Abstract
The Notch signaling pathway is required to maintain renin expression within juxtaglomerular (JG) cells. However, the specific ligand which activates Notch signaling in renin-expressing cells remains undefined. In this study, we found that among all Notch ligands, Jagged1 is differentially expressed in renin cells with higher expression during neonatal life. We therefore hypothesized that Jagged1 was involved in renin expression and/or vascular integrity. We used a conditional knockout approach to delete Jagged1 in cells of the renin lineage. Deletion of Jagged1 specifically within renin cells did not result in decreased renin production within the kidney. However, animals with conditional deletion of Jagged1 did develop focal kidney fibrosis and elevated blood urea nitrogen. Our data demonstrate that Jagged1-mediated Notch signaling is dispensable in renin cells of the kidney in regard to renin expression. However, deletion of Jagged1 in renin cells descendants affects perivascular–interstitial integrity leading to focal fibrosis and diminished renal function.
Keywords: jagged1, notch, renin
Introduction
Renin-expressing cells are crucial in the control of blood pressure and fluid-electrolyte homeostasis and nephrovascular development (Gomez et al. 2014). While the mechanisms that maintain renin expression have not been fully identified, they are likely dependent on the direct interaction between renin and adjacent cells. The Notch signaling pathway is an evolutionarily conserved pathway, which is vital for cell–cell communication during fundamental developmental processes (Tanigaki and Honjo 2010). Members of the Notch signaling pathway, including receptors, ligands, and the final common effector, RBP-J, are expressed in renin cells of the kidney (Brunskill et al. 2011). Therefore, we hypothesized that the Notch signaling pathway, conveyed by cell–cell signaling, is important in maintaining the renin cell identity. To that end, our laboratory previously used a conditional knockout approach to delete the final effector of Notch signaling, RBP-J, specifically within cells of the renin lineage (Castellanos Rivera et al. 2011). Conditional deletion of RBP-J in renin cells resulted in a severe reduction in the number of renin-positive cells in the kidney. Furthermore, it was determined that this effect is via canonical Notch signaling, as treatment of mice with a gamma secretase inhibitor (which represses the Notch pathway) nearly phenocopies the effect of RBP-J deletion (unpublished work from our laboratory). Together, this work showed that Notch/RBPJ signaling is required to maintain renin expression within juxtaglomerular (JG) cells. However, how the Notch pathway is activated in renin cells remains undefined. Therefore, we questioned which Notch ligand is responsible for providing the instructive cues to renin cells, triggering the Notch signaling pathway, and thus responsible for maintaining the identity of renin cells and their descendants. In this study, we investigated the expression of the Notch ligand Jagged1 in renin cells and the effects of conditional Jagged1 deletion.
Materials and Methods
Mice
To investigate whether expression of Jagged1 is critical for renin cell development, we generated mice with conditional deletion of Jagged1 specifically in renin cells. We crossed Jagged1fl/fl mice (Kiernan et al. 2006) which contain LoxP sites flanking exon 4, the DSL domain of the Jagged1 gene, with EIIa-Cre mice which express cre in the early embryo, resulting in germline deletion of the loxP-flanked Jagged1 allele (Jagged1del/+) (Lakso et al. 1996). These mice were then crossed with Jagged1fl/fl mice and Ren1d cre/+ mice (Sequeira-Lopez et al. 2004), ultimately generating mice with one deleted Jagged1 allele, one floxed Jagged1 allele, and one copy of Ren1d cre/+ (Jagged1del/fl;Ren1d cre/+) termed “mutant” animals in this study. Control animals either lacked cre recombinase (Jagged1del/fl;Ren1d +/+) or had a wild-type allele of Jagged1 (Jagged1+/fl;Ren1d cre/+) and are termed “control” animals in this study. Ren1d cre animals were generated previously in our laboratory (Sequeira-Lopez et al. 2004), and Jagged1fl/fl mice were obtained from Jackson Laboratories. All procedures were performed following the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Virginia Animal Care and Use Committee.
