Abstract
Objectives The aim of the study was to evaluate the efficacy of different dosages of estetrol (E4) combined with one of two progestins in suppressing the pituitary–ovarian axis and ovulation in healthy premenopausal women.
Methods This was an open, parallel, phase II, dose-finding, pilot study performed in healthy women aged 18 to 35 years with a documented ovulatory cycle before treatment. For three consecutive cycles in a 24/4-day regimen, participants received 5 mg or 10 mg E4/3 mg drospirenone (DRSP); 5 mg, 10 mg or 20 mg E4/150 μg levonorgestrel; or 20 μg ethinylestradiol (EE)/3 mg DRSP as comparator. Pituitary–ovarian axis activity and the occurrence of ovulation were evaluated by monitoring follicular size, serum levels of follicle-stimulating hormone, luteinising hormone, estradiol and progesterone during treatment cycles 1 and 3. Endometrial thickness was evaluated throughout the trial, and the return of ovulation was evaluated after the last intake of medication.
Results A total of 109 women were included in the trial. No ovulation occurred in any treatment group. Ovarian activity inhibition seemed proportional to the E4 dosage: the highest suppression was observed in the 20 mg E4 group and was very similar to that observed with EE/DRSP. Endometrial thickness was suppressed to the same extent in all groups. Post-treatment ovulation occurred in all participants between 17 and 21 days after the last active treatment. The study combinations were well tolerated and safe.
Conclusions Combined with a progestin, E4 adequately suppresses ovarian activity, particularly when given at a dosage above 10 mg/day.
Chinese Abstract
摘要
目的 这项研究的目的是评估不同剂量的雌四醇联合两种孕激素中的其中一种对垂体-卵巢轴以及健康的绝经前妇女的排卵方面的抑制疗效。
方法 这是一个在18到35岁的健康女性中进行的开放的、平行的,关于II期药物剂量探索的初步研究,这些女性均有治疗前的排卵周期记录。连续三个周期的24/4-天方案,参与者接受5毫克或10毫克的雌四醇/3毫克屈螺酮;5毫克,10毫克或20毫克的雌四醇/150 μg左炔诺孕酮;或用20 μg炔雌醇/3毫克屈螺酮作比较。在第1和第3个治疗周期,通过监测卵泡的大小,血清促卵泡激素、黄体生成素、雌二醇和孕激素的水平对垂体-卵巢轴的活动和排卵进行评估。对子宫内膜厚度的评估贯穿于整个试验过程中,对排卵抑制的评估在用完药物之后。
结果 总共有109名妇女参与试验。任何一个治疗组都没有出现排卵。对卵巢活动的抑制似乎与雌四醇的剂量成正比:最严重的抑制出现在20毫克的雌四醇组,与在炔雌醇/屈螺酮组观察到的情况非常相似。所有组的子宫内膜厚度的抑制程度是相同的。在最后一次积极治疗后的第17到21天之间,所有参与者均出现了治疗后的排卵。所有的试验组合均有良好的耐受性和安全性。
结论 雌四醇联合一种雌激素能够充分的抑制卵巢活动,尤其是当雌四醇的剂量大于10毫克/天的时候。
关键词
雌四醇;雌激素;口服避孕药;排卵抑制;孕激素
KEYWORDS: Estetrol, Estrogen, Oral contraception, Ovulation inhibition, Progestin
INTRODUCTION
Estetrol (E4) is a naturally occurring estrogen discovered in 19651. E4 is produced exclusively and in large amounts by the human fetal liver. E4 has a relatively low affinity for the estrogen receptor (ER), but this is largely compensated by its high oral bioavailability (80% in contrast to 1% for estradiol [E2]) and a long half-life of approximately 28 h (in contrast to 3.6 h for E2). It binds to both ERα and ERβ, with a four- to fivefold preference for ERα. After its initial discovery, research on E4 was performed for approximately 20 years in unsuccessful attempts to discover its function or to correlate its maternal plasma levels with fetal well-being. Thereafter, scientific interest in the hormone declined. In recent years, preclinical and clinical therapeutic studies have shown that E4 might be an effective drug for several indications, including contraception, as it was notably shown to inhibit ovulation in cycling rats in a dose-dependent manner2,3.
Some evidence suggests that E4 may be suitable as a daily oral contraceptive and has several benefits in comparison with the currently available estrogen. Most marketed combined oral contraceptives (COCs) contain the potent synthetic estrogen ethinylestradiol (EE). EE has been shown to be safe but causes subjective side effects and increases the hepatic production of several coagulation factors, resulting in a prothrombotic status4. The most serious adverse effects of EE are cardiovascular complications, both arterial and venous, and in particular an increased risk of venous thromboembolism (VTE)5,6. These cardiovascular complications are rare but serious, especially when they occur in young, healthy women. The risk of VTE has been reduced by decreasing the EE dosage in COCs and it could also be lowered by replacing EE with the natural estrogen E2. There are currently two COCs on the market that contain E2 instead of EE: a sequential COC containing estradiol valerate (E2V) and dienogest (DNG) and a monophasic COC containing E2 and nomegestrol acetate. Recent epidemiological data suggest that the risk of VTE for users of COCs containing E2V and DNG is similar to that for users of COCs containing second-generation progestins7. Because E4 has minimal impact on the hepatic production of coagulation factors, it is hypothesised that the VTE risk will also be reduced by using the natural estrogen E4 instead of EE [Kluft C, et al., submitted].
In addition, in contrast to EE or E2, E4 does not inhibit the cytochrome P450 enzymes and should consequently not interfere with the metabolism of other drugs8. It is excreted in the urine as inactive sulfo- and glucurono-conjugates that do not interfere with the biliary system and therefore would not increase the incidence of gallbladder diseases as do classical COCs9. E4 metabolism has not been shown to produce active metabolites, in contrast to E2, whose metabolism leads to the production of carcinogenic catechol estrogen metabolites10. Finally, recent clinical and experimental in vitro and animal studies demonstrate a minimal impact of E4 on normal and malignant breast cells11–13.
The present study was performed to investigate the effects of different doses of E4 in combination with two different progestins, drospirenone (DRSP) and levonorgestrel (LNG), on ovarian follicular activity and ovulation, in comparison to the registered COC EE/DRSP (Yaz; Bayer HealthCare Pharmaceuticals, Berlin, Germany). In addition, pituitary–ovarian function, the effect on endometrial thickness and the return of ovulation were investigated.
METHODS
This single centre, open, parallel, phase II, dose-finding pilot study was performed on a limited number of healthy female volunteers. The study was conducted in a clinical research centre (Dinox BV) in Groningen, the Netherlands. The trial was registered in the Netherlands Trial Register (www.trialregister.nl) under the registration number NTR2102. Compliance with Good Clinical Practice and the statistical and clinical study report were verified by an independent auditor.
