Abstract
Introduction: Inhaled corticosteroids (ICS) (in fixed combinations with long-acting β2-agonists [LABAs]) are frequently prescribed for patients with chronic obstructive pulmonary disease (COPD), outside their labeled indications and recommended treatment strategies and guidelines, despite having the potential to cause significant side effects.
Areas covered: Although the existence of asthma in patients with asthma–COPD overlap syndrome (ACOS) clearly supports the use of anti-inflammatory treatment (typically an ICS/LABA combination, as ICS monotherapy is usually not indicated for COPD), the current level of ICS/LABA use is not consistent with the prevalence of ACOS in the COPD population. Data have recently become available showing the comparative efficacy of fixed bronchodilator combinations (long-acting muscarinic antagonist [LAMA]/LABA with ICS/LABA combinations). Additionally, new information has emerged on ICS withdrawal without increased risk of exacerbations, under cover of effective bronchodilation.
Expert opinion: For patients with COPD who do not have ACOS, a LAMA/LABA combination may be an appropriate starting therapy, apart from those with mild disease who can be managed with a single long-acting bronchodilator. Patients who remain symptomatic or present with exacerbations despite effectively delivered LAMA/LABA treatment may require additional drug therapy, such as ICS or phosphodiesterase-4 inhibitors. When prescribing an ICS/LABA, the risk:benefit ratio should be considered in individual patients.
Keywords: chronic obstructive pulmonary disease, inhaled corticosteroid, long-acting muscarinic antagonist, long-acting β2-agonist
1. Introduction
Because chronic obstructive pulmonary disease (COPD) is viewed as an inflammatory disease, inhaled corticosteroids (ICS) have come to be a widely used treatment. Before the development of long-acting inhaled bronchodilators, ICS were prescribed to patients with COPD. Following the Towards a Revolution in COPD Health (TORCH) study, in which ICS monotherapy demonstrated an unfavorable risk:benefit ratio [1,2], ICS are now generally administered in fixed combinations with long-acting β2-agonist (LABA) bronchodilators, and are not recommended for use as monotherapy in COPD. Although indicated for patients with repeated exacerbations and/or severe airflow limitation, and in patients with asthma–COPD overlap syndrome (ACOS), ICS-based treatments are often used unnecessarily and contrary to recommendations, as well as their labeled indications [3-6]. Furthermore, the benefits of these agents need to be weighed against the risk of side effects, particularly pneumonia and diabetes [1,7-9].
In recent years, there has been increasing recognition of the benefits of long-acting bronchodilators in a number of important outcomes – lung function, symptoms, health status and exacerbations. This has led to the introduction of new agents and fixed combinations of bronchodilators that provide comparable efficacy to that of ICS/LABA, without the risk of ICS-related side effects. ICS-related side effects reported in patients with COPD include pneumonia [7,8,10,11], increased bone fracture risk [12,13], skin bruising and delayed healing [14], tuberculosis (in endemic areas) [15,16], diabetes [9], cataracts [17,18], dysphonia and oropharyngeal candidiasis [1]. The risks of ICS have been fully reviewed elsewhere by Price et al., 2013 [19] and Ernst et al., 2014 [20]. Patients with COPD are likely to be more vulnerable to side effects than those with asthma as they are older, more likely to have received oral steroids (lifetime cumulative dose) and the doses of ICS used in COPD are higher, especially in Europe [19,21,22].
This review describes how ICS are currently overused and how treatment with these agents is evolving, with newer data illustrating the benefits of bronchodilators and bronchodilator combinations and the possibility of reducing or withdrawing ICS under cover of effective bronchodilation. Where possible we will focus on the effect of treatment on prevention of exacerbations, an important management goal in COPD [4].
1.1. The use of ICS and ICS/LABA in COPD
The use of ICS in patients with COPD arose from the recognition of the need for anti-inflammatory treatment in asthma and the possibility that the inflammatory component of COPD might be similarly amenable to such treatment [23]. However, the inflammatory processes of asthma and COPD were shown to be fundamentally different [23], and some of the early studies of ICS in patients with COPD demonstrated no clinical benefit [24]. Other trials, whereas failing to show any effect on the decline in lung function (as primary outcome), revealed some benefit of ICS on health status and exacerbations [25,26], the latter particularly in patients with more severe disease and frequent exacerbations [27].
Patients with frequent exacerbations are more likely than those with infrequent exacerbations to have eosinophilic inflammation [28,29], and selection of such a population can act as a potential source of bias. For this reason, care is needed when extrapolating data from randomized controlled trials with ICS. Other overt and hidden inclusion criteria that have the potential to influence outcomes include existing ICS users, who may be more prone than others to exacerbations based on application of treatment strategies such as GOLD [4], and specialist care patients, who are likely to have a prior history of exacerbations. Additionally, adverse consequences are more likely in patients with COPD with high versus low co-morbidity.
The combined use of an ICS/LABA, which provides greater improvement in lung function and symptom control than doubling the ICS dose in asthma [30-32], has also been investigated in COPD [33]. Many studies have since demonstrated the efficacy of ICS/LABA combinations against placebo in preventing exacerbations and improving lung function and health status in patients with COPD [1,34,35]. Set against these benefits, however, ICS are associated with significant and potentially serious side effects.
2. ICS/LABA versus a single bronchodilator
2.1. ICS/LABA versus LABA
Most studies of ICS/LABA combinations have compared the efficacy of the ICS/LABA versus the LABA monocomponent (rather than a long-acting muscarinic antagonist [LAMA]). In general, the ICS/LABA combination has demonstrated greater efficacy in preventing exacerbations compared with the LABA monocomponent, especially in more recent trials [1,7,36,37]. In the early TRial of Inhaled STeroids ANd LABAs (TRISTAN) study, there was no statistical difference in exacerbation rate between an ICS/LABA (fluticasone/salmeterol combination [FSC] 500/50 µg twice daily [b.i.d.]) and the LABA salmeterol alone [34]. In the 3-year TORCH study, FSC 500/50 µg b.i.d. was more effective than salmeterol in preventing moderate and severe exacerbations combined (rate ratio 0.88, i.e., 12% reduction), but not severe exacerbations (rate ratio 1.02) [1]. Pneumonia was reported in 19.6% of the FSC group, 18.3% of the fluticasone group, 13.3% of the salmeterol group and 12.3% of the placebo group. In a recently published study, FSC 250/50 µg b.i.d. was no better than salmeterol alone in preventing relapse during the 6 months after an admission for an exacerbation of COPD, or for patient-reported health outcomes [38].
In the 44-week VIVACE (Impact of Salmeterol/Fluticasone Propionate versus Salmeterol on Exacerbations in Severe COPD) study, FSC 500/50 µg b.i.d. reduced moderate or severe exacerbations by 35% compared with the LABA alone in patients with severe disease and a history of frequent exacerbations [7,28,29]. Exacerbation rates were also lower with FSC 500/50 µg b.i.d. in patients with more severe forced expiratory volume in 1 second [FEV1] (< 50% predicted) versus mild-to-moderate disease (FEV1 > 50% predicted) at baseline in the TRISTAN trial [34].
The ICS/LABA combination of budesonide/formoterol has been shown to be more effective in preventing exacerbations compared with formoterol alone [36,37]. In addition, some have suggested that budesonide has less propensity to cause pneumonia than fluticasone, which may have a higher, dose-related risk [11,39,40]. However, the two drugs have not been compared directly in randomized controlled trials, and diagnostic criteria for pneumonia can vary between studies.
The newer ICS/LABA formulation of fluticasone furoate and vilanterol (100/25 µg once daily [q.d.]) significantly reduced the rate of moderate and severe exacerbations compared with vilanterol alone (p < 0.0001), an effect that was driven mainly by a reduction in moderate exacerbations [41,42]. A significant reduction was also observed in a subgroup of patients with frequent exacerbations (p = 0.0005). However, an increased frequency of radiographically confirmed pneumonia was observed with the ICS/LABA (4% for the 100/25 µg q.d. dose approved for COPD) versus the LABA alone (2%) [41,42].
In a population-based cohort study by Gershon et al. [43] comparing new users of ICS/LABAs and LABAs, no significant difference for the composite outcome of hospitalization or death was observed between LABA/ICS and LABA alone in the overall population. In a subgroup with coexistent asthma, a significantly better outcome was observed with LABA/ICS compared with LABA treatment. Rodrigo et al. performed a systematic review of 18 randomized controlled trials of ICS/LABA versus LABA and showed no significant difference in relative risk for the number of severe exacerbations or mortality (all-cause, respiratory or cardiovascular) [44]. ICS/LABA treatment was associated with serious adverse events (pneumonia) and the magnitude of their benefits (on health status and moderate exacerbations) was regarded as below the level of clinical importance. Small benefits on lung function and health status were reported in a network meta-analysis evaluating efficacy of an ICS/LABA versus a LABA or LAMA [45]. It is important to note that pivotal landmark studies in COPD (such as Inhaled Steroids in Obstructive Lung Disease in Europe [ISOLDE] and TORCH), whereas highlighting the merits of ICS and ICS/LABA treatment, also revealed that many patients receiving these therapies withdrew from study participation prematurely for various reasons, including a lack of perceived benefit.
