Abstract
Background
Although transcatheter aortic valve replacement (TAVR) is an effective treatment for aortic stenosis, long-term mortality after TAVR remains high and challenging to predict. The Kansas City Cardiomyopathy Questionnaire (KCCQ) is a health status measure, assessed directly from patients, that integrates two clinically relevant factors (symptoms and functional status) that may predict TAVR outcomes.
Methods and Results
Among 7769 patients from 286 sites in the STS-ACC TVT Registry, we examined the association between pre-procedure (baseline) patient health status, as assessed by the KCCQ, and 1-year mortality after TAVR. The KCCQ Overall Summary Score was categorized as very poor: <25, poor: 25–49, fair: 50–74, or good: ≥75. Prior to TAVR, health status was rated as very poor in 28%, poor in 38%, fair in 24%, and good in 10%. Patients with worse health status were more likely to be female and had more comorbidities and higher STS mortality risk scores. Compared with those with good health status prior to TAVR, and after adjusting for a broad range of baseline covariates, patients with very poor health status had a 2-fold increased hazard of death over the first year after TAVR (adjusted HR 2.00, 95% CI 1.58–2.54), while those with poor and fair health status had intermediate outcomes (adjusted HRs 1.54, 95% CI 1.22–1.95 and 1.20, 95% CI 0.94–1.55, respectively).
Conclusions
In a national, contemporary practice cohort, worse pre-procedure patient health status, as assessed by the KCCQ, was associated with greater long-term mortality after TAVR. These results support the measurement and integration of the KCCQ into mortality risk assessments for patients considering TAVR.
Keywords: transcatheter aortic valve replacement, quality of life, mortality
Transcatheter aortic valve replacement (TAVR) offers substantial reductions in mortality as compared with medical therapy among inoperable patients,1 and at least equivalent outcomes to surgical AVR among high-risk patients.2, 3 However, given that elderly patients with multiple comorbidities are the primary candidates for TAVR, mortality after TAVR remains high and difficult to predict.4–7 Across different study populations and settings, some factors have been consistently found to be prognostically important among patients undergoing TAVR including worse heart failure symptoms (assessed by New York Heart Association [NYHA]) and poor functional capacity (assessed with a 6-minute walk test).4, 7–9 However, each of these measures has challenges in its assessment. NYHA class, as a physician-reported measure, can be an unreliable assessment of patient-reported symptoms and provides only a coarse assessment of symptoms.10, 11 Six-minute walk tests, while providing an objective measure of functional status, can be difficult to routinely collect in clinical settings.
The Kansas City Cardiomyopathy Questionnaire (KCCQ) is a disease-specific instrument originally developed to describe and monitor health status in patients with heart failure.12–14 The KCCQ integrates patients’ symptoms, functional status, and quality of life into a single measure and has been shown to predict mortality in various heart failure populations15 and has also been shown to be prognostically significant in patients with medically managed aortic stenosis.16 To date, however, the association of the KCCQ with mortality after TAVR has not been evaluated. If health status is an independent prognostic risk factor for TAVR patients, its inclusion in risk models seeking to compare the quality of TAVR across centers would enhance the fairness and quality of these efforts. To determine whether pre-procedure health status is associated with survival after TAVR, we used data from the Society of Thoracic Surgeons (STS)/American College of Cardiology (ACC) Transcatheter Valve Therapy (TVT) Registry to examine whether baseline KCCQ scores are associated with short- and long-term mortality after TAVR.
