Abstract
Background
Myocardial perfusion scintigraphy (MPS) is used widely to assess cardiovascular risk in patients with chest pain. The utility of carotid intima-media thickness (CIMT) and endothelial function as assessed by reactive hyperemia-peripheral arterial tonometry index (RHI) in risk stratifying patients with angina-like symptom needs to be defined. We investigated whether addition of CIMT and RHI to Framingham Cardiovascular Risk Score (FCVRS) and MPS improves comprehensive cardiovascular risk prediction in patients presenting with angina-like symptom.
Methods
We enrolled 343 consecutive patients with angina-like symptom suspected of having stable angina. MPS, CIMT, and RHI were performed and patients were followed for cardiovascular events for a median of 5.3 years (range 4.4-6.2). Patients were stratified by FCVRS and MPS.
Results
During the follow-up, 57 patients (16.6%) had cardiovascular events. Among patients without perfusion defect, low RHI was significantly associated with cardiovascular events in the intermediate and high FCVRS groups (Hazard ratio (HR) [95% confidence interval (CI)] of RHI≤2.11 was 6.99 [1.34-128] in the intermediate FCVRS group and 6.08 [1.08-114] in the high FCVRS group). Furthermore, although MPS did not predict, only RHI predicted hard cardiovascular events (cardiovascular death, myocardial infarction, and stroke) independent from FCVRS, and adding RHI to FCVRS improved net reclassification index (20.9%, 95% CI 0.8-41.1, p=0.04). Especially, RHI was significantly associated with hard cardiovascular events in the high FCVRS group (HR [95% CI] of RHI≤1.93 was 5.66 [1.54-36.4], p=0.007).
Conclusions
Peripheral endothelial function may improve discrimination in identifying at-risk patients for future cardiovascular events when added to FCVRS-MPS-based risk stratification.
Keywords: cardiovascular disease, Framingham Cardiovascular Risk Score, myocardial perfusion, noninvasive, endothelial function, carotid intima-media thickness
Introduction
Atherosclerotic cardiovascular disease is the leading cause of mortality in the world [1]. The management of atherosclerotic cardiovascular disease is based on the absolute risk of adverse cardiovascular outcomes [2]. Current practice guidelines recommend classifying individuals as high, intermediate, or low risk, for example, using by Framingham Cardiovascular Risk Score (FCVRS), SCORE risk score by European Society of Cardiology, PROCAM risk score, or other similar risk prediction models which are based on identifying the established risk factors for atherosclerotic diseases [3,4,5]. The cardiovascular risk score recently published by the Framingham study group includes the risk for future overall cardiovascular events, including stroke and heart failure [6]. Stress myocardial perfusion scintigraphy (MPS) is an established, widely used method in detecting coronary artery disease and predicting future cardiovascular events in patients complaining of angina-like symptom, and is also recommended by current guidelines [7,8].
Carotid intima-media thickness (CIMT) and reactive hyperemia-peripheral arterial tonometry (RH-PAT) index (RHI) have shown promise in improving risk stratification for cardiovascular events [9,10,11]. MPS reflects myocardial perfusion reserve including microvascular and macrovascular diseases, whereas these 2 techniques assess different aspects of atherosclerotic vascular disease: CIMT reflects structural changes in the arterial wall, and RHI reflects peripheral endothelial function [12,13,14]. It has been reported that peripheral endothelial function as assessed by RHI well correlates with coronary artery endothelial function as invasively assessed by catheterization [15]. A comprehensive cardiovascular evaluation using assessment of myocardial perfusion, arterial wall structural change, and endothelial function might improve cardiovascular risk stratification. However, it is not clear whether CIMT and RHI when added to the established Framingham risk model and myocardial perfusion assessment are effective in improving risk prediction of future cardiovascular events.
Accordingly, the purpose of the present study was to examine whether a combination of CIMT and RHI with FCVRS-MPS-based risk stratification improves predictive value for cardiovascular events in patients presenting with stable angina-like symptoms.
Methods
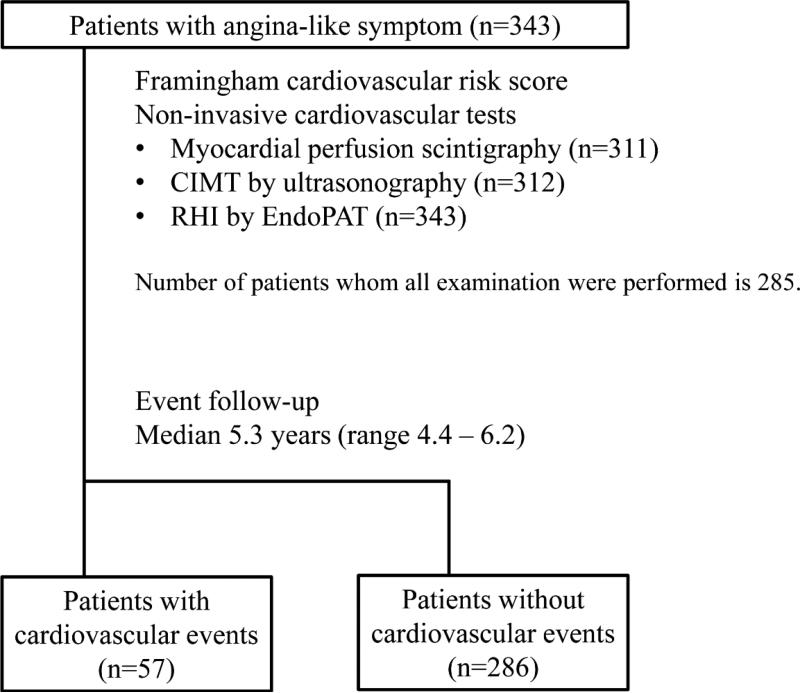
This is a prospective observational study, conducted at the Department of Clinical Physiology, Sahlgrenska University Hospital, Sweden from February 2006 to November 2008. Three hundred and forty three patients with angina-like symptom who were suspected of having stable angina pectoris, but without past history of angiographically proven coronary artery disease, were consecutively enrolled and MPS, carotid ultrasound, and RH-PAT were performed within a week after enrolment (Fig. 1). Physicians referring to MPS examinations were blinded to results of CIMT and RHI which therefore did not alter the clinical decision process in this study. The study was approved by the Local Ethics Committee in Gothenburg, and complies with the Declaration of Helsinki.
Figure 1. Study design.
CIMT: carotid intima media thickness, and RHI: reactive hyperemia-peripheral arterial tonometry index.
