Abstract
High-risk human papillomaviruses (e. g., HPV16, HPV18) are closely associated with the development of head and neck cancers including oral/oropharyngeal squamous cell carcinoma (OSCC). We previously demonstrated immortalization of normal human oral keratinocytes by introducing high-risk HPV whole genome, suggesting that HPV infection plays an important role in the early stage of oral carcinogenesis. Although HPV infection may occur in different stages of cancer development, roles of HPV in exacerbating malignant phenotypes in already-transformed cells in the context of cancer stemness are not clearly defined. In this study, we investigated the role of HPV16 in promoting the virulence of HPV-negative OSCC. Introducing HPV16 whole genome in HPV-negative OSCC increased malignant growth and self-renewal capacity, a key characteristic of cancer stem cells (CSCs). HPV16 also enhanced other CSC properties, including aldehyde dehydrogenase 1 (ALDH1) activity, migration/invasion, and CSC-related factor expression. Mechanistically, we found that HPV16 inhibited the expression of miR-181a and miR-181d (miR-181a/d) at the transcriptional level. Ectopic expression of miR-181a/d decreased anchorage independent growth and CSC phenotype of HPV16-transfected OSCC. Furthermore, silencing of miR-181a/d target genes, i. e., K-ras and ALDH1, abrogated the effects of HPV16 in HPV16-transfected OSCC, supporting the functional importance of HPV16/miR-181a/d axis in HPV-mediated oral carcinogenesis. Our study suggests that high-risk HPV infection further promotes malignancy in HPV-negative OSCC by enhancing cancer stemness via miR-181a/d regulation. Consequently, miR-181a/d may represent a novel therapeutic agent for the treatment of HPV-positive OSCC.
Abbreviations: HPV, Human papillomavirus; CSCs, Cancer stem cells; OSCC, Oral/oropharyngeal squamous cell carcinomas; miRNA, MicroRNA; IL4, ALDH1, Aldehyde dehydrogenase 1; siRNA, small interfering RNA
Keywords: HPV, OSCC, cancer stem cells, miR-181
Highlights
-
•
HPV16 directs malignant progression of OSCC by enhancing stemness properties of OSCC.
-
•
HPV16 represses miR-181 by inhibiting its promoter activity.
-
•
The HPV-induced phenotypes are epigenetically mediated by miR-181a/d.
-
•
miR-181a/d may represent a novel therapeutic agent for HPV-positive OSCC.
1. Introduction
OSCC is the sixth most common cancer and an important public health concern worldwide [1]. Risk factors for OSCC development in the elderly are typically associated with a life-long history of cigarette smoking and heavy alcohol consumption [2], [3]. Recent studieshaveshown that there is anincreasing incidence of oral cancer among young adults [4], [5] and that a significant percentage of OSCC cases in young adults were non-habitués [6]. Indeed, 34% of the cases in young adults did not show any tobacco/alcohol related habits [7], but rather, changes in sexual behaviors was one of the contributing factors for this increasing incidence of oral cancer in young adults.
HPV is a DNA virus that is typically transmitted through sexual contacts. Many studies revealed that high-risk HPV (e. g., HPV16, HPV18) is an additional independent risk factor for oral cancer [8], [9]. HPV infection is closely linked to benign and malignant oral lesions [10] and up to 30–40% of oral cancer biopsies contain the viral DNA [11]. Despite the saturated understanding of HPV-inducing immortalization mechanisms in normal human epithelial cells, not much is known about the possible role and the underlying mechanisms of HPV in promoting the virulence of already transformed cells, such as HPV-negative OSCC.
Recent studies have unveiled and validated the pathophysiologic role of CSCs (alternatively cancer initiating cells) in long-term sustenance of cancers [12]. CSCs are small subpopulations of tumor cells with self-renewal capacity and share many molecular similarities to embryonic and normal adult stem cells. CSCs have been isolated from various primary tumors and established cancer cell lines, including OSCC [13], [14]. They are also potentially responsible for drug resistance, metastasis, and recurrence of cancers. Therefore, CSCs drive the perpetuity of the disease while producing cellular heterogeneity of cancer tissues, making them strategically plausible targets for cancer therapies. The phenotypes of CSC have been reported to be maintained by several endogenous signaling pathways, such as Notch, Hedgehog, and Wnt [15], [16], which are frequently activated in human cancers [16], [17], [18]. In addition, increasing numbers of publications reported that CSCs can be epigenetically regulated by microRNAs (miRNAs) [19], [20].
miRNAs are one of major epigeneticregulators of eukaryotic gene expression [21]. miRNAs are 21–25 nucleotide non-coding RNAs that bind to the target mRNAs at their 3′ untranslated region (UTR) and suppress the gene expression. They are estimated to regulate at least one-third of all human genes and play key roles in many cellular processes, including cancer development and stemness maintenance [22], [23]. Many studies identified various miRNAs affecting CSC phenotype [20], [24]. For instance, miR-34a suppresses CSCs by repressing CD44 [25], and miR-200 inhibits CSC self-renewal and properties by suppressing diverse target genes [26], [27]. Interestingly, various studies showed that miRNA expression pattern is affected by HPV status in human SCC [28], [29]. These observations suggest the potential role of HPV/miRNA axis in oral cancer progression in the context of epigenetic regulation of CSCs.
In the present study, we provide evidence that high-risk HPV16 enhances malignant growth and CSC phenotype of HPV-negative OSCC cells by suppressing tumor suppressive miRNAs, miR-181a and miR-181d. Our study suggests that HPV infection in precancerous and cancerous lesions may potentiate malignant phenotypes by affecting CSC-enriched populations through miRNA-mediated epigenetic regulation and it can ultimately dictate the cancer progression.
2. Materials and methods
2.1. Cells and cell culture
Four HPV-negative OSCC cell lines (SCC66, SCC105, UM6, UM10b [30]) were cultured in DMEM/Ham's F12 (Invitrogen) supplemented with 10% FBS (Gemini Bioproducts) and 0.4 µg/ml hydrocortisone (Sigma-Aldrich).
2.2. Transfection with HPV16 whole genome
Approximately 70% of confluent OSCC cells grown in 60 mm Petri dishes were transfected with pMHPV-16d, a head-to-tail dimer of HPV-16 DNA inserted into the plasmid pdMMTneo or pdMMTneo by using the Lipofectin reagent [31]. To select cells transfected with the pMHPV-16d or pdMMTneo plasmid, the cells were incubated in a culture medium containing 200 µg/ml of G418 (GIBCO, Grand Island, NY) and G418-resistant cells were isolated, subcultured and used for experiments.
2.3. Anchorage-independent growth
To determine colony-forming efficiency in semi-solid medium, 1×104 cells were plated in culture medium containing 0.3% agarose over a base layer of serum-free medium containing 0.5% agarose. Two weeks after incubation, the colonies were counted. The experiment was performed in triplicates with 60-mm dishes.
2.4. Organotypic raft cultures
One million cells were seeded on the submucosal equivalents consisting of type I collagen and normal human oral fibroblasts. The cells were grown to confluence, submerged in the culture medium, and then exposed to the liquid–air interface by lowering the medium level. The cultures were maintained in this “rafting” fashion for 14 days and were harvested by fixing in 10% buffered formalin. Subsequently, hematoxylin–eosin (H&E) staining was performed on thick (6 μm) sagittal sections of each reconstructs to reveal the histological features. Sample processing, paraffin-embedding, sectioning, and H&E staining were performed at UCLA’s Translational Pathology Core Laboratory (TPCL).
2.5. In vivo xenograft tumor assay
Four million cells were subcutaneously injected into the flank of immunocompromised mice (strain nu/nu, Charles River Laboratories). The animal study was performed according to the protocol approved by UCLA Animal Research Committee. The kinetics of tumor growth was determined by measuring the volume in three perpendicular axes of the nodules using micro-scaled calipers.
