Abstract
Objective. Activation of membrane receptor guanylate cyclase-C (GC-C) is implicated in gastrointestinal fluid and electrolyte balance, preservation of intestinal barrier integrity, anti-trophic effects and inhibition of pain sensation. To evaluate GC-C signaling, we examined the regulation of GC-C (GUCY2C/Gucy2c) and its endogenous ligands guanylin (GN/GUCA2A/Guca2a) and uroguanylin (UGN/GUCA2B/Guca2b) in colonic Crohn’s disease (CD), ulcerative colitis (UC) and in rats with 2,4,6-Trinitrobenzene sulphonic acid (TNBS) colitis. Correlation analyses between expression of GUCA2A and GUCY2C and expression of inflammatory cytokines (IL1A, IL1B, TNFA and IFNG) were conducted. Additionally, expression of transcription factors for GUCA2A and GUCY2C, and the GC-C signaling pathway, were examined. Material and methods. Biopsies from active UC/CD, un-inflamed UC/CD and healthy controls, and inflamed and healthy rat colon were investigated with gene expression microarray, immunohistochemistry (IHC) and in situ hybridization (ISH). Results. GUCA2A/Guca2a, GUCA2B, GUCY2C/Gucy2c, transcription factors, as well as several cyclic guanosine-3′,5′-monophosphate downstream mediators were all significantly down-regulated in both inflamed colonic inflammatory bowel disease (IBD) mucosa and TNBS colitis. Expression of GUCA2A and GUCY2C negatively correlated to expression of inflammatory cytokines. IHC and ISH confirmed microarray results for GUCA2A/Guca2a and GUCY2C/Gucy2c in inflamed samples. We identified a highly significant positive correlation between the expression of the transcription factor caudal type homeobox 2 (CDX2) and the expression of the downstream target gene GUCY2C. Conclusions. GUCA2A, GUCA2B and GUCY2C as well as several steps of the GC-C signaling pathway are down-regulated in IBD. This may have implications in IBD pathogenesis.
Key Words: cyclic GMP, guanylate cyclase-C, guanylin, inflammatory bowel disease, uroguanylin
Introduction
The two main types of idiopathic inflammatory bowel disease (IBD), ulcerative colitis (UC) and Crohn’s disease (CD), have a fluctuating disease pattern characterized by remissions and relapses. The prevailing understanding is that IBD has a multifactorial etiology where interaction between environmental and microbial triggers in the gut lumen and a dysfunctional intestinal barrier lead to an inappropriate immunological response in genetically susceptible individuals [1,2]. The intestinal epithelium, mucus layer and antimicrobial peptides serve as protection against the luminal environment [3]. Breaches in the intestinal barrier lead to an immunological response with activated T-cells and macrophages and secretion of pro-inflammatory cytokines including interleukin (IL) 1, IL6, IL7, IL17, TNFα and IFNγ [4]. Persistent defects in this protective barrier could sustain inflammation. A large proportion of patients with IBD experience pain, and long-lasting disease is associated with an increased risk for intestinal malignancy [1,2]. Current treatments are only symptomatic and aimed at suppressing the inflammatory response. Consequently, a continuous search for more specific etiological factors and identification of new therapeutic targets is required.
Dysfunctional guanylate cyclase-C (GC-C/GUCY2C) signaling has been implicated in diarrhea, constipation, abdominal pain, dysfunctional epithelial barrier function, and polyp and tumor growth [5]. Endogenous ligands for the membrane receptor GC-C are the peptides guanylin (GN/GUCA2A) and uroguanylin (UGN/GUCA2B). GN and UGN are expressed in several tissues, with high expression in the gastrointestinal (GI) epithelium which is the main source for both GN and UGN [6]. GN and UGN have a similar peptide structure to bacterial derived heat-stable enterotoxin (STa). GC-C signaling is mediated through increased cellular cyclic guanosine-3′,5′-monophosphate (cGMP). Downstream mediators of cGMP include intracellular phosphodiesterases (PDE), protein kinases (PK) and membrane-bound proteins and ion channels [7]. In the GI-tract, cGMP activation leads to secretion of chloride and bicarbonate through activation of the cystic fibrosis transmembrane conductance regulator (CFTR). Efflux of cGMP predominantly occurs through basolateral transporters in intestinal cells. Transporters for nucleotide analogues (cGMP) are the multidrug resistance proteins (MRP) 4 and 5 [8], which are expressed throughout the GI-tract [9]. MRP4 is localized predominantly in the apical cellular membrane, whereas MRP5 is localized basolaterally [8,10].
GN and UGN seem to be inversely distributed in the GI tract where UGN is abundant in the upper part with decreasing amounts towards the rectum and vice versa for GN [5]. Several potential transcription factor binding sites have been identified within the GUCA2A (GN), GUCA2B (UGN) and GUCY2C (GC-C) genes. GUCY2C transcription is e.g., shown to be regulated by hepatocyte nuclear factor 4-alpha (HNF-4α/HNF4A/Hnf4a) [11] and caudal type homeobox 2 (CDX2/CDX2) [12], the latter regulates intestine-specific GUCY2C expression. GUCA2A transcription is regulated by hepatocyte nuclear factor 1-alpha (HNF-1α/HNF1A/Hnf1a) [13].
The possible coupling of aberrant GC-C signaling with dysfunctional epithelial barrier function is intriguing in the pathogenesis of IBD. Treatment of a dysfunctional intestinal barrier is an emerging topic and the use of GC-C agonists in UC has been suggested [7]. In a study from 1995, circulating GN was not altered in IBD compared to healthy controls [14], while gene expression data for GUCA2A, GUCA2B and GUCY2C in IBD have only been infrequently investigated. Down-regulation of GUCA2A and GUCA2B in UC has been suggested to have implications for dysfunctional epithelial electrolyte regulation [15].
We hypothesized that GC-C signaling is dysregulated in IBD. We therefore examined the gene expression of GUCA2A/Guca2a (GN), GUCA2B/Guca2b (UGN) and GUCY2C/Gucy2c (GC-C), proposed transcription factors for GN and GC-C encoding genes, as well as the expression of downstream mediators of cGMP, and cGMP transporters MRP4/Mrp4 and MRP5/Mrp5 in IBD and in chemically induced colitis (rats) with 2,4,6-Trinitrobenzene sulphonic acid (TNBS).
Material and methods
Human IBD samples
The human samples were collected from an IBD cohort at Trondheim University Hospital, Trondheim, Norway, approved by the Regional Medical Research Ethics Committee (approval no 5.2007.910) and registered in the Clinical Trials Protocol Registration System (identifier NCT00516776). Written informed consent was obtained from all patients and control individuals.
