Methamphetamine modulates T cell immune responses via TAAR1.
Keywords: drug abuse, immune response, T cells, HIV infection
Abstract
The novel transmembrane G protein-coupled receptor, trace amine-associated receptor 1 (TAAR1), represents a potential, direct target for drugs of abuse and monoaminergic compounds, including amphetamines. For the first time, our studies have illustrated that there is an induction of TAAR1 mRNA expression in resting T lymphocytes in response to methamphetamine. Methamphetamine treatment for 6 h significantly increased TAAR1 mRNA expression (P < 0.001) and protein expression (P < 0.01) at 24 h. With the use of TAAR1 gene silencing, we demonstrate that methamphetamine-induced cAMP, a classic response to methamphetamine stimulation, is regulated via TAAR1. We also show by TAAR1 knockdown that the down-regulation of IL-2 in T cells by methamphetamine, which we reported earlier, is indeed regulated by TAAR1. Our results also show the presence of TAAR1 in human lymph nodes from HIV-1-infected patients, with or without a history of methamphetamine abuse. TAAR1 expression on lymphocytes was largely in the paracortical lymphoid area of the lymph nodes with enhanced expression in lymph nodes of HIV-1-infected methamphetamine abusers rather than infected-only subjects. In vitro analysis of HIV-1 infection of human PBMCs revealed increased TAAR1 expression in the presence of methamphetamine. In summary, the ability of methamphetamine to activate trace TAAR1 in vitro and to regulate important T cell functions, such as cAMP activation and IL-2 production; the expression of TAAR1 in T lymphocytes in peripheral lymphoid organs, such as lymph nodes; and our in vitro HIV-1 infection model in PBMCs suggests that TAAR1 may play an important role in methamphetamine -mediated immune-modulatory responses.
Introduction
Among the various substances of abuse, METH is gaining increasing popularity among drug-abusing populations worldwide for its addictive psychostimulant effects [1, 2]. Recreational METH use is one of the fastest growing substance-abuse problems in the United States [3, 4]. METH use is associated with high-risk sexual behavior and high rates of HIV acquisition [5]. Animal and human studies have demonstrated that METH suppresses innate and adaptive immunity [6–8]. More insights into the mechanism of action of METH have been revealed in the last decade with the discovery of the receptor(s) for METH that belong to specific families of GPCRs [9, 10]. Members of this large family of GPCRs are now referred to as TAARs, and TAAR1 is the prototypical member [11, 12], which is activated by a broad range of monoamines and amphetamine-related psychostimulants, including METH [13, 14].
There is substantial evidence for TAAR1 mRNA expression in the rodent and primate brain [15–17]. TAAR1 mRNA and protein expression have also been shown in other peripheral tissues, such as liver, kidney, spleen, pancreas, heart, and gastrointestinal tract, in rodent and primate models [9], as well as in leukocyte subpopulations of mice and humans [18]. TAAR1 mRNA has been shown to be up-regulated in human PBMCs following in vitro stimulation with PHA [18]. Miller et al. [15] have pharmacologically characterized rhesus monkey TAAR1 in HEK293 cells by use of a highly sensitive CRE-Luc (cAMP response element - luciferase) assay. Activation of TAAR1 has been shown to induce cAMP, which involves phosphorylation-dependent, downstream effects of PKA and PKC pathways [9, 16].
METH abuse has seriously impacted management of HIV-1 infection globally [8, 19–21], as evidenced by studies of various cohorts in the United States [4, 22, 23] and around the world [24, 25]. METH abuse has been shown to be associated with high viral loads, especially in the CNS [26, 27], development of antiretroviral resistance, and rapid progression to AIDS [28–31]. METH-induced responses mediated through TAAR1 signaling have never been studied in the context of HIV-1 infection in peripheral immune cells.
We have shown that METH induces T cell dysfunction via induction of oxidative stress and mitochondrial injury [7]. Mitochondrial dysfunction paralleled reduced IL-2 secretion and T cell-proliferative responses after TCR-CD28 stimulation, indicating impaired T cell function. In this study, we focused on the mechanisms of METH action via TAAR1 in resting (nonactivated) human T cells from normal donors. Our studies show, for the first time, a significant induction of TAAR1 mRNA and protein expression in resting T lymphocytes in response to METH. Similar to neuronal and non-neuronal cells, resting T cells induced cAMP and cAMP-dependent PKA activation upon METH treatment. With the use of a gene-silencing technique, we confirm that the signaling is mediated via TAAR1. Previously, we have reported [7] that METH decreased IL-2 production in T cells upon CD3/CD28 stimulation. In the current study, we show that METH via TAAR1 signaling modulates IL-2 production. These data suggest that METH has an important immunomodulatory effect that is likely attributable to the activity of the receptor. Furthermore, increased TAAR1 expression was seen in T cells from PBMC upon METH treatment, followed by HIV-1 infection in an in vitro infection model. We also show increased TAAR1 expression in human lymph nodes from HIV-1-infected patients with a history of METH abuse compared with non-METH abusers.
In summary, the current study clearly demonstrates the following: that 1) METH activates TAAR1 in vitro, 2) METH regulates cAMP and IL-2 production in T cells, 3) HIV-infected METH abusers express TAAR1 in T lymphocytes in peripheral lymphoid organs, such as lymph nodes, and 4) HIV-infected, METH-exposed T cells in vitro express increased TAAR1. Taken together, the study suggests that TAAR1 may play an important role in METH-mediated, immune-modulatory responses.
MATERIALS AND METHODS
Sample collection
PBMCs were isolated from leukopheresis packs (Pall, Port Washington, NY, USA) from normal donors obtained from the American Red Cross (Philadelphia, PA, USA) within 2–6 h after blood collection.
Reagents and antibodies
METH, β-PEA, and tyramine are from Sigma-Aldrich (St. Louis, MO, USA). Antibodies to human TAAR1 were obtained from MBL International (Woburn, MA, USA; LS-A2041 and LS-A2042). Fluorochrome-tagged antibodies were procured from eBioscience (San Diego, CA, USA) and BioLegend (San Diego, CA, USA; anti-human CD3, CD4, and CD8) and secondary antibodies from Invitrogen, Life Technologies (Carlsbad, CA, USA). Reagents for PCR were from Applied Biosystems (Life Technologies, Carlsbad, CA, USA).
T cell isolation and culture
PBMCs were obtained by Ficoll-gradient centrifugation of blood eluted from leukophoresis packs. T cells were isolated by use of a Pan T Cell Isolation Kit (Miltenyi Biotec, San Diego, CA, USA; purity was >98% by this method; data not shown). T cells (1 × 106/ml) were cultured in X-Vivo 20 medium (Lonza, Walkersville, MD, USA), supplemented with 1% heat-inactivated normal human serum, 20 μg/ml gentamycin, and 2 mM glutamine. The concentration of METH used (100 μM) was based on our previously published dose-response studies that produced a maximum biologic response without causing toxicity [7].
qRT-PCR
Pan-isolated T cells (1 × 106/ml) were stimulated with 100 µM METH or 100 µM β-PEA for 4, 6, and 8 h and harvested for RNA extraction by use of an RNA Extraction Kit (Qiagen, Germantown, MD, USA). RNA was extracted, per the manufacturer’s protocol, including DNase treatment. cDNA was made by use of 1 µg RNA template and proceeded to real-time PCR by use of TaqMan probes for TAAR1 (Applied Biosystems, Life Technologies). Real-time PCR was run in a StepOnePlus PCR thermocycler (Applied Biosystems, Life Technologies). GAPDH was used as the housekeeping gene. The ΔΔ comparative threshold method was used to calculate the fold change in TAAR1 expression compared with untreated control.
Confocal imaging
Total PBMCs were treated with METH, β-PEA, or tyramine for 24 h and were harvested and plated on poly-d-lysine-coated coverslips and allowed to adhere for 1 h at 37°C. Cells on coverslips were fixed with methanol/acetone (1:1) for 20 min at −20°C. Cells were then blocked with 3% BSA + 0.1% Triton X-100 in PBS for 15 min at room temperature. After blocking, cells were incubated with mouse anti-human CD3 (1:100; BioLegend) and rabbit anti-human TAAR1 (10 µg/ml; MBL International; LS-A2041) primary antibodies for 1 h at room temperature. Cells were then incubated with donkey anti-mouse Alexa Fluor 594 (1:400; Invitrogen, Life Technologies) and donkey anti-rabbit Alexa Fluor 488 (1:400; Invitrogen, Life Technologies) secondary antibodies for 1 h at room temperature. Samples were washed and mounted on glass slides by use of ProLong Gold + DAPI reagent. Immunofluorescently labeled T lymphocytes were imaged at the Temple University Confocal Imaging Facility by use of a Leica SP5 confocal microscope.