2.2. ICS/LABA versus LAMA
The comparative efficacy of ICS/LABAs versus LAMAs has been less extensively investigated [46]. In the INSPIRE study of patients with severe or very severe COPD, no difference was observed between an ICS/LABA (FSC 500/50 µg b.i.d.) and a LAMA (tiotropium) in reducing the risk of all exacerbations, despite patients in the LAMA arm undergoing ICS withdrawal to participate in the study [47]. Tiotropium was more effective in preventing exacerbations requiring treatment with antibiotics, whereas FSC was more effective for exacerbations requiring systemic steroids. A Canadian study reported by Aaron et al. [48] has also observed that addition of an ICS/LABA (FSC 500/50 µg b.i.d.) to the LAMA tiotropium did not improve COPD exacerbations rates compared with LAMA monotherapy in patients with moderate-to-severe disease. However, lung function, quality of life and hospitalization rates were improved with triple therapy versus LAMA treatment alone in this study (see also further discussion of triple ICS/LAMA + LABA therapy below). Subsequently, the authors of a 2013 Cochrane systematic review concluded that they were unable to determine whether ICS/LABA or LAMA treatment had the lower mortality rate based on the large INSPIRE trial and two smaller studies comparing ICS/LABA with tiotropium [49]. Additionally, it was not clear which therapeutic approach was better in terms of reducing COPD exacerbations, hospitalizations and serious adverse events or improving health status. This conclusion was primarily due to missing outcome data for patients who withdrew from INSPIRE and the resulting possibility of bias in treatment effect.
3. ICS/LABA versus dual bronchodilation
Three fixed-dose LAMA/LABA combinations are currently available: q.d. glycopyrronium/indacaterol 50/110 µg (QVA149; Ultibro Breezhaler; Novartis) and umeclidinium/vilanterol 62.5/25 µg (Laventair/Anoro Ellipta; GlaxoSmithKline), and a b.i.d. combination of aclidinium/formoterol 400/12 µg (Duaklir Genuair; AstraZeneca). Each has been shown to be more effective in improving lung function, compared with placebo or a single long-acting bronchodilator alone [50-55]. A combination of tiotropium and olodaterol, filed for approval in 2014, also improved lung function relative to its monocomponents [56]. Although all published LAMA/LABA studies have not shown consistent significant improvement in other important outcomes (such as dyspnea, moderate/severe exacerbations and clinically relevant improvements in health status vs LAMA monotherapy), overall trends are positive for the efficacy of LAMA/LABA combinations as a class in these outcomes [57].
3.1. ICS/LABA versus LAMA/LABA
Before the fixed-dose LAMA/LABA combinations were developed, the concurrent administration of a LAMA (tiotropium) and LABA (formoterol) using separate inhalers was shown to improve lung function to a greater extent than FSC 500/50 µg b.i.d. over 6 weeks of treatment in patients with moderate COPD [58]. Similarly, Magnussen et al. reported a greater effect on markers of hyperinflation in an 8-week study comparing the free combination of tiotropium and salmeterol with FSC 500/50 µg b.i.d. [59]. Lending indirect support to the use of combined bronchodilators, the large population-based cohort study by Gershon et al. referred to earlier showed no difference in primary outcome (hospitalization or death) between new users of ICS/LABA and LABA when the latter were also receiving a LAMA [43]. However, the possibility of a type II error (false negative) cannot be excluded.
Current comparisons of fixed LAMA/LABA combinations with ICS/LABA include two fully published studies comparing QVA149 and FSC, and two abstracts comparing umeclidinium/vilanterol with FSC in three studies (Table 1). In the ILLUMINATE study in symptomatic patients with COPD and no moderate-to-severe exacerbations in the prior year, q.d. QVA149 provided significant improvements in lung function versus b.i.d. FSC 500/50 µg (p < 0.0001), with significant improvement in dyspnea at 6 months (p = 0.0031) [60]. In a post-hoc analysis of the ILLUMINATE data, there was a 20% reduction in the rate of moderate or severe exacerbations with QVA149 versus FSC, although this was not significantly different [61].
Table 1.
Summary of studies comparing fixed-dose combinations of LAMA/LABAs and ICS/LABAs.
| Study/(Year) | Duration | N randomized | FEV1 % predicted (GOLD stage) | Entry criteria | Treatment (randomization) | Exacerbation RR for LAMA/LABA versus FSC |
Treatment difference for LAMA/LABA versus FSC |
||
|---|---|---|---|---|---|---|---|---|---|
| Lung function | Other outcomes | Pneumonia incidence | |||||||
| ILLUMINATE Vogelmeier et al. (2013) [60,61] |
26 weeks | 523 | 51 (II/III) | Symptomatic; no moderate/severe exacerbation in the previous year | QVA149 110/50 μg q.d. FSC 500/50 μg b.i.d. (1:1) |
All exacerbations: RR 0.69 (95% CI 0.44, 1.07); p = 0.098 Moderate/severe exacerbations: RR 0.80 (95% CI 0.41, 1.56); p = 0.512 |
FEV1 AUC0–12h 0.138 l (95% CI 0.100, 0.176); p < 0.0001; primary end point at 26 weeks Trough FEV1 0.103 l (95% CI 0.065, 0.141); p < 0.0001 |
TDI total score 0.76 (95% CI 0.26, 1.26); p = 0.0031 SGRQ total score −1.24 (95% CI −3.33, 0.85); NS (clinically significant improvement from baseline with both treatments) Rescue medication use −0.39 (95% CI −0.71, −0.06) puffs/day; p = 0.019 |
0 versus 1.5% |
|
LANTERN Zhong et al. (2015) [66] |
26 weeks |
744 |
52 (II/III) |
Symptomatic; no more than 1 moderate/severe exacerbation in the previous year (21% had one in the previous year) |
QVA149 110/50 μg q.d. FSC 500/50 μg b.i.d. (1:1) |
Moderate/severe exacerbations: RR 0.69 (95% CI 0.48, 1.00); p < 0.05 |
Trough FEV1 0.075 l (95% CI 0.044, 0.107); p < 0.001; primary endpoint at 26 weeks |
TDI total score 0.13 (95% CI −0.2, 0.47); NS SGRQ total score -0.69 (95% CI −2.38, 1.00); NS Rescue medication use −0.03 puffs/day (95% CI −0.26, 0.21); NS |
0.8 versus 2.7% |
| Donohue et al. (2015) [65,106] | 12 weeks | 717 | FEV1 ≥ 30 and ≤ 70% | Symptomatic; no moderate/severe exacerbation in prior year | UMEC/VI 62.5/25 μg q.d. FSC 500/50 μg b.i.d. (1:1) |
– | Change from baseline in 24-h weighted-mean serial FEV1 0.080 l (95% CI 0.046, 0.113); p < 0.001; primary endpoint at 12 weeks Change from baseline in trough FEV1 0.151 ± 0.0126 versus 0.062 ± 0.0125 l |
With both treatments, changes in TDI total score (> 1 point) and change from baseline in SGRQ total score (> 4 units) clinically meaningful over 12 weeks | NR (no serious events) |
|
NCT01817764 Donohue et al. (2014) (abstract) [62] |
12 weeks | 707 | FEV1 ≥ 30 and ≤ 70% | Symptomatic; no moderate/severe exacerbation in prior year | UMEC/VI 62.5/25 μg q.d. FSC 250/50 μg b.i.d. (1:1) |
– | Change from baseline in 24-h weighted-mean serial FEV1 0.074 l (95% CI 0.038, 0.110); p < 0.001; primary end point at 12 weeks Change from baseline in trough FEV1 0.154 ± 0.0133 versus 0.072 ± 0.0134 l |
With both treatments, changes in TDI total score (> 1 point) and change from baseline in SGRQ total score (> 4 units) clinically meaningful over 12 weeks in both studies; no treatment differences between UMEC/VI and FSC | Overall NR Pneumonia as SAE: 0 versus 0.85% |
|
NCT01879410 Donohue et al. (2014) (abstract) [62] |
12 weeks |
700 |
FEV1 ≥ 30 and ≤ 70% |
Symptomatic; no moderate/severe exacerbation in prior year |
UMEC/VI 62.5/25 μg q.d. FSC 250/50 μg b.i.d. (1:1) |
– |
Change from baseline in 24-h weighted-mean serial FEV1 0.101 l (95% CI 0.063, 0.139); p < 0.001; primary end point at 12 weeks Change from baseline in trough FEV1 0.185 ± 0.0138 versus 0.087 ± 0.0140 l |
With both treatments, changes in TDI total score (> 1 point) and change from baseline in SGRQ total score (> 4 units) clinically meaningful over 12 weeks in both studies; no treatment differences between UMEC/VI and FSC |
Overall NR Pneumonia as SAE: 0.29 versus 1.15% |
b.i.d.: Twice daily; CI: Confidence interval; FEV1: Forced expiratory volume in 1 second; FSC: Fluticasone–salmeterol combination; GOLD: Global initiative for chronic Obstructive Lung Disease; HR: Hazard ratio; ICS: Inhaled corticosteroids; LABA: Long-acting β2-agonist; LAMA: Long-acting muscarinic antagonist; NR: Not reported; NS: Not significant; q.d.: Once daily; RR: Rate ratio; SAE: Serious adverse event; SGRQ: St George’s respiratory questionnaire; TDI: Transition dyspnea index; UMEC/VI: Umeclidinium/vilanterol.