The previously described [16] sample population consists of 37 active UC, 7 active CD, 44 un-inflamed UC (UC-U), 19 un-inflamed CD (CD-U) and 20 healthy controls (denoted Healthy in tables and figures). Participant data are shown in Table I. Of 44 patients with active inflammation, 15 (34.1%) were not on IBD medication. All participants underwent colonoscopy, and biopsies were collected from endoscopically assessed, maximally inflamed colonic mucosa. Samples from healthy controls and un-inflamed mucosa in IBD patients (when applicable) were taken from the hepatic flexure. Inflamed UC samples were mostly collected from the left colon (descending, sigmoid colon and rectum), as 28 of 37 patients had predominantly left-sided colitis. Un-inflamed IBD (IBD-U) samples were defined as no endoscopical or histological signs of inflammation.
Table I.
Number and characteristics of the subjects in the IBD study [16].
| Ha | UCb | CDc | UC-Ud | CD-Ue | |
|---|---|---|---|---|---|
| Subjects | 20 | 37 | 7 | 44 | 19 |
| Age, median years (range) | 45 (19–71) | 38 (19–72) | 31 (20–41) | 45 (21–71) | 39 (20–61) |
| Female/Male | 9/11 | 22/15 | 2/5 | 24/20 | 6/13 |
| Duration of disease, median years (range) | NAf | 9 (0–40) | 7 (1–12) | 13 (0–40) | 6 (0–28) |
| 5-ASAg/sulphasalzine (%) | 0 | 23 (62) | 2 (29) | 27 (61) | 6 (32) |
| Systemic corticosteroids (%) | 0 | 9 (26) | 2 (29) | 4 (9) | 8 (42) |
aHealthy, bUlcerative colitis, cCrohn’s disease, dUn-inflamed UC, eUn-inflamed CD, fNot applicable, g5-aminosalisylic acid.
Healthy controls were defined from clinical and endoscopical assessment and had no sign of GI disease. Four samples from each area were collected. Three biopsies were snap-frozen in liquid nitrogen, before processing. One biopsy was formalin-fixed and thereafter embedded in paraffin. Hematoxylin-eosin stained samples were evaluated by an experienced pathologist and samples with divergent endoscopic and histological assessment were removed.
Rat TNBS colitis samples
Data were generated from a genome-wide gene expression study comparing TNBS colitis in rats with IBD [17]. Five female Sprague Dawley rats weighing 200–250 g were used (Taconic Farms, Inc., Hallingore, Denmark). The animal studies were approved by the National Animal Research Authority. The general care and use of the animals were in accordance with the European Convention for the protection of Vertebrate Animals used for Experimental and other Scientific purposes.
We followed colitis in individual rats sequentially with colonoscopy and endoscopic biopsies [17]. Briefly, colitis was induced by a rectal enema with TNBS dissolved in 50% ethanol to a concentration of 30 mg/ml in a total volume of 0.6 ml. Colonoscopy was performed and five endoscopic biopsies were collected for both histopathological and gene expression analyses before and at Days 3, 7 and 12 after colitis induction. Colitis was assessed using a modified Murine Endoscopic Index of Colitis severity (MEICS, 0–12).
Genome-wide gene expression analysis
RNA extraction, processing, microarrays from human and rat endoscopic biopsies and corresponding statistical analyses were done as previously described [17,18]; the specific methodology is also available in Supplementary Methods. The data are accessible at ArrayExpress, E-MTAB-184 and ArrayExpress, E-MTAB-1263, respectively.
In the present study, we specifically examined gene expression profiles of GUCA2A/Guca2a (GN), GUCA2B/Guca2b (UGN) and GUCY2C/Gucy2c (GC-C), transcription factors HNF4A/Hnf4a, HNF1A/Hnf1a and CDX2/Cdx2, as well as downstream mediator proteins of cGMP (PRKG2/Prkg2 [PKGII]; CNGA1/Cnga1 and CNGA2/Cnga2 [CNGA]; PDE3A/Pde3a and PDE3B/Pde3b (PDE3), PDE5/Pde5, CFTR/Cftr, SLC9A3/Slc9a3 [NHE3]) and cGMP transporters MRP4/Mrp4 and MRP5/Mrp5. To determine whether there was a correlation between degree of inflammation and expression of GUCA2A and GUCY2C, we did regression and correlation analyses for the expression of these genes and the expression of inflammatory cytokines IL1A (IL1α), IL1B (IL1β), TNFA (TNFα) and IFNG (IFNγ). Additionally, we investigated whether there was any differential gene expression of GUCA2A, GUCA2B and GUCY2C in inflamed tissue sampled from the left (descending, sigmoid and rectum) and right (cecum, ascending and transverse) colon in UC and CD (analyzed together).
Immunohistochemistry and in situ hybridization
We used anti-GN (HPA018215, Atlas Antibodies AB, Stockholm, Sweden; dilution 1:100) and anti-GC-C (HPA037655, Atlas Antibodies AB; dilution 1:50) to perform IHC on human endoscopic biopsies from healthy and inflamed colon. A previously described [19] immunoreactivity score (IRS) (0–12) was calculated from the product of the percentage of positive epithelial cells (4: >80%, 3: 51–80%, 2: 10–50%, 1: <10%) and the intensity of the staining (0: non, 1: weak, 2: moderate and 3: strong). IHC against UGN could not be done due to a lack of appropriate antibodies.
In situ hybridization (ISH) was performed using the RNAscope® 2.0 assay kit (Advanced Cell Diagnostics, Inc., Hayward, CA) with probes for GUCA2A/Guca2a, GUCA2B/Guca2b and GUCY2C/Gucy2c.
A more detailed description is provided in Supplementary Methods.
Statistical analyses
Gene expression analyses were performed as described in Supplementary Methods. Statistical analyses were performed in Prism® Version 6.03 (GraphPad Software, Inc., La Jolla, CA). Statistics for IRS were performed with the nonparametric Kruskal–Wallis test and Dunn’s multiple comparisons test with adjusted p-Values. Kruskal-Wallis test and Dunn’s multiple comparisons test were also used for analyses of differential expression of GUCA2A, GUCA2B and GUCY2C between healthy controls, IBD-U, IBD Left colon, and IBD Right colon, as not all data sets of individually expressed genes were normally distributed according to D’Agostino-Pearson omnibus normality test. Correlation and regression analyses of gene expression (IL1A and GUCA2A/GUCY2C, IL1B and GUCA2A/GUCY2C, TNFA and GUCA2A/GUCY2C, IFNG and GUCA2A/GUCY2C, HNF1A and GUCA2A, CDX2 and GUCY2C, and HNF4A and GUCY2C) were done with computation of Pearson correlation coefficient, r.
p-Values of <0.05 were considered significant.
Results
Genes involved in GC-C signaling are down-regulated in IBD and TNBS colitis
Genome-wide gene expression analysis of human IBD samples revealed that mRNAs of the ligands GUCA2A, GUCA2B and the receptor GUCY2C were all significantly down-regulated in IBD with active inflammation compared to healthy controls. Fold change (FC) for GUCA2A was −4.91 and −2.86, for GUCA2B −6.06 and −4.64 and for GUCY2C −1.61 and −1.36, in UC and CD, respectively (Table II).
Table II.