Flow cytometry
Total PBMCs (1 × 106 /ml) were cultured in complete RPMI-1640 medium, supplemented with 10% heat-inactivated FBS, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 20 mM HEPES (Life Technologies, Carlsbad, CA, USA) for 24 h, with or without 100 µM METH, tyramine, or β-PEA. Cells were harvested and stained with purified rabbit anti-human TAAR1 (1:100 dilution; MBL International), followed by allophycocyanin-tagged anti-rabbit secondary, along with FITC-tagged anti-human CD3, PE-tagged anti-human CD4, and eFlour 450-tagged anti-human CD8. Cells were washed in FACS buffer, fixed in 1% paraformaldehyde/PBS, and read in a BD FACSCanto II flow cytometer (BD Biosciences, San Jose, CA, USA). Data were analyzed by use of FlowJo software (TreeStar, Ashland, OR, USA).
cAMP analysis and Phosflow detection of phosphorylated PKA
METH-induced cAMP on T cells was detected by use of an antibody to cAMP after stimulation of Pan-isolated T cells with METH or forskolin for 30 min–1 h. Cells were permeabilized, per the manufacturer’s protocol, and stained with mouse monoclonal cAMP (SPM486; Abcam, Cambridge, MA, USA) at room temperature for 1 h, followed by anti-mouse secondary PE antibody for a further 15 min. Dead cells were analyzed by Fixable Viability Dye 780 dye (eBioscience). Intracellular Phosflow assay was used to analyze PKA phosphorylation on T cells by use of anti-PKA RIIα (pS99) antibody (a regulatory subunit; BD Biosciences) [32] by flow cytometry. Total PBMCs were treated with 100 µM METH or forskolin (positive control), harvested after 15 min treatment, and processed for flow cytometric analysis by use of the BD Biosciences Phosflow-staining protocol. Cells were counterstained with FITC-conjugated anti-human CD3 antibody to analyze phophorylated PKA. Cells were analyzed in a BD FACSCanto II cytometer. Approximately 10,000 events were acquired for each sample. Data were analyzed by use of FlowJo software.
TAAR1 siRNA knockdown
TAAR1 expression in total PBMCs was silenced by siRNA transfection for ∼44 h. We chose the TAAR1 siRNA (CAGAATATATCTTATCGCTAA) that has been used by Babusyte et al. [33]. INTERFERin transfection reagent (Polyplus-Transfection; Bioparc, Illkirch, Cedex, France) was used to transfect directly the siRNA in RPMI medium. In brief, fresh PBMCs, isolated by the Ficoll-Hypaque method, were seeded between 3 and 5 × 105 in a 12-well tissue-culture plate in complete RPMI with 10% FBS. TAAR1 siRNA was obtained from Qiagen (FlexiTube gene solution) or scrambled siRNA (ON-TARGETplus nontargeting siRNA (Fisher Scientific, Pittsburg, PA, USA); 20 nM in buffer was allowed to form siRNA duplexes with the INTERFERin transfection reagent in nonserum medium. TAAR1 or scrambled duplex solution, 200 μl each, was added in the respective wells and mixed well by swirling the plate. Cells were incubated at 37°C, 5% CO2, for 4 h, and 1 ml complete RPMI was added to each well. Cells were incubated for up to 44 h and then tested for other functional readouts. Cells in medium alone served as controls. TAAR1-specific siRNA selectively knocked down the target, as assessed by qPCR, by use of GAPDH control. The knockdown was up to 90%.
cAMP analysis of siRNA-treated cells
METH-induced cAMP was measured to test whether cAMP was regulated by TAAR1. Scrambled and TAAR1 siRNA conditioned cells were treated with METH (100 μM) or forskolin (100 μM) for 30 min–1 h. Cells were harvested and stained for CD3 surface marker (CD3-FITC; eBioscience). Cells were permeabilized by use of the manufacturer’s protocol and stained with mouse monoclonal cAMP (SPM486; Abcam), followed by anti-mouse secondary PE antibody, as described earlier. Dead cells were analyzed by Fixable Viability Dye 780 dye from eBioscience. Cells were analyzed in a BD FACSCanto II cytometer. Approximately 10,000 events were acquired for each sample. Data were analyzed by use of FlowJo software.
IL-2 measurement
siRNA-treated cells were treated further after ∼44 h, with or without METH, for 30 min and then cross-linked with soluble CD3 (LEAF Purified anti-human CD3, clone HIT3a; BioLegend) and CD28 (LEAF Purified anti-human CD28, clone CD28.2; BioLegend). After 24 h of CD3/CD28 stimulation, supernatants were collected. As the volume of the medium for the number of cells was high, we concentrated the supernatants by use of Amicon Ultra centrifugal filters (EMD Millipore, Billerica, MA, USA) by use of 3 kDa cutoff. The samples were then tested by use of an MSD human IL-2 detection kit (Meso Scale Diagnostics, Rockville, MD, USA). Fold change in the IL-2 levels between the scrambled and TAAR1 siRNA-treated cells, stimulated with METH and CD3/CD28, was analyzed.
Immunohistochemistry and image analysis
Immunohistochemistry analysis was performed to determine the expression of TAAR1 receptors in lymph nodes from HIV-1-infected patients, with or without a history of METH abuse. Paraffin-embedded samples were procured from the core facility at the Medical Center at the University of California, San Diego (San Diego, CA, USA), from which 5 μm serial sections were obtained and glass-slide mounted. Sections were heat-induced epitope retrieval antigen retrieved by use of pH 6.0 citrate buffer (Cat. #S1700; Dako, Carpenteria, CA, USA) and subsequently probed for p24, TAAR1, and CD3 expression in a sequential manner by use of Vector Laboratories-labeled polymer ImmPRESS HRP and ImmPRESS-AP detection systems (Vector Laboratories, Burlingame, CA, USA). As a result of the use of multiple systems, both endogenous peroxidase and alkaline phosphatase activity were quenched (Bloxall; Vector Laboratories) before normal serum blocking. Sections were then incubated overnight at 4°C in mouse anti-human p24 (1:8 dilution, Cat. #M0857, Clone Kal-1; Dako), followed by detection with anti-mouse ImmPRESS HRP and visualization with ImmPACT DAB (Vector Laboratories). Sections were reblocked and incubated in rabbit anti-human TAAR1 (1:1200 dilution, Cat. #MC-2041; MBL International) for 45 min at room temperature, followed by detection with anti-rabbit ImmPRESS-AP and visualization with Vector Blue (Vector Laboratories). Sections were then reblocked and incubated overnight at 4°C in mouse anti-human CD3 (Ready-to-Use, Clone PS1; Novocastra, Buffalo Grove, IL, USA), followed by detection with anti-mouse ImmPRESS-AP and visualization with Vector Red. Sections were rinsed, dehydrated, cleared, and permanently mounted (VectaMount; Vector Laboratories) for bright-field microscopy. Slides were reviewed by use of a Nikon 80i upright microscope configured to a DS-Fi2 color camera (Nikon Instruments, Melville, NY, USA). Images were acquired by use of the NIS Elements imaging software (Nikon).
Image analysis was performed with an automated cell-counting macro by use of ImageJ 1.48v image processing and analysis software (U.S. National Institutes of Health, Bethesda, MD, USA; http://rsb.info.nih.gov/ij/). Particle counting was performed on high-resolution images (3/case, taken at 10× objective magnification) on double (CD3 and TAAR1)- and triple (CD3, TAAR1, and p24)- immunolabeled cells. In ImageJ, the images were calibrated and then sequentially processed for background subtraction, color threshold segmentation, and binary conversion (with application of the watershed function). Cells identified from the above parameters in an area of 5.61 × 105 μm2 were counted by use of the analysis particles function (selected on the basis of particle area and circularity).
HIV-1 infection of PBMC
Ficoll-separated total PBMCs (1 × 106/ml) from normal donors were first activated for 72 h with PHA (2.5 µg/ml) and then infected with HIV-1-1ADA at a multiplicity of infection of 0.01–0.1 virus particles/target cell for 4 h [34] with polybrene (10 µg/ml) [35]. Following incubation, cells were washed and cultured in fresh RPMI-1640 medium, supplemented with 10% FBS, antibiotics, and 20 U/ml IL-2 (Advanced Biotech, Totowa, NJ, USA) in the presence or absence of 100 µM METH for 6 days. In some experiments, PBMCs were also treated with METH, 24 h before HIV-1 infection. Treatments were maintained for 6 d, with half-medium replacement on d 3 after HIV-1 was added to the cells.