2.6. Tumor sphere formation assay
Three thousand cells were grown in 3 ml of serum-free DMEM/F12 media supplemented with 1:50 B27 (Invitrogen), 20 ng/ mL EGF, 20 ng/ mL, 10 μg/ mL insulin, penicillin, streptomycin, and amphotericin B in Ultra-Low Attachment 6-well Plates (Corning) for 6–10 days. The assay was performed in triplicate and the number of tumor spheres that formed were observed and counted under a microscope.
2.7. Quantitative real-time PCR (qPCR)
cDNA was synthesized from 5 μg of total RNA using SuperScript first-strand synthesis system (Invitrogen). We used 1 μl cDNA for qPCR amplification with SYBR Green I Master mix (Roche) and LightCycler 480 II (Roche). The primer sequences were obtained from the Universal Probe Library (Roche), which are available upon request. Second derivative Cq value determination method was used to compare fold-differences according to the manufacturer’s instructions.
2.8. ALDH1 assay
We used Aldehyde Dehydrogenase-Based Cell Detction Kit (STEMCELL) to determine ALDH1 enzymatic activity. A total of 1×106 cells were re-suspended in 1 mL of ALDEFLUOR Assay Buffer. Fluorescent nontoxic ALDEFLUOR Reagent BODIPYTM (1.25 µl) was added as a substrate to measure ALDH1 enzymatic activity in intact cells. Immediately after adding the substrate reagent, 0.5 ml of the cell suspension was transferred into the control tube which contained a specific inhibitor for ALDH1, diethylaminobenzaldehyde (DEAB) for calculating background fluorescence. Then, the cells were incubated at 35 °C for 30 min and fluorescence data acquisition was made by using a BD FACScan flow cytometer (BD Biosciences).
2.9. Migration and invasion assay
Cell migration was measured using transwell chambers with polycarbonate membranes (Corning) as described in our previous publication [32]. Cell invasion was measured using Matrigel Basement Membrane Matrix (BD Biosciences) according to the method as described in manufacture protocol.
2.10. Overexpression of miR-181
10μM of pre-miR-181a and 181d (Ambion) was used individually or combined together with Lipofectamin 2000 (Invitrogen). Initially 8x105 cells were seeded on 60 mm dish for 6 h prior to transfection. Transfecting solution was prepared by mixing the 10 μl of Lipofectamin 2000 with 20 μl of siRNA and 470 μl of culture medium. After 5 min of incubation at room temperature, the transfecting solution was added to the cell for overnight incubation at 37 °C. For the negative control, we used 5nM of negative control #1 pre-miR (Ambion).
2.11. qPCR analysis of miR-181
Total RNA was extracted from cell cultures using Trizol Reagent (Invitrogen). A cDNA pool of miRNAs was synthesized by QuantiMir cDNA Kit (System Biosciences) according to the manufacturer's protocol. Next, miRNAs were amplified using the SYBR Green I Master PCR Mix (Roche) with the LightCycler 480 II real time PCR system (Roche). For miR-181a amplification, one primer is mature miR-181a specific (5′-aacattcaacgctgtcggtgagt-3′) and the other is the company-provided universal primer. U6 small nuclear RNA (snRNA) level was used as an internal control for the starting amount of cDNA.
2.12. Luciferase report assays
The pGL3-615 plasmid containing a minimal regulatory region of miR-181a’s promoter (−615 to +1) was used in this study [33]. Transfection and luciferase assay were carried out as described in previous publications. For normalization of transfection efficiency, the cells were also co-transfected with pRL-SV40 (Promega) containing the renilla luciferase gene under SV40 promoter.
2.13. Knockdown of K-ras and ALDH1 using siRNA
K-ras and ALDH1 were knocked down with duplex siRNA targeting K-ras (Santa Cruz Biotech) and ALDH1 (Santa Cruz Biotech), respectively. The cells were also transfected with scrambled siRNA (Santa Cruz Biotech) as the control. Transfection was carried out using Lipofectamine RNAiMAX (Invitrogen). OSCC cells (2×105) were plated in 60-mm dishes and transfected with 15 μg siRNA. Finally, the cultures were harvested after two days post-transfection for expression and functional analyses.
2.14. Western blotting
Western blotting was performed as previously described [32]. We used the following primary antibodies for this study: K-ras (C-19; Santa Cruz Biotech) and GAPDH (Santa Cruz Biotech).
3. Results
3.1. HPV16 enhances tumor growth of HPV-negative OSCC cells both in vitro and in vivo
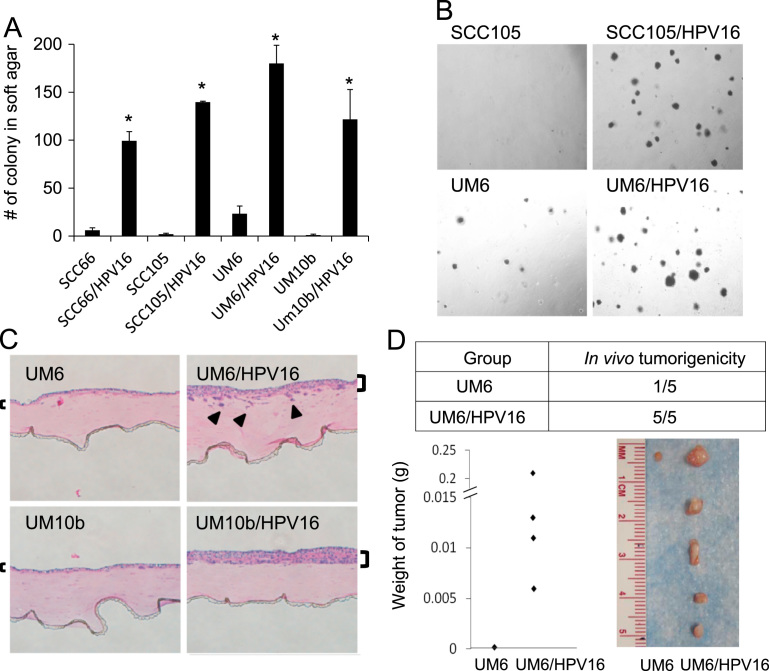
To investigate whether high-risk HPV can further promote the malignant phenotype of HPV-negative OSCC, we first transfected four HPV-negative OSCC cell lines (SCC66, SCC105, UM6, UM10b) with HPV16 whole genome. All of the HPV16-transfected OSCC cell lines expressed HPV16 E6 and E7 (data not shown). Then, we examined the effect of HPV16 on the anchorage independent growth ability of HPV-negative OSCC by performing soft agar assays. All HPV16-transfected OSCC cell lines showed robust induction of anchorage independent growth compared to their empty vector-transfected controls (Fig. 1A and B). We also employed an in vivo-like 3-demensional organotypic cell culture system where we could reconstitute squamous epithelium in vitro [32], [33]. Organotypic cultures of the HPV16-transfected cells showed malignant histomorphology with invasive characteristics into the subepithelial layer and formed thicker squamous epithelium than those of their control cells (Fig. 1C). To extend these observations, we investigated the effect of HPV16 on in vivo tumorigenicity of HPV-negative OSCC cells by performing xenograft tumor assay in nude mice. The HPV16-transfected cells (UM6/HPV16) exhibited robust increase in xenograft tumor growth and tumor forming ability compared to their control cells (UM6) (Fig. 1D). These findings collectively indicate that high-risk HPV16 further enhances tumorigenicity of HPV-negative OSCC.
Fig. 1.
HPV16 increases tumor growth of HPV-negative OSCC both in vitro and in vivo. Four HPV-negative OSCC cell lines (SCC66, SCC105, UM6, and UM10b) were transfected with pMHPV-16d containing a head-to-tail dimer of HPV-16 whole genome or with pdMMTneo, an empty vector as the control. Stable transfected cells were selected in culture medium containing 200 µg/ml of G418, then subcultured and used for experiments. (A) Effect of HPV16 on anchorage independent growth of HPV-negative OSCC was determined by soft agar assay. Data are means±SD of triplicate experiments. ⁎P<0.001 by two-tailed Student's t test. (B) Representative images of colonies formed by HPV16-transfected OSCC cell lines (SCC105/HPV16 and UM6/HPV16) and their empty vector-transfected controls (SCC105 and UM6). The photographs were taken at a magnification of 40×. (C) Organotypic raft cultures were established with HPV16-transfected OSCC cell lines (UM6/HPV16 and UM10b/HPV16) and their controls (UM6 and UM10b). Brackets indicate epithelial thickness, while arrows indicate infiltrating islands of cells through the basement membrane. (D) Effect of HPV16 on in vivo tumorigenicity of HPV-negative OSCC was determined by xenograft tumor assay. UM6 and UM6/HPV16 were injected subcutaneously into five nude mice. Mice were killed at week 5, and tumors were surgically removed from all animals, weighted, and photographed.