Participants
All trial participants gave their written informed consent, and the study was approved by the independent ethics committee Stichting Therapeutische Evaluatie Geneesmiddelen (Duivendrecht, the Netherlands). The main inclusion criteria were as follows: age 18 to 35 years; ovulation in the pretreatment cycle between cycle day 9 (± 1) and day 24 (± 1), with a subsequent progesterone concentration ≥ 16 nmol/l and a luteal phase duration of at least 6 (± 1) days; body mass index (BMI) of 18 to 30 kg/m2; and good physical and mental health. Exclusion criteria were as follows: contraindication for contraceptive steroids; clinically relevant abnormal laboratory results; a long duration of the washout cycle after stopping hormonal contraception for more than 42 days; pregnancy; lactation; pregnancy during accurate hormonal contraceptive use in the past; history of breast cancer; abnormalities of the uterus or ovaries; abnormal cervical smear in the last 3 years or at screening; renal insufficiency; hepatic dysfunction; adrenal insufficiency; status postpartum or postabortion in the last 2 months; and a history (within 12 months) of alcohol or drug abuse. Use of the following drugs within two cycles prior to the start of study medication were also exclusion criteria: hepatic enzyme-inducing medicinal products; sex steroids; herbal remedies containing St John's Wort; antihypertensive drugs; phytoestrogens; investigational drugs in the last 2 months; and an injectable hormonal method of contraception in the last 6 months.
Before inclusion in the study, all participants underwent a general physical and gynaecological examination, including electrocardiogram, transvaginal ultrasonography (TVUS), breast examination and cervical smear (if no smear result had been obtained within the last 3 years). Haematological and clinical chemical blood parameters were determined, and urinalysis was performed.
The participants received financial compensation for their participation in the trial.
Study design
Participants who were using hormonal contraception at the start of the study discontinued its use after completion of the current cycle and then had a washout cycle. All participants had to use barrier contraception methods throughout the study. The pretreatment cycle started on the first day of spontaneous menstrual blood loss after the washout cycle (if any). Participation in the study was accepted only if ovulation occurred on or before day 24 (± 1) of the pretreatment cycle, if the progesterone concentration was ≥ 16 nmol/l and if the next menstruation did not start within 6 (± 1) days after ovulation. Eligibility was evaluated by monitoring follicular growth in the pretreatment cycle by TVUS, which was performed every 3 (± 1) days. After ovulation was documented by TVUS, a blood sample was taken 2 (± 1) days later to determine the progesterone concentration. If the progesterone concentration was in the postovulatory range but below 16 nmol/l another blood sample was taken 4 (± 1) days after ovulation.
During the first and the third treatment cycles, TVUS and blood sampling were performed every third (± 1) day from day 3 (± 1) to day 24 (± 1). TVUS and blood sampling were also performed on day 3 (± 1) of the second cycle. If a follicle with a diameter ≥ 13 mm was observed at day 24 (± 1) of the first or third cycle or at day 3 (± 1) of the second cycle, TVUS and blood sampling were continued every 3 (± 1) days until the follicle disappeared.
During the spontaneous cycle following the three treatment cycles, TVUS was performed every third (± 1) day from day 3 onwards until ovulation was observed. A blood sample was taken 2 (± 1) days after ovulation to determine the progesterone concentration. If the progesterone concentration was in the postovulatory range but below 16 nmol/l, another blood sample was taken 4 (± 1) days after ovulation. A follow-up visit was performed on day 3 (± 1) of the cycle after the post-treatment cycle. At the follow-up visit, physical and gynaecological examinations were performed.
Urine pregnancy tests were performed before the first intake of study medication and several times during the course of the study. Haematological and clinical chemical blood determinations and urinalysis were performed at screening and during the post-treatment cycle. At all visits during the study, participants were questioned for adverse events and use of concomitant medication.
Treatment
There were six treatment groups: (i) 5 mg E4 combined with 3 mg DRSP (5 mg E4/DRSP); (ii) 10 mg E4 combined with 3 mg DRSP (10 mg E4/DRSP); (iii) 20 μg EE combined with 3 mg DRSP (EE/DRSP); (iv) 5 mg E4 combined with 150 μg LNG (5 mg E4/LNG); (v) 10 mg E4 combined with 150 μg LNG (10 mg E4/LNG); and (vi) 20 mg E4 combined with 150 μg LNG (20 mg E4/LNG). All participants were stratified according to the day of ovulation in the pretreatment cycle and then assigned to one of the treatment groups. E4 was supplied as tablets of 5 or 10 mg, in blister packs. DRSP was supplied as tablets of 3 mg, and LNG as tablets of 150 μg, both in blister packs. EE/DRSP was supplied as tablets in the original blister pack. Blinding was therefore not possible.
Production, packaging and labelling of the study medication were performed according to Good Manufacturing Practice guidelines (Haupt Pharma, Münster, Germany). The chemical synthesis of E4 was performed by Cambridge Major Laboratories Europe (Weert, the Netherlands). A quality control of the tablets was performed at their release, and studies were conducted to evaluate the stability of the products for periods of time beyond the duration of the study. Oral treatment was started on the first day of menstruation following the pretreatment cycle and was administered for three cycles once daily in the morning at approximately the same time, which was recorded in a diary. In each cycle, participants treated with E4 used the study medication for 24 days, followed by 4 days without medication. Participants treated with EE/DRSP used 24 active tablets followed by four placebo tablets.
Measurements
TVUS was performed using a Voluson E8 device (GE Healthcare, Kretztechnik GmbH & Co OHG, Zipf, Austria). The mean diameter of the bidirectional measurement of the largest follicle in each ovary and the double-layer endometrial thickness were assessed. Serum levels of follicle-stimulating hormone (FSH), luteinising hormone (LH), E2 and progesterone were determined in each blood sample. Blood samples were processed to serum and stored at − 20°C until assays were performed. FSH, LH and progesterone levels in serum were determined by the Immulite 2000 immunoassay system (Siemens Healthcare GmbH, Erlangen, Germany). Because of significant crossreactivity between E4 and E2 using the commercially available ligand-binding assay, the E2 concentrations were determined using the API 4000 LC/MS/MS system (Applied biosystems/MSD Sciex, Waltham, MA, USA).
At several time points in the study, extra blood samples were taken to measure various liver parameters. In addition, bone turnover markers and growth endocrine parameters were determined, and pharmacokinetic parameters were measured in the blood and urine. The methods and results of these assessments will be reported separately.
