Abstract
The study investigated the effect of bright blue-enriched versus blue-suppressed indoor light on sleep and wellbeing of healthy participants over 65 years. Twenty-nine participants in 20 private houses in a uniform settlement in Copenhagen were exposed to two light epochs of 3 weeks with blue-enriched (280 lux) and 3 weeks blue-suppressed (240 lux) indoor light or vice versa from 8 to 13 pm in a randomized cross-over design. The first light epoch was in October, the second in November and the two light epochs were separated by one week. Participants were examined at baseline and at the end of each light epoch. The experimental indoor light was well tolerated by the majority of the participants. Sleep duration was 7.44 (95% CI 7.14–7.74) hours during blue-enriched conditions and 7.31 (95% CI 7.01–7.62) hours during blue-suppressed conditions (p = 0.289). Neither rest hours, chromatic pupillometry, nor saliva melatonin profile showed significant changes between blue-enriched and blue-suppressed epochs. Baseline Pittsburgh Sleep Quality Index (PSQI) was significantly worse in females; 7.62 (95% CI 5.13–10.0) versus 4.06 (95% CI 2.64–5.49) in males, p = 0.009. For females, PSQI improved significantly during blue-enriched light exposure (p = 0.007); no significant changes were found for males. The subjective grading of indoor light quality doubled from participants habitual indoor light to the bright experimental light, while it was stable between light epochs, although there were clear differences between blue-enriched and blue-suppressed electrical light conditions imposed. Even though the study was carried out in the late autumn at northern latitude, the only significant difference in Actiwatch-measured total blue light exposure was from 8 to 9 am, because contributions from blue-enriched, bright indoor light were superseded by contributions from daylight.
Keywords: Actiwatch, circadian rhythm, light for elderly, light intensity, melatonin, pupillometry
INTRODUCTION
Disturbances in circadian rhythms and disturbed sleep are important problems in modern society and the prevalence is increasing (Pallesen, 2014). Both hypersomnia (Jennum, 2014) and insomnia (Bin, 2012) are associated with increased socioeconomic costs related to reduced productivity, in- or outpatient treatment, medications, etc. Poor sleep quality is associated with increased risk of mortality from all causes (Ensrud, 2012), from cardiovascular events (Li, 2014), from stroke (Pan, 2014) and from suicide (Bernert, 2014). Daytime sleepiness is an independent risk factor for stroke and other vascular events (Boden-Albala, 2012), and insomnia is a risk factor for cardiovascular events (Canivet, 2014) and heart failure (Laugsand, 2014). Insomnia is more prevalent in older subjects (Gindin, 2014) and women (Pallesen, 2014; Peretti-Watel, 2009). Most biological functions have some diurnal variation, e.g. cortisol levels, blood pressure and triglycerides (Skene, 2006). Variability in night-time blood pressure and lipid profiles may account for some of the association between circadian rhythm disturbances and increased risks of cardiovascular events and mortality (Palatini, 2014).
Circadian rhythms are maintained by external cues. The most important zeitgeber is ambient light conditions. In modern life many hours are spent indoors with a markedly lower light intensity than outdoors. During the evening, television and computer are frequently used and during night many bedrooms are polluted by light coming from outdoor sources. These conditions differ from the evolutionary adaptation of man where life was spent outdoors with very low light intensity at night. The lack of a timely sequence of high intensity light during the day and too much light at night may affect circadian rhythms and hence influence human mood and health, increasing the risk of hypertension and diabetes (Gordijn, 2012; Lee, 1997; Sharkey, 2013).
The photometer of the human body is located in a small number of cells in the retina (Berson, 2002). These cells, also called intrinsic photosensitive retinal ganglion cells (ipRGC), express a light sensitive photopigment: melanopsin. When illuminated by intense blue light, the ipRGC cell transmit impulses to the brain, in particular the suprachiasmatic nucleus (SCN), which in turn acts as the body’s master clock, synchronising the timing of our physiology to the surroundings (Beaulé, 2003; Cajochen, 2005).
The classical photoreceptors, i.e. rods and cones, also contribute to the ipRGC’s and studies have shown, that even low levels of light might stimulate the system due to the input from photoreceptors at light levels that are too low to activate melanopsin (Dacey, 2005; Glickman 2002).
Through the connection to the SCN, stimulation of ipRGC’s suppresses the release of the hormone melatonin. The ipRGC’s also projects to the brain centre for pupillary responses in the pretectal nuclei (Allen, 2011). In mammals, blue light has a significant influence on melatonin release, behaviour and pupil responses (Gamlin, 2007; Gooley 2012; Güler, 2008; Hattar, 2002; Kardon, 2009). In humans, different light regimes have been found to have beneficial effects on sleep parameters in many studies, ranging from younger people during controlled conditions in laboratory settings to elderly in nursing homes (Lockley, 2003; Lockley, 2006; Mishima, 1994; Schochati, 2000; van Someren, 2000; Vitiello, 2004). High intensity white light is used in the treatment of seasonal affective disorder (Gordijn, 2012; Martiny, 2004; Thompson, 1990).
The relation between light, melatonin and sleep has also been investigated for evening light. Evening exposure to blue-enriched light leads to phase-delays with suppression of the evening rise of melatonin and subjective lack of sleepiness (Chang, 2015). The effect on mood may not always be correlated to melatonin as daylight seems to influence alertness though melatonin is nearly absent (Sahin, 2014). Also, a recent study suggests that the melatonin concentration may adapt to long-term changes in light levels (Giménez, 2014).
Whereas the impact of light on the circadian rhythm in human is unquestionable, the optimal light conditions are difficult to define. Both intensity of light, proportions of blue light and timing to the solar cycle has to be considered and in addition, the internal rhythm of the individual subject. In particular, the focus has been on the proportion of blue light due to the major importance of the blue part of the spectrum of visible light for entrainment of the circadian rhythm (Brainard, 2001; Thapan, 2001). It seems that blue light is especially important during the morning and early hours of the day and detrimental late in the evening and during the night (Chang, 2015; Nuutinen, 2014). With increasing age, blue light is transmitted to a lower degree through the lens of the eye (Brøndsted, 2013; Kessel, 2010; Turner, 2008) which may help explain the increase of sleeping disorders in the elderly population (Kessel, 2011; Kim, 2012; Stone, 2014).
The aim of the present study was to investigate the effect of indoor lighting on sleep and circadian photoentrainment. In contrast to most studies, where the participants have been elderly in nursing homes and hospitals, the present study investigated the lighting conditions in the private homes of healthy elderly citizens. Participants were randomized in a cross-over design to blue-enriched indoor light versus blue-suppressed light in the morning hours. The primary outcome was sleep duration as reported by the participants, based on the hypothesis that improvement in circadian rhythms leads to a longer sleep.
METHODS
The study was a randomized cross-over design including healthy subjects above 65 years. Randomization to blue-enriched or blue-suppressed light first was performed with a computerized random number procedure, taking into account gender and whether participants shared home or not. The experimental indoor light was applied for 3 weeks in October 2013 followed by 1 week pause, and then the participants were subjected to the other light condition for 3 weeks in November 2013. In Denmark, daylight saving time ends on the last Sunday in October, and this was at the beginning of the one week pause. Due to this shift, sunrise was fairly equal for the two light epochs. The day length was less than 10.15 hours during the last of the 3 weeks in October and less than 8.25 hours during the last week in November.
