Abstract
The relationships between litter moisture, footpad dermatitis (FPD) and pain in medium-heavy turkey strains was studied by gait analysis in two medium-heavy with and without analgesia (betamethasone or bupivacaine).
The relationship between FPD and litter moisture was linear above a breakpoint of 49% litter moisture, and there were no differences between the two breeds in susceptibility to FPD.
Gait analysis showed higher impulse, single support time, stride time and stance time in breed A compared to breed B. Significant interactions between breed, litter and analgesic for impulse, single support time and stride time were associated with higher means for breed A given saline injection on wet litter.
Data from betamethasone analgesia in Experiments 1 and 3 were combined for analysis. Peak vertical force was higher in saline- compared to betamethasone-treated birds. Compared to the wet (high FPD) litter treatments, birds on dry (low FPD) litter had greater speed and lower double support time and longer stride length. Turkeys kept on wet litter had a longer stride length compared to dry litter when given saline, whereas in betamethasone-treated birds the means were similar.
There were no differences between birds with or without bupivacaine analgesia. Peak vertical force was higher in breed A than B and in birds with a low FPD compared to a high FPD score.
It was concluded that breeds A and B did not differ in susceptibility to develop FPD when housed on wet litter but may have natural gait differences. Significant changes in gait parameters were associated with wet litter and with analgesic treatments. The results showed that FPD affected the gait of the turkeys and, combined with evidence of behavioural changes when given analgesia, suggest that footpad lesions are painful.
INTRODUCTION
Footpad dermatitis (FPD) is a contact dermatitis of poultry associated with an immune response to wet litter (Mayne et al., 2007a ) that affects the plantar aspect of the feet. It is a common condition in commercially grown turkeys and broiler chickens with a prevalence that varies between 20% and 41% of severe lesions (ulcers) and 78% to 59% of mild lesions (discoloration, erosion) (Ekstrand and Algers, 1997; Allain et al., 2013). Lesions commonly begin with skin discoloration, followed by inflammation, hyperkeratosis and erosions of the skin that may develop into ulcers and necrosis of the epidermis (Martland, 1984; Greene et al., 1985).
More recent research has shown that water alone is sufficient to develop FPD (Mayne et al., 2007a ) and that the response to wet litter was linear above a certain minimum (Wu and Hocking, 2011). However, other factors such as diet, age and sex may affect the prevalence of FPD (Mayne, 2005) and there is anecdotal evidence that different commercial lines differ in their susceptibility to FPD. The objectives of the study were therefore first to obtain confirmatory evidence that the response to wet litter was linear above a specific minimum and second that two similar lines of turkeys differed in their susceptibility to FPD.
Gentle et al. (2001) showed that mechanothermal nociceptors are present in the scaly skin of chicken feet, and Martland (1984) suggested that FPD might be painful. Furthermore, turkeys with FPD induced by wet litter were less active and showed reduced behavioural complexity than birds on dry litter (Hocking and Wu, 2013). Differences in body weight and reluctance to place the feet on the ground in turkeys with FPD on wet litter might also indicate that lesions are painful (Mayne et al., 2007a ). However, conclusive proof of pain requires more critical methods and the main purpose of this study was to assess the painfulness of FPD lesions by a combination of objective gait assessment in turkeys with and without lesions with and without analgesic intervention. Gait assessment was conducted on a tactile force and pressure measurement system that consists of plates of rubber-like material containing piezoelectric sensors to determine the force and pressure distribution exerted by each foot (Naas et al., 2010).
MATERIALS AND METHODS
Animals and husbandry
Experiment 1
A total of 240 male poults from breeder flocks of the same age (33 weeks) and from two medium-heavy commercial hybrids (A and B) were obtained from a commercial hatchery. The poults were housed on wood shavings in 24 pens containing 10 poults and from hatch to d 26 they were brooded under a heat lamp. The pens were 1.77 m × 1.25 m and were sealed with mastic at the junction of the pen wall and the floor to make them watertight. On arrival, poults were given water for 1 h followed by feed for 2 h and then 3 h darkness followed by 3 h light that was repeated until the onset of the planned dark period. Thereafter the birds were fed ad libitum on a commercial wheat and soya-based turkey starter feed presented in a feed trough; water was supplied in suspended bell drinkers and the photoperiod was 16 h light (07:30–23:30) and 8 h darkness per 24 h. Light intensities at d 1, 2 and from d 4 to the end of the experiment were 100, 50 and 12 lux, respectively. Ambient temperatures were 28oC, 26oC and 23oC, respectively, in weeks 1, 2 and 3–5. Relative humidity averaged 38% (range 31–53%) during the course of the experimental treatments. On d 27, the poults were reallocated in groups of 4 birds of one breed to 48 pens. The existing litter was removed and replaced with fresh wood shavings (4.6 kg) and the remaining birds were culled.
Experiment 2
Male poults (n = 60) of breed B were beak trimmed at the hatchery by an infrared method. They were housed at a rate of 10 or 11 per pen on white wood shavings and the feed, ambient temperature, photoperiod and light intensities were as described for Experiment 1, whereas water was supplied ad libitum in nipple drinkers (10 nipples in 80 cm, http://www.quillproductions.co.uk). Relative humidity averaged 42% (range 23–44%).