In a similar population of symptomatic but stable patients (no exacerbations in previous year), two 12-week studies comparing the umeclidinium/vilanterol 62.5/25 µg combination and FSC (250/50 µg b.i.d.) reported improved lung function with the LAMA/LABA and no difference between the two treatments in dyspnea or health status [62-64]. Similar findings were reported in a 12-week study comparing umeclidinium/vilanterol 62.5/25 µg q.d. with FSC 500/50 µg b.i.d. [65].
The patient population in the 6-month LANTERN study comparing QVA149 with FSC 500/50 µg b.i.d. included patients with moderate-to-severe COPD and a history of up to one exacerbation in the previous year. QVA149 demonstrated better effects on lung function, similar effects on patient-reported outcomes (dyspnea and health status) and significantly reduced the rate of moderate or severe exacerbations by 31% [66]. In the subgroup of patients with a history of exacerbations (21% of total patients), the annualized rate of all exacerbations was significantly lower with QVA149 (0.78) versus FSC (1.81), with a rate ratio of 0.43 (95% CI; 0.25, 0.76; p = 0.003). The annualized rate of moderate or severe exacerbations was similar for each treatment among patients with an exacerbation history (rate ratio 0.60, 95% CI 0.33, 1.08; p = 0.086).
The studies comparing LAMA/LABA combinations with an ICS/LABA have generally been conducted in non-exacerbators, rather than in patients with a history of frequent exacerbations for whom ICS would be appropriately prescribed, and in whom ICS might be expected to be more effective in preventing exacerbations [7]. The efficacy of a LAMA/LABA versus an ICS/LABA on exacerbations in patients with moderate-to-very-severe COPD and a history of exacerbations is currently under investigation with QVA149 in the 1-year FLAME study [67]. Another study, currently recruiting, will enable comparison of a LAMA/LABA (umeclidinium/vilanterol) and an ICS/LABA (fluticasone furoate/vilanterol 100/25 µg q.d.) over 1 year in patients with a history of exacerbations [68].
4. Current use of ICS: the impact of dose
Many of the side effects of ICS, including pneumonia [11], bone fracture risk [12], tuberculosis [15] and diabetes [9], are dose-related, in terms of both daily dose and lifetime exposure. Despite this, FSC is approved in the EU for use in COPD only at a daily fluticasone propionate dose of 1000 µg, which is double the 500 µg daily dose approved for COPD in the US and Japan. In their review of data on FSC 500/50 and 250/50 µg b.i.d. for use in COPD, the US FDA reported both a higher frequency of adverse events (78 vs 70%) and no treatment benefit for the higher versus the lower dose [69]. The dosage of budesonide in combination with the LABA formoterol is also lower in the US than that approved in the EU (200 and 400 µg b.i.d., respectively). Evidence to support the use of the higher rather than the lower doses in the EU is lacking [21,22]. Comparative efficacy data are also lacking for other ICS agents, as a 2014 Cochrane network meta-analysis identified only a few prospective head-to-head trials evaluating different doses of the same ICS (administered alone or with a LABA) on relevant COPD outcomes [45]. Additionally, the majority of studies powered to investigate the effects of ICS on important COPD outcomes used a single ICS dose that was the highest dose given among studies included in the meta-analysis. Exceptions include trials of the newer formulation of fluticasone, the furoate salt, which is approved for COPD in the EU and the US at the same q.d. dose of 100 µg (in combination with the LABA vilanterol), after studies showed no additional benefit on lung function or exacerbation frequency of a combination containing a higher (200 µg) dose of fluticasone furoate [42]. Short-term (12-week) head-to-head comparisons showed similar bronchodilator efficacy and also suggested a comparable incidence of pneumonia between FSC 500/50 µg b.i.d. and fluticasone furoate/vilanterol 100/25 µg q.d. [70]. In duplicate 1-year studies in patients with a history of exacerbations, the incidence of pneumonia with fluticasone furoate/vilanterol 100/25 µg q.d. was higher than with vilanterol 25 µg q.d. alone (6 vs 3%) [42], and similar to the incidence of 5% with FSC 500/50 µg b.i.d. reported by Kardos et al. in a comparable patient population [7].
Preparations of ICS of small particle size may offer the prospect of similar efficacy at a reduced dose compared with standard particle size ICS. Indeed, a recent observational study reported comparable exacerbation rates over 2 years when extrafine beclometasone was compared with a 1.2 – 1.4-fold higher dose of fluticasone (relative doses were calculated based on 1:1 equivalency) [71].
5. Which patients should receive ICS?
Some phenotypes are beginning to be identified among patients with COPD and are an important consideration for physicians when selecting treatment. This is acknowledged in the Spanish guideline for COPD (GesEPOC) in which diagnosis and management of COPD are based upon four clinical phenotypes: a non-exacerbator phenotype; a mixed COPD-asthma/ACOS phenotype; an exacerbator phenotype with emphysema and an exacerbator phenotype with chronic bronchitis (Figure 1) [72].
Figure 1.
Clinical phenotypes of COPD proposed by the Spanish guideline for COPD (Guía Española de la EPOC; GesEPOC).
Reprinted from [72], © 2014 with permission from Elsevier.
COPD: Chronic obstructive pulmonary disease.
Two phenotypes are particularly relevant for physicians in determining which patients should receive ICS: ACOS and frequent exacerbators.
5.1. Asthma–COPD overlap syndrome
Some patients present with features of both COPD and asthma, described as ACOS. As yet, there is no universally accepted definition of ACOS and no validated diagnostic criteria [73], although the GINA/GOLD collaboration provides a clinical description: “ACOS is characterized by persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD. ACOS is therefore identified by the features that it shares with both asthma and COPD”. The definition of ACOS used in the COPDGENE study is similar and is increasingly used: COPD based on usual spirometric criteria and a history of smoking, plus history of asthma before the age of 40 years [74]. The reported prevalence rates of ACOS have varied widely, likely as a result of differences in definition, but 10 – 20% of patients with COPD may have ACOS [73]. Although reliable biomarkers for ACOS are not currently available, it is important to identify the syndrome due to different therapeutic strategies for ACOS and COPD [4,73].
In patients with ACOS, the default is to start treatment as for asthma, that is, an ICS at low or moderate dose plus a LABA [4], in recognition of the potentially life-saving role of ICS in asthma. LAMA treatment can also be considered in addition to ICS/LABA. In the previously mentioned cohort study by Gershon et al. comparing new users of ICS/LABA or LABA, there was only a modest overall benefit for ICS/LABA but the greatest difference was observed among COPD patients with a co-diagnosis of asthma (difference of −6.5% in the composite outcome of hospitalization or death at 5 years), supporting the use of ICS in this population [43].
5.2. Patients with COPD who experience frequent exacerbations
The frequent exacerbator phenotype is important, because it is quite stable over time and is the strongest predictor of an individual’s future risk of exacerbations [75]. Additionally, as preventative treatments are available, identification of this phenotype based on medical history is of paramount importance. ‘Frequent exacerbations’ is generally taken to mean at least two exacerbations per year and defines a patient as high risk in the GOLD assessment scheme (the definition may also be based on at least one hospitalization for exacerbation per year) [4]. Initial treatment may be ICS-based, although GOLD includes LAMAs as another preferred choice, and a LAMA/LABA is an alternative option [4]. In the Spanish phenotype-based approach, a bronchodilator would be the initial choice with anti-inflammatory treatment employed if exacerbations persist despite bronchodilation [72]. Recently, the addition of roflumilast to ICS/LABA (with or without background LAMA) treatment has been shown to further reduce exacerbation rates and hospital admissions in patients who also have chronic cough and sputum production, compared with ICS/LABA or triple ICS/LAMA/LABA therapy [76].
It has been reported that up to 70% of patients with COPD are treated with high-dose ICS in many European countries [21]. In practice, the burden of exacerbations does not support such widespread use of ICS. Among 9219 patients with diagnosed COPD in a UK primary care database, 28% met the GOLD criteria for high-risk, frequent exacerbators [77]. Similarly, a German real-life study of 6209 patients reported that exacerbations were experienced by 28% of patients over a 6-month period [78]. In the first year of follow-up in the ECLIPSE cohort, frequent exacerbators comprised only 22, 33 and 47% of those with moderate, severe and very severe disease, respectively (using the GOLD pre-2011 definitions of severity), with 7, 18 and 33% of these groups hospitalized for an exacerbation [75]. There were no exacerbations in 55% of patients with moderate disease, 41% of those with severe disease and in 29% of the very severe group, in this cohort [75]. Thus, irrespective of the severity of airflow limitation, the majority of all patients with COPD are at low risk of exacerbations. Although this reinforces the traditional recommendation to prescribe ICS-based treatment according to an individual’s exacerbation history, the ICS withdrawal studies described below suggest that this issue is more complex than previously thought.
