Abstract
Objective. Iron isomaltoside 1000 (Monofer®) is a high-dose intravenous (IV) iron, which in a recent 8 weeks trial in inflammatory bowel disease (IBD) subjects with iron deficiency anemia (IDA) demonstrated good tolerability and efficacy. The present trial is an extension to this trial, which evaluates the need for additional high IV iron doses to maintain a stable hemoglobin (Hb) ≥12.0 g/dl. Material and methods. This was a prospective, open-label, 12 months trial of European IBD subjects willing to participate after completing the lead-in trial. Subjects were allowed re-dosing with 500–2000 mg single doses of iron isomaltoside 1000 infused over ∼15 min at 3 months intervals depending on a predefined algorithm. Outcome measures included Hb, safety parameters and need for additional iron dosing. Results. A total of 39 subjects were enrolled of which 34 subjects required re-dosing with a median cumulative 1-year dose of 1.8 g (mean cumulative dose 2.2 g). The mean (SD) Hb was 12.3 (1.5) g/dl at baseline, 12.8 (1.6) g/dl at 3 months, 12.8 (1.6) g/dl at 6 months, 12.9 (1.4) g/dl at 9 months and 12.9 (1.6) g/dl at 12 months. Seventy-four percent of subjects who had an Hb ≥12.0 g/dl at baseline were able to maintain Hb ≥12.0 g/dl till the end of the trial at 12 months. Nonserious probably related hypersensitivity reactions without significant hypotension were reported at the beginning of the infusion in two subjects, who recovered without sequelae. Conclusion. Repeated treatment of iron deficiency with iron isomaltoside 1000 could avoid episodes of IDA without major safety issues.
Trial registration: ClinicalTrial.gov identifier: NCT01410435.
Key Words: anemia, ferric derisomaltose, inflammatory bowel disease, intravenous iron, iron deficiency
Introduction
Iron deficiency anemia (IDA) is one of the most common causes of anemia in patients with inflammatory bowel disease (IBD), leading to a significant deterioration in patient’s quality of life (QoL) [1–5]. The prevalence of IDA has been reported to be 36–76% in IBD [6]. Poor absorption and intolerance often limit the use of oral iron supplementation in IBD patients [5,7]. Therefore, the intravenous (IV) route is the preferred route of iron supplementation in these patients with moderate-to-severe IDA [8–11]. Systemic iron treatment not only normalizes the hemoglobin (Hb) but also improves the iron status (s-ferritin and transferrin saturation [TSAT]) of an anemic patient [12]. However, the frequency of IDA recurrence has been found quite high in IBD patients even after systemic iron treatment [10,13]. Hence, after initial resolution of anemia and repletion of iron stores, the patient’s hematological and iron parameters should be carefully and periodically monitored, and maintenance iron treatment should be initiated as required [14]. The present trial is an extension to the PROCEED lead-in trial [7] where the long-term iron need as well as the safety of iron isomaltoside 1000 (Monofer, Pharmacosmos A/S, Holbaek, Denmark) and its ability to maintain stable Hb in iron-treated IBD subjects have been evaluated over a 12 months period.
Methods
Ethical considerations
The trial protocol was approved by local ethics committees and competent authorities and the trial was conducted in accordance with International Conference on Harmonization guideline for good clinical practice and the Declaration of Helsinki of 1975, as revised in 1983. The trial was registered on ClinicalTrial.gov (NCT01410435). The subjects were informed by the investigator of the risks and benefits of the trial. The subjects were informed that they could withdraw from the trial at any time for any reason. Informed consent was obtained in writing prior to any trial-related activities.
Trial design
This prospective, open-label, nonrandomized, Good Clinical Practice trial was an extension of the PROCEED lead-in trial [7]. The trial was conducted at one site in Austria and two sites in Hungary from June 2011 to July 2013. The enrolment period of the trial was 6 months (June–November 2011) and the projected trial duration for an individual subject was 12 months where each subject attended five visits – one screening/baseline visit (visit 1), three treatment/follow-up visits (visit 2 [3 months], visit 3 [6 months], visit 4 [9 months]) and one end-of-trial (EOT) visit (visit 5 [12 months]). Subjects were allowed re-dosing with 500–2000 mg single-dose infusions of iron isomaltoside 1000 over ∼15 min at the above-mentioned visits depending on a predefined algorithm (Table I) based upon Hb, TSAT and s-ferritin levels. Iron isomaltoside 1000 was administered according to either an IDA dosing regimen based on Hb and body weight or a maintenance ID dosing regimen if Hb was ≥12.0 g/dl based on TSAT and s-ferritin (Table I). The infusion was prepared by diluting iron isomaltoside 1000 in 100 ml normal saline (0.9% sodium chloride).
