Abstract
The past decade saw the advent of a number of promising biomarkers to detect pregnancies at risk for preeclampsia (PE), the foremost being those associated with an imbalance of angiogenic factors. In late pregnancy, these are useful for the detection of imminent cases of PE, while earlier they were more predictive for early- than late-onset PE. This suggests that there may be fundamental differences between the underlying pathology of these two PE forms. Therefore, it is possible that such a biological premise may limit the development of biomarkers that will permit the efficacious detection of both early- and late-onset PE via an analysis of first-trimester maternal blood samples. Consequently, a significant increase in our understanding of the underlying pathology of PE, using a variety of approaches ranging from systems biology to animal models, will be necessary in order to overcome this obstacle.
Keywords: animal model, early-onset/late-onset, etiology, placenta, preeclampsia, screening
It has recently been suggested that a concerted effort should be undertaken to invert the pyramid of prenatal care [1]. In this manner, more emphasis would be placed on the early detection of pregnancy complications, such as preeclampsia (PE), preterm labor (PTL), intrauterine fetal growth restriction (IUGR) or fetal genetic anomalies such as Down syndrome (DS) in the first, rather than in the second or third trimester of pregnancy [1,2].
The rationale behind such a proposed paradigm shift is the observation that it may be possible to extend the scope of the current first trimester integrated or combined test, conducted at 11–13 weeks of gestation, to detect not only fetal chromosomal anomalies, but also pregnancies at risk for an array of complications including PE, IUGR and PTL [1,2].
Such a practice would effectively break with a nearly century-old tradition. It would also lead to a change in therapeutic strategy, namely the long-term intervention or amelioration rather than the fait accompli of severe manifest symptoms clinicians are currently presented with, frequently leaving no other option than emergency delivery of a very premature baby. Hence, in this new first trimester screening setting, at-risk pregnancies would pursue a separate path of dedicated specialist care, while normal low-risk pregnancies would undergo standard routine examinations in the second and third trimester as per usual [1].
The success of such a scheme would depend on the detection rate of at-risk pregnancies, and possible therapeutic options, that is, if only a fraction could be detected and no treatment option existed, then what would be the point? [3].
PE – syndrome with multiple etiologies?
PE is a relatively frequent disorder of pregnancy, affecting between 3 and 8% of all pregnant women, with a higher predisposition for certain ethnic groups [4–8]. It is characterized by a sudden elevation in blood pressure (BP) in previously normotensive pregnant women, a feature accompanied by proteinuria and frequently edema [4–7]. If left untreated, PE can progress to eclampsia, characterized by epileptic-like seizures. This condition is frequently fatal, especially in developing countries, where mortality rates may be as high as 15% [4–7,9].
Despite best clinical practice in developed countries, PE remains a leading cause of fetal and maternal morbidity and mortality, especially with regard to preterm delivery [6,10].
The clinical presentation of PE can be confusing and need not follow the general pattern of elevated BP followed by proteinuria, but can occur in a reverse order or with obscured clinical symptoms [9,11]. Furthermore, the advent of eclampsia can be sudden, without noticeable signs of PE, and may even occur very early in the second trimester [9]. An additional confounding condition is that PE can occur postpartum, by a period as late as 3 weeks [9,12]. These cases are frequently severe, and can be associated with maternal mortality [12].
For this reason, the pathophysiology leading to the development of PE has been deemed so complex that PE has frequently been labeled a disease of theories [13–15]. This facet has changed significantly in the past decade when a significant understanding in the underlying etiology was gained [7]. These include the imbalance in angiogenic factors [16], the contribution by placental galectin PP13 (placental protein 13) [17], the activation of the complement cascade [18] and the excessive presence of neutrophil extracellular traps (NETs) directly in the intervillous space of affected placentae [19,20].
Further immunological factors suggested to play a role in PE include reduced expression of the tolerance molecule HLA-G on the trophoblast [21], inappropriate action of uterine NK cells [22], an imbalance in Treg [23], reduced placental expression of the tryptophan catabolizing enzyme indoleamine 2,3-dioxygenase (IDO) [24], altered distribution of placental macrophages [25] or occurrence of autoantibodies against the type 1 angiotensin II receptor [26] as well as thrombin and platelet activation [27].
PE – two forms depending on time of onset?
A fundamental contribution to our understanding was the segregation of PE into early (<34 weeks gestation, ePE) and late-onset forms (>34 weeks gestation, lPE), with the implication that these two forms may be disparate and the result of different or independent lesions [8,28]. This tenet is supported by a number of lines of evidence, including differences in placental pathology between ePE and lPE [29], the association of ePE but not lPE with certain genetic loci such as the activin A receptor IIA [30], or evidence of ischemic stress and endoplasmic reticulum stress-induced apoptosis in ePE placentae [31,32].
On the other hand, lPE appears to be associated with factors which are not directly placenta associated and not evident in ePE, such as reduced levels of 25-hydroxyvitamin D [33] or ficolin-2, a carbohydrate pattern recognition receptor [34]. Additionally, the incidence of lPE but not ePE, may be enhanced by certain environmental factors, such as air pollution or especially obesity [35,36].
A further noteworthy factor to bear in mind is that the incidence of lPE is almost 10-fold higher than that of ePE [37,38], and although the symptoms for the former may be less severe than for the latter, they are still of considerable clinical concern, especially in tertiary settings [39].
Following almost century-old reports of familial clusters of PE [40], a considerable effort has been made to determine genetic factors that contribute to the development of PE [41], which has resulted in the detailing of almost 180 genetic loci [42]. Although currently no clear data exist concerning genetic predisposition for ePE rather than lPE or vice-versa, epidemiological data indicate that pre-existing chronic hypertension appears to be a significant risk factor for the development of ePE, rather than lPE [38].
Differences also appear to exist on the postpartum influence of ePE or lPE on mother and child. In this context, data from a recent study indicate that ePE is associated with a significantly higher occurrence of postpartum cardiovascular disorders, such as hypertension, than lPE [43].
Furthermore, ePE leads to an increased risk of childhood asthma, not evident in the children born from lPE pregnancies [44]. The issue of ePE or lPE can also lead to confusion, as is evident from studies testing the efficacy of low-dose aspirin, where some studies indicate that this can reduce the incidence of lPE [45], while more recent data suggest that this may be more useful for cases at risk of ePE [46].
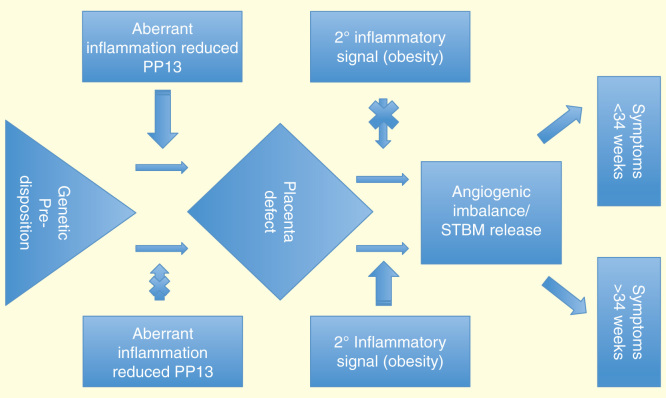
These diverse facets suggesting different underlying etiologies are explored in more detail below and referred to in Figure 1.
Figure 1.
Illustration of putative differences in the underlying etiology of ePE versus lPE. The primary lesion in deep placentation plays a leading role in the development of ePE. Failure of spiral artery modification may involve aberrant maternal inflammation due to reduced PP13 expression. By leading to placental insufficiency, this results in the liberation of inflammatory trophoblast micro-debris and imbalance in pro- and anti-angiogenic factors, events which precede clinical symptoms. In lPE, the placental lesion is insufficient to trigger development of clinical symptoms, bur rather renders the maternal system highly susceptible to secondary signals, such as obesity, which then initiate the etiological cascade leading to onset of symptoms.
ePE: Early preeclampsia; lPE: Late preeclampsia.
Development of PE – a dual role of the placenta?
While it is generally accepted that the placenta is the main culprit triggering the development of PE, it is still unclear what exact contribution in each instance it has [7,29,47]. In this regard, the placenta in ePE is frequently characterized by inadequate modification of the maternal spiral arteries by invasive fetal cytotrophoblast cells [29].
During early embryo development, the placenta has limited contact to the maternal circulation since the mouths of the maternal spiral arteries are plugged by invading trophoblast cells [48]. These relatively hypoxic conditions are suggested to protect the fetus from potential teratogenic effects of reactive oxygen species [49]. It is of interest to note that this condition does not lead to hypoxic or metabolic stress in these placental tissues, suggesting adequate nutrition and support by the maternal endometrial glands during this phase of fetoplacental development [50].
At the end of the first trimester these plugs are removed, leading to a sudden exposure of the hypoxic placenta to maternal circulation and to a threefold rise in oxygen concentration [49]. Aberrancies during this crucial phase of development, resulting in toxic oxygen levels due to too rapid or uncoordinated opening of these plugs can lead to fetal loss [48].
During the second trimester, the maternal spiral arteries are widened by the action of endovascular cytotrophoblast cells, which replace the maternal endothelial cells [51]. This results in a low-pressure system whereby the placental villi are bathed in a continuous slow flow of maternal blood. A number of studies have indicated that this modification during this crucial phase of placenta development is defective in PE [51]. The underlying mechanism for this defect is not clear, but may involve an incapacity of the extravillous trophoblast cells to differentiate correctly [52], or it may result from the failure of the maternal endothelial glands not producing critical cytokines in the required amounts [48].