5.3. Can ICS responders be identified pre-treatment?
Targeting ICS to those patients who are likely to respond would minimize unnecessary exposure and costs while increasing the chance of improved outcomes. Some of the indicators of a probable ICS response in patients with obstructive lung disease (such as a previous history of asthma, atopy, positive bronchodilator test [reversibility], bronchial hyperresponsiveness, high levels of the fraction of exhaled nitric oxide and eosinophilia in sputum or blood) [28,79-86] may reflect aspects of a COPD phenotype with eosinophilic inflammation. For example, patients with a positive bronchodilator test have more bronchial eosinophilic inflammation than those who are non-reversible [84]. A large bronchodilator response (≥ 12% and ≥ 400 ml) may be required to predict treatment response [20].
One study has reported that the frequency of exacerbations and hospitalizations in patients with COPD was lower in a group of patients who received ICS treatment based on their level of sputum eosinophilia (anti-inflammatory treatment was initiated or escalated if sputum eosinophils were > 3%, and reduced or removed if sputum eosinophils were < 1%) compared with those who received ICS treatment according to traditional (British Thoracic Society) guidelines [87]. A post-hoc cluster analysis of clinical trial data with FSC 250/50 µg b.i.d. versus salmeterol alone identified three groups of patients with different responses to FSC versus salmeterol treatment, based on annual rates of moderate/severe exacerbations. Patients with a positive bronchodilator test (baseline bronchodilator reversibility ≥ 12%) and those receiving diuretics had a greater reduction in exacerbations with FSC versus salmeterol, whereas no significant difference was seen between treatments in the third cluster without bronchodilator reversibility who were not receiving diuretics [88]. The use of diuretics may have an effect on respiratory disease, or it may reflect the existence of cardiovascular disease and the possibility of a generalized inflammatory state that benefits from ICS. Recently, a post-hoc analysis of data from two clinical trials stratified patients by baseline blood eosinophil levels and compared the effect of fluticasone furoate/vilanterol versus vilanterol alone on exacerbation rates [28]. Combination treatment was more effective than vilanterol in patients with higher baseline levels of blood eosinophils (29% reduction in exacerbations in those with blood eosinophils ≥ 2% compared with a non-significant reduction of 10% in those with eosinophils < 2%) [28]. These studies excluded patients with a current diagnosis of asthma, but did not exclude patients with eosinophilia or those with a prior history of asthma, suggesting that undiagnosed ACOS may have been present in some patients [42].
Blood or sputum eosinophilia during an exacerbation have also been identified as biomarkers for a particular (eosinophilia-associated) exacerbation phenotype [89], and the usefulness of blood eosinophilia as a marker for directing corticosteroid treatment of exacerbations has been explored [86]. Sputum eosinophilia was identified as one of a range of markers (along with longer duration of symptoms, smoking history < 40 pack-years and ICS withdrawal in winter months) predicting a higher risk of exacerbation following ICS withdrawal [90]. A database study by Freeman et al. reported that patients with frequent COPD exacerbations and admissions could be predicted by female gender, asthma, blood eosinophilia (≥ 450/μl) in non-smokers, nasal polyps and COPD Assessment Test (CAT) score, suggesting the viability of developing a risk assessment tool using variables typically included in primary care patient records [91].
Although blood eosinophil measurements may prove useful for selecting patients for different therapeutic approaches, the work is exploratory and a number of factors need to be investigated further before it can be validated. In the ECLIPSE cohort, 37% of patients with COPD had blood eosinophil counts persistently ≥ 2% (as did a similar proportion of healthy control subjects) [92]. However, a single measurement may not be adequate: in the ECLIPSE cohort, for example, blood eosinophils varied above and below the 2% cut-off over the 3-year follow-up in 49% of patients [92]. The most appropriate cut-off is not known, nor whether it should be expressed as a percentage or absolute number of cells.
6. ICS withdrawal to LAMAs, LABAs or combination
Early studies showed that abrupt withdrawal of ICS under cover of short-acting bronchodilators or theophylline alone led to an increased risk of exacerbations compared with continued ICS treatment [93-95]. This phenomenon may also be a confounding factor in exacerbation studies when ICS are given pre-treatment and then withdrawn at baseline [96,97]. However, recent studies in which patients were supported by more effective bronchodilation have suggested ICS withdrawal may not increase the risk of exacerbation, even in patients with severe COPD.
In the 12-month WISDOM study, patients with severe COPD and at least one exacerbation in the previous year received tiotropium, salmeterol and fluticasone propionate (500 µg b.i.d.) during a 6-week run-in and were randomized either to continue this treatment or to step down the fluticasone dose gradually and continue with tiotropium and salmeterol alone [98]. The gradual withdrawal of ICS in patients receiving LABAs and LAMAs did not result in an increased risk of exacerbation. These patients had a clear indication for ICS based on severe or very severe airflow limitation (mean FEV1 34% predicted), but not necessarily based on exacerbation history alone. The mean exacerbation frequency in this population was around one per year. Patients in the ICS-withdrawal group had small reductions in FEV1 and health status during the stepped ICS withdrawal, although the clinical importance of these changes is not clear [99]. Interestingly, the lowest ICS dose in this study (fluticasone propionate 100 µg b.i.d.) provided comparable lung function to that observed with the 500 µg b.i.d. dose, during a stepwise reduction in ICS dose and concurrent LABA/LAMA treatment [98].
In the relatively small, real-life OPTIMO study, patients with symptomatic COPD, moderate airflow limitation, fewer than two exacerbations in the year prior to the study, and who were receiving long-acting bronchodilators and ICS were either continued on this treatment or maintained mostly on long-acting bronchodilator(s) alone, at the discretion of the treating physician [100]. The withdrawal of ICS was not associated with any deterioration in symptoms, lung function or exacerbation rate during 6 months of observation.
In the 26-week INSTEAD study, patients with moderate COPD and no exacerbation in the past year, who had been receiving FSC 500/50 µg b.i.d. for at least 3 months, were either maintained on FSC or switched to a LABA alone (indacaterol 150 µg q.d.) [101]. The two groups did not differ in terms of lung function, symptoms, health status or exacerbation rate over the course of the study, and study discontinuation rates were similar. As in the OPTIMO study, this population of patients did not have an indication for ICS, although such treatment reflects the common inappropriate use of these agents [21].
It may also be noted that in the ILLUMINATE study comparing QVA149 and FSC in patients with stable COPD, 33% of patients in the QVA149 group had their previous ICS treatment withdrawn at baseline [60]. During the run-in following ICS withdrawal, the mean pre-bronchodilator FEV1 decreased only by 35 ml or 1.8%. In these patients, after 6 months, lung function was significantly better compared with FSC (difference in FEV1 AUC0–12h 145 ml; p < 0.0001) and they experienced no increase in the incidence of exacerbations after withdrawal. Similarly, the decrease in exacerbation risk with QVA149 versus FSC in the LANTERN study (in which patients could have had a recent exacerbation) was achieved despite the fact that 55% of the QVA149-treated patients were on ICS that was withdrawn at baseline [66].
7. Concluding remarks
Maintaining patients who are not frequent exacerbators on ICS solely in an attempt to reduce exacerbation risk may not be necessary, if they are also receiving effective bronchodilator LAMA/LABA therapy. Newly diagnosed patients with frequent exacerbations can be initiated on dual LAMA/LABA therapy that may provide better bronchodilation, and in many cases similar exacerbation protection, to an ICS/LABA. Other preventative measures, as well as potential contraindications for ICS, should also be considered. Despite the recommended strategy for the use of ICS/LABA fixed combinations and their approved indications, these agents (whereas the mainstay of treatment for non-phenotypic asthma) are greatly overused in COPD. There is growing evidence that ICS treatment, if not indicated, can gradually be withdrawn under cover of effective (LAMA/LABA) bronchodilation without increased exacerbation risk and with only a very small decrease in FEV1. Low-dose ICS combination therapy based on US-approved dosages or data from randomized controlled trials can also be considered.
The recent evidence of the relative efficacy and safety of a LAMA/LABA versus an ICS/LABA suggests that a LAMA/LABA combination could be first-line therapy in most patients with COPD, the exceptions being the ‘mildest’ patients (who may need only one bronchodilator) and those in whom ACOS is suspected. In the future, blood eosinophilia testing could be a useful way of selecting those patients who may benefit from ICS treatment, but this approach requires further prospective clinical evaluation. Patients who remain symptomatic or who present with exacerbations despite LAMA/LABA treatment should receive additional treatment, such as ICS or phosphodiesterase-4 inhibitors. Another alternative might be the use of macrolides or mucolytics; however, no study has been conducted thus far with macrolides and mucolytics specifically on top of a LAMA/LABA and further research is required.
8. Expert opinion
8.1. Where do ICS/LABA and LAMA/LABA combinations fit in the treatment strategy?
ACOS is a special case in which the asthmatic element presents airway inflammation that is amenable to intervention with ICS, and these patients should receive first-line ICS-based therapy in accordance with recommendations for asthma treatment [4,72]. In patients with COPD only, there is evidence to support the use of an ICS-based regimen (i.e., ICS/LABA) for patients with a history of repeated exacerbations, who have significant symptoms despite regular therapy with long-acting bronchodilators, in line with the EU indications for ICS/LABA (Table 2). However, an approach of ‘lowest effective dose’ may be preferable over the high EU-approved doses of fluticasone propionate or budesonide [22]. For patients with frequent exacerbations, the Spanish guideline recommends a stepwise approach, starting with a single bronchodilator and progressing with increasing severity to the addition of an anti-inflammatory agent and/or second bronchodilator [72].