Recruitment
The study was announced by direct mail to all residents of the chosen community and was followed by information meetings. Those who expressed an interest in participation were invited for initial evaluation and further information at the Department of Ophthalmology at Glostrup Hospital. A total of 38 subjects expressed interest, 2 subjects were too young and further 6 subjects did not continue to informed consent. One subject withdrew from the study before light installation and was substituted by another, leaving 29 participants in 20 households for the study. Subjects were excluded in case of depressions and/or medication considered to influence the circadian rhythm.
The study was approved by a local ethical review board and written informed consent was obtained from all participants. Also, the study was conducted in accordance with the policies of international chronobiological standards (Portaluppi et al., 2010).
Participants were encouraged to spend as much time at home until midday as possible, but strict restrictions on daily life were not imposed and for two male participants the daily routine included continuing working out of house, although at a reduced amount for the study period. All participants lived in uniform houses in a community in the town of Albertslund, in the greater Copenhagen area, Denmark. The sitting-room and dining-room faced South-West and in the opposite direction all walls were windowless. There were some differences in size of the individual houses and some houses were mirrored, i.e. the bedroom could be left or right of the dining-room.
Light conditions
All homes were visited prior to the study epochs to identify potential problems. Despite the uniform architecture of the homes, the visits revealed rather large differences in the indoor daylight levels due to furnishing, wall colors, and floor coverings as well as plants inside and outside the home. The indoor daylight level may have influenced the individual participant's perception and acceptance of the experimental light. However, we assessed the indoor daylight levels to be of little importance for the experiment. A lack of correlation between indoor daylight levels and measured light exposures on Actiwatches supports this statement (data not shown).
Existing luminaires which were to be replaced by experimental light were hanging pendants over the dining tables and generally these lamps distributed only limited light at the position of the cornea (approximately below 100 lux). The experimental light was Philips Hue light bulbs © (http://www2.meethue.com/en-us/this-is-hue/), with 3 bulbs placed in globe-shaped frosted glass pendants in the central area of the house, i.e. where the dining table and/or living room was located and in close connection to the kitchen. This light position was chosen to maximise the hours spent by the participants near the light in each house during meals and other activities, especially in the morning hours.
To increase the light exposure and to avoid contrast problems, an LED uplight was mounted (Philips, SkyRibbon IntelliHUE Wall Washing Powercore © Eindhoven, the Netherlands) to illuminate the ceiling and add some indirect light to the exposure zone. Light from uplights and the HUE light bulbs was set to follow the same light sequence. From 8 am to 1 pm, the intensity of the light system was during the blue-enriched and blue-suppressed conditions controlled to reach approx. 280 and 240 lux, respectively, at the position of the cornea, measured half a meter from the frosted glass pendants. After 1 pm, the light intensity was decreased to approx. 140 lux blue-suppressed in both light epochs. The equivalent total irradiance was 920 mW/m2 for blue-enriched, 710 mW/m2 for blue-suppressed, and 392 mW/m2 for the afternoon set-up (Table 1). From 6 pm until bedtime the setting was approx. 100 lux blue-suppressed. During the night the participants could switch the light off or on when they wanted. If they wanted light turned on during night time, the light level was controlled at 100 lux blue-suppressed. In the bedroom of the homes, blackout curtains were installed. The light settings were measured on location (after sunset) with a handheld spectrometer from UPRtek (MK350, Zhunan, Taiwan).
TABLE 1. Total irradiance from on location measurements of the experimental light for the scenario applied from 8 am to 6 pm among four different houses.
| Light Intensity | |
|---|---|
| Light condition | mW/m2 |
| Morning: Blue-enriched | |
| Mean (SD) | 920 (170) |
| Total range | 351 |
| Morning: Blue-suppressed | |
| Mean (SD) | 710 (110) |
| Total range | 225 |
| Afternoon: | |
| Mean (SD) | 392 (48) |
| Total range | 105 |
The morning scenario were applied from 8 am to 1 pm and were either blue-enriched or blue-suppressed. The afternoon scenario was applied from 1 pm to 6 pm and was blue-suppressed.
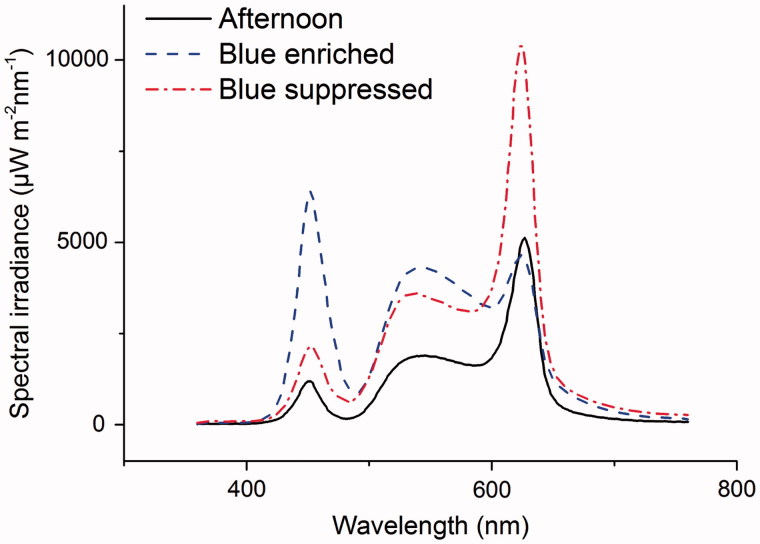
The blue-enriched light from the system had a correlated color temperature (CCT) at 5100 K with a wavelength peak (λp) at 450 nm (Figure 1). The blue-suppressed light CCT was measured to 2800 K and with a λp of 625 nm (Figure 1). The light spectra were chosen to achieve a color rendering index (CRI) above 80.
FIGURE 1.
Spectral irradiance for blue-enriched, blue-suppressed and afternoon light. The blue-enriched and blue-suppressed light conditions were applied with total irradiance approximately 700–900 mW/m2 during morning until 1 pm in the participant’s living room. Based on a cross-over design, half of the participants were exposed to blue-enriched light and the other half to blue-deprived light during the first 3-weeks experimental period, and switched to the opposite in the second 3-weeks experimental period. From 1pm, the afternoon light conditions (total irradiance approximately 400 mW/m2) were applied in all cases.
Ophthalmological examinations
Ophthalmological examinations were performed by the same experienced ophthalmologist in all participants. The examination included measurement of intraocular pressure, biomicroscopic evaluation of both the anterior and posterior segment of the eye including an evaluation of the status of the lens and any presence of cataract, glaucoma or retinal changes. In addition to the clinical evaluation of the lens, the light transmittance of the lens was measured by an ocular fluorometer (Ocumetrics, Mountain View, CA). The lens was excited by blue light (488 nm) and the emitted autofluorescence was detected in the axial direction from the anterior to the posterior surface of the lens. The transmittance of the lens was calculated as the ratio between the signal intensity for the anterior lens divided by the signal from the posterior lens (Brøndsted, 2011). The visual acuity was measured with the ETDRS chart.
Questionnaires
A diary was filled in during the two light epochs. Participants reported the time when they went to bed, when they fell asleep and what time they woke up. The questionnaire also included the number and duration of naps during the day and the hours of television and/or computer usage after 6 pm. Self-reported diary is considered to be a valid method for assessment of sleeping pattern and time (Levenson, 2013).
The Pittsburg sleep index (PSQI) and the Morningness–Eveningness questionnaire were registered at baseline and after both light epochs (Buysse, 1988). Participants were instructed to refer only to the 3 foregoing weeks of experimental light when filling out the questionnaires. Poor sleepers were defined by a PSQI ≥ 5. The Morningness–Eveningness (MEscore) scheme questionnaire was used to grade participants as morning types, score >59, or evening types, score <41, while neutral corresponds to a score in the range 42–58 (Horne, 1976).