This feature is, however, also present in IUGR pregnancies, and in a proportion of cases with PTL. In the latter instance, the degree of spiral artery modification is significantly lower than in PE or IUGR. This has led to the premise that the major obstetrical concerns may have similar defects in deep placentation [29].
On the other hand, recent evidence suggests that placental lesions consistent with maternal underperfusion and spiral artery anomalies are less frequent in cases with lPE than ePE, but that these are still significantly more prevalent in lPE than in healthy controls at term [53,54].
Due to the decreased incidence of these lesions in cases with lPE, it was proposed that maternal systemic inflammation and other factors may play more substantial roles besides placental lesions in the development of this form of PE. The conundrum is that both forms of PE have similar clinical characteristics with regard to BP and proteinuria, reflecting the ‘common pathway’ of PE [53,54].
Although the placenta in lPE may more frequently appear healthy and normal, there are other lines of evidence suggesting that it may be altered in other ways. The first of these relies on a century-old observation that trophoblast deportation is elevated in cases with PE, in that trophoblast cells could be detected in the lungs of women who had died from eclampsia [55], a feature recently confirmed by the detection of transcriptionally active placenta fragments [56].
An examination of cross-placental fetal cell traffic indicated that this was elevated in cases with manifest PE [57]. Furthermore, enhanced fetal cell trafficking was observed early in gestation prior to the onset of symptoms [58]. As these study cohorts included both cases with ePE and lPE, this would suggest that the lesion permitting cross-placental trafficking occurs in both forms [59].
A further suggestion that the placenta is affected in both forms of PE is provided by the quantitative analysis of placental cell-free DNA (cfDNA), where elevations in both forms were noted [60,61]. As this material is largely released by the syncytiotrophoblast (STB), this would suggest that turnover of this tissue is dysregulated in both forms [59]. Of interest is that the levels of placental cfDNA were greater in ePE cases than those with lPE, despite the latter having a significantly larger placenta. This indicates that the placental lesions in ePE leading to the liberation of cfDNA are more severe [60].
In addition, it was observed that placental cfDNA levels were elevated in cases at risk for PE well in advance of the development of symptoms, indicating that the disturbance of the trophoblast involved in cfDNA release is an early event in the development of PE [62,63]. This underscores the hypothesis that PE is a disorder of early implantation or placentation, with a long asymptomatic phase, before these anomalies culminate in the clinical symptoms [9,59].
To account for this disparity, a model has recently been proposed with two different placental causes leading to the development of PE, with placental oxidative stress playing a central role [9].
In the development of ePE, placental oxidative stress is mediated via the failure to adequately modify the maternal spiral arteries, whereas in lPE, it is suggested to result predominantly as a consequence of abnormal uteroplacental perfusion [9]. It is, however, possible that lPE may result from a number of other biological or environmental stresses, especially secondary inflammatory lesions, as indicated in Figure 1.
As a consequence of such stress conditions, the STB produces an excess of the anti-angiogenic factor soluble fms-like tyrosine kinase-1 (sFlt-1), which leads to a concomitant decrease in pro-angiogenic placental growth factor (PlGF) in maternal blood. This imbalance in pro- and anti-angiogenic factors has been shown to be a major contributor to the widespread systemic endothelial damage evident in cases with PE [16].
It is interesting that recent studies examining placental stress pathways in PE found that ePE is characterized by the significant activation of stress kinase pathways, while similar phenomenon was not observed in lPE [32,64]. These data suggest that factors other than those related to oxidative stress may be key in the activation of the common pathway and angiogenic imbalance in lPE.
PE – the role of the innate immune system
PE involves more than an imbalance in angiogenic factors, and is for instance characterized by an excessive activation of the maternal innate immune system, specifically neutrophils [65]. Indeed, the degree of neutrophil activation assessed via the production of reactive oxygen species was greater in PE than in cases with sepsis [66]. This activation is mediated via the excessive release of microparticles by STB, and are hence, termed STBM [67].
Previous studies have indicated that STBM can trigger the release of NETs by isolated neutrophils, and that high numbers of NETs can be detected directly in the intervillous space of affected placentae [19]. Since NETs can promote coagulation, acting as a scaffold for the coagulation process [68], the excessive presence of these structures could lead to maternal underperfusion and consequent placental occlusion or infarction [20]. These would contribute to hypoxic and/or ischemic conditions frequently detected in PE placentae [48].
In addition to neutrophils, PE is also associated with overt activation of the complement system, as is evident by the ready detection of complement products such as C5a and C4a in maternal plasma, or C5b-9 and adipsin (complement factor D) in maternal urine [18,69,70]. Activation of the complement system was shown to occur in affected placentae, for instance, by staining for C4d deposition, which was rarely observed in normal placentae, while it was frequently detected on the STB of PE placentae [71]. Furthermore, PE was associated with increased expression of CD55 and CD59, two complement regulatory proteins [70]. In a more recent study, this aspect has been examined in more detail in both cases with ePE and lPE, where it was observed that C1q deposition was greater in the former than in the latter [71]. The authors also noted that maternal C4 deficiencies were more frequent in the ePE group [71]. Mutations in the complement C3F gene are associated with an increased susceptibility for PE [72]. Further evidence implicating a role for complement activation in PE is the report that treatment of a patient with severe ePE/HELLP syndrome with eculizumab, a targeted inhibitor of complement protein C5, which resulted in a significant reduction of symptoms and permitted a prolongation of pregnancy by 17 days, in itself is a remarkable achievement [73].
Most of these findings support that the activation of the complement pathway in the second half of pregnancy plays a role in the development of PE. Interestingly, a prospective study showed that women with high levels of complement activation fragment Bb before 20 weeks of gestation had increased risk to develop PE later in pregnancy [74]. This finding suggests that the alternative pathway of complement was activated in these patients in early pregnancy. Moreover, studies on pentraxin 3 (PTX3), a multifunctional pattern recognition receptor that also binds to complement component C1q and modulates complement activation [75], revealed that patients who presented with PE had higher maternal plasma levels of PTX3 than controls [76,77], and maternal plasma PTX3 concentrations at 11–13 weeks were already higher in patients subsequently developing ePE but not lPE [78,79]. These findings point to an innate immune imbalance in the development of PE, especially ePE, in early pregnancy.
Incidence of ePE versus lPE – the influence of secondary inflammatory stimuli such as air pollution, diabetes or obesity & possible lack of scavenger molecules
It is highly probable that the large number of anecdotes concerning possible etiological or risk factors for PE contributed to this enigmatic disorder being stigmatized as ‘disease of theories’ [14]. Of these is the increased incidence of PE with certain weather conditions.
Credible evidence for such a phenomenon was provided in a study conducted in Zimbabwe, where a seasonal influence on the incidence of PE was noted, in that there was a peak at the onset of the rainy season [80]. In Cape Town, at the tip of South Africa, increased numbers of cases with PE were noted in winter [81]. In an extended study of almost 5000 pregnant women, of whom almost half were affected by PE, an increased incidence was noted in autumn [82].
Unfortunately, in none of these studies was an attempt made to stratify cases with PE into those with early or late onset. However, it is to be assumed that a large proportion of these were cases with lPE. Although it is unclear what the seasonal noxin was triggering this increased onset of PE, it is possible that it is related to air pollution, since the rural population in South Africa rely on the burning of biofuels for heating in winter. Such a facet is supported by a number of studies suggesting that high levels of air pollutants are associated with elevations in the incidence of PE [35,83]. In a large-scale analysis of more than 8000 pregnant women, it was noted that exposure airborne particulate matter in the first trimester led to an increase in the incidence of cases with ePE, while similar exposure in the third trimester was associated with an increase in the number of lPE cases [84]. In a smaller scale study, exposure to airborne particulate matter during a particularly dusty dry autumn was shown to be associated with an increase in the number of lPE cases [85]. On a similar note, smoking during pregnancy was shown to be a major risk factor for the development of both ePE and lPE [38]. In this context, it is worth noting that previous studies suggesting that smoking may appear protective or diminish the incidence of PE are misleading due to left truncation bias in the statistical analysis [86].
Another set of well-described risk factors for PE are diabetes, obesity or chronic hypertension [6,7,36,38]. In an examination of diabetes-associated markers in ePE and lPE cases, it was determined that lPE was associated with a significant increase in insulin resistance and reduced serum adiponectin concentrations [87]. These data indicated that adiponectin levels were reduced in the first trimester in cases that developed IPE compared to ePE. In the third trimester, adiponectin levels were only elevated in cases that developed IPE. Therefore, these data provide further evidence for an etiological difference between ePE and IPE [87,88].
The influence of obesity on the development of PE was clearly demonstrated in a retrospective analysis of over one million live births, where a striking association with the increasing BMI and the incidence of lPE was noted [36]. In this regard, super obesity having a BMI >50 was shown to be associated with a greater than fourfold increase in lPE [36]. No significant influence of obesity on the presence of ePE was detected [36].