Table 2.
Approved COPD indications in the EU and US for some LABA/ICS combinations.
| Drug | Daily dose |
Indication |
|
|---|---|---|---|
| EU | US | ||
| Fluticasone propionate/salmeterol | 500/50 µg × 2 | Symptomatic treatment of patients with COPD, with a FEV1 < 60% predicted normal (pre-bronchodilator) and a history of repeated exacerbations, who have significant symptoms despite regular bronchodilator therapy | Not approved |
| Fluticasone propionate/salmeterol | 250/50 µg × 2 | Not approved | Twice-daily maintenance treatment of airflow obstruction in patients with COPD, including chronic bronchitis and/or emphysema Also indicated to reduce exacerbations of COPD in patients with a history of exacerbations |
| Budesonide/formoterol | 400/12 µg × 2 | Symptomatic treatment of patients with severe COPD (FEV1 < 50% predicted normal) and a history of repeated exacerbations, who have significant symptoms despite regular therapy with long-acting bronchodilators | Not approved |
| Budesonide/formoterol | 200/6 µg × 2 | Not approved | Maintenance treatment of airflow obstruction in patients with COPD including chronic bronchitis and emphysema |
| Fluticasone furoate/vilanterol | 100/25 µg | Symptomatic treatment of adults with COPD with a FEV1 < 70% predicted normal (post-bronchodilator) with an exacerbation history despite regular bronchodilator therapy | Long-term, once-daily, maintenance treatment of airflow obstruction in patients with COPD, including chronic bronchitis and/or emphysema Also indicated to reduce exacerbations of COPD in patients with a history of exacerbations |
COPD: Chronic obstructive pulmonary disease; FEV1: Forced expiratory volume in 1 second; LABA: Long-acting β2-agonist; LAMA: Long-acting muscarinic antagonist; ICS: Inhaled corticosteroids.
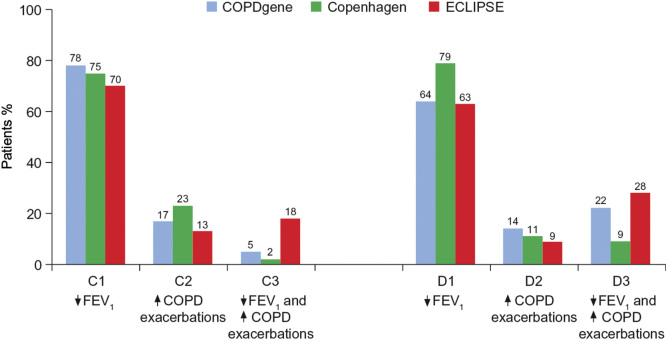
In the GOLD strategy, in which treatment is based on risk rather than phenotypes, ICS/LABA is a recommended first-choice treatment for high-risk patients (groups C and D), risk being determined by frequency of exacerbations and/or airflow limitation [4]. However, lung function is a relatively poor predictor of exacerbation risk [91], and most patients in groups C and D are classified as such on the basis of low airflow limitation only (70 – 78% in group C; 63 – 79% in group D), rather than frequent exacerbations (9 – 23%) or both (2 – 28%; Figure 2). Similarly, in a survey of patients in UK general practice, 70% of 2282 patients with severe airflow limitation had a low exacerbation risk profile (≤ 1 exacerbation) [102]. Subdivision of the GOLD groups C and D may be warranted in order to target ICS-based treatment more precisely to the appropriate frequent exacerbator phenotype, in accordance with the current EU labels of the ICS/LABA combinations (Table 2).
Figure 2.
Prevalence of GOLD group C and D subtypes, defined by FEV1 < 50% predicted only (C1 and D1), exacerbation history only (C2 and D2) or both (C3 and D3), in the large COPD gene [107], Copenhagen [108] and ECLIPSE [109] trials. In each study, most patients were categorized as high risk on the basis of low FEV1 alone.
Adapted and reproduced with permission of the European Respiratory Society [110].
COPD: Chronic obstructive pulmonary disease; FEV1: Forced expiratory volume in 1 second.
Another difficulty is in the case of patients with concomitant bronchiectasis who are unlikely to benefit from ICS. Bronchiectasis is present in up to approximately half of the patients with severe COPD, and is associated with severe airflow limitation, severe exacerbations and chronic bacterial colonization [103-105].
8.2. ICS withdrawal: unanswered questions
Although ICS withdrawal under cover of effective bronchodilation appears to be a realistic possibility for patients at low risk of exacerbation, several questions remain unanswered. In the studies described in the previous section, ICS were withdrawn during either single or dual long-acting bronchodilator treatment. There are currently no guideline recommendations for ICS withdrawal, but the clinician would need to judge what constitutes effective bronchodilation for an individual patient, either a single long-acting bronchodilator or, probably more likely, a dual LAMA/LABA. For patients at low risk of exacerbation receiving a high EU-approved ICS dose plus a LABA, gradual ICS withdrawal under dual bronchodilation could be considered.
The interplay between lung function and exacerbation risk requires further study, and many patients who meet GOLD C and D criteria are not necessarily exacerbators. With the exception of the WISDOM trial in patients with severe-to-very severe airflow limitation [98], the ICS withdrawal studies described previously were conducted in patients with COPD who did not have a clear indication for ICS [100,101]. The WISDOM trial included patients with at least one exacerbation [98], but ICS withdrawal in a specific population of frequent exacerbators (defined as at least two exacerbations per year) has not been explored. In frequent exacerbators, the dose of ICS may be reduced, but these agents should not be completely withdrawn except where contraindicated (for example, due to pneumonia, bronchiectasis or candidiasis), until broadly accepted biomarker or phenotype characterizations that identify patients who will benefit from ICS treatment are available. Withdrawal of ICS might be a potential consideration in a patient who suffers an episode of pneumonia, even if there is a history of frequent exacerbations. Dual LAMA/LABA treatment, roflumilast if indicated, and/or long-term macrolides, as well as vaccinations and other preventative measures should be offered in this context. As discussed above, an alternative strategy would be to initiate treatment with a LAMA/LABA and add or subtract further treatment as the patient’s condition requires.
Until recently, most ICS studies have looked at mean treatment effects across patient populations. This approach may mix results in different patient subgroups (such as previous ICS users and ICS-naive patients) and show a treatment effect in the overall population that is only present in one subgroup [97]. Future studies are needed that look at interactions between patient baseline characteristics and response to therapy a priori.
Article highlights.
Inhaled corticosteroids (ICS; in fixed combinations with long-acting β2-agonists [LABAs]) are often prescribed to patients with COPD outside labeled indications and recommended treatment strategies and guidelines, despite the potential for side effects.
Available data indicate that LABA/long-acting muscarinic antagonist (LAMA) combination therapy provides generally comparable efficacy to ICS/LABA treatment in patients with stable COPD.
ICS dose reduction or withdrawal under cover of effective long-acting bronchodilator therapy does not increase exacerbation rates in patients at low risk of exacerbation.
Two phenotypes are particularly relevant in determining which patients should receive ICS: asthma–COPD overlap syndrome (ACOS) and frequent exacerbators.
A LAMA/LABA combination may be an appropriate starting therapy for patients with COPD who do not have ACOS, although patients with mild disease may be managed with a single long-acting bronchodilator. Additional drug therapy may be required for patients who remain symptomatic or present with exacerbations despite effectively delivered LAMA/LABA treatment.
This box summarizes key points contained in the article.
Declaration of interest
The authors were assisted in the preparation of the manuscript by Sarah Filcek and Molly Heitz (of CircleScience, Tytherington, UK, an Ashfield company, part of UDG Healthcare plc). J Donohue has received consulting fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Teva, and Sunovion. A D’Urzo has received research, consulting, and lecturing fees from Almirall SA, Altana, AstraZeneca, Boehringer Ingelheim (Canada) Ltd, Forest Laboratories, GlaxoSmithKline, KOS Pharmaceuticals, Merck Canada, Methapharm, Ono Pharmaceuticals, Novartis Canada/US, Pfizer Canada, Schering Plough, Sepracor, and SkyePharma. P Kardos has received consulting and lecturing fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Menarini, Takeda and Teva. M Miravitlles has received speaker fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, Glaxo-SmithKline, Grifols, Menarini, Pfizer, and Novartis, and consulting fees from Almirall, Boehringer Ingelheim, CSL Behring, Gebro Pharma, GlaxoSmithKline, Grifols, MedImmune, Novartis, Pfizer, and Takeda. D Price has board membership with Aerocrine, Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, and Teva; and has received consultancy fees from Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Pfizer, and Teva; grants and unrestricted funding for investigator-initiated studies from UK National Health Service, British Lung Foundation, Aerocrine, AKL Ltd, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Eli Lilly, GlaxoSmithKline, Meda, Merck, Mundipharma, Napp, Novartis, Orion, Pfizer, Respiratory Effectiveness Group, Takeda, Teva, and Zentiva; payments for lectures/speaking from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Meda, Merck, Mundipharma, Novartis, Pfizer, SkyePharma, Takeda, and Teva; payment for manuscript preparation from Mundipharma and Teva; payment for the development of educational materials from GlaxoSmithKline, Novartis; payment for travel/accommodations/meeting expenses from Aerocrine, Boehringer Ingelheim, Mundipharma, Napp, Novartis, and Teva; and funding for patient enrolment or completion of research from Almirall, Chiesi, Teva, and Zentiva. He owns shares in AKL Ltd, which produces phytopharmaceuticals, and owns 80% of Research in Real Life Ltd and its subsidiary social enterprise Optimum Patient Care, and holds patents (planned, pending or issued) with AKL Ltd. Prof Price has acted as a peer reviewer for the following grant committees: Medical Research Council (2014), Efficacy and Mechanism Evaluation programme (2012), HTA (2014). Writing assistance was utilized in the production of this manuscript and funded by Novartis.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–89. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- Niewoehner DE. TORCH and UPLIFT: what has been learned from the COPD “mega-trials”? COPD. 2009;6:1–3. doi: 10.1080/15412550902723984. [DOI] [PubMed] [Google Scholar]
- Vestbo J, Vogelmeier C, Small M, et al. Understanding the GOLD 2011 Strategy as applied to a real-world COPD population. Respir Med. 2014;105:729–36. doi: 10.1016/j.rmed.2014.03.002. [DOI] [PubMed] [Google Scholar]
- •• .Global initiative for chronic Obstructive Lung Disease (GOLD 2015) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2015. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015.pdf. Available from: [Last accessed 13 February 2015]; •• This document outlines the global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (COPD).
