Abstract
Maternal intake of dietary methyl-micronutrients (e.g. folate, choline, betaine and vitamin B-12) during pregnancy is essential for normal maternal and fetal methionine metabolism, and is critical for important metabolic processes including those involved in developmental programming. Maternal obesity and nutrient excess during pregnancy influence developmental programming potentially predisposing adult offspring to a variety of chronic health problems. In the present study, we hypothesized that maternal obesity would dysregulate the maternal and fetal methionine cycle. To test this hypothesis, we developed a nulliparous baboon obesity model fed a high fat, high energy diet (HF-HED) prior to and during gestation, and examined methionine cycle biomarkers (e.g., circulating concentrations of homocysteine, methionine, choline, betaine, key amino acids, folate, and vitamin B-12). Animals were group housed allowing full physical activity and social interaction. Maternal prepregnancy percent body fat was 5% in controls and 19% in HF-HED mothers, while fetal weight was 16% lower in offspring of HF-HED mothers at term. Maternal and fetal homocysteine were higher, while maternal and fetal vitamin B-12 and betaine were lower in the HF-HED group. Elevations in circulating maternal folate were evident in the HF-HED group indicating impaired folate metabolism (methyl-trap) as a consequence of maternal vitamin B-12 depletion. Finally, fetal methionine, glycine, serine, and taurine were lower in the HF-HED fetuses. These data show that maternal obesity disturbs the methionine cycle in primate pregnancy, providing a mechanism for the epigenetic changes observed among obese pregnant women and suggesting diagnostic and therapeutic opportunities in human pregnancies complicated by obesity.
Keywords: Baboon; folate; maternal obesity; methionine cycle; vitamin B-12, one-carbon metabolism
Introduction
Nutritional imbalance and obesity during pregnancy predispose adult offspring to a variety of chronic health problems through a process known as developmental (or fetal) programming (Barker 1999; Armitage et al. 2004; Fernandez-Twinn and Ozanne 2010; Alfaradhi and Ozanne 2011; Sullivan et al. 2011; Shasa et al. 2015). Fetal adaptation to an abnormal nutritional environment is accompanied by changes in gene expression that are mediated (at least in part) by epigenetic modifications such as DNA and histone methylation (Saffery 2014). Epigenetic modification of the fetal genome can have profound effects on gene function, organ development, and metabolic processes throughout the lifespan of the offspring.
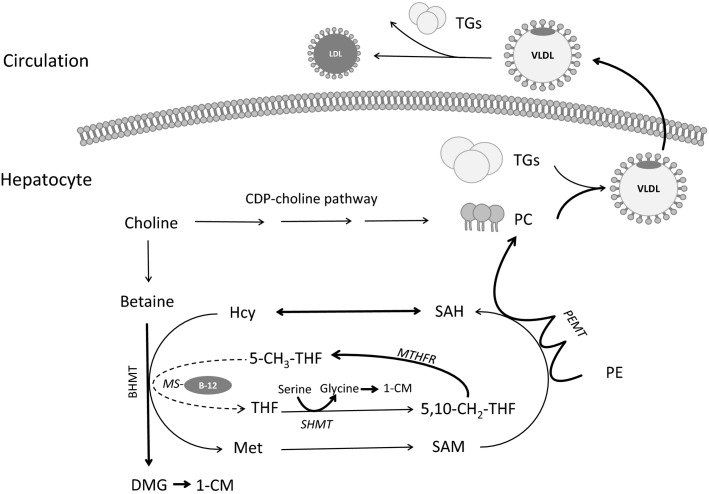
Within the one-carbon metabolic network, the methionine cycle (Fig.1) provides methyl groups from methionine and other dietary methyl-micronutrients (e.g. folate, vitamin B-12, choline, and betaine) to several important metabolic processes (Kalhan and Marczewski 2012) including those involved in developmental programming. While there are studies showing disturbances in the methionine cycle and gene specific DNA methylation as a result of maternal nutritional imbalances in rodent pregnancy (Lillycrop et al. 2010), few studies have been conducted in pregnant nonhuman primates (Blocker et al. 1989; Burbacher et al. 2004; McCurdy 2009). The need for data in primate pregnancy to translate to the human situation is evident from observed differences in the one-carbon and methionine cycle pathways between rodents and primates. For example, reduction of folic acid to tetrahydrofolate (THF; the active form of folate) by dihydrofolate reductase in the human liver occurs at less than 2% the rate observed in rat liver (Bailey and Ayling 2009). This lower rate of reduction in humans may result in the accumulation of dihydrofolate, which can compete with THF as a substrate for polyglutamyl synthase, resulting in diminished levels of the active folate polyglutamate cofactors required for one-carbon metabolism.
Figure 1.
