a diversity of intracellular microbial ligands and endogenous danger signals and activate caspase-1, thus initiating maturation and release of the proinflammatory cytokines interleukin-1β and interleukin-18. These events, although crucial to the innate immune response, have also been linked to the pathology of several inflammatory and autoimmune disorders. The natural isothiocyanate sulforaphane, present in broccoli sprouts and available as a dietary supplement, has gained attention for its antioxidant, anti-inflammatory, and chemopreventive properties. We discovered that sulforaphane inhibits caspase-1 autoproteolytic activation and interleukin-1β maturation and secretion downstream of the nucleotide-binding oligomerization domain-like receptor leucine-rich repeat proteins NLRP1 and NLRP3, NLR family apoptosis inhibitory protein 5/NLR family caspase-1 recruitment domain-containing protein 4 (NAIP5/NLRC4), and absent in melanoma 2 (AIM2) inflammasome receptors. Sulforaphane does not inhibit the inflammasome by direct modification of active caspase-1 and its mechanism is not dependent on protein degradation by the proteasome or de novo protein synthesis. Furthermore, sulforaphane-mediated inhibition of the inflammasomes is independent of the transcription factor nuclear factor erythroid-derived 2-like factor 2 (Nrf2) and the antioxidant response-element pathway, to which many of the antioxidant and anti-inflammatory effects of sulforaphane have been attributed. Sulforaphane was also found to inhibit cell recruitment to the peritoneum and interleukin-1β secretion in an in vivo peritonitis model of acute gout and to reverse NLRP1-mediated murine resistance to Bacillus anthracis spore infection. These findings demonstrate that sulforaphane inhibits the inflammasomes through a novel mechanism and contributes to our understanding of the beneficial effects of sulforaphane.
Introduction
The inflammasomes are large, cytosolic, multiprotein complexes that form in response to diverse intracellular microbial ligands and endogenous danger signals (reviewed in [1]). Inflammasome activation follows signaling through a cytosolic sensor, which results in recruitment of the cysteine protease caspase-1 (formerly interleukin-1β-converting enzyme) to the inflammasome platform and its autoproteolytic or conformational activation. Activated caspase-1 initiates maturation and secretion of the proinflammatory cytokines IL-1β and IL-18 and, in some situations, induces pyroptosis, a rapid inflammatory cell death.
Several different NLRPs act as inflammasome receptors, each being activated through a different mechanism. For example, murine NLRP1b and rat NLRP1 are activated after cleavage of an N-terminal domain by the Bacillus anthracis LT protease [2, 3]. NLRP1 is also activated through an unknown mechanism by the parasite Toxoplasma gondii [4–6]. Direct binding by bacterial flagellin leads to activation of NAIP5/NLRC4 [7, 8]. NLRP3 is not known to engage in direct ligand binding but detects diverse activating “danger” signals, including potassium efflux, mitochondrial damage, ATP, and uric acid (reviewed in [1, 9]). In addition to the NLR family of inflammasome sensors, absent in melanoma 2 activates the inflammasome in response to cytosolic double-stranded DNA [10–13]. Although some NLR sensors, such as NLRP3, require up-regulation through a “signal 1,” such as LPS/NF-κB signaling [14], NLRP1 activation by LT and induction of pyroptosis does not require signal 1 [15, 16].
Although the inflammasomes have been shown to have an important role in defense against pathogens and infection (reviewed in [9]), they have also been implicated in inflammatory and autoimmune disorders, including colitis, type I diabetes, multiple sclerosis, vitiligo, and atherosclerosis (reviewed in [17, 18]). For this reason, targeting the inflammasomes may be an effective means of controlling these and similar disorders.
SFN is an isothiocyanate found in broccoli sprout extracts [19]. Isothiocyanates, such as SFN, are known to react with sulfhydryl groups and thus modify proteins at cysteine residues. SFN has been studied for its antioxidant, apoptosis-inducing, and anti-inflammatory effects (reviewed in [20, 21]). It has been suggested to have cytoprotective roles in cardiovascular, neurologic, and other diseases (reviewed in [21]) as well as potential for cancer prevention or treatment [20, 22]. To better understand the underlying mechanism responsible for these effects, SFN has been extensively studied for its role in inducing the Nrf2/ARE antioxidant pathway [23]. SFN stabilizes and activates the Nrf2 transcription factor by reacting with cysteine residues of its repressor, Keap1 [24]. Keap1 facilitates proteasomal degradation of Nrf2 under normal cellular conditions, and its cysteine residues act as sensors for oxidative stress [25, 26]. Nrf2 activation by SFN induces expression of antioxidant and detoxification genes, such as glutathione S-transferase, heme oxygenase (decycling) 1, NAD(P)H, quinone oxidoreductase, and UDP glucuronosyltransferase [27]. Nrf2-independent effects of SFN, such as induction of cell cycle arrest and apoptosis, and inhibition of angiogenesis, histone deacetylases, and cytochrome P450 may also contribute to its cytoprotective and cancer chemopreventive abilities (reviewed in [20]). Additionally, evidence suggests SFN may react directly with other cellular targets, such as TLR4 [28] and tubulin [29].
In the current study, we discovered a novel, previously unknown, cytoprotective and anti-inflammatory role for SFN. We found that SFN inhibits multiple inflammasomes in an Nrf2-independent manner. This discovery may lead to a better understanding of the mechanisms by which SFN protects against inflammatory diseases.
MATERIALS AND METHODS
Reagents and toxins
SFN and erucin were purchased from Cayman Chemical (Ann Arbor, MI, USA). Cycloheximide, actinomycin D, NAC, buthionine sulfoximine, butylated hydroxyanisole, melatonin, propidium iodide, ATP, PEITC, and thiazolyl blue tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Nigericin, lactacystin, Boc-D-CMK, trolox, and ultrapure LPS were purchased from Calbiochem (San Diego, CA, USA). Poly(dA:dT) was purchased from InvivoGen (San Diego, CA, USA). TurboFect was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Active recombinant murine caspase-1 was purchased from MBL International Corporation (Woburn, MA, USA).
B. anthracis spore preparation
B. anthracis spores were prepared from the nonencapsulated, toxigenic strain Ames 35 [30] grown on sporulation agar at 37°C for 1 d, then 5 d at 30°C, and inspected by microscopy for >95% sporulation. Spores were purified by 4 rounds of centrifugation and sterile water washes, followed by 2 treatments at 70°C for 30 min to kill remaining vegetative bacteria. Spore quantification was performed using a Petroff Hausser bacterial counting chamber (Hausser Scientific, Horsham, PA, USA), and CFUs were verified.
Antibodies
Anti-MEK1 NT (44492) was purchased from Calbiochem, anti-MEK3 NT (sc-959) and anti-caspase-1 p10 (sc-514) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and anti-IL-1β (AF-401-NA) was purchased from R&D Systems (Minneapolis, MN, USA). The secondary antibody, infrared dye 800CW-conjugated anti-rabbit IgG (926-32211), was purchased from LI-COR Biosciences (Lincoln, NE, USA), and infrared dye 800CW-conjugated anti-goat IgG (605-731-125) was purchased from Rockland Immunochemicals (Gilbertsville, PA, USA).
Anthrax LT is made of 2 proteins: a receptor-binding component PA and a protease LF. PA and LF were purified from B. anthracis, as previously described [31]. Concentrations of LT describe the concentrations of each toxin component (i.e., 1 μg/ml LT is 1 μg/ml LF and 1 μg/ml PA). LFn-Fla is a fusion protein of LFn, the PA-binding moiety of LF (aa 1–254), to full-length flagellin from Legionella pneumophila (a gift from Dr. Russel Vance, University of California at Berkeley, Berkeley, CA, USA) [32]. PA delivers LFn-Fla into cells. FlaTox is a mixture of LFn-Fla and PA at a mass ratio of 1:2. Concentrations of FlaTox refer to the concentrations of LFn-Fla (i.e., 1 μg/ml FlaTox is 1 μg/ml LFn-Fla and 2 μg/ml PA). LF protease inhibitor (Hawaii Biotech PT-168541-1) was a kind gift from Alan Johnson (Hawaii Biotech, Honolulu, HI, USA). FP59 is a fusion protein of LFn to the Pseudomonas aeruginosa exotoxin A catalytic domain [31]. This chimeric toxin is transported to the cytosol by PA and ADP-ribosylates elongation factor 2, inhibiting protein synthesis.
Animals
All animal studies were performed in accordance with U.S. National Institutes of Health and Animal Welfare Act guidelines and protocols LPD8E and LPD9E, approved by the Animal Care and Use Committee of the U.S. National Institute of Allergy and Infectious Disease, U.S. National Institutes of Health. C57BL/6J, Balb/cJ and Nrf2−/− mice (on the C57BL/6 background) were purchased from Jackson laboratories (Bar Harbor, ME, USA).
Cell culture
Murine RAW264.7 macrophage and L929 fibroblast cells were cultured in DMEM supplemented with 10% FBS, 20 mM HEPES, and 100 μg/ml gentamicin (all purchased from Life Technologies, Grand Island, NY, USA). Mouse primary bone marrow cells were cultured in DMEM complete (as above) supplemented with 50% L929 supernatant for 7–10 d to allow for differentiation into BMDMs. All cells were cultured at 37°C and 5% CO2.