Table I.
Dosing regimen.
| Iron deficiency anemia dosing regime | Body weight <70 kg | Body weight ≥70 kg |
|---|---|---|
| Hemoglobin | ||
| 10.0 g/dl ≤Hb <12.0 g/dl | 1000 mg | 1500 mg |
| Hb <10.0 g/dl | 1500 mg | 2000 mg |
| Iron deficiency dosing regime | Body weight <70 kg | Body weight ≥70 kg |
|---|---|---|
| Iron status | ||
| TSAT <20% and 100 µg/l <s-ferritin <500 µg/l | 500 mg | 1000 mg |
| TSAT <20% and s-ferritin ≤100 µg/l | 1000 mg | 1500 mg |
Abbreviations: Hb = Hemoglobin; TSAT = Transferrin saturation.
Note: Subjects with Hb <12.0 g/dl, TSAT <20%, and s-ferritin <500 µg/L at any visit except at end of study visit 5 were administered a single dose as iron deficiency anemia dosing regime and subjects with Hb ≥12.0 g/dl, TSAT <20%, and s-ferritin <500 µg/L at any visit except visit 5 were administered a dose as per the iron deficiency dosing regimen.
Participants
Subjects who either completed the lead-in trial or were discontinued from the lead-in trial due to intolerance to oral iron, and had life expectancy beyond 18 months by investigator’s judgment, together with willingness to provide written informed consent were considered eligible to participate in the trial. The exclusion criteria were discontinuation from the lead-in trial (unless the reason was intolerance to oral therapy), any major protocol deviation in the lead-in trial, pregnant or nursing women, any other medical condition that in the opinion of the investigator may have caused the subject to be unsuitable for completion of the trial or placed the subject at potential risk from being in the trial, or had Harvey–Bradshaw Index (HBI) >8 or partial Mayo score (pMS) (excluding endoscopy sub-score) >6 at the EOT visit of the lead-in trial.
During the trial, any concomitant medications or treatments deemed necessary to provide adequate supportive care were allowed. The subjects were prohibited from having a blood transfusion, erythropoiesis-stimulating agent treatment and any iron supplementation other than the investigational drug as this would influence the outcome measures of the trial.
Outcomes
The primary end point of the trial was to assess the number of subjects who maintained Hb ≥12.0 g/dl. Subjects with an Hb ≥12.0 g/dl at baseline needed to maintain Hb ≥12.0 g/dl at all trial visits, whereas subjects with an Hb <12.0 g/dl at baseline needed to achieve and maintain Hb ≥12.0 g/dl from month 3 and onward at all visits in order to meet the primary end point. The secondary end points included dosage of iron isomaltoside 1000 re-administered (if required), frequency of additional dosing of iron isomaltoside 1000 (if required), change in concentrations of s-iron, s-ferritin total iron-binding capacity (TIBC), and TSAT from baseline to 12 months, change in total QoL score from baseline to 6 months and 12 months as measured by IBD questionnaire, and change in disease activity status using HBI for Crohn’s disease or pMS for ulcerative colitis from baseline to 6 and 12 months. The safety end points included the assessment of adverse events (AEs), vital signs, electrocardiogram, s-phosphate and other safety biochemistry parameters.
Sample size
No sample size calculations were performed. The extension trial was only offered to subjects from participating sites in Austria and Hungary in the lead-in trial.
Statistical methods
The following data sets were used in the analyses:
The full analysis set (FAS) consisted of all subjects who were included in the trial and had at least one post-baseline Hb assessment. The per protocol set (PP) consisted of all subjects in the FAS who did not have any major protocol deviation. The safety analysis set consisted of all subjects who attended visit 1 and were enrolled in the trial.