At the end of each light period the participants were interviewed to grade their impression of the indoor light during the last 3 weeks. The interview was based on a visual analogue scale (VAS, scale from 1 to 10) and carried out by one of the authors. The aim of the interview was to obtain a subjective evaluation of the change in light and therefore, the participants at the first interview rated the experimental light (either blue-enriched or blue-suppressed as per randomisation) to the light in their home before the study. At the second interview, participants rated blue-enriched versus blue-suppressed light. The participants were encouraged to comment on the light.
Actiwatch
Activity and light exposure was measured with Actiwatch (Actiwatch Spectrum, Respironics, Philips Healthcare, Eindhoven, the Netherlands) worn on the wrist of the participants. Measurements were logged every 30 seconds around the clock in the last 7 days of each light epoch. With Actiwatch, the white light response is derived from a sensor measuring light in three wavelength bands (red, green and blue). The white light response does not follow the spectral photopic luminous efficiency function, V(λ) as described earlier (Figuerio et al., 2013; Price, 2012). Therefore, we corrected the “white light” output of all the Actiwatches against a calibrated illuminance photometer (Hagner® EC1-X, Instr. Nr. 54211, Solna, Sweden) using a side-by-side calibration method (Markvardt, 2015: http://dx.doi.org/10.1080/15502724.2015.1020948). Daylight at noon-time during overcast sky condition was used as the reference light-source. The red and blue Actiwatch light output was calibrated towards the average output of all the Actiwatches during the side-by-side calibration test. The Actiwatch calibrated white light output will in the following be termed photopic illuminance.
Chromatic pupillometry
Pupillary responses were measured with a chromatic pupillometer. In short, the pupillary contraction during and following light stimulation was video-filmed by an infrared camera and the pupil diameter expressed relative to the initial, dark adapted, undilated pupil (Herbst, 2011, 2012). Light exposure was 20 seconds of red light (660 nm) at 300 cd/m2, followed by a 5 minutes pause, and a new exposure to blue light (470 nm) at 300 cd/m2. During exposure to red light, the pupil redilates to some degree, and approximately 5 seconds after the light is turned off the baseline diameter is reached. At the light intensities used, the response to red light reflects cone activation, i.e. the photoreceptors which are also a part of our visual forming system. The pupil response to blue light is maintained both during light on and also after light off. Based on the properties of melanopsin activation the post-exposure response to blue light was chosen as the primary outcome for activation of the circadian system (Kankipati, 2010; Kardon, 2009). The outcome was expressed as the mean pupil contraction in % relative to the dark-adapted pupil-size (100%) and was measured from 0 seconds to 10 seconds and from 10 to 30 seconds after light off.
Melatonin
Saliva samples were collected at the end of each lighting epoch, from approximately 2 hours before estimated dim light onset, with 7 samples every 45 minutes (Aoki, 1998, Sharkey, 2013, Wahnschaffe, 2013). The samples for each participant were classified into 4 groups based on the maximal concentration at any time point: no sample above 2 pg/ml, no sample larger than 4 pg/ml, no sample larger than 8 pg/ml and cases with at least one sample larger than 8 pg/ml. The lower limit of detection from the laboratory was 0.5 pg/ml (Analyses by Stichting Ziekenhuis Gelderse Vallei, Ede, The Netherlands).
Statistics
The data are reported as the mean, SD (standard deviation) or standard errors (SE) and 95% confidence limits (95% CI). Assuming a normal distribution, paired t-tests were used to compare blue-enriched and blue-suppressed outcomes. A nonparametric signed rank test confirmed the parametric analysis. Chi-square was used for qualitative data and proportions. The major outcome of the study was the self-reported sleep duration, i.e. the number of hours from falling asleep to awakening in the test periods. Sleep duration, rest-time and other data from the diary and Actiwatch were determined from the mean of the last week of each light epoch. For further analysis, a general linear model was applied to evaluate if the outcomes were significantly affected by explanatory variables (gender, age and transmission of the lens). Backward elimination of non-significant covariates was used for the analysis. Data from married couples were treated statistically as independent observations. Visual acuity was measured on the ETDRS chart, and for comparison, also given as equivalent Snellen decimal.
Actiwatch data from 7 consecutive 24-hour days at the end of each light epoch was used for light-exposure analyses. The statistical analyses were performed with SAS (version 9.4, SAS Institute, Cary, NC) and a p value below 5% was considered significant. Separating epoch 1 versus 2 and blue-enriched versus blue-suppressed light conditions, light exposure averages per minute during the 24-hour day were calculated for each experimental day and afterwards this data-set was analysed using the mixed model procedure. The model included epoch and type of light condition as explanatory variables and random effects of experimental day. The same analyses were also performed only using data from the time between 8 am and 1 pm and between 8 am and 9 am.
RESULTS
A total of 29 participants in 20 homes were included in the study. The mean age was 69.7 years (95% CI 68.2 to 71.1) and 16 (55%) were male. The mean age was equal for the two genders, 69.5 years (95% CI 67.5–71.4) for males and 69.9 years (95% CI 67.5–72.4) for females. Nine married couples were included. In accordance with the randomization procedure, there was the same distribution of males and females receiving blue-enriched light in the first or second period; 8 males and 6 females received blue-enriched light in the first period and 8 male and 7 females received blue-enriched light in the second period (p > 0.8).
Sleep and rest
The sleep duration during blue enriched light was 7.44 hours (95% CI 7.14–7.74) for blue suppressed light the duration was 7.31 hours (95% CI 7.01–7.62), the difference was not significant (p = 0.29, Table 2). No advance in the phase of sleep timing was found, as the difference between the two light conditions on the diary reported sleep time was less than 5 minutes (p = 0.85). The rest duration, i.e. the time from going to bed to awakening in the morning including reading, television etc. in bed, was 8.00 hours (95% CI 7.59–8.41) for blue enriched light and 7.88 hours (95% CI 7.47–8.29) during blue-suppressed light, the difference was not significant (p = 0.257, Table 2).
TABLE 2. Results for 29 subjects at baseline and after each type of experimental light exposure.
| Baseline | Blue-enriched | Blue-suppressed | t-Value DF | p value | |
|---|---|---|---|---|---|
| Sleep and rest | |||||
| Diary, sleep hours, mean (SE) | 7.44 (0.14) | 7.31 (0.15) | 1.08 | ||
| 95% clm | 7.14–7.74 | 7.01–7.62 | 27† | 0.289 | |
| Diary, rest hours, mean (SE) | 8.00 (0.20) | 7.88 (0.20) | 1.16 | ||
| 95% clm | 7.59–8.41 | 7.47–8.29 | 27† | 0.257 | |
| Actiwatch, rest hours, mean (SE) | 7.91 (0.16) | 7.75 (0.18) | 1.36 | ||
| 95% clm | 7.58–8.24 | 7.40–8.11 | 28 | 0.184 | |
| Sleep Quality | |||||
| PSQI, total score | |||||
| mean (SE) | 5.66 (0.07)* | 4.90 (0.70) | 4.93 (0.66) | 0.13 | |
| 95% clm | 4.22–7.09 | 3.47–6.32 | 3.58–6.28 | 27 | 0.901 |
| Poor sleepers | |||||
| PSQI ≥ 5, no. | 17/29 | 13/29 | 11/28 ‡ | ||
| % | (59%) | (45%) | (39%) | 0.672 | |
| Circadian rhytm | |||||
| Morningness-Eveningness | |||||
| mean (SE) | 61.14 (1.36) | 59.62 (1.52) | 60.29 (1.38) | 1.00 | |
| 95% clm | 58.35–63.92 | 56.51–62.73 | 57.47–63.12 | 28 | 0.324 |
| Melatonin | |||||
| max concentration (pg/ml) | 8/7/7/6 | 8/6/9/5 | 0.937 | ||
| ≤2/>2≤4/>4≤8/>8 | |||||
| Pupillometry | |||||
| % contraction, 0–10 seconds, mean (SE) | 29.92 (1.23) | 27.95 (0.95) | 29.54 (1.46) | 1.26 | |
| 95% clm | 27.39–32.44 | 26.00–29.90 | 26.55–32.53 | 28 | 0.217 |
| % contraction, 10–30 seconds, mean (SE) | 13.23 (1.61) | 11.39 (1.33) | 13.54 (1.79) | 1.43 | |
| 95% clm | 9.94–16.52 | 8.67–14.2 | 9.87–17.21 | 28 | 0.165 |
Data given as mean, standard error (SE) and 95% confidence limits (95% clm). For paired t-tests, the t-value, degrees of freedom (DF) and the p value are included. p values are for the comparison of blue-enriched and blue-deprived light, where all were found non-significant. At baseline, the PSQI index was significantly increased in female gender (*: p = 0.009). Data separated by gender are shown in Table 3. Data were missing form one participant for the diary and one participant from the PSQI as indicated by † and ‡.