Genotyping
Tail biopsies were sent to Transnetyx for genotyping (Transnetyx Genotyping Services, Cordova, TN).
Histological and immunohistochemical analysis
Mice were anesthetized with tribromoethanol (300 mg/kg). Kidneys were harvested at indicated time points, weighed, fixed overnight in Bouin’s fixative, and embedded in paraffin. Sections (5 μm) were deparaffinized in xylenes and graded alcohols and stained with hematoxylin and eosin to examine overall kidney morphology and Masson’s Trichrome to identify areas of fibrosis as described previously (Gomez et al. 2009; Sequeira-Lopez et al. 2010).
We performed immunohistochemistry for renin (1:500 dilution of rabbit polyclonal anti-mouse antibody) to determine the distribution of renin-expressing cells, Jagged1 (1:100 dilution of goat polyclonal antibody; Santa Cruz, Dallas, TX) to demonstrate conditional deletion, and α-smooth muscle actin (SMA, 1:10,000 dilution of mouse monoclonal antibody; Sigma, St. Louis, MO) to evaluate vascular architecture, as described previously (Sequeira-Lopez et al. 2001; Lin et al. 2014). To quantitate the occurrence of fibrosis in control and mutant animals, we reviewed midsagittal sections of kidneys from control and mutant animals at each of the indicated ages. Fibrosis was scored as areas of increased αSMA staining of interstitial cells and dilated tubules.
The juxtaglomerular apparatus (JGA) index was calculated as the number of renin-positive JGA per total number of glomeruli and expressed as a percentage. To determine the length of renin staining, we measured the maximal length of each renin-staining JGA.
RNA extraction and PCR analysis
Whole kidneys from control and mutant animals were removed and placed in RNAlater (Ambion, Austin, TX) overnight in 4°C and then stored in −20°C. Total RNA was isolated using Trizol extraction (Life Technologies, Grand Island, NY) according to the manufacturer’s instructions. cDNA was prepared from 2 μg of RNA using Maloney murine leukemia virus reverse transcriptase (Life Technologies, Grand Island, NY) and an oligo(dT) primer according to the manufacturer’s instructions. Quantitative reverse transcription polymerase chain reaction (RT-PCR) was performed using SYBR Green I (Invitrogen Molecular Probes, Eugene, OR) in a CFXConnect system (BioRad, Hercules, CA). Renin and Jagged1 mRNA expression was normalized to GAPDH expression, and the changes in expression were determined by the ∆∆Ct method and are reported as relative expression compared to control mice. The following primers were used: renin forward 5’-ACAGTATCCCAACAGGAGAGACAAG-3’ and renin reverse 5’-GCACCCAGGACCCAGACA-3’; Jagged1 forward 5’-GCAACGACCGTAATCGCATC-3’ and Jagged1 reverse 5’-CCATTGCCGGCTAGGGTTTA-3’; GAPDH forward 5’-TTGATGGCAACAATCTCCAC-3’ and GAPDH reverse 5’-CGTCCCGTAGACAAAATGGT-3’.
Plasma renin
Plasma was collected from animals at the time of sacrifice. Plasma renin concentration was determined using Renin ELISA following the manufacturer’s instructions (RayBiotech, Norcross, GA).
Statistical analysis
Values are expressed as mean ± standard error of the mean (SEM). Statistical significance between groups was evaluated using Student’s t-test and two-way analysis of variance. Differences were considered statistically significant at *P < 0.05, **P < 0.01, and ***P < 0.001 levels.