Cell viability assays
RAW264.7 or Balb/cJ BMDM cells were plated in 96-well plates and grown to 90% confluence, then treated with various drugs (doses and timing described in figure legends) or vehicle (≤0.36% vol/vol ethanol). Unless otherwise stated, drugs and SFN were not washed off before treatment with LT (1 μg/ml) or media for 2 h. Cell viability was assessed by MTT staining, as previously described [33]. For assays that included trolox or NAC treatment, the following changes were made: DMEM complete without phenol red was used, and cells were treated with propidium iodide (5 μM) for 15 min to assess cell death. Emission at 615 nm was measured using a Victor3 plate reader (PerkinElmer, Waltham, MA, USA).
MEK, caspase-1, and IL-1β cleavage
Balb/cJ, C57BL/6, or Nrf2−/− BMDM cells were treated with LPS (1 μg/ml) for 2 h, followed by various drugs (doses and timing described in figure legends) or a vehicle. Vehicle for all studies described in this work was 0.089% ethanol, unless otherwise indicated. LPS, drugs, and SFN were not washed off unless otherwise stated. Inflammasome activators were then added (LT, FlaTox, nigericin, ATP) or transfected [poly(dA:dT)] at indicated doses and timing. Cells were lysed with radioimmunoprecipitation assay buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% SDS in 1× PBS) containing protease inhibitors, including LF protease inhibitor (PT-168541-1), and Western blotting was performed as previously described [33].
In vitro caspase-1 assay
In vitro caspase-1 activity assays were performed as previously described [34]. Briefly, Balb/cJ BMDMs were treated with LPS (1 μg/ml) for 2 h to up-regulate pro-IL-1β expression. Cells were treated with SFN (50 μM) or vehicle for 30 min. Sucrose buffer (250 mM sucrose, 10 mM HEPES pH 7.4) lysates were prepared. SFN (6.25–50 μM), caspase-1 inhibitor Boc-D-CMK (400 μM), or the vehicle was added to lysates that were not pretreated with SFN. Active recombinant mouse caspase-1 (1 U) was added to 50 μl of cell lysate and was incubated for 3 h at 37°C. Caspase-1 activity was evaluated by assessing cleavage of pro-IL-1β by Western blot as previously described [33].
Evaluation of caspase-1 sequestration in a high molecular mass complex
Balb/cJ BMDMs were treated with SFN (50 μM) or vehicle. SFN-treated cells were incubated at 37°C and untreated cells were incubated at 37°C or 42°C for 1 h. Sucrose lysates were prepared (as described above) and centrifuged at 15,000 g for 10 min. Cytosolic (supernatant) and membranous (pellet) fractions were analyzed for caspase-1 by Western blotting, as previously described [33].
In vivo peritonitis model
A peritoneal model of MSU crystal-induced acute gout was performed [35]. MSU crystals were prepared by crystallization of uric acid as previously described [36]. C57BL/6J mice were injected i.p. with SFN (25 mg/kg SFN) or with vehicle (2.4% vol/vol ethanol in PBS) 5 min before injection of MSU crystals (4 mg in 250 μl PBS, i.p.), or vehicle, as described in the figure legends. At 4 h, mice were again treated with SFN or vehicle. At 6 h post-MSU treatment, mice were euthanized, and a peritoneal lavage was performed (6 ml/mouse). After erythrocyte lysis using ACK buffer (Life Technologies), cells recruited to the peritoneum were counted. IL-1β secretion in peritoneal lavage fluids was measured by ELISA (R&D Systems).
B. anthracis spore challenge
B. anthracis spore-resistant Balb/cJ and C57BL/6NTac-Nlrp1S/S mice were treated with SFN (30–32 mg/kg, i.p.) or vehicle (2.4% vol/vol ethanol in PBS, i.p.) 2 h before spore challenge, and with the same dose of drug s.c. at a site distal to spores at the time of challenge. Mice were challenged with B. anthracis Ames 35 (2–5×107 spores, s.c.). Spore-susceptible C57BL/6J and C57BL/6NTac-Nlrp1bR/R mice were challenged with 2–5×107 spores as controls. Animals were monitored for survival.
Statistical analysis
Statistical analysis was performed with either a Student’s t test or 1-way ANOVA with a Bonferroni correction using GraphPad Prism software (version 6.0; GraphPad Software, La Jolla, CA, USA).
RESULTS
SFN and related isothiocyanates protect RAW264.7 cells and primary BMDMs from LT-induced pyroptosis
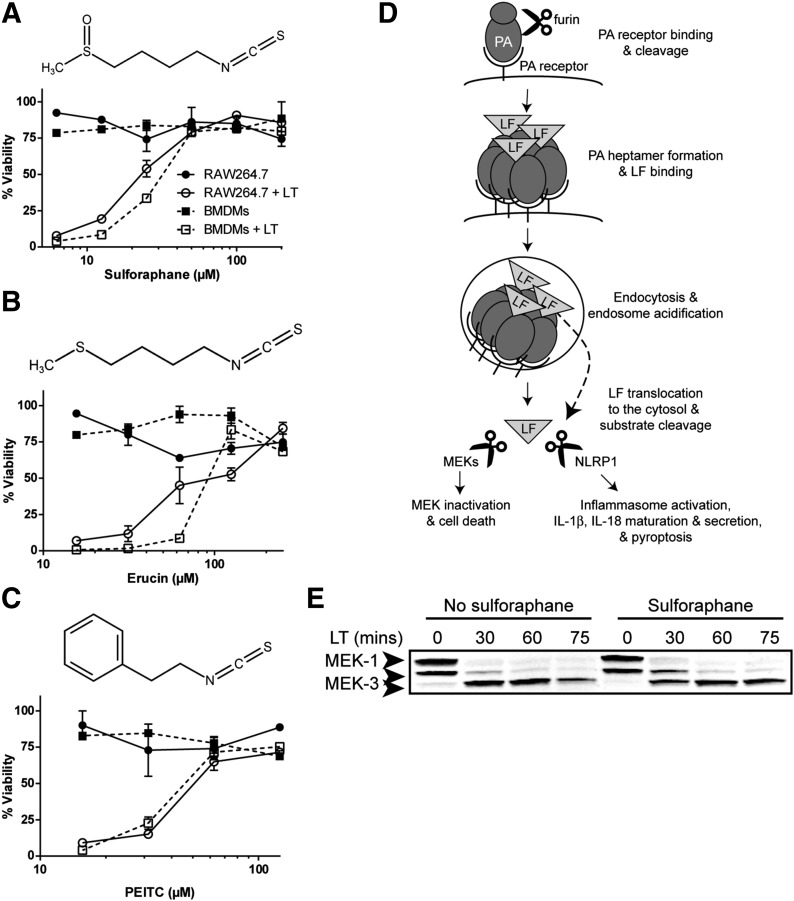
LT activates the NLRP1b inflammasome in a signal 1-independent manner [15, 16] in macrophages from certain inbred mouse strains, such as Balb/cJ and the Balb/cJ-derived RAW264.7 cell line [15, 37] by cleaving the N terminus of this sensor [2, 3]. This activation results in a rapid toxin-induced lysis of macrophages (pyroptosis), quantifiable by assessment of mitochondrial activity [15, 38, 39], which can be used as an assay to screen for inhibitors of inflammasome activation that lead to protection from cell death. SFN treatment was found to inhibit LT-induced pyroptosis over a range of concentrations (50–200 μM) in both RAW264.7 and primary BMDM cells (Fig. 1A). Other isothiocyanates, including erucin [1-isothiocyanato-4-(methylthio)-butane] and PEITC also inhibited LT-induced pyroptosis, although with less potency, achieving complete protection at 125 μM (Fig. 1B and C).
Figure 1. SFN inhibits LT-induced pyroptosis but does not inhibit LF release into the cytosol or protease activity.
(A–C, top) Chemical structure of SFN (A), erucin (B), and PEITC (C). (A–C, bottom) RAW264.7 or primary Balb/cJ BMDM cells were treated with or without SFN (A) for 30 min or erucin (B) or PEITC (C) for 1 h over a range of concentrations. Cells were then treated with or without LT (1 μg/ml) for 2 h, and viability was assessed by MTT staining. Data are means ± sem of 2 biologic replicates and are representative of 2 or more independent experiments. Legend is shown in (A). (D) Steps required in macrophage entry and substrate cleavage by B. anthracis LF: PA binds its receptor, is cleaved by the furin protease, and oligomerizes to form a heptamer. LF binds the PA heptamer, the complex is endocytosed, and endosome acidification triggers PA pore formation and LF translocation to the cytosol. LF cleaves its substrates, the MEKs, initiating cell-cycle arrest and apoptosis, and NLRP1 (in select rodent strains), leading to caspase-1 recruitment and activation, IL-1β and IL-18 maturation and secretion, and pyroptosis (modified from Liu et al. [40]). LF translocation to the cytosol and protease activity can be monitored by MEK cleavage. (E) Balb/cJ BMDM cells were treated with or without SFN (50 μM) for 30 min then with or without LT (1 μg/ml) for 30, 60, or 75 min and MEK1 and MEK3 cleavage in cell lysates was assessed by Western blot. The N-terminal epitope recognized by the anti-MEK1 Ab is lost upon LF cleavage. The N-terminal epitope recognized by the anti-MEK3 Ab is not lost, and cleavage is observed by a change in mobility. The same gel that was probed for MEK1 was probed for MEK3, demonstrating equal protein loading. Data are representative of 2 independent experiments.
