Abstract
The polio eradication endgame aims to bring transmission of all polioviruses to a halt. To achieve this aim, it is essential to block viral replication in individuals via induction of a robust mucosal immune response. Although it has long been recognized that inactivated poliovirus vaccine (IPV) is incapable of inducing a strong mucosal response on its own, it has recently become clear that IPV may boost immunity in the intestinal mucosa among individuals previously immunized with oral poliovirus vaccine. Indeed, mucosal protection appears to be stronger following a booster dose of IPV than oral poliovirus vaccine, especially in older children. Here, we review the available evidence regarding the impact of IPV on mucosal immunity, and consider the implications of this evidence for the polio eradication endgame. We conclude that the implementation of IPV in both routine and supplementary immunization activities has the potential to play a key role in halting poliovirus transmission, and thereby hasten the eradication of polio.
Keywords: environmental surveillance, eradication, inactivated poliovirus vaccine, mucosal immunity, oral poliovirus vaccine, poliovirus
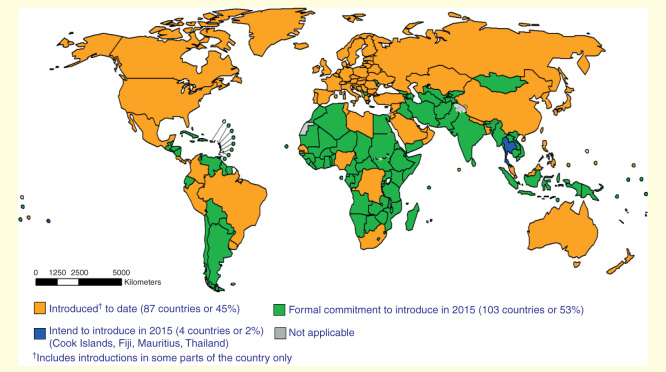
As we draw closer to the eradication of poliomyelitis, the Global Polio Eradication Initiative (GPEI) has outlined an endgame strategy that includes a transition from using oral poliovirus vaccine (OPV) to inactivated poliovirus vaccine (IPV). To support this goal, the WHO has recommended that all countries introduce at least one dose of IPV into routine immunization programs by the end of 2015 (Figure 1) [1]. This will be followed by the sequential and globally synchronized removal of the three strains of OPV, starting with serotype 2, until the use of OPV is halted entirely.
Figure 1.
Map of global inactivated poliovirus vaccine use and planned dates for its introduction. Data source: WHO/IVB Database, as of 1 May 2015. Map production: Immunization Vaccines and Biologicals (IVB), WHO.
Reproduced with permission from [73].
The need for such a transition relates to the differing strengths and weaknesses of the two available polio vaccines. As a live-attenuated vaccine, OPV induces an immune response that is virtually identical to that produced by wild-type infection [2]. The live virus stimulates both humoral immunity, which prevents transit of poliovirus to the central nervous system and thus protects against paralysis, and mucosal immunity, which halts poliovirus replication at the nasopharyngeal and gastrointestinal mucosal surfaces (the major entry points of the pathogen) [3,4]. By contrast, IPV is a killed vaccine in which the antigen dose is administered via intramuscular injection. Although more effective than OPV in inducing a humoral immune response in low-income countries [5,6], IPV induces only limited mucosal immunity. In particular, it is less effective in preventing poliovirus replication and shedding in the intestine. Despite being protected from paralysis, IPV-immunized individuals may, therefore, contribute to fecal–oral poliovirus transmission, substantially diminishing the herd effects of immunization [7].
Since its inception in 1988, the GPEI has recommended the use of OPV (mainly trivalent OPV [tOPV]) to protect against all serotypes of poliovirus owing to its low cost, ease of administration, ability to produce a strong mucosal immune response and potential to indirectly immunize secondary contacts. Following the elimination of wild type 2 poliovirus (one of the three poliovirus serotypes), the GPEI developed new formulations of OPV – including monovalent OPV (mOPV) in 2005 and bivalent OPV (bOPV) in 2009 – with the aim of improving protective efficacy against the two remaining serotypes (1 and 3) by reducing interference between the three vaccine strains [8,9]. These new formulations have been used in mass campaigns (supplementary immunization activities [SIAs]) to boost population immunity in regions with persistent poliovirus transmission. The program has achieved great success with OPV, reducing the global number of wild poliovirus cases by >99% since its launch. Currently, 145 countries use tOPV to vaccinate children against polio in routine immunization programs [10].
Although OPV has served the GPEI well, the vaccine has certain limitations owing to its genetic instability. First, on very rare occasions, cases of vaccine-associated paralytic poliomyelitis (VAPP) may occur among OPV recipients or their close contacts [11]. It is estimated that there are one to two cases of VAPP per million primary immunizations with OPV [12]. Second, vaccine polioviruses shed from OPV recipients can lose their attenuating mutations during viral replication and can regain transmissibility and neurovirulence [13]. In rare instances, these vaccine-derived polioviruses (VDPVs) may circulate widely in the population (circulating VDPVs [cVDPVs]), showing high levels of divergence from the OPV parental strains and transmission dynamics similar to those of wild-type viruses [14]. Since 2000, outbreaks of cVDPV have occurred in 18 different countries, producing more than 720 cases of poliomyelitis [13]. Serotype 2 has caused more than 85% of these cases, which may be attributed to deficits in population immunity to type 2 poliovirus following the replacement of tOPV with mOPV and bOPV targeting serotypes 1 and 3 in SIAs since 2005 [15].
A major concern with transitioning from OPV to IPV is the limited intestinal mucosal immunity provided by the latter. By failing to halt poliovirus replication in the gut mucosa, the use of IPV may enable fecal–oral transmission to continue – a scenario at odds with the eradication endgame. However, recent evidence has called into question the traditional view that IPV offers limited mucosal protection by showing that administering the vaccine to individuals who have previously received OPV and are ‘mucosally primed’ significantly boosts mucosal immunity [16,17].
Given the risk of VAPP and cVDPVs, the use of OPV must be phased out from all immunization activities if the eradication of polio is to be achieved [1]. To fill the gap in poliovirus immunity, IPV must first be introduced into routine immunization activities globally. However, the extent to which IPV can fill the role of its live counterpart remains to be seen. A delicate balance between the two vaccines must be achieved to eliminate wild polioviruses and prevent cVDPVs. The impact of IPV on mucosal immunity is key to this balance. In this review, we examine the available evidence regarding the influence of IPV on mucosal protection. In doing so, we address the potentially vital role that IPV may play in halting viral transmission during the polio eradication endgame.
Measuring mucosal immunity to poliovirus
When considering vaccine-induced protection against poliomyelitis, the distinction between humoral and mucosal immunity is crucial. Humoral immunity refers to the presence of poliovirus-specific neutralizing antibodies in blood (predominantly IgG) and can be measured using a standardized microneutralization assay [18]. Administration of antibody has been shown to protect against poliomyelitis and the detection of antibodies at a titer of 1 in 8 or greater is considered a good correlate of protection [19]. However, immunity at mucosal surfaces – in particular, the nasopharyngeal and intestinal mucosae, which form the two primary sites of poliovirus replication – is required to prevent viral replication and shedding, and thus halt transmission. In this review, we refer to mucosal immunity as the degree of protection against poliovirus replication at these surfaces. The immune mechanisms responsible for this protection may involve both mucosally targeted IgA responses and transudation of serum antibodies.