Results
Jagged1 is expressed in renin cells during kidney development
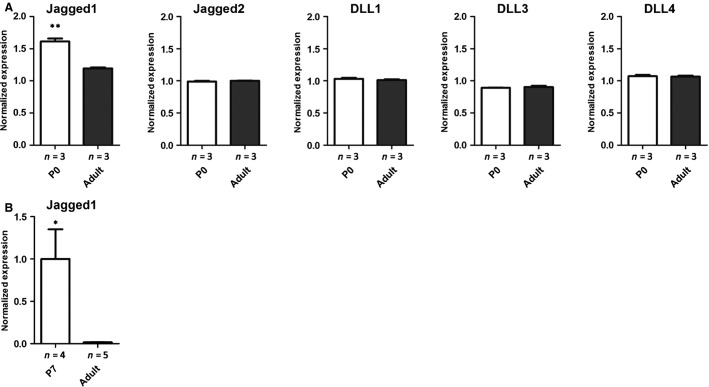
To determine which Notch ligands are expressed in renin cells during kidney development, we first examined previously published and publicly available gene microarray data generated in our laboratory for expression of the Notch ligands Jagged1, Jagged2, DLL1, DLL3, and DLL4 within JG cells (Brunskill et al. 2011). This analysis demonstrated that Jagged1 is upregulated in the renin cells during neonatal life compared to adult (Fig.1A). Furthermore, Jagged1 is the most highly expressed gene of the five Notch signaling ligands within renin cells.
Figure 1.
Jagged1 is upregulated in renin cells during neonatal life compared to adult. (A) The expression of all five Notch ligands within renin cells at P0 and adult ages was assessed from a publicly available database (Brunskill et al. 2011) (**P < 0.01, n = 3 for each condition). (B) Quantitative PCR for Jagged1 was performed at P7 and adult ages (*P < 0.05, n = 4 for P7 and n = 5 for adult time points).
We then performed quantitative RT-PCR on RNA extracted from whole kidney to confirm the expression pattern of Jagged1 in neonatal and adult kidney. This demonstrated increased expression of Jagged1 shortly after birth with decreasing expression with age (Fig.1B). Together, these data demonstrate that Jagged1 is expressed in renin cells of the kidney during neonatal life and suggests this ligand may be the key pathway member triggering Notch signaling.
Deletion of Jagged1 in renin cells
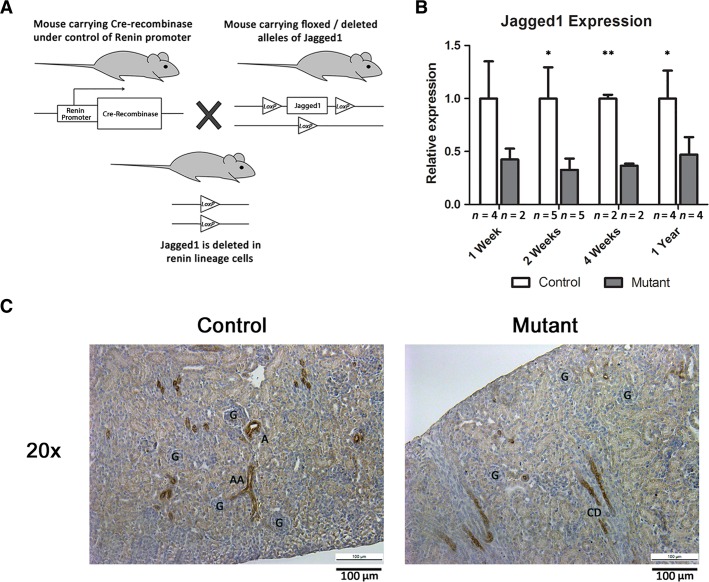
To investigate whether expression of Jagged1 is critical for renin cell development, we generated mice with conditional deletion of Jagged1 specifically in renin cells. We crossed mice that express Cre recombinase under control the renin locus (Sequeira-Lopez et al. 2004) with mice that have the Jagged1 allele either deleted or floxed (see Materials and Methods section) (Lakso et al. 1996; Kiernan et al. 2006) (Fig.2A). Control mice (Jagged1+/fl;Ren1d cre/+ or Jagged1del/fl;Ren1d +/+) and mutant mice (Jagged1del/fl;Ren1d cre/+) were then studied at indicated time points.
Figure 2.