Analysis of HIV-1 infection
Culture supernatant was collected on d 6 and stored at −80°C until it was assayed for HIV-1 RT activity. The HIV-1 RT assay is a radiometric assay designed for the quantitative measurement of RT activity in cell culture and was performed as described previously [35]. HIV-1 p24 expression was analyzed by flow cytometry. Total PBMCs were stained for HIV-1 p24 antigen by use of BD Coulter KC57-FITC antibody (Beckman Coulter, Indianapolis, IN, USA) and for CD3 by use of mouse anti-human CD3-PE/Cyanine7 (eBioscience). Cell death staining was performed by use of Fixable Viability Dye eFluor 780 from eBioscience. Twenty-thousand events were collected for each sample and analyzed by FlowJo software.
ELISA for p24 detection was also performed on the HIV-infected culture supernatants to confirm infection and how METH modulated the p24 levels. The HIV p24 antigen capture assay kit was purchased from ABL (Rockville, MD), and assay was performed per the manufacturer’s instructions.
Western blotting
PBMCs were harvested on d 6 post-HIV-1 infection and analyzed for TAAR1 by Western blotting. In brief, whole-cell lysate of total PBMC was made with CelLytic M (Sigma-Aldrich) buffer. Protein concentration was measured by bicinchoninic acid assay (Pierce, Rockford, IL, USA). SDS-PAGE and Western blot analysis were performed as described previously [7, 36]. TAAR1 was detected by use of anti-human rabbit polyclonal TAAR1 antibody.
Statistical analysis
Data were analyzed by use of Prism software (GraphPad Software, San Diego, CA, USA). One sample t test, two-tailed Student’s t test, or one-way ANOVA, as appropriate, was used as statistical test for different sets of experiments and considered significant values at P < 0.05 (see figures for other significance values).
RESULTS
METH increases TAAR1 gene expression in resting human T cells
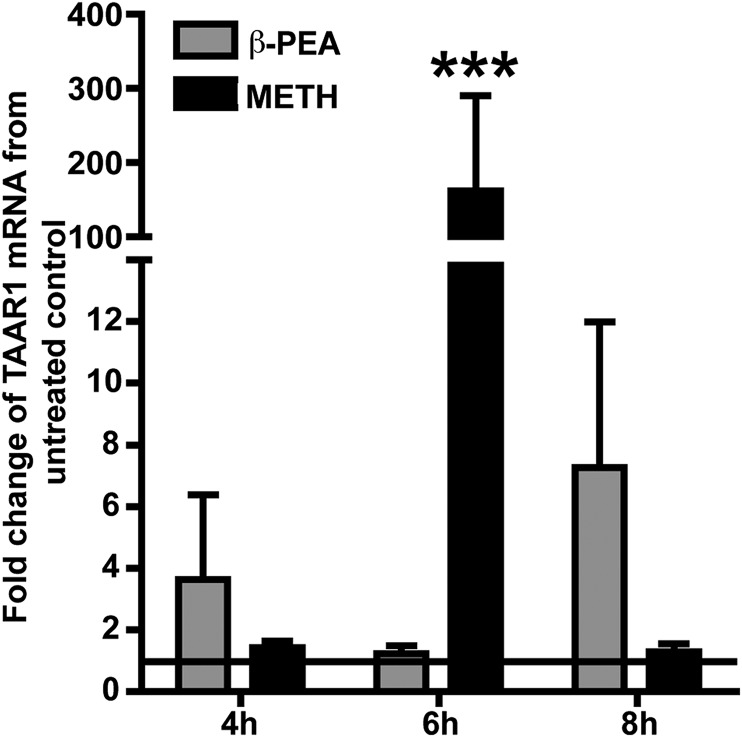
TAAR1 expression has been classically demonstrated primarily in the brain [12, 16]. Subsequent studies have shown evidence in different cell types in rodent, primate, and human models [18, 37–39]. We followed a time course of up to 8 h to test induction of TAAR1 mRNA in Pan-isolated T cells from PBMCs from different donors after METH treatment (Fig. 1). Although there were appreciable differences in the level of TAAR1 mRNA in the different donors tested (n = 5), the increase in TAAR1 expression at 6 h was highly significant (P < 0.001; Fig. 1). Interestingly, in all of the donors tested, we consistently observed a huge induction of TAAR1 mRNA upon METH treatment at 6 h (up to ∼300-fold up-regulation over untreated control), which dropped drastically at 8 h (Fig. 1). β-PEA, a well-known ligand of TAAR1, consistently induced high TAAR1 expression in T cells at 8 h. The TAAR1 gene was constitutively expressed in 3 out of 5 donors tested (data not shown), confirming the observations by Babusyte et al. [33]. The huge variation in TAAR1 gene expression induced by METH was also attributable to the variation in the baseline-level expression of TAAR1 in the different individuals.
Figure 1. METH induces TAAR1 mRNA expression in resting human T lymphocytes.
Human T cells were treated with β-PEA (100 μM) and METH (100 μM) over a time course (4–8 h) to see how these treatments altered TAAR1 gene expression. TAAR1 mRNA was quantified by use of TaqMan probe reagents by real-time PCR. Data represent the mean fold changes ± sem of the mRNA from at least 3 different experiments in triplicate compared with untreated control cells. The horizontal line in the graph represents the baseline of 1 for the untreated control, from which the fold change of expression was calculated. There was a significant increase in TAAR1 mRNA after 6 h of METH treatment and 8 h of β-PEA treatment. ***P < 0.001 by one-way ANOVA.
METH increases TAAR1 protein expression in human T cells from normal donors
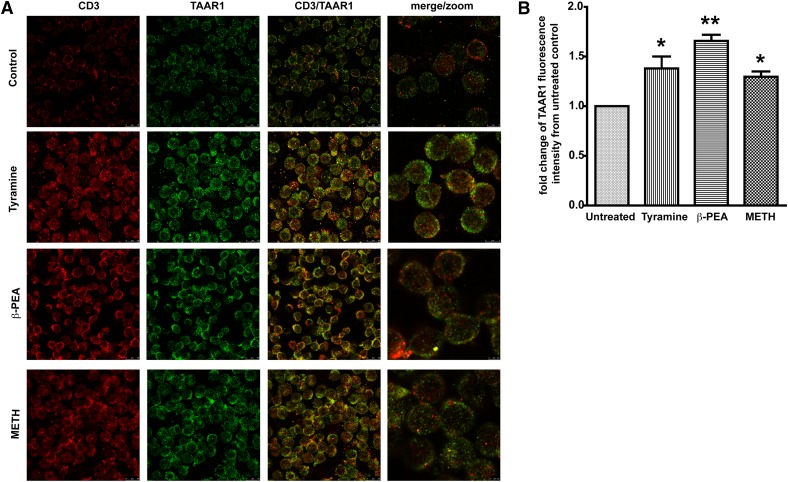
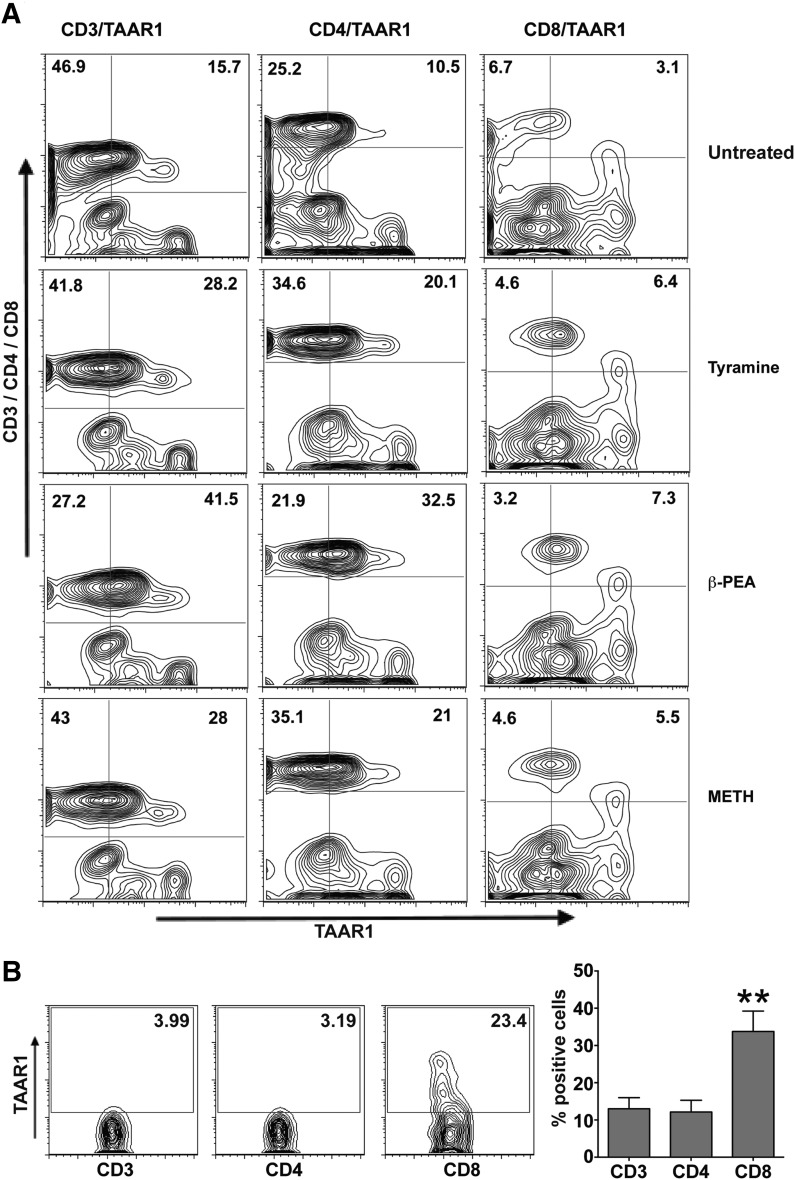
Increased expression in TAAR1 has been shown in total lymphocytes and in B cell lines [16, 18, 33] upon stimulation with biogenic amines [17]. CD4+ T cells are prime targets for HIV-1 infection [40, 41]. It is now well established that METH abuse increases HIV-1 infection [42, 43]. Therefore, we first sought to explore effects of METH on TAAR1 expression on resting T cells, that is, the primary signaling pathway downstream of METH stimulation. We analyzed the expression of TAAR1 on primary human PBMCs stimulated with METH or known, potent agonists of TAAR1, β-PEA, or tyramine [44] by use of confocal microscopy and flow cytometry. PBMCs treated with tyramine (100 μM), β-PEA (100 μM), and METH (100 μM) showed significant increases in TAAR1 expression in T cells compared with untreated control cells (Fig. 2). Flow cytometric analyses (Fig. 3) also