Myocardial perfusion scintigraphy (MPS)
Gated single-photon emission computed tomography images were acquired using two different dual-head cameras (Infinia or Millennium VG, GE Healthcare, Milwaukee, Wisconsin, USA) with standard clinical 2-day stress / rest protocol using 99mTc-sestamibi. Stress test was performed according to the physician's discretion; either by symptom-limited exercise test on an ergometric bike or by standard pharmacological challenge using adenosine infusion (6-minute infusion at 140 μg/kg/min). Reversible myocardial ischemia was detected by the software ECT-tool box.
Carotid intima media thickness (CIMT)
Carotid ultrasound was performed by experienced sonographers using the Acuson Sequoia 512 ultrasound system (Siemens Medical Solutions Inc.) with an 8 MHz transducer (Sequoia 8L5C). CIMT was measured with a standardized protocol recommended by “Mannheim Carotid IMT consensus update (2004–2006)” [16]. CINE-looped images of common carotid arteries and carotid bifurcations were obtained using B-mode real-time ultrasound and were stored for offline analysis. CIMT was defined as the distance from the lumen-intimal interface to the medial-adventitial border. Mean CIMT was averaged for both right and left common carotid arteries. The reproducibility of the CIMT measurement has been reported to be adequate [17].
Reactive hyperemia-peripheral arterial tonometry index (RHI)
EndoPAT 2000 device (Itamar Medical Ltd., Caesarea, Israel) was used to evaluate peripheral arterial endothelial function, as described previously [10,14,18,19,20,21]. Pneumatic probes were applied to the tip of one finger on each hand to measure digital volume changes accompanying arteriolar tone changes. After a 5-minute equilibration period, the blood pressure cuff was inflated on one arm to 60 mmHg above systolic pressure or 200 mmHg for 5 minutes, and then deflated to induce reactive hyperemia. RH-PAT data was analyzed by a computer in an operator-independent manner and RHI was calculated as the ratio of average amplitude of the PAT signal over a 1-minute time interval, starting 1.5 minutes after cuff deflation, divided by its average amplitude over a 2.5-minute time period before cuff inflation (baseline), through a computer algorithm. Previous studies have demonstrated good reproducibility (intra-class correlation coefficient 0.61 to 0.78) of the RH-PAT data recorded by this procedure [22,23,24].
Coronary risk factors and atherosclerotic disease risk scores
Coronary risk factors were defined as current smoking (within one year), diabetes mellitus (patients history and/or need for insulin or oral hypoglycemic agents), and presence of a family history of cardiovascular disease in first-degree relatives <55 years (male) or <65 years (female). Low density lipoprotein cholesterol was calculated by using the equation of Friedewald et al. [25]. Ten-year general cardiovascular disease risk was calculated using FCVRS, which includes age, diabetes, smoking, treated or untreated systolic blood pressure, total cholesterol, and high-density lipoprotein cholesterol [6]. The Framingham Heart Study defines cardiovascular diseases as a composite of coronary death, myocardial infarction, coronary insufficiency, angina, stroke, peripheral arterial disease, and heart failure, the end-points of our study. Patients were classified accordingly as low (<6%), intermediate (6-20%), or high (>20%) risk [6]. In order to assess the additional value of non-invasive tests in predicting hard cardiovascular events, 10-year atherosclerotic cardiovascular disease (ASCVD) risk score by the Pooled Cohort Equation was calculated, and patients were classified accordingly as low (<7.5%) or high (≥7.5%) [5]. ASCVD in the Pooled Cohort Equation is defined as a composite of coronary death, myocardial infarction, and stroke, the hard end-points of our study.
Follow-up
The cardiovascular events were adjudicated by physicians caring for the patients. All physicians were blinded to results of CIMT and RHI. No patients were lost to follow-up. Our primary endpoint was a composite of cardiovascular death, nonfatal myocardial infarction, unstable angina, nonfatal stroke, coronary revascularization, and heart failure. Hard cardiovascular events were defined as cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. The cause of death was established by data from the Swedish National Board of Health registries. Cardiovascular death was defined as death due to acute myocardial infarction, congestive heart failure, stroke, or documented sudden death without apparent non-cardiovascular causes. Acute myocardial infarction was diagnosed by a rise or fall in cardiac biomarkers above the 99th percentile of upper limit of normal range and at least one of the following: electrocardiogram changes (new ST-T changes, left bundle branch block, or pathological Q wave), imaging evidence of new viable myocardium loss, and new regional wall motion abnormality. A diagnosis of unstable angina pectoris was made by new or accelerating myocardial ischemia symptoms accompanied by new ischemic ST-T-wave changes. Stroke diagnosis was based on neurological deficits lasting more than 24 hours and verified either by a neurologist and/or radiological evidence of brain infarction. Hospitalization for heart failure decompensation was defined if the patient was admitted with typical heart failure symptoms and had objective signs of a worsening disease that required intravenous drug administration.
Statistical analysis
Statistical analysis was performed using JMP version 9.0.0 (SAS Institute, Inc. Cary, NC). The Shapiro-Wilk test was used to verify that the data followed a normal distribution. Continuous variables were presented as mean ± standard deviation or median (25th, 75th percentile). Differences among continuous variables were analysed using unpaired t-test or Mann-Whitney U test, as appropriate. Categorical variables were presented as frequencies and percentages, and intergroup comparisons were analysed by Fisher's exact test. Univariate and multivariate time-to-event analyses were performed using the Cox proportional hazard model. Kaplan-Meier curves were compared using log-rank test. Prediction of events was evaluated by receiver operating characteristic curve analysis. We defined optimal thresholds of CIMT and RHI by maximizing the sum of sensitivity and specificity for cardiovascular events and hard cardiovascular events, respectively [26]. Incremental discriminative value, by adding MPS, CIMT, or RHI to Framingham risk score, was estimated using net reclassification index [27]. For net reclassification index calculations, the intermediate risk group was defined by a lower limit of 6% and an upper limit of 20%. To calculate net reclassification index for hard cardiovascular events, predicted risk categories were adjusted by the ratio of hard cardiovascular events of total cardiovascular events. Corresponding intermediate risk for hard cardiovascular events was 2-6%. All analyses were two-sided and a P value of <0.05 was considered statistically significant.