Gene expression data for GUCA2A/Guca2a, GUCA2B/Guca2b and GUCY2C/Gucy2c in IBD and TNBS colitis.
| FC with p-Values | ||||
|---|---|---|---|---|
| Human | UC vs. Healthy | CD vs. Healthy | ||
| FC | p | FC | p | |
| GUCA2A | −4.91 | 3.28 × 10−13 | −2.86 | 0.0037 |
| GUCA2B | −6.06 | 1.01 × 10−16 | −4.64 | 7.12 × 10−6 |
| GUCY2C | −1.61 | 1.27 × 10−11 | −1.36 | 0.010 |
| TNBS colitis | Day 3 |
Day 7 |
Day 12 |
|||
|---|---|---|---|---|---|---|
| FC | P | FC | p | FC | p | |
| Guca2a | −3.47 | 0.0003 | −2.40 | 0.0006 | −1.79 | 0.0009 |
| Guca2b | −1.05 | 0.82 | 2.22 | 0.0032 | 1.78 | 0.034 |
| Gucy2c | −2.88 | 0.0002 | −2.36 | 0.0008 | −2.02 | 0.0024 |
| Log2 gene expression in Healthy, un-inflamed IBD (IBD-U) and IBD Left colon and IBD Right colon | ||||||
| Gene | Healthy (n = 20) |
IBD-U (n = 61a) |
IBD Left colon (n = 33) |
IBD Right colon (n = 11) |
pb, f | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Log2 expression (SD) | Log2 expression (SD) | pb, c | Log2 expression (SD) | FC vs. Healthy | pb, d | Log2 expression (SD) | FC vs. Healthy | pb, e | ||
| GUCA2A | 13.42 (0.21) | 13.32 (0.29) | >0.99 | 11.29 (1.37) | −4.08 | <0.0001 | 11.11 (1.78) | −4.63 | <0.0001 | >0.99 |
| GUCA2B | 10.85 (0.33) | 10.90 (0.56) | >0.99 | 8.30 (1.21) | −6.06 | <0.0001 | 8.30 (1.44) | −6.06 | 0.0003 | >0.99 |
| GUCY2C | 9.92 (0.20) | 9.92 (0.25) | >0.99 | 9.26 (0.42) | −1.59 | <0.0001 | 9.34 (0.38) | −1.51 | 0.0019 | >0.99 |
Abbreviations: CD = Crohn’s disease; FC = Fold change; IBD = Inflammatory bowel disease; TNBS = 2,4,6-Trinitrobenzene sulphonic acid; UC = Ulcerative colitis.
aDenotes the number of samples from both IBD patients in remission without inflammatory changes, as well as samples from endoscopically and histologically un-inflamed hepatic flexure of IBD patients with inflammatory changes elsewhere in the colon.
bData analyzed with the Kruskal–Wallis test and Dunn’s multiple comparisons test.
cComparison between Healthy and IBD-U.
dComparison between IBD Left colon and Healthy.
eComparison between IBD Right colon and Healthy.
fComparison between IBD Left colon and IBD Right colon.
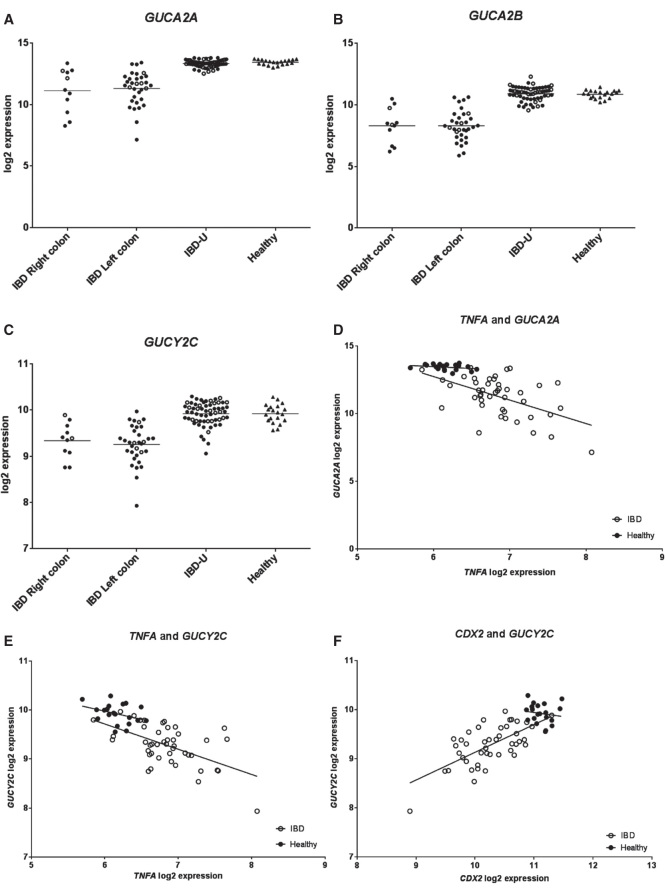
Animal studies have suggested that Guca2a expression is decreasing in caudal direction within the colon [20]. To our knowledge, studies regarding potential differential expression of GUCA2A between different colonic segments in humans are lacking. We therefore examined whether there was differential gene expression of GUCA2A, GUCA2B and GUCY2C in inflamed mucosa from the left (descending, sigmoid and rectum) compared to right (cecum, ascending and transverse) colon in both UC and CD patients (analyzed together), and compared to IBD-U and healthy controls. Analysis of individual log2 intensity values showed that gene expression profiles for GUCA2A, GUCA2B and GUCY2C were similar in biopsies sampled from IBD mucosa in predominantly right- and left-sided colitis (Table II and Figure 1A–C).
Figure 1.
Individual log2 expression for (A) GUCA2A, (B) GUCA2B and (C) GUCY2C for IBD Right colon, IBD Left colon, IBD-U and Healthy. Log2 expressions are significantly different for all genes between both IBD Right colon and Healthy and IBD Left colon and Healthy. There are no significant differences in log2 expressions neither between IBD-U and Healthy nor between IBD Right colon and IBD Left colon. Filled circles represent UC (n = 37) or UC-U (n = 42) samples and open circles represent CD (n = 7) or CD-U (n = 19) samples in A-C. (D) and (E) show individual log2 expression values for TNFA and GUCA2A and TNFA and GUCY2C, respectively. In IBD (both UC and CD) there is negative correlation between both TNFA and GUCA2A (r2 = 0.27, p = 0.0003) and TNFA and GUCY2C (r2 = 0.31, p <0.0001). (F) In IBD samples (both UC and CD) log2 expression of CDX2 positively correlates with log2 expression of GUCY2C (r2 = 0.43, p <0.0001). Figures in D-F are shown with regression lines; open circles represent individual log2 expression in IBD (both UC and CD) samples, while filled circles represent individual log2 expression in Healthy.
Expression of inflammatory cytokines (IL1A, IL1B, TNFA and IFNG) was significantly increased in IBD compared to healthy controls: IL1A with FC 2.74 and 2.01, IL1B with FC 8.38 and 5.19, TNFA with FC 1.65 and 1.41 and IFNG with FC 2.13 and 1.70, in UC and CD, respectively (S1). Individual log2 expression values of GUCA2A and GUCY2C negatively correlated with log2 expression of all inflammatory cytokines, however, not significantly for IL1A and GUCY2C, in IBD (Figure 1D, E and S2A-F, open circles).