The rest duration measured by Actiwatch was 7.91 hours (95% CI 7.58–8.24) during blue-enriched conditions and 7.75 hours during blue-suppressed (95% CI 7.40–8.11, p = 0.184, Table 2). The rest duration measured by Actiwatch was shorter (non-significant) than the diary reported rest (Table 2).
The number of nightly awakenings ranged from 0 to 4 throughout the study period with a mean number of 1.00 during blue-enriched and 1.22 during blue-suppressed conditions (p = 0.137). The number of naps during day ranged from 0 to 1, with a mean of 0.33 during blue-enriched and 0.40 during blue-suppressed conditions (p = 0.241). Evening use of computer and television was also recorded in the diary. The daily time spent using computers and/or television during the study was 3.51 hours (range 1.56 – 6.63) for the blue-enriched and 3.54 hours (range 2.10 – 6.94) for the blue-suppressed conditions (p = 0.843).
Sleep quality
The Pittsburgh Sleep Quality Index (PSQI) at baseline with the participants’ habitual lighting conditions was 5.66 (95% CI 4.22–7.09, Table 2). The mean score was significantly different for males and females: 7.62 (95% CI 5.13–10.0) for females versus 4.06 (95% CI 2.64–5.49) for males (p = 0.0091, gender-specific data presented in Table 3). Correspondingly, the number of poor sleepers at baseline (PSQI ≥ 5) was 3 out of 16 men (19%) and 8 out of 13 females (=62%). The PSQI for the blue-enriched and blue-suppressed conditions were not significantly different, neither for all participants (p > 0.9) nor when analysed by gender (p > 0.4).
TABLE 3. PSQI for the subjects at baseline during blue-enriched and blue-suppressed light.
| Baseline | Blue-enriched | Blue-supressed | |
|---|---|---|---|
| Sleep quality by gender | |||
| Male: PSQI, total score | |||
| Mean (SE) | 4.06 (0.67) | 4.31 (1.04) | 4.13 (0.97) |
| 95% clm | 2.64–5.49 | 2.09–6.54 | 2.06–6.20 |
| Female: PSQI, total score | |||
| Mean (SE) | 7.62 (1.14) | 5.62 (0.87) | 5.85 (0.88) |
| 95% clm | 5.13–10.00 | 3.71–7.52 | 4.02–7.67 |
| p value for comparison of genders | 0.009 | 0.361 | 0.198 |
| Male: comparison to baseline | |||
| p value | 0.721 | 0.784 | |
| t-Value | 0.36 | 0.28 | |
| DF | 15 | 14‡ | |
| Female: comparison to baseline | |||
| p value | 0.007 | 0.079 | |
| t-Value | 3.22 | 1.92 | |
| DF | 12 | 12 | |
Data are presented as the mean, standard error (SE) and 95% confidence limits (95% clm). At baseline the PSQI was significantly higher for women than in men, thereafter the PSQI was similar for both genders. For females, a significant decrease was found from baseline to blue-enriched conditions, for the change to blue-deprived conditions we also found a decrease, but this did not reach statistical significance.
The PSQI improved significantly in females after installation of the brighter experimental light. On average, the PSQI score decreased by 2.0 (range 0 to −8, p = 0.007) from baseline to blue-enriched light and decreased by 1.76 (range 5 to −7, p = 0.079, Table 3) for blue-suppressed light. For males, no significant changes were found from baseline to the experimental light conditions (p > 0.5). The PSQI values were also analysed for the comparison of baseline visit to the first light epoch (October 2013), irrespective of light condition, and from baseline to the second light epoch (November 2013). The pattern was as that above, i.e. for females a decrease was found from baseline (p = 0.018 to the first period and p = 0.053 to the second light period), while no differences were found for males (p > 0.5).
Circadian rhythm
At baseline, the mean MEscore was 61.14 (95% CI 58.35–63.92, Table 2), i.e. moderate morning. As for the PSQI, a significant difference between genders was found at baseline with a mean score of 58.00 (95% CI 52.82–63.18) in women and 63.69 in men (95% CI 60.97–66.41, p = 0.035), The MEscore was neither significantly different for the comparison of blue-enriched to blue-suppressed conditions for all participants (p > 0.3) nor when analysed by gender (p > 0.2).
When baseline was compared to blue-enriched or blue-suppressed light, the change was non-significant both for males and females (p > 0.1). For both genders, the MEscore decreased by 1.5 for the change to blue-enriched indoor light which was just on the border of statistical significance (p = 0.0517). For the change to blue-suppressed indoor light there was no significant change in the MEscore (p = 0.2). As for the baseline, the MEscore for the last light epoch was significantly lower for women compared to men (56.81 vs 62.41, p = 0.040).
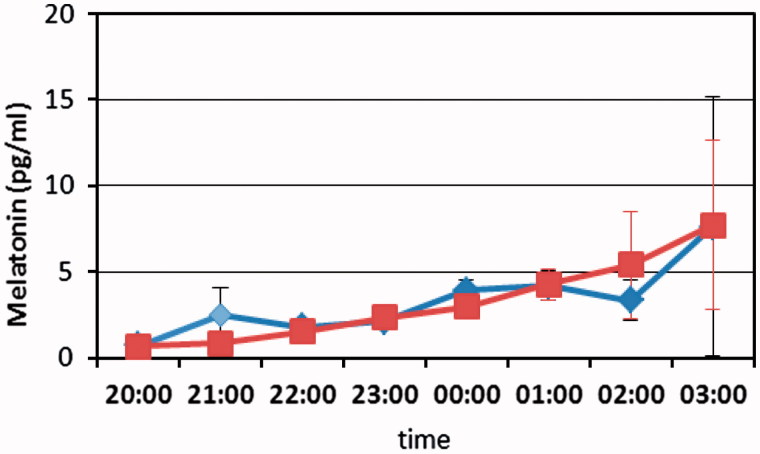
Saliva melatonin concentration was measured at the end of each light epoch. No significant difference was found between blue-enriched and blue-suppressed light (Table 2, Figure 2). The variation between subjects was large, ranging from a concentration below 2 pg/ml during all hours with no detectable nightly rise, to a distinct increase during late evening and early night to a level approaching 50 pg/ml. No significant changes were found for the type of light or for the comparison of light epochs (p > 0.5).