Results
Patients and cardiovascular events
Among the 343 patients enrolled in the study, MPS was performed in 311 patients, carotid ultrasound in 312 patients, and RH-PAT in 343 patients (Fig. 1). The median duration of follow-up was 5.3 years (range 4.4 – 6.2). During this time, 57 (16.6%) patients experienced cardiovascular events, 6 (1.7%) died due to cardiovascular disease, 9 (2.6%) had acute myocardial infarction, 11 (3.2%) unstable angina, 5 (1.5%) stroke, 44 (12.8%) coronary revascularization, and 5 (1.5%) heart failure.
Baseline characteristics and results of non-invasive cardiovascular tests
As shown in Table 1, patients with cardiovascular events were older (p=0.04), and had higher body mass index (p=0.04) and lower high-density lipoprotein cholesterol (p=0.002). Male sex (p<0.001), diabetes (p=0.006), and use of anti-hypertensive drugs (p<0.001) were more frequent in patients with cardiovascular events than in those without events. In addition, patients with events had higher mean CIMT (p=0.002), lower RHI (p=0.02), and higher prevalence of perfusion defect on scintigraphy (p<0.001) than those without events.
Table 1.
Baseline characteristics and non-invasive cardiovascular assessments.
| All (n = 343) | With CV events (n = 57) | Without CV events (n = 286) | P | |
|---|---|---|---|---|
| Age, years | 61 ± 9 | 63 ± 9 | 61 ± 9 | 0.04* |
| Male | 133 (38.8%) | 35 (61.4%) | 98 (34.3%) | <0.001* |
| Body mass index | 26 (23-28) | 27 (24-30) | 25 (23-28) | 0.04* |
| Smoking | 41 (12.0%) | 6 (10.5%) | 35 (12.2%) | 0.83 |
| Family history of CAD | 127 (37.0%) | 24 (42.1%) | 103 (36.0%) | 0.45 |
| Diabetes | 34 (9.9%) | 12 (21.1%) | 22 (7.7%) | 0.006* |
| Anti-hypertensive drugs | 163 (47.5%) | 41 (71.9%) | 122 (42.7%) | <0.001* |
| Statins | 108 (31.5%) | 20 (35.1%) | 88 (30.8%) | 0.53 |
| Systolic BP, mmHg | 130 (115-145) | 135 (115-149) | 130 (115-145) | 0.44 |
| Total cholesterol, mg/dl | 211 (180-244) | 195 (169-233) | 214 (184-245) | 0.07 |
| HDL cholesterol, mg/dl | 58 (49-67) | 51 (43-63) | 59 (50-69) | 0.002* |
| LDL cholesterol, mg/dl | 130 ± 40 | 126 ± 40 | 131 ± 40 | 0.42 |
| Triglycerides, mg/dl | 105 (73-149) | 107 (85-163) | 104 (70-145) | 0.12 |
| hsCRP, mg/dl (n=184) | 0.87 (0.25-2.06) | 1.11 (0.42-2.28) | 0.81 (0.25-2.06) | 0.46 |
| FCVRS, % | 12.0 (6.7-21.3) | 20.3 (10.7-31.1) | 11.5 (6.5-18.8) | <0.001* |
| Low FCVRS (<6%) | 69 (20.1%) | 5 (8.8%) | 64 (22.4%) | <0.001* |
| Intermediate FCVRS (6≤ <20%) | 182 (53.1%) | 23 (40.4%) | 159 (55.6%) | |
| High FCVRS (20%≤) | 92 (26.8%) | 29 (50.9%) | 63 (22.0%) | |
| PC_ASCVDRS, % | 7.7 (3.2-15.8) | 11.9 (7.4-22.1) | 6.8 (3.0-13.4) | <0.001* |
| Low PC_ASCVDRS (<7.5%) | 171 (49.9%) | 15 (26.3%) | 156 (54.6%) | <0.001* |
| High PC_ASCVDRS (≥7.5%) | 172 (50.2%) | 42 (73.7%) | 130 (45.5%) | |
| Perfusion defect on MPS (n=311) | 78 (25.1%) | 32 (60.4%) | 46 (17.8%) | <0.001 |
| CIMT, mm (n=312) | 0.58 (0.51-0.69) | 0.62 (0.58-0.77) | 0.58 (0.51-0.68) | 0.002 |
| RHI (n=343) | 1.95 (1.59-2.51) | 1.74 (1.48-2.24) | 2.01 (1.61-2.60) | 0.02 |
Data are expressed as mean ± SD, median (IQR), or no. (%). Significance was assessed between patients with CV events and patients without CV events.
indicates P<0.05.
BP: blood pressure, CIMT: carotid intima-media thickness, CV: cardiovascular, FCVRS: Framingham Cardiovascular Risk Score, HDL: high density lipoprotein, hsCRP: high sensitivity C reactive protein, IQR: interquartile range, LDL: low density lipoprotein, PC_ASCVDRS: atherosclerotic cardiovascular disease risk score by the Pooled Cohort Equation, SD: standard deviation, MPS: myocardial perfusion scintigraphy, and RHI: reactive hyperemia-peripheral arterial tonometry index.
Association of non-invasive tests with cardiovascular events
In univariate Cox hazard analysis, each of the non-invasive cardiovascular tests was associated with cardiovascular events; however, after adjusting for Framingham risk score, the association with RHI and CIMT was no longer significant (Table 2). By Cox hazard analyses across Framingham risk categories, RHI was a significant predictor of cardiovascular events in the high Framingham risk group. The presence of perfusion defect was significantly associated with cardiovascular events in all Framingham risk categories. The addition of MPS, CIMT, and RHI to Framingham risk score improved area under the curve for cardiovascular events (Online Fig. 1), and MPS showed the highest increment. The optimal cut-off values of CIMT and RHI that predicted future cardiovascular events were 0.57mm and 2.11 respectively. Online Figure 2 demonstrates Kaplan-Meier estimates of the probability of cardiovascular events according to Framingham risk categories and perfusion defect on MPS. In each Framingham risk category, patients with perfusion defect had more events compared with those without perfusion defect. Furthermore, the addition of MPS to Framingham risk score resulted in the largest improvement of net reclassification index (31.6%, 95% confidence interval (95%CI) 11.8-51.5%, p=0.002) (Table 3).
Table 2.