SFN does not inhibit LF translocation or protease activity
Because SFN protected against LT-induced pyroptosis with the greatest potency, it was selected for further studies. SFN could be acting at any step in LT host-cell entry or LF protease activity, upstream of inflammasome activation. These steps include PA receptor binding, cleavage, and oligomerization, LF binding to the PA oligomer, endocytosis of LT, acidification of the endosome, PA pore formation, translocation of LF into the cytosol, and LF proteolytic cleavage of its cellular substrates ([40], as summarized in Fig. 1D). Upon release into the cytosol, LF cleaves its cellular substrates: multiple MEKs [41, 42] and NLRP1 [2, 3]. Inhibition of LT-mediated cell death may result from either inhibition of the inflammasome or any of the steps upstream of inflammasome activation mentioned above (Fig. 1D). To distinguish between these possibilities, LF entry into the cytosol and cleavage of its MEK substrates in the presence and absence of SFN were assayed. We tested for cleavage of MEK1, resulting in loss of an N-terminal epitope, and for cleavage of MEK3, by a change in mobility in SFN- and LT-treated Balb/cJ BMDMs [15, 33, 43]. SFN treatment was not found to inhibit LT cleavage of MEK1 or MEK3 (Fig. 1E), demonstrating that LT translocates to the cytosol and cleaves its substrates with equal efficiency in the presence or absence of SFN. Thus, the drug inhibits the NLRP1 inflammasome and not LF translocation or protease activity.
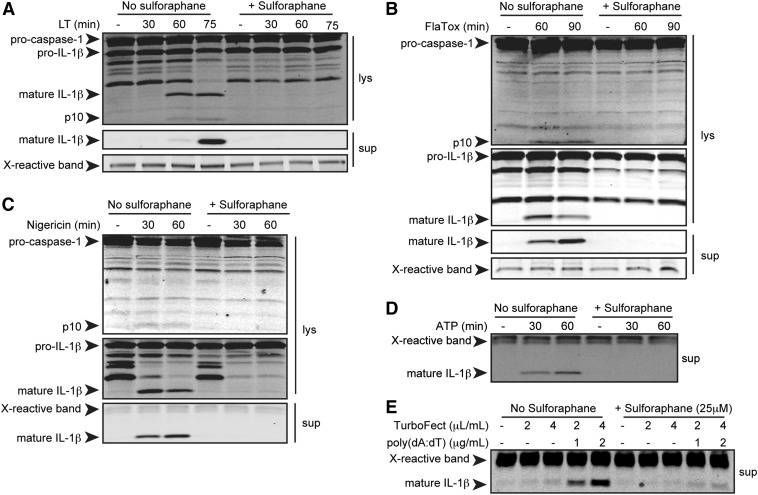
SFN inhibits caspase-1 cleavage and IL-1β maturation for the NLRP1b, NLRP3, NAIP5/NLRC4, and AIM2 inflammasomes
To determine whether SFN-mediated inhibition of LT-induced pyroptosis was due to inhibition of inflammasome activation, we tested the effects of SFN on caspase-1 autoproteolytic activation and IL-1β cleavage. SFN has been reported to inhibit NF-κB signaling at doses ranging from 5 to 50 μM [44–46]. Inhibition of this transcription factor could affect pro-IL-1β or NLR levels. Therefore, to ensure observed effects of SFN treatment were due to the inhibition of inflammasome activation and not to priming, BMDMs were treated with the TLR4 agonist LPS for 2 h to up-regulate expression of inflammasome components and pro-IL-1β before treatment with SFN. Cells were then treated with SFN followed by LT (an NLRP1b agonist), nigericin or ATP (NLRP3 agonists), or FlaTox (a NAIP/NLRC4 agonist). For activation of the AIM2 inflammasome, cells were transfected with poly(dA:dT) using doses of a cationic polymer transfection agent that would not activate the NLRP3 inflammasome through induction of general ion fluxes (Fig. 2E). IL-1β and caspase-1 maturation in cell lysates, supernatants, or both, prepared at various times, was assessed by Western blot. SFN was found to inhibit caspase-1 and IL-1β processing following activation of each tested inflammasome sensor (Fig. 2). This suggests that SFN inhibits the 4 inflammasomes through a common mechanism that is not inflammasome receptor-specific. Inhibition of the NLRP1 inflammasome, which does not require induction through a priming signal and functions in an NF-κB-independent manner, also supports the hypothesis that SFN acts at a step downstream of inflammasome priming. Furthermore, SFN inhibited NLRP1b-dependent pyroptosis and IL-1β processing in RAW264.7 cells (Fig. 1A and data not shown). These cells are deficient in the common inflammasome adaptor, ASC [47]. Thus, SFN also acts in an ASC-independent manner.
Figure 2. SFN inhibits caspase-1 and IL-1β cleavage downstream of multiple inflammasome receptors.
Balb/cJ primary BMDMs were primed with LPS (1 μg/ml) for 2 h then incubated with or without SFN (A–D, 50 μM; E, 25 μM) for 30 min, followed with or without LT (A; 1 μg/ml) for 30, 60, or 75 min; FlaTox (B; 1 μg/ml) for 60 or 90 min; nigericin (C; 50 μM); ATP (D; 5 mM) for 30 or 60 min; or poly(dA:dT) (E; 1 or 2 μg/ml) with TurboFect transfection agent (0.2 or 0.4% vol/vol) or TurboFect alone for 6 h. Cell lysates (lys) and supernatants (sup) were analyzed for caspase-1 and IL-1β cleavage by Western blot. Data are representative of 2 or more independent experiments. X-reactive band is a nonspecific, cross-reactive band to demonstrate equal protein loading.
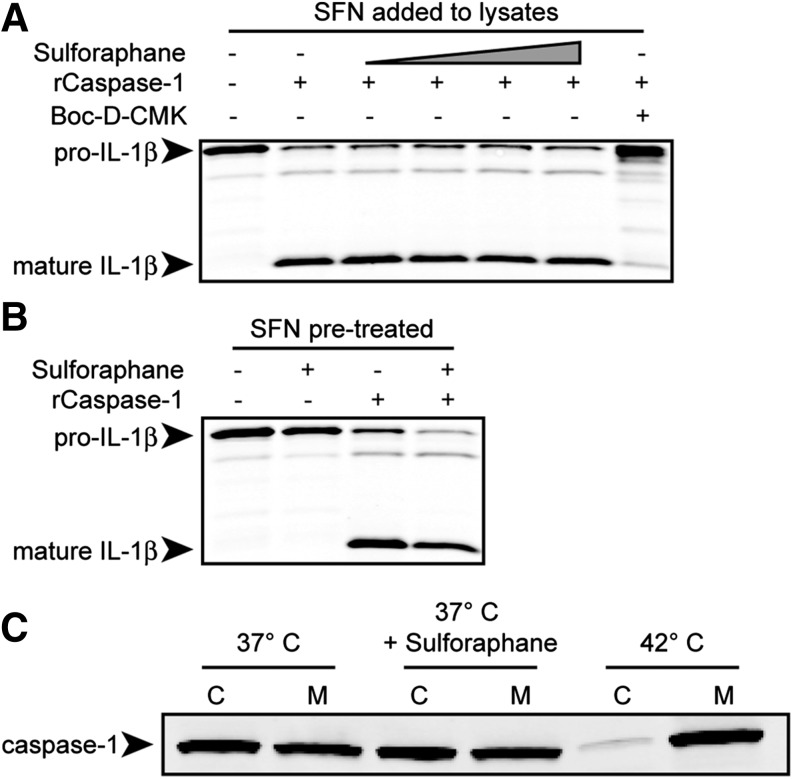
SFN does not inhibit caspase-1 enzymatic activity
SFN is known to directly modify cellular targets at cysteine residues. Because caspase-1 is a cysteine protease, we hypothesized that SFN may directly inhibit caspase-1 activity. Sucrose lysates, prepared from cells in which pro-IL-1β was up-regulated, were treated with SFN over a range of concentrations. SFN was added to cell lysates at concentrations ranging from 6.25 to 50 µM and up to 2500-fold molar excess over recombinant caspase-1. The amount of SFN added to the lysates was expected to greatly exceed the amount that was able to enter cells in previous experiments. Active recombinant caspase-1 was added to the lysates, and IL-1β cleavage was assessed as a measure of caspase-1 activity. Boc-D-CMK, a caspase-1 inhibitor, was used as a positive control for caspase-1 inhibition. SFN addition to the lysates did not inhibit IL-1β processing, demonstrating that SFN cannot directly inhibit the protease activity of caspase-1 under these conditions (Fig. 3A).
Figure 3. SFN does not inhibit recombinant caspase-1 (rCaspase-1) directly, induce a cellular state inhibitory to rCaspase-1 activity, or sequester procaspase-1 into a high-molecular-weight complex.