3.2. HPV16 promotes self-renewal capacity and CSC properties of HPV-negative OSCC cells
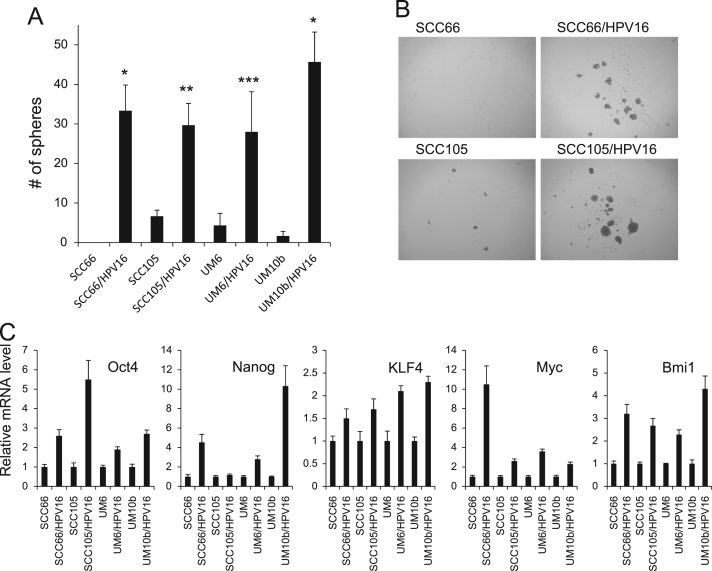
A key feature of CSCs is self-renewal capacity, which appears to be a driving force for the initiation and maintenance of tumorigenicity [12]. CSCs have been previously isolated from HPV-positive SCC [34]. To determine the effect of high-risk HPV on self-renewal capacity of HPV-negative OSCC, we employed tumor sphere formation assay in which CSCs can be enriched in non-adherent tumor spheres [32]. Abundance and the growth kinetics of tumor spheres are indicative of CSC content and self-renewal capacity in a given culture of heterogeneous cancer cells. All HPV16-transfected OSCC cell lines displayed robust increase in tumor sphere-forming ability compared to their empty vector-transfected controls (Fig. 2A and B). The effect of HPV16 on self-renewal was further confirmed by evaluating the expression of stemness transcription factors (Fig. 2C). All HPV16-transfected OSCC cell lines consistently exhibited higher gene expression levels of Oct4, Nanog, KLF4, c-Myc, and Bmi1 than the empty vector controls. Our findings indicate that HPV16 promotes self-renewal capacity of HPV-negative OSCC.
Fig. 2.
HPV16 promotes self-renewal capacity of HPV-negative OSCC. (A) Effect of HPV16 on self-renewal capacity of HPV-negative OSCC was determined by tumor sphere formation assay. Data are means±SD of triplicate experiments. ⁎P<0.001, ⁎⁎P<0.01, and ⁎⁎⁎P<0.05 by two-tailed Student's t test. (B) Representative images of tumor spheres formed by HPV16-transfected OSCC cell lines (SCC66/HPV16 and SCC105/HPV16) and their corresponding controls (SCC66 and SCC105). The photographs were taken at a magnification of 40×. (C) Effect of HPV16 on stemness transcription factor expression in HPV-negative OSCC was determined by real-time qPCR. Levels of the factors were normalized with the level of GAPDH. Their levels in HPV16-transfected OSCC cell lines were plotted as fold induction against those in their corresponding controls.
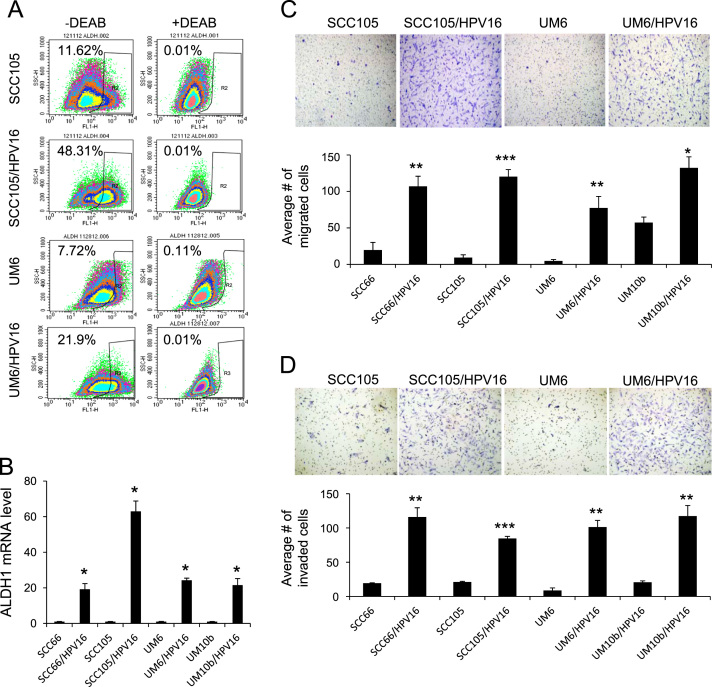
Next, we further investigated the effect of HPV16 on CSC properties in HPV-negative OSCC. Aldehyde dehydrogenase 1 (ALDH1) activity is an important CSC marker and is enriched in CSCs of head and neck cancer [34]. The flow cytometric analysis revealed a significant increase in ALDH1+ (or ALDH1 high) cell population in the HPV16-transfected cells compared to their corresponding controls (48.13% versus 11.62% and 21.9% versus 7.72%; Fig. 3A). We also observed robust induction of ALDH1 mRNA in the HPV16-transfected cells (Fig. 3B). Because another well-known property of CSCs is their metastatic potential [12], we examined the effect of HPV16 on migration and invasion of HPV-negative OSCC in vitro. As demonstrated by a transwell migration assay (Fig. 3C), all HPV16-transfected OSCC cell lines migrated significantly faster than their corresponding controls. We also found a significant increase in invasion in all HPV16-transfected OSCC cell lines as shown by Matrigel invasion assay (Fig. 3D). Overall, our data indicate that high-risk HPV16 enriches CSC population and properties in HPV-negative OSCC.
Fig. 3.
HPV16 promotes CSC properties of HPV-negative OSCC. (A) Effect of HPV16 on ALDH1 activity in HPV-negative OSCC was determined by Aldefluor assay. Cells were labeled with Aldefluor combined with or without the ALDH1 inhibitor DEAB and analyzed by flow cytometry. The gate for ALDH1 + cells is determined in relation to the DEAB control (+DEAB) and shows the brightly fluorescent ALDH1 population versus the side scatter, a population that is absent/decreased in the presence of DEAB. The number shown in each panel reflects the percentage of ALDH1+ cells in each cell type. (B) Effect of HPV16 on ALDH1 mRNA was determined by real-time qPCR. ALDH1 levels in HPV16-transfected OSCC cell lines were plotted as fold induction against those in their corresponding controls. ⁎P<0.001 by two-tailed Student’s t test. (C) Effect of HPV16 on migration ability in HPV-negative OSCC was determined by transwell migration assay. Migration ability was described as number of migrated cells per field with data as mean±SD for 3 randomly selected fields. ⁎P<0.05, ⁎⁎P<0.01, and ⁎⁎⁎P<0.001 by two-tailed Student's t test. Representative images of transwell migration assay are shown above the graph. (D) Effect of HPV16 on invasion ability of HPV-negative OSCC was determined by Matrigel invasion assay. Invasion ability was described as the number of invaded cells per field with the data as mean±SD for 3 randomly selected fields. ⁎⁎P<0.01 and ⁎⁎⁎P<0.001 by two-tailed Student’s t test. Representative images of Matrigel invasion assay are shown above the graph.
