Abstract
Tumor necrosis factor receptor-associated factor 3 (TRAF3), a member of the TRAF family of cytoplasmic adaptor proteins with E3 ligase activity, is ubiquitously expressed in various cell types of the immune system. It is shared for signaling by a variety of adaptive and innate immune receptors as well as cytokine receptors. Previous studies examining conditional TRAF3-deficient mouse models that have the Traf3 gene specifically deleted in B lymphocytes or T lymphocytes have revealed the diverse and critical in vivo functions of TRAF3 in adaptive immunity. Although in vitro evidence points to a pivotal and indispensable role for TRAF3 in type I interferon production induced by pattern recognition receptors in macrophages and dendritic cells, the in vivo functions of TRAF3 in the innate immune system had long remained unclear. Three laboratories have recently addressed this gap in knowledge by investigating myeloid cell-specific TRAF3-deficient (genotype: TRAF3flox/floxLysM+/Cre) mice. The new evidence together demonstrates that specific ablation of TRAF3 in myeloid cells leads to inflammatory diseases, altered progression of diabetes, and spontaneous development of different types of tumors and infections in mice. These new findings indicate that TRAF3 acts as an anti-inflammatory factor and is required for optimal innate immunity in myeloid cells. Strikingly, the new evidence also identifies TRAF3 as a novel tumor suppressor gene in macrophages and other myeloid cells. In this review, we discuss and summarize the new findings and current knowledge about the multi-faceted regulatory roles and complex signaling mechanisms of myeloid cell TRAF3 in inflammation, innate immunity, and tumor development.
Introduction
Tumor necrosis factor receptor-associated factor 3 (TRAF3), a member of the TRAF family of cytoplasmic adaptor proteins with E3 ligase activity, is ubiquitously expressed in various cell types of the immune system [1, 2]. It is broadly employed in signaling by a variety of adaptive and innate immune receptors as well as cytokine receptors [1, 2]. TRAF3 binds directly to almost all members of the tumor necrosis factor receptor (TNF-R) superfamily that do not contain death domains, including CD40, BAFF-R, TACI, BCMA, LT-βR, CD27, CD30, RANK, HVEM, EDAR, XEDAR, 4-1BB, OX-40, and GITR [1]. TRAF3 is indirectly recruited to the signaling complexes of pattern recognition receptors (PRRs) of the innate immune system through interactions with additional adaptor proteins, including MyD88 and TRIF for Toll-like receptors (TLRs), RIP2 for NOD-like receptors (NLRs), and MAVS for RIG-I-like receptors (RLRs) [1, 3–5]. TRAF3 also directly or indirectly regulates signaling by cytokine receptors, including receptors for M-CSF, GM-CSF, IL-2, IL-15, and IL-17 [1, 6, 7]. Consistent with the shared usage of TRAF3 by such a variety of immune receptors, increasing evidence from studies of conditional TRAF3-deficient mouse models demonstrates the diverse and critical in vivo functions of TRAF3 in B and T lymphocytes of the adaptive immune system [1, 7].
We and Gardam et al. previously reported that B cell-specific TRAF3-deficient (B-TRAF3−/−; TRAF3flox/floxCD19+/Cre) mice exhibit severe peripheral B cell hyperplasia and autoimmunity, due to vastly prolonged B cell survival and constitutive activation of the NIK-NF-κB2 pathway [8, 9]. These mice eventually develop splenic marginal zone lymphomas (MZL) or B1 lymphomas by 18 months of age [10]. We also found that TRAF3−/− B cells display enhanced production of cytokines and type I interferons (IFN) as well as elevated Ig isotype switching in response to signaling by TLR3, 4, 7/8, and 9 [11]. These observations indicate that TRAF3 is a critical regulator of peripheral B cell homeostasis and autoimmunity, and serves as an important tumor suppressor in B lymphocytes.
In contrast, T cell-specific TRAF3-deficient (T-TRAF3−/−; TRAF3flox/floxCD4-Cre) mice exhibit normal homeostasis of CD4 and CD8 T cells, but are defective in T cell-dependent IgG1 responses and in T cell-mediated immunity to infection with Listeria monocytogenes [12]. The defects in T cell-mediated immune responses are caused by compromised T cell receptor (TCR)/CD28 signaling in both TRAF3−/− CD4 and CD8 T cells [12]. Interestingly, T-TRAF3−/− mice contain markedly increased frequency and numbers of regulatory T cells (Treg) cells [12], but decreased numbers of CD8 central memory T (Tcm) cells [13] and invariant natural killer T (iNKT) cells [14]. It was revealed that TRAF3 inhibits IL-2 signaling by facilitating the recruitment of the tyrosine phosphatase TCPTP to the IL-2 receptor complex to dephosphorylate Jak1, Jak3 and STAT5, thereby restraining thymic Treg development [15]. On the other hand, TRAF3 is required for TCR-induced expression of T-bet and CD122, two molecules required for IL-15 signaling, and as a consequence, IL-15-mediated homeostasis of CD8 Tcm cells and development of iNKT cells are impaired in T-TRAF3−/− mice [13, 14]. Furthermore, Treg cell-specific TRAF3-deficient (Treg-TRAF3−/−; TRAF3flox/floxFoxp3-Cre) mice exhibit heightened formation of germinal centers (GCs) and increased production of high-affinity IgG antibodies, resulting from decreased numbers of follicular Treg cells (TFR cells) and increased numbers of follicular T helper cells (TFH cells) [16]. It is found that TRAF3 signaling in Treg cells is required to maintain high level expression of the inducible co-stimulator (ICOS), which in turn is essential for TFR cell generation in GCs and inhibition of antibody responses [16]. Both T-TRAF3−/− and Treg-TRAF3−/− mice have increased numbers of CD4 effector/memory T cells, suggesting that TRAF3−/−Treg cells might have defects in suppression of Th1 responses [7]. Collectively, the findings obtained from B-TRAF3−/−, T-TRAF3−/−, and Treg-TRAF3−/− mice indicate that TRAF3 is a highly versatile regulator of different lymphocyte subpopulations in the adaptive immune system and thus adaptive immune responses.
Different from adaptive immune responses, inflammation and innate immunity are mainly mediated by myeloid cells, including granulocytes, monocytes, macrophages and dendritic cells (DCs) [17]. These cells constitutively or inducibly express a number of receptors of the TNF-R, TLR, NLR, and RLR families as well as cytokine receptors, whose signals are regulated by TRAF3 [1, 2, 6, 7, 17, 18]. In particular, macrophages represent a major type of innate immune cells that initiate inflammatory responses and host defense against infections by producing pro-inflammatory cytokines and type I IFN [18]. Although in vitro evidence indicates that TRAF3 regulates pro-inflammatory cytokine and type I IFN production in macrophages and DCs [19, 20] almost a decade ago, the in vivo functions of TRAF3 in the innate immune system had remained elusive. Now three laboratories have addressed this gap in knowledge by investigating myeloid cell-specific TRAF3-deficient (M-TRAF3−/−; TRAF3flox/floxLysM+/Cre) mice [21–23]. It is shown that young adult M-TRAF3−/− mice have normal sized lymphoid organs and also normal frequency and numbers of myeloid cell populations in various hematopoietic compartments [21]. This indicates that specific ablation of TRAF3 in myeloid cells neither affects the development nor alters the homeostasis of myeloid cells in young adult mice [21]. However, evidence from all three groups indeed demonstrates that TRAF3 is a crucial intrinsic regulator of myeloid cell functions [21–23]. Here we review the new findings about the multi-faceted regulatory roles of myeloid cell TRAF3 in inflammation, innate immunity, and tumor development, which identify TRAF3 as a novel tumor suppressor in macrophages.
TRAF3-mediated regulation of inflammatory responses in macrophages
The intensity and duration of macrophage-mediated inflammatory responses need to be tightly controlled to avoid tissue damage and inflammatory diseases [24]. Previous in vitro evidence suggests that TRAF3 acts as an anti-inflammatory factor in macrophages, as TLR-induced pro-inflammatory cytokine production is enhanced by TRAF3 deficiency in bone marrow-derived macrophages (BMDMs) and DCs [19, 20]. Consistent with the in vitro observations, M-TRAF3−/− mice display elevated serum levels of the pro-inflammatory cytokines IL-6 and IL-12 but decreased serum levels of the anti-inflammatory cytokine IL-10 in response to i.p. injection with LPS (an agonist for TLR4) or polyI:C (an agonist for TLR3) [21]. Thus, TRAF3 deficiency in myeloid cells appears to favor pro-inflammatory responses following in vivo challenge with LPS or polyI:C. In support of this notion, aging M-TRAF3−/− mice (15–22 months old) spontaneously develop chronic inflammation often affecting multiple organs, including the liver, gastrointestinal (GI) tract, lung, kidney, pancreas, and heart [21]. Furthermore, in the dextran sulfate sodium (DSS)-induced colitis model, M-TRAF3−/− mice exhibit exacerbated colon inflammation, as demonstrated by reduced survival rate, worsened bodyweight loss, as well as more severe mucosal damage and colon shortening [22]. Indeed, colonic macrophages purified from the DSS-treated M-TRAF3−/− mice express significantly higher levels of proinflammatory cytokines, including IL-6, IL-12 and IL-23[22]. Taken together, these in vivo data demonstrate that TRAF3 normally inhibits inflammation in macrophages and other myeloid cells.