The primary analysis on primary end point was conducted on the FAS and PP analysis sets. The analyses on secondary end points and two exploratory efficacy analyses not specified in the protocol (efficacy analysis on maintenance of Hb ≥12.0 g/dl in subjects with baseline Hb ≥12.0 g/dl or reaching Hb ≥12.0 g/dl during the trial and the maintenance of change in Hb ≥2.0 g/dl in subjects that had a response of Hb ≥2.0 g/dl at any visit in the lead-in trial) were conducted on the FAS, whereas dosage and frequency of additional iron isomaltoside 1000 and the safety analyses were conducted on the safety analysis set.
The primary, secondary and exploratory analyses were summarized descriptively. Kaplan–Meier plot was used to display results of primary and exploratory analyses. Paired t-test was used to assess changes in concentrations of s-iron, s-ferritin, TIBC, TSAT, total QoL score and C-reactive protein (exploratory analysis). A two-sample t-test was used to assess differences in single doses and cumulative doses, and a Fisher’s exact test was used to assess differences in number of doses between patients with baseline HB </≥12 g/dl (exploratory analyses).
All statistical tests were two-sided and the significance level was 0.05.
Results
Subjects
A total of 39 subjects (31 with Crohn’s disease and 8 with ulcerative colitis) from the lead-in trial provided their consent and were enrolled for this extension trial. The first subject first visit was on June 7, 2011 and the last subject last visit was on July 11, 2013. Of 39 enrolled subjects, 24 (61.5%) subjects completed the trial and 15 (38.5%) subjects were discontinued due to: withdrawal of consent (7 subjects), lost to follow-up (3 subjects), withdrawn due to an AE (hypersensitivity reaction) (1 subject), received investigational drug from another clinical trial (1 subject), as per investigator’s decision (1 subject), intolerance to iron isomaltoside 1000 (i.e., hypersensitivity) (1 subject), or were unable to attend the scheduled visits (1 subject). None of the seven subjects who withdrew their consent experienced an AE that was related to the trial drug. A total of 39 subjects were included in the safety analysis set, 35 subjects in the FAS and 25 in the PP analysis set.
Four subjects were excluded from the FAS as they did not provide a post-baseline Hb measurement due to the following reasons: three subjects withdrew consent before a post baseline Hb and one subject was receiving another investigational drug concurrently and therefore was withdrawn from the trial before a post-baseline Hb.
The subject demographics are summarized in Table II. The median age of the trial population was 36 years (range 19–67 years). Overall, more women (76.9%) than men (23.1%) participated in the trial and the majority of the subjects were white (92.3%). A higher proportion of subjects had Crohn’s disease than ulcerative colitis (79.5% vs. 20.5%).
Table II.
Subject demographics.
| Parameters | Statistics/category | Overall (N = 39) |
|---|---|---|
| Gender |
Men | 9 (23.1) |
| Women | 30 (76.9) | |
| Age (years) |
n | 39 |
| Median (range: min:max) | 36 (19:67) | |
| Ethnic origin, n (%) | White | 36 (92.3) |
| Black | 1 (2.6) | |
| Asian | 1 (2.6) | |
| Hispanic | 1 (2.6) | |
| Weight (kg) |
n | 39 |
| Median (range: min:max) | 65 (46:110) | |
| Disease type and score, n (%) | Crohn’s disease | 31 (79) |
| Ulcerative colitis | 8 (21) | |
| Harvey-Bradshaw index: median (range: min:max) | 2 (0:5) | |
| Partial Mayo score: median (range: min:max) | 2 (0:6) | |
| C-reactive protein (mg/L, reference range: 0–5 mg/l) | ||
| Mean (SD) | 8.8 (10.8) | |
Abbreviations: Max: Maximum; Min: Minimum.