3.3. HPV16 increases anchorage independent growth and CSC phenotype of HPV-negative OSCC cells by suppressing miR-181a and miR-181d
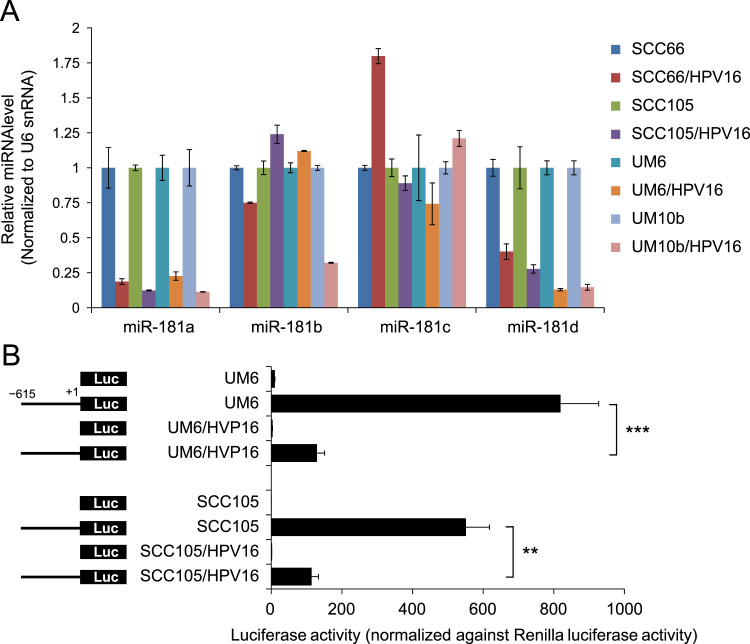
Recent studies clearly demonstrated that HPV status affect the miRNA expression pattern in human SCC [28], [29]. To understand the role of miRNAs that are specific for high-risk HPV16, we compared global miRNA expression profiles of HPV16-transfected OSCC cells (SCC105/HPV16 and UM10b/HPV16) with their empty vector controls (SCC105 and UM10b) using miRCURY LNATM miRNA Array (Exiqon). We identified 34 miRNAs that were altered greater than 2-fold, p<0.05 by HPV16 in both cell lines (data not shown). Among them, 15 miRNAs were downregulated and 19 miRNAs were upregulated by HPV16. We then compared the expression of these 34 miRNAs in the four HPV16-transfected cell lines versus their corresponding controls (data not shown). Two miR-181 family members, miR-181a and miR-181d, were consistently downregulated in all of the HPV16-transfected cell lines (Fig. 4A). However other miR-181 members, miR-181b and miR-181c, were not consistently altered by HPV16 (Fig. 4A). To further understand the mechanism of miR-181 downregulation by HPV16, we utilized the miR-181a promoter construct, whose luciferase activity is driven by the 615-bp minimal promoter sequence of miR-181a [33]. When transfected with the miR-181a promoter constructs, the HPV16-positive cells showed markedly lower luciferase activity compared to their corresponding control cells, indicating that miR-181a is transcriptionally repressed by HPV16 (Fig. 4B).
Fig. 4.
HPV16 downregulates the expression of miR-181a and miR-181d by inhibiting miR-181 promoter activity. (A) The levels of miR-181 family members (miR-181a, miR-181b, miR-181c, and miR-181d) in the HPV16-transfected cell lines and their controls were measured by real-time qPCR and normalized to U6 snRNA. (B) Effect of HPV16 on miR-181a promoter activity was determined by luciferase promoter assay. Cells were transfected with pGL3-Basic (promoter-less) or pGL3 vectors containing the minimal miR-181a promoter region (-615 to +1). The cells were also cotransfected with pRL-SV40 to monitor the transfection efficiency. ⁎⁎P < 0.01 and ⁎⁎⁎P < 0.001 by two-tailed Student’s t test.
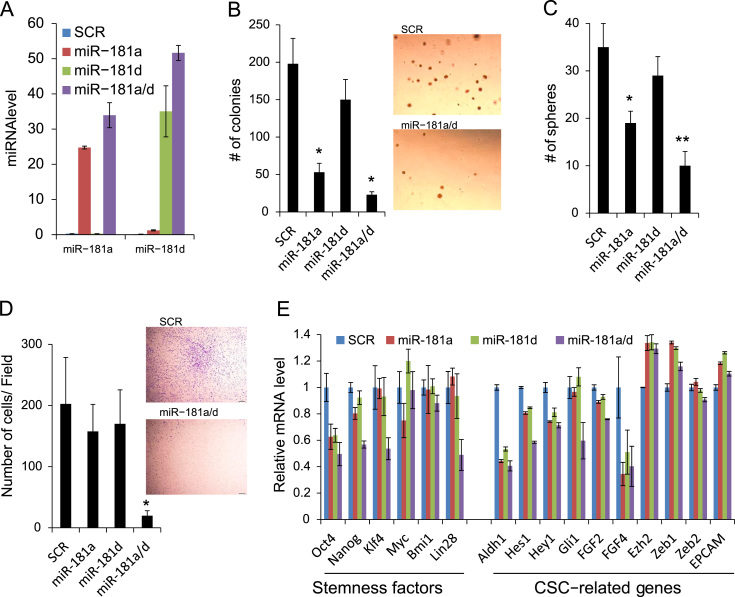
Having determined that miR-181a and miR-181d are transcriptional targets of HPV16, we next sought to demonstrate the HPV16’s enhancing capacity of tumor growth and CSC phenotype by repressing miR-181. We overexpressed miR-181a and miR-181d in the HPV16-transfected cells (UM6/HPV16) by transfecting with pre-miR-181a, pre-miR-181d or both (Fig. 5A). These transfected pre-miRNAs do not contain their natural promoters; therefore, these miRNAs were expected to reconstitute their expressions in the presence of HPV. Consistent with our previous finding [33], overexpression of miR-181a and miR-181d suppressed anchorage independent growth ability of the HPV16-transfected cells (Fig. 5B). miR-181a and miR-181d also decreased the tumor sphere-forming ability of UM6/HPV16 (Fig. 5C). Interestingly, the combination of miR-181a and miR-181d (miR-181a/d) showed the most potent inhibitory effect on anchorage independent growth and self-renewal capacity of the cells. We also found the strongest inhibitory effect of miR-181a/d on the migratory ability of UM6/HPV16 (Fig. 5D). The inhibitory effect of miR-181a/d on CSC phenotype was further validated by qPCR analysis of stemness transcription factors and CSC-related genes (Fig. 5E). We observed that miR181a/d suppressed stemness transcription factors (i. e., Oct4, Nanog, KLF4, Lin28) and CSC-related genes (i.e., ALDH1, Hes1, Hey1, Gli1, FGF4) in UM6/HPV16. However, miR-18a/d did not alter viral gene expression such as E6 and E7 (data not shown). Our findings indicate that HPV16 enhances tumor growth and CSC phenotype of HPV-negative OSCC cells by transcriptionally suppressing miR-181a/d.
Fig. 5.
Ectopic expression of miR-181a/d suppresses anchorage independent growth and CSC phenotype in HPV16-transfected OSCC. (A) Real-time qPCR analysis of ectopic overexpression of miR-181a and miR-181d in UM6/HPV16 transfected with pre-miR-miR-181a (miR-181a), pre-miR-181d (miR-181d), or both (miR-181a/d). Scramble oligonucleotides (SCR) were used as a control transfection. Levels of miRNAs were measured 3 day posttransfection and normalized to that of U6 snRNA. (B) Anchorage independent growth assay. ⁎Significantly different (P<0.01, Student's t test) from the SCR-transfected cells. Representative images of colonies formed by UM6/HPV16 transfected with miR-181a/d and SCR are shown on the right side. (C) Tumor sphere formation assay. Significantly different (⁎P<0.05 and ⁎⁎P<0.01, Student's t test) from the SCR-transfected cells. (D) Transwell migration assay. ⁎Significantly different (P<0.01, Student's t test) from other transfected cells. Representative images of transwell migration assay from UM6/HPV16 transfected with miR-181a/d and SCR are shown on the right side. (E) Effects of miR-181a and miR-181d on stemness transcription factors and CSC-related genes in UM6/HPV16 were examined by real-time qPCR.