To understand the mechanisms underlying TRAF3-mediated inhibition of proinflammatory cytokine production, several groups have carefully compared the early signaling events of TLR4 engagement in macrophages in the presence or absence of TRAF3. It was previously proposed that TRAF3 inhibits TLR4-induced activation of mitogen-activated protein kinases (MAPKs) in a model whereby TRAF3 degradation is required for the release of the TAK1 signaling complex from the membrane receptor into the cytosol to activate the MAPK signal cascades [2, 25]. However, TLR4-induced activation of MAPKs, including JNK1/2, ERK1/2, and p38, is neither enhanced nor prolonged but appears to be normal in different models of TRAF3-deficient macrophages [19, 21, 22]. These include BMDMs derived from TRAF3−/− chimeric mice, BMDMs and peritoneal exudate macrophages (PEMs) derived from M-TRAF3−/− mice, and BMDMs derived from TRAF3flox/floxCreER mice and treated with the CreER inducer tamoxifen [19, 21, 22]. Therefore, the elevated production of the proinflammatory cytokines IL-6, IL-12 and IL-23 in response to TLR4 stimulation observed in TRAF3-deficient macrophages could not be attributed to the hyperactivation of MAPKs. These data argue against an inhibitory role for TRAF3 in TLR4-induced activation of MAPKs as previously proposed.
In contrast to the unaltered MAPK activation, constitutive activation of the noncanonical NF-κB (NF-κB2) pathway was consistently detected in TRAF3-deficient macrophages and neutrophils as demonstrated by enhanced accumulation of NIK and processing of NF-κB2 (from p100 to p52) as well as increased nuclear levels of the p52-RelB dimer [21, 22]. In fact, constitutive processing of NF-κB2 from the inactive precursor p100 to the active p52 observed in TRAF3-deficient macrophages and neutrophils is as robust as that observed in TRAF3-sufficient counterparts after TLR4 stimulation [21]. Constitutive processing of NF-κB2 results from accumulation of NIK, as NIK was previously demonstrated to be targeted for K48-linked ubiquitination and proteasome-mediated degradation by the TRAF3-TRAF2-cIAP1/2 complex in resting cells [1, 2]. The NIK-NF-κB2 pathway has been shown to mediate inflammation in epithelial cells and hepatocytes [26, 27]. However, Jin et al. demonstrated that the enhanced TLR4-induced proinflammatory cytokine production is not reversed by the ablation of NIK, with the exception of IL-23 that is partially downregulated in the NIK-null background [22]. Thus, constitutive activation of the NIK-NF-κB2 pathway only plays a minor role in mediating the hyper-induction of the proinflammatory cytokine IL-23 in TRAF3-deficient macrophages in response to TLR4 stimulation [22](Fig. 1).
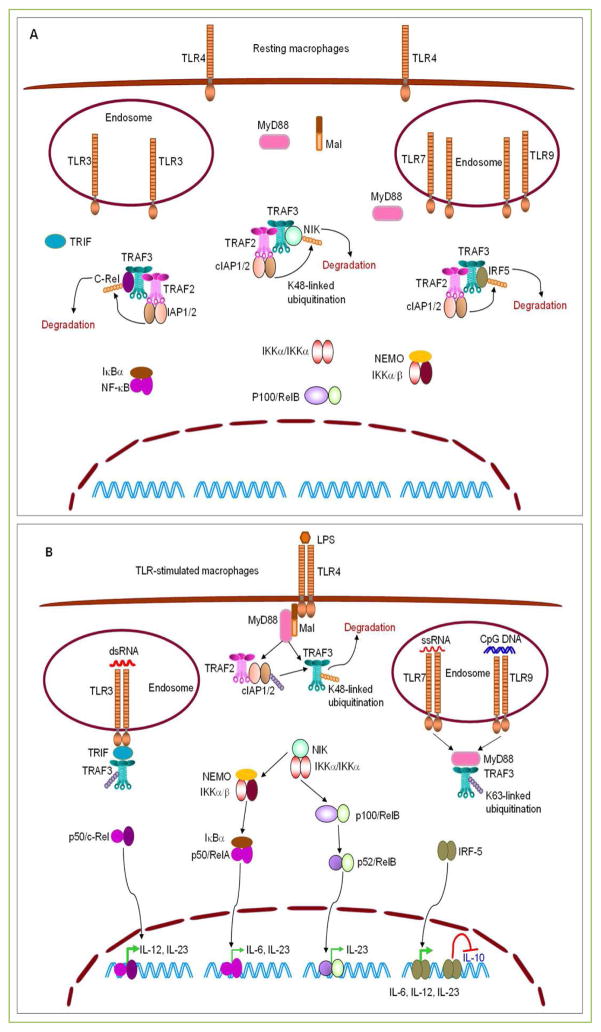
Figure 1. TRAF3-mediated inhibition of proinflammatory cytokine production induced by TLRs in macrophages.
(A) TRAF3 targets NIK, c-Rel and IRF5 for degradation in resting macrophages. In the absence of stimulation, TRAF3 constitutively binds to NIK, c-Rel, and IRF5, and bring them to the TRAF3-TRAF2-cIAP1/2 complexes. In these complexes, the E3 ligases cIAP1/2 catalyze the K48-linked ubiquitination on NIK, c-Rel, and IRF5, thereby targeting them for proteasome-mediated degradation. Thus, TRAF3 prevents the activation of NF-κB2, NF-κB1, and IRF5 in resting macrophages. (B) TRAF3 inhibits TLR-induced proinflammatory cytokine production in macrophages. In response to LPS stimulation, dimerized or oligomerized TLR4 recruits Mal and MyD88, which in turn recruits TRAF3 and the associated TRAF2-cIAP1/2 complex to the receptor signaling complex at the plasma membrane. This allows the activation of cIAP1/2 to target TRAF3 for K48-linked ubiquitination and degradation. Similarly, stimulation of TLR7 by ssRNA or stimulation of TLR9 by CpG DNA also recruits TRAF3 via MyD88, while engagement of TLR3 by dsRNA recruits TRAF3 via TRIF to the receptor signaling complexes at the endosome membrane. Recruitment of TRAF3 by dimerized or oligomerized TLRs via MyD88 or TRIF disrupts the interaction between TRAF3 and NIK, c-Rel, or IRF5. This results in the accumulation of NIK, c-Rel and IRF5, and subsequent activation of NF-κB2 (p52/RelB), p50/c-Rel, and IRF5, which promote the expression of the proinflammatory cytokines IL-6, IL-12, and IL-23 in stimulated macrophages. In addition, nuclear IRF5 also inhibits the expression of the anti-inflammatory cytokine IL-10. Ablation of TRAF3 from macrophages mimics TLR engagement and also releases NIK, c-Rel and IRF5 from the TRAF2-cIAP1/2 complexes, allowing the accumulation of NIK, c-Rel and IRF5. Therefore, TLR agonists induce enhanced production of the proinflammatory cytokines IL-6, IL-12 and IL-23 but decreased production of the anti-inflammatory cytokine IL-10 in TRAF3−/− macrophages. Transcription factors (p50/c-Rel and IRF5) that play major roles in driving the production of IL-6, IL-12 and IL-23 are shown in bold green arrows. TRAF3-independent TLR signaling pathways, including TRAF6- or TRAF2-induced activation of ERK1/2, p38, JNK1/2 and NF-κB1 (p50/RelA), are not depicted in the figures.
Similar to NIK, two transcription factors essential for the expression of proinflammatory cytokines, the NF-κB member c-Rel [28, 29] and the interferon regulatory factor 5 (IRF5) [30], are also targeted for degradation by TRAF3 in resting macrophages [22]. Both IRF5 and c-Rel have been associated with human inflammatory diseases [22, 28, 30]. As revealed by Jin et al., TRAF3 constitutively binds to c-Rel and IRF5, and therefore is responsible for recruiting c-Rel and IRF5 to the TRAF3-TRAF2-cIAP1/2 complexes [22]. In these complexes, the E3 ligases cIAP1/2 catalyze the K48-linked ubiquitination on c-Rel and IRF5, thereby targeting them for proteasome-mediated degradation in resting macrophages [22] (Fig. 1). Indeed, elevated protein levels of c-Rel and IRF5 are observed in both TRAF3- and TRAF2-deficient macrophages in the absence of stimulation [22]. TRAF2−/− macrophages also exhibit hyper-induction of proinflammatory cytokines in response to TLR4 stimulation and M-TRAF2−/− mice are also more susceptible to DSS-induced colon inflammation [22]. In line with these observations, the cIAP inhibitor SMAC mimetics (SM) causes marked accumulation of c-Rel and IRF5, and has proinflammatory actions on macrophages [22, 31]. It is known that c-Rel is specifically required for TLR-stimulated expression of IL-12 and IL-23 [22, 28, 29]. Similarly, IRF5 mediates the expression of multiple proinflammatory cytokines, including IL-6, IL-12, and IL-23, and inhibits the expression of the anti-inflammatory cytokine IL-10 [22, 30] (Fig. 1). Taken together, the above findings support the model that stabilized c-Rel and IRF5 are the major transcription factors that drive the hyper-induction of the proinflammatory cytokines IL-6, IL-12, and IL-23 in TRAF3- or TRAF2-deficient macrophages in response to TLR agonists (Fig. 1).