A possible explanation for this increased susceptibility to secondary inflammatory stimuli may be a reduction in suitable scavenger molecules, such as anti-oxidant selenoproteins in PE, which play an important role in the removal of hydroperoxides and oxidized lipoproteins [89]. This hypothesis is supported by recent data indicating that PE is associated with decreased levels of α1-microglobulin (A1M), crucial for the removal of radicals and heme and furthermore, that treatment with A1M infusions can reduced PE symptoms in a large animal model [90,91]. Consequently, the use of A1M is being explored for the treatment of human cases with PE, which if successful would indeed be a novel development in the clinic [92]. It, however, remains to be seen if this therapy will be useful for both cases with ePE and lPE, or only for the latter.
In this context, a recent large-scale study concerning folic acid supplementation of over 10,000 pregnant women indicated that while this did lead to a reduction in PE, it was most pronounced for cases with mild or lPE [93].
Taken together, these data support the hypothesis that the development of ePE is primarily driven by an underlying placental lesion with negligible influence from environmental or secondary inflammatory stimuli, whereas in lPE the contribution by the placenta is not as pronounced, but the maternal immune system has become more sensitive to secondary inflammatory stimuli (Figure 1).
PP13 – only a biomarker or a crucial factor involved in placenta development?
Similar to the above findings, several studies conducted in the first trimester have shown that maternal blood concentrations of the immunoregulatory PP13 are changed in PE [17,94]. Some of these revealed that PP13 concentrations were lower in ePE compared with controls, while this change was not significant in lPE [95–97]. Of interest, PP13 is a uniquely placental expressed protein, predominantly produced by the STB during differentiation and syncytialization, from where it is released into the maternal circulation [98–100]. Thus, decreased maternal blood PP13 concentrations reflect decreased syncytiotrophoblastic PP13 production and impaired syncytialization [98–100]. Indeed, a study that examined villous trophoblastic cells laser-captured from first trimester CVS samples found the downregulation of PP13 mRNA expression in samples from women who later developed PE compared with normal pregnant women [101]. All these data point to impaired early placentation events in PE, especially in cases with early onset.
In this context, it is interesting that PP13 is member of the multifunctional galectin family that regulates innate and adaptive immune responses and confer immune tolerance [17,98]. Functional studies have shown that PP13 may also have important regulatory role on both arms of the immune system, as it induced the apoptosis of activated T cells [98] and the cytokine production of macrophages in vitro [102]. In addition, according to the in situ observations of the latter study, perivenous aggregates of PP13 formed in the decidua in the first trimester may facilitate trophoblast invasion and spiral artery conversion via attracting, activating and killing maternal immune cells. These findings are also important from an evolutionary aspect, since the gene encoding PP13 – along with related galectin genes in a cluster on chromosome 19 – has emerged in anthropoid primates, species with deep placentation and long gestation [98]. Further studies need to address the immunobiological questions on how PP13 regulates maternal-fetal immune interactions during early placentation, and how these processes are impaired in various PE subforms by various disease etiologies.
Multiple independent or linked etiologies – what are the implications for biomarker development?
A caveat of the above findings is that PE could be the result of multiple etiological factors. Such a facet was suggested by a recent murine-human translational proteomic study, which suggested three independent diverging pathways [103]. The first group included dysfunctions in angiogenesis, the second group included changes in MAPK signaling, while the last group showed evidence for metabolic or hormonal changes. Of interest is that GNA12, a guanine nucleotide binding protein detected in subgroup 2, was overexpressed in PE placentae that were coincident with chronic hypertension [103]. Although highly detailed, no evidence for activation of the maternal immune system was noted in this study.
In order to gain insight into possible differences between the two PE forms, Chaiworapongsa et al. examined the maternal blood leukocyte transcriptional signature in 25 cases with ePE, 47 with lPE in comparison with 61 uncomplicated pregnancies [104]. This study showed that 43 genes were differentially expressed between ePE and controls, versus 28 between lPE and controls. The expression of 20 genes involved in coagulation, immune regulation and inflammation was enhanced only in ePE, while only 7 genes were altered in lPE. The expression of 13 genes was determined to be altered in both PE forms in comparison with controls [104].
In a similar microarray examination of maternal lymphocytes, a different pattern was noted between PE cases and controls, mainly related to complement and protein translation [105]. When the PE cases were stratified into severe ePE and non-severe lPE, eight genes were found to be specifically upregulated in the former. It is of interest that in both studies, V-set and Ig domain-containing 4, related to the B7 family of co-stimulatory molecules, showed the clearest discrimination between ePE and lPE [105]. Apart from the above gene transcript findings, there is, however, additional evidence suggesting that the underlying etiological pathways may converge into a single mechanism initiating the maternal symptoms. These include recent observations that urinary excretion of complement factors is associated with an imbalance in angiogenic factors in cases with severe PE [106].
A tentative link between the complement system and neutrophil activation has been proposed via the action of C5a, which would further underscore a convergence of pathways [107].
These diverse findings raise the question of whether a biomarker-based screen would detect both PE forms equally well. Furthermore, it is unclear if such a screen would detect only a subset of PE cases initiated by a specific etiological factor, and be blind to other etiological triggers.
Early detection of PE – the current status – is there a need for new additional biomarkers?
Numerous studies confirmed the high performance of the sFlt-1/PlGF ratio in the second and third trimester of pregnancy [16,108–112]. The ratio can be used in asymptomatic women at high risk of developing PE, as well as in women with signs and symptoms of PE [109–111]. So far, sFlt-1/PlGF ratio has not been evaluated as a screening test for PE in widespread clinical practice. Furthermore, the timeframe of the use of the sFlt-1/PlGF ratio lies after the established time point of starting low-dose aspirin (before 16th week of gestation) to have a beneficial effect on the occurrence of early-onset PE [6,113,114].
There is good evidence that a combination of different bio- and ultrasound-markers pregnancy-associated plasma protein A, PlGF, mean maternal arterial pressure (MAP), with the pulsatility index (PI) of the uterine arteries has a detection rate of 93% for early-onset PE and 61% for cases before 37th week of gestation, at a false-positive rate (FPR) of 5% [115].
However, because the majority of the PE cases are considered as late-onset forms, there is an urgent need for additional biomarkers [37,38].
Development of new biomarkers for early detection of PE – the issue of early versus late events
A major gap in our understanding of PE is what the initiating lesions are [7,9]? Indeed, it may be that the key events triggering the cascade leading to PE occur so early in pregnancy and are so subtle that they are not readily detectable by current technologies. This renders the quest for biomarkers suitable for detection of at-risk pregnancies at the integrated 11- to 13-week screen difficult. This deficit becomes evident when examining the utility of current screening tools, such as those implicated in the angiogenic imbalance, where significant changes in sFlt-1 levels are only noted after 20 weeks of gestation [112]. It is also evident based on publications to date that changes in the innate immune system, such as neutrophil [19,66], or complement activation [70], are likely to be mostly late events in the etiological cascade, and may not be evident in the late first trimester of at-risk pregnancies.
Furthermore, the functional role of biomarkers included in current or suggested screens such as α-fetoprotein, human chorionic gonadotropin, pregnancy-associated plasma protein A, inhibin-A, activin-A, pentraxin 3 (PTX3) or P-selectin in PE etiology is unknown or questionable. It is perhaps for this reason that a list of over 70 unrelated biochemical analytes has been proposed for inclusion in future screening assays, which would not be feasible in clinical practice [115]. It is, therefore, a tenet of several researchers in the field that the development of effective screening markers or therapeutic approaches will only be possible once a more complete understanding of the biological cascade leading to the development of manifest PE is known [6,7].
Development of new biomarkers – which approach is best?
Due to this knowledge deficit concerning the key initiating steps driving the development of PE, it is unclear what the best strategy for the development of new markers should be [116,117]. It is, however, evident that by examining affected placentae but whatever sophisticated molecular biological means, that this will only lead to an increasingly intimate knowledge of the final state of the disorder, but not provide more than an inkling of initiating steps. The same caveat would appear to exist for maternal blood transcriptomic analyses, as most PE-specific changes have been seen so far as a relatively late event. As such, any potential biomarkers obtained by these means will largely only be useful for the detection of imminently threatening PE cases [6,7].
Since it is not possible to readily obtain placental material early in gestation, it has been suggested to establish a biobank of villi obtained by CVS for the detection of fetal aneuploidy [118,119]. As this is usually carried out at the end of the first trimester, this could be an ideal source of vital material. Samples obtained from pregnant women who develop PE could then be compared with those with normal outcome. Although to be lauded such an approach is hampered by the low incidence of PE, especially that of ePE, thereby necessitating a very large biobank of material which is not readily attainable. Furthermore, due to the high sensitivity of current screening programs for fetal aneuploidy, very few of the CVS samples will have a normal fetal genotype [120].
Since most biomarkers used in clinical screening are blood based, it has been suggested that maternal plasma obtained in the first trimester could be a good source of starting material [116]. This could be examined by quantitative proteomic technologies using isobaric labeling isobaric tags for relative and absolute quantification, whereby patient samples are labeled with one set of tags and the reference samples with another discrete set [116,121,122]. This permits highly precise quantitative comparison of specific peptide concentrations between cases and controls by mass spectrometry. By such means, an examination of first trimester maternal plasma samples obtained from pregnant women who subsequently developed PE revealed significant quantitative differences in 10 peptides, including fibronectin, angiotensinogen, galectin-3-binding protein, plasminogen and transferrin [121]. It is noteworthy that some of these potential biomarkers were corroborated by a parallel proteomic study using a different proteomic methodology, 2D difference gel electrophoresis [Than NG et al., Unpublished Data]. It is currently not clear how useful or specific these markers are for early PE detection. Since a similar analysis for the development of DS screening markers detected known screening analytes such as α-fetoprotein and human chorionic gonadotropin, it would appear that the isobaric tags for relative and absolute quantification approach may prove to be a useful tool [116,121,122].