3.4. Silencing of miR-181a/d target genes abrogates the effect of HPV16 in HPV16-transfected OSCC cells
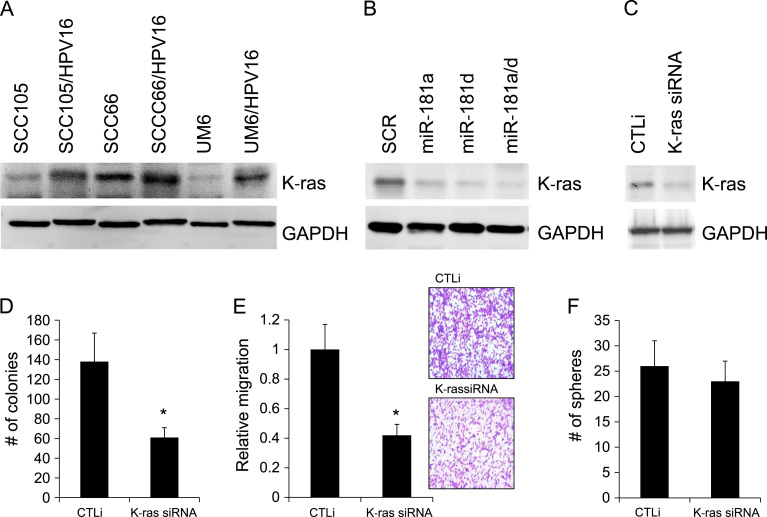
Previously, we reported that miR-181a elicits tumor suppressive activity against OSCC by downregulating K-ras [33]. miR-181a inhibits K-ras translation by regulating the 3′-UTR of K-ras gene [33]. miR-181d also acts as tumor suppressor by targeting K-ras [35]. To investigate whether HPV16/miR-181a/d axis enhances tumor growth and CSC phenotype by regulating target gene(s) of miR-181a/d, we first examined the role of K-ras. We confirmed that K-ras was increased in the HPV16-transfected OSCC cell lines (Fig. 6A) and then tested to see if increased K-ras is attributable to the downregulation of miR-181a and miR-181d in the HPV16-transfected cells. Overexpression of miR-181a and miR-181d notably decreased the level of K-ras expression in UM6/HPV16, indicating that K-ras was indeed increased by HPV16 via the downregulation of miR-181a and miR-181d (Fig. 6B). Furthermore, we examined if knockdown of K-ras would abrogate the effects of HPV16 on UM6/HPV16. We successfully knocked down K-ras in UM6/HPV16 using siRNA (Fig. 6C). K-ras siRNA significantly inhibited anchorage independent growth and migration ability of UM6/HPV16 (Fig. 6D and E). However, knockdown of K-ras marginally suppressed tumor sphere-forming ability of the cells (Fig. 6F), suggesting that multiple targets of miR-181a/d may be responsible for the HPV16-induced CSC phenotype in OSCC cells.
Fig. 6.
Silencing of K-ras, a miR-181a/d target, inhibits anchorage independent growth and migration ability in HPV16-transfected OSCC cells. (A) Decreased expression of K-ras in HPV16-tranfected OSCC cell lines was determined by Western blot analysis. GAPDH was used as controls. (B) Ectopic expression of miR-181a, miR-181d, and miR-181a/d decreased K-ras in UM6/HPV16 as determined by Western blot analysis. (C) Endogenous K-ras in UM6/HPV16 was knocked down using siRNA against K-ras (K-ras siRNA). The cells transfected with control siRNA (CTLi) were included for comparison. (D) Effect of K-ras knockdown on anchorage independent growth in UM6/HPV16 was determined by soft agar assay. ⁎P<0.05 by two-tailed Student’s t test. (E) Effect of K-ras knockdown on migration ability of UM6/HPV16 was determined by transwell migration assay. ⁎P<0.05 by two-tailed Student’s t test. Representative images of transwell migration assay from UM6/HPV16 transfected with CTLi and K-ras siRNA are shown on the right side. (F) Effect of K-ras knockdown on self-renewal capacity of UM6/HPV16 was determined by tumor sphere formation assay.
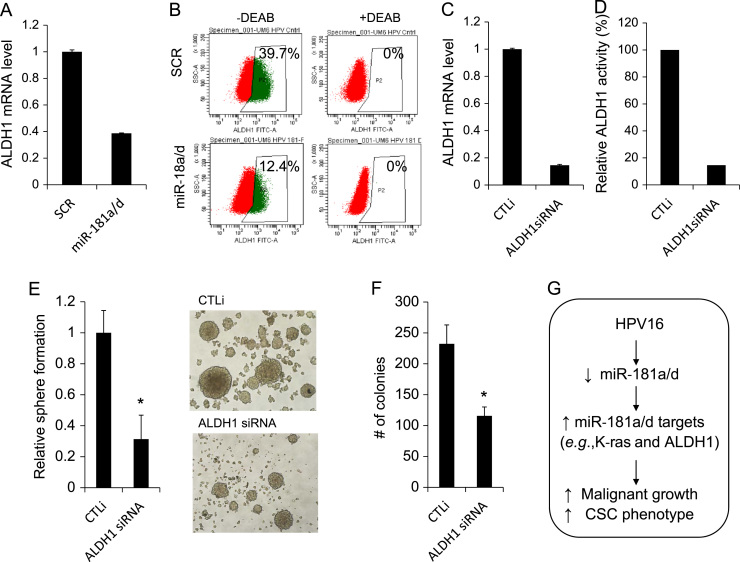
To test this possibility, we sought to identify other target(s) of miR-181a/d involved in the HPV16-induced CSC phenotype. Increased ALDH1 by HPV16 (Fig. 3A and B) and decreased ALDH1 by miR-181a/d in the HPV16-transfected cells (Fig. 5E) suggest a possible role of ALDH1 in the CSC regulation by the HPV16/miR-181 axis. Indeed, we demonstrated that miR-181a/d suppressed both ALDH1 mRNA (Fig. 7A) and ALDH1+ cell population (Fig. 7B) in UM6/HPV16 cells, indicating that ALDH1 is a downstream target of HPV16/miR181a/d axis. To investigate the functional role of ALDH1 in the CSC regulation by the HPV16/miR-181 axis, we knocked down ALDH1 in UM6/HPV16 cells using siRNA (Fig. 7C and D). ALDH1 siRNA significantly suppressed self-renewal capacity, as demonstrated by tumor sphere formation assay (Fig. 7E). Knockdown of ALDH1 also inhibited anchorage independent growth ability of UM6/HPV16 (Fig. 7F). These findings signify the importance of ALDH1 in the CSC phenotype increased by the HPV16/miR-181 axis. Taken together, our findings indicate the functional role of miR-181a/d target genes under the HPV16/miR-181a/d axis in further malignant progression of HPV-negative OSCC by high-risk HPV16.
Fig. 7.