Interestingly however, Chen et al. reported that the anti-inflammatory function of TRAF3 in macrophages is not static, but is dynamically modulated according to the metabolic states [23]. Macrophages in adipose tissue and the liver are the major mediators of metabolic inflammation, promoting insulin resistance and metabolic disease progression in obesity [32]. Obesity is associated with chronic, low-grade inflammation, which contributes to insulin resistance and metabolic disease [32]. Chen et al. found that myeloid cell-specific deletion of TRAF3 has opposite effects on inflammation between lean and obese mice [23]. In lean mice, myeloid cell-specific deletion of TRAF3 increases the expression of proinflammatory cytokines in the liver and adipose tissue [23]. In contrast, TRAF3 deficiency in myeloid cells decreases the expression of proinflammatory cytokines in the liver and adipose tissue of obese mice, and largely prevents high-fat diet (HFD)-induced inflammation in these metabolic tissues [23]. Consequently, M-TRAF3−/− mice exhibit significantly attenuated insulin resistance and hepatic steatosis in models of either genetic (ob/ob) or HFD-induced obesity [23]. Chen et al. also showed evidence to suggest that in obese state, TRAF3 may promote metabolic inflammation by increasing the expression of proinflammatory cytokines in myeloid cells and by facilitating macrophage infiltration into metabolic tissues [23]. Thus, during metabolic inflammation and obesity progression, TRAF3 functionally switches its activity from anti-inflammatory to pro-inflammatory modes in macrophages, thereby coupling overnutrition to metabolic inflammation, insulin resistance, and metabolic disease [23].
In addition to TLRs, other receptors that directly interact with TRAF3 or indirectly recruit TRAF3 are also likely involved in and contribute to the inflammatory phenotypes observed in M-TRAF3−/− mice, including the spontaneous inflammatory diseases of aging M-TRAF3−/− mice, exacerbated colitis of DSS-treated M-TRAF3−/− mice, and attenuated metabolic inflammation and hepatic steatosis of obese M-TRAF3−/− mice [21–23]. Under the above disease conditions, cytokines secreted by inflammatory cells, toxic metabolites and damage-associated molecular patterns (DAMPs) released from aberrant or dead cells, and pathogen-associated molecular patterns (PAMPs) derived from commensal microbiota would engage their cognate receptors. These may trigger multiple signaling pathways that converge at TRAF3 to regulate inflammation. For example, markedly elevated serum levels of IL-17, a potent proinflammatory cytokine, were detected in aging M-TRAF3−/− mice with inflammatory diseases [21]. It has been previously shown that TRAF3 inhibits IL-17 receptor signaling by interfering with the IL-17R-Act1-TRAF6 complex formation [1, 33] and that IL-17 receptors are up-regulated in inflammatory macrophages [34]. This suggests that TRAF3 may inhibit IL-17-induced inflammatory responses in macrophages in disease progression. Interestingly, TRAF3 has also been reported to participate in inflammation-related signaling by CD40 [35], lymphotoxin-β receptor (LTβR) [36], IL-1R [37], and NLRP12 [38] in macrophages. Therefore, engagement of multiple TRAF3-employing receptors on macrophages likely occurs simultaneously or sequentially in the pathogenesis of inflammatory diseases, which represents an important area for future investigation.
Requirement of TRAF3 in type I IFN production and innate immunity
Innate immunity provides the first line of protection against infectious microorganisms such as bacteria and viruses, which are recognized by pattern recognition receptors (PRRs), including TLRs, NLRs, and RLRs [1, 39, 40]. Detection of microorganisms triggers signaling cascades leading to the production of type I IFN, which is pivotal for the initiation of an anti-microbial state of the host [1, 39, 40]. Previous in vitro evidence indicates that TRAF3 is required for the innate anti-viral responses and type I IFN production triggered by TLRs or RLRs in macrophages and DCs [19, 20, 41]. In response to engagement of TLRs or RLRs, TRAF3 serves as a critical link between the upstream adaptor proteins of receptors (TRIF or MyD88 for TLRs; MAVS for RLRs) and downstream activating kinases TBK1-IKKε or IRAK1-IKKα [1, 25, 39] (Fig. 2). Recruitment of TRAF3 by TLR or RLR signaling complexes activates the E3 ligase activity of TRAF3 to catalyze its own K63-linked ubiquitination, and subsequently mediates the activation of TBK1-IKKε or IRAK1-IKKα, which in turn phosphorylate and activate key transcription factors IRF3 and IRF7 to drive type I IFN production [1, 25, 39] (Fig. 2). Consistent with previous observations, TLR4-induced expression of Ifnb and Ifna4 is almost abolished and phosphorylation of IRF3 is markedly reduced in BMDMs and PEMs derived from M-TRAF3−/− mice [21, 22]. However, we noticed that phosphorylation of IRF3 is still significantly induced by TLR4 stimulation in TRAF3−/− BMDMs and PEMs [21], suggesting that reduced IRF3 activation is not the sole contributor of defective type I IFN production observed in these cells and additional mechanism(s) are involved.
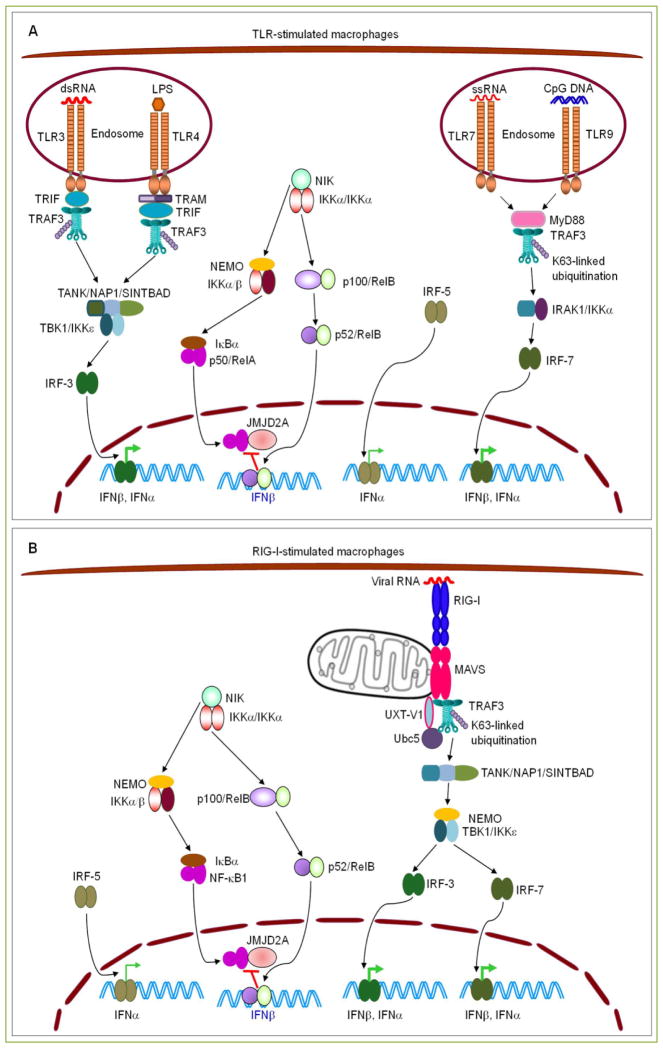
Figure 2. Requirement of TRAF3 in TLR- or RIG-I-induced type I IFN production in macrophages.
(A) Roles of TRAF3 in type I IFN production induced by TLR3, 4, 7 and 9 signaling. Upon ligand binding in endosomes, TLR3 recruits TRAF3 via TRIF, while TLR7 and TLR9 recruit TRAF3 via MyD88. In response to LPS stimulation, internalized TLR4 also recruits TRAF3 via TRAM-TRIF to the endosome membrane. Recruitment of TRAF3 by endosomal TLRs activates the E3 ligase activity of TRAF3 to catalyze its own K63-linked ubiquitination, and subsequently mediates the activation of TBK1/IKKε in TRIF-dependent signaling or IRAK1/IKKα in MyD88-dependent signaling. This eventually leads to the phosphorylation, dimerization, and nuclear translocation of IRF3 or IRF7 to promote the expression of IFNβ and IFNα in stimulated macrophages. In addition, recruitment of TRAF3 by engaged TLRs via TRIF or MyD88 also allows the accumulation of NIK and IRF5. Activation and nuclear translocation of IRF5 promotes the expression of IFNα, while nuclear NF-κB2 (p52/RelB) inhibits the expression of IFNβ by preventing the binding of NF-κB1 (p50/RelA) and the RelA-interactor histone demethylase JMJD2A to the Ifnb promoter region. (B) Roles of TRAF3 in type I IFN production induced by RIG-I signaling. Upon viral RNA binding, RIG-I recruits TRAF3 via MAVS to the receptor signaling complex at the mitochondrial membrane. This induces the self-ubiquitination of TRAF3 and subsequent activation of TBK1/IKKε, leading to the activation and nuclear translocation of IRF3 and IRF7 to promote the expression of IFNβ and IFNα in infected macrophages. Similar to TLR signaling, recruitment of TRAF3 by engaged RIG-I via MAVS also allows the accumulation of IRF5 and NIK, which promotes the expression of IFNα and inhibits the expression of IFNβ, respectively. Therefore, TRAF3−/− macrophages are defective in producing type I IFN in response to TLR agonists or viral infections, due to both impaired activation of stimulatory IRFs (IRF3 and IRF7) and constitutive activation of repressive NIK-NF-κB2. TRAF3-independent TLR or RIG-I signaling pathways, including TRAF6- or TRAF2-induced activation of MAPKs and NF-κB1, are not depicted in the figures.