showed increases in TAAR1 expression in CD4+ and CD8+ T cell subsets after 24 h stimulation. Stimulation with METH potently increased TAAR1 expression in T cells, similar to β-PEA and tyramine. The strongest induction was with β-PEA (Fig. 3A). In our hands, TAAR1 antibodies LS-A2041 and -A2042 performed optimally for flow with good cell-surface staining, as well as good detection by confocal microscopy. Interestingly, a greater percentage of CD8+ T cells constitutively expressed TAAR1 on their cell surface rather than CD4+ T cells without any stimulation (Fig. 3B). Together, the data for the protein expression of TAAR1 suggest that TAAR1 plays a role in modulating T cell responses to METH, as other biogenic amines, such as tyramine and β-PEA [44].
Figure 2. METH increases TAAR1 protein expression in resting human T cells.
(A) Immunofluorescent staining and confocal imaging of human T lymphocytes showing expression of TAAR1. Representative photomicrographs of CD3 (red) and TAAR1 (green). One million T lymphocytes were left untreated or incubated for 24 h at 37°C with 100 μM of the one following treatments: β-PEA (Sigma-Aldrich), tyramine (Sigma-Aldrich), or MET (Sigma-Aldrich). (B) Data represent the fold change of the average of mean gray-pixel values ± sem from 5 separate images/treatment were taken at 63×. Images were analyzed for mean gray pixels/cells in the field by use of ImageJ software. T cells treated with tyramine (100 μM), β-PEA (100 μM), and METH (100 μM) showed a significant increase in TAAR1 expression compared with untreated control cells. *P < 0.05 for METH and tyramine; **P < 0.01 for β-PEA.
Figure 3. METH enhances expression of TAAR1 protein in CD4+ and CD8+ T cell subsets.
Two million PBMCs were treated with tyramine (100 μM), β-PEA (100 μM), and METH (100 μM) for 24 h and then stained with CD3-, CD4-, CD8-, and TAAR1-specific antibody and run on an LSRII (BD Biosciences) flow cytometer. (A) Representative flow cytometric analysis of TAAR1 expression in CD4 and CD8 T cells treated with tyramine (100 μM), β-PEA (100 μM), and METH (100 μM). Plots show percentage of CD3+, CD4+, CD8+ T cell subsets positive for TAAR1 staining, respectively. (B, left) Representative of TAAR1 expression on CD3, CD4, and CD8 T cells from a normal donor; (right) bar graph shows percentage of CD3+, CD4+, and CD8+ T cells expressing TAAR1 constitutively. Data are means ± sem of 3 independent testings. Significant constitutive increase in CD8 among the other subsets is represented by **P < 0.01, as analyzed by one-way ANOVA.
METH induced cAMP and cAMP-dependent protein kinase activity in resting T cells from normal donors via TAAR1
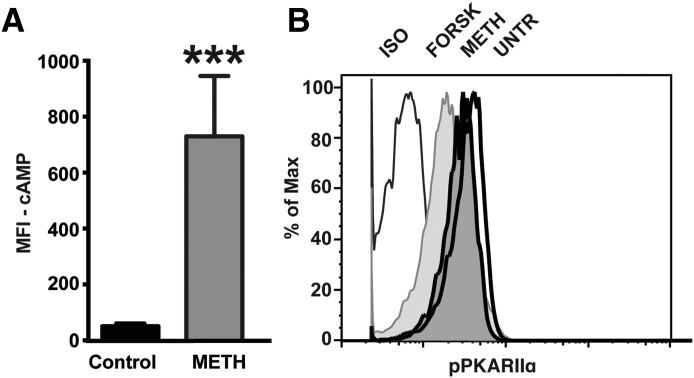
An increase in cAMP and cAMP-dependent PKA activation is a classic response to TAAR1 activation and has been demonstrated in HEK293 cell lines, immortalized rhesus monkey B cells, PHA-activated rhesus monkey lymphocytes [38], and neurologic cell types [45]. TAAR1-dependent phosphorylation of PKA and PKC has been shown in these studies. Whereas other groups have shown PKA activation upon METH in PHA-activated total lymphocytes, in this report, we demonstrate that METH, upon TAAR1 signaling, increased cAMP levels in resting human T cells. cAMP levels increased on T cells stimulated with METH for 30 min–1 h, as indicated by the increase in the MFI compared with control (Fig. 4A). Forskolin was used as a positive control. Phosflow analysis of METH-induced cAMP increase and subsequent PKA activation on T cells was measured by use of an antibody to pPKA RIIα (pS99), which is a regulatory subunit of PKA. An increase in cAMP decreases phosphorylation of PKA RIIα. This is demonstrated by the shift in fluorescence intensity to the left in the histogram, showing decreased MFI (Fig. 4B). Treatments with METH and the positive control forskolin decreased the MFI compared with the control, suggesting that METH induces activation of cAMP on T cells.
Figure 4. METH-induced TAAR1 activates cAMP and cAMP-dependent PKA phosphorylation.
(A) Induction of cAMP was detected by flow cytometry in MACS-isolated T cells after METH stimulation for 30 min–1 h by use of a purified mouse mAb, followed by an anti-mouse secondary, as described in materials and methods. Results are average MFI of cAMP from 3 different normal donors, each condition performed in duplicate. ***P < 0.001. (B) Phosflow analysis for pPKA RIIα (pS99) was done on total PBMC. Cells were counterstained for CD3 and analyzed. Histograms show fluorescence intensities of pPKA RIIα on CD3+ T cells at 15 min after stimulation. Decrease in pPKA RIIα is indicated by a shift of the curve to the left, indicating increased cAMP-dependent protein kinase activity. One representative of 2 independent testings done in duplicate. ISO, isotype; FORSK, forskolin; UNTR, untreated.