The most widely adopted measure of poliovirus-specific mucosal protection involves administration of a challenge dose of OPV. The shedding of vaccine polioviruses in nasopharyngeal and stool samples collected after challenge signifies viral replication in the nasopharynx and gut, respectively, while the absence of shedding can be viewed as an indicator of mucosal protection. During the early stages of poliomyelitis control, both oral–oral and fecal–oral transmission were thought to be common. Community transmission was ascribed to the former and familial transmission to the latter [20]. Today, fecal–oral transmission is thought to predominate in countries at risk of poliomyelitis. Our main focus here is therefore on fecal shedding. Outcomes of challenge studies may include one or more of the following: the proportion of individuals who shed OPV, the duration of viral shedding and the titer of shedding. Typically, a full or partial dose of OPV is administered as challenge. However, the degree to which this approach accurately reflects natural poliovirus exposure (at a significantly lower dose) is uncertain.
Alternative assays for mucosal immunity include the measurement of poliovirus-specific antibody in the nasopharynx, saliva or feces. This has been achieved using a variety of methods, of which the two most common are ELISA for poliovirus-specific IgA [21,22] and neutralization assays [21,23,24]. Although higher titers of nasal IgA have been linked with lower quantities and shorter duration of nasopharyngeal shedding after OPV challenge [25], other studies have not found such correlations [3] and the level of secretory antibody required for protection against poliovirus replication is unknown. Moreover, unlike the microneutralization assay for serum antibodies, a standardized approach for detecting secretory poliovirus-specific antibodies has not been widely adopted, making it difficult to compare findings among studies. Other potential indicators of mucosal immunity include the presence of circulating poliovirus-specific IgA- and IgG-producing cells expressing gut-homing markers (such as α4β7 integrin), which may be examined via fluorescence-activated cell sorting and enzyme-linked immunosorbent spot assay [22]. Again, the relationship between these circulating cells and mucosal protection against poliovirus remains uncertain. Response to OPV challenge is, therefore, viewed as the gold standard for measuring mucosal immunity to poliovirus.
When considering any of the measures above, it is apparent that OPV is capable of inducing a strong mucosal immune response. OPV-immunized individuals typically begin to secrete IgA in the nasopharynx and duodenum 1–3 weeks after vaccine administration [23]. This provides a strong barrier to poliovirus re-infection: in one of the earliest challenge studies, Ghendon and Sanakoyeva observed shedding after mOPV type 1 challenge in 37% of individuals previously vaccinated with three doses of mOPV (in the order type 1, type 3, type 2), with a mean shedding duration of 5 days and a mean viral titer of 150 median tissue culture infectious doses per gram of stool (results similar to those observed after exposure to wild type 1 poliovirus) [2]. By contrast, shedding was documented in 80% of seronegative unvaccinated individuals, with a mean duration of >20 days and a mean viral titer of 141,000 median tissue culture infectious doses per gram. The mucosal protection afforded by OPV has been confirmed by many studies since (reviewed in [26]). However, the duration of this immunity remains equivocal: Grassly et al. documented significant waning of mucosal protection within 1 year of OPV administration among Indian infants [27].
Impact of IPV on mucosal immunity
IPV-only schedules
When administered to naïve individuals, IPV does not induce a classic, class-switched, mucosally targeted IgA response [28]. It has repeatedly been shown that children given IPV without prior exposure to OPV remain susceptible to intestinal poliovirus infection (e.g., [29,30]). In a recent meta-analysis, Hird and Grassly found no significant difference in the odds of viral shedding following OPV challenge among IPV-immunized compared with unvaccinated individuals [26]. By contrast, the odds of shedding were significantly reduced among OPV-immunized individuals, both in comparison to unvaccinated and IPV-immunized individuals.
Although IPV does not protect against poliovirus replication in the intestine, several challenge studies have documented a reduction in the duration and/or titer of viral shedding in IPV-immunized individuals [2,30–34], albeit to a much lesser extent than in OPV-immunized individuals [2,3,32]. In the challenge study described above, Ghendon and Sanakoyeva observed shedding after OPV challenge in 74% of individuals immunized with two doses of IPV, with a mean shedding duration of 12.3 days and a mean viral titer of 13,000 median tissue culture infectious doses per gram of stool (a 10-fold decrease in mean titer compared with unvaccinated individuals) [2]. IPV has also been shown to prevent shedding in the nasopharynx upon either natural exposure to wild virus [20,29,35] or challenge with OPV [33,36,37]. However, evidence to support nasopharyngeal protection remains limited to a small number of studies with few subjects. Higher serum antibody titers following IPV administration have been linked with increased protection against shedding in both the intestine [32,33] and nasopharynx [20,33], although Onorato et al. [3] found no evidence for this trend. Since the majority of these studies were carried out in settings either endemic for poliovirus or concurrently using OPV, prior exposure to live poliovirus and consequent mucosal priming cannot be ruled out. However, the findings suggest that administration of IPV alone may, at least partially, limit the duration and titer of virus excretion in the nasopharynx and intestine following subsequent poliovirus exposure.
Studies of secretory antibodies following IPV administration are generally consistent with the results of challenge studies. Several trials have documented poliovirus-specific IgA [3,21,24] and neutralizing antibodies [21,24] in nasopharyngeal secretions among individuals immunized with enhanced-potency IPV, in some instances at titers comparable to those induced by OPV [3]. However, prior exposure to circulating wild-type or vaccine polioviruses cannot be ruled out in these studies, and Herremans et al. [22] observed no IgA in saliva or stool among 14 IPV-immunized adults from the Netherlands, where prior exposure to OPV or wild virus would be limited. The same group also observed no IgA in the serum of 104 children 4 months after they received their sixth dose of IPV [28]. Onorato et al. documented similar titers of poliovirus-specific IgA in stool samples obtained from infants previously immunized with OPV or IPV; however, significantly fewer OPV than IPV recipients shed poliovirus after challenge [3]. Notably, fecal IgA titers in OPV recipients correlated with protection against viral shedding in stool, while the same was not true for IPV recipients. These findings may relate to differences in the specificity of secretory antibodies induced by the two vaccines, with OPV having been shown to promote a more potent antibody response against poliovirus antigens that have been treated with trypsin (simulating the enzymatic modifications that occur during gastrointestinal transit) [21]. Other mechanisms may also be involved in poliovirus-specific immunity at mucosal sites; in particular, it has been suggested that the reduction in duration and titer of viral shedding following OPV challenge in IPV-immunized individuals may be caused, in part, by transudation of serum IgG rather than the induction of IgA-secreting cells at the mucosal surface [38]. However, the extent of this effect remains uncertain.
Despite the absence of strong mucosal immunity following IPV administration, several industrialized countries – including Sweden, Finland, Iceland and the Netherlands – implemented IPV-only schedules in their routine immunization programs in the late 1950s and subsequently eliminated polio [39]. These countries have never or only occasionally used OPV. The success of IPV-only schedules in these regions has been attributed to their high standards of hygiene and sanitation, which may limit fecal–oral transmission in favor of pharyngeal spread. In such conditions, a reduction in the titer and duration of poliovirus shedding in the nasopharynx among IPV recipients may be sufficient to eliminate viral circulation.