Unfortunately, of the large number of proteomic studies carried out for PE biomarker discovery, only a few of these focused on maternal blood samples obtained prior to onset of symptoms, and of these only two examined first trimester samples [123–125]. There is thus a huge need for well-designed clinical studies geared toward biomarker discovery, such as the EU-funded Improved Pregnancy Outcomes by Early Detection (IMPROVED) study [126].
A major concern with the detection of new proteomic biomarkers is the validation of their usefulness [116]. For this purpose, it would be the best to use methods developed for systems biology analyses, such as selective reaction monitoring or its further bioinformatics development of SWATH MS [127,128].
Development of new biomarkers – can a translational approach be used?
Since access to human material indicative of the early initiating lesion is high or impossible to obtain, the question arises whether it is not possible to use an animal model system to gain insight into these key steps [129–133]. The main issue with commonly used rodent model systems, such as the mouse, is that the placenta is very different from that of humans, especially with regard to spiral artery modification [51,130]. On the other hand, even though evidence of such features may be present in guinea pigs, this model system is hampered by the lack of specific reagents, especially antibodies. Nevertheless, the murine hemochorial placenta may be useful to examine certain facets, especially as the placental tissue is in direct contact with maternal immune cells [134]. As such, a translational approach may be possible for the study of PE [129], especially when relying on a systems biology approach [133].
In this context, it has been shown that factors contributing to an angiogenic imbalance in PE such as sFlt-1 or endoglin can be used to induce PE-like symptoms in mice or rats [135–138]. Furthermore, activation of the complement cascade by either anti-phospholipid antibody treatment, induction of ischemia or genetic knock-out of the C1q gene has similarly been shown to induce PE-like conditions in mice or rats [107,139,140].
It has further been shown that the CBA/J x DBA/2 mouse model of recurrent miscarriages shares a number of PE-like features, including proteinuria, endothelial cell damage, increased sensitivity to angiotensin II and elevated plasma leptin levels [129]. Akin to human PE, this spontaneous mouse model involves an imbalance in angiogenic factors, including VEGF and sFlt-1. Its clinical usefulness is underscored by the observation that treatment with pravastatin restored the angiogenic imbalance, and ameliorated glomerular damage, thereby paving the way for the recent similar treatment of a PE patient with anti-phospholipid syndrome.
Animal models have also been developed to test the functional role of proteins identified in proteomic screens such as transthyretin, the serum level of which is reduced in PE [141]. In a humanized murine model for PE, application of exogenous transthyretin leads to a reversal of PE symptoms, including proteinuria, elevated BP, glomerular endotheliosis and production of anti-angiogenic factors [141]. These findings lead to the suggestion that transthyretin can serve as a new biomarker for PE [142].
In a recent publication, it has been shown that mice lacking the tryptophan catabolizing enzyme IDO develop symptoms consistent with those of PE [143]. IDO has previously been suggested to play a key role in maintaining fetomaternal tolerance by regulating maternal T-cell activity [24]. A decrease in its activity has also been implicated in development of PE [144].
A further interesting report indicates that inappropriate inflammation, induced by low-dose lipopolysaccharide in pregnant rats, inhibits spiral artery remodeling, thereby leading to IUGR and PE-like features [145], which are mirrored by altered placenta morphometrics [146].
Although these results may appear exciting or encouraging, the fundamental problem with such animal models is that it is unclear how they compare the early initiating lesions leading to PE, which is considered to be a disease mainly of humans and is very rarely observed in a few evolutionarily closely related anthropoid primates [147,148]. Consequently, their analysis may once again simply increase our understanding of late events that appear imminently prior to clinical symptoms. A further concern is that they may lead to a skewed appraisal of the events occurring in humans, as seems to be the case in a complex comparative murine-human translational proteomic analysis, in which no evidence of inflammation was obtained [103].
Early- versus late-onset PE – which disorder do we need to detect?
When considering the development of biomarkers for the early (first trimester) detection of PE, it is important to consider whether the emphasis will be placed on either ePE or lPE [1,6]. This deliberation should include an analysis of the difference in incidence between the two forms, the associated fetomaternal morbidity/mortality and the possibility of therapeutic intervention [1,6].
A recent examination of 607,120 singleton deliveries indicated that the incidence of ePE was 0.3%, while that for lPE was 2.7% [37,38]. ePE was associated with a higher rate of maternal mortality (42.1/100,000 deliveries), while that of lPE (11.2/100,000 deliveries), albeit this latter was still significantly higher than in normal healthy pregnancies (4.2/100,000 deliveries) [37,38]. Furthermore, maternal morbidity while higher in ePE than lPE cases was still higher in the latter than in uncomplicated pregnancies [37,38]. This confirms previous observations that cases with lPE need optimized care, and that failure to do so can have severe consequences, especially in rural settings [39].
It would, therefore, appear that under ideal settings, that newly developed tests would detect both PE forms with equal, or similar, efficacy. An analysis of current attempts to achieve this, however, indicates that such a goal may be difficult to achieve, in that pregnancies at-risk for ePE are detected with much greater efficacy than those that develop lPE [115,149]. In this context, recent reports suggest that 95% of cases with ePE can be detected with a FPR of 10%, while approximately only 50–70% of cases with lPE with the same FPR [115]. Since the ability to detect lPE decreases with increasing gestational age of onset, these data are once again indicative of multiple, shared and disproportionate initiating etiological lesions leading to the development of ePE and lPE.
For this reason, it has been suggested to split PE screening into two arms, an early phase incorporated into the 11- to 13-week integrated test, and second screen carried out at 30–33 weeks to detect cases with lPE [1]. The latter test would rely on current screens assessing an imbalance in angiogenic factors (PlGF/sFlt-1), which should detect all cases with lPE for a FPR of 5% [1].
Expert commentary
A major facet to consider before initiating the chase to development of new biomarkers for PE detection is what the uptake of current screens into widespread clinical practice has been [150]. Following the observation that an imbalance in angiogenic factors (PlGF/sFlt-1) precedes the development of PE [112], a number of very large-scale studies have been carried out to examine the usefulness of this approach. This included studies carried out under the auspices of the NIH, WHO as well as several carried out in conjunction with leading pharmaceutical companies [108–111]. These studies have clearly shown that a quantitative assessment of these factors has a very high predictive value for detecting the onset of PE post 34 weeks of gestation [110]. Indeed, it has also been shown that they can be useful to detect cases of PE that develop during triage [111].
Despite these promising results and generally low cost of the ELISA assays, it is astounding that no published report exists to date documenting clinical implementation.
This is in widespread contrast to the rapid uptake of newly developed non-invasive prenatal testing assays examining fetomaternal cfDNA by complex next-generation sequencing technologies, where a constantly high demand exists [151,152]. This is all the more astonishing granted the high costs of these tests, which are frequently not reimbursed by healthcare insurers [153].
It would thus appear that the detection of a DS fetus has a higher priority in our society than the detection of a grievous disorder associated with high rates of maternal mortality. Furthermore, it should be noted that PE is associated with an increased risk of severe cardiovascular and metabolic complications for both mother and child later in life, thereby placing a considerable burden on already stretched healthcare budgets [154]. Under these conditions, it seems invidious to suggest that there are ethical concerns with the implementation of new PE screens [155], rather it would appear unethical to ensure the widespread implementation of such assays, especially in developing countries where access to high-quality care is limited [10,39].
Five-year view
An a priori requirement for the development of new biomarkers for the early detection of PE is a solid understanding of the key initiating placental lesions [6,7]. This will require a concerted multicenter or even multinational effort harnessing a wealth of experience from a variety of scientific and clinical disciplines. The newly established NIH Human Placenta Project is a step in the right direction [156].
Another requirement is to ensure the efficacious translation of current laboratory developed assays, such as those for PlGF/sFlt-1, which have been shown to be reliable and robust into widespread clinical practice. This will help in assisting the similar transfer of the next generation of assays.
In order to achieve this, the perception of PE, its devastating consequences and the hope of new developments need to be firmly communicated to the general public. Such a lobbying force will also ensure adequate funds are available, a vital requirement in scientific field known to be under-resourced financially and in dire need to new effective therapies [92,157].
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
Key issues.
Current attempts at the early detection of preeclampsia (PE) via first trimester screening systems, preferentially detect cases with early-onset PE rather than late-onset PE.
This may reflect upon fundamental etiological differences between these two forms of PE.
The etiology ePE appears to involve a distinct early placental lesion that is the key driving force for the development of PE symptoms, with little or no contribution by secondary inflammatory incidents.
Although significant when compared with control pregnancies, the placental lesion in lPE serves in a priming capacity, rendering the maternal innate immune system highly susceptible to secondary inflammatory signals (e.g., obesity), which then triggers the development of clinical symptoms.
Deficiency in scavenger molecules, such as seleno-proteins or A1M may contribute to increased susceptibility to secondary inflammatory signals in lPE. These form the basis for novel therapies.
Animal models and systems biology approaches could be exploited to obtain clearer insight into etiological differences between ePE and lPE. These include studies on the role of complement activation, therapeutic use of A1M to counter PE symptoms, or data indicating that inflammation during early placentation hinders spiral artery modification.