ALDH1 is a downstream target of miR-181a/d, and its knockdown inhibits self-renewal and anchorage independent growth of HPV16-transfected OSCC cells. (A) Effect of miR-181a/d on ALDH1 mRNA in UM6/HPV16 was determined by miR-18a/d transfection followed by real-time qPCR. (B) Effect of miR-181a/d on ALDH1 activity in UM6/HPV16 was determined by Aldefluor assay. The number shown in each panel reflects the percentage of ALDH1+ cells in each cell type. (C) Endogenous ALDH1 in UM6/HPV16 was knocked down using siRNA against ALDH1 (ALDH1 siRNA). Decreased ALDH1 mRNA was determined by real-time qPCR. (D) Decreased ALDH1 activity was determined by Aldefluor assay. Relative ALDH1 activity was obtained from ALDH1+ cell population. Percentage of ALDH1+ cells in the CTLi-transfected cells is 100%. (E) Effect of ALDH1 knockdown on self-renewal capacity of UM6/HPV16 was determined by tumor sphere formation assay. ⁎P<0.05, Student's t test. Representative images of tumor spheres formed by UM6/HPV16 transfected with CTLi and ALDH1 siRNA were shown on the right side. (F) Effect of ALDH1 knockdown on anchorage independent growth of UM6/HPV16 was determined by soft agar assay. ⁎P<0.05, Student's t test. (G) Schema depicting HPV16-induced suppression of miR-181a/d, thereby induction of miR-181a/d targets and promotion of malignant growth and CSC phenotype in HPV-negative OSCC.
4. Discussion
Here, we demonstrated for the first time that high-risk HPV16 enhances tumor growth and cancer stemness of HPV-negative OSCC via miR-181a/d regulation. HPV16 represses miR-181 by inhibiting its promoter activity. We also provide the evidence that miR-181a/d regulate tumor growth and CSC phenotype by regulating their multiple target genes in HPV16-positive OSCC cells, indicating the importance of miR-181/miR-181 targets axis in HPV-mediated oral carcinogenesis (Fig. 7G). Thus, our study highlights the important role of high-risk HPV in malignant progression of HPV-negative OSCC by enriching cancer stemness via epigenetic regulators, miRNAs.
Previously, we have studied the role of HPV in oral carcinogenesis, particularly focusing on the mechanisms of the transforming capacity of high-risk HPVs (i.e., HPV16 and HPV18) in normal human oral keratinocytes (NHOKs). We reported successful immortalization of NHOK by transfection with high-risk HPV whole genome, but failed to yield neoplastic conversion of the normal cells [31], [36]. This suggested that HPV infection plays an important role in the early stage of oral carcinogenesis. However, the possible role of HPV in malignant progression of already transformed cells, i.e., HPV-negative OSCC has not been well documented. Our study clearly demonstrated that high-risk HPV16 further promotes malignant growth of HPV-negative OSCC cells in vivo and in vitro. This finding is consistent with the results of a previous study reporting that HPV16 induced significant increases in proliferation of an HPV-negative OSCC line in vitro [37]. Surprisingly, we found that HPV16 led to robust increase in self-renewal capacity and stemness transcription factors in multiple HPV-negative OSCC cell lines. Our findings suggest that HPV16 further promotes malignant progression of HPV-negative OSCC by increasing self-renewal capacity, a key feature of CSCs.
Furthermore, we demonstrate that HPV16 promotes other CSC properties, ALDH1 activity and in vitro metastatic potential. HPV16 markedly increases ALDH1+ cell population in multiple HPV-negative OSCC cell lines. ALDH1 has been found to be a marker for CSCs in different types of cancer, including OSCC [38], [39]. ALDH1+ cancer cells displayed high self-renewal, migration, and in vivo tumor-forming capacity compared to ALDH1- cells [38], [39]. HPV16 also increases motility and invasion ability of HPV-negative OSCC cells. Our findings are consistent with previous observations that high-risk HPV oncoprotein promoted migration of cervical cancer cells [38], [39], [40]. However, the underlying mechanism by which HPV16 regulates OSCC migration is not understood, warranting further investigations on the effects of HPV16 on epithelial-to-mesenchymal transition (EMT) and metastasis-related gene expression [41], [42]. Thus, we hypothesize that high-risk HPV infection further promotes malignant progression of HPV-negative OSCC by enriching CSC phenotype. Our study further suggests that HPV infection plays a pivotal role during any stage of cancer development, supporting the potential benefit of HPV vaccination inolder adults.
miRNAs are epigenetic regulators of gene expression and their deregulation is closely linked to cancer initiation and progression. Recent studies clearly demonstrated that HPV status affect the miRNA expression pattern in human SCC [29], [31], [43]. A number of cellular miRNAs have been found to be modulated by HPV proteins and to play important roles in HPV-mediated carcinogenesis [39]. A recent study reported that miR-181 family members are the most downregulated miRNAs in HPV16-positive compared to HPV-negative HNSCC cell line [29]. Consistently, our study showed that HPV16 suppresses miR-181 expression by inhibiting its promoter activity, indicating for the first time that miR-181a and miR-181d are novel transcription targets of HPV16. Several biomarkers have been identified and used for diagnosing HPV-associated cancers, such as p16INK4a, TOP2A, and surviving [44]. Among these putative biomarkers, p16INK4a is shown to be a reliable surrogate marker for the presence of HPV [45]. Although p16INK4a is a known tumor suppressor, its expression is believed to be high in HPV-associated cancers due to E7-mediated inactivation of Rb, a negative regulator of p16INK4a [45]. However, its sensitivity is still questionable because HPV-negative cancer patients have been shown to express high level of p16INK4a [46]. Therefore, miR-181a and miR-181d could be used as potential diagnostic biomarkers, either alone or together, for HPV-associated oral cancer. It is important to note that high-risk HPV E6 inactivates p53 tumor suppressor, which is known to function as a transcription factor in regulating miRNAs [47]. However, we are able to rule out the possible involvement of p53 in the regulation of miR-181a/d because HPV16 suppresses the expressions of miR-181a/d in oral keratinocytes containing wild-type p53 (data not shown) and the OSCC cell lines containing defective p53 gene [30].
Previously, we reported that miR-181a elicits tumor suppressive activity against OSCC by downregulating K-ras [33]. miR-181d also acts as tumor suppressor by targeting K-ras [35], indicating that K-ras is a common target of miR-181a and miR-181d. Activation of K-ras is implicated in the pathogenesis of oral cancer [48] and CSC in mammalian cancer [49], [50]. These results suggest that miR-181a/d could be important epigenetic regulators for CSC phenotype via their target gene(s). In our study, overexpression (restoration) of miR-181a/d suppressed various CSC properties increased by HPV16, indicating their CSC-suppressive role in HPV16-positive OSCC. Furthermore, K-ras knockdown resulted in suppression of migration and anchorage independent growth, but not self-renewal capacity of HPV16-positive OSCC. Because of the redundant function of miRNA and multiple targets of miR-181a/d, it is plausible that multiple targets of miR-181a/d in the HPV16/miR-181 axis may cooperatively contribute to CSC phenotype. Indeed, we found that ALDH1 is a novel downstream target of miR-181a/d; it was suppressed by miR-181a/d in HPV16-positive OSCC. Moreover, knockdown of ALDH1 in HPV16-positive OSCC suppressed self-renewal and anchorage independent growth ability, supporting the functional role of ALDH1 in HPV-mediated oral carcinogenesis. We hypothesize that the HPV16/miR-181 axis regulates tumor growth and CSC phenotype via multiple targets of miR-181. Our data also suggest that miR-181a/d may be novel CSC inhibitors and targeting miR-181a/d may represent a novel therapeutic approach to suppress CSCs of OSCC.
In conclusion, high-risk HPV directs malignant progression of OSCC by enhancing stemness properties of OSCC. The HPV-induced phenotypes are epigenetically mediated by miRNAs. Our results identified novel HPV-regulated miRNAs, i.e., miR-181a/d, whose expression and promoter activity are suppressed by HPV16. Furthermore, we showed that K-ras and ALDH1, targets of miR-181a/d, are important players in driving cancer stemness in HPV-positive OSCC. Thus, our study supports the role of HPV in oral cancer progression in the context of epigenetic regulation of CSCs. Moreover, our finding suggests that HPV may have multiple roles in various stages of oral carcinogenesis; inducing immortalization of normal oral epithelial cells and promoting malignant progression of existing OSCC through enriching cancer stemness via the miR-181/miR181 targets axis. Future studies should investigate the correlation between HPV status and miR-181a/d expression, and the biological outcome of low miR-181a/d expression in vivo.
Funding
This work was supported in part by the Grant UCLA School of Dentistry faculty seed Grant (44190169749SHIN to K.H.S.), the Grant (R01DE18295 to M.K.K.), the Grant (R01DE023348 to R.H.K.) from NIDCR/NIH and the Grant from UCLA Chancellor’s Office (to N.H.P.).