It is unlikely, however, that IPV-only schedules would be sufficient to halt poliovirus transmission in settings where fecal–oral transmission predominates. This has been highlighted on several occasions by poliovirus importations into polio-free countries using IPV-only schedules. The most recent example is Israel, which was certified as polio-free in June 2002 and implemented an IPV-only schedule from 2005 onward. Despite high routine immunization coverage (estimated at approximately 95% in 2012), wild type 1 poliovirus was detected in sewage samples collected in southern Israel between February and July 2013, and subsequent surveillance indicated country-wide spread of the virus [40,41]. This has been attributed to the lack of intestinal mucosal immunity in cohorts of children born after OPV withdrawal who were exclusively immunized with IPV [40,41]. No cases of acute flaccid paralysis were detected, probably owing to the high population immunity against paralysis induced by IPV immunization. Notably, control of the outbreak was achieved through the re-introduction of OPV in routine and campaign-based immunization activities, and OPV is once again part of the routine immunization schedule in Israel (together with IPV).
IPV/OPV mixed schedules
In contrast to IPV-only schedules, the impact of IPV on mucosal immunity when administered alongside OPV in mixed vaccination schedules remains equivocal. Recently, two clinical trials conducted in India demonstrated that IPV has the potential to significantly boost mucosal protection in children previously immunized with OPV. John et al. compared viral excretion following a challenge dose of bOPV among children 1–4 years of age who had received either one dose of IPV, one dose of bOPV, or no vaccine 4 weeks earlier [17]. The proportion of individuals shedding vaccine polioviruses after bOPV challenge was significantly lower among IPV recipients (12 and 8% for serotypes 1 and 3, respectively) compared with those who had received no vaccine (19 and 26% for serotypes 1 and 3, respectively). By contrast, no significant reduction in the proportion of shedders was observed among individuals who received bOPV.
A similar approach was adopted by Jafari et al., who administered either one dose of IPV, one dose of bOPV, or no vaccine to children aged 6–11 months, 5 years or 10 years and compared subsequent response to a challenge dose of bOPV [16]. Prior to enrollment, the children would have received OPV during routine immunization and SIAs. Again, delivery of IPV significantly reduced the proportion of subjects excreting virus after challenge. The reduction in shedding was greatest at 10 years of age and was significantly greater in IPV than OPV recipients in all age groups. These results suggest not only that IPV has the potential to significantly boost mucosal immunity in OPV-primed children but also that this boosting effect may exceed that of an additional dose of OPV. By targeting children aged >6 months, the trials by John et al. [17] and Jafari et al. [16] best capture the potential impact of introducing IPV in SIAs (which target all children up to 5 years of age).
The results of these studies have helped to clarify the impact of IPV on mucosal immunity; the boosting effect of IPV in OPV-primed individuals has previously been explored with inconclusive findings. Herremans et al. compared poliovirus-specific IgA response after administration of IPV among adults previously exposed to either IPV or OPV and observed a boost in mucosal (salivary) IgA response only among individuals previously immunized with OPV [22]. Administration of IPV also induced circulating IgA-producing cells in OPV-primed but not in IPV-primed volunteers. By contrast, studies by Parent du Chatelet et al. [42] and the WHO [43] compared response to a challenge dose of mOPV among infants immunized either with OPV-only schedules or a mixed schedule involving administration of up to three doses of IPV to OPV-primed infants at timings consistent with routine immunization. No significant difference in the proportion of individuals shedding after challenge was observed in either study. The failure to find a mucosal boosting effect of IPV in these studies may be due to the low proportion of subjects excreting virus (range 0.5–8.6% and 4–13%, respectively), limiting the potential to detect significant differences between groups. Another study performed in Oman compared response to a challenge dose of type 3 mOPV at 15 months of age among infants who had received either IPV, tOPV or type 3 mOPV at 9 months (along with five preceding doses of OPV) [44]. No significant differences were observed between the groups, but once again the proportion of individuals shedding the challenge virus was low (13% overall). The proportion of children excreting the challenge virus in the recent studies from India was considerably higher, which is likely due to differing immune profiles in these study populations, the inclusion of older children and the lapse in time since these children were last vaccinated with OPV. Older age groups may be more susceptible to poliovirus re-infection owing to the waning of OPV-induced mucosal immunity [27]. Thus, while concomitant or recent OPV administration may mask the beneficial impact of IPV on mucosal immunity, the boosting effect of IPV may be more apparent when OPV-induced mucosal protection has waned. Overall, the findings suggest that the use of IPV in SIAs will provide a significant boost in mucosal immunity, while the presence of a boosting effect during routine immunization has yet to be confirmed. Table 1 provides a summary of the overall impact of OPV and IPV on humoral and mucosal immunity, and on the risk of VAPP and type 2 cVDPV emergence (the serotype of greatest concern ahead of the upcoming withdrawal of type 2 OPV). Notably, the precise effects of the vaccines will depend on whether they are being used in routine or supplementary immunization efforts and on the vaccination history of the recipients.
Table 1.
Effects of different vaccination strategies on humoral and mucosal immunity to poliovirus types 1, 2 and 3, and the risk of vaccine-associated paralytic poliomyelitis and type 2 circulating vaccine-derived poliovirus emergence.
|
Humoral immunity |
Mucosal immunity |
VAPP risk | Risk of type 2 cVDPV emergence† | |||||
|---|---|---|---|---|---|---|---|---|
| PV1 | PV2 | PV3 | PV1 | PV2 | PV3 | |||
|
Routine immunization | ||||||||
| IPV–IPV–IPV | +++ | +++ | +++ | – | – | – | – | – |
| tOPV–tOPV–tOPV | +++ | +++ | +++ | +++ | +++ | +++ | + | + |
| bOPV–bOPV–bOPV | +++ | ?‡ | +++ | +++ | ?‡ | +++ | + | – |
| tOPV–tOPV–tOPV+IPV | +++ | +++ | +++ | +++ | +++ | +++ | + | + |
| IPV–tOPV–tOPV | +++ | +++ | +++ | +++ | +++ | +++ | – | + |
| bOPV–bOPV–bOPV+IPV | +++ | ?‡ | +++ | +++ | ?‡ | +++ | + | – |
| IPV–bOPV–bOPV | +++ | ?‡ | +++ | +++ | ?‡ | +++ | – | – |
|
Supplementary immunization activities | ||||||||
| tOPV | +++ | +++ | +++ | +++ | +++ | +++ | + | + |
| bOPV | +++ | ?‡ | +++ | +++ | ?‡ | +++ | + | – |
| IPV (low tOPV routine immunization coverage) | +++ | +++ | +++ | + | + | + | – | – |
| IPV (high tOPV routine immunization coverage) | +++ | +++ | +++ | +++ | +++ | +++ | – | – |
–: Absent or negligible; +: Low; ++: Moderate; +++: Strong; bOPV: Bivalent oral poliovirus vaccine; cVDPV: Circulating vaccine-derived poliovirus; IPV: Inactivated poliovirus vaccine; PV1: Type 1 poliovirus; PV2: Type 2 poliovirus; PV3: Type 3 poliovirus; tOPV: Trivalent oral poliovirus vaccine; VAPP: Vaccine-associated paralytic poliomyelitis.
†Refers to the likelihood of a Sabin type 2 virus regaining neurovirulence and transmissibility. Any vaccination strategy involving the administration of type 2 Sabin strains (such as those including tOPV) will carry a risk of type 2 cVDPV emergence.