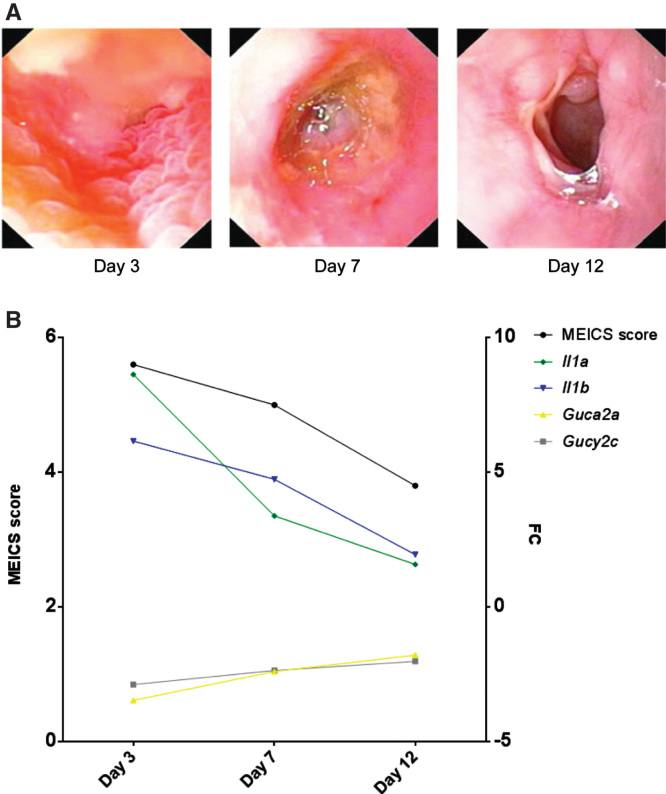
The degree of inflammation in the rats with TNBS colitis was moderate according to the MEICS score. Average MEICS score (0–12 ± SD) peaked at day 3 (5.6 ± 0.9) and day 7 (5.0 ± 1.2). Guca2a and Gucy2c were down-regulated in TNBS colitis with FC at Days 3, 7 and 12 of −3.47, −2.40 and −1.79 for Guca2a and −2.88, −2.36 and −2.02 for Gucy2c. Guca2b, was dissimilarly regulated in TNBS colitis compared to IBD, and was significantly up-regulated at Days 7 and 12, with FC of 2.22 and 1.78 (Table II). Severity of TNBS colitis assessed endoscopically (as depicted and from MEICS score) and from microarray gene expression of Il1a and Il1b, demonstrated an inverse relationship with expression of Guca2a and Gucy2c (Figure 2A, B).
Figure 2.
(A) Representative endoscopic pictures from one rat at Days 3, 7 and 12 after induction of colitis with TNBS. (B) Average MEICS score is portrayed with corresponding fold change (FC) of Guca2a, Gucy2c, Il1a and Il1b relative to baseline (two days before colitis induction). FC of Guca2a and Gucy2c is inversely related to both MEICS score and FC of Il1a and Il1b.
Since mRNAs for GN and GC-C were down-regulated in both IBD and TNBS colitis, gene expression of transcription factors that have been related to control of expression of GN (HNF1A/Hnf1a) and GC-C (HNF4A/Hnf4a and CDX2/Cdx2) encoding genes were identified [11–13]. HNF1A/Hnf1a was significantly down-regulated in UC (FC −1.08), CD (FC −1.10) and TNBS colitis (FC −2.24); similarly CDX2/Cdx2 was significantly down-regulated in UC (FC −1.92), in CD (FC −1.37) and in TNBS colitis (FC −1.91). HNF4A was significantly down-regulated in UC (FC −1.24) and CD (FC −1.23), while Hnf4a was significantly up-regulated (FC 1.23) in TNBS colitis (Table III). We further examined whether there were significant correlations between the expression levels of these transcription factors and possible target genes in IBD. Although HNF4A and HNF1A were significantly down-regulated in IBD (CD and UC) compared to healthy controls, we found no correlation neither between expression of HNF4A and GUCY2C nor between HNF1A and GUCA2A (S2G and H). However, linear regression analysis showed that there was a significant positive linear relation (p < 0.0001) between CDX2 expression and GUCY2C expression in IBD (Figure 1F, open circles). Such significant correlation was not observed in healthy controls (Figure 1F, filled circles), suggesting that CDX2 is an important transcriptional regulator of intestine-specific membrane receptor GC-C in IBD.
Table III.
Gene expression FC and p-Values for transcription factors, cGMP mediators and transporters in IBD and TNBS colitis.
| Gene/protein | UC vs. Healthy |
CD vs. Healthy |
TNBS colitis vs. Healthy rat colon |
|||
|---|---|---|---|---|---|---|
| FC | p | FC | p | FC | p | |
| Transcription factors | ||||||
| HNF4A/Hnf4a | −1.24 | 1.37 × 10−7 | −1.23 | 0.003 | 1.23 | 0.05 |
| CDX2/Cdx2 | −1.92 | 4.55 × 10−17 | −1.37 | 0.01 | −1.91 | 0.004 |
| HNF1A/Hnf1a | −1.08 | 0.04 | −1.10 | 0.004 | −2.24 | 0 |
| cGMP downstream mediators | ||||||
| PRKG2/Prkg2 | −2.14 | 1.2 × 10−15 | −2.19 | 2.7 × 10−7 | −30.87 | 0.001 |
| CNGA1/Cnga1 | −1.93 | 2.5 × 10−8 | −1.80 | 0.004 | No data | No data |
| CNGA2/Cnga2 | 1.004 | 0.92 | 1.04 | 0.50 | No data | No data |
| PDE3A/Pde3a | −1.17 | 0.001 | −1.13 | 0.21 | −1.08 | 0.21 |
| PDE3B/Pde3b | −1.05 | 0.30 | 1.07 | 0.56 | −1.08 | 0.39 |
| PDE5/Pde5 | −1.34 | 0.007 | −1.15 | 0.59 | 1.33 | 0.05 |
| CFTR/Cftr | −1.84 | 2.2 x 10−10 | −1.59 | 0.004 | −2.64 | 0.0002 |
| SLC9A3/Slc9a3 | −1.21 | 2.6 x 10−5 | −1.17 | 0.06 | −4.59 | 0.002 |
| cGMP transporters | ||||||
| MRP4/Mrp4 | 1.06 | 0.94 | 1.02 | 0.52 | No data | No data |
| MRP5/Mrp5 | −1.23 | 0.12 | −1.25 | 0.007 | −1.15 | 0.29 |
Abbreviations: CD = Crohn’s disease; CFTR = Cystic fibrosis transmembrane conductance regulator; cGMP = Cyclic guanosine-3′,5′-monophosphate; FC = Fold change; IBD = Inflammatory bowel disease; MRP = Multidrug resistance protein; PDE = Phosphodiesterase; TNBS = 2,4,6-Trinitrobenzene sulphonic acid; UC = Ulcerative colitis.