METH-induced cAMP and IL-2 production by T cells is TAAR1 dependent
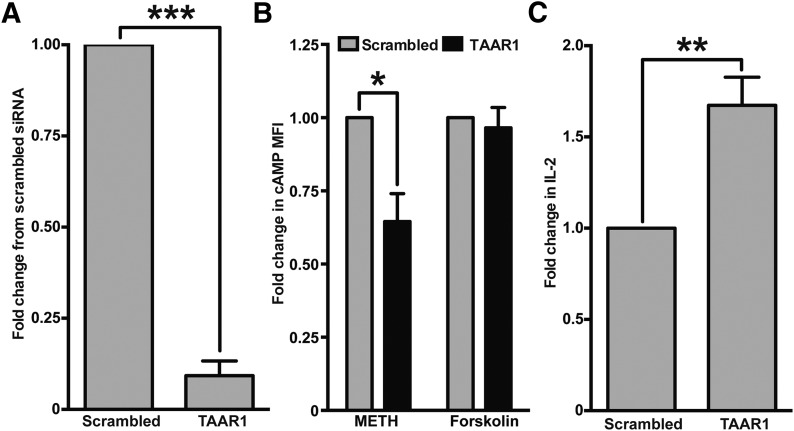
To demonstrate that TAAR1 regulated METH-induced responses in T cells, we resorted to a gene-silencing technique and used cAMP production and IL-2 production, the 2 major classic responses of T cells upon METH stimulation, as readouts. We used the TAAR1 siRNA sequence referenced in Babusyte et al. [33] and used INTERFERin transfection reagent and obtained a very good TAAR1 gene knockdown (up to 90%; Fig. 5A). Gene expression was assessed by qPCR by use of the GAPDH housekeeping gene as reference. By the gene-silencing technique, we confirm that the induction of cAMP is mediated via TAAR1 (Fig. 5B), as seen by a significant decrease in cAMP induction in the absence of TAAR1 compared with scrambled control. Forskolin, which increases intracellular levels of cAMP by activating enzyme adenylyl cyclase, remained unaltered, further confirming that METH via TAAR1 induces cAMP levels in human T cells.
Figure 5. The silencing of TAAR1 decreases METH-induced cAMP and increases IL-2 production in T cells.
Total PBMCs were treated with scrambled or TAAR1 siRNAs and cultured for ∼44 h in complete RPMI medium. (A) Cells were harvested at 44 h, and RNA was isolated for qPCR check for TAAR1 silencing. TAAR1 siRNA significantly knocked down the expression in PBMC. TAAR1 expression in scrambled siRNA versus TAAR1 siRNA; ***P < 0.001. (B) PBMC treated with siRNA was stimulated with METH (100 μM) or forskolin (100 μM) for 30 min–1 h, harvested and stained for CD3 and cAMP, and analyzed by flow cytometry. Results are expressed as fold change in cAMP MFI on CD3+ T cells in TAAR1 siRNA-treated PBMC versus scrambled siRNA-treated PBMC for each treatment. TAAR1 knocked down PBMCs showed significantly less cAMP expression compared with scrambled control siRNA (*P < 0.05), whereas forskolin-treated cells responded the same to both scrambled and TAAR1 siRNA. (C) Cells that were treated with siRNA were further treated with METH (100 μM) for 30 min and then stimulated with soluble CD3/CD28 antibodies and cultured for another 24 h. Supernatants were concentrated and analyzed for IL-2 by use of the MSD human IL-2 ELISA kit. The bar graph represents fold change from scrambled control. A significant increase (**P < 0.01) in the IL-2 levels was observed in the absence of TAAR1.
As a measure of T cell effector function, we measured IL-2 in the supernatant of siRNA-treated cultures that were stimulated with soluble CD3/CD28 in the presence or absence of 100 μM METH. Earlier, we have shown that METH decreased IL-2 production by T cells upon CD3/CD28 stimulation [7]. Knockdown of TAAR1 siRNA reversed the effect of METH on IL-2 as opposed to the scrambled siRNA control (Fig. 5C), suggesting that IL-2 production, a key cytokine produced by T cells, is regulated by TAAR1, implicating a very important role of TAAR1 in modulating T cell responses.
METH abusers with HIV-1 infection have increased TAAR1 expression in their lymph nodes
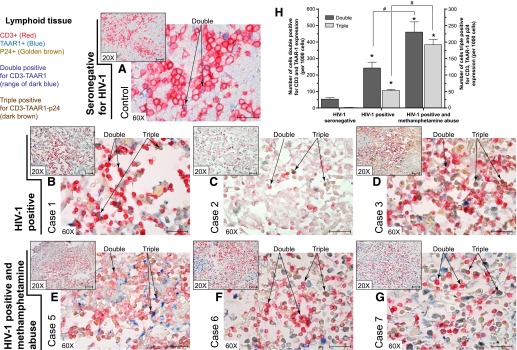
METH-induced modulation of immune responses in HIV-1 infection has a huge impact on treatment interruption and on the pathogenesis of HIV-1 infection itself [5, 46]. It is important to study the effects of this drug on the immune response in the context of understanding the biology, as well as in viral infections, especially HIV-1 infection, along with chronic drug abuse. Paraffin-embedded lymph node tissue was sectioned at 5 μ and immunostained for CD3, TAAR1, and the HIV-1 core protein p24, shown in Fig. 6. As shown in the multiplexed images (Fig. 6), evaluation of TAAR1 expression (blue) on the CD3 T cells (red) and p24 (golden brown) was performed on paraffin-embedded lymph node sections of HIV-1-infected patients (10 male patients and 1 female patient), obtained from the California NeuroAIDS Tissue Network, University of California, San Diego. The mean age of patients with and without a history of METH abuse was the following: METH-negative group, 54 ± 10 yr; METH-positive group, 39 ± 9 yr, respectively. The mean HIV-1 plasma viral load (Log 10) was the following: METH-negative group, 3.85 ± 2.07; METH-positive group, 4.41 ± 1.70. Among the METH-positive, HIV-infected patients, 3 cases had no alcohol dependence nor other drug dependence, whereas the other 4 cases had past alcohol dependence; 1 of these patients also had other drug dependence. One of the 4 HIV patients from the METH-negative group was alcohol dependent at the time the samples were collected but had no history of other drugs of abuse.
Figure 6. TAAR1 expression in lymph nodes of HIV-1-infected patients, with or without a history of METH abuse.
Paraffin-embedded lymph node tissue was sectioned at 5 μm and immunostained for CD3, TAAR1, and the HIV-1 core protein p24. As shown in the multiplexed images, the T lymphocyte marker CD3 appears in red (Vector Red), TAAR1 expression is observed in blue (Vector Blue), and p24 appears as golden brown (ImmPACT DAB). Representative images of (A) HIV-1 seronegative control, (B–D) HIV-1-positive cases (n = 3) with no history of METH abuse, and (E–G) HIV-1-positive individuals with a known history of METH abuse. Small insert images were taken under 20× objective magnification (original scale bars, 50 μm); the larger images were taken under 60× objective magnification (original scale bars, 25 μm). (H) Graph showing image analysis (with the use of segmentation, color threshold, and particle counting) results of the number of cells showing double (plotted on the left y-axis) and triple (plotted on the right y-axis) labeling. Results are shown as the averages ± sem. Statistical analyses were performed by use of one-way ANOVA with Dunnett’s multiple comparisons. Statistical significance differences, *P < 0.05, comparisons with the control (seronegative for HIV-1); #P < 0.05, analysis of those particular group comparisons.
Numerous T lymphocytes in the paracorticular area had detectable TAAR1 expression in the cytosolic, membranous, or both cellular compartments (examples are denoted as “Double” positive by the arrows). Individual T cells, stained double positive for CD3 and TAAR1 in the seronegative HIV-1 control, did not show any p24 immunostaining (Fig. 6A). In HIV-1 (Figures 6B–D) and HIV-1 with METH abuse cases (Fig. 6E–G), there was an appreciable increase in TAAR1 detection (number of cells) and in expression (strength of staining). Cases from HIV-1-positive individuals with a known history of METH abuse showed a greater number of double- and triple-positive cells (indicated by arrows under “Triple” positive) compared with HIV-1-only cases (Fig. 6H). These findings of increased expression of TAAR1 in T lymphocytes in the peripheral lymphoid organs, such as lymph nodes, suggest that TAAR1 may play a role in METH-mediated, immune-modulatory responses.