Experiment 3
A further 72 male poults of breed B were also beak trimmed at the commercial hatchery by an infrared method. Husbandry of the birds was as described for Experiment 2 except that the birds were fed on one of 4 diets based on maize or wheat as the main energy source and soya or non-soya ingredients as the main source of protein. The diets were fed as starter crumbs (0–4 weeks), rearer pellets (5–8 weeks) and grower pellets (9–12 weeks) based on commercial recommendations for diet composition at these three phases. Ambient temperatures were reduced from 23oC at 6 weeks to 20oC at 7 weeks, 18oC at 8 weeks and 16oC at 11 weeks of age. A total of 18 birds from each dietary treatment consisting of 6 from each of 3 pens were housed in a double pen (1.77 m × 1.25 m) at 64 d of age and were fed on the same diet that they had previously been given. Feed and water were provided ad libitum in a suspended tubular feeder and bell drinker. Relative humidity averaged 60% (range 45–75%).
Treatments
Experiment 1
Experimental procedures were staggered over 2 days (24 pens on the described day and 24 on the next). Pens were randomly allocated to one of the following treatments: 1W1, 1W2 and 1W3, initially adding 1.0, 2.0 and 3.3 kg water/pen, respectively, in order to reach a target water content of approximately 30%, 40% and 50% and a dry (no water added) control, 1D. The same quantity of water was applied daily with a garden watering can during 7 d to the corresponding pens, starting from d 29. On the last day, after water was added and also 24 h later, litter samples were taken from all pens. Litter material was sampled at 30 cm from the 4 corners of each pen, mixed in a bucket and a sub-sample of approximately 100 g was placed in a disposable container and dried in a fan oven (Gallenkamp size one fan incubator) at 60oC for at least 7 d.
Two days after litter water addition started, a pair of birds from each pen was randomly selected to be injected into the breast muscle with either betamethasone (Betnesol Injection, RPH Pharmaceuticals AB, Haninge, Sweden) at a rate of 0.04 mg/kg or the same volume of saline (0.4 ml) for 5 consecutive days as described by Hocking et al. (2001). Treated birds were marked with an animal-safe spray on their back.
On d 37, 8 turkeys that had not been used for betamethasone and saline injections were randomly selected from each breed (4 with footpad score 0 and 4 with footpad score 6/7). Each bird was injected with 0.7 ml of either bupivacaine (3 mg/kg, Marcain, AstraZeneca, Luton, UK) or saline into the footpad using the dose recommended by Hocking et al. (1997). A delay of at least 15 min was observed for the bupivacaine to take effect before assessing gait on the Walkway, which was completed within one hour of the injection.
Experiment 2
On d 35, the turkeys were distributed to 12 pens of 5 birds per pen, and the litter was replaced with clean wood shavings. Each pen was randomly allocated to one of three water treatments (2D, 2W1 and 2W2), which started on d 38 with the litter water addition and continued for 7 consecutive days. The amount of water added daily was either 2.5 kg (2W1) or 4.0 kg (2W2) or half of this quantity in order to maintain the target moisture contents based on the litter scoring system of Tucker and Walker (1999) of score 2 (slightly damp) in 2W1 or score 3 (damp/tacky) in 2W2.
Experiment 3
Three different litter water treatments were allocated at random (3D, 3W1 and 3W2) within each diet from d 64 to d 79. The target moisture contents were 55% for 3W1 and 75% for 3W2. Litter was assessed to maintain a score of 3 (3W1, damp or tacky litter) or 5 (3W2, soggy, very wet and greasy litter, leaving a durable imprint when compressed or very slippery) as described by Tucker and Walker (1999).
On d 74, two birds from each pen were randomly selected and marked with an animal-safe spray and injected intramuscularly with either betamethasone (0.04 mg/kg) or saline for 6 consecutive days as described for Experiment 1.
Experimental observations
Experiment 1
Turkeys were weighed on the first and last day of water addition, and water and food consumption for the experimental period (d 29–35) was also recorded. Footpad lesions were scored by one person on the last day of each experiment on an 8-point scale as described previously (Mayne et al., 2007a ) and the highest score for both feet was recorded.
Gait analysis was conducted on d 36 on a tactile force and pressure measurement system (Walkway Research v. 7.02, Tekscan Inc., Boston, USA) using treated (betamethasone) and control (saline) turkeys from 1D and 1W3 pens (n = 48 birds). Prior to data collection, each turkey was weighed individually and given 2–5 min for habituation to the runway. The runway was 240 cm long x 60 cm high x 60 cm wide and contained the pressure platform (112 cm long × 59 cm wide) covered by a black rubber mat 5 mm thick at one end. A pen mate was placed at the other end of the walkway and the other bird was encouraged to walk through it as many times as necessary in order to get at least three good recordings (steady walking rhythm, with at least 4 steps on the sensor plates). Walkway data were collated in a laptop computer linked to the Walkway and gait parameters were subsequently extracted for analysis (Table 1).