(A) RAW264.7 (pictured) or Balb/cJ BMDM cells were treated with LPS (1 μg/ml) for 2 h, and sucrose lysates were prepared. Boc-D-CMK (400 μM) or SFN at varying concentrations (0, 6.25, 12.5, 25, or 50 μM) was added to cell lysates. (B) RAW264.7 (pictured) or Balb/cJ BMDM cells were treated with LPS (1 μg/ml) for 2 h, then with SFN (50 μM) for 1 h before preparation of sucrose lysates. (A and B) Murine active recombinant caspase-1 (rCaspase-1) was added to the lysates. Lysates were incubated at 37°C for 3 h. To measure rCaspase-1 activity, IL-1β processing was evaluated by Western blot. (C) Balb/cJ BMDMs were pretreated with or without SFN (50 μM) at 37°C or without SFN at 42°C for 1 h. Sucrose lysates were prepared and fractionated by centrifugation into cytosolic (C) or membranous (M) fractions. Western blotting for caspase-1 was performed to determine its localization to either the cytosol or membrane-bound, high-molecular-weight protein complex. All data are representative of 2 or more independent experiments.
Next, it was hypothesized that SFN induces a cellular state that is inhibitory to active caspase-1. This could include modification, up-regulation, or degradation of a cellular protein that can directly modulate caspase-1 activity. To test this hypothesis, BMDMs were treated with LPS to up-regulate pro-IL-1β, with or without SFN, before preparing cell lysates. Active recombinant caspase-1 was added to lysates, and IL-1β cleavage was assessed. IL-1β processing was not inhibited when cells were pretreated with SFN, indicating that SFN does not induce a cellular state or protein that inhibits caspase-1 enzymatic activity (Fig. 3B). Neither pretreatment of cells with SFN nor addition of the drug to cell lysates could inhibit IL-1β processing, which suggests that SFN has no effect on the cysteine residues involved in caspase-1 activity. However, SFN may bind directly to other cysteine residues in procaspase-1 to affect its activation in cells.
SFN does not lead to sequestration of caspase-1 into large, membrane-bound protein complexes
Heat shock induces procaspase-1 sequestration into large, membrane-bound protein complexes, which prevent its activation [43], and SFN induces a heat shock-like response [48]. Thus, we hypothesized that SFN may induce procaspase-1 sequestration, preventing its activation through a heat shock-like mechanism. We found that although heat shocking BMDMs for 1 h at 42°C caused caspase-1 to be detected largely in the membranous fraction of cell lysates, treatment with SFN did not, demonstrating that SFN does not inhibit the inflammasomes through a heat shock-like mechanism (Fig. 3C).
SFN does not require proteasomal breakdown of specific proteins or de novo protein synthesis for inflammasome inhibition
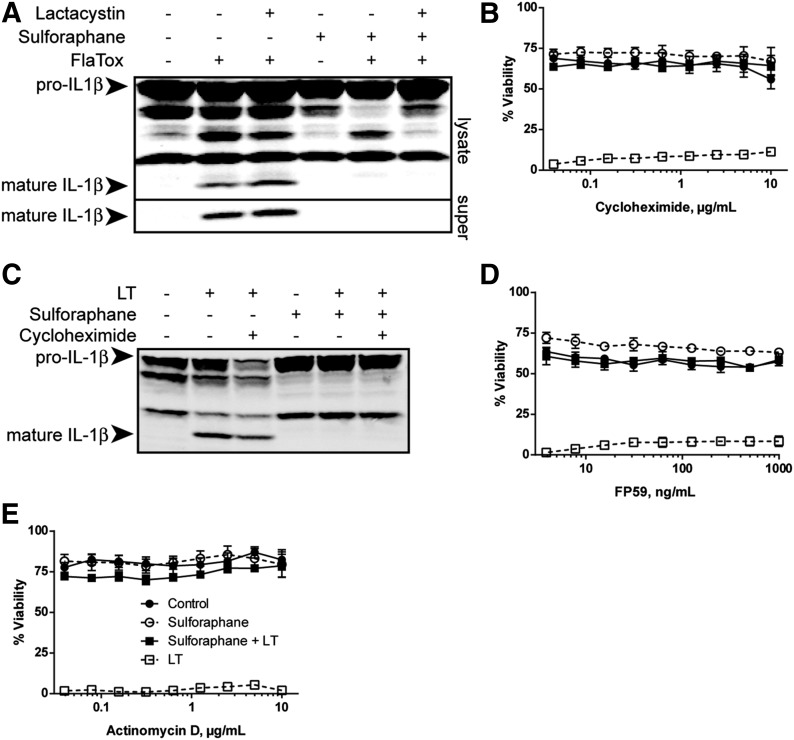
SFN treatment at low concentrations and activation of the Nrf2 pathway has been reported to up-regulate proteasome expression [49–51]. We investigated whether protein degradation by the proteasome or de novo protein synthesis was required for SFN-mediated inflammasome inhibition. Unlike NLRP3 and NAIP5/NLRC4 inflammasomes, activation of the NLRP1b inflammasome requires proteasome degradation activity [15, 16, 52]. Therefore, we tested the effect of the proteasome inhibitor lactacystin on SFN-mediated inhibition of the NLRC4 inflammasome. Lactacystin did not reverse SFN-mediated inhibition of FlaTox-induced IL-1β processing by the NLRC4 inflammasome (Fig. 4A).
Figure 4. SFN-mediated inflammasome inhibition requires neither proteasomal breakdown of specific proteins nor de novo protein synthesis.
(A) Balb/cJ BMDMs were treated with or without lactacystin (20 μM) for 1 h, then with or without SFN (50 μM) for 30 min, followed with or without FlaTox (1 μg/ml) for 90 min to activate the NAIP5/NLRC4 inflammasome. Western blotting of cell lysates and supernatants (super) was performed to assess IL-1β processing. (C) Balb/cJ BMDMs were treated with or without cycloheximide (10 μg/ml) for 1 h, then with or without SFN (50 μM) for 30 min, followed with or without LT (1 μg/ml) for 75 min. Western blotting of cell lysates was performed to assess IL-1β processing. (A and B) Data are representative of 2 independent experiments. (B, D, and E) RAW264.7 cells were treated with cycloheximide (B) for 1 h, FP59 (D) for 2 h with 10 ng/ml PA, or actinomycin D (E) for 1 h over a range of concentrations. After 2 h, FP59 was washed off cells. Cells were then treated with or without SFN (50 μM) for 30 min and with or without LT (1 μg/ml) for 2 h (B and E) or 4 h (D). Cell viability was assessed by MTT staining. Data represent means ± sem of 4 or more replicates. Legend is shown in (E).
SFN manifests many of its effects through activation of the Nrf2 transcription factor and by up-regulating antioxidant and detoxification proteins [53]. Cycloheximide, an inhibitor of protein translation, did not reverse protection by SFN against LT-induced pyroptosis (Fig. 4B) or rescue LT-induced IL-1β processing in SFN-treated BMDMs (Fig. 4C). Similarly, FP59, a potent enzymatic inhibitor of protein translation, did not reverse SFN-mediated protection against pyroptosis (Fig. 4D). Furthermore, actinomycin D, a transcription inhibitor, did not reverse the protective effects of SFN on LT-induced pyroptosis (Fig. 4E). Collectively, these data indicate that neither proteasome-dependent proteolysis nor de novo protein synthesis is required for SFN-mediated inhibition of the inflammasomes.
SFN-mediated inhibition of the inflammasomes is not affected by ROS modulation
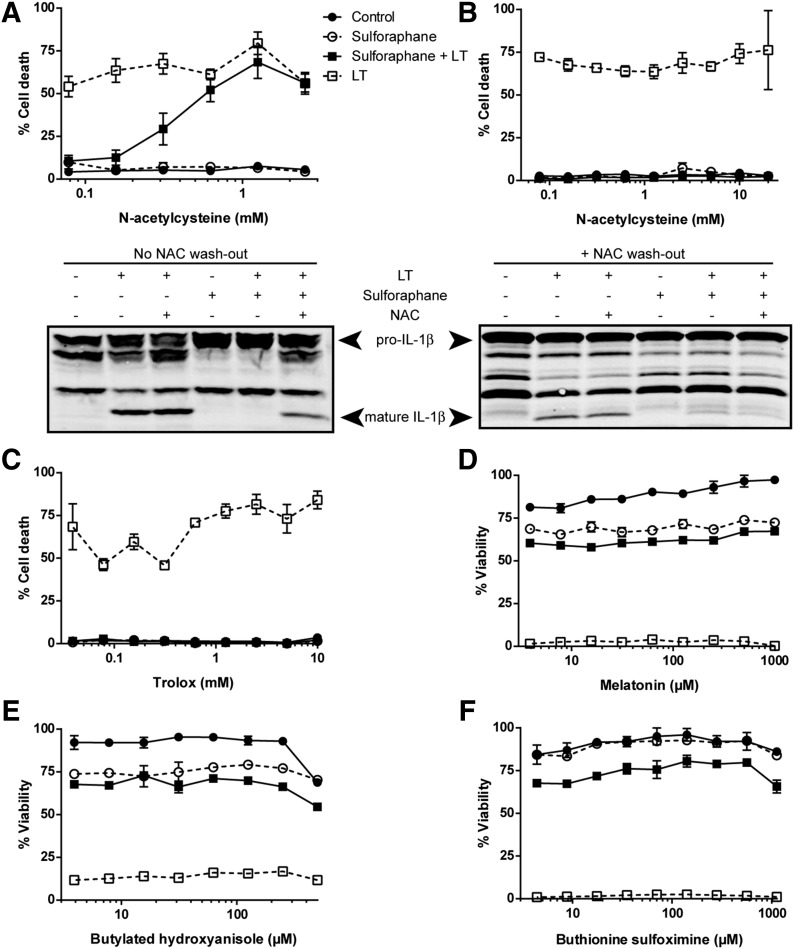
Because SFN is known to have an important role in the antioxidant response, it was hypothesized that SFN-mediated inhibition of the inflammasomes may be affected by modulation of ROS. Several studies have suggested that SFN modifies cellular activities in an ROS-dependent manner and that NAC, a ROS scavenger, can reverse SFN-mediated effects [54–57]. We found that treatment with NAc-cys also reversed the protective effects of SFN on LT-induced pyroptosis in a dose-dependent manner (Fig. 5A, upper panel) and partially reversed SFN-mediated inhibition of LT-induced IL-1β processing in BMDMs (Fig. 5A, lower panel). Although NAc-cys is often used to implicate ROS in isothiocyanate-mediated actions [54–57], the NAC sulfhydryl can react directly with isothiocyanates to form NAC-isothiocyanate thiocarbamates [58] and inhibit their cellular uptake [59]. Thus, direct modification of SFN by NAC may alter its protective abilities. In support of this hypothesis, when NAC was washed off cells before treatment with SFN, protection against LT-induced pyroptosis was restored (Fig. 5B, upper panel). Washing NAC off cells before SFN treatment also partially restored inhibition of LT-induced IL-1β processing (Fig. 5B, lower panel). This partial effect may be due to residual amounts of NAC inhibiting cellular uptake of SFN or decreased activity of the NAC–SFN thiocarbamate. Furthermore, ROS scavengers trolox, butylated hydroxyanisole, and melatonin were unable to reverse the protective effects of SFN against LT-induced pyroptosis (Fig. 5C–E), suggesting that generation of ROS is not the basis for SFN-mediated inhibition of the inflammasomes. Buthionine sulfoximine, a glutathione inhibitor, also did not affect SFN-mediated inhibition of LT-induced pyroptosis (Fig. 5F), demonstrating that SFN induction of glutathione S-transferase and glutathione reductase [27] is not involved in the SFN effects on the inflammasomes. Together, these data demonstrate that ROS modulation does not affect the ability of SFN to inhibit the inflammasomes.