METHODS
Study Sample and Protocol
Details of the design, structure, and data elements for the TVT Registry have been previously published.17, 18 In brief, the registry is a joint initiative of the STS and ACC and was launched in 2011, following Food and Drug Administration approval of the Sapien Transcatheter Heart Valve. The TVT Registry was originally developed in response to the Centers for Medicare & Medicaid Services’ requirement for national registry participation of all TAVR centers and now includes more than 300 clinical sites. Registry activities have been approved by a central institutional review board, and the Duke University School of Medicine institutional review board granted a waiver of informed consent for this study. Participating sites collect data on patient demographics, comorbidities, hemodynamics, functional status, patient-reported health status, and outcomes. Data quality is maintained through site training and support by TVT Registry staff, data integrity checks by the analytic centers, auditing portions of data at the site level, and adjudication of selected 30-day and 1-year outcomes. The TVT Registry has been linked to Medicare administrative claims using direct patient identifiers by the Centers for Medicare & Medicaid Services in order to evaluate long-term patient outcomes, including hospitalizations and survival.19
Health Status Assessment
The Kansas City Cardiomyopathy Questionnaire (KCCQ) is a patient-reported disease-specific health status survey originally developed to describe and monitor health status in patients with heart failure.12 The KCCQ has undergone extensive reliability and validity testing in various heart failure populations,12, 20 as well as those with severe aortic stenosis.16 Recently, a 12-item version of the KCCQ has been developed and found to be psychometrically valid,21 and this version was collected in the TVT Registry. For this study, we focused on the overall summary score of the KCCQ-12, which ranges from 0 to 100 with higher scores indicating less symptom burden, less physical and social limitations, and better quality of life. Linguistically and culturally validated translations of the KCCQ were provided to non-English speakers. As in previous work, we categorized the KCCQ Overall Summary Score as very poor health status (KCCQ <25), poor (KCCQ 25–49), fair (KCCQ 50–74), and good (KCCQ ≥75).15, 16
Statistical Analysis
Baseline characteristics and in-hospital outcomes were compared across health status groups using the Cochran-Armitage test for categorical variables and the Jonckheere-Terpstra test for continuous variables. The mortality risk of the patients was estimated using STS Predicted Risk of Operative Mortality score, which includes 24 variables and has been validated for predicting mortality from surgical aortic valve replacement.22 The association of health status with mortality over the year following TAVR was assessed with the Kaplan-Meier method and log-rank test (for unadjusted comparisons) and Cox proportional hazards models (for risk-adjusted comparisons). Covariates in our adjusted model were selected from a recently developed TVT risk-adjustment model for in-hospital mortality and included age, body surface area, sex, race, estimated glomerular filtration rate, left ventricular ejection fraction, hemoglobin, platelet count, left main coronary artery stenosis ≥50%, proximal left anterior descending artery stenosis, prior myocardial infarction, current dialysis, endocarditis, prior transient ischemic attack, prior stroke, carotid stenosis, peripheral artery disease, current smoker, diabetes mellitus, atrial fibrillation or flutter, conduction defects, severe chronic lung disease, home oxygen, hostile chest, porcelain aorta, access site (femoral vs. other), permanent pacemaker, implantable defibrillator, prior percutaneous coronary intervention, prior coronary artery bypass graft surgery, prior cardiac operations (2 or more vs. 1 vs. 0), prior aortic procedure, prior non-aortic procedure, aortic stenosis etiology (degenerative vs. other), valve morphology (tricuspid vs. other), aortic insufficiency (moderate/severe vs. other), mitral insufficiency (moderate/severe vs. other), tricuspid insufficiency (moderate/severe vs. other), and patient acuity (urgent vs. shock/inotropes/assist device vs. emergency/salvage/cardiac arrest).
The characteristics of patients who were missing baseline KCCQ data were compared with those in the analytic cohort using the chi-square test for categorical variables and the Mann-Whitney test for continuous variables. Unadjusted and adjusted mortality were compared between groups using similar methods as above (log-rank test and Cox proportional hazards models, respectively). There were modest differences in baseline characteristics as well as outcomes between those with and without missing KCCQ data (Supplemental Table 1). For purposes of sensitivity analysis, missing independent variable data were assumed to be missing at random conditional on observed patient demographic and clinical factors and outcomes. We estimated baseline missing KCCQ data using sequential regression modeling and 20 imputed data sets23. The models were re-run on the imputed data sets and the results pooled. All analyses were performed with SAS version 9.3 (SAS Institute, Cary, North Carolina), and statistical significance was defined as a 2-sided p-value of <0.05.