Deletion of Jagged1 within renin cells. (A) A schematic of the breeding strategy for conditional deletion of Jagged1 in renin cells. (B) Jagged1 deletion efficiency as measured by quantitative PCR. There was >50% reduction in Jagged1 expression levels at each time point (*P < 0.05; **P < 0.01, n = 4, 5, 2, and 4 for control and 2, 5, 2, and 4, for mutant kidneys at 1 week, 2 weeks, 4 weeks, and 1 year, respectively). (C) Immunostaining for Jagged1 (brown staining) in 1-week-old control and mutant kidney sections. Scale bar: 100 μm. G = glomeruli, A = artery, AA = afferent arteriole, CD = collecting duct.
To assess the effectiveness of Jagged1 deletion, we performed quantitative RT-PCR for Jagged1. Compared to control mice, expression of Jagged1 mRNA was reduced in mutant mice at 7 days, 2 weeks, 4 weeks, and 1 year of age as shown by quantitative PCR studies (Fig.2B). Of note, RNA was extracted from whole kidney, and thus the efficiency of Jagged1 deletion specifically within renin cells may be underestimated.
In addition, we performed immunohistochemistry for Jagged1 in kidney sections of control and mutant animals. We examined Jagged1 immunostaining at P7, because Jagged1 is at its peak expression during neonatal life and it is easier to observe differences between control and mutant animals, whereas later in life, Jagged1 expression is downregulated. At 1 week of life, we found that there was Jagged1 staining in the arteries, afferent arterioles, and JG cells as well as collecting ducts of control mice. However, in mutant mice, there was decreased Jagged1 staining in the afferent arterioles and JG regions (Fig.2C). In both control and mutant kidneys, Jagged1 is expressed in the collecting ducts, which may be the reason we see only 50–60% reduction in Jagged1 mRNA levels in whole kidneys from mutant mice. Together, these data demonstrate conditional deletion of Jagged1 in renin-expressing and renin-lineage cells.
Renin expression is unaffected by deletion of Jagged1 in renin cells
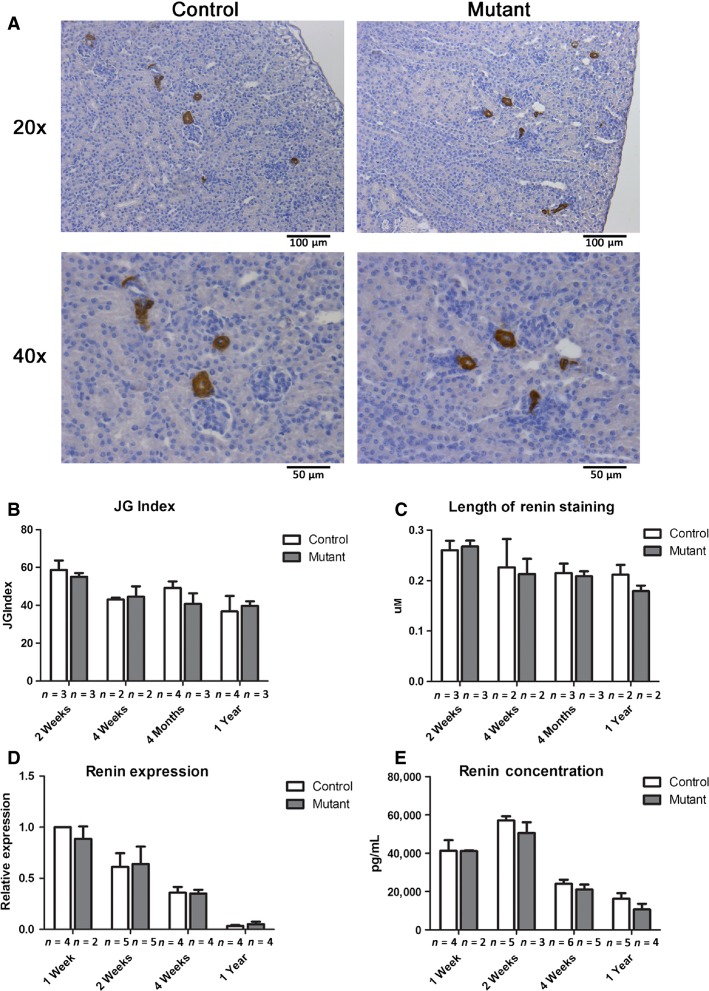
Control and mutant kidneys were sectioned and stained for renin, and the number of renin-positive JGA was examined (Fig.3A). There was no reduction in the number of renin-positive JGAs when corrected for the total number of glomeruli (JGA index) in mutant animals compared to controls at 2 weeks, 4 weeks, 4 months, and 12 months (Fig.3B). Renin production can be increased through the recruitment of additional renin-synthesizing JG cells (which would increase the JG index) or through recruitment of smooth muscle cells along the afferent arteriole (which would not be reflected by the JG index). Therefore, we measured the length of renin staining from control and mutant animals to determine if there was decreased extension of staining in mutants. There was no significant difference between control and mutant animals (Fig.3C).