These fundamental etiological differences between ePE and lPE may, however, hinder the reliable detection of lPE using first trimester screening, or render it impossible.
The development of reliable point-of-care tools for the imminent detection of lPE in tertiary rural settings would be a life-saver and should not be neglected.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- •• .Nicolaides KH. Turning the pyramid of prenatal care. Fetal Diagn Ther. 2011;29(3) doi: 10.1159/000324320. [DOI] [PubMed] [Google Scholar]; •• This report discusses the possibility for a ground-breaking change in prenatal screening and care, thereby froming the basis for current strategies.
- Nicolaides KH. A model for a new pyramid of prenatal care based on the 11 to 13 weeks’ assessment. Prenat Diagn. 2011;31(1):3–6. doi: 10.1002/pd.2685. [DOI] [PubMed] [Google Scholar]
- Lees C. First-trimester screening for pre-eclampsia and fetal growth restriction: a test seeking both a treatment and an optimal timing. Ultrasound Obstet Gynecol. 2010;35(6):647–9. doi: 10.1002/uog.7686. [DOI] [PubMed] [Google Scholar]
- Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36(1):56–9. doi: 10.1053/j.semperi.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Redman CW. Preeclampsia: a multi-stress disorder. Rev Med interne. 2011;32(Suppl 1):S41–4. doi: 10.1016/j.revmed.2011.03.331. [DOI] [PubMed] [Google Scholar]
- Chaiworapongsa T, Chaemsaithong P, Korzeniewski SJ, et al. Pre-eclampsia part 2: prediction, prevention and management. Nat Rev Nephrol. 2014;10(9):531–40. doi: 10.1038/nrneph.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10(8):466–80. doi: 10.1038/nrneph.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376(9741):631–44. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- •• .Redman CW, Sargent IL, Staff AC. IFPA Senior Award Lecture: making sense of pre-eclampsia - two placental causes of preeclampsia? Placenta. 2014;35(Suppl):S20–5. doi: 10.1016/j.placenta.2013.12.008. [DOI] [PubMed] [Google Scholar]; •• A report detailing current view on the underlying etiology of preeclampsia (PE).
- Sibai BM. What to expect from expectant management in severe preeclampsia at <34 weeks gestation: pregnancy outcomes in developed vs developing countries. Am J Obstet Gynecol. 2013;209(5):400–1. doi: 10.1016/j.ajog.2013.08.024. [DOI] [PubMed] [Google Scholar]
- Sibai BM. Imitators of severe pre-eclampsia. Semin Perinatol. 2009;33(3):196–205. doi: 10.1053/j.semperi.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol. 2012;206(6):470–5. doi: 10.1016/j.ajog.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Higgins JR, Brennecke SP. Pre-eclampsia – still a disease of theories? Curr Opin Obstet Gynecol. 1998;10(2):129–33. doi: 10.1097/00001703-199804000-00009. [DOI] [PubMed] [Google Scholar]
- Jeffcoate TN. Pre-eclampsia and eclampsia: the disease of theories. Proc R Soc Med. 1966;59(5):397–404. doi: 10.1177/003591576605900503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlembach D. Pre-eclampsia – still a disease of theories. Fukushima J Med Sci. 2003;49(2):69–115. [PubMed] [Google Scholar]
- Naljayan MV, Karumanchi SA. New developments in the pathogenesis of preeclampsia. Adv Chronic Kidney Dis. 2013;20(3):265–70. doi: 10.1053/j.ackd.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than NG, Balogh A, Romero R, et al. Placental Protein 13 (PP13) - a placental immunoregulatory galectin protecting pregnancy. Front Immunol. 2014;5:348. doi: 10.3389/fimmu.2014.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch AM, Salmon JE. Dysregulated complement activation as a common pathway of injury in preeclampsia and other pregnancy complications. Placenta. 2010;31(7):561–7. doi: 10.1016/j.placenta.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • .Gupta AK, Hasler P, Holzgreve W, et al. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol. 2005;66(11):1146–54. doi: 10.1016/j.humimm.2005.11.003. [DOI] [PubMed] [Google Scholar]; • First report of the involvement of neutrophil extracellular traps in a human pathology.
- Hahn S, Giaglis S, Hoesli I, Hasler P. Neutrophil NETs in reproduction: from infertility to preeclampsia and the possibility of fetal loss. Front Immunol. 2012;3:362. doi: 10.3389/fimmu.2012.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynge Nilsson L, Djurisic S, Hviid TV. Controlling the Immunological Crosstalk during Conception and Pregnancy: HLA-G in Reproduction. Front Immunol. 2014;5:198. doi: 10.3389/fimmu.2014.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratsep MT, Felker AM, Kay VR, et al. Uterine natural killer cells: supervisors of vasculature construction in early decidua basalis. Reproduction. 2015;149(2):R91–R102. doi: 10.1530/REP-14-0271. [DOI] [PubMed] [Google Scholar]
- Hsu P, Nanan RK. Innate and adaptive immune interactions at the fetal-maternal interface in healthy human pregnancy and pre-eclampsia. Front Immunol. 2014;5:125. doi: 10.3389/fimmu.2014.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlmayr P, Blaschitz A, Stocker R. The role of placental tryptophan catabolism. Front Immunol. 2014;5:230. doi: 10.3389/fimmu.2014.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk MR, Troeger C, Brinkhaus R, et al. Severely reduced presence of tissue macrophages in the basal plate of pre-eclamptic placentae. Placenta. 2001;22(4):309–16. doi: 10.1053/plac.2001.0624. [DOI] [PubMed] [Google Scholar]
- Zhou CC, Zhang Y, Irani RA, et al. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14(8):855–62. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner C, Tannetta DS, Simms CA, et al. Syncytiotrophoblast microvesicles released from pre-eclampsia placentae exhibit increased tissue factor activity. PLoS One. 2011;6(10):e26313. doi: 10.1371/journal.pone.0026313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• .von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22(2):143–8. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]; •• Pioneering exposé on the possible stratification of PE into two forms, with different underlying etiologies.
- •• .Brosens I, Pijnenborg R, Vercruysse L, Romero R. The "Great Obstetrical Syndromes" are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204(3):193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Important overview illustrating that defects in deep placenation are not limited to PE.
- Lokki AI, Klemetti MM, Heino S, et al. Association of the rs1424954 polymorphism of the ACVR2A gene with the risk of pre-eclampsia is not replicated in a Finnish study population. BMC Res Notes. 2011;4:545. doi: 10.1186/1756-0500-4-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Zhao L, Wang L, Zhu X. Expression of markers of endoplasmic reticulum stress-induced apoptosis in the placenta of women with early and late onset severe pre-eclampsia. Taiwan J Obstet Gynecol. 2015;54(1):19–23. doi: 10.1016/j.tjog.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Yung HW, Atkinson D, Campion-Smith T, et al. Differential activation of placental unfolded protein response pathways implies heterogeneity in causation of early- and late-onset pre-eclampsia. J Pathol. 2014;234(2):262–76. doi: 10.1002/path.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Fernandez I, Prieto B, Rodriguez V, et al. Role of vitamin D and sFlt-1/PlGF ratio in the development of early- and late-onset preeclampsia. Clin Chem Lab Med. 2014 doi: 10.1515/cclm-2014-1039. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Halmos A, Rigo J, Jr, Szijarto J, et al. Circulating ficolin-2 and ficolin-3 in normal pregnancy and pre-eclampsia. Clin Exp Immunol. 2012;169(1):49–56. doi: 10.1111/j.1365-2249.2012.04590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorifuji T, Naruse H, Kashima S, et al. Residential proximity to major roads and obstetrical complications. Sci Total Environ. 2015;508:188–92. doi: 10.1016/j.scitotenv.2014.11.077. [DOI] [PubMed] [Google Scholar]
- •• .Mbah AK, Kornosky JL, Kristensen S, et al. Super-obesity and risk for early and late pre-eclampsia. BJOG. 2010;117(8):997–1004. doi: 10.1111/j.1471-0528.2010.02593.x. [DOI] [PubMed] [Google Scholar]; •• Highly interesting report illustrating the influence of obesity on the incidence of late PE (lPE), but not early PE (ePE).
- Lisonkova S, Sabr Y, Mayer C, et al. Maternal morbidity associated with early-onset and late-onset preeclampsia. Obstet Gynecol. 2014;124(4):771–81. doi: 10.1097/AOG.0000000000000472. [DOI] [PubMed] [Google Scholar]
- •• .Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209(6):544 e541–12. doi: 10.1016/j.ajog.2013.08.019. [DOI] [PubMed] [Google Scholar]; •• Important epidemiological analysis of factors contributing to development of ePE and lPE.
- • .Kenneth L, Hall DR, Gebhardt S, Grove D. Late onset preeclampsia is not an innocuous condition. Hypertens Pregnancy. 2010;29(3):262–70. doi: 10.3109/10641950902777697. [DOI] [PubMed] [Google Scholar]; • Important report indicating that lPE can be dangerous, especially in tertiary settings.