Cox proportional hazards analysis for cardiovascular events according to Framingham cardiovascular risk category.
| Univariate | Adjusted for FCVRS | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| All patients | (number of patients =343, number of events = 57) | ||||||
| FCVRS | Per 1% | 1.036 | 1.020 – 1.052 | <0.001 | – | ||
| CIMT | Per 0.1mm | 1.176 | 1.030 – 1.309 | 0.019 | 1.056 | 0.888 – 1.212 | 0.50 |
| RHI | Per 1 | 0.649 | 0.411 – 0.978 | 0.038 | 0.685 | 0.432 – 1.036 | 0.07 |
| Perfusion defect on MPS | yes | 5.779 | 3.353 – 10.17 | <0.001 | 4.911 | 2.816 – 8.742 | <0.001 |
| Low FCVRS group | (number of patients =69, number of events = 5) | ||||||
| CIMT | Per 0.1mm | 1.357 | 0.621 – 2.468 | 0.40 | - | - | - |
| RHI | Per 1 | 1.008 | 0.261 – 2.408 | 0.99 | - | - | - |
| Perfusion defect on MPS | yes | 14.19 | 2.345 – 108.02 | 0.005 | - | - | - |
| Intermediate FCVRS group | (number of patients =182, number of events = 23) | ||||||
| CIMT | Per 0.1mm | 1.133 | 0.849 – 1.420 | 0.37 | - | - | - |
| RHI | Per 1 | 0.872 | 0.445 – 1.546 | 0.66 | - | - | - |
| Perfusion defect on MPS | yes | 3.351 | 1.350 – 8.099 | 0.01 | - | - | - |
| High FCVRS group | (number of patients =92, number of events = 29) | ||||||
| CIMT | Per 0.1mm | 1.046 | 0.840 – 1.226 | 0.65 | - | - | - |
| RHI | Per 1 | 0.506 | 0.243 – 0.949 | 0.03 | - | - | - |
| Perfusion defect on MPS | yes | 5.263 | 2.397 – 12.73 | <0.001 | - | - | - |
CI: confidence interval, CIMT: carotid intima media thickness, FCVRS: Framingham Cardiovascular Risk Score, HR: hazard ratio, MPS: myocardial perfusion scintigraphy, and RHI: reactive hyperemia-peripheral arterial tonometry index.
Table 3.
Net reclassification improvement for incident cardiovascular events with addition of CIMT, RHI, and MPS to the Framingham Cardiovascular Risk Score.
| Risk category | ||||||||
|---|---|---|---|---|---|---|---|---|
| Low | Intermediate | High | % Net Correct Reclassification | NRI | 95% CI | p | ||
| FCVRS + CIMT | Events Nonevents |
0 0 |
32 217 |
18 45 |
−2.00 1.15 |
−0.85 | −7.85 – 6.14 | 0.81 |
| FCVRS + RHI | Events Nonevents |
0 8 |
29 223 |
28 55 |
8.77 2.10 |
10.87 | 2.31 – 19.43 | 0.013 |
| FCVRS + MPS | Events Nonevents |
2 26 |
16 182 |
35 50 |
20.75 10.85 |
31.61 | 11.76 – 51.45 | 0.002 |
CI: confidence interval, CIMT: carotid intima media thickness, FCVRS: Framingham Cardiovascular Risk Score, MPS: myocardial perfusion scintigraphy, NRI: net reclassification index, and RHI: reactive hyperemia-peripheral arterial tonometry index.
Additional tests in patients without perfusion defect
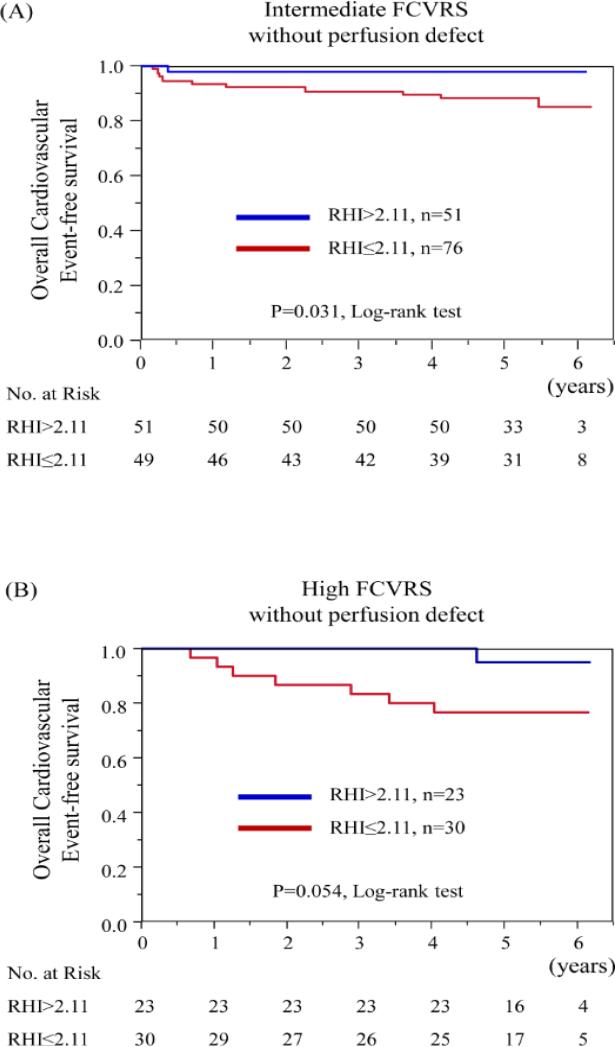
Table 4 shows Cox hazard analyses using the cut-off values for CIMT and RHI in subgroups divided by Framingham risk categories and myocardial perfusion defect on MPS. Among patients without perfusion defect, we found that low RHI was significantly associated with cardiovascular events in the intermediate and high Framingham risk groups (Table 4). When the intermediate Framingham risk category patients without perfusion defect were subdivided into 2 groups according to the cut-off value for RHI, Kaplan-Meier curves showed an increased risk of cardiovascular events in low RHI group (p=0.03, by log-rank test) (Fig. 2A). Although the difference did not reach statistical significance, Kaplan-Meier analysis showed that patients with RHI ≤ 2.11 experienced higher rate of cardiovascular events in the high Framingham risk category patients without perfusion defect (p=0.054, by log-rank test) (Fig. 2B).
Table 4.