‡There may be some heterotypic immunity among the poliovirus strains. The extent of this immunity remains equivocal, but is likely to be short-lived and of limited significance [55–57].
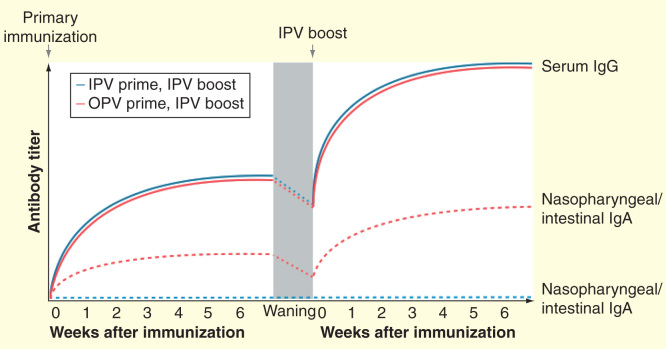
Although mechanisms by which IPV may boost mucosal immunity remain unclear, prior exposure to live poliovirus appears to be a requirement. Several studies have examined the impact of mixed vaccination schedules in which IPV was administered prior to OPV and observed no significant impact of IPV on mucosal protection, as measured either by a challenge dose of OPV [45] or nasopharyngeal IgA response [24,38]. Thus, prior exposure to IPV does not appear to alter the mucosal immunity induced by OPV. The immunological pathway for the boosting effect of IPV in OPV-primed individuals may rely on activation of memory cells formed after exposure to the live virus (i.e., OPV). Dendritic cells located in the gut mucosa have been shown to promote the gut-homing receptor α4β7 integrin on activated B and T cells, whereas this marker is not induced by dendritic cells isolated from the spleen [46]. Herremans et al. observed that a large percentage of circulating IgA-producing cells induced following administration of IPV to OPV-immunized adults expressed α4β7 integrin [22]. Accordingly, the authors concluded that presentation of antigens from IPV at peripheral lymph nodes may induce the proliferation of memory cells formed after OPV exposure, resulting in a circulating population of IgA-producing cells expressing gut-homing receptors. IPV has also been shown to induce an increase in poliovirus-specific CD4+ T cells expressing α4β7 in adults previously immunized with OPV [47], while a boost in secretory IgA response was observed following administration of IPV to individuals previously exposed to wild-type poliovirus [48]. Figure 2 illustrates the different effects of IPV boosting in individuals previously immunized with OPV or IPV, based on the findings reported above.
Figure 2.
Schematic representation of serum and secretory antibody responses following administration of IPV to OPV- or IPV-primed individuals. Primary immunization with OPV induces both a humoral response (serum IgG) and a mucosal response (intestinal and nasopharyngeal IgA), whereas IPV induces only a humoral response and potentially a limited mucosal response in the nasopharynx. However, following the waning of OPV-induced mucosal immunity, administration of IPV is capable of boosting both humoral and mucosal immunity in OPV-primed individuals. The same boosting of humoral immunity does not occur among individuals without prior OPV exposure.
IPV: Inactivated poliovirus vaccine; OPV: Oral poliovirus vaccine.
Informed by [16,17,22,23].
IPV in the polio eradication endgame
There are two contexts in which IPV will be used as the GPEI seeks to secure the eradication of polio: routine immunization and SIAs. In each of these settings, the potential boosting of protection against intestinal poliovirus replication among OPV-immunized children has significant benefits for the eradication endgame, as discussed below.
IPV in routine immunization
The WHO now recommends that all countries introduce at least one dose of IPV in routine immunization [1], a measure primarily motivated by the need to provide adequate poliovirus-specific immunity by alternative means prior to OPV withdrawal. In countries presently immunizing infants with OPV at 6, 10 and 14 weeks of age and opting to introduce one dose of IPV, it is recommended that this dose be co-administered with OPV at 14 weeks of age. This strategy avoids the need for additional vaccination visits. Moreover, by administering IPV later in infancy, interference by maternal antibodies will be diminished, thus improving immunogenicity [43,49–51]. An additional benefit of introducing IPV in routine immunization may be a boosting of the mucosal immune response induced by preceding OPV doses. To date, this boosting effect has been demonstrated only among older children (>6 months of age) [16,17], while observations from younger infants remain equivocal [42,43]. However, it is possible that a similar boosting of mucosal immunity may occur in early infancy, either in the strength or duration of protection. Several ongoing trials may help to clarify this possibility. In particular, studies currently underway in Latin America (ClinicalTrials.gov identifier NCT01831050) and Pakistan (NCT02189811) will assess the impact of mixed OPV/IPV administration (according to the schedule outlined above) on the response to OPV challenge.
In countries presently immunizing infants with OPV at 2, 4 and 6 months of age, the WHO recommends that IPV be co-administered at either 4 or 6 months of age. Again, the administration of IPV to OPV-primed infants in this schedule may enhance mucosal immunity, although such an effect has yet to be demonstrated. An alternative strategy being investigated by a trial in Chile (NCT01841671) is to immunize infants with IPV prior to OPV during routine immunization. This strategy may reduce the risk of VAPP, which is highest at the first exposure to OPV. However, it is unlikely that the IPV dose(s) will have any significant beneficial effects on mucosal immunity owing to the lack of prior live virus exposure in such a schedule. In ideal circumstances, a routine immunization schedule of IPV–OPV–IPV, or IPV followed by co-administration of IPV and OPV at subsequent visits, might minimize the risk of VAPP while maximizing humoral and mucosal protection. The WHO recommendation is that ‘at least one dose of IPV’ should be introduced in countries currently relying solely on OPV in advance of the withdrawal of serotype 2 OPV [1]. Financial constraints mean that most countries will introduce just a single IPV dose, although this may be a prelude to adoption of a more extensive IPV schedule in routine programs in the future, perhaps through the use of combination products.
A major challenge with the planned introduction of IPV is the persistently low routine immunization coverage in many countries either endemic for polio or at high risk of virus importation. Coverage at 14 weeks of age has been identified as a particular problem owing to dropout across sequential routine vaccination visits [52], which poses a challenge to the proposed introduction of IPV at this dose. High-risk countries typically administer tOPV in routine immunization and rely heavily on SIAs (mainly using bOPV) to improve coverage, leaving large populations vulnerable to type 2 poliovirus. These countries have also experienced repeated type 2 cVDPV emergences and prolonged outbreaks [53,54]. A primary focus of the GPEI strategic plan is to strengthen routine immunization, so as to ensure adequate protection against type 2 poliovirus in the form of both humoral and mucosal immunity prior to the switch from tOPV to bOPV (accompanying the withdrawal of serotype 2 OPV). There may be some protection against type 2 virus in populations given bOPV due to heterotypic immunity (Table 1); specifically, antibodies induced by poliovirus types 1 and/or 3 may have some degree of cross-reactivity with epitopes on type 2 poliovirus [55,56]. However, heterotypic protection against shedding and poliomyelitis is likely to be short-lived and of limited significance [57].