- Chesley LC, Annitto JE, Cosgrove RA. The familial factor in toxemia of pregnancy. Obstet Gynecol. 1968;32(3):303–11. [PubMed] [Google Scholar]
- Williams PJ, Broughton Pipkin F. The genetics of pre-eclampsia and other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):405–17. doi: 10.1016/j.bpobgyn.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jebbink J, Wolters A, Fernando F, et al. Molecular genetics of preeclampsia and HELLP syndrome - a review. Biochim Biophys Acta. 2012;1822(12):1960–9. doi: 10.1016/j.bbadis.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Veerbeek JH, Hermes W, Breimer AY, et al. Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension. 2015;65(3):600–6. doi: 10.1161/HYPERTENSIONAHA.114.04850. [DOI] [PubMed] [Google Scholar]
- Liu X, Olsen J, Agerbo E, et al. Maternal Preeclampsia and Childhood Asthma in the Offspring. Pediat Allergy Immunol. 2015 doi: 10.1111/pai.12344. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Moore GS, Allshouse AA, Post AL, et al. Early initiation of low-dose aspirin for reduction in preeclampsia risk in high-risk women: a secondary analysis of the MFMU High-Risk Aspirin Study. J Perinatol. 2014 doi: 10.1038/jp.2014.214. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park F, Russo K, Williams P, et al. Prediction and prevention of early onset pre-eclampsia: the impact of aspirin after first trimester screening. Ultrasound Obstet Gynecol. 2015 doi: 10.1002/uog.14819. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Redman CW, Tannetta DS, Dragovic RA, et al. Review: does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta. 2012;33(Suppl):S48–54. doi: 10.1016/j.placenta.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Invest. 2004;11(6):342–52. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Watson AL, Hempstock J, et al. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol. 2000;157(6):2111–22. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cindrova-Davies T, van Patot MT, Gardner L, et al. Energy status and HIF signalling in chorionic villi show no evidence of hypoxic stress during human early placental development. Mol Hum Reprod. 2015;21(3):296–308. doi: 10.1093/molehr/gau105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnenborg R, Robertson WB, Brosens I, Dixon G. Review article: trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta. 1981;2(1):71–91. doi: 10.1016/s0143-4004(81)80042-2. [DOI] [PubMed] [Google Scholar]
- Genbacev O, Joslin R, Damsky CH, et al. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest. 1996;97(2):540–50. doi: 10.1172/JCI118447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogge G, Chaiworapongsa T, Romero R, et al. Placental lesions associated with maternal underperfusion are more frequent in early-onset than in late-onset preeclampsia. J Perinat Med. 2011;39(6):641–52. doi: 10.1515/JPM.2011.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto E, Romero R, Kusanovic JP, et al. Late-onset preeclampsia is associated with an imbalance of angiogenic and anti-angiogenic factors in patients with and without placental lesions consistent with maternal underperfusion. J Matern Fetal Neonatal Med. 2012;25(5):498–507. doi: 10.3109/14767058.2011.591461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapaire O, Holzgreve W, Oosterwijk JC, et al. Georg Schmorl on trophoblasts in the maternal circulation. Placenta. 2007;28(1):1–5. doi: 10.1016/j.placenta.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Buurma AJ, Penning ME, Prins F, et al. Preeclampsia is associated with the presence of transcriptionally active placental fragments in the maternal lung. Hypertension. 2013;62(3):608–13. doi: 10.1161/HYPERTENSIONAHA.113.01505. [DOI] [PubMed] [Google Scholar]
- Holzgreve W, Ghezzi F, Di Naro E, et al. Disturbed feto-maternal cell traffic in preeclampsia. Obstet Gynecol. 1998;91(5 Pt 1):669–72. doi: 10.1016/s0029-7844(98)00068-4. [DOI] [PubMed] [Google Scholar]
- Holzgreve W, Li JJ, Steinborn A, et al. Elevation in erythroblast count in maternal blood before the onset of preeclampsia. Am J Obstet Gynecol. 2001;184(2):165–8. doi: 10.1067/mob.2001.108861. [DOI] [PubMed] [Google Scholar]
- Hahn S, Huppertz B, Holzgreve W. Fetal cells and cell free fetal nucleic acids in maternal blood: new tools to study abnormal placentation? Placenta. 2005;26(7):515–26. doi: 10.1016/j.placenta.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Zhong XY, Gebhardt S, Hillermann R, et al. Parallel assessment of circulatory fetal DNA and corticotropin-releasing hormone mRNA in early- and late-onset preeclampsia. Clin Chem. 2005;51(9):1730–3. doi: 10.1373/clinchem.2005.053959. [DOI] [PubMed] [Google Scholar]
- Hahn S, Giaglis S, Buser A, et al. Cell-free nucleic acids in (maternal) blood: any relevance to (reproductive) immunologists? J Reprod Immunol. 2014;104-105:26–31. doi: 10.1016/j.jri.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Zhong XY, Holzgreve W, Hahn S. The levels of circulatory cell free fetal DNA in maternal plasma are elevated prior to the onset of preeclampsia. Hypertens Pregnancy. 2002;21(1):77–83. doi: 10.1081/PRG-120002911. [DOI] [PubMed] [Google Scholar]
- Leung TN, Zhang J, Lau TK, et al. Increased maternal plasma fetal DNA concentrations in women who eventually develop preeclampsia. Clin Chem. 2001;47(1):137–9. [PubMed] [Google Scholar]
- Szabo S, Mody M, Romero R, et al. Activation of Villous Trophoblastic p38 and ERK1/2 Signaling Pathways in Preterm Preeclampsia and HELLP Syndrome. Pathol Oncol Res. 2015 doi: 10.1007/s12253-014-9872-9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks G, Sargent I, Redman C. An innate view of human pregnancy. Immunol Today. 1999;20(3):114–18. doi: 10.1016/s0167-5699(98)01393-0. [DOI] [PubMed] [Google Scholar]
- Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179(1):80–6. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta. 2000;21(7):597–602. doi: 10.1053/plac.2000.0560. [DOI] [PubMed] [Google Scholar]
- Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107(36):15880–5. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Zhou R, Gao L, et al. Elevation of urinary adipsin in preeclampsia: correlation with urine protein concentration and the potential use for a rapid diagnostic test. Hypertension. 2014;64(4):846–51. doi: 10.1161/HYPERTENSIONAHA.113.02688. [DOI] [PubMed] [Google Scholar]
- Buurma A, Cohen D, Veraar K, et al. Preeclampsia is characterized by placental complement dysregulation. Hypertension. 2012;60(5):1332–7. doi: 10.1161/HYPERTENSIONAHA.112.194324. [DOI] [PubMed] [Google Scholar]
- Lokki AI, Heikkinen-Eloranta J, Jarva H, et al. Complement activation and regulation in preeclamptic placenta. Front Immunol. 2014;5:312. doi: 10.3389/fimmu.2014.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim MS, Meddeb S, Kaabia O, et al. C3F gene mutation is involved in the susceptibility to pre-eclampsia. Arch Gynecol Obstet. 2014 doi: 10.1007/s00404-014-3515-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Burwick RM, Feinberg BB. Eculizumab for the treatment of preeclampsia/HELLP syndrome. Placenta. 2013;34(2):201–3. doi: 10.1016/j.placenta.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Lynch AM, Murphy JR, Byers T, et al. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am J Obstet Gynecol. 2008;198(4):385 e381–9. doi: 10.1016/j.ajog.2007.10.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inforzato A, Doni A, Barajon I, et al. PTX3 as a paradigm for the interaction of pentraxins with the complement system. Semin Immunol. 2013;25(1):79–85. doi: 10.1016/j.smim.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Cetin I, Cozzi V, Pasqualini F, et al. Elevated maternal levels of the long pentraxin 3 (PTX3) in preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2006;194(5):1347–53. doi: 10.1016/j.ajog.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Rovere-Querini P, Antonacci S, Dell’Antonio G, et al. Plasma and tissue expression of the long pentraxin 3 during normal pregnancy and preeclampsia. Obstet Gynecol. 2006;108(1):148–55. doi: 10.1097/01.AOG.0000224607.46622.bc. [DOI] [PubMed] [Google Scholar]
- Akolekar R, Casagrandi D, Livanos P, et al. Maternal plasma pentraxin 3 at 11 to 13 weeks of gestation in hypertensive disorders of pregnancy. Prenat Diagn. 2009;29(10):934–8. doi: 10.1002/pd.2311. [DOI] [PubMed] [Google Scholar]
- Cetin I, Cozzi V, Papageorghiou AT, et al. First trimester PTX3 levels in women who subsequently develop preeclampsia and fetal growth restriction. Acta Obstet Gynecol Scand. 2009;88(7):846–9. doi: 10.1080/00016340902971441. [DOI] [PubMed] [Google Scholar]
- Wacker J, Schulz M, Fruhauf J, et al. Seasonal change in the incidence of preeclampsia in Zimbabwe. Acta Obstet Gynecol Scand. 1998;77(7):712–16. [PubMed] [Google Scholar]
- Immink A, Scherjon S, Wolterbeek R, Steyn DW. Seasonal influence on the admittance of pre-eclampsia patients in Tygerberg Hospital. Acta Obstet Gynecol Scand. 2008;87(1):36–42. doi: 10.1080/00016340701743066. [DOI] [PubMed] [Google Scholar]
- Khojasteh F, Safarzadeh A, Burayri T. Survey of Correlation between Preeclampsia and Season & Some of its Risk Factor In Pregnant Women. J Women’s Health Care. 2012;1(3):114. [Google Scholar]
- Proietti E, Roosli M, Frey U, Latzin P. Air pollution during pregnancy and neonatal outcome: a review. J Aerosol Med Pulm Drug Deliv. 2013;26(1):9–23. doi: 10.1089/jamp.2011.0932. [DOI] [PubMed] [Google Scholar]
- •• .Dadvand P, Figueras F, Basagana X, et al. Ambient air pollution and preeclampsia: a spatiotemporal analysis. Environ Health Perspect. 2013;121(11-12):1365–71. doi: 10.1289/ehp.1206430. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Novel insight into the contribution of air pollution on development of ePE and lPE.