Univariate Cox proportional hazards analysis for cardiovascular events according to Framingham cardiovascular risk category and myocardial perfusion defect.
| Perfusion defect negative on MPS (patients number 233, events number 21) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low FCVRS patients number 53, events number 2 | Intermediate FCVRS patients number 127, events number 11 | High FCVRS patients number 53, events number 8 | |||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| CIMT ≥ 0.57 | <0.001 | <0.001 – 7.435 | 0.38 | 1.334 | 0.381 – 5.215 | 0.65 | >9999 | 0.751 - >9999 | 0.084 |
| RHI ≤ 2.11 | 0.909 | 0.036 – 22.97 | 0.95 | 6.987 | 1.338 – 128.2 | 0.017 | 6.075 | 1.080 – 113.6 | 0.039 |
| Perfusion defect positive on MPS (patients number 78, events number 32) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low FCVRS patients number 7, events number 3 |
Intermediate FCVRS patients number 35, events number 9 |
High FCVRS patients number 36, events number 20 |
|||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| CIMT ≥ 0.57 | 1.029 | 0.098 – 22.28 | 0.98 | 1.983 | 0.479 – 13.31 | 0.37 | 1.157 | 0.325 – 7.357 | 0.84 |
| RHI ≤ 2.11 | 0.474 | 0.022 – 5.053 | 0.53 | 0.902 | 0.238 – 3.646 | 0.88 | 0.731 | 0.293 – 2.067 | 0.53 |
CI: confidence interval, CIMT: carotid intima media thickness, FCVRS: Framingham Cardiovascular Risk Score, HR: hazard ratio, MPS: myocardial perfusion scintigraphy, and RHI: reactive hyperemia-peripheral arterial tonometry index.
Figure 2. Kaplan-Meier analysis for the probability of cardiovascular events according to RHI in each group by Framingham risk category and myocardial perfusion defect.
(A) Kaplan-Meier curves according to RHI in intermediate FCVRS with PD (−) group. (B) Kaplan-Meier curves according to RHI in high FCVRS with PD (−) group. FCVRS: Framingham Cardiovascular Risk Score, PD: perfusion defect, and RHI: reactive hyperemia-peripheral arterial tonometry index.
Additional tests in patients with perfusion defect
Among patients with perfusion defect on MPS, both CIMT and RHI did not show additional predictive value in each Framingham risk category (Table 4). The event rate in patients with perfusion defect was considerably higher compared with those without (41% versus 9%, p<0.001 by log-rank test), suggesting those patients need to be considered at high-risk without additional tests.
Hard cardiovascular events
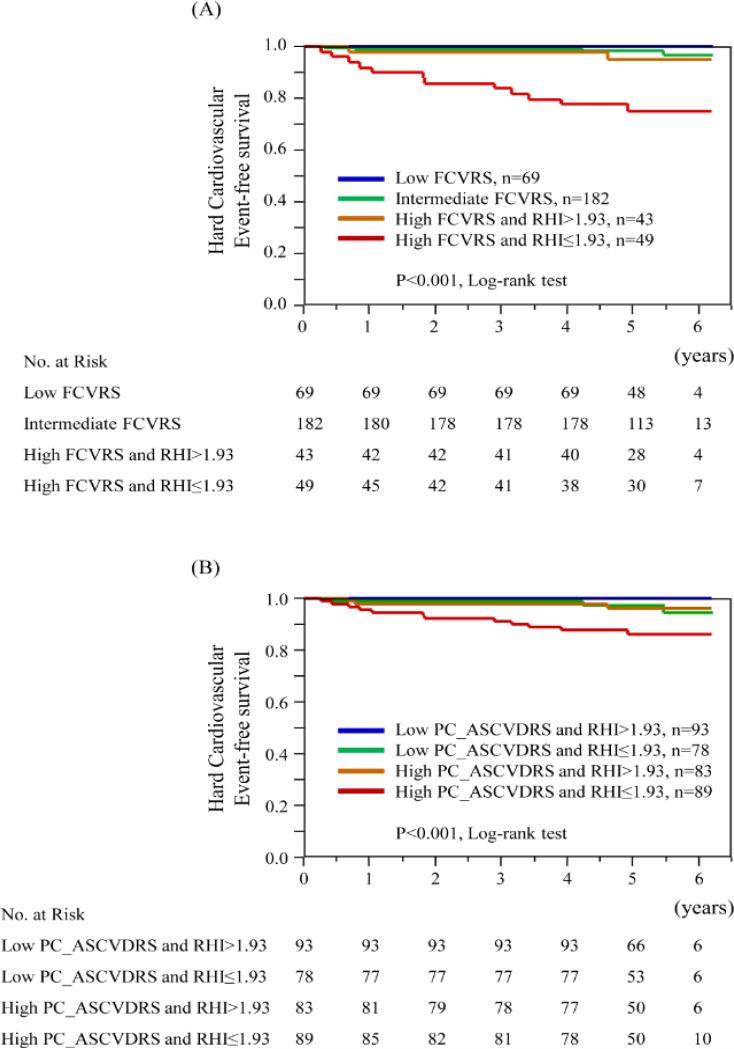
Hard cardiovascular events, consisting of cardiovascular death, myocardial infarction, and stroke, were observed in none of patients in the low Framingham risk group, 4 (2.2%) patients in the intermediate Framingham risk group, and 14 (15.2%) patients in the high Framingham risk group. In univariate Cox proportional hazard model, only RHI was significantly associated with hard cardiovascular events, and remained significant after adjusting for FCVRS (Table 5). The addition of RHI to Framingham risk score showed the highest increment for incident hard cardiovascular events by receiver operating characteristic curve analysis (Online Fig. 3). The optimal cut-off value of RHI to predict hard cardiovascular events was 1.93, and patients with RHI ≤ 1.93 had a 5.3-fold higher risk of hard cardiovascular events than those with RHI > 1.93 (Table 5). Net reclassification index was significant with the addition of RHI to Framingham risk score [5.6% for patients with events, 15.4% for patients without events, and 20.9% (95%CI 0.8-41.1) for overall, p=0.042]. By Cox hazard analyses, according to Framingham risk categories, RHI was significantly associated with hard cardiovascular events in the high Framingham risk group (Table 5). Figure 3A shows Kaplan-Meier estimates of the probability of hard cardiovascular events divided by Framingham risk category and RHI of 1.93 (p<0.001, by log-rank test). Thus, RHI can contribute to further risk stratification in the high Framingham risk groups (Fig. 4).
Table 5.