Figure 3.
Renin expression is unaffected by deletion of Jagged1 in renin cells. (A) Immunostaining for renin (brown staining) in 2-week-old control and mutant kidney sections at low and high magnification. Scale bar: 100 μm top panel and 50 μm bottom panel. (B) Quantification of the number of juxtaglomerular (JG) apparatuses that stain positive for renin demonstrates no difference between control and mutant kidneys at indicated time points (n = 3, 2, 4, and 4 for control animals and n = 3, 2, 3, and 3 for mutant animals at 2 weeks, 4 weeks, 4 months, and 1 year, respectively). (C) The length of renin staining was measured from positively stained JG cells along the afferent arteriole. A minimum of 12 measurements were taken for each sample (n = 3, 2, 3, and 2 for both control and mutant kidneys at 1 week, 2 weeks, 4 weeks, and 1 year, respectively). (D) Relative renin mRNA levels for control and mutants animals (n = 4, 5, 4, and 4 for control animals and n = 2, 5, 4, and 4 for mutant animals at 1 week, 2 weeks, 4 weeks, and 1 year, respectively). (E) Circulating plasma renin levels in control and mutant animals (n = 4, 5, 6, and 5 for control animals and n = 2, 3, 5, and 4 for mutant animals at 1 week, 2 weeks, 4 weeks, and 1 year, respectively).
In agreement with these data, there was also no difference in renin mRNA expression in whole kidney as measured by qRT-PCR between control and mutant animals at 7 days, 2 weeks, 4 weeks, and 1 year of age (Fig.3D). Finally, the concentration of circulating renin was measured in the plasma of control and mutant mice, and there was no difference in the levels of renin at the same time points (Fig.3E). As expected, renin expression and circulating levels of renin decreased with age in both control and mutant animals (Gomez et al. 1991). Together, these data demonstrate that conditional deletion of Jagged1 within renin cells does not result in diminished renin production.