IPV in SIAs
Wide-scale and frequent SIAs targeting children under 5 years of age have been rolled out to compensate for low routine immunization coverage in infected countries. In Pakistan and Nigeria, in particular, there has been a heavy reliance on SIAs. Traditionally, tOPV has been used in SIAs; however, the transition to mOPVs and bOPVs targeting serotypes 1 and 3 in recent years has taken place in endemic and high-risk countries to target these remaining wild virus serotypes. To compensate for the limited use of type 2 OPV in these countries and to boost mucosal immunity to serotypes 1 and 3, IPV has recently been used alongside bOPV in parts of Kenya, Nigeria and Pakistan. The introduction of IPV into SIAs poses particular challenges: in addition to the increased costs of the vaccine, the campaigns require extra training for healthcare workers, are limited to fixed health posts because of the challenges in administering the vaccine through house-to-house visits and require more intensive social mobilization efforts to promote acceptance of the vaccine. Despite these challenges, SIAs involving IPV to date have achieved high coverage and good acceptance [58], leading the GPEI to plan further IPV SIAs in high-risk areas of Pakistan, Afghanistan and Nigeria in 2015. In light of the potential boosting of mucosal protection among OPV-primed children [16,17], the introduction of IPV into SIAs may contribute to the reduction in viral transmission which is required to halt poliovirus circulation in endemic regions. To date, the boosting effect of IPV on mucosal immunity has been demonstrated only among individuals enrolled in clinical trials. With appropriate surveillance, the use of IPV in SIAs may enable the population-level impacts of this boosting effect on poliovirus transmission to be determined.
IPV may also play an important role in boosting mucosal immunity to type 2 poliovirus prior to the withdrawal of serotype 2 OPV (currently planned for 2016). To minimize the risk of type 2 cVDPV emergence, several tOPV campaigns will likely be conducted prior to the synchronized withdrawal of type 2 OPV. Including IPV alongside OPV in these supplementary campaigns may have a greater boosting effect on mucosal immunity compared to OPV alone, thereby decreasing the likelihood of a type 2 VDPV emerging and spreading following the final doses of tOPV. A major challenge, however, will be the supply of sufficient quantities of IPV, most likely limiting IPV use to key high-risk areas.
Environmental surveillance
Following the globally coordinated withdrawal of OPV (starting with serotype 2) [1], IPV will be the only poliovirus vaccine in use. At this point, the beneficial boosting effects of IPV on mucosal immunity will be lost, leading to an increased risk of silent transmission of wild polioviruses or cVDPVs. The recent observation of silent poliovirus circulation in Israel emphasizes the importance of environmental surveillance in detecting viral transmission within populations immunized with IPV alone. This will be of particular importance in countries with poor standards of hygiene and sanitation, where there is greater potential for fecal–oral transmission. Currently, fewer than 30 countries systematically report the results of environmental surveillance to the GPEI. The expansion of this surveillance system will be necessary to help establish that wild poliovirus transmission has been brought to a halt after the cessation of paralytic cases and, subsequently, to monitor for vaccine virus circulation after OPV withdrawal [1,59]. Response to the detection of wild polioviruses or cVDPVs may involve the temporary re-introduction of OPV in the affected regions. However, the appropriate scale of such a response is unclear, and represents an important topic of current research.
Alternative IPV formulations
Currently, the cost of full-dose intramuscular IPV for Gavi-supported countries is nearly seven-times that of tOPV (US$0.15 for tOPV and US$1 for IPV) [60,61], posing an immense challenge to the financial feasibility of wide-scale introduction. Also, the time required for manufacturers to expand their IPV production capacity will likely exceed the immediate demand for the vaccine, limiting its use in SIAs, particularly in the period preceding type 2 OPV withdrawal. Numerous cost- and antigen-reduction strategies for the short to medium term are therefore being explored, and these strategies may impact the boosting effect of IPV on mucosal immunity.
One option to reduce the cost and meet the rapid increase in demand is to administer fractional (1/5) doses of IPV intradermally. The use of fractional IPV (fIPV) as a substitute for full-dose IPV would reduce the amount of antigen required, thereby increasing the number of available doses and reducing the cost per vaccination. Evidence regarding the immunogenicity of fIPV is inconclusive: while several studies have demonstrated that fIPV induces similar seroconversion rates to full-dose vaccine [62–64], others have found that fIPV induces significantly lower seroconversion rates [50,65,66], and antibody titers have generally been found to be lower following fIPV compared with full-dose IPV [50,64]. Data regarding the impact of fIPV on mucosal immunity are currently limited. However, Mohammed et al. observed shedding in a significantly greater proportion of children who had received three intradermal doses of fIPV compared with recipients of three full intramuscular doses of IPV following type 1 mOPV challenge [64]. Further evaluation of both humoral and mucosal immunogenicity following receipt of fIPV is required to determine whether this vaccine is able to provide adequate population protection in the polio eradication endgame.
Alternative approaches include the development of IPV from attenuated Sabin strains or further attenuated polioviruses. The switch to Sabin IPV (sIPV) would eliminate the large volumes of wild polioviruses generated during traditional IPV production, thereby enabling the safe production of IPV in developing countries [67]. sIPV demonstrated comparable immunogenicity to conventional IPV (i.e., Salk IPV) in a randomized, controlled dose-escalation trial carried out in Poland [68], and production of the first sIPV was approved in China in January 2015. However, whether sIPV produces a similar mucosal boosting effect to Salk IPV is yet to be determined. Other strategies being explored include the addition of mucosal adjuvants to IPV with the aim of improving intestinal response to the vaccine [69] and the development of stable Sabin strains that are less likely to regain neurovirulence [70].
In the longer term, many countries may choose to incorporate IPV into routine immunization schedules within combination vaccines (a strategy currently adopted in many high-income countries). Although several existing vaccines combine IPV, diphtheria, acellular pertussis, tetanus, Haemophilus influenzae type B and (in some cases) hepatitis B antigens, efforts are underway to develop hexavalent vaccines that include both IPV and whole-cell pertussis (wP) [71]. wP vaccines are cheaper to produce than acellular pertussis vaccines, and are therefore the preferred formulation in many low-income countries. However, alternative methods of wP inactivation will be required for vaccines combining wP alongside IPV, as thimerosol (which is currently used to inactivate wP) has been shown to negatively impact IPV potency [72]. The potential impact of these combination vaccines on poliovirus-specific mucosal immunity will be an important consideration, particularly if they enter the market prior to OPV cessation.
Expert commentary
IPV is playing a key role in the eradication of polio, and its potential influence on mucosal immunity may well be crucial to the success of the eradication endgame. While previously thought to have only a minimal influence on mucosal immunity, it is now recognized that IPV significantly boosts the mucosal protection of individuals whose primary response to OPV has waned. Moreover, this effect may exceed the protection conferred by additional doses of OPV. Although the significant boosting effect of IPV has currently been demonstrated only in children aged >6 months, the incorporation of at least one dose of IPV in routine immunization programs (as recommended by the WHO) may enhance protection against subsequent intestinal infection. Where resources permit, there is also a strong rationale for using IPV alongside OPV in SIAs (as currently being carried out in high-risk endemic areas); it is likely that doing so will have a greater effect on both humoral and mucosal protection than the use of OPV alone.