Figure 5. ROS modulation does not affect SFN-mediated inhibition of the NRLP1b inflammasome.
RAW264.7 cells were treated with the ROS scavengers NAC (A and B), trolox (C), melatonin (D), butylated hydroxyanisole (E), or the glutathione inhibitor buthionine sulfoximine (F) over a range of concentrations for 1 h. After 1 h, NAC was washed out in (B) but not in (A). No other drugs were washed out. Cells were then treated with or without SFN at 50 μM for 30 min, followed by treatment with or without LT (1 μg/ml) for 2 h. (A–C) Cell death was assessed by propidium iodide staining, and (D–F) cell viability was assessed by MTT staining. Data are the means ± sem of 2 or more biologic replicates. Legend is shown in (A). (A and B, lower panels) RAW264.7 cells were treated with or without NAC (1 mM) for 1 h. NAC was washed out in (B) but not in (A). Cells were then treated with or without SFN (50 μM) for 30 min, followed with or without LT (1 μg/ml) for 75 min. Western blotting of cell lysates was performed to assess IL-1β processing.
SFN inhibits the NLRP3 inflammasome in an Nrf2-independent manner
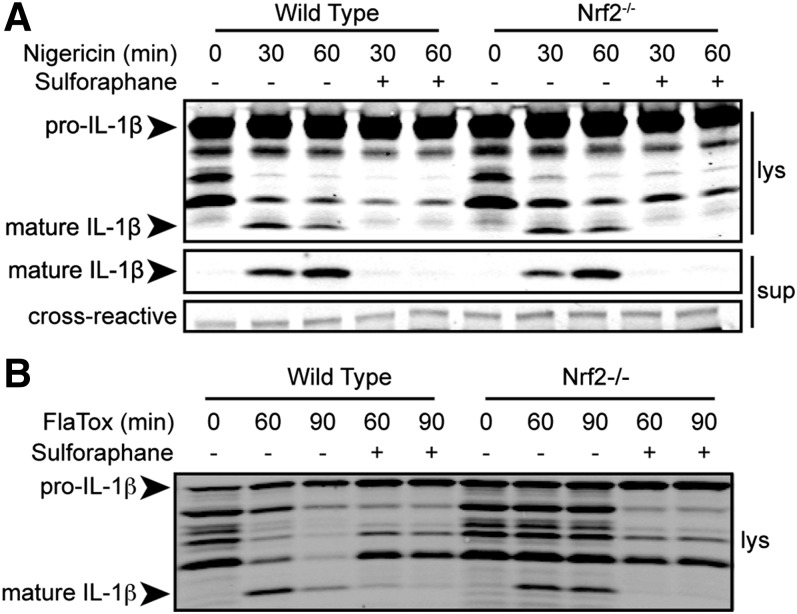
SFN is best-studied for its role in inducing the Nrf2/ARE antioxidant pathway [23]. However, SFN-mediated inhibition of the NLRP1 inflammasome does not require de novo protein synthesis and is unaffected by ROS modulation. Together, these data suggest that Nrf2 is not important in SFN-mediated inhibition of the inflammasomes. To confirm this, Nrf2−/− and wild-type BMDMs were treated with SFN, and then, with nigericin or FlaTox to activate the NLRP3 and NRLC4 inflammasomes, respectively. SFN-mediated inhibition of NLRP3- and NLRC4-dependent IL-1β processing and secretion was not reversed in Nrf2−/− BMDMs (Fig. 6), demonstrating that SFN inhibits the inflammasome in an Nrf2-independent manner.
Figure 6. SFN inhibits the NLRP3 and NAIP5/NLRC4 inflammasomes in an Nrf2-independent manner.
Nrf2−/− or wild-type C57BL/6J BMDMs were treated with LPS (1 μg/ml) for 2 h, then with or without SFN (50 μM) for 30 min, followed with or without nigericin (A; 50 μM) for 30 or 60 min or FlaTox (B; 1 μg/ml) for 60 or 90 min. Cell lysates were prepared and IL-1β processing was assessed by Western blotting of cell lysates (lys) and supernatants (sup). Cross-reactive is a nonspecific cross-reactive band to demonstrate equal protein loading.
SFN inhibits the inflammasome in an in vivo peritonitis model
To determine whether SFN can inhibit the inflammasome in an NLRP3-dependent peritoneal model of acute gout, cell recruitment induced by MSU crystals was compared in SFN-treated and untreated mice. MSU injection induced a robust recruitment of cells to the peritoneum, and this effect was significantly reduced in SFN-treated mice (Fig. 7A). Furthermore, MSU crystal induction of IL-1β was significantly reduced in SFN-treated animals (Fig. 7B). These data demonstrate that SFN can inhibit the NLRP3 inflammasome in a peritonitis model of acute gout.
Figure 7. SFN inhibits the inflammasome in vivo.
(A–B) C57BL/6J mice were injected i.p. with 25 mg/kg SFN or the vehicle, followed after 5 min by injection of MSU crystals (i.p.; 4 mg in 250 μl PBS) or the vehicle. After 4 h, mice were again treated with SFN or the vehicle. At 6 h after MSU treatment, a peritoneal lavage was performed and cell recruitment to the peritoneum (A) and IL-1β levels (B) were measured. N.D., not detected. (A–B) Data are pooled means ± sem of 2 independent experiments. Vehicle + MSU, n = 9; MSU + SFN, n = 9; SFN, n = 7; vehicle, n = 4. ***P < 0.001 and ****P < 0.0001. The differences seen between SFN-treated and vehicle-treated groups were not significant (P = 0.067). (C) B. anthracis-resistant Balb/cJ mice (n = 5/group) were injected with 32 mg/kg SFN or the vehicle (i.p.) at 2 h before infection and subcutaneously at the time of challenge at a site distal to spores. All mice were challenged with 2 × 107 spores (s.c.). Anthrax-susceptible C57BL/6J mice (n = 5) were challenged as controls. (D) B. anthracis-resistant C57BL/6JNTac-Nlrp1bS/S mice were injected with 30 mg/kg SFN or vehicle (i.p.) at 2 h before the infection, and s.c. at the time of the challenge at a site distal to the spores. All mice were challenged with 5 × 107 spores (s.c.). Anthrax-susceptible C57BL/6J and C57BL/6JNTac-Nlrp1bR/R mice were challenged as controls. C57BL/6NTac-Nlrp1bS/S + vehicle, n = 5; C57BL/6NTac-Nlrp1bS/S + SFN, n = 7; C57BL/6NTac-Nlrp1bR/R + vehicle, n = 7; C57BL/6 + vehicle, n = 5.