Since GC-C signaling is mediated through cGMP [6], gene expression of cGMP downstream mediators (PRKG2/Prkg2 [PKGII]; CNGA1/Cnga1 and CNGA2/Cnga2 [CNGA]; PDE3A/Pde3a and PDE3B/Pde3b (PDE3), PDE5/Pde5, CFTR/Cftr, SLC9A3/Slc9a3 [NHE3]) were also examined and all human genes, except CNGA2 and PDE3B, were found significantly down-regulated (Table III).
Expression of cGMP transporter MRP4 was not significantly changed, while MRP5 was significantly down-regulated in CD (FC −1.25) and down-regulated in UC (FC −1.23), but not significantly. From the TNBS colitis study, we have no data for Mrp4 and Mrp5 was not significantly changed (Table III).
Overall, gene expression of GUCA2A/Guca2a (GN), GUCA2B/Guca2b (UGN), GUCY2C/Gucy2c (GC-C), relevant transcription factors as well as downstream targets for GC-C signaling were mostly down-regulated in IBD and TNBS colitis. Expression of GUCA2A/Guca2a and GUCY2C/Gucy2c in both IBD and TNBS colitis seems to be inversely related to degree of colitis (MEICS score and/or inflammatory cytokines). Expressions of CDX2 and GUCY2C are highly correlated in IBD.
Protein (IHC) and mRNA (ISH) in mucosal biopsies support gene expression data
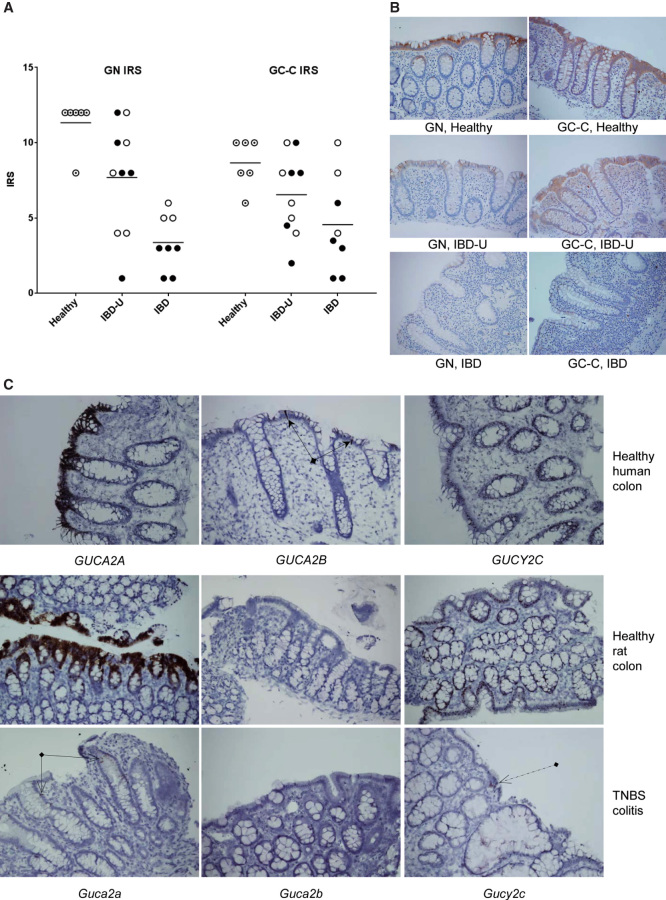
We further used IHC and ISH to investigate localization and expression levels of relevant peptides/proteins and mRNAs in colonic biopsies. IHC demonstrated a markedly less positivity for GN and GC-C in inflamed mucosa compared to healthy controls. A non-significant reduction in IRS compared to control was also observed in biopsies collected from un-inflamed parts of the colon of IBD patients (Figure 3A, B).
Figure 3.
Detection of peptides/proteins (IHC) and mRNA (ISH). (A) Immunoreactivity score (IRS) for GN and GC-C in healthy mucosa, un-inflamed IBD patients (IBD-U) and inflamed IBD. Healthy (n = 6) is indicated with target circle, UC (n = 5) and UC-U (n = 5) with filled circles and CD (n = 3) and CD-U (n = 5) with open circles. Mean IRS for GN was 11.33, 7.70 and 3.38 for Healthy, IBD-U and IBD. Mean rank difference for GN was 6.50 for Healthy vs. IBD-U (p = 0.14) and 13.63 for Healthy vs. IBD (p = 0.0006). Mean IRS for GC-C was 8.67, 6.55 and 4.56 for Healthy, IBD-U and IBD. Mean rank difference for GC-C was 5.05 for Healthy vs. IBD-U (p = 0.32) and 9.44 for Healthy vs. IBD (p = 0.02). (B) Representative GN and GC-C stained tissue specimens from Healthy, IBD-U and IBD (Magnification, 200×). (C) Mucosal expression of GUCA2A/Guca2a, GUCA2B/Guca2b and GUCY2C/Gucy2c in endoscopic biopsies from healthy human colon and from endoscopic biopsies from healthy and inflamed rat colon after induction of TNBS colitis. There is marked expression of GUCA2A/Guca2a and quite significant expression of GUCY2C/Gucy2c in healthy colonic mucosa. After induction of TNBS colitis only a few cells are positive for Guca2a (arrows with open arrowheads) and Gucy2c (dashed arrow). The pattern of expression is similar in healthy human and rat colon. GUCA2A/Guca2a and GUCY2C/Gucy2c are expressed in both goblet and columnar cells. Guca2b has no noticeable expression in rat samples neither before nor after colitis induction, whereas in human colonic mucosa GUCA2B is strongly expressed in solitary epithelial cells (arrows with solid arrowheads) and weaker in other epithelial cells (Magnification, 200×).
GN and GC-C are expressed in the epithelial cells and it is possible that mucosal denudation could have reduced immunoreactivity. However, biopsies were not collected from ulcerated areas and histopathological examination demonstrated that the epithelium was intact with goblet cells and enterocytes. A detailed description of IRS scores, sample location and patient characteristics is included in S3.
GUCA2A/Guca2a, GUCA2B/Guca2b and GUCY2C/Gucy2c were all expressed in the colonic epithelium of healthy human and rat samples assessed by ISH. GUCA2A/Guca2a and GUCY2C/Gucy2c expression was localized to both goblet and columnar cells in healthy colonic mucosa. GUCA2B, however, was mainly expressed in solitary epithelial cells of the human colon. No expression of Guca2b was detectable in rat colon. In the inflamed rat colon, expression of Guca2a and Gucy2c was decreased and was only detectable in a few cells (Figure 3C).