FIGURE 2.
Melatonine concentration (mean and error bars) for all subjects during either blue-enriched light (blue) light or blue-suppressed light (red). Data are plotted in hourly intervals and the connecting lines show linear interpolation between data points. No significant differences of melatonine concentrations were found between the two experimental light conditions.
Chromatic pupillometry did not show any differences in the pupil responses driven by photoreceptors (red light stimulation) or melanopsin containing ganglion cells (blue light stimulation) neither when comparing genders, spectrum of light (blue-enriched or blue-suppressed, p > 0.3) nor light epoch (p > 0.6).
Light conditions
Several participants reported that they found the experimental light to be brighter or much brighter during the morning hours. Subjective assessments of the pre-experimental light installations support this, revealing little light distributed on the cornea compared to after installation (data not shown). During the last week of each light epoch, the light exposure of the participants was monitored with Actiwatches.
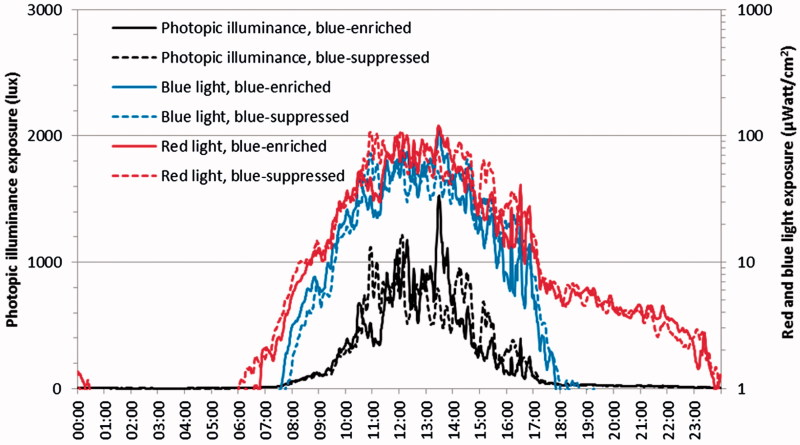
Using data for full 24-hour days, light exposure analyses showed significant difference between the two epoch periods (October and November), with more light during the first epoch (p < 0.001). This relates to the analyses of the overall mean differences for red light, blue light and photopic illuminance. Comparing blue-supressed to blue-enriched conditions, a significant difference was found for the red light with more red light under the blue-suppressed conditions compared to the blue-enriched (p = 0.024). The estimated exposures of blue light (p = 0.26) and photopic illuminance (p = 0.56) were comparable for the two light conditions (Figure 3). Light exposure data from the Actiwatches, mounted on the wrist of participants living in their own homes, included the experimental indoor lighting as well as light from outside through windows and light received during time spent outside their homes. The daylight intensity was high compared to the imposed electrical light and may account for the lack of difference found for blue light and photopic illuminance exposure between blue-enriched and blue-suppressed light condition (Figure 3).
FIGURE 3.
Average diurnal light exposure measured with Actiwatch. In the evening, the amount of blue light exposure decreased to almost zero unlike red light exposure (right axis). Significantly more red light exposure was found in the blue-suppressed treatment compared to the blue-enriched treatment (p = 0.024), while no significant difference was found for the measured blue light (p = 0.26, right axis) nor photopic illuminance (p = 0.56, left axis) for the two experimental light conditions.
Limiting the data to the time-of-day from 8 am to 1 pm, the amount of red light was significantly increased during blue-suppressed exposure (p = 0.0004), whereas no significant difference was found for blue light (p = 0.56) or photopic illuminance (p = 0.25), neither when analysing blue-suppressed versus blue-enriched per se nor when the sequence of light-epochs was considered. Analysing only data from 8 am to 9 am the amount of red light was significantly increased during blue-suppressed exposure (p < 0.0001) and for this restricted time period, both the blue light and photopic illuminance was significantly decreased (p < 0.0001) during blue-suppressed exposure compared to the blue-enriched treatment. The results of the analysis, including estimates of the light exposure per light condition, can be found in Table 4.
TABLE 4. Results of the statistical analysis for differences between blue-enriched and blue-suppressed light condition concerning Actiwatch red light, blue light and photopic illuminance for all participants.
| Period 8 am to 1 pm |
||||||
|---|---|---|---|---|---|---|
| Actiwatch measurement analysis | Blue-enriched Estimate | Blue-suppressed Estimate | SE | F-value | DF | p Value |
| Actiwatch red light | 40.396 | 45.038 | (10.686) | 12.48 | 8385 | 0.0004 |
| Actiwatch blue light | 28.621 | 28.050 | (7.225) | 0.33 | 8385 | 0.564 |
| Photopic illuminance* | 444.28 | 461.25 | (115.03) | 1.34 | 8385 | 0.247 |
| Period 8 am to 9 am |
||||||
| Actiwatch red light | 8.892 | 10.303 | (1.139) | 32.84 | 1665 | <0.0001 |
| Actiwatch blue light | 5.376 | 3.592 | (0.782) | 118.61 | 1665 | <0.0001 |
| Photopic illuminance* | 89.005 | 77.039 | (12.111) | 24.73 | 1665 | <0.0001 |
Red and blue light levels in µWatt/cm2 (nominal value) and photopic illuminance is the daylight calibrated Actiwatch “white light” output for approximate lux (*). Actiwatch measurements from 8 am to 1 pm time-of-day period and 8 am to 9 am time-of-day period were analysed. 24-hour time-of-day period gave comparable non-significant differences to the 8 am to 1 pm analysis. The Actiwatch recordings for red light showed a significantly lower intensity during blue-enriched light conditions, both during the day from 8 am to 1 pm and in the early morning (from 8 am to 9 am). Only in the early morning (from 8 am to 9 am), the recordings for blue light and photopic illumination showed a significant difference between the light conditions with higher intensity found for the blue-enriched light condition.
Subjective evaluation
Participants were asked to rate the quality of the light on a visual analogue scale ranging from 0 to 10 with 5 being neutral. The participants preferred the experimental light (first epoch) compared to their habitual light, with a mean score of 7.84 (range 2.5–10). There was no preference of blue-enriched or blue-suppressed light, mean score 4.95 (range 1–9). As mentioned, several participants reported that they found the experimental light to be brighter or much brighter during the morning hours. Some participants expressed that the blue-enriched light was acceptable but too bluish and less cozy than their usual indoor light. One participant, in particular, disliked the blue light, while a few other participants favoured the blue-enriched light, feeling much more energetic, whereas the blue suppressed light made them feel less energetic.
Ocular status
The mean visual acuity (logMAR) was 0.03 (95% CI 0.09 to −0.02), corresponding to 1.0 Snellen (total range 0.25 to 1.6). The mean lens transmission was 0.474 (95% CI 0.44–0.53), range 0.21–0.65), i.e. less than half of the blue light was transmitted through the lens to the retina. Out of the 58 eyes in 29 participants, 3 eyes had previously been operated for cataract and had an implant intraocular lens. During the study, 2 participants were diagnosed with cataract on both eyes and surgery was recommended, but performed after the study had finished. Glaucoma was diagnosed before the study in 2 eyes of 2 participants, for one of which the diagnosis and anti-glaucomatous treatment was changed as a consequence of the eye examinations. In addition, 1 eye was diagnosed with glaucoma. Retinal changes and a case of corneal scarring were found in additional 4 eyes in 4 participants, one of which was treated for peripapillary choroidal neovascularization after the study since observation during the study period showed progression of subretinal fluid towards the fovea.