Deletion of Jagged1 in renin cells leads to focal fibrosis
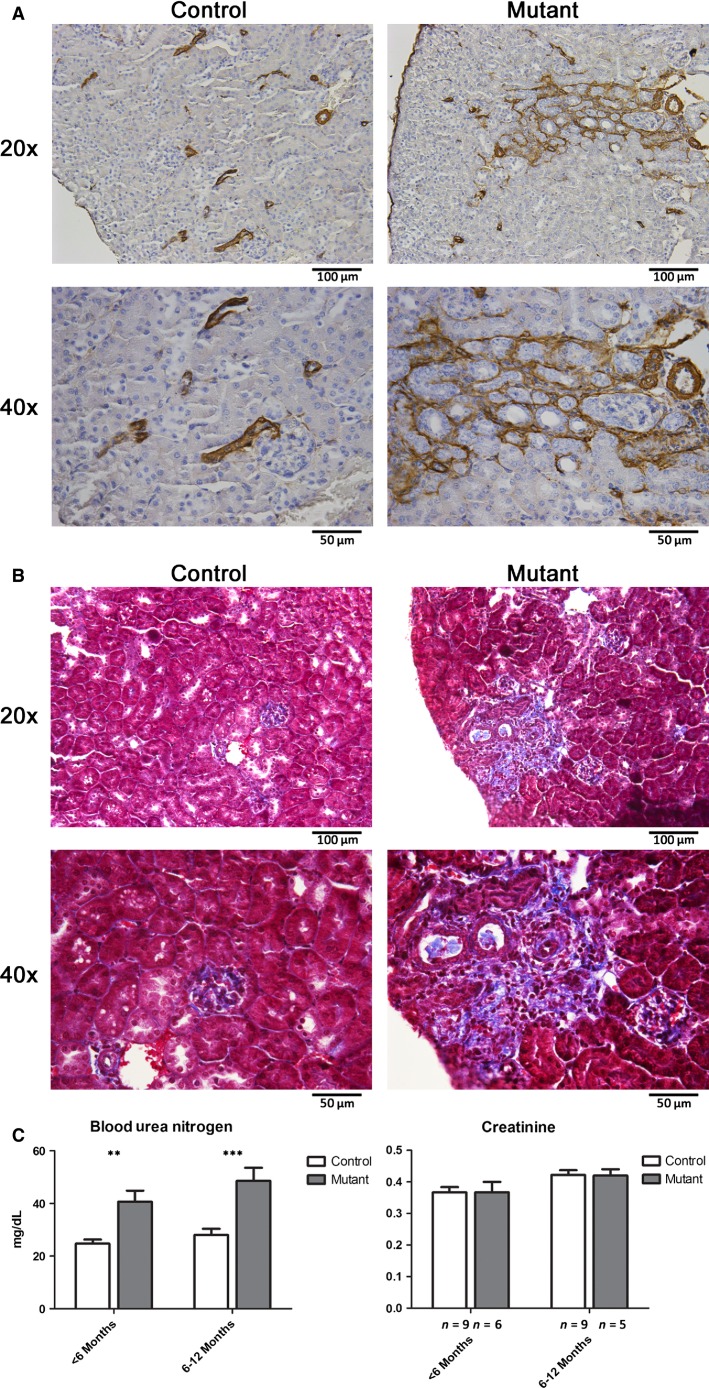
Immunostaining for αSMA demonstrated an overall normal vascular anatomy in both control and mutant kidneys. However, in mutant kidneys, there were focal areas containing activated interstitial cells and dilated tubules expressing αSMA, indicating an active fibrotic process (Fig.4A). Masson’s trichrome staining demonstrated increased collagen fibers in mutant kidneys, consistent with areas of interstitial fibrosis (Fig.4B). Overall, we found an increased incidence of focal areas of fibrosis in mutant animals compared to control animals, and this appeared to worsen with age (Table1). In accordance with the presence of focal fibrosis, we also found that mutant animals had decreased renal function as measured by blood urea nitrogen, however with preserved creatinine (Fig.4C). Similar to the pattern of focal fibrosis, renal function appeared to worsen with age.
Figure 4.
Deletion of Jagged1 in renin cells leads to focal areas of fibrosis and decreased renal function. (A) Immunostaining for α-smooth muscle actin (αSMA) demonstrates focal areas of injured interstitial cells and dilated tubules which express αSMA in mutant animals (age 4 months). (B) Masson’s Trichrome staining demonstrates areas of increased collagen fibers in mutant animals (age 4 months). (C) Blood urea nitrogen and creatinine measured from peripheral blood of control and mutant animals at indicated ages (**P < 0.01; ***P < 0.001, n = 9 and 9 for control animals and n = 6 and 5 for mutant animals at age <6 months and 6–12 months, respectively).
Table 1.