Discussion
This descriptive gene expression study of GN, UGN and GC-C encoding genes and the GC-C signaling pathway in IBD illuminates the molecular premise for an evolving research topic in IBD. A reduced transcription of GUCA2A (GN) and GUCA2B (UGN) in IBD has previously been demonstrated [15] and is confirmed in the current study. In addition, the receptor for GN, UGN and STa, GUCY2C (GC-C), is down-regulated in IBD, which to our knowledge, has not previously been reported. Consequently, the combined reduction of both the agonists and their corresponding receptor should lead to a pronounced downstream reduction in colonic GC-C signaling. Semi-quantitative determination of GN and GC-C by IHC and ISH confirmed the microarray findings for GUCA2A/Guca2a and GUCY2C/Gucy2c.
GC-C signaling has received increasing interest in GI disease in general and in the study of IBD in particular. It was recently demonstrated that the GC-C agonist Linaclotide, used to treat constipation, also has an analgesic effect through increased production and release of cGMP. cGMP is transported through the basolateral cellular membrane and acts on and inhibits colonic afferent nociceptors [21]. Interestingly MRP5, one of the proposed transporters for cGMP localized in the basolateral membrane [10], is among the down-regulated genes in our IBD cohort. It is thus possible that this in conjunction with reduced expression of GUCA2A, GUCA2B and GUCY2C and diminished cGMP release to the submucosa may affect pain sensation also in IBD.
We demonstrated that Guca2a and Gucy2c were down-regulated in rats with moderate TNBS colitis. The expression profiles of Guca2a and Gucy2c were inversely related to the MEICS score and the expression of inflammatory cytokines Il1a and Il1b. Similarly, Guca2a down-regulation has been demonstrated in inflamed colon of Il10 −/− mice and in cell culture after TNFα stimulation [22]. The human data also showed a negative correlation between increased expression of inflammatory cytokines (IL1A, IL1B, TNFA and IFNG) and expression of both GUCA2A and GUCY2C. Thus, it seems that down-regulation of GUCA2A/Guca2a and GUCY2C/Gucy2c is a common feature during colonic mucosal inflammation. This could be a phylogenetically conserved mechanism to prevent fluid and electrolyte loss – as described in loss-of-function mutations for GUCY2C [23]. However, other mechanisms of diarrhea in IBD seem to override this [24]. The results are, nevertheless, in accordance with previous results showing that net absorption of chloride, sodium and fluid is decreased in IBD (without increased chloride secretion) through, for example, cytokine-mediated (TNFα, IFNγ and IL1β) down-regulation of NHE and epithelial sodium channels [24]. Additionally, reduced GC-C signaling through down-regulation of GUCY2C in intestinal inflammation might also facilitate tissue regeneration and healing by reducing the anti-proliferative effect of GN and UGN [5].
The mechanism for the regulation of transcription of GUCA2A/Guca2a, GUCA2B/Guca2b and GUCY2C/Gucy2c probably involves the role of several transcription factors. We studied the expression of the transcription factors CDX2, HNF4A and HNF1A. Our gene expression data demonstrated that the expression of CDX2/Cdx2 was significantly down-regulated in both UC, CD and TNBS colitis. CDX2 together with HNF4A could explain the reduced transcription of GUCY2C in IBD [11,12], while down-regulation of HNF1A is in accordance with reduced expression of GUCA2A [13]. In addition to down-regulation of GUCY2C, we found a positive correlation between CDX2 and GUCY2C expression in IBD. This corresponds with previous studies investigating CDX2 in intestinal inflammation and as an intestine-specific transcription factor for GUCY2C/Gucy2c [12,25]. Both HNF4A and CDX2 have been found decreased in IBD [26,27] and Hnf4a deficient mice are susceptible to dextran sulfate sodium (DSS) colitis [26]. Both HNF-4α and CDX2 have been postulated to function as tumor suppressors in the colon [28,29], which in conjunction with their role as transcription factors for GUCY2C corresponds with the anti-proliferative effect of increased GC-C signaling. In accordance with this, an increased number of intestinal crypts and proliferation of epithelial cells as well as increased tumorigenesis and attenuated apoptosis has been observed in Gucy2c −/− mice and cell lines [30–32], supporting the concept that reduced GC-C signaling may be favorable for growth and regeneration of a damaged intestinal epithelium. Moreover, GUCY2C is expressed in colonic adenoma and cancer [33]. The GC-C ligand GN, abundantly expressed in the colonic epithelium, however, is lost in colon cancer, leading to reduced GC-C stimulation and oncogenesis [34]. Due to the chronic nature of UC and CD, these mechanisms may be both a necessity for tissue regeneration but may also be a disadvantage if similar mechanisms drive dysplasia and cancer development.
Sustained loss of GC-C signaling in IBD might lead to a vicious cycle, demonstrated by Gucy2c −/− mice that have disrupted tight junction assembly, increased intestinal permeability and susceptibility to colitis [35]. Some conflicting data exist, as DSS colitis has been found to be less severe in Gucy2c −/− and Guca2a −/− mice [36], and in a described gain of function mutation of GUCY2C with increased activation of GC-C, susceptibility for intestinal obstruction and ileal inflammation were observed [37]. Such discrepancies are difficult to explain. It seems likely that GC-C signaling is important in intestinal homeostasis, with delicate regulation of growth, epithelial renewal and barrier integrity, required for a healthy epithelium.
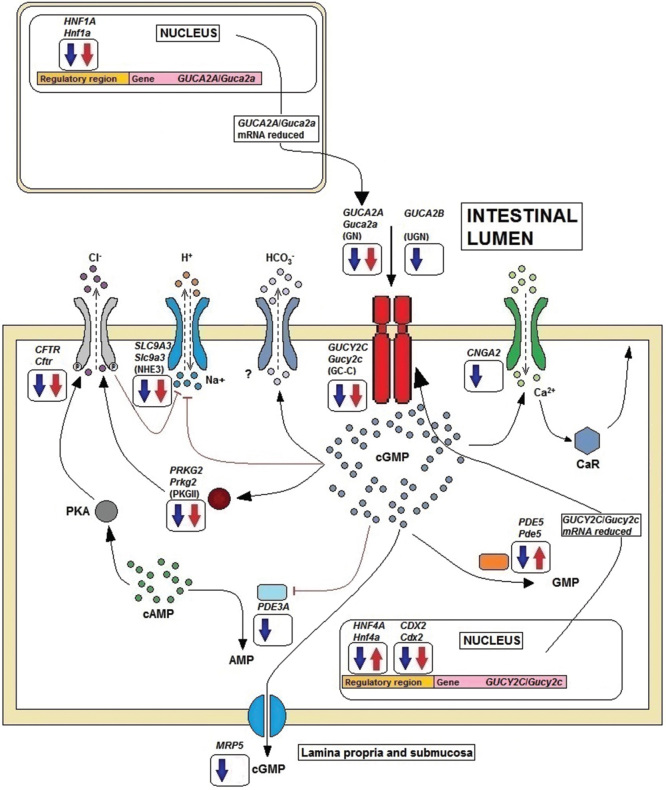
We also found down-regulation of several genes encoding cGMP downstream mediators, which are involved in processes including the regulation of electrolyte balance and water secretion [7]. Altogether, genes encoding for GN, UGN and GC-C, together with effector proteins involved in downstream signaling of cGMP, as well as transcription factors associated with transcription of GN and GC-C are down-regulated in IBD. Figure 4 displays proposed GC-C signaling pathway aberrations in colitis. The dysregulatory mechanisms include reduced levels of transcription factors HNF-1α for GUCA2A, and HNF-4α and CDX2 for GUCY2C, with reduced levels of ligand (GN) and reduced receptor (GC-C) availability. Lower cGMP levels diminish activation of PKGII and reduce activation of CFTR. Likewise, reduced availability of cGMP reduces activation of CNG calcium channels. Finally, the expression of several downstream mediators, ion channels and also the cGMP transporter MRP5 are down-regulated.