Indeed, Jin et al. obtained the interesting finding that another signaling pathway affected by TRAF3 deletion, constitutive activation of NIK-IKKα-NF-κB2, also contributes to the defective type I IFN production observed in TRAF3−/− macrophages [6]. A variety of mouse models genetically deficient in this pathway all exhibit hyper-induction of type I IFN in macrophages in response to TLR or RLR ligands and are substantially more resistant to vesicular stomatitis virus (VSV) infection [6]. These mouse models include Map3k14−/− (NIK-deficient) mice, Chuk−/− (IKKα-deficient) mice, Nfkb2xdr (deficient in NF-κB2 expression because of a splicing-disruptive mutation of the Nfkb2 gene) mice, and Nfkb2lym1 (deficient in NF-κB2 processing because of a mutation of the Nfkb2 gene that causes the loss of the C-terminal phosphorylation site on p100) mice [6]. In contrast, macrophages derived from NIK T3-transgenic mice overexpressing a stable form of NIK, which lacks its TRAF3-binding motif and thus results in constitutive activation of IKKα-NF-κB2, show remarkably impaired production of type I IFN in response to TLR agonists [6]. Jin et al. further revealed that NF-κB2 suppresses TLR or RLR-induced histone modifications at the Ifnb promoter, an action that involves attenuated recruitment of the transactivator RelA and the histone demethylase JMJD2A [6]. It is known that JMJD2A, recruited to the Ifnb promoter by RelA, induces activating modifications of histone H3 such as trimethylation of H3K4 (H3K4me3) and H3 acetylation (H3Ac), and also decreases the repressive histone modifications such as H3K9me2 and H3K9me3 [6, 42]. Elevated nuclear levels of NF-κB2 (p52-RelB) lead to inhibitionof RelA -JMJD2A recruitment to the Ifnbpromoter, as p52-RelB bind to the Ifnb promoter more strongly than RelA [6] (Fig. 2). Consequently, RelA-JMJD2A-mediated activation of chromatin structures at the Ifnb promoter is suppressed by constitutive NF-κB2 activation, which is present in TRAF3−/− macrophages [21, 22]. Taken together, TRAF3 promotes TLR- or RLR-induced type I IFN production by facilitating the phosphorylation of IRF3 and IRF7 via its adaptor function and E3 ligase activity, and also by suppressing the inhibitory roles of the NIK-IKKα-NF-κB2 pathway via targeting NIK for degradation (Fig. 2).
In addition to its requirement in type I IFN production, TRAF3 also plays a critical role in mediating the apoptosis-associated specklike protein (ASC)-dependent inflammasome activation in macrophages upon RNA virus infection as demonstrated in a recent study by Guan et al. [43]. It is revealed that engagement of RIG-I by RNA viruses induces the formation of a new MAVS-TRAF3-ASC complex, in which TRAF3 serves as a direct E3 ligase to catalyze K63-linked ubiquitination on Lys174 of ASC [43]. Ubiquitination of ASC by TRAF3 is vital for ASC protein stabilization, speck formation and inflammasome activation, leading to caspase 1 activation and the subsequent processing and secretion of IL-1β and IL-18 [43]. Deficiency in TRAF3 or MAVS impairs ASC ubiquitination and cytosolic speck formation, resulting in reduced inflammasome activation and decreased IL-1β secretion in macrophages upon infection with VSV or influenza virus [43]. Intriguingly, TRAF3 and MAVS appear to be specifically required for inflammasome activation induced by RNA virus infection, but dispensable for inflammasome activation induced by NLRP3 activators such as calcium pyrophosphate dihydrate (CPPD) and monosodium urate (MSU) [43]. Therefore, TRAF3 plays multiple positive roles in innate anti-viral responses mediated by macrophages.
Under normal conditions and in healthy individuals, the magnitude and duration of induction of both type I IFN and pro-inflammatory cytokines are tightly controlled in response to either endogenous ligands or infectious microbes to prevent tissue injury [1, 2, 24, 40]. Imbalanced production of pro-inflammatory cytokines versus type I IFNs has been implicated in the pathogenesis of immunological disorders, such as inflammatory diseases, autoimmune diseases, and infectious diseases [1, 2, 24, 40]. The unique positive roles of TRAF3 in type I IFN production but negative roles in proinflammatory cytokine production poise it at the center for fine-tuning of these responses [1, 2]. In support of this notion, the expression, stability, activity, and subcellular localization of TRAF3 are subjected to delicate regulation by a number of cellular proteins, including receptors, K48 or K63-linked E3 ubiquitin ligases, deubiquitinating enzymes (DUBs), kinases, phosphatases and other adaptor proteins [1, 40]. Signaling via TLR2 remarkably up-regulate TRAF3 at both mRNA and protein levels in macrophages [44], while activation of CD40, LTβR, TLR4, estrogen receptor α (ERα), or M-CSFR results in the degradation of TRAF3 proteins [1, 2, 6, 45]. Upon different receptor signaling, E3 ligases that catalyze the ubiquitination of TRAF3 include cIAP1/2, Triad3A, and TRAF3 itself, and DUBs that remove the ubiquitin chains from TRAF3 include DUBA, OTUB1, OTUB2, MCPIP1, and USP25 [1, 46]. In innate immune responses, the kinases c-Src and Syk as well as the protein tyrosine phosphatase nonreceptor type 22 (PTPN22) can directly bind to TRAF3 and modulate its activity [47–49]. Furthermore, numerous adaptor proteins either facilitate or interfere with the complex formation of MAVS-TRAF3-TBK1-IKKε or TRIF-TRAF3-TBK1-IKKε in response to infections. Examples of such adaptor proteins include TANK [50, 51], TRADD [52], UXT-V1 [53], DOK3 [54], A20 [55] , TAX1BP1 [55], UBXN1 [56], optineurin [57], TRAM [58], and FLN29 [59]. Therefore, TRAF3 is a central regulatory point for optimal innate immune responses.
Although in vivo infection studies on M-TRAF3−/− mice are still lacking, we found that some aging M-TRAF3−/− mice spontaneously develop bacterial or entamoeba infections in the intestine/colon, liver, and lung, which are most likely caused by opportunistic strains of commensal microbiota (termed “pathobionts”) [21]. Pathobionts may trigger TRAF3-dependent signaling pathways via TLRs in macrophages, neutrophils and DCs [60–62]. Defective type I IFN production in TRAF3−/− myeloid cells in response to TLR signaling may occasionally allow colonization of commensal bacteria or entamoeba following opportunistic penetration of protective mucosal and epithelial barriers [21]. This indicates that innate immunity is evidently altered by ablation of TRAF3 from myeloid cells. The importance of TRAF3 in innate immunity is also highlighted by the evidence that a variety of viral and bacterial proteins target TRAF3 for inactivation. These include Lb(pro) of foot-and-mouth disease virus, X protein (HBx) of hepatitis B virus, UL36 of herpes simplex virus 1 (HSV-1), Tat protein of HIV-1, Gn protein of NY-1 hantavirus, M protein of severe acute respiratory syndrome coronavirus, and YopJ of the Gram- bacterium Yersinia pestis [1]. All these pathogenic proteins target TRAF3 and thus inhibit IRF3 phosphorylation and type I IFN production [1]. Given the above evidence, future studies are required to determine the in vivo responses of M-TRAF3−/− mice to infectious agents, and especially co-infections or sequential infections by different pathogens, such as bacteria and viruses that engage different innate immune receptors.
TRAF3: a novel tumor suppressor gene in macrophages
Of particular interest, we found that the majority of M-TRAF3−/− mice, but none of the TRAF3-sufficient littermate control mice, spontaneously develop tumors that often infiltrate multiple organs at the age of 15 – 22 months [21]. Tumors observed in M-TRAF3−/− mice include histiocytic sarcomas, B cell lymphomas, and hepatocellular adenoma [21]. Among these, histiocytic sarcomas are derived from TRAF3-deficient tissue-resident macrophages [21]. Histiocytic sarcoma (HS) in humans is a rare malignancy with a dismal prognosis, and its pathologic and cytogenetic data are sparse [63–65]. Because the genetic etiology of HS is largely unknown and patients with HS respond poorly to conventional chemotherapy, there is no standard therapy for human HS [63–65]. In M-TRAF3−/− mice with histiocytic sarcomas, whitish nodules of tumors are observed in the liver, and tumor cells are also identified as the major cell type in the spleen and disrupt the splenic architecture [21]. TRAF3−/− histiocytic sarcomas have the typical morphology of HS cells with abundant eosinophilic cytoplasm, and accompanied by frequent mitotic figures and occasional multinucleated giant cells and erythrophagocytosis [21] (Lalani and Xie, unpublished data). Immunophenotypically, TRAF3−/− HS cells are identified as CD68+MHC class II+CD11blowGr-1low, and are negative for markers of the B and T lymphoid lineages, including B220, CD19, IgM, CD3, CD4, and CD8 [21] (Lalani and Xie, unpublished data). How TRAF3 deficiency leads to malignant transformation of histiocytes remains to be investigated. We speculate that TRAF3 deficiency may gradually cause prolonged survival or increased proliferation of histiocytes, and eventually result in the development of HS in M-TRAF3−/− mice. Regardless of the detailed mechanisms, the spontaneous malignant transformation of tissue-resident macrophages observed in M-TRAF3−/− mice points to a tumor suppressive role of TRAF3 in macrophages.