Cox proportional hazards analysis for hard cardiovascular events according to Framingham cardiovascular risk category.
| Univariate | Adjusted for FCVRS | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| All patients | (number of patients =343, number of events = 18) | ||||||
| FCVRS | Per 1% | 1.056 | 1.030 – 1.082 | <0.001 | - | - | - |
| CIMT | Per 0.1mm | 1.231 | 0.983 – 1.446 | 0.068 | 0.978 | 0.665 – 1.260 | 0.89 |
| RHI | Per 1 | 0.268 | 0.083 – 0.689 | 0.004 | 0.307 | 0.096 – 0.792 | 0.012 |
| Perfusion defect on MPS | Yes | 2.663 | 0.999 – 6.969 | 0.0502 | 1.847 | 0.686 – 4.891 | 0.22 |
| RHI ≤ 1.93 | 5.362 | 1.769 – 23.14 | 0.002 | 4.685 | 1.540 – 20.27 | 0.005 | |
| Low FCVRS group | (number of patients = 69, number of events = 0) | ||||||
| Intermediate FCVRS group | (number of patients = 182, number of events = 4) | ||||||
| CIMT ≥ 0.72 | 2.669 | 0.124 – 27.86 | 0.45 | - | - | - | |
| RHI ≤ 1.93 | 3.212 | 0.411 – 64.93 | 0.28 | - | - | - | |
| Perfusion defect on MPS | <0.001 | <0.001 – 2.897 | 0.20 | - | - | - | |
| High FCVRS group | (number of patients = 92, number of events = 14) | ||||||
| CIMT ≥ 0.72 | 2.236 | 0.713 – 7.559 | 0.17 | - | - | - | |
| RHI ≤ 1.93 | 5.662 | 1.544 – 36.38 | 0.007 | - | - | - | |
| Perfusion defect on MPS | 2.037 | 0.708 – 6.191 | 0.18 | - | - | - | |
CI: confidencial interval, CIMT: carotid intima media thickness, FCVRS: Framingham Cardiovascular Risk Score, HR: hazard ratio, MPS: myocardial perfusion scintigraphy, and RHI: reactive hyperemia-peripheral arterial tonometry index.
Figure 3. Kaplan-Meier analysis for the probability of hard cardiovascular events according to established risk prediction models and RHI.
FCVRS: Framingham Cardiovascular Risk Score, PC_ASCVDRS; atherosclerotic cardiovascular disease risk score by the Pooled Cohort Equation, and RHI: reactive hyperemia-peripheral arterial tonometry index.
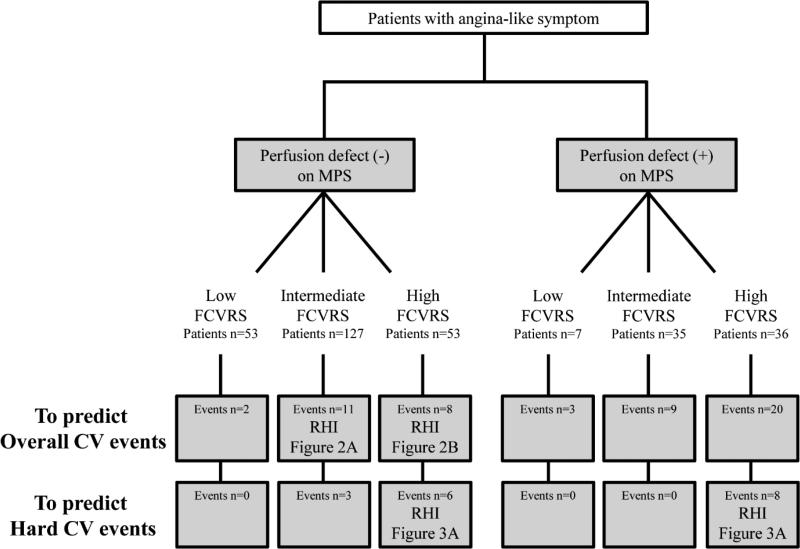
Figure 4. Proposed sequence of tests for cardiovascular risk assessment in patients with angina-like symptom.
FCVRS: Framingham Cardiovascular Risk Score, MPS: myocardial perfusion scintigraphy, and RHI: reactive hyperemia-peripheral arterial tonometry index.
Only RHI was independently associated with hard cardiovascular events from ASCVD risk score by the Pooled Cohort Equation (adjusted hazard ratio per 1.0 increase in RHI 0.32, 95%CI 0.10 to 0.81, p=0.01). The addition of RHI to ASCVD risk score showed the highest increment in C-statistics for incident hard cardiovascular events (C-statistics; from 0.779 to 0.812), and net reclassification index was significant (28.9% for patients without events, 5.6% for patients with events, and 34.5% for overall, 95%CI 9.0 to 59.9%, p=0.008). Using the cut-off value of RHI 1.93, lower RHI was significantly associated with hard cardiovascular events in the both low (<7.5%) and high (≥7.5%) ASCVD risk groups (in the low ASCVD risk group; hazard ratio >9999, 95%CI 1.30 to >9999, p=0.03, in the high ASCVD risk group; hazard ratio 3.80, 95%CI 1.21 to 16.7, p=0.02). Figure 3B shows Kaplan-Meier estimates of the probability of hard cardiovascular events divided by ASCVD risk score of 7.5% and RHI of 1.93 (p<0.001, by log-rank test).
Proposed sequence of tests for cardiovascular risk stratification
Figure 4 demonstrates a proposed sequence of tests for risk stratification of patients presenting with angina-like symptoms based on our results.
Overall cardiovascular events
MPS can be applied as the first step for risk stratification of patients presenting with angina-like symptoms in all Framingham risk categories. As the second step, among patients without perfusion defect, RHI can contribute to further risk stratification in the intermediate and high Framingham risk groups.
Hard cardiovascular events
Regardless MPS results, RHI can contribute to further risk stratification for hard cardiovascular events in the high Framingham risk group.
Discussion
The current study demonstrates that MPS is a predictor of cardiovascular events across all Framingham risk categories and improved reclassification when added to FCVRS. MPS is commonly used as the first step in risk stratification among all Framingham risk categories, and subsequently, among patients without perfusion defects, RHI can contribute to further risk stratification in the intermediate and high Framingham risk groups. In addition, the combination of RHI with established risk scores was useful in predicting hard cardiovascular events. These findings indicate that the sequence of the peripheral endothelial function assessment provides additional predictive value when added to FCVRS-MPS-based risk stratification.
While an increasing body of evidence supports the usefulness of a single method strategy to predict cardiovascular events [9,10,11], several recent studies reported that a combination of multiple measurements may strengthen the predictive power for future cardiovascular events [28,29,30]. Furthermore, in clinical practice, several methods are often used in combination to evaluate risk, and efficacy of each method is critically dependent on target groups. Our study focused on this aspect of implementation by examining usefulness of a combined strategy in identifying at-risk patients. To our knowledge, this is the first study to assess the predictive value of both carotid arterial wall structural change and peripheral endothelial dysfunction in patients with angina-like symptom after stratification by Framingham risk category and myocardial perfusion.