The data were also analysed for additional effects of age, gender, lens transmission. As stated above, gender was significant for PSQI at the baseline. The time of sleep and rest increased significantly with age, both measured with diary and Actiwatch (all p values <0.01), with increases of 0.14 hour per year for rest time and 0.10 hours for sleep time as reported by the diary and 0.11 hours per year as evaluated from the Actiwatch rest times. The lens transmission was not significant for the outcomes.
DISCUSSION
In the present study, we investigated the effect of blue-enriched versus blue-suppressed indoor lighting in private homes of senior citizens in a randomized cross-over design. We did not find any significant differences between the two indoor lighting conditions in sleep or rest duration measured by diary and Actiwatch, or sleep quality measured with the Pittsburg Sleep Quality Index. Neither did we find significant effects of light conditions on the circadian rhythm parameters, i.e. morning-evening type, melatonin concentration or chromatic pupillometry.
We did, however, find that females had significant worse baseline PSQI score than males, and this improved significantly when the habitual indoor lighting was changed to the brighter blue-enriched experimental light. An improvement was also found for the change to blue-suppressed light, but the difference failed to reach statistical significance. We did not find any changes in PSQI scores for males. This probably reflects a ceiling-effect, as males had normal PSQI scores at baseline and hence no major improvements could be anticipated. The gender differences in sleep quality have been reported for a similar age group (Vitiello, 2004) and for patients with heart disease (Assari, 2013).
Blue-enriched light is considered the optimum for stimulation of the circadian rhythm and thus regulation of sleep (Brainard, 2001). Most studies of lighting conditions in the elderly has been conducted in nursing homes and hospitals, where the participants are subjected to daily routines set by the institution and many participants have severe health problems. Other studies have been conducted in the workplace showing significant effects on concentration and alertness (Mills, 2007; Viola, 2008). In these studies, a high color temperature of 17 000 K has been used and this may be more effective in stimulation the circadian system but also leads to low color discrimination and a bluish appearance of the surroundings which are not acceptable for elderly in their homes. Head to head studies of blue-enriched to blue-suppressed light at approximately the same intensity outside laboratory conditions are not frequent and a direct comparison of our study to previous studies is difficult. The intensity of the experimental light might have been too low to find a difference. When analysing Actiwatch data, we found a significantly increased amount of blue in the morning from 8 to 9, however the amount of blue light was not significantly higher later during the day. This may be due to the active life-style of the participants, as a social life with daily activities and also outdoor time are important time keepers. Also the amount of sunlight during the study is important, and unfortunately the weather in Copenhagen was dominated by unusual amount of clear sky, in particular during the first light epoch. In order to ensure that the participants were exposed to the experimental light for as long as possible each day, our study was placed in the autumn when most people normally spent the majority of the day indoors but we did, however, not impose any restrictions on the daily activities of the participants.
The concentration of melatonin was highly variable, ranging from below 2 pg/ml for all samples in one-fourth of the participants to peak concentrations up to 50 pg/ml with a marked increase during early night. The differences in melatonin concentration between participants could not be explained by the changes in indoor light conditions or any of the other parameters in the study. It is noteworthy that a quarter of the subjects were below 2 pg/ml. Low melatonin concentrations have previously been described in older age groups and a lack of nocturnal rise has been reported both with age and in patients with dementia, a low concentration seems to be associated with a high level during daytime (Aoki, 1998; Cajochen, 2005; Karasek, 2004). Thus, the low melatonin concentration in a large group of our participants is probably related to age.
We performed chromatic pupillometry in all participants at baseline and after each experimental light epoch. Chromatic pupillometry was used as a measurement of activation of intrinsically photosensitive retinal ganglion cells (ipRGCs) by blue light. Stimulation of ipRGCs by blue light is the initial step in photoentrainment of circadian rhythms. We did not find any significant changes during the study suggesting that ipRGCs ability to be activated by blue light was not affected by changes in the indoor electrical lighting conditions.
Overall, the different parameters showed comparable results, both for the sleep duration, sleep quality and circadian rhythm parameters and did not support the concept of beneficial effects of blue light at least not in our healthy, active, elderly participants. A large difference in blue-enriched and blue-suppressed light was achieved for the isolated experimental indoor electrical lighting, with similar light intensities and acceptable color quality (Figure 1). However, the light measurements by the Actiwatch showed a high contribution from outdoor light hiding the contribution of blue light in the blue-enriched condition when analysing all 24-hour data and data between 8 am and 1 pm. This finding was not expected. We had specifically aimed for an autumn test period where the amount of sunlight normally is low in Denmark (latitude 55.7° N). However in 2013 the weather was unusually sunny (153.9 hours of sunshine in October and November 2013 versus an expected average of 145, source: the Danish Meteorological Institute: www.dmi.dk). These meteorological conditions are likely to have influenced our findings. If performed in December–January, the outdoor condition would have been darker, however the Danish tradition for Christmas celebration favours light candles and reddish illumination and the application of the blue-enriched light would be hard to tolerate. In February, vacation abroad is very popular and due to these reasons, we chose late autumn. However, in the morning between 8 am and 9 am the contribution of daylight was limited and a significant difference between the blue-enriched and blue-suppressed light was found.
We included a detailed ophthalmic evaluation as part of our study design since ocular diseases such as glaucoma, age-related macular degeneration and cataract increases markedly with age and all may influence the visual and non-visual perception of light (Asplund, 2004, Ayaki, 2013; Feigl, 2011; Kankipati, 2011; Kessel, 2010, 2011; Nissen, 2014; Tanaka, 2010). As a consequence of the study, a number of participants were diagnosed with eye disease and were offered treatment for those diseases. This was felt as a positive side-effect of the study by the participants. No participants experienced any adverse events during the study.
The major part of the participants experienced a large subjective improvement in indoor light quality during the study. The amount of blue light was close to zero at evening, regardless of the daytime conditions. The increased light intensity during the mornings and/or low evening light might explain the change in sleep quality for women after initiation of the experimental light.
In conclusion, with a simplified scheme of blue-enriched or blue-suppressed lighting conditions from morning to midday, no difference was found for the sleep duration or other parameters of sleep quality. Daylight intensity was high during the test period and may have blunted responses to the experimental light and the comparison of blue-enriched to blue-suppressed light. The well-known poor sleep quality in females was found to improve by the bright experimental indoor morning light, suggesting that healthy elderly females with decreased sleep quality may benefit from brighter indoor light to improve sleep quality.
Acknowledgements
Study group: In addition to the authors, the study group included; Albertslund Kommune, Gate 21, VISO systems, AF Lighting, Philips Lighting.
The authors thank Palle R. Jensen for constructing the custom-made chromatic pupillometer. The authors thank optometrist Hajer Ahmad Al-Abaijdi for enthusiastic participation in the procedures performed at Glostrup Hospital.
Declaration of interest
The project was supported by The Danish Energy Association, the ELFORSK-programme, ref. 345-026 and The VELUX Foundation.