Sample size
The study was explorative. Its aim was to gather information that would help to decide which dose regimen should be selected for future studies. Therefore, the sample size was not calculated but arbitrarily assigned to 18 women per group. Based on this sample size, the upper limit of the unidirectional confidence interval of the ovulation rate in the absence of ovulation would be 5% when considering no intra-subject correlation (i.e., no ovulation in any of the three treatment cycles for the same participant) or 14% when considering perfect intra-subject correlation (i.e., one ovulation in each treatment cycle for the same participant). This sample size was considered acceptable for a dose-finding pilot study.
Analysis
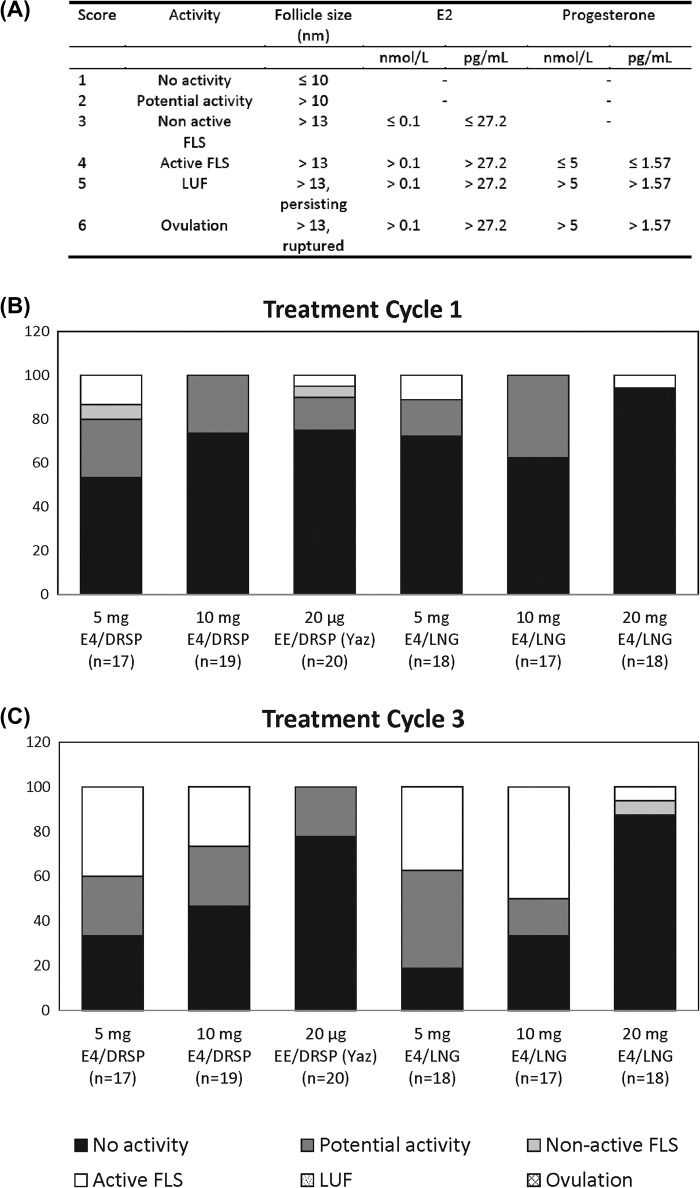
The primary efficacy variable was the ovulation rate, i.e., the number of ovulations per number of cycles per treatment group. Ovulation was defined using the Hoogland score, which is based on the combination of maximum follicular diameter and concentrations of E2 and progesterone during a treatment cycle14 (Figure 1A). Hoogland scores were determined for treatment cycles 1 and 3. In addition, summary statistics of the largest follicle size per time point and the maximum follicle size per participant over the entire treatment period were calculated.
Figure 1. Ovulation inhibition according to the Hoogland score (A). Hoogland scores obtained during cycle 1 (B) and cycle 3 (C) with the different combinations tested during the trial. Results are expressed in percentage of participants.
Secondary study objectives were to investigate pituitary–ovarian function, effect on endometrial thickness and return of ovulation. The mean and maximum serum concentrations of E2, progesterone, FSH and LH per cycle were calculated. Summary statistics were calculated for the maximum endometrial thickness per woman per cycle. The return of ovulation was evaluated by assessing the day of ovulation in the post-treatment cycle.
Differences in the maximum follicle diameter and endometrial thickness for treatment cycles 1 and 3, comparing the E4 groups versus EE/DRSP, the different E4 dose groups and DRSP versus LNG, were analysed using a random effects repeated measures model. Hormone concentrations on day 3 of cycle 1 and pooled results on day 24 in cycles 1 and 3 were analysed statistically using a random effects repeated measures analysis model with pretreatment day 3 values as covariate after logarithmic transformation. Differences in the return of ovulation day comparing the E4 groups with the EE/DRSP group, comparing the different E4 doses, and comparing DRSP with LNG were analysed by analysis of covariance with day of ovulation in the pretreatment cycle as the covariate. In the statistical analyses, p < 0.01 was used as the criterion for statistical significance.
RESULTS
Study population
The study was performed between November 2009 and November 2010. In total, 210 women were screened: 99 were screening failures and 111 were included and assigned to a treatment group. The most common reasons for screening failure were menstrual cycle deviations, in particular a washout cycle of more than 42 days after stopping COC, no ovulation until cycle day 24 in the pretreatment cycle, or a low progesterone concentration after ovulation in the pretreatment cycle. The participant disposition is shown in Figure 2.
Figure 2. Participant disposition by treatment group.
The demographic and pretreatment cycle characteristics were generally similar across the treatment groups (Table 1). However, compared with the other groups, the mean BMI was lower in the 5 mg E4/LNG and 10 mg E4/LNG groups, and the percentage of smokers was lower in the 10 mg E4/LNG and 20 mg E4/LNG groups.