Simplified diagram of one-carbon metabolism (1-CM) including the methionine cycle, the roles of one-carbon nutrients, and links to lipid metabolism. Methionine (Met) can be synthesized from homocysteine (Hcy) in a reaction catalyzed by betaine-homocysteine methyltransferase (BHMT) using the choline metabolite betaine as the methyl donor. Alternatively, the remethylation of homocysteine to methionine can proceed via the folate and vitamin B-12 dependent methionine synthase (MS) reaction. Choline, betaine, folate, and vitamin B-12 have critical roles in lipid metabolism because they contribute to the production of phosphatidylcholine (PC) via the phosphatidylethanolamine-N-methyltransferase (PEMT) pathway, which is necessary for the formation of very-low-density-lipoproteins (VLDL) and the removal of triglycerides (TGs) from the liver. DMG, dimethylglycine; LDL, low density lipoprotein; MTHFR, 5,10-methylenetetrahydrofolate reductase; PE, phosphatidylethanolamine; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; SHMT, serine hydroxymethyltransferase; THF, tetrahydrofolate.
In the present study, a baboon obesity model was developed to investigate the effects of maternal obesity combined with a high fat, high energy diet (HF-HED) before and during pregnancy on maternal and fetal indicators of one-carbon metabolism and the methionine cycle. We hypothesized that several metabolic abnormalities would be detected including alterations in: (1) the maternal and fetal circulating profile of B-vitamins involved in the methionine cycle; (2) methionine cycle intermediates (e.g., homocysteine); and (3) amino acids that play a role in one-carbon metabolism (e.g., serine). Since the methionine cycle is involved in hepatic lipid metabolism and transport (Fig.1) (Caudill et al. 2012), we also measured biomarkers of fetal and maternal lipid status.
Methods
Subjects and diets
All animal procedures were approved by the Texas Biomedical Research Institute and University of Texas Health Science Center at San Antonio Institutional Animal Care and Use Committee, and conducted in AAALAC International approved facilities. Animals were grouped up to 16 animals per cage which allowed free movement and full social interaction. Healthy, nonpregnant female nulliparous baboons of similar weight, body dimensions, and age were randomly assigned to either a control diet (n = 22) or a combination of the control diet and a high fat-high energy diet (HF-FED) with free access to a sugar-containing drink (Kool Aid) (n = 5). The control group had continuous access to the Southwest National Regional Primate Research Center biscuits (Purina Monkey Diet and Monkey Diet Jumbo, Purina LabDiets, St Louis, MO) containing 12% energy from fat, 0.29% from glucose, 0.32% from fructose, and a metabolizable energy content of 3.07 kcal/g. The HF-HED group had continuous access to both the control diet and the HF-HED regimen containing 45% energy from fat, 4.6% from glucose, 5.6% from fructose, and a metabolizable energy content of 4.03 kcal/g as described previously (Maloyan et al. 2013). Both diets were made available to the HF-HED group because baboons ate more HF-HED when it was provided together with the control diet. HF-HED baboons were also given free and continuous access to a sugar containing Kool Aid drink. The control diet and HF-HED provided the same amount of vitamin B-12 (33 μg/kg) and choline chloride (1200 ppm). However, folic acid was provided at levels of 2.2 ppm in the control diet, and 1.3 ppm in the HF-HED diet, while methionine comprised 0.31% of the ration in control diet and 0.33% in the HF-HED. The sugar-containing beverage (Kool Aid) contained no folate, choline, betaine, vitamin B-12, or amino acids.
Because all animals in the group cage had continuous access to the food, individual food intake was not assessed. Instead the daily intake per cage was measured and divided by the number of animals in the cage. Thus, food intake measurements are estimates per animal – a limitation inevitable in all studies using group housing to allow full activity and socialization. Females were maintained on either the control diet for 37 ± 0.3 months (mean ± SEM) or the combined control diet and HF-HED for 35 ± 0.8 months prior to breeding at 7.1 ± 0.1 (control) and 7.3 ± 0.1 (HF-HED; ns) years of age. Maternal morphometrics were performed at the time of group assembly and again after 3, 9, and 18 months; Dual Beam X-ray absorptiometry (DEXA) was performed at the time of group assembly and again after 6, 12, and 18 months; and measures of serum LDL-cholesterol and triglycerides in venous blood samples were performed at the time of group assembly and again after 3 months. No additional assessments were conducted after 18 months of the prepregnancy period.
Breeding and animal management has been described in detail (Schlabritz-Loutsevitch et al. 2004). Control and HF-HED baboons were housed in groups in separate cages to prevent control females from consuming components of the HF-HED. All animals remained on their prepregnancy diet throughout pregnancy. Fetuses were delivered at 0.9 gestation (G; Term 184 days) via cesarean section under general anesthesia as previously described (Cox et al. 2006). Prior to removal, the fetus was exsanguinated while still under general anesthesia, a method approved by the American Veterinary Medical Association, and checked for response to a footpad pinch with a forcep. Morphometric measurements were made on mothers at baseline and delivery, and on the fetus at delivery. Blood samples for metabolite measurements were taken from the maternal uterine vein and fetal umbilical vein at C-Section, and processed to provide serum and plasma which were stored at −80°C.