Frequency of focal kidney fibrosis in control and mutant animals
| Age | Control | Mutant |
|---|---|---|
| 2 Weeks | 0/3 | 1/3 |
| 4 Weeks | 0/5 | 2/5 |
| 4 Months | 0/4 | 4/4 |
| 1 Year | 1/5 | 2/3 |
Discussion
In this study, we found that the Notch ligand Jagged1 is expressed in renin cells during neonatal life. Conditional deletion of Jagged1 specifically within renin cells did not result in decreased renin expression in JG cells. However, animals with loss of Jagged1 in renin cells did develop focal kidney fibrosis and decreased renal function.
The Notch pathway is a highly conserved signaling pathway which plays a critical role in cell fate decisions in a variety of tissue and cell types (Andersson et al. 2011). In mammals, there are five Notch ligands (Jagged1, 2, DLL1, 3, 4) and four Notch receptors (Notch1–4). The Notch pathway is activated when two cells come into contact, facilitating Notch ligand–receptor binding between adjacent cells and leading to liberation of the intracellular component of Notch (ICN). ICN then translocates to the nucleus where it forms a transcriptional activation complex with RBP-J and ultimately influences Notch target gene expression. Previous studies from our laboratory and others have demonstrated that the Notch signaling pathway plays a crucial role in renin cell development and regulation (Pan et al. 2005; Castellanos Rivera et al. 2011, 2015). Conditional deletion of RBP-J, the central mediator of Notch signaling, results in decreased renin expression (Castellanos Rivera et al. 2011). However, which Notch ligand and receptor provide the signal to RBP-J remains unknown. In this work, we found that the Notch ligand Jagged1 is more highly expressed in renin cells during development when compared to other Notch ligands. Thus, we hypothesized that Jagged1-initiated juxtacrine signaling between neighboring renin cells plays a crucial role in renin cell development. Surprisingly, conditional deletion of Jagged1 had no effect on renin expression, suggesting that Jagged1-mediated Notch signaling is dispensable for renin expression within JG cells. It is possible that other Notch ligands may compensate for loss of Jagged1 within renin cells. Indeed, a precedent for redundancy in Notch signaling has been demonstrated in other tissue and organ systems. Demehri and Kopan (2009) demonstrated that deletion of a single notch receptor within hair follicle stem cells was insufficient to cause disease, however deletion of multiple receptors created a severe phenotype of follicle destruction which phenocopied deletion of the common effector RBP-J. Thus, it is possible that inhibition of the Notch pathway in renin cells is dosage dependent, where deletion of a single ligand (or receptor) is compensated, but deletion of multiple components or the final common effector RBP-J creates more striking disease. Further studies will be necessary to determine if other Notch ligands compensate for loss of Jagged1 in preserving renin expression and if deletion of multiple Notch ligands (or receptors) in renin cells will have a more dramatic phenotype.
The pathogenesis of the focal fibrosis seen in this model is not completely understood, however, it is likely due to abnormal differentiation of renal vessels within the kidney leading to areas of decreased perfusion and hypoxia. In addition to the role of renin-synthesizing cells in control of blood pressure and fluid–electrolyte homeostasis, renin progenitor cells are precursors for a subset of arteriolar smooth muscle cells. Thus, deletion of Jagged1 in renin precursors may lead to abnormal differentiation of renal vessels, then patchy hypoperfusion, ischemia, and ultimately fibrosis. More work will be needed to elucidate the mechanism of fibrosis, however these findings suggest an important role of Jagged1 in the maintenance of the morphologic integrity of the kidney vasculature and parenchyma.
Acknowledgments
We thank Danielle Stumbo for expert technical assistance and animal care. This research was supported by the University of Virginia Children’s Health Research Center internal grant support (B. C. B.) and the National Institutes of Health (grants R01 DK 091330 to M. L. S. S.-L., Merit Award R37 HL 066242 to R. A. G., Center of Excellence in Pediatric Nephrology P50 DK 096373 to R. A. G. and M. L. S. S.-L., and Center of Excellence in Pediatric Nephrology Pilot Program P50 DK 096373-01 to B.C.B.). A portion of this work was presented at the 2014 American Heart Association High Blood Pressure Research Scientific Session.