The first author (Birgit Sander) is married to Palle R Jensen, constructor of the custom made pupillometer. The authors reports no other conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Allen AE, Brown TM, Lucas RJ. A distinct contribution of short-wavelength-sensitive cones to light-evoked activity in the mouse pretectal olivary nucleus. J Neurosci. 2011;31:16833–43. doi: 10.1523/JNEUROSCI.2505-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki H, Yamada N, Ozeki Y, et al. Minimum light intensity required to suppress nocturnal melatonin concentration in human saliva. Neurosci Lett. 1998;252:91–4. doi: 10.1016/s0304-3940(98)00548-5. [DOI] [PubMed] [Google Scholar]
- Asplund R, Lindblad BE. Sleep and sleepiness 1 and 9 months after cataract surgery. Arch Gerontol Geriatr. 2004;38:69–75. doi: 10.1016/j.archger.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Ayaki M, Muramatsu M, Negishi K, et al. Improvements in sleep quality and gait speed after cataract surgery. Rejuven Res. 2013;16:35–42. doi: 10.1089/rej.2012.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assari S, Moghani Lankarani M, Kazemi Saleh D, et al. Gender modifies the effects of education and income on sleep quality in patients with coronary heart disease. Int Cardiovasc Res J. 2013;7:141–6. [PMC free article] [PubMed] [Google Scholar]
- Beaulé C, Amir S. The eyes suppress a circadian rhythm of FOS expression in the suprachiasmatic nucleus in the absence of light. Neuroscience. 2003;121:253–7. doi: 10.1016/s0306-4522(03)00420-2. [DOI] [PubMed] [Google Scholar]
- Bernert RA, Turvey CL, Conwell Y. Association of poor subjective sleep quality with risk for death by suicide during a 10-year period: A longitudinal, population-based study of late life. JAMA Psychiatry. 2014;71:1129–37. doi: 10.1001/jamapsychiatry.2014.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–3. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Bin YS, Marshall NS, Glozier N. The burden of insomnia on individual function and healthcare consumption in Australia. Aust NZ J Pub Health. 2012;36:462–8. doi: 10.1111/j.1753-6405.2012.00845.x. [DOI] [PubMed] [Google Scholar]
- Boden-Albala B, Roberts ET, Bazil C, et al. Daytime sleepiness and risk of stroke and vascular disease: Findings from the northern Manhattan study (NOMAS) Circ Cardiovasc Qual Outcomes. 2012;5:500–7. doi: 10.1161/CIRCOUTCOMES.111.963801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci. 2001;21: 6405–12. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburg sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1988;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Brøndsted AE, Lundeman JH, Kessel L. Short wavelength light filtering by the natural human lens and IOLs – implications for entrainment of circadian rhythm. Acta Ophthalmol. 2013;91:52–7. doi: 10.1111/j.1755-3768.2011.02291.x. [DOI] [PubMed] [Google Scholar]
- Brøndsted AE, Hansen MS, Lund-Andersen H, et al. Human lens transmission of blue light: A comparison of autofluorescence-based and direct lens transmission. Ophthalmic Res. 2011;46:118–24. doi: 10.1159/000323576. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Münch M, Kobialka S, et al. High sensitivity of human melatonin, alertness, thermoregulation and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–16. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- Canivet C, Nilsson PM, Lindeberg SI, et al. Insomnia increases risk for cardiovascular events in women and in men with low socioeconomic status: A longitudinal, register-based study. J Psychosom Res. 2014;76:292–9. doi: 10.1016/j.jpsychores.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Chang AM, Aeschbach D, Duffy JF, Czeisler CA. Evening use of e-Readers negatively affects sleep, circadian timing and Nex-morning alertness. Proc Natl Acad Sci. 2015;112:1232–7. doi: 10.1073/pnas.1418490112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM., Liao HW, Peterson BB, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–54. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Blackwell TL, Ancoli-Israel S, et al. Sleep disturbances and risk of frailty and mortality in older men. Sleep Med. 2012;13:1217–25. doi: 10.1016/j.sleep.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigl B, Mattes D, Thomas R, et al. Intrinsically photosensitive (melanopsin) retinal ganglion cell function in glaucoma. Invest Ophthalmol Vis Sci. 2011;52:4362–7. doi: 10.1167/iovs.10-7069. [DOI] [PubMed] [Google Scholar]
- Figuerio MG, Hamner R, Bierman A, et al. Comparisons of three practical field devices used to measure personal light exposures and activity levels. Lighting Res Technol. 2013;45:421–34. doi: 10.1177/1477153512450453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamlin P.D, McDougal DH, Pokorny J, et al. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47:946–54. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giménez MC, Beersma DG, Bollen P, et al. Effects of a chronic reduction of short-wavelength light input on melatonin and sleep patterns in humans: Evidence for adaptation. Chronobiol Int. 2014;31:690–7. doi: 10.3109/07420528.2014.893242. [DOI] [PubMed] [Google Scholar]
- Gindin J, Shochat T, Chetrit A, et al. Insomnia in long-term care facilities: A compararison of seven European countires and Israel: The services and health for elderly in long term care study. J Am Geriatr Soc. 2014;62:2033–9. doi: 10.1111/jgs.13099. [DOI] [PubMed] [Google Scholar]
- Glickman G, Levin R, Brainard GC. Ocular input for human melatonin regulation: Relevance to breast cancer. Neuroendocrinol Lett. 2002;23:17–22. [PubMed] [Google Scholar]
- Gooley JJ, Ho Mien I, St Hilaire MA, et al. Melanopsin and rod-cone photoreceptors play different roles in mediating pupillary light responses during exposure to continuous light in humans. J Neurosci. 2012;32:14242–53. doi: 10.1523/JNEUROSCI.1321-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordijn MC, ‘t Mannetje D, Meesters Y. The effects of blue-enriched light treatment compared to standard light treatment in seasonal affective disorder. J Affect Disord. 2012;136:72–80. doi: 10.1016/j.jad.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Güler AD, Ecker JL, Lall GS, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–5. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, et al. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science. 2002;8;295:1065–70. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst K, Sander B, Milea D, et al. Test–retest repeatability of the pupil light response to blue and red light stimuli in normal human eyes using a novel pupillometer. Front Neurol. 2011;2:10. doi: 10.3389/fneur.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst K, Sander B, Lund-Andersen H, et al. Intrinsically photosensitive retinal ganglion cell function in relation to age: A pupillometric study in humans with special reference to the age-related optic properties of the lens. BMC Ophthalmol. 2012;12:4. doi: 10.1186/1471-2415-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms, Int. J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Jennum P, Ibsen R, Avlund K, Kjellberg J. Health, social and economic consequences of hypersomnia: A controlled national study from a national registry evaluating the societal effect on patients and their partners. Eur J Health Econ. 2014;15:303–11. doi: 10.1007/s10198-013-0491-2. [DOI] [PubMed] [Google Scholar]