Metabolite measurements
Amino acids: Serum amino acids were determined by HPLC with precolumn derivatization with o-phthaldialdehyde (Wu and Knabe 1994). All amino acids in the samples were quantified on the basis of authentic standards (Sigma Chemicals, St. Louis, MO) using Millenium-32 Software (Waters, Milford, MA).
Maternal and fetal serum vitamin B12, folate and homocysteine were measured on an Immulite 1000.
Holotranscobalamin: Plasma holotranscobalamin was measured by the monoclonal antibody capture assay (Ulleland et al. 2002).
Lipid measures: Serum triglycerides and LDL-cholesterol were measured enzymatically (Rainwater et al. 2002a, 2007). Concentrations of serum apolipoproteins A1 (ApoA1) and B (ApoB) were quantified immunoturbidometrically (Kammerer et al. 2002; Rainwater et al. 2002b).
Free choline, betaine, and dimethylglycine (DMG) were quantified in plasma using liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Holm et al. 2003) with modifications based on the instrumentation (Yan et al. 2011). The metabolites and internal standards (d9-choline, d9-betaine, and d3-dimethylglycine) were detected as positive ions by tandem mass spectroscopy in the multiple-reaction monitoring mode, using molecular transitions of m/z 104→60 (choline), m/z 113→69 (d9-choline), m/z 118→59 (betaine), m/z 127→68 (d9-betaine), m/z 104→58 (DMG), and m/z 107→61 (d3-DMG). For all three metabolites, the within- and between day imprecision (CVs) were <6%.
Statistical analysis
Analysis was conducted by either Student’s t-test or repeated measures ANOVA; Bonferroni multiple comparison test was used for mean group separation. Differences were considered statistically significant at P ≤ 0.05, while 0.05 < P ≤ 0.10 was indicative of borderline significance. Since the sex distribution of the five HF-HED fetuses was three females and two males, no attempt was made to analyze the data according to fetal sex. Data are presented as means ± SEM.
Results
Food intake and weight changes during the prepregnancy period
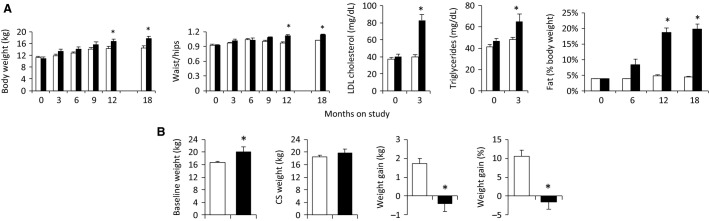
Total weight gain in the first year prior to pregnancy was significantly higher (P < 0.001) in the HF-HED group (5.8 kg) as compared to the control group (3.1 kg) (Fig.2A). During the prepregnancy period control baboons ate 22 g/kg body weight per day (68 kcal/kg/day) of the control diet while HF-HED mothers ate 13.6 g/kg per day of the control diet (42 kcal/kg/day) and 3.8 g/kg per day of the HF-HED diet (15 kcal/kg/day) for a total calorie intake of 57 kcal/kg/day, which is numerically lower than the control group. However, when expressed as an absolute value, daily caloric intake from the diets was similar among the groups (∼1000 kcal/day), and was further increased in the HF-HED group via their consumption of the high calorie Kool-Aid. The HF-HED group also exhibited higher waist-hip circumferences, higher LDL-cholesterol and triglycerides, and a greater percentage body fat during the prepregnancy period (P < 0.05, Fig.2A).
Figure 2.
(A) Maternal prepregnancy anthropometrics over the first 18 months on the high fat–high energy diet (HF-HED), and circulating lipids at 0 and 3 months in control (CTR, open, n = 7) and HF-HED (filled, n = 5) animals. (B) Baseline body weight before pregnancy, weight at C-section (CS), and weight gained during pregnancy in CTR (open, n = 22) and HF-HED animals (filled, n = 5). Data are mean ± SEM; *P < 0.05 versus CTR.
Weight changes during pregnancy
Baseline body weight at pregnancy was higher (P < 0.05) in HF-HED versus control (Fig.2B). During pregnancy, HF-HED mothers failed to gain weight, while the control mothers gained 1.8 kg (Fig.2B). Otherwise pregnancies in both groups were uneventful.
Fetal morphometrics at 0.9 gestation (G)
Table1 presents the morphometric characteristics of the placenta and fetuses of control and HF-HED mothers at Cesarean section. Although there were no differences in placental weight or volume, placental efficiency expressed as weight of fetal mass supported per unit placental mass, was lower in HF-HED pregnancies (Table1). Fetal body weight was 16.3% lower (P < 0.05) in HF-HED pregnancies (vs. control) and was also lower (P < 0.05) than control as a percent of maternal weight. The only fetal organ difference to approach significance in absolute weight between the two groups was lower fetal liver weight (P = 0.07) in the HF-HED group (vs. control). When fetal organ weights were expressed as a percentage of fetal weight, fetal brain and thymus weights were higher (P < 0.05) in HF-HED pregnancies suggesting some degree of tissue sparing for these organs.