Figure 4.
Proposed GC-C signaling in colitis. Direction of filled arrows in frames indicates significantly down- or up-regulated expression of genes encoding transcription factors, GC-C, GC-C ligands (GN and UGN) or cGMP downstream mediators in IBD (blue) and TNBS colitis (red). Down-regulation of transcription factors leads to reduced synthesis of GN (and UGN), which both act in a paracrine, luminocrine and autocrine manner, and reduced synthesis of GC-C, with subsequent decreased intracellular cGMP generation. Reduced cGMP and down-regulation of cGMP-dependent protein kinase II (PKGII) reduce phosphorylation of CFTR and diminish chloride efflux. In addition bicarbonate efflux through an unknown channel decreases. The ion channels CFTR and sodium-hydrogen exchanger (NHE) are also down-regulated. These processes have implications for fluid and ion homeostasis. Both lower cGMP levels and down-regulation of cyclic nucleotide-gated channels (CNG) lead to diminished calcium influx and calcium-sensing receptor (CaR) signaling, with reduced anti-proliferative effects as a consequence [38]. cGMP-dependent phosphodiesterase (PDE5) which facilitates conversion of cGMP to GMP is down-regulated in IBD and up-regulated in TNBS colitis. cGMP is transported through the basolateral cell membrane, possibly by multidrug-resistance transporter (MRP) 5. Reduced availability of cGMP in the lamina propria and submucosa could hamper inhibition of nociceptors. The figure was adapted and modified from Basu et al. and Fiskerstrand et al. [37,39].
A possible limitation of the current study is related to the IBD cohort as the number of CD patients is low, making comparison to UC difficult. The data would also have been strengthened if a larger proportion of patients were treatment naïve and if clinical activity index and endoscopic activity were recorded.
In conclusion, we have demonstrated reduced expression of the GC-C activating peptides GN and UGN in IBD. Furthermore, the GC-C signaling pathway and corresponding down-stream signaling is reduced. This indicates possible pathogenetic significance given cGMP’s multiple cellular downstream mediators and effects.
Supplementary Material
Acknowledgments
This study was supported by grants from the Liaison Committee between the Central Norway Regional Health Authority (RHA) and the Norwegian University of Science and Technology (NTNU). Øystein Brenna has been awarded scholarships from Ferring Pharmaceuticals and MSD after board decisions of the Norwegian Gastroenterological Association. We want to thank Bjørn Munkvold for help with rat endoscopy and for technical assistance with tissue processing and sectioning. Clinical Trial Registration: NCT00516776.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Supplementary material available online
References
- Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- Ordas I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606–19. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15:100–13. doi: 10.1002/ibd.20539. [DOI] [PubMed] [Google Scholar]
- Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–42. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- Camilleri M. Guanylate Cyclase C agonists: emerging gastrointestinal therapies and actions. Gastroenterology. 2015;148:483–7. doi: 10.1053/j.gastro.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Beltowski J. Guanylin and related peptides. J Physiol Pharmacol. 2001;52:351–75. [PubMed] [Google Scholar]
- Pitari GM. Pharmacology and clinical potential of guanylyl cyclase C agonists in the treatment of ulcerative colitis. Drug Des Devel Ther. 2013;7:351–60. doi: 10.2147/DDDT.S32252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnholds J, Mol CAAM, van Deemter L, de Haas M, Scheffer GL, Baas F, et al. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci USA. 2000;97:7476–81. doi: 10.1073/pnas.120159197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann C, Gutmann H, Hruz P, Gutzwiller J-P, Beglinger C, Drewe J. Mapping of multidrug resistance gene 1 and multidrug resistance-associated protein isoform 1 to 5 mRNA expression along the human intestinal tract. Drug Metab Dispos. 2005;33:219–24. doi: 10.1124/dmd.104.001354. [DOI] [PubMed] [Google Scholar]
- Silos-Santiago I, Hannig G, Eutamene H, Ustinova EE, Bernier SG, Ge P, et al. Gastrointestinal pain: Unraveling a novel endogenous pathway through uroguanylin/guanylate cyclase-C/cGMP activation. Pain. 2013;154:1820–30. doi: 10.1016/j.pain.2013.05.044. [DOI] [PubMed] [Google Scholar]
- Swenson ES, Mann EA, Jump ML, Giannella RA. Hepatocyte nuclear factor-4 regulates intestinal expression of the guanylin/heat-stable toxin receptor. Am J Physiol. 1999;276:G728–36. doi: 10.1152/ajpgi.1999.276.3.G728. [DOI] [PubMed] [Google Scholar]
- Park J, Schulz S, Waldman SA. Intestine-specific activity of the human guanylyl cyclase C promoter is regulated by Cdx2. Gastroenterology. 2000;119:89–96. doi: 10.1053/gast.2000.8520. [DOI] [PubMed] [Google Scholar]
- Hochman JA, Sciaky D, Whitaker TL, Hawkins JA, Cohen MB. Hepatocyte nuclear factor-1alpha regulates transcription of the guanylin gene. Am J Physiol. 1997;273:G833–41. doi: 10.1152/ajpgi.1997.273.4.G833. [DOI] [PubMed] [Google Scholar]
- Kuhn M, Kulaksiz H, Cetin Y, Frank M, Nold R, Arnold R, et al. Circulating and tissue guanylin immunoreactivity in intestinal secretory diarrhoea. Eur J Clin Invest. 1995;25:899–905. doi: 10.1111/j.1365-2362.1995.tb01964.x. [DOI] [PubMed] [Google Scholar]
- Wu F, Dassopoulos T, Cope L, Maitra A, Brant SR, Harris ML, et al. Genome-wide gene expression differences in Crohn’s disease and ulcerative colitis from endoscopic pinch biopsies: insights into distinctive pathogenesis. Inflamm Bowel Dis. 2007;13:807–21. doi: 10.1002/ibd.20110. [DOI] [PubMed] [Google Scholar]