Table 1. Demographic and baseline characteristics.
| Parameter | 5 mg E4/DRSP (n = 19) | 10 mg E4/DRSP (n = 19) | 20 μg EE/DRSP (n = 20) | 5 mg E4/LNG (n = 18) | 10 mg E4/LNG (n = 17) | 20 mg E4/LNG (n = 18) | Overall (n = 111) |
|---|---|---|---|---|---|---|---|
| Mean age, years (SD) | 24.3 (3.11) | 23.7 (3.67) | 23.4 (3.87) | 22.3 (2.65) | 22.4 (2.42) | 21.1 (2.30) | 22.9 (3.20) |
| BMI, kg/m2 | |||||||
| Mean (SD) | 22.54 (2.33) | 23.20 (3.21) | 23.03 (2.93) | 21.51 (1.70) | 21.78 (2.52) | 24.28 (3.37) | 22.74 (2.83) |
| Range | 18.3–26.1 | 18.8–30.0 | 19.2–28.3 | 18.2–24.5 | 18.7–27.4 | 19.1–29.8 | 18.2–30.0 |
| Race, n (%) | |||||||
| White or Caucasian | 16 (84.2) | 18 (94.7) | 19 (95.0) | 16 (88.9) | 17 (100) | 16 (88.9) | 102 (91.9) |
| Black or African American | 1 (5.3) | 0 | 0 | 0 | 0 | 2 (11.1) | 3 (2.7) |
| Asian | 2 (10.5) | 0 | 1 (5.0) | 0 | 0 | 0 | 3 (2.7) |
| Other | 0 | 1 (5.3) | 0 | 2 (11.1) | 0 | 0 | 3 (2.7) |
| Mean duration of menstrual cycle, days (SD) | 28.8 (1.81) | 28.3 (0.75) | 28.7 (1.29) | 27.8 (2.13) | 28.0 (0.38) | 28.5 (3.22) | 28.4 (1.86) |
| Gravidity, n (%) | |||||||
| 0 | 14 (73.7) | 18 (94.7) | 19 (95.0) | 18 (100.0) | 16 (94.1) | 16 (88.9) | 101 (91.0) |
| ≥ 1 | 5 (26.3) | 1 (5.3) | 1 (5.0) | 0 (0.0) | 1 (5.9) | 2 (11.1) | 10 (9.0) |
| Parity, n (%) | |||||||
| 0 | 16 (84.2) | 19 (100) | 19 (95.0) | 18 (100.0) | 17 (100.0) | 17 (94.4) | 106 (95.5) |
| ≥ 1 | 3 (15.8) | 0 (0.0) | 1 (5.0) | 0 (0.0) | 0 (0.0) | 1 (5.6) | 5 (4.5) |
| Smoking habits, n (%) | |||||||
| Non-smoker | 12 (63.2) | 13 (68.4) | 12 (60.0) | 11 (61.1) | 14 (82.4) | 15 (83.3) | 77 (69.4) |
| Smoker | 5 (26.3) | 5 (26.3) | 7 (35.0) | 6 (33.3) | 3 (17.6) | 3 (16.7) | 29 (26.1) |
| Former smoker | 2 (10.5) | 1 (5.3) | 1 (5.0) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 5 (4.5) |
SD, standard deviation
Ovulation rate
The distribution of the Hoogland scores in treatment cycles 1 and 3 in the different treatment groups is depicted in Figure 1B & 1C. In none of the treatment cycles was the Hoogland score higher than 4, so there were no luteinised unruptured follicles (LUFs) or ovulations. During treatment cycle 1, in all treatment groups, the majority (80% or more) of participants had no ovarian activity (Hoogland score 1) or potential activity (Hoogland score 2). The remaining participants had a non-active follicle-like structure (FLS) (Hoogland score 3) or active FLS (Hoogland score 4). For the E4 treatment groups, the number of participants with non-active FLS or active FLS was higher in treatment cycle 3 compared with treatment cycle 1. During treatment cycle 3, the majority (50% or more) of the participants had no activity or potential activity. Despite the low number of participants in each group, it appears that increasing the dose of E4 was associated with an increased suppression of ovarian activity, particularly in treatment cycle 3, during which the percentage of participants with non-active FLS or active FLS was lowest in the 20 mg E4/LNG group (12.6%) and comparable to the EE/DRSP group (0%).
Ovarian and pituitary function
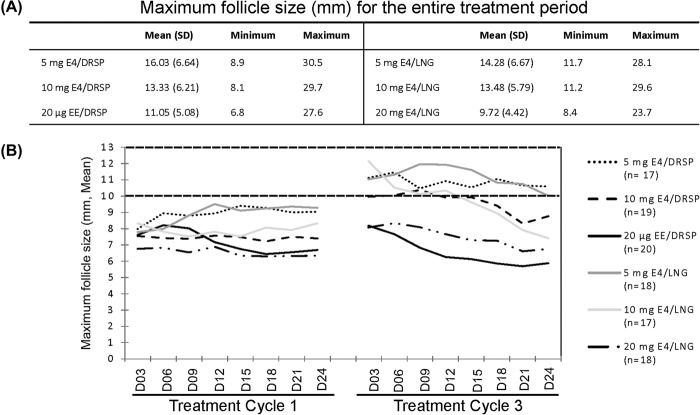
The maximum values of the largest follicular diameter during the entire treatment period are shown in Figure 3A. The mean values of the largest follicular diameter at each time point during treatment cycles 1 and 3 are depicted in Figure 3B.
Figure 3. Mean (± SD), minimum and maximum value of the largest follicular diameter per participant in each group over the entire treatment period (A). Mean diameter of the largest follicle (mm) measured in each treatment group every 3 days during cycles 1 and 3 (B).
The mean maximum follicular diameter in treatment cycle 1 and 3 decreased significantly with increasing E4 dose (p < 0.0001) and did not differ between the DRSP and the LNG groups. The mean maximum follicular diameter in the 5 mg E4 groups was higher than in the EE/DRSP group (p < 0.0001). The difference between the 10 mg E4 groups and the EE/DRSP group almost reached significance (p = 0.0133).
Table 2 shows the mean and maximum FSH, LH, E2 and progesterone concentrations in treatment cycles 1, 2 and 3. Pooled FSH and LH concentrations on day 24 of cycle 1 and cycle 3 were significantly lower with increasing E4 dose (p < 0.0001 and p = 0.0078, respectively). There were no statistically significant differences in FSH and LH concentrations when the DRSP and the LNG groups were compared. Pooled FSH and LH concentrations on day 24 of cycle 1 and cycle 3 were significantly higher in the 5 mg E4 and 10 mg E4 groups than in the EE/DRSP group (p < 0.0001).