Table 1.
Placental and fetal morphometric characteristics at cesarean section (0.9 gestation) in control (n = 20–22) and HF-HED (n = 5) group*
| Control | HF-HED | |
|---|---|---|
| Placenta Measures | ||
| Weight (g) | 210 ± 9 | 214 ± 9 |
| Efficiency (g fetus/g placenta) | 3.9 ± 0.1 | 3.2 ± 0.2† |
| Volume (cm3) | 159 ± 18 | 111 ± 26 |
| Fetal Measures | ||
| Body weight (g) | 806 ± 24 | 675 ± 36† |
| Length (cm) | 38 ± 0.6 | 37 ± 1 |
| BMI (kg/m2) | 5.7 ± 0.1 | 5.1 ± 0.4‡ |
| Ponderal index (kg/m3) | 15 ± 0.5 | 14 ± 1 |
| Brain (g) | 82 ± 2 | 80 ± 2 |
| Heart (g) | 4.9 ± 0.2 | 4.2 ± 0.2 |
| Kidneys (g) | 4.5 ± 0.2 | 4.0 ± 0.2 |
| Liver (g) | 24 ± 0.9 | 20 ± 1.2‡ |
| Lung (g) | 17 ± 0.6 | 17 ± 1.5 |
| Pancreas (g) | 0.56 ± 0.06 | 0.44 ± 0.05 |
| Thymus (g) | 3.4 ± 0.20 | 3.4 ± 0.46 |
| Fetal measures as percent fetal weight | ||
| Placenta | 26 ± 0.8 | 32 ± 2.5† |
| Brain | 11 ± 0.3 | 12 ± 0.6† |
| Heart | 0.61 ± 0.02 | 0.63 ± 0.04 |
| Kidneys | 0.56 ± 0.02 | 0.59 ± 0.03 |
| Liver | 3.0 ± 0.06 | 3.0 ± 0.125 |
| Lungs | 2.2 ± 0.07 | 2.4 ± 0.12‡ |
| Pancreas | 0.07 ± 0.01 | 0.07 ± 0.01 |
| Thymus | 0.42 ± 0.02 | 0.52 ± 0.05† |
| Fetal measures as percent maternal weight | ||
| Fetal weight | 4.3 ± 0.1 | 3.5 ± 0.2† |
| Placenta | 1.13 ± 0.04 | 1.09 ± 0.05 |
Data are mean ± SEM.
P < 0.05 versus control.
0.05 < P < 0.10 versus control.
Maternal and fetal methionine cycle metabolites
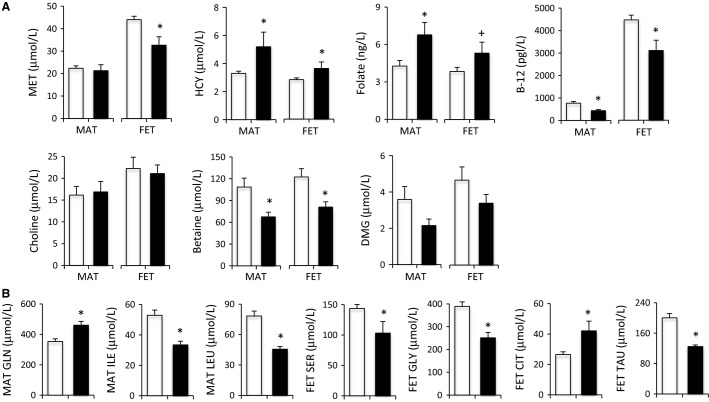
Methionine cycle metabolites in the maternal and fetal baboon at Cesarean section are shown in Figure3A. While methionine was unchanged in maternal plasma, concentrations were 28 percent lower in fetuses of the HF-HED mothers than controls (P < 0.05) (Fig.3A). Homocysteine was 36% higher in maternal blood and 28% higher in fetal blood in the HF-HED group (vs. control) (P < 0.05). Vitamin B-12 was lower (P < 0.05) in mothers (−44%) and fetuses (−31%) in the HF-HED group (vs. control), while lower, but nonsignificant levels of holotranscobalamin were observed in maternal blood (−36%) and in fetal blood (−21%) in the HF-HED group. In contrast, folate was 59% higher (P < 0.05) in maternal blood in the HF-HED group (vs. control) and tended (P < 0.10) to be higher (+38%) in fetal blood. Betaine, which is derived from choline and serves as an alternate to folate as a source of methyl groups for conversion of homocysteine to methionine, was lower in both the maternal (−39%; P < 0.02) and fetal (−36%, P < 0.02) blood in the HF-HED group (vs. control). Dimethylglycine, the metabolite produced when betaine is used as a methyl donor and source of one-carbon units for folate-mediated one-carbon metabolism, tended to be lower (−45%) in maternal blood (P < 0.1) of the HF-HED group (vs. control); fetal blood also exhibited lower dimethylglycine but statistical significance was not achieved (Fig.3). Circulating choline was unchanged by the HF-HED diet in either mother or fetus.