Similar to M-TRAF3−/− mice, several other mouse models were previously reported to spontaneously develop histiocytic sarcomas, including p21−/− [66], Cyp1b1−/− [67], p19ARF−/−Bax−/− [68], PTEN−/−INK4a/ARF−/− [63], Dok1−/−Dok2−/−Dok3−/− [69], and humanized TLR7/TLR8 transgenic [70] mice, implicating these genes in the pathogenesis of HS. Among these, TRAF3 is functionally linked to TLR7, TLR8, and DOK3 [1, 54]. TRAF3 is recruited to the TLR7 and TLR8 signaling complex through direct interaction with MyD88 [1] (Fig. 2). Snyder et al. found that transgenic expression of human TLR7/TLR8 in mice deficient for endogenous TLR7/TLR8 drives proliferative histiocytosis with multisystemic infiltration of histiocytes that efface normal tissue architecture [70]. Compound deletion of MyD88 in humanized TLR7/TLR8 transgenic mice prevents the inflammatory phenotype and the development of HS, suggesting that the illness is caused by constitutive activation of humanized TLR7/TLR8 and exuberant MyD88-mediated signaling [70]. Interestingly, a recent study by Kim et al. identified TRAF3 as a new interacting protein for DOK3, a negative regulator of protein tyrosine kinase-mediated signaling [54]. As observed in TRAF3−/− macrophages, DOK3−/− macrophages are also impaired in IRF3 phosphorylation and IFNβ production upon influenza virus infection or polyI:C stimulation [54]. Some DOK3−/− mice exhibit abnormal accumulation of macrophages in the lung, and Dok1−/−Dok2−/−Dok3−/− mice succumb to spontaneous HS at a high incidence [69]. Taken together, the above findings reinforce the notion that dysregulation of TRAF3-dependent signaling pathways in macrophages contributes to the pathogenesis of histiocytic sarcoma.
We noticed that tumors are not only derived from TRAF3-deficient histiocytes, but also originate from other TRAF3-sufficient cell types that are not affected by LysM-Cre-mediated deletion, including B cells and hepatocytes, in M-TRAF3−/− mice [21]. This is in sharp contrast to that observed in B-TRAF3−/− mice, in which tumor development is limited to TRAF3-deficient B cells but is not observed in other TRAF3-sufficient cell types [10]. These findings suggest that myeloid cell TRAF3 may contribute to tumor surveillance and tumor immunity. Interestingly, B cell lymphomas observed in M-TRAF3−/− mice include diffuse large B cell lymphomas (DLBCLs) and follicular lymphomas (FLs), which are derived from GC or post-GC B cells [21]. During GC reaction, both somatic hypermutation (SHM) and class switch recombination (CSR) of the Ig genes generate double strand DNA breaks (DSBs), which increase the risk of genomic instability in B cells [71–73]. It is conceivable that B cells acquired oncogenic alterations during GC passage may escape the compromised tumor surveillance and develop into lymphomas in M-TRAF3−/− mice. In this regard, defective type I IFN production in TRAF3−/− myeloid cells in response to TLR-MyD88/TRIF or RLR-MAVS signaling, triggered by DAMPs derived from necrotic cancer cells, may result in compromised tumor surveillance and tumor immunity [1, 74–76]. Paradoxically however, TRAF2, another TRAF molecule that has many overlapping functions with TRAF3, was recently reported to play negative roles in myeloid cell-mediated tumor immunity [22]. Jin et al. showed that myeloid cell-specific TRAF2-deficient (M-TRAF2−/−; TRAF2flox/floxLysM+/Cre) mice are more potent in controlling the growth of inoculated B16 melanomas than wild type mice [22]. M-TRAF2−/− mice contain increased frequency and numbers of tumor-infiltrating IFN-γ+ CD4 and CD8 effector T cells, and TRAF2−/− tumor-associated macrophages exhibit elevated levels of iNOS production [22]. These changes lead to more effective inhibition on the growth of inoculated tumor in M-TRAF2−/− mice [22]. In this context, it would be interesting to examine the roles of myeloid cell TRAF3 in tumor surveillance and tumor immunity using M-TRAF3−/− mice as model systems in future studies.
In addition to its effects on tumor surveillance/immunity, myeloid cell TRAF3 may suppress tumor development through its functions on containing overt inflammation and preventing chronic inflammation. Consistent with this possibility, M-TRAF3−/− mice with spontaneous tumors often also contain chronic inflammation in multiple organs [21]. For example, several M-TRAF3−/− mice with B lymphomas displayed lung, liver or intestine/colon inflammation, and an M-TRAF3−/− mouse with hepatocellular adenoma also had pancreatitis and pericardial inflammation [21] (Lalani and Xie, unpublished data). It is known that chronic inflammation is a strong risk factor for cancer [77–80]. We detected remarkably elevated levels of a number of cytokines and chemokines in M-TRAF3−/− mice with tumors, including CXCL-13, G-CSF, CCL1, IL-16, IL-17, IP-10, MCP-1, MCP-5, CXCL9, TIMP-1, and TREM-1, which have been implicated in the pathogenesis of inflammatory diseases and cancers [81–86]. These proinflammatory cytokines and chemokines may stimulate tumor growth and also promote angiogenesis to accelerate tumor progression, invasion, and metastasis [77–86]. Furthermore, the chronic inflammatory environment of M-TRAF3−/− mice may also induce mutations [77–80] that facilitate malignant transformation of TRAF3-sufficient cells, such as GC B cells and hepatocytes.
Increasing evidence also suggests that myeloid cell TRAF3 may control tumor development by inhibiting the expansion of CD11b+Gr-1+ myeloid derived suppressor cells (MDSCs), which in turn suppress the anti-tumor immunity mounted by natural killer (NK) cells and CD8 cytotoxic T cells. We consistently observed a striking expansion of CD11b+Gr-1+ myeloid cells (most likely MDSCs) in M-TRAF3−/− mice with spontaneous tumors or chronic inflammation [21]. Although the increased population of CD11b+Gr-1+ MDSCs may be a consequence of spontaneous inflammation and tumor development [87–90], emerging new evidence suggests that TRAF3 may regulate the expansion and function of MDSCs. First, Parker et al. found that an endogenous agonist of the TRAF3-dependent receptor TLR4, HMGB1, is commonly present in the tumor microenvironment and potently promotes the generation and suppressive activity of MDSCs [91]. Second, Jin et al. demonstrated that upon ligand stimulation, TRAF3 is recruited to M-CSFR and GM-CSFR, which are known to play essential roles in regulating the development and differentiation of MDSCs [6, 92, 93]. Signaling by M-CSFR or GM-CSFR induces the degradation of TRAF3 and subsequent accumulation of c-Rel and p52 NF-κB2 [6, 22]. Third, Yu et al. reported that c-Rel and p52 NF-κB2 cooperatively bind to the promoter region of the Csf2 gene to induce the production of GM-CSF in Th17 cells, raising the interesting possibility that this may also occur in certain myeloid cell populations [94]. Increased GM-CSF production has been shown to cause the expansion of MDSCs [95]. Finally, constitutive activation of NF-κB2 has been shown to promote the immuno-suppressive activity of MDSCs by mediating the expression of IDO, an enzyme that catalyzes the degradation of tryptophan through the kynurenine pathway to suppress T cell proliferation and activation [96]. Collectively, the above evidence suggests the importance of TRAF3 in regulating the expansion and function of MDSCs, which are recognized as crucial drivers of tumor progression and chronic inflammation in the tumor microenvironment [87, 88, 90, 97, 98].
In summary, TRAF3 may function as a tumor suppressor in myeloid cells directly by acting in an intrinsic manner to inhibit the malignant transformation of histiocytes, or indirectly by controlling tumor development via multiple mechanisms. These indirect mechanisms of myeloid cell TRAF3-mediated tumor suppression include participating in tumor surveillance and tumor immunity, restricting the magnitude of inflammation and preventing chronic inflammation, and suppressing the expansion and function of MDSCs. All of these potential mechanisms are exciting areas for future exploration.
TRAF3 in human tumors, inflammatory diseases, and infectious diseases
The new findings obtained from in vivo studies of M-TRAF3−/− mice by all three laboratories indicate that aberrant functions of TRAF3 in myeloid cells may contribute to the pathogenesis of tumors, inflammatory diseases, metabolic diseases, and infectious diseases [21–23]. However, evidence of TRAF3 mutations or malfunctions in myeloid cells of human patients is still very limited. Previous efforts on human Traf3 gene mutations have been mainly focused on B cell malignancies considering the instrumental roles of TRAF3 in B cell survival/apoptosis as revealed by investigation of B-TRAF3−/− mice [1, 99]. Somatic biallelic deletions and inactivating mutations of Traf3 have been detected in a variety of human B cell neoplasms, including multiple myeloma, MZL, B cell chronic lymphocytic leukemia, mantle cell lymphoma, DLBCL, Waldenström’s macroglobulinemia, and Hodgkin lymphoma [1, 99–101]. Deletions and missense mutations of Traf3 have also been reported recently in two types of human carcinomas, the Epstein Barr virus-associated nasopharyngeal carcinoma and papillomavirus-associated head and neck squamous cell carcinomas [102, 103]. Additionally, a genome-wide association study (GWAS) identified Traf3 as a high-confidence candidate gene associated with multiple sclerosis, a neurological disease with prominent inflammation [104]. However, to date only one publication reports a germline mutation of human Traf3 that directly affects the function of macrophages and DCs [105]. In this case, a heterozygous Traf3 autosomal dominant mutation (R118W in the first zinc-finger domain) was found in a young adult with a history of herpes simplex virus-1 encephalitis in childhood. This Traf3 mutation results in impaired TLR3-induced type I IFN production in macrophages, DCs, and fibroblasts [105]. Together, available evidence suggests that most functions of TRAF3 are conserved between mice and humans.
Given the stringent control of TRAF3 protein levels and activities observed under normal circumstances, altered protein levels or activation of the TRAF3 protein in myeloid cells may also contribute to disease pathogenesis. Indeed, elevated protein levels of TRAF3, TRAF2, and TRAF5 were reported in human patients with inflammatory bowel diseases, and increased TRAF2 was identified as a predictor of relapse in patients with ulcerative colitis [106, 107]. In contrast, expression of TRAF3 is significantly decreased in peripheral blood mononuclear cells of patients chronically infected with hepatitis B virus as compared to that observed in healthy controls [108]. Overall, available information in this area is scattered. Therefore, further efforts are required to systematically examine the existence and frequency of alterations of TRAF3 and other components of TRAF3-dependent signaling pathways at the genetic, epigenetic, and protein as well as activity levels in myeloid cells in human patients with tumors, inflammatory diseases, metabolic diseases, and infectious diseases. Such studies will provide groundwork for therapeutic development of new targeted therapies to manage human diseases and improve patient outcome.