Table 2. Effects of treatment on pituitary–ovarian axis parameters*.
| Parameter | 5 mg E4/DRSP (n = 17) | 10 mg E4/DRSP (n = 19) | 20 μg EE/DRSP (n = 20) | 5 mg E4/LNG (n = 18) | 10 mg E4/LNG (n = 17) | 20 mg E4/LNG (n = 18) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Maximum | Mean (SD) | Maximum | Mean (SD) | Maximum | Mean (SD) | Maximum | Mean (SD) | Maximum | Mean (SD) | Maximum | |
| LH, IU/l | ||||||||||||
| Baseline | 4.84 (3.67) | 5.83 (3.24) | 4.31 (1.30) | 4.47 (1.39) | 4.79 (1.72) | 4.08 (1.58) | ||||||
| Treatment cycle 1 | 5.50 (2.85) | 7.28 (3.18) | 6.26 (2.87) | 8.55 (3.41) | 3.38 (1.75) | 5.43 (2.13) | 5.77 (2.62) | 8.05 (3.40) | 5.52 (1.83) | 8.01 (2.24) | 3.15 (1.56) | 4.87 (2.18) |
| Treatment cycle 2 | 6.38 (3.95) | 7.01 (3.65) | 7.14 (3.89) | 7.57 (3.68) | 4.46 (2.40) | 4.89 (2.73) | 7.69 (4.78) | 7.82 (4.73) | 7.01 (2.71) | 7.10 (2.81) | 3.94 (1.81) | 4.11 (1.98) |
| Treatment cycle 3 | 5.69 (3.25) | 7.92 (3.88) | 6.77 (3.77) | 8.92 (4.55) | 2.77 (2.31) | 5.01 (3.27) | 5.71 (2.27) | 8.49 (3.06) | 5.89 (2.06) | 8.51 (3.12) | 4.13 (1.76) | 6.44 (2.52) |
| FSH, IU/l | ||||||||||||
| Baseline | 6.23 (1.57) | 6.71 (1.70) | 5.82 (1.48) | 5.50 (1.32) | 6.10 (2.08) | 5.12 (1.36) | ||||||
| Treatment cycle 1 | 6.66 (1.78) | 7.91 (2.08) | 6.39 (1.76) | 7.64 (2.40) | 4.19 (1.83) | 5.94 (1.85) | 5.82 (1.27) | 7.22 (1.37) | 6.17 (1.75) | 7.56 (2.16) | 3.67 (1.70) | 4.58 (1.87) |
| Treatment cycle 2 | 6.34 (1.65) | 6.87 (1.67) | 6.99 (1.66) | 7.18 (1.42) | 5.49 (1.95) | 5.89 (2.16) | 6.59 (1.73) | 6.77 (1.69) | 6.72 (1.75) | 6.76 (1.78) | 4.82 (1.75) | 4.98 (1.76) |
| Treatment cycle 3 | 6.59 (1.59) | 8.35 (1.37) | 6.63 (1.52) | 7.89 (1.65) | 3.14 (2.01) | 5.72 (2.00) | 5.75 (1.77) | 7.06 (1.79) | 5.97 (1.71) | 7.50 (2.11) | 4.60 (1.63) | 6.18 (2.10) |
| E2, pmol/l | ||||||||||||
| Baseline | 170 (210) | 130 (60) | 130 (170) | 100 (60) | 120 (50) | 100 (50) | ||||||
| Treatment cycle 1 | 80 (30) | 120 (50) | 60 (20) | 110 (70) | 50 (20) | 140 (120) | 80 (60) | 80 (40) | 50 (20) | 80 (40) | 50 (10) | 80 (60) |
| Treatment cycle 2 | 200 (150) | 320 (350) | 140 (120) | 190 (220) | 100 (150) | 170 (160) | 150 (90) | 180 (170) | 180 (170) | 180 (170) | 60 (50) | 70 (110) |
| Treatment cycle 3 | 120 (120) | 270 (270) | 110 (150) | 200 (260) | 40 (10) | 280 (290) | 120 (110) | 170 (110) | 70 (40) | 170 (110) | 50 (10) | 90 (90) |
| Progesterone, nmol/l | ||||||||||||
| Baseline | 1.50 (0.54) | 1.66 (0.87) | 1.50 (0.42) | 1.57 (0.48) | 1.69 (1.02) | 1.53 (0.69) | ||||||
| Treatment cycle 1 | 1.34 (0.34) | 1.83 (0.49) | 1.39 (0.46) | 2.36 (1.14) | 1.35 (0.41) | 2.04 (0.64) | 1.39 (0.39) | 1.91 (0.53) | 1.16 (0.35) | 1.74 (0.57) | 1.39 (0.55) | 1.87 (0.79) |
| Treatment cycle 2 | 1.30 (0.34) | 1.47 (0.50) | 1.16 (0.54) | 1.20 (0.57) | 1.25 (0.57) | 1.34 (0.62) | 1.34 (0.56) | 1.36 (0.54) | 1.10 (0.53) | 1.11 (0.53) | 1.50 (0.69) | 1.51 (0.69) |
| Treatment cycle 3 | 1.33 (0.30) | 1.91 (0.54) | 1.15 (0.39) | 1.69 (0.73) | 1.23 (0.48) | 1.73 (0.72) | 1.28 (0.34) | 1.89 (0.61) | 1.04 (0.35) | 1.41 (0.50) | 1.41 (0.54) | 1.95 (0.79) |
SD, standard deviation.
*Mean (± SD) and maximum (mean ± SD) values exposed for all parameters measured during treatment cycles 1, 2 and 3.
Mean and maximum E2 concentrations decreased with increasing E4 concentration in the study medication combinations, and the lowest mean E2 concentration was observed in the 20 mg E4/LNG group (50 ± pmol/l at cycle 3) and was comparable to that of the EE/DRSP combination (40 ± 10 pmol/l at cycle 3). The pooled E2 concentrations on day 24 of cycle 1 and cycle 3 were significantly lower with increasing E4 dose (p < 0.0001). There was no statistically significant difference in E2 concentrations between the DRSP and LNG groups. The pooled E2 levels on day 24 of cycles 1 and 3 were significantly higher in the 5 mg E4 groups than in the EE/DRSP group (p = 0.0066 and p = 0.0001, respectively); no significant difference was observed when comparing the 10 mg E4 groups and the EE/DRSP group (p = 0.0708).
There were no discernible differences in mean or maximum progesterone concentrations among the treatment groups. All measured progesterone concentrations during treatment cycles 1, 2 and 3 were below 5 nmol/l, indicating absence of a LUF or ovulation, except for one measurement (a participant included in the 10 mg E4/DRSP group had a progesterone concentration of 5.69 nmol/l on day 3 of treatment cycle 1, probably due to incomplete regression of a corpus luteum from the pretreatment cycle). Progesterone concentrations did not statistically differ between the E4 groups and the EE/DRSP group, nor between the different E4 dose groups, nor between the DRSP and the LNG groups.