- Granlund AV, Flatberg A, Østvik AE, Drozdov I, Gustafsson B, Kidd M, et al. Whole genome gene expression meta-analysis of inflammatory bowel disease colon mucosa demonstrates lack of major differences between crohn’s disease and ulcerative colitis. PLoS One. 2013;8:e56818. doi: 10.1371/journal.pone.0056818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenna Ø, Furnes MW, Drozdov I, van Beelen Granlund A, Flatberg A, Sandvik AK, et al. Relevance of TNBS-Colitis in rats: a methodological study with endoscopic, histologic and transcriptomic characterization and correlation to IBD. PLoS One. 2013;8:e54543. doi: 10.1371/journal.pone.0054543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granlund AB, Beisvag V, Torp SH, Flatberg A, Kleveland PM, Ostvik AE, et al. Activation of REG family proteins in colitis. Scand J Gastroenterol. 2011;46:1316–23. doi: 10.3109/00365521.2011.605463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto F, Feelders RA, van der Pas R, Kros JM, Waaijers M, Sprij-Mooij D, et al. Immunoreactivity score using an anti-sst2A receptor monoclonal antibody strongly predicts the biochemical response to adjuvant treatment with somatostatin analogs in acromegaly. J Clin Endocrinol Metab. 2013;98:E66–71. doi: 10.1210/jc.2012-2609. [DOI] [PubMed] [Google Scholar]
- Qian X, Prabhakar S, Nandi A, Visweswariah SS, Goy MF. Expression of GC-C, a receptor-guanylate cyclase, and its endogenous ligands uroguanylin and guanylin along the rostrocaudal axis of the intestine. Endocrinology. 2000;141:3210–24. doi: 10.1210/endo.141.9.7644. [DOI] [PubMed] [Google Scholar]
- Castro J, Harrington AM, Hughes PA, Martin CM, Ge P, Shea CM, et al. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3’,5’-monophosphate. Gastroenterology. 2013;145:1334–46. doi: 10.1053/j.gastro.2013.08.017. e1-11. [DOI] [PubMed] [Google Scholar]
- Harmel-Laws E, Mann EA, Cohen MB, Steinbrecher KA. Guanylate cyclase c deficiency causes severe inflammation in a murine model of spontaneous colitis. PLoS One. 2013;8:e79180. doi: 10.1371/journal.pone.0079180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romi H, Cohen I, Landau D, Alkrinawi S, Yerushalmi B, Hershkovitz R, et al. Meconium ileus caused by mutations in GUCY2C, encoding the CFTR-activating guanylate cyclase 2C. Am J Hum Genet. 2012;90:893–9. doi: 10.1016/j.ajhg.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder HJ. Mechanisms of diarrhea in inflammatory bowel diseases. Ann N Y Acad Sci. 2009;1165:285–93. doi: 10.1111/j.1749-6632.2009.04039.x. [DOI] [PubMed] [Google Scholar]
- Coskun M, Troelsen JT, Nielsen OH. The role of CDX2 in intestinal homeostasis and inflammation. Biochim Biophys Acta BBAMol Basis Dis. 2011;1812:283–9. doi: 10.1016/j.bbadis.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Ahn SH, Shah YM, Inoue J, Morimura K, Kim I, Yim S, et al. Hepatocyte nuclear factor 4alpha in the intestinal epithelial cells protects against inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:908–20. doi: 10.1002/ibd.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun M, Olsen AK, Holm TL, Kvist PH, Nielsen OH, Riis LB, et al. TNF-alpha-induced down-regulation of CDX2 suppresses MEP1A expression in colitis. Biochim Biophys Acta BBAMol Basis Dis. 2012;1822:843–51. doi: 10.1016/j.bbadis.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Bonhomme C, Duluc I, Martin E, Chawengsaksophak K, Chenard M-P, Kedinger M, et al. The Cdx2 homeobox gene has a tumour suppressor function in the distal colon in addition to a homeotic role during gut development. Gut. 2003;52:1465–71. doi: 10.1136/gut.52.10.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saandi T, Baraille F, Derbal-Wolfrom L, Cattin AL, Benahmed F, Martin E, et al. Regulation of the tumor suppressor homeogene Cdx2 by HNF4[alpha] in intestinal cancer. Oncogene. 2013;32:3782–8. doi: 10.1038/onc.2012.401. [DOI] [PubMed] [Google Scholar]
- Lin JE, Li P, Snook AE, Schulz S, Dasgupta A, Hyslop TM, et al. The hormone receptor GUCY2C suppresses intestinal tumor formation by inhibiting AKT signaling. Gastroenterology. 2010;138:241–54. doi: 10.1053/j.gastro.2009.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Schulz S, Bombonati A, Palazzo JP, Hyslop TM, Xu Y, et al. Guanylyl cyclase C suppresses intestinal tumorigenesis by restricting proliferation and maintaining genomic integrity. Gastroenterology. 2007;133:599–607. doi: 10.1053/j.gastro.2007.05.052. [DOI] [PubMed] [Google Scholar]
- Basu N, Saha S, Khan I, Ramachandra SG, Visweswariah SS. Intestinal cell proliferation and senescence are regulated by receptor guanylyl cyclase C and p21. J Biol Chem. 2014;289:581–93. doi: 10.1074/jbc.M113.511311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbe R, Palazzo JP, Walters R, Weinberg D, Schulz S, Waldman SA. Guanylyl cyclase C is a marker of intestinal metaplasia, dysplasia, and adenocarcinoma of the gastrointestinal tract. Hum Pathol. 2005;36:170–9. doi: 10.1016/j.humpath.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Wilson C, Lin JE, Li P, Snook AE, Gong J, Sato T, et al. The Paracrine Hormone for the GUCY2C tumor suppressor, guanylin, is universally lost in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2014;23:2328–37. doi: 10.1158/1055-9965.EPI-14-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JE, Snook AE, Li P, Stoecker BA, Kim GW, Magee MS, et al. GUCY2C opposes systemic genotoxic tumorigenesis by regulating AKT-dependent intestinal barrier integrity. PLoS One. 2012;7:e31686. doi: 10.1371/journal.pone.0031686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrecher KA, Harmel-Laws E, Garin-Laflam MP, Mann EA, Bezerra LD, Hogan SP, et al. Murine guanylate cyclase C regulates colonic injury and inflammation. J Immunol. 2011;186:7205–14. doi: 10.4049/jimmunol.1002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskerstrand T, Arshad N, Haukanes BI, Tronstad RR, Pham KD, Johansson S, et al. Familial diarrhea syndrome caused by an activating GUCY2C mutation. N Engl J Med. 2012;366:1586–95. doi: 10.1056/NEJMoa1110132. [DOI] [PubMed] [Google Scholar]
- Pitari GM, Lin JE, Shah FJ, Lubbe WJ, Zuzga DS, Li P, et al. Enterotoxin preconditioning restores calcium-sensing receptor-mediated cytostasis in colon cancer cells. Carcinogenesis. 2008;29:1601–7. doi: 10.1093/carcin/bgn148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu N, Arshad N, Visweswariah SS. Receptor guanylyl cyclase C (GC-C): regulation and signal transduction. Mol Cell Biochem. 2010;334:67–80. doi: 10.1007/s11010-009-0324-x. [DOI] [PubMed] [Google Scholar]
- Gene expression data: E-MTAB-184. https://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-184/ Available from.
- Gene expression data: E-MTAB-1263. https://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-1263/ Available from.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.