Conclusions
Substantial progress has recently been made in elucidating the in vivo functions of TRAF3 in macrophages and other myeloid cells. Evidence obtained by three laboratories together demonstrates that specific ablation of TRAF3 in myeloid cells leads to inflammatory diseases, altered progression of diabetes, and spontaneous development of different types of tumors and infections in mice. These new findings indicate that myeloid cell TRAF3 acts as an anti-inflammatory factor, and is required to resist infections and control development of hematopoietic and solid tumors. In conclusion, these studies identify TRAF3 as a critical regulator of inflammation and innate immunity, and notably, also a novel tumor suppressor in macrophages. Although information about TRAF3 mutations or malfunctions in human macrophages is limited, available evidence indicates that TRAF3 mutation and aberrant expression exist in myeloid cells of human patients with viral infectious diseases and inflammatory bowel diseases. Therefore, the functions of TRAF3 in myeloid cells appear to be conserved between mice and humans, suggesting that findings obtained from M-TRAF3−/− mice may be extrapolated to human diseases and merit further systematic investigations of TRAF3 in human patients. Furthermore, TRAF3 is now recognized as a converging point of numerous signaling pathways, including the TNF-R superfamily, TLRs, RLRs, and cytokine receptors. It would thus be especially interesting to further decipher how TRAF3 integrates or modulates different signals in situations that simultaneous or sequential engagement of multiple receptors occurs on macrophages and DCs, such as chronic inflammation, co-infections, or tumorigenesis. Deeper mechanistic insights into TRAF3 signaling pathways will be valuable for understanding the molecular pathogenesis of TRAF3-associated diseases, and will provide new opportunities for developing effective therapeutic modalities for chronic inflammation, infection, and cancer.
Acknowledgments
This study was supported by a Pilot Award from the Cancer Institute of New Jersey through Grant Number P30CA072720 from the National Cancer Institute (P. Xie), the Department of Defense grant W81XWH-13-1-0242 (P. Xie), a Busch Biomedical Grant (P. Xie), and the Arthur Herrmann Endowed Cancer Research Fund (P. Xie).
Footnotes
Competing interests statement
The authors declare that they have no competing financial interests.
References
- 1.Xie P. TRAF molecules in cell signaling and in human diseases. J Mol Signal. 2013;8:7. doi: 10.1186/1750-2187-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hacker H, Tseng PH, Karin M. Expanding TRAF function: TRAF3 as a tri-faced immune regulator. Nat Rev Immunol. 2011;11:457–468. doi: 10.1038/nri2998. [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 4.Saleh M. The machinery of Nod-like receptors: refining the paths to immunity and cell death. Immunol Rev. 2011;243:235–246. doi: 10.1111/j.1600-065X.2011.01045.x. [DOI] [PubMed] [Google Scholar]
- 5.Yu M, Levine SJ. Toll-like receptor, RIG-I-like receptors and the NLRP3 inflammasome: key modulators of innate immune responses to double-stranded RNA viruses. Cytokine Growth Factor Rev. 2011;22:63–72. doi: 10.1016/j.cytogfr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin J, Hu H, Li HS, Yu J, Xiao Y, Brittain GC, et al. Noncanonical NF-kappaB pathway controls the production of type I interferons in antiviral innate immunity. Immunity. 2014;40:342–354. doi: 10.1016/j.immuni.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yi Z, Wallis AM, Bishop GA. Roles of TRAF3 in T cells: many surprises. Cell Cycle. 2015;14:1156–1163. doi: 10.1080/15384101.2015.1021524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie P, Stunz LL, Larison KD, Yang B, Bishop GA. Tumor necrosis factor receptor-associated factor 3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity. 2007;27:253–267. doi: 10.1016/j.immuni.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardam S, Sierro F, Basten A, Mackay F, Brink R. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity. 2008;28:391–401. doi: 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Moore CR, Liu Y, Shao CS, Covey LR, Morse HC, 3rd, Xie P. Specific deletion of TRAF3 in B lymphocytes leads to B lymphoma development in mice. Leukemia. 2012;26:1122–1127. doi: 10.1038/leu.2011.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie P, Poovassery J, Stunz LL, Smith SM, Schultz ML, Carlin LE, et al. Enhanced Toll-like receptor (TLR) responses of TNFR-associated factor 3 (TRAF3)-deficient B lymphocytes. J Leukoc Biol. 2011;90:1149–1157. doi: 10.1189/jlb.0111044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie P, Kraus ZJ, Stunz LL, Liu Y, Bishop GA. TNF Receptor-Associated Factor 3 Is Required for T Cell-Mediated Immunity and TCR/CD28 Signaling. J Immunol. 2011;186:143–155. doi: 10.4049/jimmunol.1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi Z, Stunz LL, Lin WW, Bishop GA. TRAF3 regulates homeostasis of CD8+ central memory T cells. PLoS One. 2014;9:e102120. doi: 10.1371/journal.pone.0102120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi Z, Stunz LL, Bishop GA. TNF receptor associated factor 3 plays a key role in development and function of invariant natural killer T cells. J Exp Med. 2013;210:1079–1086. doi: 10.1084/jem.20122135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yi Z, Lin WW, Stunz LL, Bishop GA. The adaptor TRAF3 restrains the lineage determination of thymic regulatory T cells by modulating signaling via the receptor for IL-2. Nat Immunol. 2014;15:866–874. doi: 10.1038/ni.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang JH, Hu H, Jin J, Puebla-Osorio N, Xiao Y, Gilbert BE, et al. TRAF3 regulates the effector function of regulatory T cells and humoral immune responses. J Exp Med. 2014;211:137–151. doi: 10.1084/jem.20131019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawamoto H, Minato N. Myeloid cells. Int J Biochem Cell Biol. 2004;36:1374–1379. doi: 10.1016/j.biocel.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12:1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hacker H, Redecke V, Blagoev B, Kratchmarova I, Hsu LC, Wang GG, et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 20.Oganesyan G, Saha SK, Guo B, He JQ, Shahangian A, Zarnegar B, et al. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. 2006;439:208–211. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 21.Lalani AI, Moore CR, Luo C, Kreider BZ, Liu Y, Morse HC, 3rd, et al. Myeloid Cell TRAF3 Regulates Immune Responses and Inhibits Inflammation and Tumor Development in Mice. J Immunol. 2015;194:334–348. doi: 10.4049/jimmunol.1401548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin J, Xiao Y, Hu H, Zou Q, Li Y, Gao Y, et al. Proinflammatory TLR signalling is regulated by a TRAF2-dependent proteolysis mechanism in macrophages. Nat Commun. 2015;6:5930. doi: 10.1038/ncomms6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Z, Shen H, Sun C, Yin L, Tang F, Zheng P, et al. Myeloid cell TRAF3 promotes metabolic inflammation, insulin resistance, and hepatic steatosis in obesity. Am J Physiol Endocrinol Metab. 2015;308:E460–469. doi: 10.1152/ajpendo.00470.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 25.Tseng PH, Matsuzawa A, Zhang W, Mino T, Vignali DA, Karin M. Different modes of ubiquitination of the adaptor TRAF3 selectively activate the expression of type I interferons and proinflammatory cytokines. Nat Immunol. 2010;11:70–75. doi: 10.1038/ni.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, Banerjee S, LeJeune WS, Choudhary S, Tilton RG. NF-kappaB-inducing kinase increases renal tubule epithelial inflammation associated with diabetes. Exp Diabetes Res. 2011;2011:192564. doi: 10.1155/2011/192564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang B, Shen H, Chen Z, Yin L, Zan L, Rui L. Carboxyl terminus of HSC70-interacting protein (CHIP) down-regulates NF-kappaB-inducing kinase (NIK) and suppresses NIK-induced liver injury. J Biol Chem. 2015;290:11704–11714. doi: 10.1074/jbc.M114.635086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goriely S, Neurath MF, Goldman M. How microorganisms tip the balance between interleukin-12 family members. Nat Rev Immunol. 2008;8:81–86. doi: 10.1038/nri2225. [DOI] [PubMed] [Google Scholar]