METH increases TAAR1 expression in the presence of HIV-1 infection
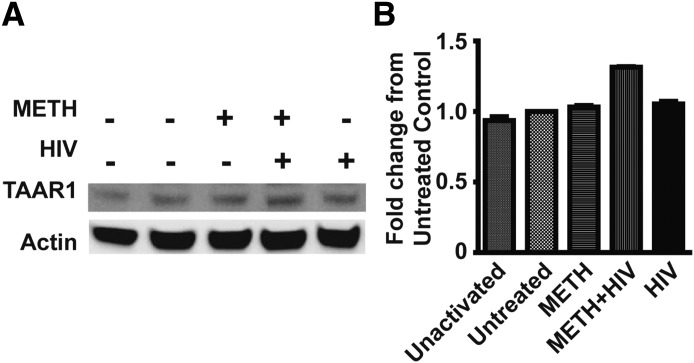
Several studies have examined the effects of METH during HIV-1 infection in different models [27, 40, 47]. We report, for the first time, an increase in TAAR1 expression in total PBMCs that were exposed to METH before infection with HIV-1 (Fig. 7). Western blot analysis of TAAR1 in total PBMC infected with HIV-1 in the presence or absence of METH for 24 h revealed an increase in TAAR1 expression (Fig. 7A and B). With the use of p24 analysis by ELISA from supernatants of HIV-infected PBMC in the presence or absence of METH, we confirm that p24 levels were increased in the HIV-infected cells exposed to METH (Supplemental Fig. 1A). HIV-RT analysis also showed an increase in HIV-1 RT activity in METH-treated and HIV-1-infected cells compared with HIV-1-infected cells alone (Supplemental Fig. 1B). METH increased HIV-1 p24 expression in CD3+ T cells, as seen by the increase in the percentage of p24-positive T cells, as assessed by flow cytometry (2.9% in HIV-1 alone vs. 4.5% in METH + HIV-1; Supplemental Fig. 1C). Overall, these data highlight a plausible association among TAAR1 expression, METH, and HIV-1 infection. Whereas these observations are noteworthy, further investigation is warranted to define the role of TAAR1 in HIV-1 infection, if any.
Figure 7. METH increases TAAR1 expression in the presence of HIV-1 infection.
PBMCs were treated with or without METH for 24 h and then infected with HIV-1ADA for 4 h, washed. and cultured in complete RPMI medium with IL-2. PBMCs were harvested on d 6 postinfection, and TAAR1 expression was analyzed by Western blot. (A) TAAR1 was detected as a 39–40 kDa protein. Actin was used as a loading control. Bands were analyzed from the same blot with 1 sample lane rearranged for the figure. (B) The bar graph represents fold change in band intensity from untreated control after normalization with actin. Unactivated control was without any PHA stimulation and without any treatment. The rest of the conditions were PHA activated and then treated with or without METH or HIV-1ADA. The blot is representative of 3 independent testings.
DISCUSSION
Since its discovery in 2001, TAAR1, a GPCR that is potently activated by trace amines, has gained a lot of attention, especially as a potential therapeutic target for psychiatric disorders [14, 48]. Among the various TAARs, TAAR1 is the most extensively studied molecule; its potential agonists, cellular expression, signaling cascades, brain regional distribution, and modulatory function in monoaminergic systems are very well established [9, 10, 15, 49]. Whereas a lot of studies in the last decade have focused on analyzing TAAR1 expression in cells of the CNS [12, 16, 17], it is only recently that the importance of TAAR1 in non-neuronal cells and immune cells in the periphery has been addressed [33, 38]. However, so far, no study has investigated the role of TAAR1 in resting/nonactivated primary human T cells exposed to METH and in the context of HIV-1 infection. Our findings are novel in this regard.
The TAAR family is comprised of 9 mammalian TAAR subtypes, with intact genes and pseudogenes differing considerably, even between closely related species [50]. Mouse and human TAAR1 is expressed in many tissues except olfactory epithelium, where other TAARs may play a role [44]. TAAR1 and TAAR2 are expressed in human lymphocytes from peripheral blood [33]. Miller and colleagues [51] have studied the functional evolution of TAAR1 and report expansion (in rodents) or reduction (in primates) of members of the gene family. In this study, we report, for the first time, TAAR1 expression and kinetics in resting human T cells upon METH stimulation. We observe that METH potentially increases TAAR1 gene and protein expression, as do other amines, such as β-PEA or tyramine, whereas the kinetics of induction may be different. A recent study shows constitutive expression of TAAR1 and TAAR2 in peripheral mononuclear cells and T and B cells [33]. Some functions, such as chemotaxis, have been tested by use of an siRNA knockdown system [33]. It will be interesting to study the expression of TAAR2 upon METH treatment and if it coexpresses with TAAR1 to bring about immune responses in T cells.
Several studies reveal that METH profoundly interferes with immunologic networks and affects diverse leukocyte subsets, thereby increasing susceptibility to infection [6, 7, 52, 53]. However, there has been no systematic approach to dissect out further the role of the primary receptor TAAR1 in METH-induced alterations in immune cell subsets in humans or in animal models. Our study, for the first time, reveals that TAAR1 protein is increased in CD4+ and CD8+ T cells upon METH stimulation, as well as with other classic biogenic amines that signal through TAAR1. Interestingly, there is an increased constitutive expression of TAAR1 in CD8+ T cells compared with CD4+ or CD3+ subsets. The biologic significance and functional consequence of this increased expression of TAAR1 in CD8+ T cells are unknown and need further investigation. As this constitutively increased TAAR1 expression on CD8 T cells may have important relevance on the ability of CD8 to fight viral infections, the observation warrants further investigation. How TAAR1 regulates CD8 T cell function in a viral infection may be important to understand, as our study shows that METH increases TAAR1 in CD4 and CD8 subsets, but we speculate that they may be differentially regulating the T cell functions in both subsets.
TAAR1 activation is followed by a very high induction of cAMP, which has been demonstrated by several groups [12, 13, 15]. Significant increases in the phosphorylation of PKA and PKC following the METH–TAAR1 interaction have been demonstrated in activated total lymphocytes [38].
In our earlier study, we showed that METH increased reactive oxygen species in T cells that are downstream of the cAMP pathway and inhibited the ability of the T cells to proliferate in response to stimulation, as assayed by measuring IL-2 secretion [7]. In this report, we demonstrate direct activation of cAMP and cAMP-dependent PKA activation in resting T cells upon METH stimulation by studying the induction of cAMP and phosphorylation of protein kinase that is immediately downstream in the signaling pathway. With the use of TAAR1 knockdown experiments, we demonstrate further that induction of cAMP, the classic downstream event after METH stimulation in T cells, is regulated by TAAR1. We also confirm, with the use of TAAR1 gene silencing, that METH stimulation via TAAR1 regulates the production of IL-2 in T cells. This is a very important finding and points to a pivotal role of TAAR1 in regulating T cell immune responses.
METH- and HIV-1-regulated TAAR1 expression and increased cAMP levels have been demonstrated recently in astrocytes [42]. Intracellular cAMP is thought to inhibit T cell activation and proliferation via activation of the cAMP-dependent PKA, and among the several immunologic factors that contribute to susceptibility to HIV infection, increasing evidence suggests that cAMP and downstream signaling via PKA play a crucial role [54]. In addition, PKA regulates cell proliferation via alteration of the cyclin/cyclin-dependent kinase complex [55, 56]. Interestingly, our unpublished data from characterization of the METH effects on T cell cycle entry and progression show that it results in altered cell-cycle entry and progression and decreased expression of cell-cycle progression markers (cyclin and cyclin-dependent kinase) of CD4+ and CD8+ T cells. This study underscores the effects of METH on the primary peripheral T cells and the probable role of TAAR1 to alleviate further susceptibility to HIV infection by increasing cAMP. Future TAAR1 molecular studies in primary human T cells could further corroborate the role of TAAR1 receptor-mediated immune regulation in the context of HIV.