Table 1.
Definition of the gait parameters recorded on the Tekscan Walkway System (Walkway User Manual v.7.02x, pp. 208–213)
| Variable | Definition |
|---|---|
| Speed (m/s) | Gait distance (from posterior heel of first stance to posterior heel of last stance) divided by gait time (time of first contact of first step to time of first contact of the last step registered on the sensor) |
| Peak vertical force (% of BW1) | The peak vertical force value reported as a percentage of the bird’s body weight |
| Impulse (% BW1/s) | The impulse value (force × time it acts over) reported as a percentage of the bird’s body weight impulse |
| Single support time (s) | The time the foot is in contact with the sensor, measured from the last contact of the opposing foot’s preceding stance to the first contact of the opposing foot’s next stance |
| Double support time (s) | Time over which the body is supported by both legs |
| Stance time (s) | The average time from first contact of the foot to last contact of the same foot |
| Stride length (cm) | Distance between the posterior heel points of two consecutive footprints of the foot in question (parallel to the line of progression) |
| Stride time (s) | Elapsed time between the first contacts of two consecutive footfalls of the foot in question |
1BW, body weight. Peak vertical force is named maximum force in the user manual.
Experiment 2
Body weight, water and food consumption were recorded as in Experiment 1 for the experimental period (d 38–45). FPD and litter moisture were also determined as described for Experiment 1 but gait analysis was not conducted.
Experiment 3
The 12 pairs of birds in 6 randomly selected pens were weighed and gait analysis was conducted as described above on d 79 and the remaining birds on d 80. The procedures were the same as in Experiment 1 except that more runs were obtained to select 5 qualifying runs for assessment of gait parameters.
Welfare inspection
All experiments and procedures were conducted after ethical approval under project licence number PPL60/45067. The health of the turkeys was inspected on a daily basis and severely affected birds were humanely killed. All turkeys were killed at the end of each experiment with an intravenous sodium pentobarbital overdose (Euthatal, Merial, Toulouse, France)
Statistical analysis
Experiment 1 was a randomised block and Experiments 2 and 3 were completely randomised designs. Analysis of variance was conducted in GenStat (13th edition, http://www.vsni.co.uk/software/genstat). Treatment effects for the analyses of FPD score, body weight, feed and water intake were water treatment (and breed in Experiment 1). The mean values for litter moisture content and FPD scores from Experiments 1 and 2 were combined and analysed by a segmented, two-line regression model to fit linear and horizontal lines compared to quadratic and cubic polynomial models.
The model for the analysis of the gait parameters of both left and right feet included pen effects as blocks and litter and drug (with breed for Experiment 1) as treatment effects. The Walkway data from Experiments 1 and 3 for the betamethasone-treated birds were combined to increase the statistical power of the analysis. Breed in Experiment 1 was ignored and treatments 3W1 and 3W2 were combined. The block structure was pen nested within experiment and the treatment structure was litter (wet vs. dry) and drug (betamethasone or bupivacaine vs. saline). All data were checked for assumptions of normality and equality of variance by inspection of the residuals and residual variance plots. No data transformations were required.
RESULTS
Production variables
There were no statistically significant production differences between the two breeds in Experiment 1. Overall means (±SD) were 1690 ± 87.2 g for live weight at 4 weeks and 76 ± 5.6 g/d for live weight gain, 169 ± 2.6 g/d for feed intake and 321 ± 38.4 ml/d for water intake from 3 to 4 weeks of age. Corresponding means in Experiment 2 were 1866 ± 134.2 g, 82 ± 11.9 g/d, 131 ± 12.3 g/d and 304 ± 111.4 ml/d. Mean body weights and weight gains were similar across the three treatments in Experiment 3 and averaged 8506 ± 748 g and 233 ± 66 g/d, respectively. Averages of production traits and tests of significance for the treatments in all three experiments are provided in Supplemental Tables 1–3.
FPD scores
Highly significant (P < 0.001) differences occurred between treatments for litter moisture content in both Experiments 1 and 2, whereas FPD score was affected (P < 0.001) only in Experiment 1. FPD score averaged over both breeds for 1D, 1W1, 1W2 and W3 were 0.8, 2.5, 4.4 and 6.2 (SED, standard error of a difference, 0.47, P < 0.001) in Experiment 1 compared to 0.56, 0.94 and 0.38 (SED 0.567, not significant) for 2D, 2W1 and 2W2. Litter moisture (%) was 36, 59, 71 and 80 (SED 2.3, P < 0.001), respectively, in Experiment 1 and 9, 30 and 43 (SED 3.70, P < 0.001) in Experiment 2.
The segmented regression model resulted in a better fit than the quadratic polynomial (RSS = 1.40, F 3,8 = 248.1 compared to RSS = 2.05, F 2,8 = 95.7) in describing the relationship between FPD score and litter moisture (Figure) and the cubic term was not significant. The linear breakpoint on the X-axis for the two-line model was 48.9% ± 2.79% moisture (95% confidence interval 41.4%, 54.2%) with a slope of 0.174 ± 0.0197 FPD score/1% moisture. The breakpoint on the Y-axis was 0.71 ± 0.020 FPD score. Mean treatment FPD score plotted against mean litter moisture is presented in the figure with the fitted two-line regression.