Figure 3.
(A) Maternal (MAT) and fetal (FET) circulating concentrations of methionine cycle metabolites and biomarkers in control (CTR, open) and high fat-high energy diet (HF-HED, closed) at 0.9 G (n = 11–22 CTR MAT; n = 5 HF-HED MAT; n = 11–21 CTR FET; n = 5 HF-HED FET). (B) Amino acids in CTR (open, n = 21–22) and HF-HED (filled, n = 5) MAT and FET blood at 0.9 G. Data are mean ± SEM; *P < 0.05 versus CTR; †0.05 < P < 0.10 versus CTR. CTT, citrulline; DMG, dimethylglycine; GLN, glutamine; GLY, glycine; HCY, homocysteine; ILE, isoleucine; LEU, leucine; MET, methionine; SER, serine; TAU, taurine; and TC, holotranscobalamin.
Maternal and fetal amino acid concentrations
HF-HED mothers had higher concentrations of plasma glutamine and lower concentrations of leucine and isoleucine, while fetuses of HF-HED mothers (vs. control) had lower plasma concentrations of glycine, serine, and taurine and higher citrulline (all P < 0.05; Fig.3B). The fetal:maternal ratio was lower (P < 0.03) in the HF-HED pregnancies (vs. control) for the following five amino acids: serine (1.46 ± 0.23 vs. 2.19 ± 0.10), glycine (1.06 ± 0.08 vs. 1.74 ± 0.08), taurine (1.10 ± 0.12 vs. 1.44 ± 0.05), methionine (1.56 ± 0.12 vs. 2.08 ± 0.07) and phenylalanine (1.09 ± 0.10 vs. 1.47 ± 0.07). The ratio was significantly higher (P < 0.01) in the HF-HED pregnancies (vs. control) for citrulline (3.76 ± 0.84 vs. 2.09 ± 0.09), isoleucine (2.25 ± 0.22 vs. 1.51 ± 0.08), and leucine (2.20 ± 0.16 vs. 1.40 ± 0.08). No other differences in amino acids were detected between the HF-HED and control pregnancies.
Maternal and fetal lipid metabolism
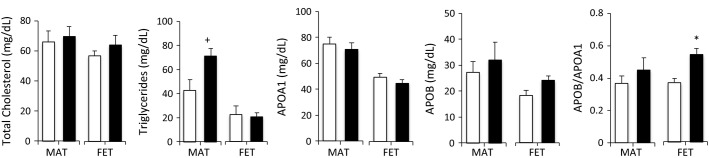
Maternal triglycerides at C-section were 67% higher (P = 0.06) in the HF-HED group (vs. control). Although fetal triglycerides were similar among groups, fetal APOB tended to be higher (+32%) in the fetuses of HF-HED mothers (P = 0.08) and the APOB/APOA1 ratio was significantly higher (P < 0.05) (Fig. 4).
Figure 4.
Lipid parameters in baboon pregnancy at 0.9 G in control (open, n = 8–11) and HF-HED (filled, n = 5) maternal (MAT) and fetal (FET) blood. Data are mean ± SEM; *P < 0.05 versus CTR; †0.05 < P < 0.10 versus CTR. APOA1, Apolipoprotein A1; APOB, Apolipoprotein B.
Discussion
Obesity and excessive nutrient intake are an ever-growing health concern in our over-consuming society. Abundant data indicate that maternal obesity in pregnancy leads to adverse developmental programming of offspring outcomes in rodents (Samuelsson et al. 2008; Alfaradhi et al. 2014), sheep (Sinclair et al. 2007; Long et al. 2012; Shasa et al. 2015), nonhuman primates (Aagaard-Tillery et al. 2008; Grayson et al. 2010; Sullivan et al. 2010; Grant et al. 2011; Suter et al. 2011; Maloyan et al. 2013), and humans (Gillman et al. 2008). However, despite the importance of one-carbon metabolism in a wide array of cellular and molecular processes fundamental to development, little is known regarding the effects of maternal obesity on the methionine cycle in mother and fetus, and there are no studies to provide guidance in this epidemic situation of obese pregnancies.