We used the Framingham risk model for general cardiovascular diseases [6], to assess additional predictive value of non-invasive tests in comprehensive cardiovascular risk stratification. The most common cardiovascular disease risk prediction models in the United States are those based on the Framingham study. Framingham risk scoring system has been developed and validated in a large prospective American cohort [31]. Practice guidelines recommend approaches to classify individuals as high, intermediate, or low risk using Framingham risk model [5]. Several non-invasive markers have been developed and clinically used to improve FCVRS-based risk classification. Stress myocardial perfusion imaging is an established method to predict coronary artery disease and future cardiovascular events, and is recommended by current guidelines [7,8]. Consistently, in this study MPS was a strong predictor for future cardiovascular events across all Framingham risk categories and improved reclassification when added to FCVRS.
Studies have shown that CIMT and RHI are important independent determinants of cardiovascular risk [9,10,11]. These 2 tests reflect different aspects of atherosclerosis; structural changes in the arterial wall (CIMT) and endothelial function (RHI). Introduction of a combined strategy that incorporates these additional tests, as appropriate, might provide a more comprehensive cardiovascular risk assessment, and improve risk discrimination based on Framingham risk scoring system and myocardial perfusion assessment by scintigraphy. The present study did not identify a significant association of CIMT with the occurrence of cardiovascular events independent from FCVRS. Our result is compatible with recent meta-analyses [32,33] which demonstrated that the addition of CIMT to the Framingham risk scoring model leads to a small improvement in predicting future cardiovascular event risk, and this increment is unlikely to provide clinical benefit. However, given its low cost [34], CIMT testing might exert proper cost-benefit performance even when taking into account its small additive predictive value. On the contrary, fingertip pneumatic probes of RH-PAT technology cannot be reused, indicating it is costly. Thus, a discussion of cost-effectiveness of these non-invasive vascular tests is moot and further cost-analysis studies are needed. Moreover, techniques of carotid ultrasound has been considerably improved in recent years, therefore newer techniques, including assessment of plaque morphology and characterization [35,36], might produce better results. On the other hand, attenuated endothelial function as assessed by RHI successfully identified at-risk patients in the intermediate and high Framingham risk patients without myocardial perfusion defects. Generally, MPS is a reliable test to predict cardiovascular events. Nevertheless, in this study, 21of 233 patients (9.0%) without perfusion defect experienced cardiovascular events. Moreover, among 17 patients with available MPS who experienced hard cardiovascular events, 8 (52.9%) patients showed no myocardial perfusion defect, and therefore predictive value of MPS for hard cardiovascular events might be smaller than that for total cardiovascular events. Thus, it is worthy of note that the peripheral endothelial function can distinguish cardiovascular event risk in patients with normal myocardial perfusion, and was the only parameter that had significant predictive value for hard cardiovascular events in this study, suggesting that introduction of RHI might contribute to avoid use of MPS. Although RHI measurements are completely non-invasive, MPS measurements have an important issue of the radiation exposure. Thus, further researches should be directed at avoiding unjustified and non-optimized use of radiation for risk stratification in these patients.
In this study, anatomical assessment of coronary plaque was not implemented. Since it was reported that the prognostic value of RHI for cardiovascular events is independent from coronary plaque complexity [10], a combination of endothelial function and coronary plaque morphological assessment, such as SYNTAX score and coronary calcium score, may provide further improvement in the risk stratification for cardiovascular events. Given its several valuable features including non-invasive, operator independent, automatic aspects, and an acceptable measuring time (15 min), in terms of risk stratification, RH-PAT examination is adequately practical. However, as mentioned above, studies on cost-effectiveness are still needed. Additionally, in order to elucidate whether endothelial function-guided therapies improve outcomes, prospective randomized studies are also needed.
Strengths and Limitations
The strengths of this study include availability of several kinds of non-invasive cardiovascular tests in comparing the predictive value, and these results were prospectively collected. CIMT and RHI results were blinded to physicians. A further strength was that the event follow-up was accomplished in all patients. Our study also has some limitations. The sample size was modest - although our results suggested that RHI were useful in some subgroups, we might have underestimated utility in other subgroups, due to low statistical power. Large-scale multicenter studies are necessary to further evaluate the importance of a combined risk stratification strategy. Although the patients were studied prospectively and consecutively there were few missing data for MPS and CIMT. This was a single-center study with experience and necessary expertise to perform all the tests described; the results may not be generalizable to all other medical settings.
Conclusion
Among patients without perfusion defects, peripheral endothelial function may improve risk assessment for overall cardiovascular events in the intermediate and high Framingham risk groups. Furthermore, the endothelial function could provide the highest predictive value for hard cardiovascular events compared to MPS and CIMT, especially in the high Framingham risk group. Thus, we should consider adding the non-invasive peripheral endothelial function test in all high Framingham risk patients and intermediate Framingham risk patients without perfusion defect. The sequence of the endothelial function test may provide additional discriminative value for cardiovascular risk assessment and optimize individualized therapeutic strategies to reduce cardiovascular morbidity and mortality in patients presenting with angina-like symptoms, when added to the current FCVRS-MPS-based risk stratification.
Supplementary Material
Acknowledgement
Funding Sources: This work was supported by the National Institute of Health (NIH Grants HL-92954 and AG-31750), the Mayo Foundation, and a research fellowship from Banyu Life Science Foundation International (Y.M. and A.T.).