- Wu M, Ries JJ, Proietti E, et al. Development of late onset preeclampsia in association with traffic-related air pollution. Zeitsch Geburt Neonatol. 2013;217 doi: 10.1159/000381802. PoO8_5. [DOI] [PubMed] [Google Scholar]
- Lisonkova S, Joseph KS. Left Truncation Bias as a Potential Explanation for the Protective Effect of Smoking on Preeclampsia. Epidemiology. 2015 doi: 10.1097/EDE.0000000000000268. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Anna R, Baviera G, Corrado F, et al. Adiponectin and insulin resistance in early- and late-onset pre-eclampsia. BJOG. 2006;113(11):1264–9. doi: 10.1111/j.1471-0528.2006.01078.x. [DOI] [PubMed] [Google Scholar]
- Masuyama H, Segawa T, Sumida Y, et al. Different profiles of circulating angiogenic factors and adipocytokines between early- and late-onset pre-eclampsia. BJOG. 2010;117(3):314–20. doi: 10.1111/j.1471-0528.2009.02453.x. [DOI] [PubMed] [Google Scholar]
- Mistry HD, Wilson V, Ramsay MM, et al. Reduced selenium concentrations and glutathione peroxidase activity in preeclamptic pregnancies. Hypertension. 2008;52(5):881–8. doi: 10.1161/HYPERTENSIONAHA.108.116103. [DOI] [PubMed] [Google Scholar]
- Akerstrom B, Gram M. A1M, an extravascular tissue cleaning and housekeeping protein. Free Radic Biol Med. 2014;74:274–82. doi: 10.1016/j.freeradbiomed.2014.06.025. [DOI] [PubMed] [Google Scholar]
- •• .Wester-Rosenlof L, Casslen V, Axelsson J, et al. A1M/alpha1-microglobulin protects from heme-induced placental and renal damage in a pregnant sheep model of preeclampsia. PLoS One. 2014;9(1):e86353. doi: 10.1371/journal.pone.0086353. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Interesting report sugegssting that scavenger supplementation could be used as a therapy for PE.
- Hahn S. Preeeclampsia - will orphan drug status facilitate innovative biological therapies? Front Surg. 2015;2:7. doi: 10.3389/fsurg.2015.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao N, Qiu J, et al. Folic acid supplementation and dietary folate intake, and risk of preeclampsia. Eur J Clin Nutr. 2015 doi: 10.1038/ejcn.2014.295. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz B, Meiri H, Gizurarson S, et al. Placental protein 13 (PP13): a new biological target shifting individualized risk assessment to personalized drug design combating pre-eclampsia. Hum Reprod Update. 2013;19(4):391–405. doi: 10.1093/humupd/dmt003. [DOI] [PubMed] [Google Scholar]
- Romero R, Kusanovic JP, Than NG, et al. First-trimester maternal serum PP13 in the risk assessment for preeclampsia. Am J Obstet Gynecol. 2008;199(2):122 e121–11. doi: 10.1016/j.ajog.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil A, Cowans NJ, Spencer K, et al. First trimester maternal serum placental protein 13 for the prediction of pre-eclampsia in women with a priori high risk. Prenat Diagn. 2009;29(8):781–9. doi: 10.1002/pd.2287. [DOI] [PubMed] [Google Scholar]
- Akolekar R, Syngelaki A, Beta J, et al. Maternal serum placental protein 13 at 11-13 weeks of gestation in preeclampsia. Prenat Diagn. 2009;29(12):1103–8. doi: 10.1002/pd.2375. [DOI] [PubMed] [Google Scholar]
- Than NG, Romero R, Goodman M, et al. A primate subfamily of galectins expressed at the maternal-fetal interface that promote immune cell death. Proc Natl Acad Sci USA. 2009;106(24):9731–6. doi: 10.1073/pnas.0903568106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than NG, Romero R, Meiri H, et al. PP13, maternal ABO blood groups and the risk assessment of pregnancy complications. PLoS One. 2011;6(7):e21564. doi: 10.1371/journal.pone.0021564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than NG, Romero R, Xu Y, et al. Evolutionary origins of the placental expression of chromosome 19 cluster galectins and their complex dysregulation in preeclampsia. Placenta. 2014;35(11):855–65. doi: 10.1016/j.placenta.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekizawa A, Purwosunu Y, Yoshimura S, et al. PP13 mRNA expression in trophoblasts from preeclamptic placentas. Reprod Sci. 2009;16(4):408–13. doi: 10.1177/1933719108328615. [DOI] [PubMed] [Google Scholar]
- •• .Kliman HJ, Sammar M, Grimpel YI, et al. Placental protein 13 and decidual zones of necrosis: an immunologic diversion that may be linked to preeclampsia. Reprod Sci. 2012;19(1):16–30. doi: 10.1177/1933719111424445. [DOI] [PubMed] [Google Scholar]; •• Novel evidence that placental protein 13 acts to establish a decoy inflammation, diverting the maternal immune system away from the spiral arteries.
- Cox B, Sharma P, Evangelou AI, et al. Translational analysis of mouse and human placental protein and mRNA reveals distinct molecular pathologies in human preeclampsia. Mol Cell Prot. 2011;10(12) doi: 10.1074/mcp.M111.012526. M111 012526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiworapongsa T, Romero R, Whitten A, et al. Differences and similarities in the transcriptional profile of peripheral whole blood in early and late-onset preeclampsia: insights into the molecular basis of the phenotype of preeclampsia. J Perinat Med. 2013;41(5):485–504. doi: 10.1515/jpm-2013-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Textoris J, Ivorra D, Ben Amara A, et al. Evaluation of current and new biomarkers in severe preeclampsia: a microarray approach reveals the VSIG4 gene as a potential blood biomarker. PLoS One. 2013;8(12):e82638. doi: 10.1371/journal.pone.0082638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guseh SH, Feinberg BB, Dawood HY, et al. Urinary Excretion of C5b-9 is Associated With the Anti-Angiogenic State in Severe Preeclampsia. Am J Reprod Immunol. 2014 doi: 10.1111/aji.12349. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Redecha P, Tilley R, Tencati M, et al. Tissue factor: a link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood. 2007;110(7):2423–31. doi: 10.1182/blood-2007-01-070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CW, Park JS, Shim SS, et al. An elevated maternal plasma, but not amniotic fluid, soluble fms-like tyrosine kinase-1 (sFlt-1) at the time of mid-trimester genetic amniocentesis is a risk factor for preeclampsia. Am J Obstet Gynecol. 2005;193(3 Pt 2):984–9. doi: 10.1016/j.ajog.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Verlohren S, Galindo A, Schlembach D, et al. An automated method for the determination of the sFlt-1/PlGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 2010;202(2):161 e161–11. doi: 10.1016/j.ajog.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Verlohren S, Herraiz I, Lapaire O, et al. New gestational phase-specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63(2):346–52. doi: 10.1161/HYPERTENSIONAHA.113.01787. [DOI] [PubMed] [Google Scholar]
- Kaufmann I, Rusterholz C, Hosli I, et al. Can detection of late-onset PE at triage by sFlt-1 or PlGF be improved by the use of additional biomarkers? Prenat Diagn. 2012;32(13):1288–94. doi: 10.1002/pd.3995. [DOI] [PubMed] [Google Scholar]
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- Sibai BM. Therapy: low-dose aspirin to reduce the risk of pre-eclampsia? Nat Rev Endocrinol. 2015;11(1):6–8. doi: 10.1038/nrendo.2014.199. [DOI] [PubMed] [Google Scholar]
- LeFevre ML. Low-Dose Aspirin Use for the Prevention of Morbidity and Mortality From Preeclampsia: U.S. Preventive Services Task Force Recommendation Statement. Ann Internal Med. 2014;161(11):819–26. doi: 10.7326/M14-1884. [DOI] [PubMed] [Google Scholar]
- Poon LC, Nicolaides KH. First-trimester maternal factors and biomarker screening for preeclampsia. Prenat Diagn. 2014;34(7):618–27. doi: 10.1002/pd.4397. [DOI] [PubMed] [Google Scholar]
- Choolani M, Narasimhan K, Kolla V, Hahn S. Proteomic technologies for prenatal diagnostics: advances and challenges ahead. Expert Rev Proteom. 2009;6(1):87–101. doi: 10.1586/14789450.6.1.87. [DOI] [PubMed] [Google Scholar]
- Lapaire O, Grill S, Lalevee S, et al. Microarray screening for novel preeclampsia biomarker candidates. Fetal Diagn Ther. 2012;31(3):147–53. doi: 10.1159/000337325. [DOI] [PubMed] [Google Scholar]
- Founds SA, Conley YP, Lyons-Weiler JF, et al. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta. 2009;30(1):15–24. doi: 10.1016/j.placenta.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Founds SA, Terhorst LA, Conrad KP, et al. Gene expression in first trimester preeclampsia placenta. Biol Res Nurs. 2011;13(2):134–9. doi: 10.1177/1099800410385448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D, Wright A, Nicolaides KH. A unified approach to risk assessment for fetal aneuploidies. Ultrasound Obstet Gynecol. 2015;45(1):48–54. doi: 10.1002/uog.14694. [DOI] [PubMed] [Google Scholar]