Five-year view
The transition from OPV to IPV poses a complex set of challenges for the GPEI. In this review, we have focused on the impact of IPV on mucosal immunity and the implications of this for the polio eradication endgame. Other important considerations include how best to recognize and respond to type 2 cVDPV emergence at the time of and after withdrawal of serotype 2 OPV. Following withdrawal, an increasing cohort of newborns will lack intestinal immunity to type 2 poliovirus, and may also have incomplete humoral protection owing to gaps in routine coverage, putting them at risk of paralytic poliomyelitis from residual circulation of type 2 cVDPV. Similar issues will also be encountered during the subsequent withdrawal of OPV serotypes 1 and 3. In the meantime, several ongoing trials may shed further light on the impact of IPV on mucosal immunity, the degree of cross-immunity induced by OPV, and the potential value of alternative vaccine formulations, including fIPV and sIPV. In preparation for the introduction of IPV, there will be a continuing emphasis on strengthening routine immunization systems, particularly in high-risk areas. Finally, given that IPV alone is insufficient to prevent the silent transmission of wild-type or vaccine-derived polioviruses, heightened environmental surveillance and clear response planning will be necessary to identify and halt poliovirus transmission following the planned withdrawal of OPV.
Acknowledgement
The authors are grateful to Hannah Slater for providing valuable comments on the manuscript.
Financial & competing interests disclosure
E Parker is a former Assistant Commissioning Editor at Expert Review of Vaccines. E Parker and K O’Reilly receive funding from the UK Medical Research Council; N Molodecky is funded by the Bill & Melinda Gates Foundation; M Pons-Salort is funded by the Wellcome Trust (106073/Z/14/Z); and N Grassly is funded by the Bill & Melinda Gates Foundation (#OPP1099374), WHO (2013/363982) and UK Medical Research Council. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Key issues.
The induction of robust mucosal immunity is necessary to halt poliovirus transmission, and is therefore crucial to the success of the polio eradication endgame.
Inactivated poliovirus vaccine (IPV) has long been thought to induce only limited mucosal immunity; however, it has recently been shown that the vaccine may significantly boost mucosal protection among individuals who have previously received oral poliovirus vaccine (OPV). This effect may exceed the boost in mucosal immunity induced by additional doses of OPV.
It is now recommended that at least one dose of IPV be included in all routine immunization programs, and the vaccine will also increasingly be used alongside OPV in supplementary immunization activities conducted in polio-endemic countries. In both these contexts, the boosting of mucosal immunity by IPV may help eliminate poliovirus transmission.
Following OPV withdrawal, environmental surveillance will be crucial for the detection of poliovirus circulation, owing to the potential for silent poliovirus circulation in populations immunized only with IPV.
References
Papers of special note has been highlighted as
• of interest
•• of considerable interest
- Global Polio Eradication Initiative 2013 Polio eradication and endgame strategic plan (2013-2018)
- Ghendon YZ, Sanakoyeva II. Comparison of the resistance of the intestinal tract to poliomyelitis virus (Sabin’s strains) in persons after naturally and experimentally acquired immunity. Acta Virol. 1961;5:265–73. [Google Scholar]
- • .Onorato IM, Modlin JF, McBean AM, et al. Mucosal immunity induced by enhanced-potency inactivated and oral polio vaccines. J Infect Dis. 1991;163:1–6. doi: 10.1093/infdis/163.1.1. [DOI] [PubMed] [Google Scholar]; • Study comparing the impact of inactivated poliovirus vaccine (IPV) and oral poliovirus vaccine (OPV) on both poliovirus-specific secretory antibodies and shedding following OPV challenge.
- Belyakov IM, Ahlers JD. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J Immunol. 2009;183(11):6883–92. doi: 10.4049/jimmunol.0901466. [DOI] [PubMed] [Google Scholar]
- Hanlon P, Hanlon L, Marsh V, et al. Serological comparisons of approaches to polio vaccination in the Gambia. Lancet. 1987;1:800–1. doi: 10.1016/s0140-6736(87)92818-2. [DOI] [PubMed] [Google Scholar]
- Moriniere BJ, van Loon FP, Rhodes PH, et al. Immunogenicity of a supplemental dose of oral versus inactivated poliovirus vaccine. Lancet. 1993;341:1545–50. doi: 10.1016/0140-6736(93)90693-b. [DOI] [PubMed] [Google Scholar]
- Stickle G. Observed and expected poliomyelitis in the United States. 1958-1961. Am J Public Health Nations Health. 1964;54:1222–9. doi: 10.2105/ajph.54.8.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter RW, John TJ, Jain H, et al. Immunogenicity of bivalent types 1 and 3 oral poliovirus vaccine: a randomised, double-blind, controlled trial. Lancet. 2010;376:1682–8. doi: 10.1016/S0140-6736(10)61230-5. [DOI] [PubMed] [Google Scholar]
- Cochi SL, Linkins RW. The final phase of polio eradication: new vaccines and complex choices. J Infect Dis. 2012;205:169–71. doi: 10.1093/infdis/jir727. [DOI] [PubMed] [Google Scholar]
- Rationale and timelines for OPV withdrawal. www.who.int/immunization/diseases/poliomyelitis/endgame_objective2/oral_polio_vaccine/planning/en/ WHO. Available from: [Last accessed 5 March 2015]
- Platt LR, Estivariz CF, Sutter RW. Vaccine-associated paralytic poliomyelitis: a review of the epidemiology and estimation of the global burden. J Infect Dis. 2014;210(Suppl 1):S380–9. doi: 10.1093/infdis/jiu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson N, Kew OM. From emergence to eradication: the epidemiology of poliomyelitis deconstructed. Am J Epidemiol. 2010;172:1213–29. doi: 10.1093/aje/kwq320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CC, Diop OM, Sutter RW, Kew OM. Vaccine-derived polioviruses. J Infect Dis. 2014;210(Suppl 1):S283–93. doi: 10.1093/infdis/jiu295. [DOI] [PubMed] [Google Scholar]
- Jenkins HE, Aylward RB, Gasasira A, et al. Implications of a circulating vaccine-derived poliovirus in Nigeria. N Engl J Med. 2010;362:2360–9. doi: 10.1056/NEJMoa0910074. [DOI] [PubMed] [Google Scholar]
- Mangal TD, Aylward RB, Mwanza M, et al. Key issues in the persistence of poliomyelitis in Nigeria: a case-control study. Lancet Glob Health. 2014;2:e90–7. doi: 10.1016/S2214-109X(13)70168-2. [DOI] [PubMed] [Google Scholar]
- •• .Jafari H, Deshpande JM, Sutter RW, et al. Polio eradication. Efficacy of inactivated poliovirus vaccine in India. Science. 2014;345:922–5. doi: 10.1126/science.1255006. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• One of two recent studies demonstrating the potential of IPV to boost mucosal immunity in OPV-primed children.
- •• .John J, Giri S, Karthikeyan AS, et al. Effect of a single inactivated poliovirus vaccine dose on intestinal immunity against poliovirus in children previously given oral vaccine: an open-label, randomised controlled trial. Lancet. 2014;384:1505–12. doi: 10.1016/S0140-6736(14)60934-X. [DOI] [PubMed] [Google Scholar]; •• One of two recent studies demonstrating the potential of IPV to boost mucosal immunity in OPV-primed children.