The association of METH with risky sexual behavior and spread of sexually transmitted diseases, such as HIV-1, is a major public health concern [4, 22]. Considerable evidence exists linking drug abuse to immune dysregulation and enhanced susceptibility to the progression of chronic infections, such as HIV-1 [21]. METH has been shown to increase HIV-1 replication in dendritic cells [43] and monocyte-derived macrophages [47]. METH has also been suggested to activate HIV-1 long terminal repeat promoter-mediated transcription [57]. Mantri et al. [40] recently reported that increased doses of METH inhibited HIV-1 replication in primary CD4+ T cells by induction of microRNAs. The time of METH exposure varied in all of these studies [40, 43, 47]. None of these studies addressed the role of TAAR1, and therefore, we studied the expression of METH-induced TAAR1 in the context of HIV infection in T cells. In our model, we exposed total PBMCs to METH for 24 h and then infected them with HIV-1 and continued to give METH every other day to simulate chronic exposure until the cells were harvested at different time points. We used p24 as a marker of HIV infection. We did see an increase in HIV-1 p24 expression levels in T cells pretreated with METH, as assessed by ELISA and flow cytometry (Supplemental Fig. 1). We also report, for the first time in this study, that METH, in the presence of HIV-1 infection, increases TAAR1 expression in peripheral blood cells. Our analysis of lymph node sections clearly shows increased TAAR1 expression in HIV-1 patients with a history of METH abuse compared with HIV-1 patients with no history of METH abuse. The potential association between the impact of substance abuse on the host factors that affect immune response and the risk for infection with HIV and sexually transmitted diseases is dependent on multiple compounding factors, along with potential confounders. The immune system of HIV patients with drug-abuse history is notably affected by the various drugs and can greatly affect the interpretation of effects of each drug studied independently [36]. Nevertheless, the enhanced expression of TAAR1 in the lymph nodes of METH users compared with those with no METH history underscores the plausible role of TAAR1 in mediating METH-induced immune dysregulation. Taken together, our findings highlight the potential role of TAAR1 in HIV-1 infection and analyzing its role as an immune modulator.
In summary, our data suggest a role for METH-induced TAAR1 expression in regulating T cell immune responses. Further investigation of the role of TAAR1 in regulating HIV-1 pathogenesis, especially in the periphery, can have a major impact in therapeutic approaches to control HIV-1 infection, especially in the context of drug abuse. Studies with humanized mouse models can be helpful to dissect out further the TAAR1-mediated pathways.
AUTHORSHIP
All authors participated in the design of experiments, interpretation of data, and writing of the manuscript. U.S., J.M.C., B.H., N.C.F., R.R., S.F., and S.H.R. performed the experiments and analyzed the data.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) Grant R01 DA031064 and Temple Development Grant (to R.P.), NIH Grant RO3 AR065157 (to U.S.), NIH/National Institute of Neurological Disorders and Stroke Grant R01 NS086570-01, and Shriners Hospitals for Children Grant 85110-PHI-14 (to S.H.R.). The California NeuroAIDS Tissue Network is acknowledged for lymphoid tissue specimens used in this study.
Glossary
- β-PEA
β-phenylethylamine
- ADA
adenosine deaminase
- GPCR
G protein-coupled receptor
- HEK
human embryonic kidney
- METH
methamphetamine
- MFI
median fluorescence intensity
- PKA/C
protein kinase A/C
- pPKA RIIα
phospho-PKA regulatory subunit IIα
- qRT-PCR
quantitative RT-PCR
- siRNA
small interfering RNA
- TAAR1
trace amine-associated receptor 1
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1.Gold M. S., Kobeissy F. H., Wang K. K., Merlo L. J., Bruijnzeel A. W., Krasnova I. N., Cadet J. L. (2009) Methamphetamine- and trauma-induced brain injuries: comparative cellular and molecular neurobiological substrates. Biol. Psychiatry 66, 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panenka W. J., Procyshyn R. M., Lecomte T., MacEwan G. W., Flynn S. W., Honer W. G., Barr A. M. (2013) Methamphetamine use: a comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend. 129, 167–179. [DOI] [PubMed] [Google Scholar]
- 3.Rawson R. A., Anglin M. D., Ling W. (2002) Will the methamphetamine problem go away? J. Addict. Dis. 21, 5–19. [DOI] [PubMed] [Google Scholar]
- 4.Freese T. E., Obert J., Dickow A., Cohen J., Lord R. H. (2000) Methamphetamine abuse: issues for special populations. J. Psychoactive Drugs 32, 177–182. [DOI] [PubMed] [Google Scholar]
- 5.Boddiger D. (2005) Metamphetamine use linked to rising HIV transmission. Lancet 365, 1217–1218. [DOI] [PubMed] [Google Scholar]
- 6.Martinez L. R., Mihu M. R., Gácser A., Santambrogio L., Nosanchuk J. D. (2009) Methamphetamine enhances histoplasmosis by immunosuppression of the host. J. Infect. Dis. 200, 131–141. [DOI] [PubMed] [Google Scholar]
- 7.Potula R., Hawkins B. J., Cenna J. M., Fan S., Dykstra H., Ramirez S. H., Morsey B., Brodie M. R., Persidsky Y. (2010) Methamphetamine causes mitrochondrial oxidative damage in human T lymphocytes leading to functional impairment. J. Immunol. 185, 2867–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabral G. A. (2006) Drugs of abuse, immune modulation, and AIDS. J. Neuroimmune Pharmacol. 1, 280–295. [DOI] [PubMed] [Google Scholar]
- 9.Borowsky B., Adham N., Jones K. A., Raddatz R., Artymyshyn R., Ogozalek K. L., Durkin M. M., Lakhlani P. P., Bonini J. A., Pathirana S., Boyle N., Pu X., Kouranova E., Lichtblau H., Ochoa F. Y., Branchek T. A., Gerald C. (2001) Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 98, 8966–8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bunzow J. R., Sonders M. S., Arttamangkul S., Harrison L. M., Zhang G., Quigley D. I., Darland T., Suchland K. L., Pasumamula S., Kennedy J. L., Olson S. B., Magenis R. E., Amara S. G., Grandy D. K. (2001) Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol. Pharmacol. 60, 1181–1188. [DOI] [PubMed] [Google Scholar]
- 11.Lindemann L., Hoener M. C. (2005) A renaissance in trace amines inspired by a novel GPCR family. Trends Pharmacol. Sci. 26, 274–281. [DOI] [PubMed] [Google Scholar]
- 12.Lindemann L., Ebeling M., Kratochwil N. A., Bunzow J. R., Grandy D. K., Hoener M. C. (2005) Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics 85, 372–385. [DOI] [PubMed] [Google Scholar]
- 13.Berry M. D. (2004) Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J. Neurochem. 90, 257–271. [DOI] [PubMed] [Google Scholar]
- 14.Berry M. D. (2007) The potential of trace amines and their receptors for treating neurological and psychiatric diseases. Rev. Recent Clin. Trials 2, 3–19. [DOI] [PubMed] [Google Scholar]
- 15.Miller G. M., Verrico C. D., Jassen A., Konar M., Yang H., Panas H., Bahn M., Johnson R., Madras B. K. (2005) Primate trace amine receptor 1 modulation by the dopamine transporter. J. Pharmacol. Exp. Ther. 313, 983–994. [DOI] [PubMed] [Google Scholar]
- 16.Miller G. M. (2011) The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J. Neurochem. 116, 164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie Z., Westmoreland S. V., Miller G. M. (2008) Modulation of monoamine transporters by common biogenic amines via trace amine-associated receptor 1 and monoamine autoreceptors in human embryonic kidney 293 cells and brain synaptosomes. J. Pharmacol. Exp. Ther. 325, 629–640. [DOI] [PubMed] [Google Scholar]
- 18.Nelson D. A., Tolbert M. D., Singh S. J., Bost K. L. (2007) Expression of neuronal trace amine-associated receptor (Taar) mRNAs in leukocytes. J. Neuroimmunol. 192, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian H. Z., Stinnette S. E., Rebeiro P. F., Kipp A. M., Shepherd B. E., Samenow C. P., Jenkins C. A., No P., McGowan C. C., Hulgan T., Sterling T. R. (2011) The relationship between injection and noninjection drug use and HIV disease progression. J. Subst. Abuse Treat. 41, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kipp A. M., Desruisseau A. J., Qian H. Z. (2011) Non-injection drug use and HIV disease progression in the era of combination antiretroviral therapy. J. Subst. Abuse Treat. 40, 386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman H., Pross S., Klein T. W. (2006) Addictive drugs and their relationship with infectious diseases. FEMS Immunol. Med. Microbiol. 47, 330–342. [DOI] [PubMed] [Google Scholar]