- Kankipati L, Girkin CA, Gamlin PD. Post-illumination pupil response in subjects without ocular disease. Invest Ophthalmol Vis Sci. 2010;51:2764–9. doi: 10.1167/iovs.09-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankipati L, Girkin CA, Gamlin PD. The post-illumination pupil response is reduced in glaucoma patients. Invest Ophthalmol Vis Sci. 2011;52:2287–92. doi: 10.1167/iovs.10-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon R, Anderson SC, Damarjian TG, et al. Chromatic pupil responses: Preferential activation of the melanopsin-mediated versus outer photoreceptor-mediated pupil light reflex. Ophthalmology. 2009;116:1564–73. doi: 10.1016/j.ophtha.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Karasek M. Melatonin, human aging, and age-related diseases. Exp Gerontol. 2004;39:1723–9. doi: 10.1016/j.exger.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Kessel L, Lundeman JH, Herbst K, et al. Age-related changes in the transmission properties of the human lens and their relevance to circadian entrainment. J Cataract Refract Surg. 2010;36:308–12. doi: 10.1016/j.jcrs.2009.08.035. [DOI] [PubMed] [Google Scholar]
- Kessel L, Siganos G, Jørgensen T, et al. Sleep disturbances are related to decreased transmission of blue light to the retina caused by lens yellowing. Sleep. 2011;34:1215–19. doi: 10.5665/SLEEP.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Jung KI, Song CH. The effect of cataract on sleep time and quality in late adulthood. Aging Clin Exp Res. 2012;24:663–8. doi: 10.3275/8501. [DOI] [PubMed] [Google Scholar]
- Laugsand LE, Strand LB, Platou C, et al. Insomnia and the risk of incident heart failure: A population study. Eur Heart J. 2014;35:1382–93. doi: 10.1093/eurheartj/eht019. [DOI] [PubMed] [Google Scholar]
- Lee TM, Chan CC, Paterson JG, et al. Spectral properties of phototherapy for seasonal affective disorder: A meta-analysis. Acta Psychiatrica Scand. 1997;96:117–21. doi: 10.1111/j.1600-0447.1997.tb09915.x. [DOI] [PubMed] [Google Scholar]
- Levenson JC, Troxel WM, Begley A, et al. A quantitative approach to distinguishing older adults with insomnia from good sleepers controls. J Clin Sleep Med. 2013;92:125–31. doi: 10.5664/jcsm.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang X, Winkelman JW. Association between insomnia symptoms and mortality: A prospective study of U.S. men. Circulation. 2014;129:737–46. doi: 10.1161/CIRCULATIONAHA.113.004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88:4502–5. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Evans EE, Scheer FA, et al. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–8. [PubMed] [Google Scholar]
- Markvardt J, Hansen ÅM, Christoffersen J, et al. Comparison and correction of the light sensor output from 48 wearable light exposure devices by using a side-by-side field calibration method. LEUKOS. 2015;11:3:155–71. [Google Scholar]
- Martiny K, Simonsen C, Lunde M, et al. Decreasing TSH levels in patients with Seasonal Affective Disorder (SAD) responding to 1 week of bright light therapy. J Affect Disord. 2004;79:253–7. doi: 10.1016/S0165-0327(02)00361-0. [DOI] [PubMed] [Google Scholar]
- Mills PR, Tomkins SC, luc Schlangen JM. The effect of high correlated colour temperature office lighting on employee wellbeing and work performance. J Circadian Rhythms. 2007;5 doi: 10.1186/1740-3391-5-2. 2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima K, Okawa M, Hishikawa Y, et al. Morning bright light therapy for sleep and behavior disorders in elderly patients with dementia. Acta Psychiatr Scand. 1994;89:1–7. doi: 10.1111/j.1600-0447.1994.tb01477.x. [DOI] [PubMed] [Google Scholar]
- Nissen C, Sander B, Milea D, et al. Monochromatic pupillometry in unilateral glaucoma discloses no adaptive changes subserved by the ipRGCs. Front Neurol. 2014;5 doi: 10.3389/fneur.2014.00015. 15:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuutinen T, Roos E, Ray C, et al. Computer use, sleep duration and health symptoms: A cross-sectional study of 15-year olds in three countries. Int J Public Health. 2014;59:619–28. doi: 10.1007/s00038-014-0561-y. [DOI] [PubMed] [Google Scholar]
- Palatini P, Reboldi G, Beilin LJ, et al. Added predictive value of night-time blood pressure variability for cardiovascular events and mortality: The Ambulatory blood pressure-International study. Hypertension. 2014;64:487–93. doi: 10.1161/HYPERTENSIONAHA.114.03694. [DOI] [PubMed] [Google Scholar]
- Pallesen S, Sivertsen B, Nordhus IH, et al. A 10-year trend of insomnia prevalence in the adult norwegian population. Sleep Med. 2014;15:173–9. doi: 10.1016/j.sleep.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Pan A, De Silva DA, Yuan JM, Koh WP. Sleep duration and risk of stroke mortality among chinese adults: Singapore Chinese health study. Stroke. 2014;45:1620–5. doi: 10.1161/STROKEAHA.114.005181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretti-Watel P, Legleye S, Baumann M, et al. Fatigue, insomnia and nervousness:Gender disparities and roles of individual characteristics and lifestyle factors among economically active people. Soc Psychiatry Psychiatr Epidemiol. 2009;44:703–9. doi: 10.1007/s00127-008-0487-x. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Smolensky MH, Touitou Y. Ethics and methods for biological rhythm research on animals and human beings. Chronobiology. 2010;27:1911–29. doi: 10.3109/07420528.2010.516381. [DOI] [PubMed] [Google Scholar]
- Price LL, Khazova M, O’Hagan. JB. Performance assessment of commercial circadian personal exposure devices. Lighting Res Technol. 2012;44:17–26. [Google Scholar]
- Sahin L, Wood BM, Plitnick B, Figuerio MG. Daytime light exposure: Effects on biomarkers, measures of alertness, and performance. Behav Brain Res. 2014;274:176–85. doi: 10.1016/j.bbr.2014.08.017. [DOI] [PubMed] [Google Scholar]
- Schochati T, Martin J, Marler M, et al. Illumination levels in nursing home patients: Effects on sleep and activity rhythms. J Sleep Res. 2000;9:373–9. doi: 10.1046/j.1365-2869.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- Sharkey KM, Pearlstein TB, Carskadon MA. Cirdadian phase shift and mood across perinatal period in women with a history of major depressive disorder: A preliminary report. J Affect Disord. 2013;150:1103–8. doi: 10.1016/j.jad.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene DJ, Arendt J. Human circadian rhythms: Physiological and therapeutic relevance of light and melatonin. Ann Clin Biochem. 2006;43:344–53. doi: 10.1258/000456306778520142. [DOI] [PubMed] [Google Scholar]
- Stone KL, Blackwell TL, Ancoli-Israel S, et al. Osteoporotic fractures in men study group. Sleep disturbances and risk of falls in older community-dwelling men: The outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study. J Am Geriatr Soc. 2014;62:299–305. doi: 10.1111/jgs.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Hosoe K, Hamada T, et al. Change in sleep state of the elderly before and after cataract surgery. J Physiological Anthropol. 2010;29:219–24. doi: 10.2114/jpa2.29.219. [DOI] [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–7. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C, Stinson D, Smith A. Seasonal affective disorder and season-dependent abnormalities of melatonin suppression by light. Lancet. 1990;336:703–6. doi: 10.1016/0140-6736(90)92202-s. [DOI] [PubMed] [Google Scholar]
- Turner Pl, Mainster MA. Circadian photoreception: Ageing and the eye’s important role in systemic health. Br J Ophthalmol. 2008;92:1439–44. doi: 10.1136/bjo.2008.141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahnschaffe A, Haedel S, Rodenbeck A, et al. Out of the lab and into the bathroom: Evening short-term exposure to conventional light suppresses melatonin and increases alertness perception. Int J Mol Sci. 2013;14:2573–89. doi: 10.3390/ijms14022573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Someren EJ. Circadian and sleep disturbances in the elderly. Exp Gerontol. 2000;35:1229–37. doi: 10.1016/s0531-5565(00)00191-1. [DOI] [PubMed] [Google Scholar]
- Viola AU, James LM, Schlangen LJM, Dijk DJ. Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scand J Work Environ Health. 2008;34:297–306. doi: 10.5271/sjweh.1268. [DOI] [PubMed] [Google Scholar]
- Vitiello MV, Larsen LH, Moe KE. Age-related sleep change Gender and estrogen effects on the subjective–objective sleep quality relationships of healthy, noncomplaining older men and women. J Psychosomatic Res. 2004;56:503–10. doi: 10.1016/S0022-3999(04)00023-6. [DOI] [PubMed] [Google Scholar]