Abbreviations
- ASCVD
Atherosclerotic cardiovascular disease
- CI
Confidence interval
- CIMT
Carotid intima-media thickness
- FCVRS
Framingham Cardiovascular Risk Score
- MPS
Stress myocardial perfusion scintigraphy
- RHI
Reactive hyperemia-peripheral arterial tonometry index
- RH-PAT
Reactive hyperemia-peripheral arterial tonometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Conflict of interest: Author A.L. declared consulting for Itamar Medical.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2015 Update: A Report From the American Heart Association. Circulation. 2014 doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63:2889–934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Perk J, De Backer G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). European heart journal. 2012;33:1635–701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 4.Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Munster (PROCAM) study. Circulation. 2002;105:310–5. doi: 10.1161/hc0302.102575. [DOI] [PubMed] [Google Scholar]
- 5.Goff DC, Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63:2935–59. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Agostino RB, Sr., Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 7.Klocke FJ, Baird MG, Lorell BH, et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). Journal of the American College of Cardiology. 2003;42:1318–33. doi: 10.1016/j.jacc.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Task Force M. Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. European heart journal. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 9.Rubinshtein R, Kuvin JT, Soffler M, et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. European heart journal. 2010;31:1142–8. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 10.Matsuzawa Y, Sugiyama S, Sumida H, et al. Peripheral endothelial function and cardiovascular events in high-risk patients. Journal of the American Heart Association. 2013;2:e000426. doi: 10.1161/JAHA.113.000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson CM, Gerry F, Fowkes R, Price JF. Carotid intima-media thickness and the prediction of vascular events. Vascular medicine. 2012;17:239–48. doi: 10.1177/1358863X12445103. [DOI] [PubMed] [Google Scholar]
- 12.Matsuzawa Y, Guddeti RR, Kwon TG, Lerman LO, Lerman A. Treating Coronary Disease and the Impact of Endothelial Dysfunction. Progress in cardiovascular diseases. 2014 doi: 10.1016/j.pcad.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuzawa Y, Lerman A. Endothelial dysfunction and coronary artery disease: assessment, prognosis, and treatment. Coronary artery disease. 2014;25:713–24. doi: 10.1097/MCA.0000000000000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuzawa Y, Li J, Aoki T, et al. Predictive value of endothelial function by noninvasive peripheral arterial tonometry for coronary artery disease. Coronary artery disease. 2014 doi: 10.1097/MCA.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr., Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. Journal of the American College of Cardiology. 2004;44:2137–41. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 16.Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovascular diseases. 2007;23:75–80. doi: 10.1159/000097034. [DOI] [PubMed] [Google Scholar]
- 17.Peters SA, Grobbee DE, Bots ML. Carotid intima-media thickness: a suitable alternative for cardiovascular risk as outcome? European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2011;18:167–74. doi: 10.1177/1741826710389400. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Flammer AJ, Reriani MK, et al. High leukocyte count is associated with peripheral vascular dysfunction in individuals with low cardiovascular risk. Circulation journal : official journal of the Japanese Circulation Society. 2013;77:780–5. doi: 10.1253/circj.cj-12-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Flammer AJ, Lennon RJ, et al. Comparison of the effect of the metabolic syndrome and multiple traditional cardiovascular risk factors on vascular function. Mayo Clinic proceedings. 2012;87:968–75. doi: 10.1016/j.mayocp.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki H, Matsuzawa Y, Konishi M, et al. Utility of noninvasive endothelial function test for prediction of deep vein thrombosis after total hip or knee arthroplasty. Circulation journal : official journal of the Japanese Circulation Society. 2014;78:1723–32. doi: 10.1253/circj.cj-13-1325. [DOI] [PubMed] [Google Scholar]
- 21.Matsuzawa Y, Sugiyama S, Sugamura K, et al. Successful diet and exercise therapy as evaluated on self-assessment score significantly improves endothelial function in metabolic syndrome patients. Circulation journal : official journal of the Japanese Circulation Society. 2013;77:2807–15. doi: 10.1253/circj.cj-13-0549. [DOI] [PubMed] [Google Scholar]
- 22.Selamet Tierney ES, Newburger JW, Gauvreau K, et al. Endothelial pulse amplitude testing: feasibility and reproducibility in adolescents. The Journal of pediatrics. 2009;154:901–5. doi: 10.1016/j.jpeds.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 23.Brant LC, Barreto SM, Passos VM, Ribeiro AL. Reproducibility of peripheral arterial tonometry for the assessment of endothelial function in adults. Journal of hypertension. 2013;31:1984–90. doi: 10.1097/HJH.0b013e328362d913. [DOI] [PubMed] [Google Scholar]
- 24.McCrea CE, Skulas-Ray AC, Chow M, West SG. Test-retest reliability of pulse amplitude tonometry measures of vascular endothelial function: implications for clinical trial design. Vascular medicine. 2012;17:29–36. doi: 10.1177/1358863X11433188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry. 1972;18:499–502. [PubMed] [Google Scholar]
- 26.Zou KH, O'Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115:654–7. doi: 10.1161/CIRCULATIONAHA.105.594929. [DOI] [PubMed] [Google Scholar]
- 27.Pencina MJ, D'Agostino RB, Sr., D'Agostino RB, Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in medicine. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207-12. [DOI] [PubMed] [Google Scholar]
- 28.Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation. 2003;108:2093–8. doi: 10.1161/01.CIR.0000095273.92468.D9. [DOI] [PubMed] [Google Scholar]
- 29.Chan SY, Mancini GB, Kuramoto L, Schulzer M, Frohlich J, Ignaszewski A. The prognostic importance of endothelial dysfunction and carotid atheroma burden in patients with coronary artery disease. J Am Coll Cardiol. 2003;42:1037–43. doi: 10.1016/s0735-1097(03)00927-6. [DOI] [PubMed] [Google Scholar]
- 30.Nagai K, Shibata S, Akishita M, et al. Efficacy of combined use of three non-invasive atherosclerosis tests to predict vascular events in the elderly; carotid intima-media thickness, flow-mediated dilation of brachial artery and pulse wave velocity. Atherosclerosis. 2013;231:365–70. doi: 10.1016/j.atherosclerosis.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 31.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 32.Den Ruijter HM, Peters SA, Anderson TJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA : the journal of the American Medical Association. 2012;308:796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 33.van den Oord SC, Sijbrands EJ, ten Kate GL, et al. Carotid intima-media thickness for cardiovascular risk assessment: systematic review and meta-analysis. Atherosclerosis. 2013;228:1–11. doi: 10.1016/j.atherosclerosis.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 34.Zannad F, De Backer G, Graham I, et al. Risk stratification in cardiovascular disease primary prevention - scoring systems, novel markers, and imaging techniques. Fundamental & clinical pharmacology. 2012;26:163–74. doi: 10.1111/j.1472-8206.2011.01023.x. [DOI] [PubMed] [Google Scholar]
- 35.Irie Y, Katakami N, Kaneto H, et al. The utility of ultrasonic tissue characterization of carotid plaque in the prediction of cardiovascular events in diabetic patients. Atherosclerosis. 2013;230:399–405. doi: 10.1016/j.atherosclerosis.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 36.Tadokoro Y, Sakaguchi M, Yamagami H, et al. Echogenicity of Medium-to-Large Carotid Plaques Predicts Future Vascular Events. Cerebrovascular diseases. 2014;38:354–361. doi: 10.1159/000365651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.