Conflict of Interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. The raw data (markers of kidney function in control and mutant animals) for Figure 4C.
References
- Andersson ER, Sandberg R. Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138:3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- Brunskill EW, Sequeira-Lopez ML, Pentz ES, Lin E, Yu J, Aronow BJ, et al. Genes that confer the identity of the renin cell. J. Am. Soc. Nephrol. 2011;22:2213–2225. doi: 10.1681/ASN.2011040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos Rivera RM, Monteagudo MC, Pentz ES, Glenn ST, Gross KW, Carretero O, et al. Transcriptional regulator RBP-J regulates the number and plasticity of renin cells. Physiol. Genomics. 2011;43:1021–1028. doi: 10.1152/physiolgenomics.00061.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos Rivera RM, Pentz ES, Lin E, Gross KW, Medrano S, Yu J, et al. Recombination signal binding protein for Ig-kappaJ region regulates juxtaglomerular cell phenotype by activating the myo-endocrine program and suppressing ectopic gene expression. J. Am. Soc. Nephrol. 2015;26:67–80. doi: 10.1681/ASN.2013101045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demehri S. Kopan R. Notch signaling in bulge stem cells is not required for selection of hair follicle fate. Development. 2009;136:891–896. doi: 10.1242/dev.030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez RA, Pupilli C. Everett AD. Molecular and cellular aspects of renin during kidney ontogeny. Pediatr. Nephrol. 1991;5:80–87. doi: 10.1007/BF00852854. [DOI] [PubMed] [Google Scholar]
- Gomez RA, Pentz ES, Jin X, Cordaillat M. Sequeira-Lopez ML. CBP and p300 are essential for renin cell identity and morphological integrity of the kidney. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H1255–H1262. doi: 10.1152/ajpheart.01266.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez RA, Belyea B, Medrano S, Pentz ES. Sequeira-Lopez ML. Fate and plasticity of renin precursors in development and disease. Pediatr. Nephrol. 2014;29:721–726. doi: 10.1007/s00467-013-2688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Xu J. Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, et al. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl. Acad. Sci. USA. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EE, Sequeira-Lopez ML. Gomez RA. RBP-J in FOXD1+ renal stromal progenitors is crucial for the proper development and assembly of the kidney vasculature and glomerular mesangial cells. Am. J. Physiol. Renal. Physiol. 2014;306:F249–F258. doi: 10.1152/ajprenal.00313.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Glenn ST, Jones CA. Gross KW. Activation of the rat renin promoter by HOXD10.PBX1b.PREP1, Ets-1, and the intracellular domain of notch. J. Biol. Chem. 2005;280:20860–20866. doi: 10.1074/jbc.M414618200. [DOI] [PubMed] [Google Scholar]
- Sequeira-Lopez ML, Pentz ES, Robert B, Abrahamson DR. Gomez RA. Embryonic origin and lineage of juxtaglomerular cells. Am. J. Physiol. Renal. Physiol. 2001;281:F345–F356. doi: 10.1152/ajprenal.2001.281.2.F345. [DOI] [PubMed] [Google Scholar]
- Sequeira-Lopez ML, Pentz ES, Nomasa T, Smithies O. Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev. Cell. 2004;6:719–728. doi: 10.1016/s1534-5807(04)00134-0. [DOI] [PubMed] [Google Scholar]
- Sequeira-Lopez ML, Weatherford ET, Borges GR, Monteagudo MC, Pentz ES, Harfe BD, et al. The microRNA-processing enzyme dicer maintains juxtaglomerular cells. J. Am. Soc. Nephrol. 2010;21:460–467. doi: 10.1681/ASN.2009090964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigaki K. Honjo T. Two opposing roles of RBP-J in Notch signaling. Curr. Top. Dev. Biol. 2010;92:231–252. doi: 10.1016/S0070-2153(10)92007-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The raw data (markers of kidney function in control and mutant animals) for Figure 4C.