RESULTS
Study Sample
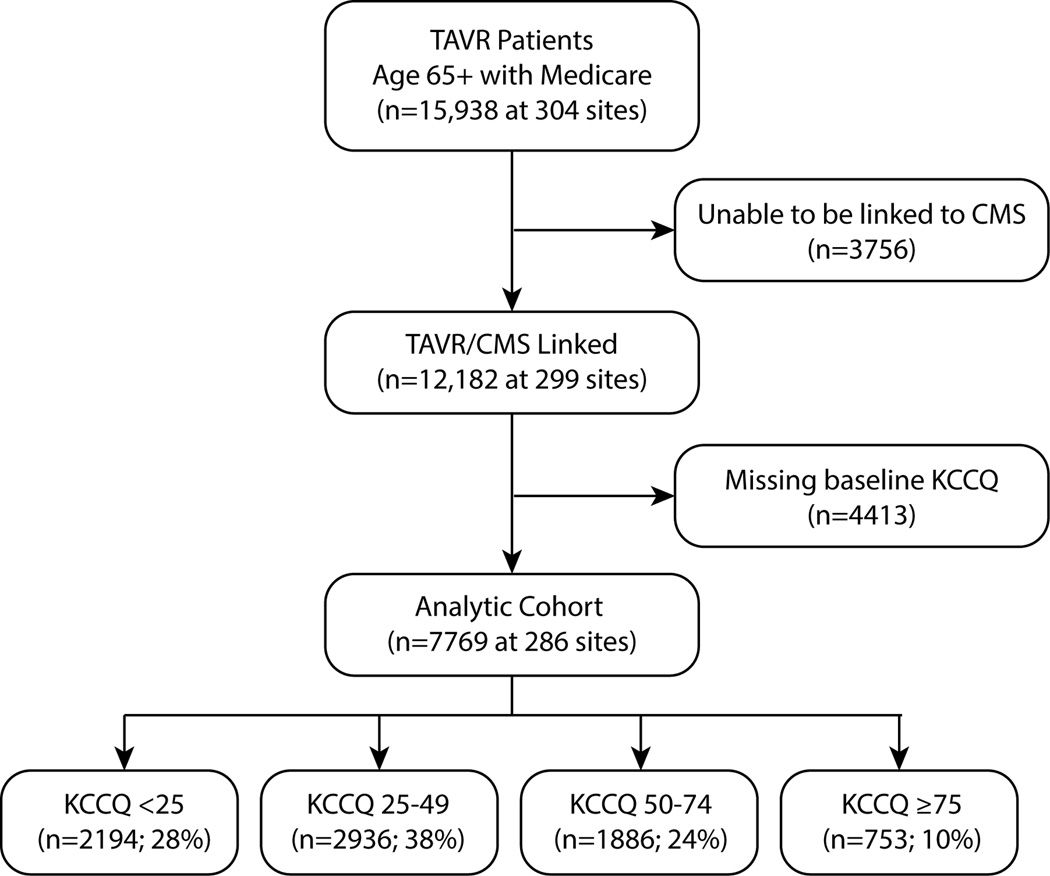
Between November 2011 and June 2014, a total of 15,938 patients aged 65 years or older underwent TAVR at 304 sites and were included in the TVT Registry. We excluded 3756 patients who were unable to be linked to Medicare claims data, resulting in an eligible cohort of 12,182 patients. After further excluding 4413 (36%) patients who were missing baseline KCCQ data, our final analytic cohort included 7769 patients who were enrolled at 286 sites (Figure 1). Patients who were missing baseline KCCQ data tended to be sicker than those in the analytic cohort, with more lung disease, more kidney disease, higher STS mortality risk scores, and were more likely to be deemed inoperable or prohibitive surgical risk (Supplemental Table 1). Over the 12 months following TAVR, patients with missing baseline KCCQ were also more likely to die as compared with those in the analytic cohort, with unadjusted mortality rates of 26.3% vs. 21.8%, respectively (p<0.001; Supplemental Figure). This increased hazard of death among those patients with missing data remained after multivariable adjustment (adjusted HR 1.21, 95% CI 1.10–1.34).