SFN reverses NLRP1b-mediated Balb/cJ resistance to B. anthracis spore challenge
NLRP1b and macrophage sensitivity to LT control mouse resistance to B. anthracis spore infection [60]. Inbred mouse strains harboring the Nlrp1bS/S allele, such as Balb/cJ, have LT-sensitive macrophages and are highly resistant to B. anthracis infection, whereas strains harboring the Nlrp1bR/R allele, such as C57BL/6J, which have LT-resistant macrophages, are susceptible [60]. Similarly, congenic C57BL/6JNTac mice, which are genetically identical at >99% of loci but harbor different Nlrp1 alleles, have different susceptibilities to B. anthracis, which are inversely correlated to their macrophage sensitivity [60]. Resistance to infection is NLRP1b-, caspase-1-, and IL-1β-mediated [60]. We found that SFN can inhibit the NLRP1b inflammasome in vivo and reverse Balb/cJ and C57BL/6JNTac-Nlrp1bS/S resistance to B. anthracis, resulting in animals succumbing to infection with the same dose and timing as B. anthracis-susceptible C57BL/6J and C57BL/6JNTac-Nlrp1bR/R mice (Fig. 7C and D).
DISCUSSION
SFN is a compound found in broccoli extracts and other cruciferous vegetables and is widely available as a dietary supplement. Its safety and lack of toxicity have been demonstrated [61]. Several clinical trials demonstrating the potential for SFN, its precursor glucoraphanin, or broccoli sprouts as a dietary supplement to ameliorate a variety of inflammation-based disorders, such as asthma and other respiratory conditions, have been completed or are currently underway. Although most effects have been attributed to activation of the Nrf2 transcription factor and the ARE antioxidant pathway (reviewed in [53]), its Nrf2-independent effects may have a previously overlooked role in many inflammatory responses.
In this study, we have shown that SFN inhibits the NLRP1b, NLRP3, NAIP/NLRC4, and AIM2 inflammasomes independent of Nrf2 and de novo protein synthesis. This inhibition occurs downstream of inflammasome priming and sensing and results in inhibition of caspase-1 activation, IL-1β processing and secretion, and macrophage pyroptosis. Although SFN inhibits multiple inflammasomes, we found that it does not directly inhibit caspase-1 activity or induce a cellular state inhibitory to this activity. The possibility remains that SFN may directly modify a cofactor responsible for procaspase-1 activation. One possible cofactor is the inflammasome adaptor ASC [1]. However, our studies also eliminated a role for ASC because this protein is dispensable for IL-1β maturation and pyroptosis downstream of the NLRP1b and NLRC4 inflammasomes [62–64]. SFN can inhibit NLRP1b-dependent pyroptosis and IL-1β maturation and secretion in ASC-deficient RAW264.7 cells [47]. Although SFN is known to induce a heat shock-like response [48] and it has been shown that thermal heat shock induces sequestration of procaspase-1 into large, membrane-bound protein complexes inhibitory to inflammasome activation [43], the heat shock-like response induced by SFN is not the basis for inflammasome inhibition.
Other possible mechanisms underlying SFN inhibition of the inflammasomes were also considered. SFN and other isothiocyanates have been shown to directly modify tubulin and alter microtubule function [29]. Microtubule dynamics have been shown to be essential for NLRP3 inflammasome activation [65]. Nevertheless, it is unlikely that SFN inhibits the inflammasomes by tubulin modification because microtubule dynamics are not required for activation of the NLRC4 or AIM2 inflammasomes [65].
We currently hypothesize that SFN acts at a step in inflammasome activation shared among the 4 different inflammasome pathways, downstream of sensing, but upstream of caspase-1 autoproteolytic activation. This step likely involves unidentified essential cofactors required for caspase-1 recruitment or conformational changes at the inflammasome platform. Alternatively, SFN may directly modify procaspase-1 before its transition to an SFN-resistant active conformation. Identifying the mechanism by which SFN inhibits inflammasomes requires further study. Proteomic approaches, such as 2-dimensional gel electrophoresis and mass spectrometry, may be used to identify candidate inflammasome cofactors or components, including procaspase-1, whose modification by SFN may lead to inflammasome inhibition.
SFN-mediated inhibition of the inflammasomes may be partially responsible for the many anti-inflammatory effects described in the literature for this compound. For example, SFN has been shown to control Helicobacter pylori colonization and IL-1β expression in both preclinical mouse models and clinical trials for gastric cancer [66–68]. Although the effect was attributed to Nrf2 in the mouse model [66], caspase-1 processing of IL-1β has been demonstrated to be a double-edged sword, important for control of H. pylori infection but also contributing to gastritis and gastric preneoplasia [69]. Thus, it is possible that an Nrf2-independent, inflammasome-dependent mechanism contributes to SFN-mediated prevention of H. pylori-associated gastric cancer.
SFN may also affect metabolic syndromes through its inhibition of the inflammasome. IL-1β has been identified as a risk factor for type II diabetes [70], and NLRP3 activation by amyloid crystals has been suggested as a mechanism for increased IL-1β in patients with type II diabetes [71]. Consuming 10 g/d broccoli sprout powder was found to significantly lower blood insulin levels in patients with type II diabetes compared with those who received placebo [72]. Similarly, SFN-mediated mitigation of the allergic response could be linked to its effects on the inflammasome. In a human nasal allergic-response model, consumption of 100 μM/d SFN in broccoli sprout extracts for 4 d before nasal exposure to diesel exhaust particles led to a significant decrease in white blood cells found in participant nasal-lavage fluid [73]. In a mouse model, diesel exhaust particle-induced lung inflammation has been shown to be a caspase-1-independent, IL-1β-dependent event, accompanied by IL-18 secretion [74]. Furthermore, in an ovalbumin-induced model of acute allergic airway inflammation, IL-1β and NLRP3 were shown to be important for eosinophil infiltration, lung tissue inflammation, and a Th2 cytokine response [75].
Interestingly, SFN has been studied for applications in cancer chemoprevention [22, 76, 77] and interception [78]. It has already been approved and used in multiple anticancer phase I and II clinical trials [both as an enriched component of broccoli sprout extracts and in the synthetic stabilized form, Sulforadex (Evgen Pharma, Liverpool, United Kingdom)] for breast cancer, prostate cancer, and acute lymphoblastic leukemia. This makes it a promising candidate for future studies in animal models and clinical trials for treatment of inflammatory disorders. It is now understood that there is a link between inflammation and the proinflammatory cytokines IL-18 and IL-1 and some cancers [79, 80]. It is possible that the chemopreventive success of SFN is linked to its anti-inflammatory effects, including inflammasome inhibition. Sulforadex has also had success in animal models of osteoarthritis and COPD and will be used in clinical trials for treatment of those diseases. Because an inflammasome has been implicated in these diseases, it is again possible that SFN inhibition of inflammatory cytokine responses has an important role in its effectiveness as a therapeutic.
Our findings demonstrate that SFN inhibits multiple inflammasomes in vitro and in vivo by a novel mechanism, independent of inflammasome priming and Nrf2 signaling. This suggests that inflammasome-mediated diseases like inflammatory bowel disease, gouty arthritis, type I and type II diabetes, atherosclerosis, and some autoimmune disorders [17, 18] may be potential targets for SFN treatment; furthermore, SFN inhibition of inflammasomes may contribute to its effects on previously described diseases. Future studies may reveal the mechanism by which SFN inhibits inflammasomes, and animal models and clinical trials may identify SFN as a promising anti-inflammatory therapy or cotherapy for diseases and disorders that are affected by inflammatory responses linked to inflammasomes.
AUTHORSHIP
A.J.G. designed and performed experiments, analyzed data, and wrote the manuscript. N.K.M. designed and performed experiments and analyzed data. S.H.L. analyzed data. M.M. designed and performed experiments, analyzed data, and wrote the manuscript.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the U.S. National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID). We thank Dr. Russell Vance for providing the LFn-Fla protein, Dr. Alan Johnson for providing the LF protease inhibitor, Devorah Crown and Brandi Butler for performing bone marrow isolation, and Rasem Fattah for protein purification of toxins. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the NIH.