Endometrium
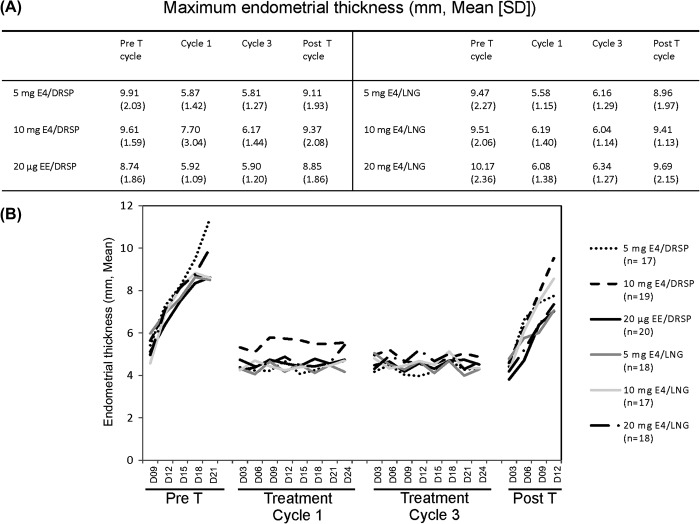
The mean endometrial thickness decreased in the treatment cycles compared with the pre- and post-treatment cycles, with no dose-related trends or significant differences between participants treated with increasing doses of E4 combined with DRSP or LNG (Figure 4A, B).
Figure 4. Maximum (mean ± SD) endometrial thickness value observed for each group during the trial (A). Mean endometrial thickness assessed in each treatment group every 3 days across the pretreatment cycle, treatment cycles 1 and 3, and post-treatment cycle (B). D, day; Pre T, pretreatment cycle; Post T, post-treatment cycle.
Return of ovulation
Return of ovulation was measured by monitoring follicular growth in the posttreatment cycle until ovulation occurred. During the post-treatment cycle, all participants ovulated within 21 days after stopping treatment. Those treated with 5 or 10 mg E4/DRSP had their first day of ovulation approximately 17 days after the last treatment (mean 17.6 and 17.1 days, respectively). The mean number of days to first ovulation was longer for participants treated with an E4/LNG combination (20.5, 20.8 and 21.0 days for the 5, 10 and 20 mg E4 groups, respectively). No difference was observed with increasing dose of E4. The mean number of days to first ovulation after the last active treatment with EE/DRSP was 20.6 days. The statistical analysis did not show any significant differences between the E4 groups and the EE/DRSP group, nor between the different E4 dose groups, nor between the DRSP and the LNG groups.
Safety and tolerability
The study combinations were well tolerated. No serious adverse events occurred. Table 3 shows the drug-related adverse events (i.e., considered possibly, probably or definitely related to treatment) reported by at least two participants in one of the treatment groups. Drug-related adverse events were reported by 50–82.4% of participants across the treatment groups. The most common drug-related adverse events were lower abdominal pain, nausea, headache, dysmenorrhoea, breast enlargement and acne. No overall trends were noted for the frequency of drug-related adverse events, when treatment groups were compared by dose of E4, between those treated with E4 and those treated with EE or between those treated with E4/DRSP or E4/LNG, except the incidence of headache, which was higher in participants treated with E4 compared with EE (not significantly different), and the incidence of acne, which was significantly higher in participants treated with E4/LNG compared with E4/DRSP.
Table 3. Drug-related adverse events reported by at least two participants in any treatment group. Data expressed in number (%) of participants.
| Parameter | 5 mg E4/DRSP (n = 17) | 10 mg E4/DRSP (n = 19) | 20 μg EE/DRSP (n = 20) | 5 mg E4/LNG (n = 18) | 10 mg E4/LNG (n = 17) | 20 mg E4/LNG (n = 18) |
|---|---|---|---|---|---|---|
| Any adverse effect | 13 (76.5) | 11 (57.9) | 12 (60.0) | 14 (77.8) | 14 (82.4) | 9 (50.0) |
| Lower abdominal pain | 4 (23.5) | 1 (5.3) | 2 (10.0) | 1 (5.6) | 1 (5.9) | 0 |
| Nausea | 2 (11.8) | 2 (10.5) | 3 (15.0) | 1 (5.6) | 1 (5.9) | 2 (11.1) |
| Irritability | 0 | 0 | 2 (10.0) | 0 | 0 | 1 (5.6) |
| Headache | 4 (23.5) | 6 (31.6) | 2 (10.0) | 4 (22.2) | 4 (23.5) | 4 (22.2) |
| Dizziness | 0 | 0 | 2 (10.0) | 0 | 0 | 1 (5.6) |
| Affect lability | 1 (5.9) | 0 | 2 (10.0) | 1 (5.6) | 0 | 0 |
| Decreased libido | 0 | 0 | 1 (5.0) | 2 (11.1) | 1 (5.9) | 0 |
| Dysmenorrhoea | 6 (35.3) | 3 (15.8) | 2 (10.0) | 1 (5.6) | 2 (11.8) | 2 (11.1) |
| Breast enlargement | 3 (17.6) | 2 (10.5) | 0 | 0 | 0 | 1 (5.6) |
| Breast tenderness/pain | 2 (11.8) | 3 (15.8) | 3 (15.0) | 0 | 1 (5.9) | 2 (11.1) |
| Acne | 0 | 1 (5.3) | 0 | 3 (16.7) | 4 (23.5) | 3 (16.7) |
| Seborrhoea | 0 | 0 | 0 | 1 (5.6) | 2 (11.8) | 0 |
| Hot flush | 2 (11.8) | 0 | 0 | 0 | 0 | 0 |
DISCUSSION
Findings and interpretation
The results of this study demonstrate that E4 in combination with DRSP or LNG effectively inhibits ovulation. There were no ovulations or LUFs during the treatment cycles in all treatment groups, showing the efficacy of 5, 10 and 20 mg E4 combined with DRSP or LNG in a regimen of 24-treatment days followed by a 4-day treatment-free period.
Ovarian suppression, as determined by maximum follicular diameter, mean and maximum E2 concentration and Hoogland score, was adequate in all E4 treatment groups. There were no discernible differences in the degree of ovarian suppression between the two progestins. However, a difference could be observed between the different E4 doses. Ovarian suppression was most pronounced at the highest E4 dose (20 mg E4). In addition, suppression of gonadotropins was most pronounced in the highest dosage group. The stronger ovarian and pituitary suppression in this group could already be observed in the first treatment cycle and was more apparent in the third treatment cycle. Ovarian suppression in the 20 mg E4/LNG group was comparable to that in the EE/DRSP group, and also with ovarian suppression reported with other registered COCs containing EE or E2 15–22.
Endometrial thickness was reduced in the treatment cycles compared with the pre- and post-treatment cycles. The results were comparable in all treatment groups, including the EE/DRSP group. Apparently, E4 in combination with a progestin has a similar effect on endometrial growth to that of EE/DRSP.
The first post-treatment ovulation occurred approximately 17 days after the last treatment day in the E4/DRSP groups, and 21 days after the last active treatment in the E4/LNG and EE/DRSP groups. An explanation for the difference between the two progestins cannot be given. For all treatment groups, the time period until the first ovulation was comparable to the duration of a normal follicular phase, confirming adequate ovarian suppression during treatment.