Figure 1. Flow Chart of the Analytic Cohort.
Median age for the analytic cohort was 84 years, 47% were male, 31% had a prior CABG, 34% had diabetes, and 13% were on home oxygen. Median STS mortality risk score was 7.0 (Q1–Q3 4.7–10.6); median mean aortic gradient was 43 mmHg; and 51% underwent TAVR via a femoral approach. Prior to TAVR, the median KCCQ score was 37.5 (Q1–Q3 22.4–56.8), and health status was rated as very poor in 28%, poor in 38%, fair in 24%, and good in 10%. The baseline characteristics of the 4 groups are shown in Table 1. Patients with poorer baseline health status were more likely to be female and were more likely to have lung disease, kidney disease, and diabetes. Poorer health status was also associated with longer times on the 5-meter walk test, lower mean aortic valve gradients, more moderate or severe mitral regurgitation, and higher estimated surgical mortality risk (per the STS mortality risk score).
Table 1.
Baseline characteristics, according to health status prior to TAVR
| KCCQ <25 (N=2194) |
KCCQ 25–49 (N=2936) |
KCCQ 50–74 (N=1886) |
KCCQ 75–100 (N=753) |
P for trend |
|
|---|---|---|---|---|---|
| Age, yr | 84 (78,88) | 84 (79,88) | 85 (80,89) | 85 (80,89) | <0.001 |
| Male sex | 41.7% | 48.1% | 48.7% | 53.4% | <0.001 |
| Body surface area, m2 | 1.8 (1.7,2.0) | 1.8 (1.6,2.0) | 1.8 (1.6,1.9) | 1.8 (1.6,1.9) | <0.001 |
| Prior PCI | 34.8% | 36.7% | 35.3% | 32.9% | 0.516 |
| Prior CABG | 28.3% | 32.6% | 32.1% | 31.4% | 0.031 |
| Peripheral arterial disease | 31.4% | 33.7% | 33.7% | 30.7% | 0.693 |
| Hypertension | 91.2% | 89.3% | 90.3% | 89.3% | 0.231 |
| Oxygen-dep lung disease | 22.0% | 13.0% | 7.3% | 4.5% | <0.001 |
| Diabetes mellitus | 39.5% | 34.1% | 30.7% | 31.0% | <0.001 |
| On insulin | 15.9% | 11.8% | 9.0% | 8.4% | <0.001 |
| Prior stroke or TIA | 19.5% | 18.8% | 18.6% | 17.6% | 0.239 |
| Hemoglobin, g/dL | 11.2 (10.1,12.5) | 11.8 (10.6,12.9) | 11.9 (10.8,13.0) | 12.1 (10.9,13.1) | <0.001 |
| Creatinine, mg/dL | 1.1 (0.9,1.5) | 1.1 (0.9,1.4) | 1.1 (0.8,1.4) | 1.0 (0.8,1.4) | <0.001 |
| Current dialysis | 4.7% | 3.6% | 2.0% | 2.1% | <0.001 |
| 5-Meter Walk Test attempted | 58.8% | 69.3% | 72.9% | 73.3% | <0.001 |
| STS Mortality Score | 8.2 (5.4,12.3) | 6.9 (4.8,10.5) | 6.5 (4.4,9.6) | 5.9 (4.0,8.7) | <0.001 |
| Aortic valve area, cm2 | 0.6 (0.5,0.8) | 0.7 (0.5,0.8) | 0.7 (0.5,0.8) | 0.7 (0.5,0.8) | 0.019 |
| Mean aortic gradient, mmHg | 43.0 (35.0,51.0) | 43.0 (36.0,52.0) | 44.0 (36.0,53.0) | 45.0 (38.0,55.0) | <0.001 |
| Aortic regurgitation (mod/sev) | 21.0% | 19.7% | 18.6% | 19.4% | 0.127 |
| Mitral regurgitation (mod/sev) | 40.7% | 34.4% | 36.2% | 30.5% | <0.001 |
Data are presented as median (Q1–Q3) or %
TAVR, transcatheter aortic valve replacement; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft surgery; TIA, transient ischemic attack; STS, Society of Thoracic Surgeons
In-Hospital Outcomes
Poorer health status was associated with a more prolonged hospital stay and an increased risk of death following TAVR (Table 2). In-hospital mortality was strongly associated with baseline health status, ranging from 6.2% among those with very poor health status to 2.8% among those with good health status (p<0.001 for test of trend). Worse baseline health status was also associated with an increased likelihood of new dialysis during the index hospitalization (p<0.001 for test of trend). Health status was not associated with the risk of other in-hospital complications. Length of stay was longer, both in the intensive care unit and in the hospital overall, for patients with poorer health status, and among those who survived the hospitalization, patients with poorer health status were less likely to be discharged to home.