- Kolla V, Jeno P, Moes S, et al. Quantitative proteomic (iTRAQ) analysis of 1st trimester maternal plasma samples in pregnancies at risk for preeclampsia. J Biomed Biotechnol. 2012;2012:305964. doi: 10.1155/2012/305964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla V, Jeno P, Moes S, et al. Quantitative proteomics analysis of maternal plasma in Down syndrome pregnancies using isobaric tagging reagent (iTRAQ) J Biomed Biotechnol. 2010;2010:952047. doi: 10.1155/2010/952047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J, Buffin-Meyer B, Mullen W, et al. Clinical proteomics in obstetrics and neonatology. Expert Rev Proteom. 2014;11(1):75–89. doi: 10.1586/14789450.2014.872564. [DOI] [PubMed] [Google Scholar]
- Baig S, Kothandaraman N, Manikandan J, et al. Proteomic analysis of human placental syncytiotrophoblast microvesicles in preeclampsia. Clin Proteom. 2014;11(1):40. doi: 10.1186/1559-0275-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JE, Tuytten R, Thomas G, et al. Integrated proteomics pipeline yields novel biomarkers for predicting preeclampsia. Hypertension. 2013;61(6):1281–8. doi: 10.1161/HYPERTENSIONAHA.113.01168. [DOI] [PubMed] [Google Scholar]
- Navaratnam K, Alfirevic Z, Baker PN, et al. A multi-centre phase IIa clinical study of predictive testing for preeclampsia: improved pregnancy outcomes via early detection (IMPROvED) BMC Pregnancy Childbirth. 2013;13:226. doi: 10.1186/1471-2393-13-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BC, Gillet LC, Rosenberger G, et al. Quantifying protein interaction dynamics by SWATH mass spectrometry: application to the 14-3-3 system. Nat Methods. 2013;10(12):1246–53. doi: 10.1038/nmeth.2703. [DOI] [PubMed] [Google Scholar]
- Selevsek N, Chang CY, Gillet LC, et al. Reproducible and consistent quantification of the Saccharomyces cerevisiae proteome by SWATH-MS. Mol Cell Proteom. 2015 doi: 10.1074/mcp.M113.035550. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Singh J, Khan Y, et al. A new mouse model to explore therapies for preeclampsia. PLoS One. 2010;5(10):e13663. doi: 10.1371/journal.pone.0013663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman WE, Carter AM, De Mestre AM, et al. IFPA Meeting 2012 Workshop Report I: comparative placentation and animal models, advanced techniques in placental histopathology, human pluripotent stem cells as a model for trophoblast differentiation. Placenta. 2013;34(Suppl):S3–5. doi: 10.1016/j.placenta.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Chaouat G, Clark DA. Are animal models useful or confusing in understanding the human feto-maternal relationship? A debate. J Reprod Immunol. 2014 doi: 10.1016/j.jri.2014.10.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Clark DA. The use and misuse of animal analog models of human pregnancy disorders. J Reprod Immunol. 2014;103:1–8. doi: 10.1016/j.jri.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Cox B, Kotlyar M, Evangelou AI, et al. Comparative systems biology of human and mouse as a tool to guide the modeling of human placental pathology. Mol Syst Biol. 2009;5:279. doi: 10.1038/msb.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai A, Cross JC. Development of the hemochorial maternal vascular spaces in the placenta through endothelial and vasculogenic mimicry. Dev Biol. 2014;387(2):131–41. doi: 10.1016/j.ydbio.2014.01.015. [DOI] [PubMed] [Google Scholar]
- Karumanchi SA, Stillman IE. In vivo rat model of preeclampsia. Methods Mol Med. 2006;122:393–9. doi: 10.1385/1-59259-989-3:393. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Ying Ma J, et al. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension. 2007;50(4):686–92. doi: 10.1161/HYPERTENSIONAHA.107.092098. [DOI] [PubMed] [Google Scholar]
- Venditti CC, Casselman R, Young I, et al. Carbon monoxide prevents hypertension and proteinuria in an adenovirus sFlt-1 preeclampsia-like mouse model. PLoS One. 2014;9(9):e106502. doi: 10.1371/journal.pone.0106502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalai G, Xu Y, Romero R, et al. In vivo experiments reveal the good, the bad and the ugly faces of sFlt-1 in pregnancy. PLoS One. 2014;9(11):e110867. doi: 10.1371/journal.pone.0110867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intapad S, Warrington JP, Spradley FT, et al. Reduced uterine perfusion pressure induces hypertension in the pregnant mouse. Am J Physiol Regul Integr Comp Physiol. 2014;307(11):R1353–7. doi: 10.1152/ajpregu.00268.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Ahmed A, Girardi G. Role of complement component C1q in the onset of preeclampsia in mice. Hypertension. 2011;58(4):716–24. doi: 10.1161/HYPERTENSIONAHA.111.175919. [DOI] [PubMed] [Google Scholar]
- Kalkunte SS, Neubeck S, Norris WE, et al. Transthyretin is dysregulated in preeclampsia, and its native form prevents the onset of disease in a preclinical mouse model. Am J Pathol. 2013;183(5):1425–36. doi: 10.1016/j.ajpath.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Chen Y, Liu C, et al. Transthyretin as a novel candidate biomarker for preeclampsia. Exp Ther Med. 2014;7(5):1332–6. doi: 10.3892/etm.2014.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo Y, Boyd CA, Sargent IL, Redman CW. Decreased tryptophan catabolism by placental indoleamine 2,3-dioxygenase in preeclampsia. Am J Obstet Gynecol. 2003;188(3):719–26. doi: 10.1067/mob.2003.156. [DOI] [PubMed] [Google Scholar]
- Liu X, Liu Y, Ding M, Wang X. Reduced expression of indoleamine 2,3-dioxygenase participates in pathogenesis of preeclampsia via regulatory T cells. Mol Med Rep. 2011;4(1):53–8. doi: 10.3892/mmr.2010.395. [DOI] [PubMed] [Google Scholar]
- •• .Cotechini T, Komisarenko M, Sperou A, et al. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J Exp Med. 2014;211(1):165–79. doi: 10.1084/jem.20130295. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Novel report suggesting that aberrant maternal inflmmation may be an early event in PE development.
- Cotechini T, Hopman WJ, Graham CH. Inflammation-induced fetal growth restriction in rats is associated with altered placental morphometrics. Placenta. 2014;35(8):575–81. doi: 10.1016/j.placenta.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Carter AM. Comparative studies of placentation and immunology in non-human primates suggest a scenario for the evolution of deep trophoblast invasion and an explanation for human pregnancy disorders. Reproduction. 2011;141(4):391–6. doi: 10.1530/REP-10-0530. [DOI] [PubMed] [Google Scholar]
- Carter AM, Pijnenborg R. Evolution of invasive placentation with special reference to non-human primates. Best Pract Res Clin Obstet Gynaecol. 2011;25(3):249–57. doi: 10.1016/j.bpobgyn.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Akolekar R, Syngelaki A, Sarquis R, et al. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11-13 weeks. Prenat Diagn. 2011;31(1):66–74. doi: 10.1002/pd.2660. [DOI] [PubMed] [Google Scholar]
- Hyde C, Thornton S. Does screening for pre-eclampsia make sense? BJOG. 2013;120(10):1168–70. doi: 10.1111/1471-0528.12309. [DOI] [PubMed] [Google Scholar]
- Bianchi DW, Wilkins-Haug L. Integration of noninvasive DNA testing for aneuploidy into prenatal care: what has happened since the rubber met the road? Clin Chem. 2014;60(1):78–87. doi: 10.1373/clinchem.2013.202663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YM. Noninvasive prenatal diagnosis: from dream to reality. Clin Chem. 2015;61(1):32–7. doi: 10.1373/clinchem.2014.223024. [DOI] [PubMed] [Google Scholar]
- Hahn S, Hosli I, Lapaire O. Non-invasive prenatal diagnostics using next generation sequencing: technical, legal and social challenges. Expert Opin Med Diagn. 2012;6(6):517–28. doi: 10.1517/17530059.2012.703650. [DOI] [PubMed] [Google Scholar]
- Seely EW, Rich-Edwards J, Lui J, et al. Risk of future cardiovascular disease in women with prior preeclampsia: a focus group study. BMC Pregnancy childbirth. 2013;13:240. doi: 10.1186/1471-2393-13-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JM, Hedley PL, Gjerris M, Christiansen M. Including ethical considerations in models for first-trimester screening for pre-eclampsia. Reprod Biomed Online. 2014;28(5):638–43. doi: 10.1016/j.rbmo.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Guttmacher AE, Maddox YT, Spong CY. The Human Placenta Project: placental structure, development, and function in real time. Placenta. 2014;35(5):303–4. doi: 10.1016/j.placenta.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk NM, Atun R. Systematic analysis of research underfunding in maternal and perinatal health. BJOG. 2009;116(3):347–56. doi: 10.1111/j.1471-0528.2008.02027.x. [DOI] [PubMed] [Google Scholar]