Figure.
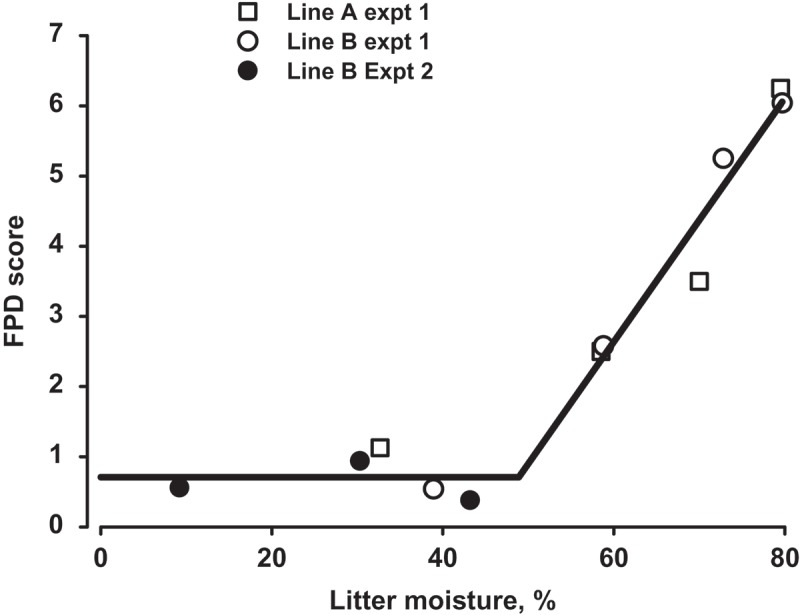
Mean FPD score of different genetic lines of turkeys reared on different litter treatments plotted against mean litter moisture for Experiments 1 and 2. The fitted line is from a segmented regression model.
Litter moisture for 3D, 3W1 and 3W2 treatments at the end of Experiment 3 averaged 36%, 59% and 63% (SED 2.9, P < 0001) and mean FPD scores were 0.5, 6.1 and 6.3 (SED 0.43, P < 0.001), respectively.
Gait analysis using betamethasone analgesia
Gait analyses were conducted on the 1D and 1W3 treatments in Experiment 1. Data for two birds for the betamethasone treatment were not available, leaving n = 46 for analysis. The correlation between left and right feet was >0.8 for all variables except peak vertical force (r = 0.4) and the mean for both left and right feet was analysed.
Marginal means for breed, litter treatment and the use of analgesic are provided in Supplemental Table 4. There was a significant interaction (P < 0.05) between breed, litter and analgesic for these same traits (impulse, single support time and stride time) that was associated with relatively high means for breed A on wet litter (high FDP) given saline injections (Supplemental Table 5). Averaged over litter and analgesic, breed A showed higher means than B for each of these variables (P < 0.05).
In Experiment 3, one betamethasone-injected bird died before being tested. The marginal means for litter treatment and analgesia are presented in Supplemental Table 6. There was a significant interaction between litter and drug for stride length where the difference between birds with low and high FPD scores (dry vs. wet litter, respectively) was similar in betamethasone-treated birds and greater in birds given a saline injection. Mean stride length (cm) for 2D, 2W1 and 2W2 birds given betamethasone were 49.4, 51.2 and 48.3 compared to 51.8, 46.0 and 46.2 for saline-treated birds (SED 1.81 for comparisons on the same litter otherwise 1.71).
Marginal means for the main effects of the combined analysis from Experiments 1 and 3 are presented in Table 2. Peak vertical force was higher in saline- compared to betamethasone-treated birds (F 1,30 = 7.25, P = 0.012). Birds on dry (low FPD) compared to the wet (high FPD) litter treatments had greater speed (F 1,33 = 4.68, P = 0.038) and lower double support time (F 1,33 = 4.81, P = 0.035). A significant interaction between litter and drug occurred for stride length: there was a greater difference in saline-treated turkeys kept on wet compared to dry litter, whereas means were similar in betamethasone-treated birds on dry and wet litter. Means (cm) for birds on dry and wet litter, respectively, were 37.1 and 36.9 for betamethasone compared to 39.1 and 25.4 on the saline treatment (SED 1.00 within analgesic and 0.76 within litter).
Table 2.