Glossary
- AIM2
absent in melanoma 2
- ARE
antioxidant response element
- Boc-D-CMK
Boc-Asp(OBzl)-chloromethylketone
- BMDM
bone marrow–derived macrophage
- Keap1
kelch-like ECH-associated protein 1
- LF
lethal factor
- LFn–Fla
fusion protein of the N terminus of LF and flagellin
- LT
lethal toxin
- MSU
monosodium urate
- NAC
N-acetylcysteine
- NAIP
NLR family apoptosis inhibitory protein
- NLR
nucleotide-binding oligomerization domain-like receptor
- NLRC4
NLR family caspase-1 recruitment domain-containing protein 4
- NLRP
nucleotide-binding oligomerization domain-like receptor leucine-rich repeat protein
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- PA
protective antigen
- PEITC
phenethyl isothiocyanate (2-isothiocyanatoethyl)benzene)
- Poly(dA:dT)
poly(deoxyadenylic:deoxythymidylic)
- ROS
reactive oxygen species
- SFN
sulforaphane (1-isothiocyanato-4-[(R)-methylsulfinyl]-butane)
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1.Lamkanfi M., Dixit V. M. (2014) Mechanisms and functions of inflammasomes. Cell 157, 1013–1022. [DOI] [PubMed] [Google Scholar]
- 2.Hellmich K. A., Levinsohn J. L., Fattah R., Newman Z. L., Maier N., Sastalla I., Liu S., Leppla S. H., Moayeri M. (2012) Anthrax lethal factor cleaves mouse Nlrp1b in both toxin-sensitive and toxin-resistant macrophages. PLoS One 7, e49741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levinsohn J. L., Newman Z. L., Hellmich K. A., Fattah R., Getz M. A., Liu S., Sastalla I., Leppla S. H., Moayeri M. (2012) Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS Pathog. 8, e1002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorfu G., Cirelli K. M., Melo M. B., Mayer-Barber K., Crown D., Koller B. H., Masters S., Sher A., Leppla S. H., Moayeri M., Saeij J. P., Grigg M. E. (2014) Dual role for inflammasome sensors NLRP1 and NLRP3 in murine resistance to Toxoplasma gondii. MBio 5, e01117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cirelli K. M., Gorfu G., Hassan M. A., Printz M., Crown D., Leppla S. H., Grigg M. E., Saeij J. P., Moayeri M. (2014) Inflammasome sensor NLRP1 controls rat macrophage susceptibility to Toxoplasma gondii. PLoS Pathog. 10, e1003927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ewald S. E., Chavarria-Smith J., Boothroyd J. C. (2014) NLRP1 is an inflammasome sensor for Toxoplasma gondii. Infect. Immun. 82, 460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kofoed E. M., Vance R. E. (2011) Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477, 592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y., Yang J., Shi J., Gong Y. N., Lu Q., Xu H., Liu L., Shao F. (2011) The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477, 596–600. [DOI] [PubMed] [Google Scholar]
- 9.Lamkanfi M., Dixit V. M. (2012) Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 28, 137–161. [DOI] [PubMed] [Google Scholar]
- 10.Bürckstümmer T., Baumann C., Blüml S., Dixit E., Dürnberger G., Jahn H., Planyavsky M., Bilban M., Colinge J., Bennett K. L., Superti-Furga G. (2009) An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10, 266–272. [DOI] [PubMed] [Google Scholar]
- 11.Hornung V., Ablasser A., Charrel-Dennis M., Bauernfeind F., Horvath G., Caffrey D. R., Latz E., Fitzgerald K. A. (2009) AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandes-Alnemri T., Yu J. W., Datta P., Wu J., Alnemri E. S. (2009) AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts T. L., Idris A., Dunn J. A., Kelly G. M., Burnton C. M., Hodgson S., Hardy L. L., Garceau V., Sweet M. J., Ross I. L., Hume D. A., Stacey K. J. (2009) HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323, 1057–1060. [DOI] [PubMed] [Google Scholar]
- 14.Bauernfeind F. G., Horvath G., Stutz A., Alnemri E. S., MacDonald K., Speert D., Fernandes-Alnemri T., Wu J., Monks B. G., Fitzgerald K. A., Hornung V., Latz E. (2009) Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wickliffe K. E., Leppla S. H., Moayeri M. (2008) Anthrax lethal toxin-induced inflammasome formation and caspase-1 activation are late events dependent on ion fluxes and the proteasome. Cell. Microbiol. 10, 332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Squires R. C., Muehlbauer S. M., Brojatsch J. (2007) Proteasomes control caspase-1 activation in anthrax lethal toxin-mediated cell killing. J. Biol. Chem. 282, 34260–34267. [DOI] [PubMed] [Google Scholar]
- 17.Lamkanfi M., Vande Walle L., Kanneganti T. D. (2011) Deregulated inflammasome signaling in disease. Immunol. Rev. 243, 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Zoete M. R., Palm N. W., Zhu S., Flavell R. A. (2014) Inflammasomes. Cold Spring Harb. Perspect. Biol. 6, a016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fahey J. W., Zhang Y., Talalay P. (1997) Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. U. S. A. 94, 10367–10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myzak M. C., Dashwood R. H. (2006) Chemoprotection by sulforaphane: keep one eye beyond Keap1. Cancer Lett. 233, 208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinkova-Kostova A. T., Kostov R. V. (2012) Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 18, 337–347. [DOI] [PubMed] [Google Scholar]
- 22.Houghton C. A., Fassett R. G., Coombes J. S. (2013) Sulforaphane: translational research from laboratory bench to clinic. Nutr. Rev. 71, 709–726. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y., Talalay P., Cho C. G., Posner G. H. (1992) A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc. Natl. Acad. Sci. U. S. A. 89, 2399–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinkova-Kostova A. T., Holtzclaw W. D., Cole R. N., Itoh K., Wakabayashi N., Katoh Y., Yamamoto M., Talalay P. (2002) Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. U. S. A. 99, 11908–11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J. D., Yamamoto M. (1999) Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 13, 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itoh K., Wakabayashi N., Katoh Y., Ishii T., O’Connor T., Yamamoto M. (2003) Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells 8, 379–391. [DOI] [PubMed] [Google Scholar]
- 27.Hu R., Xu C., Shen G., Jain M. R., Khor T. O., Gopalkrishnan A., Lin W., Reddy B., Chan J. Y., Kong A. N. (2006) Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Cancer Lett. 243, 170–192. [DOI] [PubMed] [Google Scholar]
- 28.Youn H. S., Kim Y. S., Park Z. Y., Kim S. Y., Choi N. Y., Joung S. M., Seo J. A., Lim K. M., Kwak M. K., Hwang D. H., Lee J. Y. (2010) Sulforaphane suppresses oligomerization of TLR4 in a thiol-dependent manner. J. Immunol. 184, 411–419. [DOI] [PubMed] [Google Scholar]
- 29.Mi L., Xiao Z., Hood B. L., Dakshanamurthy S., Wang X., Govind S., Conrads T. P., Veenstra T. D., Chung F. L. (2008) Covalent binding to tubulin by isothiocyanates: a mechanism of cell growth arrest and apoptosis. J. Biol. Chem. 283, 22136–22146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pomerantsev A. P., Sitaraman R., Galloway C. R., Kivovich V., Leppla S. H. (2006) Genome engineering in Bacillus anthracis using Cre recombinase. Infect. Immun. 74, 682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park S., Leppla S. H. (2000) Optimized production and purification of Bacillus anthracis lethal factor. Protein Expr. Purif. 18, 293–302. [DOI] [PubMed] [Google Scholar]
- 32.Von Moltke J., Trinidad N. J., Moayeri M., Kintzer A. F., Wang S. B., van Rooijen N., Brown C. R., Krantz B. A., Leppla S. H., Gronert K., Vance R. E. (2012) Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 490, 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newman Z. L., Sirianni N., Mawhinney C., Lee M. S., Leppla S. H., Moayeri M., Johansen L. M. (2011) Auranofin protects against anthrax lethal toxin-induced activation of the Nlrp1b inflammasome. Antimicrob. Agents Chemother. 55, 1028–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maier N. K., Crown D., Liu J., Leppla S. H., Moayeri M. (2014) Arsenic trioxide and other arsenical compounds inhibit the NLRP1, NLRP3, and NAIP5/NLRC4 inflammasomes. J. Immunol. 192, 763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinon F., Pétrilli V., Mayor A., Tardivel A., Tschopp J. (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241. [DOI] [PubMed] [Google Scholar]
- 36.Martin W. J., Walton M., Harper J. (2009) Resident macrophages initiating and driving inflammation in a monosodium urate monohydrate crystal-induced murine peritoneal model of acute gout. Arthritis Rheum. 60, 281–289. [DOI] [PubMed] [Google Scholar]
- 37.Boyden E. D., Dietrich W. F. (2006) Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 38, 240–244. [DOI] [PubMed] [Google Scholar]
- 38.Alileche A., Squires R. C., Muehlbauer S. M., Lisanti M. P., Brojatsch J. (2006) Mitochondrial impairment is a critical event in anthrax lethal toxin-induced cytolysis of murine macrophages. Cell Cycle 5, 100–106. [DOI] [PubMed] [Google Scholar]
- 39.Wickliffe K. E., Leppla S. H., Moayeri M. (2008) Killing of macrophages by anthrax lethal toxin: involvement of the N-end rule pathway. Cell. Microbiol. 10, 1352–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu S., Moayeri M., Leppla S. H. (2014) Anthrax lethal and edema toxins in anthrax pathogenesis. Trends Microbiol. 22, 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duesbery N. S., Webb C. P., Leppla S. H., Gordon V. M., Klimpel K. R., Copeland T. D., Ahn N. G., Oskarsson M. K., Fukasawa K., Paull K. D., Vande Woude G. F. (1998) Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280, 734–737. [DOI] [PubMed] [Google Scholar]