Table 2.
In-hospital events, according to health status prior to TAVR
| KCCQ <25 (N=2194) |
KCCQ 25–49 (N=2936) |
KCCQ 50–74 (N=1886) |
KCCQ 75–100 (N=753) |
P for trend |
|
|---|---|---|---|---|---|
| Procedural Details | |||||
| Femoral access | 52.0% | 50.2% | 51.6% | 51.5% | 0.923 |
| Procedure duration (hrs) | 2.18 (1.68,2.97) | 2.15 (1.65,2.83) | 2.10 (1.62,2.85) | 2.12 (1.65,2.80) | 0.003 |
| Conversion of open surgery | 1.2% | 1.5% | 1.4% | 0.8% | 0.605 |
| Need for cardiopulmonary bypass | 5.1% | 3.9% | 3.3% | 2.9% | 0.001 |
| Need for acute valve-in-valve | 4.8% | 4.3% | 3.8% | 4.5% | 0.262 |
| Contrast volume (mL) | 105 (70,150) | 105 (70,160) | 105 (70,160) | 105 (70,160) | 0.640 |
| Fluouroscopy time (min) | 16.5 (11.3,23.2) | 16.4 (11.1,23.2) | 16.0 (11.2,22.9) | 16.5 (11.8,23.4) | 0.606 |
| In-hospital Complications | |||||
| Any in-hospital valve complication | 1.6% | 2.1% | 1.9% | 2.0% | 0.452 |
| Death | 6.2% | 4.9% | 3.1% | 2.8% | <0.001 |
| Cardiac arrest | 5.9% | 5.3% | 4.2% | 4.1% | 0.005 |
| Transient ischemic attack | 0.5% | 0.2% | 0.2% | 0.4% | 0.221 |
| Stroke | 1.9% | 2.0% | 2.4% | 1.6% | 0.735 |
| Myocardial infarction | 0.7% | 0.8% | 0.8% | 0.7% | 0.876 |
| New atrial fibrillation | 5.7% | 6.2% | 7.2% | 7.6% | 0.018 |
| Transapical access complication | 0.3% | 0.2% | 0.2% | 0.4% | 0.955 |
| New renal dialysis | 2.9% | 1.3% | 1.7% | 0.7% | <0.001 |
| New post-operative Cr ≥3 mg/dL | 10.3% | 8.1% | 6.0% | 4.7% | <0.001 |
| Major bleeding | 11.8% | 11.1% | 10.9% | 9.9% | 0.125 |
| Multiple transcatheter valves used | 6.1% | 7.2% | 8.7% | 7.3% | 0.012 |
| New permanent pacemaker | 4.8% | 4.6% | 4.6% | 5.5% | 0.674 |
| Length of Stay/Discharge | |||||