- European Medicines Agency (EMA) Seretide diskus summary of product characteristics (SmPC) http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Seretide_Diskus_6_13/WC500013534.pdf Available from:
- Montuschi P, Malerba M, Santini G, et al. Pharmacological treatment of chronic obstructive pulmonary disease: from evidence-based medicine to phenotyping. Drug Discov Today. 2014;19:1928–35. doi: 10.1016/j.drudis.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Kardos P, Wencker M, Glaab T, et al. Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:144–9. doi: 10.1164/rccm.200602-244OC. [DOI] [PubMed] [Google Scholar]
- Ernst P, Gonzalez AV, Brassard P, et al. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med. 2007;176:162–6. doi: 10.1164/rccm.200611-1630OC. [DOI] [PubMed] [Google Scholar]
- Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med. 2010;123:1001–6. doi: 10.1016/j.amjmed.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Crim C, Calverley PM, Anderson JA, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009;34:641–7. doi: 10.1183/09031936.00193908. [DOI] [PubMed] [Google Scholar]
- • .Suissa S, Patenaude V, Lapi F, et al. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68:1029–36. doi: 10.1136/thoraxjnl-2012-202872. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper shows that pneumonias are just as important as exacerbations in the benefit:risk assessment of inhaled corticosteroids (ICS)-containing combination therapy in COPD.
- Loke YK, Cavallazzi R, Singh S. Risk of fractures with inhaled corticosteroids in COPD: systematic review and meta-analysis of randomised controlled trials and observational studies. Thorax. 2011;66:699–708. doi: 10.1136/thx.2011.160028. [DOI] [PubMed] [Google Scholar]
- Lee TA, Weiss KB. Fracture risk associated with inhaled corticosteroid use in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169:855–9. doi: 10.1164/rccm.200307-926OC. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Murray HE, Skeans M, et al. Skin manifestations of inhaled corticosteroids in COPD patients: results from lung health study II. Chest. 2004;126:1123–33. doi: 10.1016/S0012-3692(15)31287-3. [DOI] [PubMed] [Google Scholar]
- Lee CH, Kim K, Hyun MK, et al. Use of inhaled corticosteroids and the risk of tuberculosis. Thorax. 2013;68:1105–13. doi: 10.1136/thoraxjnl-2012-203175. [DOI] [PubMed] [Google Scholar]
- Dong YH, Chang CH, Lin Wu FL, et al. Use of inhaled corticosteroids in patients with COPD and the risk of TB and influenza: A systematic review and meta-analysis of randomized controlled trials. a systematic review and meta-analysis of randomized controlled trials. Chest. 2014;145:1286–97. doi: 10.1378/chest.13-2137. [DOI] [PubMed] [Google Scholar]
- Ernst P, Baltzan M, Deschênes J, et al. Low-dose inhaled and nasal corticosteroid use and the risk of cataracts. Eur Respir J. 2006;27:1168–74. doi: 10.1183/09031936.06.00043005. [DOI] [PubMed] [Google Scholar]
- Flynn RW, MacDonald TM, Hapca A, et al. Quantifying the real life risk profile of inhaled corticosteroids in COPD by record linkage analysis. Respir Res. 2014;15:141. doi: 10.1186/s12931-014-0141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D, Yawn B, Brusselle G, et al. Risk-to-benefit ratio of inhaled corticosteroids in patients with COPD. Prim Care Respir J. 2013;22:92–100. doi: 10.4104/pcrj.2012.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst P, Saad N, Suissa S. Inhaled corticosteroids in COPD: the clinical evidence. Eur Respir J. 2015;45:525–37. doi: 10.1183/09031936.00128914. [DOI] [PubMed] [Google Scholar]
- Izquierdo Alonso JL, Rodríguez Glez-Moro JM. The excessive use of inhaled corticosteroids in chronic obstructive pulmonary disease. Arch Bronconeumol. 2012;48:207–12. doi: 10.1016/j.arbres.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Miravitlles M. Arguments in favor of inhaled corticosteroids in COPD by phenotype instead of by severity. Arch Bronconeumol. 2011;47:271–3. doi: 10.1016/j.arbr.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–80. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- Vestbo J, Sørensen T, Lange P, et al. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 1999;353:1819–23. doi: 10.1016/s0140-6736(98)10019-3. [DOI] [PubMed] [Google Scholar]
- Burge PS, Calverley PM, Jones PW, et al. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320:1297–303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung Health Study Research Group Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343:1902–9. doi: 10.1056/NEJM200012283432601. [DOI] [PubMed] [Google Scholar]
- Jones PW, Willits LR, Burge PS, et al. Disease severity and the effect of fluticasone propionate on chronic obstructive pulmonary disease exacerbations. Eur Respir J. 2003;21:68–73. doi: 10.1183/09031936.03.00013303. [DOI] [PubMed] [Google Scholar]
- • .Pascoe S, Locantore N, Dransfield MT, et al. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3:435–42. doi: 10.1016/S2213-2600(15)00106-X. [DOI] [PubMed] [Google Scholar]; • This research suggests that blood eosinophil counts provide a biomarker of response to ICS treatment in patients with COPD.
- Price D, Rigazio A, Postma D, et al. Blood eosinophilia and the number of exacerbations in COPD patients (Abstract) Eur Respir J. 2015;44(Suppl 58):4416. [Google Scholar]
- Greening AP, Ind PW, Northfield M, et al. Added salmeterol versus higher-dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Allen & Hanburys Limited UK Study Group. Lancet. 1994;344:219–24. doi: 10.1016/s0140-6736(94)92996-3. [DOI] [PubMed] [Google Scholar]
- Condemi JJ, Goldstein S, Kalberg C, et al. The addition of salmeterol to fluticasone propionate versus increasing the dose of fluticasone propionate in patients with persistent asthma. Salmeterol study group. Ann Allergy Asthma Immunol. 1999;82:383–9. doi: 10.1016/s1081-1206(10)63288-7. [DOI] [PubMed] [Google Scholar]
- van Noord JA, Schreurs AJ, Mol SJ, et al. Addition of salmeterol versus doubling the dose of fluticasone propionate in patients with mild to moderate asthma. Thorax. 1999;54:207–12. doi: 10.1136/thx.54.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuso L, Mores N, Valente S, et al. Long-acting beta-agonists and their association with inhaled corticosteroids in COPD. Curr Med Chem. 2013;20:1477–95. doi: 10.2174/0929867311320120003. [DOI] [PubMed] [Google Scholar]
- Calverley P, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449–56. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- Nannini LJ, Poole P, Milan SJ, et al. Combined corticosteroid and long-acting beta2-agonist in one inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;11:CD003794. doi: 10.1002/14651858.CD003794.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard SI, Tashkin DP, McElhattan J, et al. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trial. Drugs. 2009;69:549–65. doi: 10.2165/00003495-200969050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calverley PM, Boonsawat W, Cseke Z, et al. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003;22:912–19. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- Ohar JA, Crater GD, Emmett A, et al. Fluticasone propionate/salmeterol 250/50 µg versus salmeterol 50 µg after chronic obstructive pulmonary disease exacerbation. Respir Res. 2014;15:105. doi: 10.1186/s12931-014-0105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin DM, Gray J, Edwards SJ, et al. Budesonide/formoterol vs. salmeterol/fluticasone in COPD: a systematic review and adjusted indirect comparison of pneumonia in randomised controlled trials. Int J Clin Pract. 2011;65:764–74. doi: 10.1111/j.1742-1241.2011.02685.x. [DOI] [PubMed] [Google Scholar]
- Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;3:CD010115. doi: 10.1002/14651858.CD010115.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crim C, Dransfield MT, Bourbeau J, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann Am Thorac Soc. 2015;12:27–34. doi: 10.1513/AnnalsATS.201409-413OC. [DOI] [PubMed] [Google Scholar]
- Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1:210–23. doi: 10.1016/S2213-2600(13)70040-7. [DOI] [PubMed] [Google Scholar]
- • .Gershon AS, Campitelli MA, Croxford R, et al. Combination long-acting β-agonists and inhaled corticosteroids compared with long-acting β-agonists alone in older adults with chronic obstructive pulmonary disease. JAMA. 2014;312:1114–21. doi: 10.1001/jama.2014.11432. [DOI] [PubMed] [Google Scholar]; • This real-world study demonstrated no benefit in preventing COPD hospitalization or death for an ICS/long-acting β2-agonist (LABA) versus a LABA, in a mixed patient population containing both COPD and asthma–COPD overlap syndrome (ACOS). In patients with ACOS only, ICS/LABA treatment reduced hospitalization or death compared with a LABA alone.