We developed a pregnant baboon model of obesity by feeding a HF-HED before and during pregnancy and comparing to controls consuming the normal primate center control diet. Nulliparous female baboons of the HF-HED (vs. control) group exhibited higher body fat percentages, higher waist-hip circumferences, and dyslipidemia (elevated LDL-C and triglycerides). Borderline higher circulating triglycerides were also detected in the HF-HED (vs. control) female baboons at delivery which is consistent with an obesogenic phenotype. The lack of significant weight gain and smaller fetuses among the baboon mothers in the HF-HED (vs. control) group has parallels in human obesity. Obese women gain less weight than their nonobese counterparts during pregnancy (Faucher and Barger 2015) and small-for-gestational-age births, which are more likely to occur when the mother fails to gain weight during pregnancy, can affect up to 14 percent of obese pregnancies (Faucher and Barger 2015).
Maternal obesity alters circulating concentrations of methyl-nutrients
Maternal and fetal blood concentrations of vitamin B-12 were depleted in the HF-HED group as compared with the control group. In contrast, blood folate concentrations were elevated in maternal blood and tended to be elevated in fetal blood. A possible explanation for the rise in folate is impaired vitamin B-12 dependent methionine synthase activity, with subsequent cellular accumulation of 5-methyl-THF (i.e., metabolic trapping of folate; methyl trap) (Caudill et al. 2012). Methionine synthase catalyzes the transfer of a methyl group from 5-methyl-THF to homocysteine generating methionine and THF. Consequently, in vitamin B-12 deficiency, cellular and circulating levels of homocysteine and 5-methyl-THF will rise. The vitamin B-12 deficiency that led to the metabolic trapping of folate in the HF-HED group may be related to the caloric/lipid load (McCurdy 2009) and increased use of vitamin B-12 as a lipotrope. By providing methyl groups for de novo synthesis of phosphatidylcholine, lipotropes facilitate VLDL biosynthesis and hepatic lipid export (Lamaziere et al. 2012) (Fig.1). In contrast, although absolute vitamin B-12 intake may have been slightly lower among the HF-HED (vs. control) group, this modestly lower intake would not be expected to cause the functional B-12 deficiency observed in the HF-HED moms.
Maternal and fetal blood concentrations of the choline metabolite, betaine, were significantly reduced in HF-HED compared with controls, while dimethylglycine tended to be lower in maternal blood of the HF-HED group. Betaine is used as a methyl donor by betaine-homocysteine methlytransferase (BHMT), an alternative to methionine synthase for conversion of homocysteine to methionine (Fig.1). Like vitamin B-12, betaine is a lipotrope and its use would be augmented under conditions of a lipid load. Similarly, although dimethylglycine is a product of the BHMT reaction, it is also a source of one-carbon units (e.g., methyl groups) which can be used in folate mediated one-carbon metabolism including the methionine cycle.
Maternal obesity alters one-carbon amino acid concentrations in fetal blood
A novel and important finding from the present study is that plasma methionine, serine, glycine, and taurine concentrations (amino acids involved in one-carbon metabolism and related pathways) were unchanged in the maternal circulation, but decreased in fetal plasma of HF-HED pregnant baboons resulting in lower fetal to maternal ratios for these amino acids. This finding may indicate placental dysfunction in relation to the activity of placental transporters for small and neutral amino acids (System ASC and SNAT) as well as taurine (System Beta). For example, placental microvilli preparations from obese pregnant women (BMI 32) exhibited reduced in vitro placental SNAT activity and SNAT4 expression compared to their lean counterparts (BMI 22) (Farley et al. 2010). The reduced placental efficiency (which has also been observed in obese human pregnancies; Wallace et al. 2012) and lower fetal weights of the HF-HED mothers are also consistent with impaired amino acid transport.
In contrast with the amino acids associated with one-carbon metabolism, fetal to maternal ratios for citrulline, leucine, and isoleucine were higher in the fetuses of the HF-HED mothers than controls, suggesting either up-regulation of transporters for branched-chain amino acids (e.g., System L) and citrulline (e.g., System N) or reduced catabolism of these amino acids in fetal tissue.
Conclusions
In our baboon nonhuman primates, maternal obesity (even without weight gain during pregnancy) disrupted the methionine cycle and reduced several one-carbon nutrients particularly in the fetal compartment. These data provide a mechanism for the epigenetic changes observed in maternal obesity, but additional studies with genomic and epigenomic endpoints are required. The reduction of one-carbon nutrients in maternal obesity suggests that nutritional strategies aimed at achieving optimal concentrations of these key components of the methionine cycle (e.g. vitamin B-12 supplementation) may be warranted.
Acknowledgments
We acknowledge assistance from Michelle Zavala with sample management, Jane VandeBerg for measurement of lipids, Susan Jenkins for statistical analysis and Karen Moore for help with the manuscript.
Conflict of Interest
None of the authors have a conflict of interest to report.