- 29.Lu YC, Kim I, Lye E, Shen F, Suzuki N, Suzuki S, et al. Differential role for c-Rel and C/EBPbeta/delta in TLR-mediated induction of proinflammatory cytokines. J Immunol. 2009;182:7212–7221. doi: 10.4049/jimmunol.0802971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunthner R, Anders HJ. Interferon-regulatory factors determine macrophage phenotype polarization. Mediators Inflamm. 2013;2013:731023. doi: 10.1155/2013/731023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lecis D, De Cesare M, Perego P, Conti A, Corna E, Drago C, et al. Smac mimetics induce inflammation and necrotic tumour cell death by modulating macrophage activity. Cell Death Dis. 2013;4:e920. doi: 10.1038/cddis.2013.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu S, Pan W, Shi P, Gao H, Zhao F, Song X, et al. Modulation of experimental autoimmune encephalomyelitis through TRAF3-mediated suppression of interleukin 17 receptor signaling. J Exp Med. 2010;207:2647–2662. doi: 10.1084/jem.20100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barin JG, Baldeviano GC, Talor MV, Wu L, Ong S, Quader F, et al. Macrophages participate in IL-17-mediated inflammation. Eur J Immunol. 2012;42:726–736. doi: 10.1002/eji.201141737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rub A, Dey R, Jadhav M, Kamat R, Chakkaramakkil S, Majumdar S, et al. Cholesterol depletion associated with Leishmania major infection alters macrophage CD40 signalosome composition and effector function. Nat Immunol. 2009;10:273–280. doi: 10.1038/ni.1705. [DOI] [PubMed] [Google Scholar]
- 36.Wimmer N, Heigl U, Klingseisen L, Schneider-Brachert W, Hehlgans T. Lymphotoxin-beta receptor signalling regulates cytokine expression via TRIM30alpha in a TRAF3-dependent manner. Mol Immunol. 2013;54:40–47. doi: 10.1016/j.molimm.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez-Navajas JM, Law J, Nguyen KP, Bhargava M, Corr MP, Varki N, et al. Interleukin 1 receptor signaling regulates DUBA expression and facilitates Toll-like receptor 9-driven antiinflammatory cytokine production. J Exp Med. 2010;207:2799–2807. doi: 10.1084/jem.20101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen IC, Wilson JE, Schneider M, Lich JD, Roberts RA, Arthur JC, et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-kappaB signaling. Immunity. 2012;36:742–754. doi: 10.1016/j.immuni.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pietras EM, Saha SK, Cheng G. The interferon response to bacterial and viral infections. J Endotoxin Res. 2006;12:246–250. doi: 10.1179/096805106X118799. [DOI] [PubMed] [Google Scholar]
- 40.Perkins DJ, Vogel SN. Space and time: New considerations about the relationship between Toll-like receptors (TLRs) and type I interferons (IFNs) Cytokine. 2015;74:171–174. doi: 10.1016/j.cyto.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saha SK, Pietras EM, He JQ, Kang JR, Liu SY, Oganesyan G, et al. Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. Embo J. 2006;25:3257–3263. doi: 10.1038/sj.emboj.7601220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whetstine JR, Nottke A, Lan F, Huarte M, Smolikov S, Chen Z, et al. Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell. 2006;125:467–481. doi: 10.1016/j.cell.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 43.Guan K, Wei C, Zheng Z, Song T, Wu F, Zhang Y, et al. MAVS Promotes Inflammasome Activation by Targeting ASC for K63-Linked Ubiquitination via the E3 Ligase TRAF3. J Immunol. 2015;194:4880–4890. doi: 10.4049/jimmunol.1402851. [DOI] [PubMed] [Google Scholar]
- 44.Perkins DJ, Polumuri SK, Pennini ME, Lai W, Xie P, Vogel SN. Reprogramming of Murine Macrophages through TLR2 Confers Viral Resistance via TRAF3-Mediated, Enhanced Interferon Production. PLoS Pathog. 2013;9:e1003479. doi: 10.1371/journal.ppat.1003479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang C, Huang Y, Sheng J, Huang H, Zhou J. Estrogen receptor alpha inhibits RLR-mediated immune response via ubiquitinating TRAF3. Cell Signal. 2015;27:1977–1983. doi: 10.1016/j.cellsig.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Zhong B, Liu X, Wang X, Li H, Darnay BG, Lin X, et al. Ubiquitin-specific protease 25 regulates TLR4-dependent innate immune responses through deubiquitination of the adaptor protein TRAF3. Sci Signal. 2013;6:ra35. doi: 10.1126/scisignal.2003708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnsen IB, Nguyen TT, Bergstroem B, Fitzgerald KA, Anthonsen MW. The tyrosine kinase c-Src enhances RIG-I (retinoic acid-inducible gene I)-elicited antiviral signaling. J Biol Chem. 2009;284:19122–19131. doi: 10.1074/jbc.M808233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin YC, Huang DY, Chu CL, Lin YL, Lin WW. The tyrosine kinase Syk differentially regulates Toll-like receptor signaling downstream of the adaptor molecules TRAF6 and TRAF3. Sci Signal. 2013;6:ra71. doi: 10.1126/scisignal.2003973. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Shaked I, Stanford SM, Zhou W, Curtsinger JM, Mikulski Z, et al. The autoimmunity-associated gene PTPN22 potentiates toll-like receptor-driven, type 1 interferon-dependent immunity. Immunity. 2013;39:111–122. doi: 10.1016/j.immuni.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gatot JS, Gioia R, Chau TL, Patrascu F, Warnier M, Close P, et al. Lipopolysaccharide-mediated interferon regulatory factor activation involves TBK1-IKKepsilon-dependent Lys(63)-linked polyubiquitination and phosphorylation of TANK/I-TRAF. J Biol Chem. 2007;282:31131–31146. doi: 10.1074/jbc.M701690200. [DOI] [PubMed] [Google Scholar]
- 51.Guo B, Cheng G. Modulation of the interferon antiviral response by the TBK1/IKKi adaptor protein TANK. J Biol Chem. 2007;282:11817–11826. doi: 10.1074/jbc.M700017200. [DOI] [PubMed] [Google Scholar]
- 52.Michallet MC, Meylan E, Ermolaeva MA, Vazquez J, Rebsamen M, Curran J, et al. TRADD protein is an essential component of the RIG-like helicase antiviral pathway. Immunity. 2008;28:651–661. doi: 10.1016/j.immuni.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 53.Huang Y, Liu H, Ge R, Zhou Y, Lou X, Wang C. UXT-V1 facilitates the formation of MAVS antiviral signalosome on mitochondria. J Immunol. 2012;188:358–366. doi: 10.4049/jimmunol.1102079. [DOI] [PubMed] [Google Scholar]
- 54.Kim SS, Lee KG, Chin CS, Ng SK, Pereira NA, Xu S, et al. DOK3 is required for IFN-beta production by enabling TRAF3/TBK1 complex formation and IRF3 activation. J Immunol. 2014;193:840–848. doi: 10.4049/jimmunol.1301601. [DOI] [PubMed] [Google Scholar]
- 55.Gao L, Coope H, Grant S, Ma A, Ley SC, Harhaj EW. ABIN1 protein cooperates with TAX1BP1 and A20 proteins to inhibit antiviral signaling. J Biol Chem. 2011;286:36592–36602. doi: 10.1074/jbc.M111.283762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang P, Yang L, Cheng G, Yang G, Xu Z, You F, et al. UBXN1 interferes with Rig-I-like receptor-mediated antiviral immune response by targeting MAVS. Cell Rep. 2013;3:1057–1070. doi: 10.1016/j.celrep.2013.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mankouri J, Fragkoudis R, Richards KH, Wetherill LF, Harris M, Kohl A, et al. Optineurin negatively regulates the induction of IFNbeta in response to RNA virus infection. PLoS Pathog. 2010;6:e1000778. doi: 10.1371/journal.ppat.1000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanimura N, Saitoh S, Matsumoto F, Akashi-Takamura S, Miyake K. Roles for LPS-dependent interaction and relocation of TLR4 and TRAM in TRIF-signaling. Biochem Biophys Res Commun. 2008;368:94–99. doi: 10.1016/j.bbrc.2008.01.061. [DOI] [PubMed] [Google Scholar]
- 59.Sanada T, Takaesu G, Mashima R, Yoshida R, Kobayashi T, Yoshimura A. FLN29 deficiency reveals its negative regulatory role in the Toll-like receptor (TLR) and retinoic acid-inducible gene I (RIG-I)-like helicase signaling pathway. J Biol Chem. 2008;283:33858–33864. doi: 10.1074/jbc.M806923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chow J, Tang H, Mazmanian SK. Pathobionts of the gastrointestinal microbiota and inflammatory disease. Curr Opin Immunol. 2011;23:473–480. doi: 10.1016/j.coi.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamada N, Chen GY, Inohara N, Nunez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013;14:685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ivory CP, Prystajecky M, Jobin C, Chadee K. Toll-like receptor 9-dependent macrophage activation by Entamoeba histolytica DNA. Infect Immun. 2008;76:289–297. doi: 10.1128/IAI.01217-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carrasco DR, Fenton T, Sukhdeo K, Protopopova M, Enos M, You MJ, et al. The PTEN and INK4A/ARF tumor suppressors maintain myelolymphoid homeostasis and cooperate to constrain histiocytic sarcoma development in humans. Cancer Cell. 2006;9:379–390. doi: 10.1016/j.ccr.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 64.Been RA, Linden MA, Hager CJ, DeCoursin KJ, Abrahante JE, Landman SR, et al. Genetic signature of histiocytic sarcoma revealed by a sleeping beauty transposon genetic screen in mice. PLoS One. 2014;9:e97280. doi: 10.1371/journal.pone.0097280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hedan B, Thomas R, Motsinger-Reif A, Abadie J, Andre C, Cullen J, et al. Molecular cytogenetic characterization of canine histiocytic sarcoma: A spontaneous model for human histiocytic cancer identifies deletion of tumor suppressor genes and highlights influence of genetic background on tumor behavior. BMC Cancer. 2011;11:201. doi: 10.1186/1471-2407-11-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin-Caballero J, Flores JM, Garcia-Palencia P, Serrano M. Tumor susceptibility of p21(Waf1/Cip1)-deficient mice. Cancer Res. 2001;61:6234–6238. [PubMed] [Google Scholar]