- 22.Cofrancesco J. Jr., Scherzer R., Tien P. C., Gibert C. L., Southwell H., Sidney S., Dobs A., Grunfeld C. (2008) Illicit drug use and HIV treatment outcomes in a US cohort. AIDS 22, 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shoptaw S., Reback C. J., Freese T. E. (2002) Patient characteristics, HIV serostatus, and risk behaviors among gay and bisexual males seeking treatment for methamphetamine abuse and dependence in Los Angeles. J. Addict. Dis. 21, 91–105. [DOI] [PubMed] [Google Scholar]
- 24.Li L., Assanangkornchai S., Duo L., McNeil E., Li J. (2014) Cross-border activities and association with current methamphetamine use among Chinese injection drug users (IDUs) in a China-Myanmar border region. Drug Alcohol Depend. 138, 48–53. [DOI] [PubMed] [Google Scholar]
- 25.Ghimire B., Suguimoto S. P., Zamani S., Ono-Kihara M., Kihara M. (2013) Vulnerability to HIV infection among female drug users in Kathmandu Valley, Nepal: a cross-sectional study. BMC Public Health 13, 1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cisneros I. E., Ghorpade A. (2012) HIV-1, methamphetamine and astrocyte glutamate regulation: combined excitotoxic implications for neuro-AIDS. Curr. HIV Res. 10, 392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Persidsky Y., Heilman D., Haorah J., Zelivyanskaya M., Persidsky R., Weber G. A., Shimokawa H., Kaibuchi K., Ikezu T. (2006) Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE). Blood 107, 4770–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellis R. J., Childers M. E., Cherner M., Lazzaretto D., Letendre S., Grant I.; HIV Neurobehavioral Research Center Group (2003) Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J. Infect. Dis. 188, 1820–1826. [DOI] [PubMed] [Google Scholar]
- 29.Smith D. M., Wong J. K., Hightower G. K., Ignacio C. C., Koelsch K. K., Petropoulos C. J., Richman D. D., Little S. J. (2005) HIV drug resistance acquired through superinfection. AIDS 19, 1251–1256. [DOI] [PubMed] [Google Scholar]
- 30.Colfax G. N., Vittinghoff E., Grant R., Lum P., Spotts G., Hecht F. M. (2007) Frequent methamphetamine use is associated with primary non-nucleoside reverse transcriptase inhibitor resistance. AIDS 21, 239–241. [DOI] [PubMed] [Google Scholar]
- 31.Moore R. D., Keruly J. C., Chaisson R. E. (2004) Differences in HIV disease progression by injecting drug use in HIV-infected persons in care. J. Acquir. Immune Defic. Syndr. 35, 46–51. [DOI] [PubMed] [Google Scholar]
- 32.Francis S. H., Corbin J. D. (1994) Structure and function of cyclic nucleotide-dependent protein kinases. Annu. Rev. Physiol. 56, 237–272. [DOI] [PubMed] [Google Scholar]
- 33.Babusyte A., Kotthoff M., Fiedler J., Krautwurst D. (2013) Biogenic amines activate blood leukocytes via trace amine-associated receptors TAAR1 and TAAR2. J. Leukoc. Biol. 93, 387–394. [DOI] [PubMed] [Google Scholar]
- 34.Gendelman H. E., Orenstein J. M., Martin M. A., Ferrua C., Mitra R., Phipps T., Wahl L. A., Lane H. C., Fauci A. S., Burke D. S. (1988) Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J. Exp. Med. 167, 1428–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbas-Terki T., Blanco-Bose W., Déglon N., Pralong W., Aebischer P. (2002) Lentiviral-mediated RNA interference. Hum. Gene Ther. 13, 2197–2201. [DOI] [PubMed] [Google Scholar]
- 36.Potula R., Persidsky Y. (2008) Adding fuel to the fire: methamphetamine enhances HIV infection. Am. J. Pathol. 172, 1467–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Andrea G., Terrazzino S., Fortin D., Farruggio A., Rinaldi L., Leon A. (2003) HPLC electrochemical detection of trace amines in human plasma and platelets and expression of mRNA transcripts of trace amine receptors in circulating leukocytes. Neurosci. Lett. 346, 89–92. [DOI] [PubMed] [Google Scholar]
- 38.Panas M. W., Xie Z., Panas H. N., Hoener M. C., Vallender E. J., Miller G. M. (2012) Trace amine associated receptor 1 signaling in activated lymphocytes. J. Neuroimmune Pharmacol. 7, 866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wasik A. M., Millan M. J., Scanlan T., Barnes N. M., Gordon J. (2012) Evidence for functional trace amine associated receptor-1 in normal and malignant B cells. Leuk. Res. 36, 245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantri C. K., Mantri J. V., Pandhare J., Dash C. (2014) Methamphetamine inhibits HIV-1 replication in CD4+ T cells by modulating anti-HIV-1 miRNA expression. Am. J. Pathol. 184, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lane H. C. (2010) Pathogenesis of HIV infection: total CD4+ T-cell pool, immune activation, and inflammation. Top. HIV Med. 18, 2–6. [PubMed] [Google Scholar]
- 42.Cisneros I. E., Ghorpade A. (2014) Methamphetamine and HIV-1-induced neurotoxicity: role of trace amine associated receptor 1 cAMP signaling in astrocytes. Neuropharmacology 85, 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nair M. P., Saiyed Z. M., Nair N., Gandhi N. H., Rodriguez J. W., Boukli N., Provencio-Vasquez E., Malow R. M., Miguez-Burbano M. J. (2009) Methamphetamine enhances HIV-1 infectivity in monocyte derived dendritic cells. J. Neuroimmune Pharmacol. 4, 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zucchi R., Chiellini G., Scanlan T. S., Grandy D. K. (2006) Trace amine-associated receptors and their ligands. Br. J. Pharmacol. 149, 967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Espinoza S., Salahpour A., Masri B., Sotnikova T. D., Messa M., Barak L. S., Caron M. G., Gainetdinov R. R. (2011) Functional interaction between trace amine-associated receptor 1 and dopamine D2 receptor. Mol. Pharmacol. 80, 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.[No authors listed] (2005) HIV & drugs. Meth use develops stronger link to HIV risk. AIDS Policy Law 20, 5. [PubMed] [Google Scholar]
- 47.Liang H., Wang X., Chen H., Song L., Ye L., Wang S. H., Wang Y. J., Zhou L., Ho W. Z. (2008) Methamphetamine enhances HIV infection of macrophages. Am. J. Pathol. 172, 1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller G. M. (2012) Avenues for the development of therapeutics that target trace amine associated receptor 1 (TAAR1). J. Med. Chem. 55, 1809–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lindemann L., Meyer C. A., Jeanneau K., Bradaia A., Ozmen L., Bluethmann H., Bettler B., Wettstein J. G., Borroni E., Moreau J. L., Hoener M. C. (2008) Trace amine-associated receptor 1 modulates dopaminergic activity. J. Pharmacol. Exp. Ther. 324, 948–956. [DOI] [PubMed] [Google Scholar]
- 50.Stäubert C., Böselt I., Bohnekamp J., Römpler H., Enard W., Schöneberg T. (2010) Structural and functional evolution of the trace amine-associated receptors TAAR3, TAAR4 and TAAR5 in primates. PLoS One 5, e11133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vallender E. J., Xie Z., Westmoreland S. V., Miller G. M. (2010) Functional evolution of the trace amine associated receptors in mammals and the loss of TAAR1 in dogs. BMC Evol. Biol. 10, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tallóczy Z., Martinez J., Joset D., Ray Y., Gácser A., Toussi S., Mizushima N., Nosanchuk J. D., Goldstein H., Loike J., Sulzer D., Santambrogio L. (2008) Methamphetamine inhibits antigen processing, presentation, and phagocytosis. PLoS Pathog. 4, e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harms R., Morsey B., Boyer C. W., Fox H. S., Sarvetnick N. (2012) Methamphetamine administration targets multiple immune subsets and induces phenotypic alterations suggestive of immunosuppression. PLoS One 7, e49897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rahmouni S., Aandahl E. M., Trebak M., Boniver J., Tasken K., Moutschen M. (2001) Increased cAMP levels and protein kinase (PKA) type I activation in CD4+ T cells and B cells contribute to retrovirus-induced immunodeficiency of mice (MAIDS): a useful in vivo model for drug testing. FASEB J. 15, 1466–1468. [DOI] [PubMed] [Google Scholar]
- 55.Matsuda S., Kominato K., Koide-Yoshida S., Miyamoto K., Isshiki K., Tsuji A., Yuasa K. (2014) PCTAIRE kinase 3/cyclin-dependent kinase 18 is activated through association with cyclin A and/or phosphorylation by protein kinase A. J. Biol. Chem. 289, 18387–18400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White P. C., Shore A. M., Clement M., McLaren J., Soeiro I., Lam E. W., Brennan P. (2006) Regulation of cyclin D2 and the cyclin D2 promoter by protein kinase A and CREB in lymphocytes. Oncogene 25, 2170–2180. [DOI] [PubMed] [Google Scholar]
- 57.Wires E. S., Alvarez D., Dobrowolski C., Wang Y., Morales M., Karn J., Harvey B. K. (2012) Methamphetamine activates nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and induces human immunodeficiency virus (HIV) transcription in human microglial cells. J. Neurovirol. 18, 400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.