| Length of stay in ICU, hours | 48.0 (25.9,96.0) | 44.0 (24.3,74.0) | 43.9 (25.0,73.0) | 41.1 (25.0,72.0) | <0.001 |
| Length of hospital stay, days | 7.0 (5.0,12.0) | 6.0 (4.0,9.0) | 6.0 (4.0,9.0) | 6.0 (4.0,8.0) | <0.001 |
| Discharge location (among survivors) | <0.001 | ||||
| Home | 52.3% | 60.1% | 63.4% | 68.0% | |
| Rehab/nursing home | 46.1% | 39.0% | 35.4% | 31.0% | |
| Other | 2.0% | 1.0% | 1.3% | 1.0% |
Data are presented as median (Q1–Q3) or %
TAVR, transcatheter aortic valve replacement; TIA, transient ischemic attack; ICU, intensive care unit
Long-term Outcomes
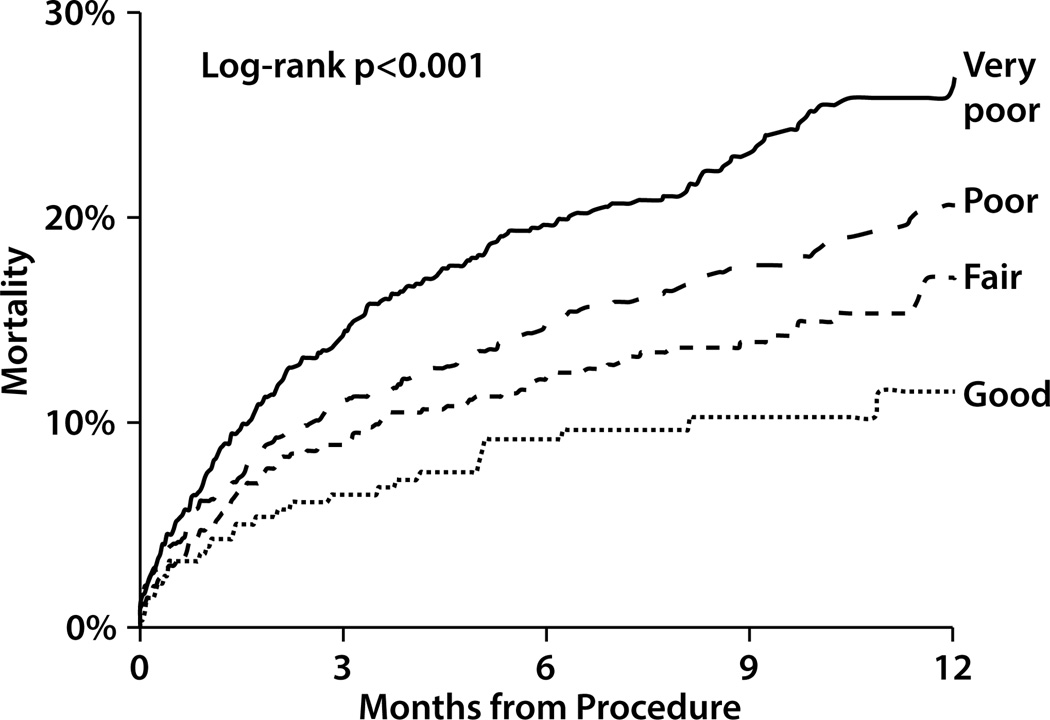
Poorer health status prior to TAVR was associated with an increased risk of mortality after the procedure—a difference that emerged early and continued to increase throughout the first year of follow-up. One year after TAVR, 29% of patients with very poor baseline health status had died, as compared with 22% with poor health status, 17% with fair health status, and 14% with good health status (p<0.001; Figure 2). After adjustment for multiple demographic and clinical characteristics, poorer health status prior to TAVR continued to be associated with an increased hazard of death after TAVR. After multivariable risk-adjustment, compared with those with good health status prior to TAVR, patients with very poor health status had a 2-fold increased hazard of death over the first year after TAVR (adjusted HR 2.00, 95% CI 1.58–2.54), while those with poor and fair health status had intermediate outcomes (adjusted HRs 1.54, 95% CI 1.22–1.95 and 1.20, 95% CI 0.94–1.55, respectively). In the sensitivity analysis where missing baseline KCCQ data were imputed, the results were consistent with the main analysis (Supplemental Table 2).