- 67.Ward JM, Nikolov NP, Tschetter JR, Kopp JB, Gonzalez FJ, Kimura S, et al. Progressive glomerulonephritis and histiocytic sarcoma associated with macrophage functional defects in CYP1B1-deficient mice. Toxicol Pathol. 2004;32:710–718. doi: 10.1080/01926230490885706. [DOI] [PubMed] [Google Scholar]
- 68.Eischen CM, Rehg JE, Korsmeyer SJ, Cleveland JL. Loss of Bax alters tumor spectrum and tumor numbers in ARF-deficient mice. Cancer Res. 2002;62:2184–2191. [PubMed] [Google Scholar]
- 69.Mashima R, Honda K, Yang Y, Morita Y, Inoue A, Arimura S, et al. Mice lacking Dok-1, Dok-2, and Dok-3 succumb to aggressive histiocytic sarcoma. Lab Invest. 2010;90:1357–1364. doi: 10.1038/labinvest.2010.121. [DOI] [PubMed] [Google Scholar]
- 70.Snyder JM, Treuting PM, Nagy L, Yam C, Yi J, Brasfield A, et al. Humanized TLR7/8 expression drives proliferative multisystemic histiocytosis in C57BL/6 mice. PLoS One. 2014;9:e107257. doi: 10.1371/journal.pone.0107257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shaffer AL, Rosenwald A, Staudt LM. Lymphoid malignancies: the dark side of B-cell differentiation. Nat Rev Immunol. 2002;2:920–932. doi: 10.1038/nri953. [DOI] [PubMed] [Google Scholar]
- 72.Kuppers R, Dalla-Favera R. Mechanisms of chromosomal translocations in B cell lymphomas. Oncogene. 2001;20:5580–5594. doi: 10.1038/sj.onc.1204640. [DOI] [PubMed] [Google Scholar]
- 73.Peled JU, Kuang FL, Iglesias-Ussel MD, Roa S, Kalis SL, Goodman MF, et al. The biochemistry of somatic hypermutation. Annu Rev Immunol. 2008;26:481–511. doi: 10.1146/annurev.immunol.26.021607.090236. [DOI] [PubMed] [Google Scholar]
- 74.Pradere JP, Dapito DH, Schwabe RF. The Yin and Yang of Toll-like receptors in cancer. Oncogene. 2014;33:3485–3495. doi: 10.1038/onc.2013.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sato Y, Goto Y, Narita N, Hoon DS. Cancer Cells Expressing Toll-like Receptors and the Tumor Microenvironment. Cancer Microenviron. 2009;2(Suppl 1):205–214. doi: 10.1007/s12307-009-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang J, Xie Y, Sun X, Zeh HJ, 3rd, Kang R, Lotze MT, et al. DAMPs, ageing, and cancer: The 'DAMP Hypothesis'. Ageing Res Rev. 2014 doi: 10.1016/j.arr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 78.Mechtcheriakova D, Svoboda M, Meshcheryakova A, Jensen-Jarolim E. Activation-induced cytidine deaminase (AID) linking immunity, chronic inflammation, and cancer. Cancer Immunol Immunother. 2012;61:1591–1598. doi: 10.1007/s00262-012-1255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hasselbalch HC. Chronic inflammation as a promotor of mutagenesis in essential thrombocythemia, polycythemia vera and myelofibrosis. A human inflammation model for cancer development? Leuk Res. 2013;37:214–220. doi: 10.1016/j.leukres.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 80.Kamp DW, Shacter E, Weitzman SA. Chronic inflammation and cancer: the role of the mitochondria. Oncology (Williston Park) 2011;25:400–410. 413. [PubMed] [Google Scholar]
- 81.Beekman R, Touw IP. G-CSF and its receptor in myeloid malignancy. Blood. 2010;115:5131–5136. doi: 10.1182/blood-2010-01-234120. [DOI] [PubMed] [Google Scholar]
- 82.Gaffen SL. Recent advances in the IL-17 cytokine family. Curr Opin Immunol. 2011;23:613–619. doi: 10.1016/j.coi.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Das S, Sarrou E, Podgrabinska S, Cassella M, Mungamuri SK, Feirt N, et al. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. J Exp Med. 2013;210:1509–1528. doi: 10.1084/jem.20111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Conti I, Rollins BJ. CCL2 (monocyte chemoattractant protein-1) and cancer. Semin Cancer Biol. 2004;14:149–154. doi: 10.1016/j.semcancer.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 85.Charo IF, Peters W. Chemokine receptor 2 (CCR2) in atherosclerosis, infectious diseases, and regulation of T-cell polarization. Microcirculation. 2003;10:259–264. doi: 10.1038/sj.mn.7800191. [DOI] [PubMed] [Google Scholar]
- 86.Liu M, Guo S, Stiles JK. The emerging role of CXCL10 in cancer (Review) Oncol Lett. 2011;2:583–589. doi: 10.3892/ol.2011.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baniyash M, Sade-Feldman M, Kanterman J. Chronic inflammation and cancer: suppressing the suppressors. Cancer Immunol Immunother. 2014;63:11–20. doi: 10.1007/s00262-013-1468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wesolowski R, Markowitz J, Carson WE., 3rd Myeloid derived suppressor cells - a new therapeutic target in the treatment of cancer. J Immunother Cancer. 2013;1:10. doi: 10.1186/2051-1426-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brandau S, Moses K, Lang S. The kinship of neutrophils and granulocytic myeloid-derived suppressor cells in cancer: cousins, siblings or twins? Semin Cancer Biol. 2013;23:171–182. doi: 10.1016/j.semcancer.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 90.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parker KH, Sinha P, Horn LA, Clements VK, Yang H, Li J, et al. HMGB1 enhances immune suppression by facilitating the differentiation and suppressive activity of myeloid-derived suppressor cells. Cancer Res. 2014;74:5723–5733. doi: 10.1158/0008-5472.CAN-13-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maenhout SK, Thielemans K, Aerts JL. Location, location, location: functional and phenotypic heterogeneity between tumor-infiltrating and non-infiltrating myeloid-derived suppressor cells. Oncoimmunology. 2014;3:e956579. doi: 10.4161/21624011.2014.956579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185:2273–2284. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu J, Zhou X, Nakaya M, Jin W, Cheng X, Sun SC. T cell-intrinsic function of the noncanonical NF-kappaB pathway in the regulation of GM-CSF expression and experimental autoimmune encephalomyelitis pathogenesis. J Immunol. 2014;193:422–430. doi: 10.4049/jimmunol.1303237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paschall AV, Zhang R, Qi CF, Bardhan K, Peng L, Lu G, et al. IFN regulatory factor 8 represses GM-CSF expression in T cells to affect myeloid cell lineage differentiation. J Immunol. 2015;194:2369–2379. doi: 10.4049/jimmunol.1402412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu J, Wang Y, Yan F, Zhang P, Li H, Zhao H, et al. Noncanonical NF-kappaB activation mediates STAT3-stimulated IDO upregulation in myeloid-derived suppressor cells in breast cancer. J Immunol. 2014;193:2574–2586. doi: 10.4049/jimmunol.1400833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khaled YS, Ammori BJ, Elkord E. Myeloid-derived suppressor cells in cancer: recent progress and prospects. Immunol Cell Biol. 2013;91:493–502. doi: 10.1038/icb.2013.29. [DOI] [PubMed] [Google Scholar]
- 99.Moore CR, Edwards SK, Xie P. Targeting TRAF3 Downstream Signaling Pathways in B cell Neoplasms. J Cancer Sci Ther. 2015;7:67–74. doi: 10.4172/1948-5956.1000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bushell KR, Kim Y, Chan FC, Ben-Neriah S, Jenks A, Alcaide M, et al. Genetic inactivation of TRAF3 in canine and human B-cell lymphoma. Blood. 2014 doi: 10.1182/blood-2014-10-602714. [DOI] [PubMed] [Google Scholar]
- 101.Zhang B, Calado DP, Wang Z, Frohler S, Kochert K, Qian Y, et al. An oncogenic role for alternative NF-kappaB signaling in DLBCL revealed upon deregulated BCL6 expression. Cell Rep. 2015;11:715–726. doi: 10.1016/j.celrep.2015.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chung GT, Lou WP, Chow C, To KF, Choy KW, Leung AW, et al. Constitutive activation of distinct NF-kappaB signals in EBV-associated nasopharyngeal carcinoma. J Pathol. 2013;231:311–322. doi: 10.1002/path.4239. [DOI] [PubMed] [Google Scholar]
- 103.Network TCGA. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Consortium IMSG. Network-based multiple sclerosis pathway analysis with GWAS data from 15,000 cases and 30,000 controls. Am J Hum Genet. 2013;92:854–865. doi: 10.1016/j.ajhg.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Perez de Diego R, Sancho-Shimizu V, Lorenzo L, Puel A, Plancoulaine S, Picard C, et al. Human TRAF3 adaptor molecule deficiency leads to impaired Toll-like receptor 3 response and susceptibility to herpes simplex encephalitis. Immunity. 2010;33:400–411. doi: 10.1016/j.immuni.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shen J, Qiao Y, Ran Z, Wang T, Xu J, Feng J. Intestinal protein expression profile identifies inflammatory bowel disease and predicts relapse. Int J Clin Exp Pathol. 2013;6:917–925. [PMC free article] [PubMed] [Google Scholar]
- 107.Shen J, Qiao YQ, Ran ZH, Wang TR. Up-regulation and pre-activation of TRAF3 and TRAF5 in inflammatory bowel disease. Int J Med Sci. 2013;10:156–163. doi: 10.7150/ijms.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Momeni M, Zainodini N, Bidaki R, Hassanshahi G, Daneshvar H, Khaleghinia M, et al. Decreased expression of toll like receptor signaling molecules in chronic HBV infected patients. Hum Immunol. 2014;75:15–19. doi: 10.1016/j.humimm.2013.09.015. [DOI] [PubMed] [Google Scholar]