Disclosure
All authors state that they have no conflicts of interest.
References
- 1.Al-Swiahb J.N., Chen C.H., Chuang H.C., Fang F.M., Tasi H.T., Chien C.Y. Clinical, pathological and molecular determinants in squamous cell carcinoma of the oral cavity. Futur Oncol. 2010;6:837–850. doi: 10.2217/fon.10.35. [DOI] [PubMed] [Google Scholar]
- 2.Lee C.H., Lee J.M., Wu D.C., Hsu H.K., Kao E.L., Huang H.L., Wang T.N., Huang M.C., Wu M.T. Independent and combined effects of alcohol intake, tobacco smoking and betel quid chewing on the risk of esophageal cancer in Taiwan. Int. J. Cancer. 2005;113:475–482. doi: 10.1002/ijc.20619. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Sayans M., Somoza-Martin J.M., Barros-Angueira F., Reboiras-Lopez M.D., Gandara Rey J.M., Garcia-Garcia A. Genetic and molecular alterations associated with oral squamous cell cancer (review) Oncol. Rep. 2009;22:1277–1282. doi: 10.3892/or_00000565. [DOI] [PubMed] [Google Scholar]
- 4.Shiboski C.H., Schmidt B.L., Jordan R.C.K. Tongue and tonsil carcinoma – increasing trends in the US population ages 20-44 years. Cancer. 2005;103:1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi A.K., Engels E.A., Pfeiffer R.M., Hernandez B.Y., Xiao W.H., Kim E., Jiang B., Goodman M.T., Sibug-Saber M., Cozen W., Liu L.H., Lynch C.F., Wentzensen N., Jordan R.C., Altekruse S., Anderson W.F., Rosenberg P.S., Gillison M.L. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iype E.M., Pandey M., Mathew A., Thomas G., Sebastian P., Nair M.K. Squamous cell carcinoma of the tongue among young Indian adults. Neoplasia. 2001;3:273–277. doi: 10.1038/sj.neo.7900172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherin N., Simi T., Shameena P., Sudha S. Changing trends in oral cancer. Indian J. Cancer. 2008;45:93–96. doi: 10.4103/0019-509x.44063. [DOI] [PubMed] [Google Scholar]
- 8.Smith E.M., Ritchie J.M., Summersgill K.F., Hoffman H.T., Wang D.H., Haugen T.H., Turek L.P. Human papillomavirus in oral exfoliated cells and risk of head and neck cancer. J. Natl. Cancer Inst. 2004;96:449–455. doi: 10.1093/jnci/djh074. [DOI] [PubMed] [Google Scholar]
- 9.Hansson B.G., Rosenquist K., Antonsson A., Wennerberg J., Schildt E.B., Bladstrom A., Andersson G. Strong association between infection with human papillomavirus and oral and oropharyngeal squamous cell carcinoma: a population-based case-control study in southern Sweden. Acta Oto-Laryngol. 2005;125:1337–1344. doi: 10.1080/00016480510043945. [DOI] [PubMed] [Google Scholar]
- 10.Kellokoski J.K., Syrjanen S.M., Chang F., Yliskoski M., Syrjanen K.J. Southern blot hybridization and PCR in detection of oral human papillomavirus (HPV) infections in women with genital HPV Infections. J. Oral. Pathol. Med. 1992;21:459–464. doi: 10.1111/j.1600-0714.1992.tb00975.x. [DOI] [PubMed] [Google Scholar]
- 11.Syrjanen S. Viral-infections in oral-mucosa. Scand. J. Dent. Res. 1992;100:17–31. doi: 10.1111/j.1600-0722.1992.tb01805.x. [DOI] [PubMed] [Google Scholar]
- 12.Beck B., Blanpain C. Unravelling cancer stem cell potential. Nat. Rev. Cancer. 2013;13:727–738. doi: 10.1038/nrc3597. [DOI] [PubMed] [Google Scholar]
- 13.Chiou S.H., Yu C.C., Huang C.Y., Lin S.C., Liu C.J., Tsai T.H., Chou S.H., Chien C.S., Ku H.H., Lo J.F. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin. Cancer Res. 2008;14:4085–4095. doi: 10.1158/1078-0432.CCR-07-4404. [DOI] [PubMed] [Google Scholar]
- 14.Ginestier C., Hur M.H., Charafe-Jauffret E., Monville F., Dutcher J., Brown M., Jacquemier J., Viens P., Kleer C.G., Liu S.L., Schott A., Hayes D., Birnbaum D., Wicha M.S., Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu S.L., Dontu G., Mantle I.D., Patel S., Ahn N.S., Jackson K.W., Suri P., Wicha M.S. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giles R.H., van Es J.H., Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochem. Biophys. Acta-Rev. Cancer. 1653;2003:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 17.Evangelista M., Tian H., de Sauvage F.J. The hedgehog signaling pathway in cancer. Clin. Cancer Res. 2006;12:5924–5928. doi: 10.1158/1078-0432.CCR-06-1736. [DOI] [PubMed] [Google Scholar]
- 18.Leong K.G., Karsan A. Recent insights into the role of Notch signaling in tumorigenesis. Blood. 2006;107:2223–2233. doi: 10.1182/blood-2005-08-3329. [DOI] [PubMed] [Google Scholar]
- 19.Liu C., Kelnar K., Liu B.G., Chen X., Calhoun-Davis T., Li H.W., Patrawala L., Yan H., Jeter C., Honorio S., Wiggins J.F., Bader A.G., Fagin R., Brown D., Tang D.A.G. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat. Med. 2011;17 doi: 10.1038/nm.2284. 211-U105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song S.J., Poliseno L., Song M.S., Ala U., Webster K., Ng C., Beringer G., Brikbak N.J., Yuan X., Cantley L.C., Richardson A.L., Pandolfi P.P. MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell. 2013;154:311–324. doi: 10.1016/j.cell.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carthew R.W., Sontheimer E.J. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia H., Hui K.M. MicroRNAs involved in regulating epithelial–mesenchymal transition and cancer stem cells as molecular targets for cancer therapeutics. Cancer Gene Ther. 2012;19:723–730. doi: 10.1038/cgt.2012.58. [DOI] [PubMed] [Google Scholar]
- 23.Ke G., Liang L., Yang J.M., Huang X., Han D., Huang S., Zhao Y., Zha R., He X., Wu X. MiR-181a confers resistance of cervical cancer to radiation therapy through targeting the pro-apoptotic PRKCD gene. Oncogene. 2013;32:3019–3027. doi: 10.1038/onc.2012.323. [DOI] [PubMed] [Google Scholar]
- 24.Yu C.C., Chen Y.W., Chiou G.Y., Tsai L.L., Huang P.I., Chang C.Y., Tseng L.M., Chiou S.H., Yen S.H., Chou M.Y., Chu P.Y., Lo W.L. MicroRNA let-7a represses chemoresistance and tumourigenicity in head and neck cancer via stem-like properties ablation. Oral Oncol. 2011;47:202–210. doi: 10.1016/j.oraloncology.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Shi Y., Liu C., Liu X., Tang D.G., Wang J. The microRNA miR-34a inhibits non-small cell lung cancer (NSCLC) growth and the CD44hi stem-like NSCLC cells. PloS One. 2014;9:e90022. doi: 10.1371/journal.pone.0090022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimono Y., Zabala M., Cho R.W., Lobo N., Dalerba P., Qian D., Diehn M., Liu H., Panula S.P., Chiao E., Dirbas F.M., Somlo G., Pera R.A., Lao K., Clarke M.F. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wellner U., Schubert J., Burk U.C., Schmalhofer O., Zhu F., Sonntag A., Waldvogel B., Vannier C., Darling D., zur Hausen A., Brunton V.G., Morton J., Sansom O., Schuler J., Stemmler M.P., Herzberger C., Hopt U., Keck T., Brabletz S., Brabletz T. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 28.Martinez I., Gardiner A.S., Board K.F., Monzon F.A., Edwards R.P., Khan S.A. Human papillomavirus type 16 reduces the expression of microRNA-218 in cervical carcinoma cells. Oncogene. 2008;27:2575–2582. doi: 10.1038/sj.onc.1210919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lajer C.B., Garns E., Friis-Hansen L., Norrild B., Therkildsen M.H., Glud M., Rossing M., Lajer H., Svane D., Skotte L., Specht L., Buchwald C., Nielsen F.C. The role of miRNAs in human papilloma virus (HPV)-associated cancers: bridging between HPV-related head and neck cancer and cervical cancer. Br. J. Cancer. 2012;106:1526–1534. doi: 10.1038/bjc.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin C.J., Grandis J.R., Carey T.E., Gollin S.M., Whiteside T.L., Koch W.M., Ferris R.L., Lai S.Y. Head and neck squamous cell carcinoma cell lines: established models and rationale for selection. Head Neck-J. Sci. Spec. Head Neck. 2007;29:163–188. doi: 10.1002/hed.20478. [DOI] [PubMed] [Google Scholar]
- 31.Park N.H., Min B.M., Li S.L., Huang M.Z., Cherick H.M., Doniger J. Immortalization of normal human oral keratinocytes with type 16 human papillomavirus. Carcinogenesis. 1991;12:1627–1631. doi: 10.1093/carcin/12.9.1627. [DOI] [PubMed] [Google Scholar]
- 32.Lee S.H., Hong H.S., Liu Z.X., Kim R.H., Kang M.K., Park N.H., Shin K.H. TNFalpha enhances cancer stem cell-like phenotype via Notch-Hes1 activation in oral squamous cell carcinoma cells. Biochem. Biophys. Res. Commun. 2012;424:58–64. doi: 10.1016/j.bbrc.2012.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin K.H., Bae S.D., Hong H.S., Kim R.H., Kang M.K., Park N.H. miR-181a shows tumor suppressive effect against oral squamous cell carcinoma cells by downregulating K-ras. Biochem. Biophys. Res. Commun. 2011;404:896–902. doi: 10.1016/j.bbrc.2010.12.055. [DOI] [PubMed] [Google Scholar]
- 34.Zhang M., Kumar B., Piao L., Xie X., Schmitt A., Arradaza N., Cippola M., Old M., Agrawal A., Ozer E., Schuller D.E., Teknos T.N., Pan Q. Elevated intrinsic cancer stem cell population in human papillomavirus-associated head and neck squamous cell carcinoma. Cancer. 2014;120:992–1001. doi: 10.1002/cncr.28538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X.F., Shi Z.M., Wang X.R., Cao L., Wang Y.Y., Zhang J.X., Yin Y., Luo H., Kang C.S., Liu N., Jiang T., You Y.P. MiR-181d acts as a tumor suppressor in glioma by targeting K-ras and Bcl-2. J. Cancer Res. Clin. 2012;138:573–584. doi: 10.1007/s00432-011-1114-x. [DOI] [PubMed] [Google Scholar]
- 36.Shin K.H., Min B.M., Cherrick H.M., Park N.H. , Combined effects of human papillomavirus-18 and N-methyl-N'-nitro-N-nitrosoguanidine on the transformation of normal human oral keratinocytes. Mol. Carcinog. 1994;9:76–86. doi: 10.1002/mc.2940090205. [DOI] [PubMed] [Google Scholar]
- 37.Reddout N., Christensen T., Bunnell A., Jensen D., Johnson D., O'Malley S., Kingsley K. High risk HPV types 18 and 16 are potent modulators of oral squamous cell carcinoma phenotypes in vitro. Infect. Agents Cancer. 2007;2:21. doi: 10.1186/1750-9378-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang S., Pang T.Y., Gao M., Kang H.X., Ding W.B., Sun X.W., Zhao Y., Zhu W., Tang X.D., Yao Y.H., Hu X.R. HPV E6 induces eIF4E transcription to promote the proliferation and migration of cervical cancer. FEBS Lett. 2013;587:690–697. doi: 10.1016/j.febslet.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 39.Liao S.J., Deng D.R., Zhang W.N., Hu X.J., Wang W., Wang H., Lu Y.P., Wang S.X., Meng L., Ma D. Human papillomavirus 16/18 E5 promotes cervical cancer cell proliferation, migration and invasion in vitro and accelerates tumor growth in vivo. Oncol. Rep. 2013;29:95–102. doi: 10.3892/or.2012.2106. [DOI] [PubMed] [Google Scholar]
- 40.Yeung C.L.A., Tsang T.Y., Yau P.L., Kwok T.T. Human papillomavirus type 16 E6 induces cervical cancer cell migration through the p53/microRNA-23b/urokinase-type plasminogen activator pathway. Oncogene. 2011;30:2401–2410. doi: 10.1038/onc.2010.613. [DOI] [PubMed] [Google Scholar]
- 41.Ranieri D., Belleudi F., Magenta A., Torrisi M.R. , HPV16 E5 expression induces switching from FGFR2b to FGFR2c and epithelial-mesenchymal transition. Int. J. Cancer. 2015;137:61–72. doi: 10.1002/ijc.29373. [DOI] [PubMed] [Google Scholar]
- 42.Jung Y.S., Kato I., Kim H.R.C. A novel function of HPV16-E6/E7 in epithelial–mesenchymal transition. Biochem. Biophys. Res. Commun. 2013;435:339–344. doi: 10.1016/j.bbrc.2013.04.060. [DOI] [PubMed] [Google Scholar]
- 43.Wald A.I., Hoskins E.E., Wells S.I., Ferris R.L., Khan S.A. Alteration of microRNA profiles in squamous cell carcinoma of the head and neck cell lines by human papillomavirus. Head Neck. 2011;33:504–512. doi: 10.1002/hed.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin C.M., Kehoe L., Spillane C.O., O'Leary J.J. Gene discovery in cervical cancer: towards diagnostic and therapeutic biomarkers. Mol. Diagn. Ther. 2007;11:277–290. doi: 10.1007/BF03256249. [DOI] [PubMed] [Google Scholar]
- 45.Chung C.H., Gillison M.L. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin. Cancer Res. 2009;15:6758–6762. doi: 10.1158/1078-0432.CCR-09-0784. [DOI] [PubMed] [Google Scholar]
- 46.Mulvany N.J., Allen D.G., Wilson S.M. Diagnostic utility of p16INK4a: a reappraisal of its use in cervical biopsies. Pathology. 2008;40:335–344. doi: 10.1080/00313020802035907. [DOI] [PubMed] [Google Scholar]
- 47.Hermeking H. MicroRNAs in the p53 network: micromanagement of tumour suppression. Nat. Rev. Cancer. 2012;12:613–626. doi: 10.1038/nrc3318. [DOI] [PubMed] [Google Scholar]
- 48.Caulin C., Nguyen T., Longley M.A., Zhou Z.J., Wang X.J., Roop D.R. Inducible activation of oncogenic K-ras results in tumor formation in the oral cavity. Cancer Res. 2004;64:5054–5058. doi: 10.1158/0008-5472.CAN-04-1488. [DOI] [PubMed] [Google Scholar]
- 49.Motohara T., Masuko S., Ishimoto T., Yae T., Onishi N., Muraguchi T., Hirao A., Matsuzaki Y., Tashiro H., Katabuchi H., Saya H., Nagano O. Transient depletion of p53 followed by transduction of c-Myc and K-Ras converts ovarian stem-like cells into tumor-initiating cells. Carcinogenesis. 2011;32:1597–1606. doi: 10.1093/carcin/bgr183. [DOI] [PubMed] [Google Scholar]
- 50.Wang Z.W., Ali S., Banerjee S., Bao B., Li Y.W., Azmi A.S., Korc M., Sarkar F.H. Activated K-Ras and INK4a/Arf deficiency promote aggressiveness of pancreatic cancer by induction of EMT consistent with cancer stem cell phenotype. J. Cell Physiol. 2013;228:556–562. doi: 10.1002/jcp.24162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]