The different combinations were safe and well tolerated. The reported adverse events were the same as those previously described with other marketed COCs. When comparing both progestins, the incidence of headache was higher in the E4/DRSP groups, whereas the incidence of acne was higher in the E4/LNG groups. No E4 dose-related trends could be observed in the frequency or severity of the reported adverse events.
Strengths and weaknesses of the study
This study represents the first attempt to combine E4 and LNG or DRSP to achieve blockade of ovulation for three cycles. The study was conducted using state-of-the-art methodologies and by an experienced scientific team. Even if the number of participants included in each treatment arm was limited, the primary objective of the study was achieved, as no ovulation occurred in any patient.
Because the primary objective of this exploratory study was to evaluate ovulation inhibition in the different groups, the sample size was not powered to perform a safety comparison between the tested combinations. Therefore, larger studies will be needed to confirm the safety profile and tolerability of an E4-containing COC.
Differences in the results and conclusions
Animal studies performed in female rats, and human studies performed in postmenopausal women, have already demonstrated the significant dose-dependent inhibitory effect of E4 on central gonadotropin secretion3,23–25. The results of the present study confirm these previous data, as with a fixed dose of progestin higher doses of E4 were associated with a more profound inhibition of ovarian activity.
A previous study showed that the 24/4-day regimen is associated with greater inhibition of ovarian function than the conventional 21/7-day regimen26. Administering the E4 combinations following that regimen might also have contributed to the total absence of Hoogland scores 5 and 6 in our study.
Finally, recent physiological studies reveal critical requirements for membrane ERα in ovarian function and thereby in fertility27. Transgenic mice lacking the membrane ERα do not ovulate, demonstrating that this receptor is essential for ovulation. E4 selectively activates the nuclear ERα but antagonises the membrane ERα28. This selective blockade of the membrane ERα could contribute to the blockade of ovulation.
Relevance of the findings: Implications for clinicians
Women with intermenstrual bleeding have significantly larger FLS and significantly higher E2 levels than those without intermenstrual bleeding. High ovarian suppression is classically positively correlated with improved cycle control characterised by less frequent intermenstrual bleeding29. When administered at a dosage above 10 mg/day, E4 appears to be a promising alternative estrogen for use in contraception. Because doses of 20 mg E4 combined with a progestin suppress ovarian activity as efficiently as 20 μg EE/DRSP or other registered COCs containing EE or E2, an additional phase II study should be able to more precisely delineate the best E4 dose regimen between 10 and 20 mg that provides an acceptable pattern of intermenstrual spotting and bleeding.
E4 exhibits several unique features that could make it suitable as an alternative estrogen for use in a COC. These advantages were evaluated in previous trials and have been reported elsewhere. First, E4 has a high oral bioavailability associated with a long half-life of approximately 30 h, allowing daily administration. Furthermore, E4 is an end-product of estrogen metabolism in the human foetus. Metabolism through oxidation does not occur. In non-pregnant women, E4 is rapidly and almost completely excreted in the urine as a conjugate (glucuronide and sulphate). In contrast to EE and E2, E4 is less subject to biliary excretion and enterohepatic recirculation30. Therefore, it is tempting to speculate that COCs containing E4 would not result in an increased risk of hepatobiliary diseases as observed among users of EE-containing medications9. Furthermore, because E4 has a minimal impact on production of coagulation factors in the liver, the VTE risk might also be reduced compared with EE-containing COCs [Kluft C. et al., submitted].
Unanswered questions and future research
In addition to ovulation inhibition, it is necessary to assess tolerability and bleeding pattern when defining the adequate dosage of a new estrogen to be incorporated in a COC. The present study, with its relatively small sample size and short treatment duration, was not designed to evaluate properly the bleeding pattern and safety aspects of the different combinations tested. A larger dose-finding study aiming at assessing the tolerability, acceptability and bleeding characteristics of different E4-containing combinations is therefore necessary.
As mentioned above, larger trials will also be needed to fully characterise the contraceptive efficacy and safety profile of an E4-containing COC among women of different ethnicities, with different health-related characteristics (e.g., BMI, smoking habits) and of different ages. Only large and sufficiently long-term studies will be able to answer these questions.
CONCLUSION
The results of this study show that E4 in combination with DRSP or LNG adequately suppresses ovarian activity and inhibits ovulation, particularly when given at a dosage above 10 mg/day. E4 appears to be a promising alternative estrogen for use in contraception.
ACKNOWLEDGEMENTS
We gratefully acknowledge the trial centre staff who conducted the study, the women who participated in the trial, and Trial Form Support (the Netherlands), who performed the data management and statistical analyses. We also gratefully acknowledge the contribution of Professor Borm, who performed additional statistical analysis.
Declaration of interest: IJMD and CK are employees of the contract organisation Dinox BV which performed the study; YZ is an employee of Pantarhei Bioscience BV; NA was an employee of Pantarhei Bioscience BV. MJ, CM, MM and J-MF are employees of Estetra SPRL; HJTCB is the CEO of Pantarhei Bioscience BV.
The study was funded by Estetra SPRL (Liège, Belgium) and by the Walloon Government [grant number C6139].
References
- Hagen AA, Barr M, Diczfalusy E. Metabolism of 17-beta-estradiol-4-14-C in Early Infancy. Acta Endocrinol. 1965;49:207–20. [PubMed] [Google Scholar]
- Visser M, Coelingh Bennink HJ. Clinical applications for estetrol. J Steroid Biochem Mol Biol. 2009;114((1–2)):85–9. doi: 10.1016/j.jsbmb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Coelingh Bennink HJ, Skouby S, Bouchard P, et al. Ovulation inhibition by estetrol in an in vivo model. Contraception. 2008;77:186–90. doi: 10.1016/j.contraception.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Cleuren AC, Van der Linden IK, De Visser YP, et al. 17alpha-ethinylestradiol rapidly alters transcript levels of murine coagulation genes via estrogen receptor alpha. J Thromb Haemost. 2010;8:1838–46. doi: 10.1111/j.1538-7836.2010.03930.x. [DOI] [PubMed] [Google Scholar]
- Lidegaard O, Nielsen LH, Skovlund CW, et al. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and estrogen doses: Danish cohort study, 2001–9. BMJ. 2011;343:d6423. doi: 10.1136/bmj.d6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidegaard O, Lokkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366((24)):2257–66. doi: 10.1056/NEJMoa1111840. [DOI] [PubMed] [Google Scholar]
- Lidegaard O. Thromboembolic complications in users of estradiolvalerate /dienogest oral contraceptives 2013, 24 May 2013. http://www.lidegaard.dk/Slides/OC%20epidem/PP-VTE%2013-05-23%20Qlaira.pdf Copenhagen. Accessed 22 December 2014. Available from.