- WHO Manual of laboratory methods for testing of vaccines used in the WHO Expanded Programme on Immunization. WHO/VSQ/9704. 1997
- Hammon WM, Coriell LL, Ludwig EH, et al. Evaluation of Red Cross gamma globulin as a prophylactic agent for poliomyelitis. 5. Reanalysis of results based on laboratory-confirmed cases. J Am Med Assoc. 1954;156:21–7. doi: 10.1001/jama.1954.02950010023009. [DOI] [PubMed] [Google Scholar]
- Marine WM, Chin TD, Gravelle CR. Limitation of fecal and pharyngeal poliovirus excretion in Salk-vaccinated children. A family study during a type 1 poliomyelitis epidemic. Am J Hyg. 1962;76:173–95. doi: 10.1093/oxfordjournals.aje.a120272. [DOI] [PubMed] [Google Scholar]
- Zhaori G, Sun M, Faden HS, Ogra PL. Nasopharyngeal secretory antibody response to poliovirus type 3 virion proteins exhibit different specificities after immunization with live or inactivated poliovirus vaccines. J Infect Dis. 1989;159:1018–24. doi: 10.1093/infdis/159.6.1018. [DOI] [PubMed] [Google Scholar]
- Herremans TM, Reimerink JH, Buisman AM, et al. Induction of mucosal immunity by inactivated poliovirus vaccine is dependent on previous mucosal contact with live virus. J Immunol. 1999;162:5011–18. [PubMed] [Google Scholar]
- Ogra PL, Karzon DT, Righthand F, MacGillivray M. Immunoglobulin response in serum and secretions after immunization with live and inactivated poliovaccine and natural infection. N Engl J Med. 1968;279:893–900. doi: 10.1056/NEJM196810242791701. [DOI] [PubMed] [Google Scholar]
- Faden H, Modlin JF, Thoms ML, et al. Comparative evaluation of immunization with live attenuated and enhanced-potency inactivated trivalent poliovirus vaccines in childhood: systemic and local immune responses. J Infect Dis. 1990;162:1291–7. doi: 10.1093/infdis/162.6.1291. [DOI] [PubMed] [Google Scholar]
- Ogra PL, Karzon DT. Formation and function of poliovirus antibody in different tissues. Prog Med Virol. 1971;13:156–93. [Google Scholar]
- • .Hird TR, Grassly NC. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog. 2012;8:e1002599. doi: 10.1371/journal.ppat.1002599. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Systematic review and meta-analysis examining the impact of OPV and IPV on response to OPV challenge.
- Grassly NC, Jafari H, Bahl S, et al. Waning intestinal immunity after vaccination with oral poliovirus vaccines in India. J Infect Dis. 2012;205:1554–61. doi: 10.1093/infdis/jis241. [DOI] [PubMed] [Google Scholar]
- Herremans T, Kimman TG, Conyn-Van Spaendonck MA, et al. Immunoglobulin A as a serological marker for the (silent) circulation of poliovirus in an inactivated poliovirus-vaccinated population. Clin Infect Dis. 2002;34:1067–75. doi: 10.1086/339489. [DOI] [PubMed] [Google Scholar]
- Davis DC, Lipson MJ, Carver DH, et al. The degree and duration of poliomyelitis virus excretion among vaccinated household contacts of clinical cases of poliomyelitis. Pediatrics. 1958;22:33–40. [PubMed] [Google Scholar]
- Cuba IPV Study Collaborative Group Randomized, placebo-controlled trial of inactivated poliovirus vaccine in Cuba. N Engl J Med. 2007;356:1536–44. doi: 10.1056/NEJMoa054960. [DOI] [PubMed] [Google Scholar]
- Enders-Ruckle G, Siegert R. [Viral excretion and antibody formation after use of a live trivalent poliomyelitis vaccine (Cox-Lederle)] Dtsch Med Wochenschr. 1961;86:1999–2008. doi: 10.1055/s-0028-1113045. [DOI] [PubMed] [Google Scholar]
- Henry JL, Jaikaran ES, Davies JR, et al. A study of poliovaccination in infancy: excretion following challenge with live virus by children given killed or living poliovaccine. J Hyg (Lond) 1966;64:105–20. doi: 10.1017/s0022172400040389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezen WP, McCollough RH, Lamb GA, Chin TD. Quantitative relationship of preexisting homotypic antibodies to excretion of poliovirus types 1, 2, and 3 following the feeding of trivalent attenuated poliovirus vaccine. Am J Epidemiol. 1969;90:146–56. doi: 10.1093/oxfordjournals.aje.a121058. [DOI] [PubMed] [Google Scholar]
- Laassri M, Lottenbach K, Belshe R, et al. Effect of different vaccination schedules on excretion of oral poliovirus vaccine strains. J Infect Dis. 2005;192:2092–8. doi: 10.1086/498172. [DOI] [PubMed] [Google Scholar]
- Wehrle PF, Carbonaro O, Day PA, et al. Transmission of polioviruses. III. Prevalence of polioviruses in pharyngeal secretions of infected household contacts of patients with clinical disease. Pediatrics. 1961;27:762–4. [PubMed] [Google Scholar]
- Dick GW, Dane DS, McAlister J, et al. Vaccination against poliomyelitis with live virus vaccines. 7. Effect of previous Salk vaccination on virus excretion. Br Med J. 1961;2:266–9. doi: 10.1136/bmj.2.5247.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezen WP, Lamb GA, Belden EA, Chin TD. Quantitative relationship of preexisting homotypic antibodies to the excretion of attenuated poliovirus type 1. Am J Epidemiol. 1966;83:224–37. doi: 10.1093/oxfordjournals.aje.a120578. [DOI] [PubMed] [Google Scholar]
- Ogra PL. Mucosal immune response to poliovirus vaccines in childhood. Rev Infect Dis. 1984;6(Suppl 2):S361–8. doi: 10.1093/clinids/6.supplement_2.s361. [DOI] [PubMed] [Google Scholar]
- Murdin AD, Barreto L, Plotkin S. Inactivated poliovirus vaccine: past and present experience. Vaccine. 1996;14:735–46. doi: 10.1016/0264-410x(95)00211-i. [DOI] [PubMed] [Google Scholar]
- Anis E, Kopel E, Singer SR, et al. Insidious reintroduction of wild poliovirus into Israel, 2013. Euro Surveill. 2013;18((38):pii/20651. doi: 10.2807/1560-7917.es2013.18.38.20586. [DOI] [PubMed] [Google Scholar]
- Kopel E, Kaliner E, Grotto I. Lessons from a public health emergency – importation of wild poliovirus to Israel. N Engl J Med. 2014;371:981–3. doi: 10.1056/NEJMp1406250. [DOI] [PubMed] [Google Scholar]
- Parent du Chatelet I, Merchant AT, Fisher-Hoch S, et al. Serological response and poliovirus excretion following different combined oral and inactivated poliovirus vaccines immunization schedules. Vaccine. 2003;21:1710–18. doi: 10.1016/s0264-410x(02)00523-6. [DOI] [PubMed] [Google Scholar]
- WHO Collaborative Study Group on Oral and Inactivated Poliovirus Vaccines Combined immunization of infants with oral and inactivated poliovirus vaccines: results of a randomized trial in The Gambia, Oman, and Thailand. Bull World Health Organ. 1996;74:253–68. [PMC free article] [PubMed] [Google Scholar]
- Sutter RW, Suleiman AJ, Malankar P, et al. Trial of a supplemental dose of four poliovirus vaccines. N Engl J Med. 2000;343:767–73. doi: 10.1056/NEJM200009143431103. [DOI] [PubMed] [Google Scholar]
- Modlin JF, Halsey NA, Thoms ML, et al. Humoral and mucosal immunity in infants induced by three sequential inactivated poliovirus vaccine – live attenuated oral poliovirus vaccine immunization schedules. J Infect Dis. 1997;175:S228–34. doi: 10.1093/infdis/175.supplement_1.s228. [DOI] [PubMed] [Google Scholar]