- 42.Vitale G., Bernardi L., Napolitani G., Mock M., Montecucco C. (2000) Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem. J. 352, 739–745. [PMC free article] [PubMed] [Google Scholar]
- 43.Levin T. C., Wickliffe K. E., Leppla S. H., Moayeri M. (2008) Heat shock inhibits caspase-1 activity while also preventing its inflammasome-mediated activation by anthrax lethal toxin. Cell. Microbiol. 10, 2434–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heiss E., Herhaus C., Klimo K., Bartsch H., Gerhäuser C. (2001) Nuclear factor κB is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J. Biol. Chem. 276, 32008–32015. [DOI] [PubMed] [Google Scholar]
- 45.Xu C., Shen G., Chen C., Gélinas C., Kong A. N. (2005) Suppression of NF-κB and NF-κB-regulated gene expression by sulforaphane and PEITC through IκBα, IKK pathway in human prostate cancer PC-3 cells. Oncogene 24, 4486–4495. [DOI] [PubMed] [Google Scholar]
- 46.Kim S. J., Kang S. Y., Shin H. H., Choi H. S. (2005) Sulforaphane inhibits osteoclastogenesis by inhibiting nuclear factor-κB. Mol. Cells 20, 364–370. [PubMed] [Google Scholar]
- 47.Pelegrin P., Barroso-Gutierrez C., Surprenant A. (2008) P2X7 receptor differentially couples to distinct release pathways for IL-1β in mouse macrophage. J. Immunol. 180, 7147–7157. [DOI] [PubMed] [Google Scholar]
- 48.Gan N., Wu Y. C., Brunet M., Garrido C., Chung F. L., Dai C., Mi L. (2010) Sulforaphane activates heat shock response and enhances proteasome activity through up-regulation of Hsp27. J. Biol. Chem. 285, 35528–35536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwak M. K., Cho J. M., Huang B., Shin S., Kensler T. W. (2007) Role of increased expression of the proteasome in the protective effects of sulforaphane against hydrogen peroxide-mediated cytotoxicity in murine neuroblastoma cells. Free Radic. Biol. Med. 43, 809–817. [DOI] [PubMed] [Google Scholar]
- 50.Kwak M. K., Kensler T. W. (2006) Induction of 26S proteasome subunit PSMB5 by the bifunctional inducer 3-methylcholanthrene through the Nrf2-ARE, but not the AhR/Arnt-XRE, pathway. Biochem. Biophys. Res. Commun. 345, 1350–1357. [DOI] [PubMed] [Google Scholar]
- 51.Kwak M. K., Wakabayashi N., Greenlaw J. L., Yamamoto M., Kensler T. W. (2003) Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol. Cell. Biol. 23, 8786–8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fink S. L., Bergsbaken T., Cookson B. T. (2008) Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc. Natl. Acad. Sci. U. S. A. 105, 4312–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kensler T. W., Egner P. A., Agyeman A. S., Visvanathan K., Groopman J. D., Chen J. G., Chen T. Y., Fahey J. W., Talalay P. (2013) Keap1–nrf2 signaling: a target for cancer prevention by sulforaphane. Top. Curr. Chem. 329, 163–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trachootham D., Zhou Y., Zhang H., Demizu Y., Chen Z., Pelicano H., Chiao P. J., Achanta G., Arlinghaus R. B., Liu J., Huang P. (2006) Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by β-phenylethyl isothiocyanate. Cancer Cell 10, 241–252. [DOI] [PubMed] [Google Scholar]
- 55.Kim H., Kim E. H., Eom Y. W., Kim W. H., Kwon T. K., Lee S. J., Choi K. S. (2006) Sulforaphane sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-resistant hepatoma cells to TRAIL-induced apoptosis through reactive oxygen species-mediated up-regulation of DR5. Cancer Res. 66, 1740–1750. [DOI] [PubMed] [Google Scholar]
- 56.Singh S. V., Srivastava S. K., Choi S., Lew K. L., Antosiewicz J., Xiao D., Zeng Y., Watkins S. C., Johnson C. S., Trump D. L., Lee Y. J., Xiao H., Herman-Antosiewicz A. (2005) Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J. Biol. Chem. 280, 19911–19924. [DOI] [PubMed] [Google Scholar]
- 57.Shankar S., Ganapathy S., Srivastava R. K. (2008) Sulforaphane enhances the therapeutic potential of TRAIL in prostate cancer orthotopic model through regulation of apoptosis, metastasis, and angiogenesis. Clin. Cancer Res. 14, 6855–6866. [DOI] [PubMed] [Google Scholar]
- 58.Podhradský D., Drobnica L., Kristian P. (1979) Reactions of cysteine, its derivatives, glutathione coenzyme A, and dihydrolipoic acid with isothiocyanates. Experientia 35, 154–155. [DOI] [PubMed] [Google Scholar]
- 59.Mi L., Sirajuddin P., Gan N., Wang X. (2010) A cautionary note on using N-acetylcysteine as an antagonist to assess isothiocyanate-induced reactive oxygen species-mediated apoptosis. Anal. Biochem. 405, 269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moayeri M., Crown D., Newman Z. L., Okugawa S., Eckhaus M., Cataisson C., Liu S., Sastalla I., Leppla S. H. (2010) Inflammasome sensor Nlrp1b-dependent resistance to anthrax is mediated by caspase-1, IL-1 signaling and neutrophil recruitment. PLoS Pathog. 6, e1001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shapiro T. A., Fahey J. W., Dinkova-Kostova A. T., Holtzclaw W. D., Stephenson K. K., Wade K. L., Ye L., Talalay P. (2006) Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr. Cancer 55, 53–62. [DOI] [PubMed] [Google Scholar]
- 62.Broz P., von Moltke J., Jones J. W., Vance R. E., Monack D. M. (2010) Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 8, 471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Opdenbosch N., Gurung P., Vande Walle L., Fossoul A., Kanneganti T. D., Lamkanfi M. (2014) Activation of the NLRP1b inflammasome independently of ASC-mediated caspase-1 autoproteolysis and speck formation. Nat. Commun. 5, 3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guey B., Bodnar M., Manié S. N., Tardivel A., Petrilli V. (2014) Caspase-1 autoproteolysis is differentially required for NLRP1b and NLRP3 inflammasome function. Proc. Natl. Acad. Sci. U. S. A. 111, 17254–17259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Misawa T., Takahama M., Kozaki T., Lee H., Zou J., Saitoh T., Akira S. (2013) Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 14, 454–460. [DOI] [PubMed] [Google Scholar]
- 66.Fahey J. W., Haristoy X., Dolan P. M., Kensler T. W., Scholtus I., Stephenson K. K., Talalay P., Lozniewski A. (2002) Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. U. S. A. 99, 7610–7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Galan M. V., Kishan A. A., Silverman A. L. (2004) Oral broccoli sprouts for the treatment of Helicobacter pylori infection: a preliminary report. Dig. Dis. Sci. 49, 1088–1090. [DOI] [PubMed] [Google Scholar]
- 68.Yanaka A., Fahey J. W., Fukumoto A., Nakayama M., Inoue S., Zhang S., Tauchi M., Suzuki H., Hyodo I., Yamamoto M. (2009) Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori–infected mice and humans. Cancer Prev. Res. (Phila.) 2, 353–360. [DOI] [PubMed] [Google Scholar]
- 69.Hitzler I., Sayi A., Kohler E., Engler D. B., Koch K. N., Hardt W. D., Müller A. (2012) Caspase-1 has both proinflammatory and regulatory properties in Helicobacter infections, which are differentially mediated by its substrates IL-1β and IL-18. J. Immunol. 188, 3594–3602. [DOI] [PubMed] [Google Scholar]
- 70.Spranger J., Kroke A., Möhlig M., Hoffmann K., Bergmann M. M., Ristow M., Boeing H., Pfeiffer A. F. (2003) Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)—Potsdam study. Diabetes 52, 812–817. [DOI] [PubMed] [Google Scholar]
- 71.Masters S. L., Dunne A., Subramanian S. L., Hull R. L., Tannahill G. M., Sharp F. A., Becker C., Franchi L., Yoshihara E., Chen Z., Mullooly N., Mielke L. A., Harris J., Coll R. C., Mills K. H., Mok K. H., Newsholme P., Nuñez G., Yodoi J., Kahn S. E., Lavelle E. C., O’Neill L. A. (2010) Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 11, 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bahadoran Z., Tohidi M., Nazeri P., Mehran M., Azizi F., Mirmiran P. (2012) Effect of broccoli sprouts on insulin resistance in type 2 diabetic patients: a randomized double-blind clinical trial. Int. J. Food Sci. Nutr. 63, 767–771. [DOI] [PubMed] [Google Scholar]
- 73.Heber D., Li Z., Garcia-Lloret M., Wong A. M., Lee T. Y., Thames G., Krak M., Zhang Y., Nel A. (2014) Sulforaphane-rich broccoli sprout extract attenuates nasal allergic response to diesel exhaust particles. Food Funct. 5, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Provoost S., Maes T., Pauwels N. S., Vanden Berghe T., Vandenabeele P., Lambrecht B. N., Joos G. F., Tournoy K. G. (2011) NLRP3/caspase-1-independent IL-1β production mediates diesel exhaust particle-induced pulmonary inflammation. J. Immunol. 187, 3331–3337. [DOI] [PubMed] [Google Scholar]
- 75.Ritter M., Straubinger K., Schmidt S., Busch D. H., Hagner S., Garn H., Prazeres da Costa C., Layland L. E. (2014) Functional relevance of NLRP3 inflammasome-mediated interleukin (IL)-1β during acute allergic airway inflammation. Clin. Exp. Immunol. 178, 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lenzi M., Fimognari C., Hrelia P. (2014) Sulforaphane as a promising molecule for fighting cancer. Cancer Treat. Res. 159, 207–223. [DOI] [PubMed] [Google Scholar]
- 77.Clarke J. D., Dashwood R. H., Ho E. (2008) Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 269, 291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Traka M. H., Melchini A., Mithen R. F. (2014) Sulforaphane and prostate cancer interception. Drug Discov. Today 19, 1488–1492. [DOI] [PubMed] [Google Scholar]
- 79.Palma G., Barbieri A., Bimonte S., Palla M., Zappavigna S., Caraglia M., Ascierto P. A., Ciliberto G., Arra C. (2013) Interleukin 18: friend or foe in cancer. Biochim. Biophys. Acta 1836, 296–303. [DOI] [PubMed] [Google Scholar]
- 80.Dinarello C. A. (2010) Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev. 29, 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]