- Visser M, Foidart JM, Coelingh Bennink HJ. In vitro effects of estetrol on receptor binding, drug targets and human liver cell metabolism. Climacteric. 2008;11((Suppl. 1)):64–8. doi: 10.1080/13697130802050340. [DOI] [PubMed] [Google Scholar]
- Thijs C, Knipschild P. Oral contraceptives and the risk of gallbladder disease: a meta-analysis. Am J Public Health. 1993;83:1113–20. doi: 10.2105/ajph.83.8.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi E, Barrett JC, Tsutsui T. The ability of four catechol estrogens of 17beta-estradiol and estrone to induce DNA adducts in Syrian hamster embryo fibroblasts. Carcinogenesis. 2001;22:1505–10. doi: 10.1093/carcin/22.9.1505. [DOI] [PubMed] [Google Scholar]
- Singer C, Coelingh BH, Natter C, et al. Anti-estrogenic effects of the fetal estrogen estetrol in women with estrogen-receptor positive early breast cancer. Carcinogenesis. 2014;35:2447–51. doi: 10.1093/carcin/bgu144. [DOI] [PubMed] [Google Scholar]
- Gérard C, Blacher S, Communal L, et al. Estetrol is a weak estrogen antagonizing estradiol-dependent mammary gland proliferation. J Endocrinol. 2015;224:85–95. doi: 10.1530/JOE-14-0549. [DOI] [PubMed] [Google Scholar]
- Visser M, Kloosterboer HJ, Coelingh Bennink HJT. Estetrol prevents and suppresses mammary tumors induced by DMBA in a rat model. Horm Mol Biol Clin Invest. 2012;9:95–103. doi: 10.1515/hmbci-2012-0015. [DOI] [PubMed] [Google Scholar]
- Hoogland HJ, Skouby SO. Ultrasound evaluation of ovarian activity under oral contraceptives. Contraception. 1993;47:583–90. doi: 10.1016/0010-7824(93)90025-3. [DOI] [PubMed] [Google Scholar]
- Coney P, DelConte A. The effects on ovarian activity of a monophasic oral contraceptive with 100 microg levonorgestrel and 20 microg ethinyl estradiol. Am J Obstet Gynecol. 1999;181((5 Pt 2)):53–8. doi: 10.1016/s0002-9378(99)70364-9. [DOI] [PubMed] [Google Scholar]
- Duijkers IJ, Klipping C, Grob P, et al. Effects of a monophasic combined oral contraceptive containing nomegestrol acetate and 17 beta-estradiol on ovarian function in comparison to a monophasic combined oral contraceptive containing drospirenone and ethinylestradiol. Eur J Contracept Reprod Health Care. 2010;15:314–25. doi: 10.3109/13625187.2010.504313. [DOI] [PubMed] [Google Scholar]
- Duijkers IJ, Klipping C, Verhoeven CH, et al. Ovarian function with the contraceptive vaginal ring or an oral contraceptive: A randomized study. Hum Reprod. 2004;19:2668–73. doi: 10.1093/humrep/deh493. [DOI] [PubMed] [Google Scholar]
- Endrikat J, Parke S, Trummer D, et al. Ovulation inhibition with four variations of a four-phasic estradiol valerate/dienogest combined oral contraceptive: results of two prospective, randomized, open-label studies. Contraception. 2008;78:218–25. doi: 10.1016/j.contraception.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Fitzgerald C, Feichtinger W, Spona J, et al. A comparison of the effects of two monophasic low dose oral contraceptives on the inhibition of ovulation. Adv Contracept. 1994;10:5–18. doi: 10.1007/BF01986524. [DOI] [PubMed] [Google Scholar]
- Rabe T, Nitsche DC, Runnebaum B. The effects of monophasic and triphasic oral contraceptives on ovarian function and endometrial thickness. Eur J Contracept Reprod Health Care. 1997;2:39–51. doi: 10.1080/13625189709049933. [DOI] [PubMed] [Google Scholar]
- Rossmanith WG, Steffens D, Schramm G. A comparative randomized trial on the impact of two low- dose oral contraceptives on ovarian activity, cervical permeability, and endometrial receptivity. Contraception. 1997;56:23–30. doi: 10.1016/s0010-7824(97)00070-x. [DOI] [PubMed] [Google Scholar]
- Thomas K, Vankrieken L. Inhibition of ovulation by low-dose monophasic contraceptive containing gestodene. Am J Obstet Gynecol. 1990;163((4 Pt 2)):1404–10. doi: 10.1016/0002-9378(90)91356-h. [DOI] [PubMed] [Google Scholar]
- de Visser J, Coert A, Feenstra H, et al. Endocrinological studies with (7 alpha, 17 alpha)-17-hydroxy-7-methyl-19-norpregn-5(10)-en-20-yn-3-one (Org OD 14) Arzneimittelforschung. 1984;34:1010–7. [PubMed] [Google Scholar]
- Visser M, Holinka CF, Coelingh Bennink HJ. First human exposure to exogenous single-dose oral estetrol in early postmenopausal women. Climacteric. 2008;11((Suppl 1.)):31–40. doi: 10.1080/13697130802056511. [DOI] [PubMed] [Google Scholar]
- Visser M, Coelingh Bennink HJ. Estetrol, the new natural estrogen for clinical use in women. Références Gynécologie Obstétrique. 2011;14:427–32. [Google Scholar]
- Klipping C, Duijkers I, Trummer D, et al. Suppression of ovarian activity with a drospirenone-containing oral contraceptive in a 24/4 regimen. Contraception. 2008;78:16–25. doi: 10.1016/j.contraception.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Abot A, Fontaine C, Raymond-Letron I, et al. The AF-1 activation function of estrogen receptor alpha is necessary and sufficient for uterine epithelial cell proliferation in vivo. Endocrinology. 2013;154:2222–33. doi: 10.1210/en.2012-2059. [DOI] [PubMed] [Google Scholar]
- Adlanmerini M, Solinhac R, Abot A, et al. Mutation of the palmitoylation site of estrogen receptor alpha in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc Natl Acad Sci USA. 2014;111:E283–E90. doi: 10.1073/pnas.1322057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endrikat J, Gerlinger C, Plettig K, et al. A meta-analysis on the correlation between ovarian activity and the incidence of intermenstrual bleeding during low-dose oral contraceptive use. Gynecol Endocrinol. 2003;17:107–14. [PubMed] [Google Scholar]
- Coelingh Bennink HJ, Holinka CF, Diczfalusy E. Estetrol review: profile and potential clinical applications. Climacteric. 2008;11((Suppl 1.)):47–58. doi: 10.1080/13697130802073425. [DOI] [PubMed] [Google Scholar]