Marginal means and significance of main effects for the gait parameters in Experiments 1 and 3 combined for birds with low or high footpad scores and treated with daily intramuscular injections of analgesic (betamethasone) or saline
| Main effect | Speed (m/s) | Peak vertical force (%BW1) | Impulse (%BW/s) | Single support time (s) | Double support time (s) | Stride length (cm) | Stride time (s) | Stance time (s) |
|---|---|---|---|---|---|---|---|---|
| Low FPD score | 38.8 | 110.9 | 46.0 | 0.440 | 0.160 | 38.3 | 1.041 | 0.585 |
| High FPD score | 34.4 | 108.5 | 48.9 | 0.444 | 0.197 | 36.2 | 1.095 | 0.640 |
| SED | 2.05 | 1.90 | 2.047 | 0.0189 | 0.0116 | 0.836 | 0.0465 | 0.0250 |
| Significance | 0.038 | NS | NS | NS | 0.035 | 0.018 | NS | 0.035 |
| Betamethasone | 36.7 | 107.9 | 47.0 | 0.446 | 0.177 | 36.9 | 1.059 | 0.614 |
| Saline | 36.1 | 111.2 | 48.3 | 0.439 | 0.183 | 37.2 | 1.083 | 0.618 |
| SED | 1.43 | 1.26 | 1.546 | 0.0134 | 0.0084 | 0.54 | 0.0332 | 0.0221 |
| Significance | NS | 0.012 | NS | NS | NS | NS | NS | NS |
The means are for the average of left and right feet except for speed and double support time. 1BW, body weight; NS, not significant (P > 0.05).
Gait assessment using bupivacaine analgesia
No statistically significant interactions between treatment effects were found when using bupivacaine. Marginal means for the main effects are presented in Table 3. Significant differences in peak vertical force were detected between breeds and litter (FPD score). Peak vertical force was higher in breed A than breed B and higher in birds with a low FPD score compared to a high FPD score. There were no differences between birds with or without analgesia.
Table 3.
Marginal means and significance of main effects for the gait parameters in Experiment 1 for birds with low or high footpad scores and treated with a local injection of bupivacaine or saline
| Main effect | Speed (m/s) | Peak vertical force (%BW1) | Impulse (%BW/s) | Single support time (s) | Double support time (s) | Stride length (cm) | Stride time (s) | Stance time (s) |
|---|---|---|---|---|---|---|---|---|
| Breed A | 32.4 | 117.1 | 49.3 | 0.508 | 0.074 | 34.2 | 1.078 | 0.575 |
| Breed B | 30.6 | 111.1 | 48.3 | 0.492 | 0.095 | 32.8 | 1.093 | 0.583 |
| Significance | NS | 0.05 | NS | NS | NS | NS | NS | NS |
| Low FPD | 30.3 | 117.2 | 50.3 | 0.518 | 0.069 | 32.9 | 1.109 | 0.576 |
| High FPD | 32.7 | 111.0 | 47.4 | 0.482 | 0.100 | 34.1 | 1.062 | 0.581 |
| Significance | NS | 0.045 | NS | NS | NS | NS | NS | NS |
| Bupivacaine | 30.7 | 113.8 | 48.8 | 0.499 | 0.084 | 33.3 | 1.098 | 0.585 |
| Saline | 32.3 | 114.4 | 48.9 | 0.501 | 0.085 | 33.6 | 1.073 | 0.573 |
| Significance | NS | NS | NS | NS | NS | NS | NS | NS |
| SED | 2.11 | 2.73 | 2.20 | 0.0251 | 0.0193 | 1.443 | 0.048 | 0.0268 |
The means are for the average of left and right feet except for speed and double support time. 1BW, body weight; NS, not significant (P > 0.10).
DISCUSSION
Litter moisture and FPD
The present study confirms the previously reported linear relationship between FPD score and litter moisture above a certain minimum (Wu and Hocking, 2011). However, the minimum moisture content at which FPD began to develop was estimated to be 30% by Wu and Hocking (2011), whereas the breakpoint established in this experiment was higher (49% moisture). In both Experiments 1 and 2 in the present research, the relative humidity was very low, leading to comparatively rapid drying of the surface of the litter. Furthermore, the litter samples were taken from the full depth of litter and may not reflect the moisture content of the surface litter actually in contact with the feet. Taken together, the results emphasise the large number of environmental variables such as ambient temperature, ventilation rate, relative humidity and litter material that affect litter moisture content and the prevalence of FPD. It is important also to note that the FPD scores refer largely to the area of affected tissue which in general had not progressed to the stage of ulceration and significant loss of epidermal tissue.
Breed and FPD
Breeds A and B are medium-heavy strains that are widely used in Europe and of similar performance characteristics. There were no differences between breeds in the first experiment, and the second hypothesis that the breeds used in this experiment differ in their susceptibility to FPD is rejected. Hocking and Wu (2013), using a similar system, found that lines of heavy turkeys developed FPD earlier than traditional lines, although the differences were not significant after 6 d. Conversely, they reported large differences between individuals within line which were not apparent in the present experiments. Nevertheless, because this is a model system, it is possible that a difference in breed susceptibility might exist in commercial situations.
FPD, feed intake and weight gain
In Experiments 1 and 2, where feed intake and weight gain were recorded, no differences among litter treatments were found. Previous studies by Wu and Hocking (2011) showed that turkeys on wet litter had a higher feed intake but similar weight gain, whereas Mayne et al. (2007a ) found lower body weights on wet litter, associated with a lower feed intake. The productivity of broiler chickens housed on wet litter was lower than in birds on dry litter (De Jong et al., 2014). It is possible that the low relative humidity affected these traits in the present experiments.