- Rodrigo GJ, Castro-Rodriguez JA, Plaza V. Safety and efficacy of combined long-acting β-agonists and inhaled corticosteroids vs long-acting β-agonists monotherapy for stable COPD: a systematic review. Chest. 2009;136:1029–38. doi: 10.1378/chest.09-0821. [DOI] [PubMed] [Google Scholar]
- • .Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014;3:CD010844. doi: 10.1002/14651858.CD010844.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This network meta-analysis by the Cochrane group showed that ICS/LABA use increased serious adverse pneumonias without providing clinically important benefits in patients with COPD.
- Montuschi P, Macagno F, Valente S, et al. Inhaled muscarinic acetylcholine receptor antagonists for treatment of COPD. Curr Med Chem. 2013;20:1464–76. doi: 10.2174/0929867311320120002. [DOI] [PubMed] [Google Scholar]
- • .Wedzicha JA, Calverley PM, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177:19–26. doi: 10.1164/rccm.200707-973OC. [DOI] [PubMed] [Google Scholar]; • This study found no difference in overall exacerbation rates between ICS/LABA and long-acting muscarinic antagonist (LAMA) treatment in patients with severe or very severe COPD.
- Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146:545–55. doi: 10.7326/0003-4819-146-8-200704170-00152. [DOI] [PubMed] [Google Scholar]
- Welsh EJ, Cates CJ, Poole P. Combination inhaled steroid and long-acting beta2-agonist versus tiotropium for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;5:CD007891. doi: 10.1002/14651858.CD007891.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Urzo AD, Rennard SI, Kerwin EM, et al. Efficacy and safety of fixed-dose combinations of aclidinium bromide/formoterol fumarate: the 24-week, randomized, placebo-controlled AUGMENT COPD study. Respir Res. 2014;15:123. doi: 10.1186/s12931-014-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • .Maleki-Yazdi MR, Kaelin T, Richard N, et al. Efficacy and safety of umeclidinium/vilanterol 62.5/25 mcg and tiotropium 18 mcg in chronic obstructive pulmonary disease: Results of a 24-week, randomized, controlled trial. Respir Med. 2014;108:1752–60. doi: 10.1016/j.rmed.2014.10.002. [DOI] [PubMed] [Google Scholar]; • This study demonstrated that the fixed-dose combination of umeclidinium/vilanterol improved lung function and reduced time to first exacerbation versus tiotropium.
- Donohue JF, Maleki-Yazdi MR, Kilbride S, et al. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 µg in COPD. Respir Med. 2013;107:1538–46. doi: 10.1016/j.rmed.2013.06.001. [DOI] [PubMed] [Google Scholar]
- • .Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1:199–209. doi: 10.1016/S2213-2600(13)70052-3. [DOI] [PubMed] [Google Scholar]; • The SPARK study showed that QVA149 reduced exacerbation rates compared with glycopyrronium monotherapy in high-risk patients with COPD.
- Bateman ED, Ferguson GT, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J. 2013;42:1484–94. doi: 10.1183/09031936.00200212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, Jones PW, Bateman ED, et al. Efficacy and safety of aclidinium bromide/formoterol fumarate fixed-dose combinations compared with individual components and placebo in patients with COPD (ACLIFORM-COPD): a multicentre, randomised study. BMC Pulm Med. 2014;14:178. doi: 10.1186/1471-2466-14-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • .Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2–4) Eur Respir J. 2015;45:969–79. doi: 10.1183/09031936.00136014. [DOI] [PMC free article] [PubMed] [Google Scholar]; • In this trial, combined tiotropium/olodaterol improved lung function and health status versus tiotropium or olodaterol alone.
- Bateman ED, Mahler DA, Vogelmeier CF, et al. Recent advances in COPD disease management with fixed-dose long-acting combination therapies. Expert Rev Respir Med. 2014;8:357–79. doi: 10.1586/17476348.2014.910457. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Timmer W, Sagkriotis A, et al. Comparison of a combination of tiotropium plus formoterol to salmeterol plus fluticasone in moderate COPD. Chest. 2008;134:255–62. doi: 10.1378/chest.07-2138. [DOI] [PubMed] [Google Scholar]
- Magnussen H, Paggiaro P, Schmidt H, et al. Effect of combination treatment on lung volumes and exercise endurance time in COPD. Respir Med. 2012;106:1413–20. doi: 10.1016/j.rmed.2012.05.011. [DOI] [PubMed] [Google Scholar]
- • .Vogelmeier CF, Bateman ED, Pallante J, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med. 2013;1:51–60. doi: 10.1016/S2213-2600(12)70052-8. [DOI] [PubMed] [Google Scholar]; • The ILLUMINATE study demonstrated that QVA149 improved lung function and patient-reported outcomes compared with salmeterol/fluticasone propionate combination treatment.
- Bateman ED, Vogelmeier C, Chen H, et al. Comparison of COPD exacerbations with once-daily QVA149 versus twice-daily salmeterol/fluticasone combination: the ILLUMINATE study (Abstract) Chest. 2014;145(3_Meeting abstracts):409A. [Google Scholar]
- Donohue J, Worsley S, Zhu CQ, et al. Efficacy and safety of umeclidinium/vilanterol (UMEC/VI) once daily (OD) vs fluticasone/salmeterol combination (FSC) twice daily (BD) in patients with moderate-to-severe COPD and infrequent COPD exacerbations (Abstract) Chest. 2014;146(4_Meeting abstracts):73A. [Google Scholar]
- Clinicaltrials.gov. NCT01817764 (DB2114930) A study to compare the efficacy and safety of umeclidinium/vilanterol and fluticasone propionate/salmeterol in subjects with chronic obstructive pulmonary disease (COPD) 2013. http://clinicaltrials.gov/ct2/show/NCT01817764. Available from: [Last accessed 5 December 2013]
- Clinicaltrials.gov. NCT01879410 (DB2114951) A study to compare the efficacy and safety of umeclidinium/vilanterol and fluticasone propionate/salmeterol in subjects with chronic obstructive pulmonary disease (COPD) 2013. http://clinicaltrials.gov/ct2/show/NCT01879410. Available from: [Last accessed 5 December 2013]
- Donohue J, Worsley S, Zhu C-Q, et al. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate-to-severe COPD and infrequent exacerbations. Respir Med. 2015;109:870–81. doi: 10.1016/j.rmed.2015.04.018. [DOI] [PubMed] [Google Scholar]
- • .Zhong N, Wang C, Zhou X, et al. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int J COPD. 2015;10:1015–26. doi: 10.2147/COPD.S84436. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This 6-month trial showed that QVA149 improved lung function and exacerbation rates and had similar effects on patient-reported outcomes, compared with fluticasone/salmeterol, in patients with moderate-to-severe COPD and a history of up to one exacerbation in the previous year.
- Clinicaltrials.gov. NCT01782326 (FLAME; A2318) QVA vs. salmeterol/fluticasone, 52-week exacerbation study 2013. http://clinicaltrials.gov/ct2/show/NCT01782326. Available from: [Last accessed 5 December 2013]
- Clinicaltrials.gov. NCT02164513 A study comparing the efficacy, safety and tolerability of fixed dose combination (FDC) of FF/UMEC/VI with the FDC of FF/VI and UMEC/VI; administered once-daily via a dry powder inhaler (DPI) in subjects with chronic obstructive pulmonary disease (COPD) 2014. http://clinicaltrials.gov/ct2/show/NCT02164513. Available from: [Last accessed 4 December 2014]
- US Food and Drug Administration Center for Drug Evaluation and Research Division of Pulmonary and Allergy Drug Products (HFD-570) Backgrounder for sNDA 21-077 - Advair Diskus 250/50, Advair Diskus 500/50. http://www.fda.gov/ohrms/dockets/ac/02/briefing/3830b1_02_Glaxo-ADVAIR.pdf. Available from: [Last accessed 20 January 2015]
- European Medicines Agency (EMA) Committee for medicinal products for human use (CHMP) assessment report. Relvar Ellipta. international non-proprietary name: fluticasone furoate/ vilanterol. Procedure No. EMEA/H/C/002673/0000 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002673/WC500157635.pdf. Available from: [Last accessed 15 December 2014]
- Postma DS, Roche N, Colice G, et al. Comparing the effectiveness of small-particle versus large-particle inhaled corticosteroid in COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:1163–86. doi: 10.2147/COPD.S68289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• .Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Spanish guideline for COPD (GesEPOC). Update 2014. Arch Bronconeumol. 2014;50(Suppl 1):1–16. doi: 10.1016/S0300-2896(14)70070-5. [DOI] [PubMed] [Google Scholar]; •• This paper provides guidelines for the treatment of stable COPD based on clinical phenotypes.