References
- Aagaard-Tillery KM, Grove K, Bishop J, Ke X, Fu Q, McKnight R, et al. Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. J. Mol. Endocrinol. 2008;41:91–102. doi: 10.1677/JME-08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaradhi MZ. Ozanne SE. Developmental programming in response to maternal overnutrition. Front. Genet. 2011;2:27. doi: 10.3389/fgene.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaradhi MZ, Fernandez-Twinn DS, Martin-Gronert MS, Musial B, Fowden AL. Ozanne SE. Oxidative stress and altered lipid homeostasis in the programming of offspring fatty liver by maternal obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;307:R26–R34. doi: 10.1152/ajpregu.00049.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage JA, Khan IY, Taylor PD, Nathanielsz PW. Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J. Physiol. 2004;561:355–377. doi: 10.1113/jphysiol.2004.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey SW. Ayling JE. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc. Natl Acad. Sci. U. S. A. 2009;106:15424–15429. doi: 10.1073/pnas.0902072106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP. Fetal origin of cardiovascular disease. Ann. Med. 1999;31(Suppl. 1):3–6. doi: 10.1080/07853890.1999.11904392. [DOI] [PubMed] [Google Scholar]
- Blocker DE, Ausman LM, Meadows CA. Thenen SW. Experimental maternal and neonatal folate status relationships in nonhuman primates. Am. J. Clin. Nutr. 1989;50:120–128. doi: 10.1093/ajcn/50.1.120. [DOI] [PubMed] [Google Scholar]
- Burbacher TM, Shen DD, Lalovic B, Grant KS, Sheppard L, Damian D, et al. Chronic maternal methanol inhalation in nonhuman primates (Macaca fascicularis): exposure and toxicokinetics prior to and during pregnancy. Neurotoxicol. Teratol. 2004;26:201–221. doi: 10.1016/j.ntt.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Caudill MA, Miller JF, III, Gregory J. Shane B. Folate, choline, vitamin B-12 and vitamin B-6. In: Stipanuk MH, Caudill MA, editors; Biochemical, physiological and molecular aspects of human nutrition. 3rd ed. St Louis, MO: Elsevier Saunders; 2012. [Google Scholar]
- Cox LA, Schlabritz-Loutsevitch N, Hubbard GB, Nijland MJ, McDonald TJ. Nathanielsz PW. Gene expression profile differences in left and right liver lobes from mid-gestation fetal baboons: a cautionary tale. J. Physiol. 2006;572:59–66. doi: 10.1113/jphysiol.2006.105726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley DM, Choi J, Dudley DJ, Li C, Jenkins SL, Myatt L, et al. Placental amino acid transport and placental leptin resistance in pregnancies complicated by maternal obesity. Placenta. 2010;31:718–724. doi: 10.1016/j.placenta.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Faucher MA. Barger MK. Gestational weight gain in obese women by class of obesity and select maternal/newborn outcomes: a systematic review. Women Birth. 2015;28:e70–e79. doi: 10.1016/j.wombi.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Fernandez-Twinn DS. Ozanne SE. Early life nutrition and metabolic programming. Ann. N. Y. Acad. Sci. 2010;1212:78–96. doi: 10.1111/j.1749-6632.2010.05798.x. [DOI] [PubMed] [Google Scholar]
- Gillman MW, Rifas-Shiman SL, Kleinman K, Oken E, Rich-Edwards JW. Taveras EM. Developmental origins of childhood overweight: potential public health impact. Obesity (Silver Spring) 2008;16:1651–1656. doi: 10.1038/oby.2008.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant WF, Gillingham MB, Batra AK, Fewkes NM, Comstock SM, Takahashi D, et al. Maternal high fat diet is associated with decreased plasma n-3 fatty acids and fetal hepatic apoptosis in nonhuman primates. PLoS ONE. 2011;6:e17261. doi: 10.1371/journal.pone.0017261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson BE, Levasseur PR, Williams SM, Smith MS, Marks DL. Grove KL. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology. 2010;151:1622–1632. doi: 10.1210/en.2009-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm PI, Ueland PM, Kvalheim G. Lien EA. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin. Chem. 2003;49:286–294. doi: 10.1373/49.2.286. [DOI] [PubMed] [Google Scholar]
- Kalhan SC. Marczewski SE. Methionine, homocysteine, one carbon metabolism and fetal growth. Rev. Endocr. Metab. Disord. 2012;13:109–119. doi: 10.1007/s11154-012-9215-7. [DOI] [PubMed] [Google Scholar]
- Kammerer CM, Rainwater DL, Cox LA, Schneider JL, Mahaney MC, Rogers J, et al. Locus controlling LDL cholesterol response to dietary cholesterol is on baboon homologue of human chromosome 6. Arterioscler. Thromb. Vasc. Biol. 2002;22:1720–1725. doi: 10.1161/01.atv.0000032133.12377.4d. [DOI] [PubMed] [Google Scholar]