Gait assessment
No major differences between treatments were observed when the FPD score on both feet were analysed and compared or the mean score was analysed and only the average is presented in the tables. In contrast to these results, Naas et al. (2010) found that peak vertical force differed between left and right legs in broiler chickens.
There is no published report of the Tekscan being used on turkeys, but in addition to the study on broiler chickens by Naas et al. (2010) there are reports of its use in cows and dogs (Lascelles et al., 2006; Shearer et al., 2013). The present research revealed differences between the two breeds in impulse, single support time, stride and stance time. It is possible that breeds A and B have naturally occurring gait differences with A taking longer in the different segments of the gait cycle compared to B. Whereas speed was not significantly different, it was numerically lower in breed B. Factors such as breed temperament might also explain these results, although, importantly, the interactions between litter, analgesia and litter treatments were one of scale rather than sign.
Most of the differences in gait parameters occurred between litter rather than drug treatments. Differences between breeds in Experiment 1 were inconsistent and the combined analysis of Experiments 1 and 3 was conducted to increase the power of the main experimental comparisons of litter (FPD score) and analgesic treatment. Wet litters led to higher footpad lesions, and gait differences are hypothesised to be due to birds experiencing pain or discomfort. In general, the results are consistent with this hypothesis. For example, walking speed was slower in birds that had been on wet litter: lame broiler chickens walked significantly slower than sound ones, and walking speed increased when an analgesic (carprofen) was administered (McGeown et al., 1999). Turkeys from wet litters spent a longer time supporting their body on both legs, which provides more stability (Caplen et al., 2013) and would be expected to increase with lameness. Stride length was shorter, reflecting shorter steps, and it has been shown that lame cows have a slower walking speed and perform shorter strides compared to sound animals (Flower et al., 2005). Finally, stance time was longer in turkeys kept on wet litter which may be interpreted as birds being more careful when placing a foot on the littered floor because of associated pain. Whereas birds with no footpad lesions kept on wet litter conditions might have altered gait in their home pen (see Sinclair et al., 2015), this is unlikely to have been carried over into the testing environment of the Walkway where the birds were evaluated under the same conditions. Nevertheless, animals with and without injuries must be observed under conditions that do not confound the behaviour of interest (Weary et al., 2006) and it will be important to confirm these results by conducting a similar study on naturally occurring lesions of different FDP scores in birds kept on the same litter.
Relatively few differences in the gait parameters were detected between saline- and betamethasone-treated turkeys. Peak vertical force showed a significant difference between breeds, consistent with the hypothesis of a natural gait difference, and between birds with low and high footpad lesions (Table 3). Peak vertical force was also higher in saline-injected birds (Table 2). Turkeys with high footpad scores may exert less force, therefore reducing stress on the musculoskeletal system produced during walking (Corr et al., 2007), a result which is consistent with that in lame broiler chickens reported by Naas et al. (2010) before and after (metanizole sodium) treatment.
Very few differences were detected between birds treated with bupivacaine and saline in Experiment 1, and this treatment was not repeated in Experiment 3: it is likely that the bupivacaine experiment was underpowered with too few birds being tested. Thus, peak vertical force was significantly higher in birds on dry litter (low FPD) scores compared to birds on wet litter (high FPD scores), a result that is inconsistent with the data from betamethasone treatment where there was no detectable difference.
Endogenous analgesia was first demonstrated in poultry by Hocking (1994) in broiler breeders with skeletal disease and by Gentle and Corr (1995) in an experimentally induced articular pain model in layer-type pullets. In addition, these authors showed that changes in motivation can supress pain by altering the attention of the animal away from pain (e.g. a novel environment). Thus, endogenous analgesia could explain the relative lack of differences between the analgesia and saline treatments in the Walkway environment if the bird was strongly motivated to reach its pen mate. Another possible explanation is that the footpad was not inflamed, preventing the analgesic from being effective, although this is inconsistent with the differences observed between the birds with and without FPD lesions (wet vs. dry litter) and previous research demonstrating an inflammatory response to wet litter in terms of both cellular and cytokine changes (Mayne et al., 2006, 2007b ).
Betamethasone was effective in turkeys and broiler breeders with hip disorders (Duncan et al., 1991; Hocking, 1994), but to our knowledge, this drug has not been used for the assessment of pain associated with FPD in birds. A disadvantage of using betamethasone is the time required for the drug to achieve effective plasma concentrations and a suitable alternative non-steroid analgesic that did not possess this limitation would be advantageous in experiments to assess pain in birds with FPD. Betamethasone is also a potent glucocorticoid that may also affect behaviour (Liu et al., 2012; Berthon et al., 2014). Alternative analgesics that have been tested in turkeys include meloxicam and flunixin (Baert and De Backer, 2003) and the opioid butorphanol (Buchwalder and Huber-Eicher, 2005). The effectiveness of bupivacaine as an analgesic has been demonstrated in young layer chicks with experimentally induced articular pain (Hocking et al., 1997). Using a local analgesic had advantages when compared to betamethasone, in terms of involving less handling of the poults (only one injection, instead of repeated administration over several days). However, concerns existed due to the high volume injected into the footpad, especially with heavier birds. Hence, a more concentrated solution or a different drug that permitted a lower injection volume would be advantageous.