Iron needs – dosage of iron isomaltoside 1000 re-administered
Of 39 subjects enrolled, 34 (92.3%) required re-dosing with iron isomaltoside 1000 at 1 or more visit(s) during the trial (median number of doses 2.0: [range: 1–4 doses]). A total of 68 doses of iron isomaltoside 1000 were given as single doses, 81% of these were ≥1000 mg and 34% were ≥1500 mg. At baseline of the extension trial, 12 subjects received a dose for IDA and 14 for ID (Table III). In total out of 68 doses, 31 (46%, including 3 doses based on the IDA criteria without fulfilling them) were given based on the IDA criteria and 37 doses (54%) were based on the ID criteria both with a median single dose of 1000 mg. Overall, the median cumulative dose re-administered was 1750 mg (mean 2.192 mg, range 30–7000 mg), where subjects with Crohn’s disease received a median cumulative dose of 2000 mg (30–7000 mg) and subjects with ulcerative colitis received a median cumulative dose of 1500 mg (range: 1000–4000 mg). Subjects who had an Hb ≥12.0 g/dl at baseline received a median number of 1 dose (range: 1–4 doses), a median single dose of 1000 mg (range: 30–2000 mg) and a median cumulative dose of 1500 mg (range: 30–4500 mg). Subjects who had an Hb <12.0 g/dl at baseline received a median number of 3 doses (range: 1–4 doses), a median single dose of 1000 mg (range: 667–1750 mg), and a median cumulative dose of 3000 mg (range: 1000–7000 mg). There was a statistical difference in the number of doses (p = 0.03) and the mean cumulative dose (p = 0.004) between the subjects who had an Hb ≥12.0 g/dl compared to those with an Hb <12.0 g/dl, whereas no statistical difference was observed in the mean single dose (p = 0.26).
Table III.
Summary of iron isomaltoside 1000 dose re-administered at each visit – safety analysis set.
| Visit | Statistics | Dosing according to IDA criteria | Dosing according to ID criteria | Total dosing* (N = 39) |
|---|---|---|---|---|
| Total dose administered (mg) | n | - | - | 34 |
| Median | - | - | 1750 | |
| Range (min: max) | - | - | (30:7000) | |
| Screening/baseline | n | 12 | 14 | 27 |
| Median | 1250 | 1000 | 1000 | |
| Range (min: max) | (1000:1500) | (500:2000) | (500:2000) | |
| At 3 months | n | 6 | 9 | 16 |
| Median | 1000 | 1000 | 1000 | |
| Range (min: max) | (1000:2000) | (30:1500) | (30:2000) | |
| At 6 months | n | 6 | 6 | 13 |
| Median | 1250 | 1000 | 1000 | |
| Range (min: max) | (1000:2000) | (500:1500) | (500:2000) | |
| At 9 months | n | 4 | 8 | 12 |
| Median | 1250 | 750 | 1000 | |
| Range (min: max) | (1000:1500) | (500:1500) | (500:1500) |
Abbreviations: ID = Iron deficiency; IDA = Iron deficiency anemia; Max: Maximum; Min: Minimum.
*One patient was dosed but did not fulfill any of the specified dosing criteria. The patient was dosed with 1000 mg iron isomaltoside 1000 at baseline, month 3, and month 6.
The median single dose across visits was 1000 mg (Table III).
Efficacy
Primary analyses
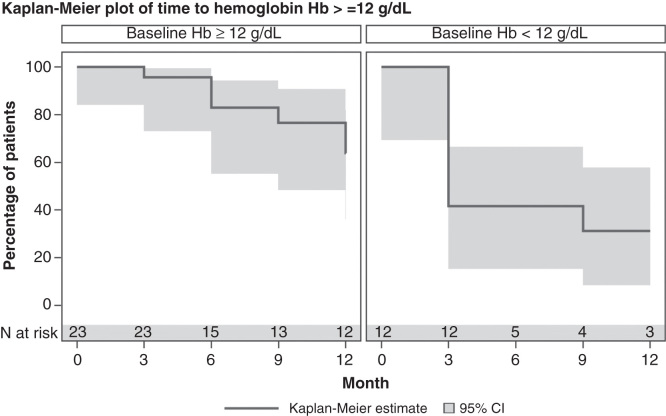
A total of 23 (65.7%) subjects had an Hb ≥12.0 g/dl and 12 (34.3%) subjects had an Hb <12.0 g/dl at baseline (FAS). Seventy-four percent of subjects who had an Hb ≥12.0 g/dl at baseline were able to maintain Hb ≥12.0 g/dl till the end of the trial at 12 months (crude last observation carried forward [LOCF] estimate). Thirty-three percent of subjects who had an Hb <12.0 g/dl at baseline were able achieve an Hb ≥12.0 g/dl at 3 month and maintain Hb ≥12.0 g/dl hereafter (crude LOCF estimate). Kaplan–Meier plots are given in Figure 1.