- Pasetti MF, Simon JK, Sztein MB, Levine MM. Immunology of gut mucosal vaccines. Immunol Rev. 2011;239:125–48. doi: 10.1111/j.1600-065X.2010.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg C, Maier R, Meyerhans A. Gut-homing (alpha(4)beta(7)(+)) Th1 memory responses after inactivated poliovirus immunization in poliovirus orally pre-immunized donors. J Gen Virol. 2004;85:1571–9. doi: 10.1099/vir.0.79919-0. [DOI] [PubMed] [Google Scholar]
- Herremans MM, van Loon AM, Reimerink JH, et al. Poliovirus-specific immunoglobulin A in persons vaccinated with inactivated poliovirus vaccine in The Netherlands. Clin Diagn Lab Immunol. 1997;4:499–503. doi: 10.1128/cdli.4.5.499-503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estivariz CF, Pallansch MA, Anand A, et al. Poliovirus vaccination options for achieving eradication and securing the endgame. Curr Opin Virol. 2013;3:309–15. doi: 10.1016/j.coviro.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resik S, Tejeda A, Sutter RW, et al. Priming after a fractional dose of inactivated poliovirus vaccine. N Engl J Med. 2013;368:416–24. doi: 10.1056/NEJMoa1202541. [DOI] [PubMed] [Google Scholar]
- Grassly NC. Immunogenicity and effectiveness of routine immunization with 1 or 2 doses of inactivated poliovirus vaccine: systematic review and meta-analysis. J Infect Dis. 2014;210(Suppl 1):S439–46. doi: 10.1093/infdis/jit601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Pallansch MA, Estivariz CF, et al. Estimating the likely coverage of inactivated poliovirus vaccine in routine immunization: evidence from demographic and health surveys. J Infect Dis. 2014;210(Suppl 1):S465–74. doi: 10.1093/infdis/jiu343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassilak S, Pate MA, Wannemuehler K, et al. Outbreak of type 2 vaccine-derived poliovirus in Nigeria: emergence and widespread circulation in an underimmunized population. J Infect Dis. 2011;203:898–909. doi: 10.1093/infdis/jiq140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif S, Abbasi BH, Khurshid A, et al. Evolution and circulation of type-2 vaccine-derived polioviruses in Nad Ali district of Southern Afghanistan during June. 2009-February 2011. PLoS One. 2014;9:e88442. doi: 10.1371/journal.pone.0088442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A, Melnick JL. Heterotypic antibody response after feeding of monovalent attenuated live poliovaccine. N Engl J Med. 1962;267:1228–30. doi: 10.1056/NEJM196212132672404. [DOI] [PubMed] [Google Scholar]
- Hovi T, Roivainen M. Peptide antisera targeted to a conserved sequence in poliovirus capsid VP1 cross-react widely with members of the genus Enterovirus. J Clin Microbiol. 1993;31(5):1083–7. doi: 10.1128/jcm.31.5.1083-1087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin AB. Transitory appearance of type 2 neutralizing antibody in patients infected with type 1 poliomyelitis virus. J Exp Med. 1952;96:99–106. doi: 10.1084/jem.96.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Implementation of inactivated polio vaccine (IPV) campaigns Global Polio Eradication Initiative. www.polioeradication.org/Portals/0/Document/Aboutus/Governance/IMB/11IMBMeeting/2.5_11IMB.pdf. Available from: [Last accessed 05 March 2015]
- Asghar H, Diop OM, Weldegebriel G, et al. Environmental surveillance for polioviruses in the Global Polio Eradication Initiative. J Infect Dis. 2014;210(Suppl 1):S294–303. doi: 10.1093/infdis/jiu384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint GPEI-GAVI statement on the availability and price of inactivated polio vaccine. www.unicef.org/media/media_72738.html. UNICEF. Available from: [Last accessed 19 March 2015]
- Vaccine price data. UNICEF. www.unicef.org/supply/index_57476.html. Available from: [Last accessed 19 March 2015]
- Samuel BU, Cherian T, Sridharan G, et al. Immune response to intradermally injected inactivated poliovirus vaccine. Lancet. 1991;338:343–4. doi: 10.1016/0140-6736(91)90480-d. [DOI] [PubMed] [Google Scholar]
- Nirmal S, Cherian T, Samuel BU, et al. Immune response of infants to fractional doses of intradermally administered inactivated poliovirus vaccine. Vaccine. 1998;16:928–31. doi: 10.1016/s0264-410x(97)00293-4. [DOI] [PubMed] [Google Scholar]
- Mohammed AJ, AlAwaidy S, Bawikar S, et al. Fractional doses of inactivated poliovirus vaccine in Oman. N Engl J Med. 2010;362:2351–9. doi: 10.1056/NEJMoa0909383. [DOI] [PubMed] [Google Scholar]
- Estivariz CF, Jafari H, Sutter RW, et al. Immunogenicity of supplemental doses of poliovirus vaccine for children aged 6-9 months in Moradabad, India: a community-based, randomised controlled trial. Lancet Infect Dis. 2012;12:128–35. doi: 10.1016/S1473-3099(11)70190-6. [DOI] [PubMed] [Google Scholar]
- Resik S, Tejeda A, Mach O, et al. Immune responses after fractional doses of inactivated poliovirus vaccine using newly developed intradermal jet injectors: a randomized controlled trial in Cuba. Vaccine. 2015;33:307–13. doi: 10.1016/j.vaccine.2014.11.025. [DOI] [PubMed] [Google Scholar]
- Bakker WA, Thomassen YE, van’t Oever AG, et al. Inactivated polio vaccine development for technology transfer using attenuated Sabin poliovirus strains to shift from Salk-IPV to Sabin-IPV. Vaccine. 2011;29:7188–96. doi: 10.1016/j.vaccine.2011.05.079. [DOI] [PubMed] [Google Scholar]
- Verdijk P, Rots NY, van Oijen MG, et al. Safety and immunogenicity of a primary series of Sabin-IPV with and without aluminum hydroxide in infants. Vaccine. 2014;32:4938–44. doi: 10.1016/j.vaccine.2014.07.029. [DOI] [PubMed] [Google Scholar]
- Steil BP, Jorquera P, Westdijk J, et al. A mucosal adjuvant for the inactivated poliovirus vaccine. Vaccine. 2014;32:448–63. doi: 10.1016/j.vaccine.2013.11.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macadam AJ, Ferguson G, Stone DM, et al. Rational design of genetically stable, live-attenuated poliovirus vaccines of all three serotypes: relevance to poliomyelitis eradication. J Virol. 2006;80:8653–63. doi: 10.1128/JVI.00370-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood K, Pelkowski S, Atherly D, et al. Hexavalent IPV-based combination vaccines for public-sector markets of low-resource countries. Hum Vaccin Immunother. 2013;9:1894–902. doi: 10.4161/hv.25407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer LA, McInnis J, Patel A, et al. Deleterious effect of thimerosal on the potency of inactivated poliovirus vaccine. Vaccine. 1994;12:851–6. doi: 10.1016/0264-410x(94)90296-8. [DOI] [PubMed] [Google Scholar]
- Status update on country planning for IPV introduction. www.who.int/entity/immunization/diseases/poliomyelitis/endgame_objective2/IPVmap_2015_May.pptx?ua=1. WHO. Available from: [Last accessed 14 May 2015]