CONCLUSIONS
The crucial role that water plays in the development of FPD is supported by the present research. However, there were no detectable differences in susceptibility to FPD in the two breeds that were tested in this experiment. The results showed that FPD affected the gait of the turkeys, which, together with further evidence of behavioural changes (Sinclair et al., 2015), is consistent with the hypothesis that footpad lesions are painful. Nevertheless, future experiments with improved analgesic intervention are necessary to obtain definitive evidence for pain and to assess the painfulness of different FPD scores. The Walkway was a useful tool that allowed objective gait assessment in turkeys to be made under experimental conditions, but birds may experience confounding motivations that compromise its use in some circumstances.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/00071668.2015.1077203.
ACKNOWLEDGEMENTS
This work includes material submitted in partial fulfilment of the MSc in Applied Animal Behaviour and Animal Welfare by CWW and AS. We are grateful to Brendan Duggan for advice on the operation of Walkway and gait analysis.
Funding Statement
The experiments were funded by the BBSRC [contract number BB/L012022/1] as part of the FP7 ERA-Net ANIHWA, “Coordination of European Research on Animal Health and Welfare” project TURKEYWELFARE (number 182). Poults and material support were kindly donated by Aviagen Turkeys Ltd Chester, UK. The Roslin Institute is supported by an Institute Core Strategic Grant from the Biotechnology and Biological Sciences Research Council.
REFERENCES
- Allain V., Huonnic D., Rouina M., Michel V. Prevalence of skin lesions in turkeys at slaughter. British Poultry Science. 2013;54:33–41. doi: 10.1080/00071668.2013.764397. [DOI] [PubMed] [Google Scholar]
- Baert K., De Backer R. Comparative pharmacokinetics of three non-steroidal anti-inflammatory drugs in five bird species. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2003;134:25–33. doi: 10.1016/S1532-0456(02)00184-9. [DOI] [PubMed] [Google Scholar]
- Berthon B.S., MacDonald-Wicks L.K., Wood L.G. A systematic review of the effect of oral glucocorticoids on energy intake, appetite, and body weight in humans. Nutrition Research. 2014;34:179–190. doi: 10.1016/j.nutres.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Buchwalder T., Huber-Eicher B. Effect of the analgesic butorphanol on activity behaviour in turkeys (Meleagris gallopavo) Research in Veterinary Science. 2005;79:239–244. doi: 10.1016/j.rvsc.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Caplen G., Colborne G.R., Hothersall B., Nicol C.J., Waterman-Pearson A.E., Weeks C.A., Murrell J.C. Lame broiler chickens respond to non-steroidal anti-inflammatory drugs with objective changes in gait function: a controlled clinical trial. TheVeterinary Journal. 2013;196:477–482. doi: 10.1016/j.tvjl.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Corr S.A., McCorquodale C., McDonald J., Gentle M., McGovern R. A force plate study of avian gait. Journal of Biomechanics. 2007;40:2037–2043. doi: 10.1016/j.jbiomech.2006.09.014. [DOI] [PubMed] [Google Scholar]
- De Jong I.C., Gunnink H., Van Harn J. Wet litter not only induces footpad dermatitis but also reduces overall welfare, technical performance, and carcass yield in broiler chickens. TheJournal of Applied Poultry Research. 2014;23:51–58. doi: 10.3382/japr.2013-00803. [DOI] [Google Scholar]
- Duncan I.J.H., Beatty E.R., Hocking P.M., Duff S.R.I. Assessment of pain associated with degenerative hip disorders in adult male turkeys. Research in Veterinary Science. 1991;50:200–203. doi: 10.1016/0034-5288(91)90106-X. [DOI] [PubMed] [Google Scholar]
- Ekstrand C., Algers B. Rearing conditions and foot-pad dermatitis in Swedish turkey poults. Acta Veterinaria Scandinavica. 1997;38:167–174. doi: 10.1186/BF03548496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower F.C., Sanderson D.J., Weary D.M. Hoof pathologies influence kinematic measures of dairy cow gait. Journal of Dairy Science. 2005;88:3166–3173. doi: 10.3168/jds.S0022-0302(05)73000-9. [DOI] [PubMed] [Google Scholar]
- Gentle M.J., Corr S.A. Endogenous analgesia in the chicken. Neuroscience Letters. 1995;201:211–214. doi: 10.1016/0304-3940(95)12181-1. [DOI] [PubMed] [Google Scholar]
- Gentle M.J., Tilston V., McKeegan D.E.F. Mechanothermal nociceptors in the scaly skin of the chicken leg. Neuroscience. 2001;106:643–652. doi: 10.1016/S0306-4522(01)00318-9. [DOI] [PubMed] [Google Scholar]
- Greene J.A., McCracken R.M., Evans R.T. A contact-dermatitis of broilers – clinical and pathological findings. Avian Pathology. 1985;14 doi: 10.1080/03079458508436205. [DOI] [PubMed] [Google Scholar]