Figure 1.
Time to hemoglobin <12.0 g/dl.
Secondary analyses
Hemoglobin
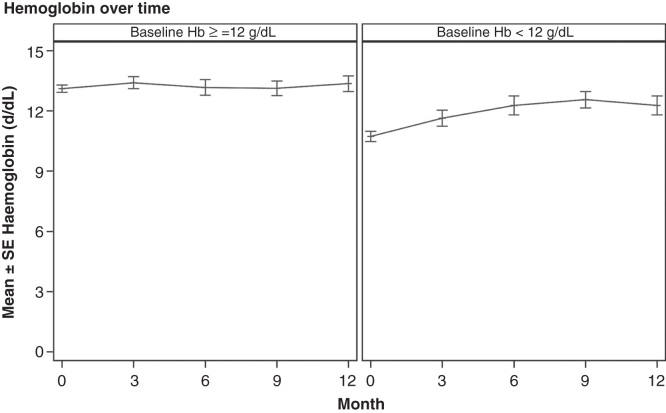
The mean (SD) Hb was 12.3 (1.5) g/dl at baseline, 12.8 (1.6) g/dl at 3 months, 12.8 (1.6) g/dl at 6 months, 12.9 (1.4) g/dl at 9 months and 12.9 (1.6) g/dl at 12 months. Hb values over time are given in Figure 2.
Figure 2.
Hemoglobin over time.
S-iron, s-ferritin, TIBC and TSAT
There was a rapid increase in s-iron, s-ferritin and TSAT concentration from baseline to 3 months followed by a gradual increase at 6, 9 and 12 months. There was a statistical significant increase in s-iron (p = 0.003), s-ferritin (p < 0.001) and TSAT (p < 0.001) concentration from baseline to 12 months. There was a decrease in TIBC concentration from baseline to 3, 6, 9 and 12 months but the decrease in TIBC concentration from baseline to 12 months was not statistical significant (p = 0.21) (Table IV).
Table IV.
Summary of serum iron, serum ferritin, total iron binding concentration, and transferrin saturation concentration at each visit – full analysis set.
| Visits | Concentration/Change in concentration |
|||
|---|---|---|---|---|
|
S-iron (µg/dl) |
S-ferritin (mcg/L) |
TIBC (µg/dl) |
TSAT (%) |
|
| At baseline | ||||
| n | 35 | 35 | 35 | 35 |
| Median (range: min:max) | 45.00 (11:191) | 32.00 (5:514) | 345.00 (229:505) | 12.00 (2:38) |
| Change in concentration from baseline to | ||||
| 3 months | ||||
| n | 34 | 34 | 34 | 34 |
| Median (range: min:max) | 10.50 (−133:170) | 46.00 (−207:464) | −17.00 (−127:88) | 5.50 (−25:53) |
| 6 months | ||||
| n | 27 | 27 | 27 | 27 |
| Median (range: min:max) | 23.00 (−34:101) | 117.00 (−12:734) | −6.00 (−135:120) | 9.00 (−9:31) |
| 9 months | ||||
| n | 25 | 25 | 25 | 25 |
| Median (range: min:max) | 19.00 (−45:98) | 102.00 (−10:568) | −16.00 (−153:119) | 5.00 (−11:31) |
| 12 months (end of study) | ||||
| n | 26 | 26 | 26 | 26 |
| Median (range: min:max) | 17.00 (−43:115) | 132.50 (−36:660) | −6.50 (−108:119) | 6.50 (−9:25) |
| p-Value | 0.003 | <0.001 | 0.21 | <0.001 |
Abbreviations: Max: Maximum; Min: Minimum; TIBC = Total iron binding capacity; TSAT = Transferrin saturation.
QoL score and disease activity status
There was a nonstatistical significant increase in total QoL score from a mean score of 175 at baseline to 178 at 6 months and 177 at 12 months (6 months: p = 0.48; 12 months: p = 0.19).