- Barrecheguren M, Esquinas C, Miravitlles M. The asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challenges. Curr Opin Pulm Med. 2015;21:74–9. doi: 10.1097/MCP.0000000000000118. [DOI] [PubMed] [Google Scholar]
- Hardin M, Cho M, McDonald ML, et al. The clinical and genetic features of COPD-asthma overlap syndrome. Eur Respir J. 2014;44:341–50. doi: 10.1183/09031936.00216013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • .Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–38. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]; • This analysis of over 2000 patients in the ECLIPSE study showed that a history of exacerbations is the most important predictor of future exacerbation risk.
- Martinez FJ, Calverley PM, Goehring UM, et al. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet. 2015;385:857–66. doi: 10.1016/S0140-6736(14)62410-7. [DOI] [PubMed] [Google Scholar]
- McGarvey L, Lee AJ, Roberts J, et al. Characterisation of the frequent exacerbator phenotype in COPD patients in a large UK primary care population. Respir Med. 2015;109:228–37. doi: 10.1016/j.rmed.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Vogelmeier C, Buhl R, Criee CP, et al. Frequency of COPD exacerbations in the real-life out-patient DACCORD cohort (Abstract) Eur Respir J. 2014;44(Suppl 58):P930. [Google Scholar]
- Bleecker ER, Emmett A, Crater G, et al. Lung function and symptom improvement with fluticasone propionate/salmeterol and ipratropium bromide/albuterol in COPD: response by beta-agonist reversibility. Pulm Pharmacol Ther. 2008;21:682–8. doi: 10.1016/j.pupt.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Brightling CE, McKenna S, Hargadon B, et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax. 2005;60:193–8. doi: 10.1136/thx.2004.032516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstjens HA, Overbeek SE, Schouten JP, et al. Airways hyperresponsiveness, bronchodilator response, allergy and smoking predict improvement in FEV1 during long-term inhaled corticosteroid treatment. Dutch CNSLD Study Group. Eur Respir J. 1993;6:868–76. [PubMed] [Google Scholar]
- Leigh R, Pizzichini MM, Morris MM, et al. Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment. Eur Respir J. 2006;27:964–71. doi: 10.1183/09031936.06.00072105. [DOI] [PubMed] [Google Scholar]
- Leuppi JD, Tandjung R, Anderson SD, et al. Prediction of treatment-response to inhaled corticosteroids by mannitol-challenge test in COPD. A proof of concept. Pulm Pharmacol Ther. 2005;18:83–8. doi: 10.1016/j.pupt.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Papi A, Romagnoli M, Baraldo S, et al. Partial reversibility of airflow limitation and increased exhaled NO and sputum eosinophilia in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:1773–7. doi: 10.1164/ajrccm.162.5.9910112. [DOI] [PubMed] [Google Scholar]
- Dummer JF, Epton MJ, Cowan JO, et al. Predicting corticosteroid response in chronic obstructive pulmonary disease using exhaled nitric oxide. Am J Respir Crit Care Med. 2009;180:846–52. doi: 10.1164/rccm.200905-0685OC. [DOI] [PubMed] [Google Scholar]
- Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186:48–55. doi: 10.1164/rccm.201108-1553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siva R, Green RH, Brightling CE, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J. 2007;29:906–13. doi: 10.1183/09031936.00146306. [DOI] [PubMed] [Google Scholar]
- • .DiSantostefano RL, Li H, Rubin DB, et al. Which patients with chronic obstructive pulmonary disease benefit from the addition of an inhaled corticosteroid to their bronchodilator? A cluster analysis. BMJ Open. 2013;3:e001838. doi: 10.1136/bmjopen-2012-001838. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This analysis identified characteristics in patients with COPD that were associated with response to ICS treatment.
- • .Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184:662–71. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]; • This study showed that sputum eosinophilia during an exacerbation acts as a biomarker for a eosinophilia-associated exacerbation phenotype.
- Liesker JJ, Bathoorn E, Postma DS, et al. Sputum inflammation predicts exacerbations after cessation of inhaled corticosteroids in COPD. Respir Med. 2011;105:1853–60. doi: 10.1016/j.rmed.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Freeman D, Price D, Jones R, et al. Predicting patients with COPD who are likely to exacerbate - is it feasible in primary care? (Abstracts from the respiratory effectiveness group’s inaugural summit, The Royal College of General Practitioners, London, 28-29 June 2014) NPJ Prim Care Respir Med. 2014;24:Abstract 06. [Google Scholar]
- Singh D, Kolsum U, Brightling CE, et al. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44:1697–700. doi: 10.1183/09031936.00162414. [DOI] [PubMed] [Google Scholar]
- Choudhury AB, Dawson CM, Kilvington HE, et al. Withdrawal of inhaled corticosteroids in people with COPD in primary care: a randomised controlled trial. Respir Res. 2007;8:93. doi: 10.1186/1465-9921-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarad NA, Wedzicha JA, Burge PS, et al. An observational study of inhaled corticosteroid withdrawal in stable chronic obstructive pulmonary disease. ISOLDE Study Group. Respir Med. 1999;93:161–6. doi: 10.1016/s0954-6111(99)90001-x. [DOI] [PubMed] [Google Scholar]
- van der Valk P, Monninkhof E, van der Palen J, et al. Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: the COPE study. Am J Respir Crit Care Med. 2002;166:1358–63. doi: 10.1164/rccm.200206-512OC. [DOI] [PubMed] [Google Scholar]
- Kardos P. Methodological issues in therapeutic trials of COPD. Eur Respir J. 2009;33:443–4. doi: 10.1183/09031936.00138808. [DOI] [PubMed] [Google Scholar]
- Suissa S, Ernst P, Vandemheen KL, et al. Methodological issues in therapeutic trials of COPD. Eur Respir J. 2008;31:927–33. doi: 10.1183/09031936.00098307. [DOI] [PubMed] [Google Scholar]
- • .Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371:1285–94. doi: 10.1056/NEJMoa1407154. [DOI] [PubMed] [Google Scholar]; • The 12-month WISDOM study demonstrated that gradual withdrawal of ICS during concomitant LABA/LAMA treatment did not increase exacerbation rates in patients at high risk of exacerbation.
- Reilly JJ. Stepping down therapy in COPD. N Engl J Med. 2014;371:1340–1. doi: 10.1056/NEJMe1409219. [DOI] [PubMed] [Google Scholar]
- Rossi A, Guerriero M, Corrado A. Withdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO) Respir Res. 2014;15:77. doi: 10.1186/1465-9921-15-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, van der Molen T, del Olmo R, et al. INSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44:1548–56. doi: 10.1183/09031936.00126814. [DOI] [PubMed] [Google Scholar]
- Haughney J, Gruffydd-Jones K, Roberts J, et al. The distribution of COPD in UK general practice using the new GOLD classification. Eur Respir J. 2014;43:993–1002. doi: 10.1183/09031936.00065013. [DOI] [PubMed] [Google Scholar]
- Martinez-Garcia MÁ, Soler-Cataluña JJ, Donat SY, et al. Factors associated with bronchiectasis in patients with COPD. Chest. 2011;140:1130–7. doi: 10.1378/chest.10-1758. [DOI] [PubMed] [Google Scholar]
- O’Brien C, Guest PJ, Hill SL, et al. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax. 2000;55:635–42. doi: 10.1136/thorax.55.8.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel IS, Vlahos I, Wilkinson TM, et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:400–7. doi: 10.1164/rccm.200305-648OC. [DOI] [PubMed] [Google Scholar]
- Singh D, Worsley S, Zhu C, et al. Umeclidinium/vilanterol (UMEC/VI) once daily (OD) vs fluticasone/salmeterol combination (FSC) twice daily (BD) in patients with moderate-to-severe COPD and infrequent COPD exacerbations (Poster P290). European Respiratory Society International Congress; 6-10 September 2014; Munich, Germany. [Google Scholar]
- Han MK, Muellerova H, Curran-Everett D, et al. GOLD 2011 disease severity classification in COPDGene: a prospective cohort study. Lancet Respir Med. 2013;1:43–50. doi: 10.1016/S2213-2600(12)70044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange P, Marott JL, Vestbo J, et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med. 2012;186:975–81. doi: 10.1164/rccm.201207-1299OC. [DOI] [PubMed] [Google Scholar]
- Agusti A, Edwards LD, Celli B, et al. Characteristics, stability and outcomes of the 2011 GOLD COPD groups in the ECLIPSE cohort. Eur Respir J. 2013;42:636–46. doi: 10.1183/09031936.00195212. [DOI] [PubMed] [Google Scholar]
- Agusti A, Hurd S, Jones P, et al. FAQs about the GOLD 2011 assessment proposal of COPD: a comparative analysis of four different cohorts. Eur Respir J. 2013;42:1391–401. doi: 10.1183/09031936.00036513. [DOI] [PubMed] [Google Scholar]