- Hocking P.M. Assessment of the welfare of food restricted male broiler breeder poultry with musculoskeletal disease. Research in Veterinary Science. 1994;57:28–34. doi: 10.1016/0034-5288(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Hocking P.M., Gentle M.J., Bernard R., Dunn L.N. Evaluation of a protocol for determining the effectiveness of pretreatment with local analgesics for reducing experimentally induced articular pain in domestic fowl. Research in Veterinary Science. 1997;63:263–267. doi: 10.1016/S0034-5288(97)90031-X. [DOI] [PubMed] [Google Scholar]
- Hocking P.M., Robertson G.W., Gentle M.J. Effects of anti-inflammatory steroid drugs on pain coping behaviours in a model of articular pain in the domestic fowl. Research in Veterinary Science. 2001;71:161–166. doi: 10.1053/rvsc.2001.0510. [DOI] [PubMed] [Google Scholar]
- Hocking P.M., Wu K. Traditional and commercial turkeys show similar susceptibility to foot pad dermatitis and behavioural evidence of pain. British Poultry Science. 2013;54:281–288. doi: 10.1080/00071668.2013.781265. [DOI] [PubMed] [Google Scholar]
- Lascelles B.D.X., Roe S.C., Smith E., Reynolds L., Markham J., Marcellin-Little D., Bergh M.S., Budsberg S.C. Evaluation of a pressure walkway system for measurement of vertical limb forces in clinically normal dogs. American Journal of Veterinary Research. 2006;67:277–282. doi: 10.2460/ajvr.67.2.277. [DOI] [PubMed] [Google Scholar]
- Liu L., Song Z., Sheikhahmadi A., Jiao H., Lin H. Effect of corticosterone on gene expression of feed intake regulatory peptides in laying hens. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 2012;162:81–87. doi: 10.1016/j.cbpb.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Martland M.F. Wet litter as a cause of plantar pododermatitis leading to foot ulceration and lameness in fattening turkeys. Avian Pathology. 1984;13:241–252. doi: 10.1080/03079458408418528. [DOI] [PubMed] [Google Scholar]
- Mayne R.K. A review of the aetiology and possible causative factors of foot pad dermatitis in growing turkeys and broilers. Worlds Poultry Science Journal. 2005;61:256–267. doi: 10.1079/WPS200458. [DOI] [Google Scholar]
- Mayne R.K., Else R.W., Hocking P.M. High litter moisture alone is sufficient to cause footpad dermatitis in growing turkeys. British Poultry Science. 2007a;48:538–545. doi: 10.1080/00071660701573045. [DOI] [PubMed] [Google Scholar]
- Mayne R.K., Hocking P.M., Else R.W. Foot pad dermatitis develops at an early age in commercial turkeys. British Poultry Science. 2006;47:36–42. doi: 10.1080/00071660500475392. [DOI] [PubMed] [Google Scholar]
- Mayne R.K., Powell F., Else R.W., Kaiser P., Hocking P.M. Foot pad dermatitis in growing turkeys is associated with cytokine and cellular changes indicative of an inflammatory immune response. Avian Pathology. 2007b;36:453–459. doi: 10.1080/03079450701639327. [DOI] [PubMed] [Google Scholar]
- Mc Geown D., Danbury T.C., Waterman-Pearson A.E., Kestin S.C. Effect of carprofen on lameness in broiler chickens. Veterinary Record. 1999;144:668–671. doi: 10.1136/vr.144.24.668. [DOI] [PubMed] [Google Scholar]
- Naas I.D.A., De Lima Almeida Paz I.C., Baracho M.D.S., De Menezes A.G., Oliveira De Lima K.A., De Freitas Bueno L.G., Mollo Neto M., De Carvalho V.C., De Lima Almeida I.C., De Souza A.L. Assessing locomotion deficiency in broiler chicken. Scientia Agricola. 2010;67:129–135. [Google Scholar]
- Shearer J.K., Stock M.L., Van Amstel S.R., Coetzee J.F. Assessment and management of pain associated with lameness in cattle. Veterinary Clinics of North America-Food Animal Practice. 2013;29 doi: 10.1016/j.cvfa.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Sinclair A., Weber Wyneken C.W., Veldkamp T., Vinco L.J., Hocking P.M. Behavioural assessment of pain in commercial turkeys (Meleagris gallopavo) with foot pad dermatitis. British Poultry Science. 2015;56(5):511–521. doi: 10.1080/00071668.2015.1077204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker S., Walker A. Hock burn in broilers. In: Wiseman J., Garnsworthy P.C., editors. Recent Developments in Poultry Nutrition. Vol. 2. Nottingham: Nottingham University Press; 1999. pp. 107–122. [Google Scholar]
- Weary D.M., Niel L., Flower F.C., Fraser D. Identifying and preventing pain in animals. Applied Animal Behaviour Science. 2006;100:64–76. doi: 10.1016/j.applanim.2006.04.013. [DOI] [Google Scholar]
- Wu K., Hocking P.M. Turkeys are equally susceptible to foot pad dermatitis from 1 to 10 weeks of age and foot pad scores were minimized when litter moisture was less than 30% Poultry Science. 2011;90:1170–1178. doi: 10.3382/ps.2010-01202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