There were no major changes in clinical disease activity from baseline (median: HBI: 1.00; pMS: 2.00) to 6 months (median: HBI: 1.00; pMS: 1.00) and 12 months (HBI: 1.00; pMS: 1.00). No significant change in C-reactive protein was observed from baseline to 12 months (p = 0.91).
Exploratory efficacy analyses
Maintenance of Hb ≥12.0 g/dl in subjects who enrolled with a baseline Hb ≥12.0 g/dl or achieved Hb ≥12.0 g/dl during the trial
Overall, 32 subjects had an Hb ≥12.0 g/dl at baseline or any follow-up visit. Of these, 24 (75%) subjects were able to maintain Hb ≥12.0 g/dl at 12 months during the trial and the probability of maintaining Hb ≥12.0 g/dl at 12 months was 62.8%. The remaining 8 (25%) subjects had an Hb <12.0 g/dl at any follow-up visit.
Achievement and maintenance of change in Hb ≥2.0 g/dl in subjects who had a response of Hb ≥2.0 g/dl at any visit in lead-in trial
A total of 23 subjects had a response of Hb ≥2.0 g/dl at any visit in the lead-in trial; 17 subjects maintained a change of Hb ≥2.0 g/dl at any visit and the remaining 6 subjects had a change in Hb <2.0 g/dl during the trial. The probability of maintaining a change in Hb ≥2.0 g/dl at 12 months was 70.7%. The 23 subjects, who had a response of Hb ≥2.0 g/dl at any visit in the lead-in trial, received a median cumulative dose of 2000 mg (range: 30–7000 mg).
Safety
All safety analyses were conducted on the safety analysis set (N = 39).
A total of 57 AEs were reported by 26 (66.7%) subjects during the trial (Table V). A total of 96.5% of the AEs were according to the investigator rated as not related or unlikely related to the trial drug and only two nonserious AEs with moderate severity (hypersensitivity reactions) were rated as probable related to iron isomaltoside 1000 by the investigators. In one case, a 31-year-old man with Crohn’s disease was scheduled to receive a dose of 1500 mg iron isomaltoside 1000 at the 3-month visit. However, the subject developed flush, dyspnea and dropped in oxygen saturation (without significant hypotension) after receiving 30 mg of iron isomaltoside 1000. In the other case, a 37-year-old woman with ulcerative colitis and a medical history of adalimumab, infliximab and penicillin allergy had previously been dosed with 1000 mg of iron isomaltoside 1000 at visit 2 without any reaction. The subject was scheduled to receive a dose of 1000 mg iron isomaltoside 1000 at the 9-month visit. However, she developed flush, nausea and chest pain (without significant hypotension) after receiving an unknown amount of iron isomaltoside 1000 at this visit. On follow-up the subjects recovered without sequelae. The trial drug was stopped in these two subjects and both subjects were withdrawn from the trial.
Table V.
Adverse events.
| Iron isomaltoside 1000 (N = 39), n (%) |
|
|---|---|
| Total number of AEs reported | 57 |
| Subjects reporting any AEs | 26 (66.7) |
| Subjects reporting 1 AE | 9 (23.1) |
| Subjects reporting >1 AE | 17 (43.6) |
| Subjects reporting no AEs | 13 (33.3) |
| Number of AEs with severity of | |
| Mild | 28 (49.1) |
| Moderate | 28 (49.1) |
| Severe | 1 (1.8) |
| Number of AEs with relationship of | |
| Probable | 2 (3.5) |
| Possible | - |
| Unlikely | 4 (7.0) |
| Not related | 51 (89.5) |
| Number of AEs by outcome | |
| Recovered without sequelae | 42 (73.7) |
| Recovered with sequelae* | 3 (5.3) |
| Ongoing, follow-up not necessary | 12 (21.1) |
| Ongoing, follow-up necessary | - |
| Unknown | - |
| Number of AEs by action taken | |
| Drug stopped permanently | 2 (3.5) |
| None | 54 (94.7) |
| Unknown | - |
| Number of AEs with seriousness | |
| Nonserious | 53 (93.0) |
| Serious | 4 (7.0) |
| Subjects reporting AEs leading to withdrawal | 1 (2.6) |
| Number of AEs with fatal outcome | - |
Abbreviation: AE = Adverse event.