- Lamaziere A, Wolf C. Quin PJ. Perturbations of lipid metabolism indexed by lipidomic biomarkers. Metabolites. 2012;2:1–18. doi: 10.3390/metabo2010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillycrop KA, Rodford J, Garratt ES, Slater-Jefferies JL, Godfrey KM, Gluckman PD, et al. Maternal protein restriction with or without folic acid supplementation during pregnancy alters the hepatic transcriptome in adult male rats. Br. J. Nutr. 2010;103:1711–1719. doi: 10.1017/S0007114509993795. [DOI] [PubMed] [Google Scholar]
- Long NM, Rule DC, Zhu MJ, Nathanielsz PW. Ford SP. Maternal obesity upregulates fatty acid and glucose transporters and increases expression of enzymes mediating fatty acid biosynthesis in fetal adipose tissue depots. J. Anim. Sci. 2012;90:2201–2210. doi: 10.2527/jas.2011-4343. [DOI] [PubMed] [Google Scholar]
- Maloyan A, Muralimanoharan S, Huffman S, Cox LA, Nathanielsz PW, Myatt L, et al. Identification and comparative analyses of myocardial miRNAs involved in the fetal response to maternal obesity. Physiol. Genomics. 2013;45:889–900. doi: 10.1152/physiolgenomics.00050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy CE. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J. Clin. Invest. 2009;119:323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainwater DL, Kammerer CM, Carey KD, Dyke B, VandeBerg JF, Shelledy WR, et al. Genetic determination of HDL variation and response to diet in baboons. Atherosclerosis. 2002a;161:335–343. doi: 10.1016/s0021-9150(01)00658-x. [DOI] [PubMed] [Google Scholar]
- Rainwater DL, Kammerer CM, Cox LA, Rogers J, Carey KD, Dyke B, et al. A major gene influences variation in large HDL particles and their response to diet in baboons. Atherosclerosis. 2002b;163:241–248. doi: 10.1016/s0021-9150(02)00015-1. [DOI] [PubMed] [Google Scholar]
- Rainwater DL, Mahaney MC, VandeBerg JL. Wang XL. Vitamin E dietary supplementation significantly affects multiple risk factors for cardiovascular disease in baboons. Am. J. Clin. Nutr. 2007;86:597–603. doi: 10.1093/ajcn/86.3.597. [DOI] [PubMed] [Google Scholar]
- Saffery R. Epigenetic change as the major mediator of fetal programming in humans: are we there yet? Ann. Nutr. Metab. 2014;64:203–207. doi: 10.1159/000365020. [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- Schlabritz-Loutsevitch NE, Howell K, Rice K, Glover EJ, Nevill CH, Jenkins SL, et al. Development of a system for individual feeding of baboons maintained in an outdoor group social environment. J. Med. Primatol. 2004;33:117–126. doi: 10.1111/j.1600-0684.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- Shasa DR, Odhiambo JF, Long NM, Tuersunjiang N, Nathanielsz PW. Ford SP. Multigenerational impact of maternal overnutrition/obesity in the sheep on the neonatal leptin surge in granddaughters. Int. J. Obes. (Lond) 2015;39:695–701. doi: 10.1038/ijo.2014.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc. Natl Acad. Sci. U. S. A. 2007;104:19351–19356. doi: 10.1073/pnas.0707258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Grayson B, Takahashi D, Robertson N, Maier A, Bethea CL, et al. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J. Neurosci. 2010;30:3826–3830. doi: 10.1523/JNEUROSCI.5560-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Smith MS. Grove KL. Perinatal exposure to high-fat diet programs energy balance, metabolism and behavior in adulthood. Neuroendocrinology. 2011;93:1–8. doi: 10.1159/000322038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter M, Bocock P, Showalter L, Hu M, Shope C, McKnight R, et al. Epigenomics: maternal high-fat diet exposure in utero disrupts peripheral circadian gene expression in nonhuman primates. FASEB J. 2011;25:714–726. doi: 10.1096/fj.10-172080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulleland M, Eilertsen I, Quadros EV, Rothenberg SP, Fedosov SN, Sundrehagen E, et al. Direct assay for cobalamin bound to transcobalamin (holo-transcobalamin) in serum. Clin. Chem. 2002;48:526–532. [PubMed] [Google Scholar]
- Wallace JM, Horgan GW. Bhattacharva S. Placental weight and efficiency in relation to maternal body mass index and the risk of pregnancy complications in women delivering singleton babies. Placenta. 2012;33:611–618. doi: 10.1016/j.placenta.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Wu G. Knabe DA. Free and protein-bound amino acids in sow’s colostrum and milk. J. Nutr. 1994;124:415–424. doi: 10.1093/jn/124.3.415. [DOI] [PubMed] [Google Scholar]
- Yan J, Wang W, Malysheva JF, 3rd, Gregory O, Brenna JT, Stabler SP, et al. MTHFR C677T genotype influences the isotopic enrichment of one-carbon metabolites in folate-compromised men consuming d9-choline. Am. J. Clin. Nutr. 2011;93:348–355. doi: 10.3945/ajcn.110.005975. [DOI] [PMC free article] [PubMed] [Google Scholar]