* A total of three unrelated AEs were recovered with sequelae: multiple sclerosis (the sequelae was numbness in fingers and feet), hemorrhoids (the sequelae was worsening in pain) and ulcerative colitis (the sequelae was bloody stools and increased bowel movement).
Four serious adverse events (perianal abscess, miliary tuberculosis, nephrolithiasis and worsening of ulcerative colitis) were observed during the trial. All serious adverse events were nonrelated to iron isomaltoside 1000. None of the AEs was fatal.
Hypophosphatemia was not reported for any of the subjects. None of the clinical significant abnormalities in C-reactive protein, alanine aminotransferase and aspartate aminotransferase (one subject), and white blood cell (one subject with elevated counts compared to previous visit) were found to be an AE. One subject was found to be pregnant at 12 months and was accordingly followed-up for safety.
Discussion
IDA, the main cause of anemia in patients with IBD, recurs frequently and rapidly even after iron-replacement therapy [10,13]. Improvement in Hb and iron status can be achieved with IV iron and is associated with improved QoL [4,7,11,12,15]. Once anemia has resolved and iron stores are replenished patients should be closely monitored for Hb and iron status, and maintenance iron treatment should be provided as required in order to avoid further anemic episodes [14,16]. Most of the trials with IV iron in IBD subjects have been of 4–12 weeks duration. However, trials assessing the need of long-term IV iron supplementation are limited. British Society of Gastroenterology guidelines on the management of IDA also recommended to monitor Hb indices and iron status every 3 months for a year and again after a year once the Hb concentration is normalized and iron stores are replenished [16]. Therefore, there is a need for more long-term trials to follow-up the iron therapy beyond complete replenishment of iron and to assess the requirement of any maintenance iron therapy.
In the present trial, 74% of the subjects with baseline Hb ≥12.0 g/dl and 33% of the subjects with baseline Hb <12.0 g/dl were able to maintain Hb ≥12.0 g/dl over the 12 months trial period, suggesting the ability of iron isomaltoside 1000 to maintain a stable Hb in the majority of IBD subjects for a period of 12 months. These data are in line with previous findings of Evstatiev et al., who showed that ∼28% of nonanemic patients treated with ferric carboxymaltose became anemic during an 8-month period [13].
A median number of 1 infusion and a median cumulative dose of 1500 mg iron isomaltoside 1000 were necessary to maintain an Hb ≥12 g/dl among subjects who entered the study with a baseline Hb ≥12 g/dl. Subjects who entered the study with a baseline Hb <12 g/dl received a statistical significant higher number of infusions and higher median cumulative dose. Despite this they were not able to maintain Hb ≥12 g/dl to the same extent. Even more aggressive dosing may be necessary in these subjects with a baseline Hb <12 g/dl. In subjects, who were not able to attain or maintain an Hb ≥12.0 g/dl, the dosing criteria applied may not have been sufficient to compensate for inflammation and iron loss, which may advocate for even higher dosing.
Repetitive long-term re-dosing of iron isomaltoside 1000 showed a good safety profile. The majority of the AEs were mild or moderate and not related to iron isomaltoside 1000. Two probable-related AEs (hypersensitivity reactions) were observed in the trial; both were nonserious, moderate in severity and led to subject discontinuation. The characteristics of these nonserious hypersensitivity reactions are in line with previously reported trials utilizing different IV iron compounds in IBD [7,12]. Interestingly, hypophosphatemia, which has been reported with other IV irons [17], was not reported in the present trial. None of the clinically significant laboratory or physical abnormalities was reported as AEs.
Although the open-label design may be considered as a potential weakness, the primary end point was biochemical and was unlikely to be affected by the subjects’ or trial personnel’s awareness of the treatment. Besides, the iron treatment strategy was strictly given. This design has been used in various other IBD trials [12,15]. A bias on the physician’s judgment of disease activity or of drug-related AEs cannot be excluded.
In conclusion, our data suggest that repeated treatment of ID in subjects with IBD could avoid episodes of IDA without major safety issues.
Acknowledgments
The authors gratefully acknowledge all the investigators and trial personnel for their contribution to the trial, the statistical support from Jens-Kristian Slott Jensen, Slott Stat, gave statistical support and Eva-Maria Damsgaard Nielsen provided medical writing assistance. Eva-Maria Damsgaard Nielsen is employed by Pharmacosmos A/S. Harald Vogelsang has received consultancy honorary from Vifor. This work was funded by Pharmacosmos A/S.
Declaration of interest: Lars L. Thomsen is an employed at Pharmacosmos A/S who sponsored the trial and the investigators/institutions received a fee per patient. Harald Vogelsang has received consultancy honorary from Vifor. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- [1].Stein J, Dignass AU. Management of iron deficiency anemia in inflammatory bowel disease - a practical approach. Ann Gastroenterol 2013;26:104–13. [PMC free article] [PubMed] [Google Scholar]
- [2].Gisbert JP, Bermejo F, Pajares R, Perez-Calle JL, Rodriguez M, Algaba A, et al. Oral and intravenous iron treatment in inflammatory bowel disease: hematological response and quality of life improvement. Inflamm Bowel Dis 2009;15:1485–91. [DOI] [PubMed] [Google Scholar]
- [3].Gomollon F, Gisbert JP. Anemia and inflammatory bowel diseases. World J Gastroenterol 2009;15:4659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wells CW, Lewis S, Barton JR, Corbett S. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm Bowel Dis 2006;12:123–30. [DOI] [PubMed] [Google Scholar]
- [5].Gasche C, Lomer MC, Cavill I, Weiss G. Iron, anaemia, and inflammatory bowel diseases. Gut 2004;53:1190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Goldberg ND. Iron deficiency anemia in patients with inflammatory bowel disease. Clin Exp Gastroenterol 2013;6:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Reinisch W, Staun M, Tandon RK, Altorjay I, Thillainayagam AV, Gratzer C, et al. A randomized, open-label, non-inferiority study of intravenous iron isomaltoside 1,000 (Monofer) compared with oral iron for treatment of anemia in IBD (PROCEED). Am J Gastroenterol 2013;108:1877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Reinisch W, Chowers Y, Danese S, Dignass A, Gomollon F, Nielsen OH, et al. The management of iron deficiency in inflammatory bowel disease - an online tool developed by the RAND/UCLA appropriateness method. Aliment Pharmacol Ther 2013;38:1109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee TW, Kolber MR, Fedorak RN, van Zanten SV. Iron replacement therapy in inflammatory bowel disease patients with iron deficiency anemia: a systematic review and meta-analysis. J Crohns Colitis 2012;6:267–75. [DOI] [PubMed] [Google Scholar]
- [10].Kulnigg S, Teischinger L, Dejaco C, Waldhor T, Gasche C. Rapid recurrence of IBD-associated anemia and iron deficiency after intravenous iron sucrose and erythropoietin treatment. Am J Gastroenterol 2009;104:1460–7. [DOI] [PubMed] [Google Scholar]
- [11].Gauche C, Berstad A, Befrits R, Beglinger C, Dignass A, Erichsen K, et al. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis 2007;13:1545–53. [DOI] [PubMed] [Google Scholar]
- [12].Kulnigg S, Stoinov S, Simanenkov V, Dudar LV, Karnafel W, Garcia LC, et al. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol 2008;103:1182–92. [DOI] [PubMed] [Google Scholar]
- [13].Evstatiev R, Alexeeva O, Bokemeyer B, Chopey I, Felder M, Gudehus M, et al. Ferric carboxymaltose prevents recurrence of anemia in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2013;11:269–77. [DOI] [PubMed] [Google Scholar]
- [14].Munoz M, Gomez-Ramirez S, Garcia-Erce JA. Intravenous iron in inflammatory bowel disease. World J Gastroenterol 2009;15:4666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Evstatiev R, Marteau P, Iqbal T, Khalif IL, Stein J, Bokemeyer B, et al. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology 2011;141:846–53. [DOI] [PubMed] [Google Scholar]
- [16].Goddard AF, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. British Society of Gastroenterology. Gut 2000;46:IV1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Blazevic A, Hunze J, Boots JM. Severe hypophosphataemia after intravenous iron administration. Neth J Med 2014;72:49–53. [PubMed] [Google Scholar]