Figure 2. Kaplan-Meier Mortality Curves for Patient After TAVR According to Baseline Health Status.
Blue line indicates those patients with very poor health status prior to TAVR (KCCQ <25); red line: poor health status (KCCQ 25–49); green line: fair health status (KCCQ 50–74); and brown line: good health status (KCCQ ≥75)
DISCUSSION
In this large, multicenter cohort of contemporary clinical practice, we found that the majority of patients who underwent commercially-available TAVR in the US between 2011–2014 had poor or very poor pre-procedure disease-specific health status. Patients with poorer baseline health status tended to have more comorbidities, higher estimated mortality risk, and worse mobility. Worse health status at baseline was associated with a greater risk of in-hospital and 1-year mortality, even after extensive adjustment for demographic and clinical characteristics. Nonetheless, even among patients with the worst health status prior to TAVR, over 70% were still alive at 1 year after their procedure, indicating that poor health status prior to TAVR should not, in isolation, be considered a contraindication for TAVR. This is a particularly important finding, as a high burden of heart failure symptoms that greatly impact quality of life is often a primary indication for TAVR. Finally, these results support the measurement and integration of the KCCQ into mortality risk assessments for TAVR and should encourage the continued collection of these data as a part of the TVT Registry and other similar quality improvement and quality assurance efforts.
The results of this study both support and extend prior research on the prognostic importance of patient health status metrics in cardiovascular populations. In patients with systolic heart failure, several previous studies have shown that lower KCCQ scores are associated with a greater risk of long-term mortality.11, 13, 15, 24 For example, in the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival (EPHESUS) Study, 18% of patients with a KCCQ score <25 at 1 month post-myocardial infarction had died by 1 year as compared with 6% of patients with KCCQ scores ≥75.15 The prognostic importance of heart failure-specific health status has also been shown among patients with severe aortic stenosis. In the PARTNER trial, among medically managed patients with severe aortic stenosis, nearly 75% of patients with baseline KCCQ scores ≤25 were dead by 1 year, as compared with only ~30% of patients with KCCQ scores >50.16
In contrast, pre-procedure KCCQ has not been found to predict mortality in heart failure patients treated with left ventricular assist devices (LVADs).25, 26 However, there are a number of potential reasons for the discrepancy between our data and those observed in the LVAD patient population. First, LVADs are often implanted when the patient is acutely ill and in cardiogenic shock. In these situations, KCCQ scores are acutely depressed and may not accurately reflect the chronic heart failure symptoms and functional capacity of the patient. This situation is in contrast to that for TAVR, which is rarely performed emergently. Second, LVADs are associated with a number of late complications and adverse events, such as gastrointestinal bleeding, stroke and infections, that impact subsequent mortality to a much greater degree than after TAVR.27
Prior work exploring factors associated with mortality after TAVR supports the hypothesis that the KCCQ would be prognostically important. Reduced functional capacity, as assessed using a 6-minute walk test, has been shown to be a strong correlate of mortality after TAVR.7, 8 Unfortunately, the 6-minute walk test can be cumbersome to complete in the clinic and, even more so, in the hospital. In addition, many patients who are being considered for TAVR have additional disabilities that may limit their ability to complete a 6-minute walk test, independent of their cardiovascular status. The KCCQ, as a patient-reported questionnaire is straightforward to administer both in the outpatient and inpatient setting, assesses functional capacity, and has been shown to retain much of the predictive ability of objective functional assessments.28
In addition to functional capacity, the KCCQ assesses the severity of heart failure symptoms, which has also been shown to be prognostically important in the form of NYHA class. However, the NYHA class is a coarse assessment of symptoms as compared with the KCCQ, which limits its ability to discriminate between patients.10, 11 Furthermore, since NYHA class is assigned by the physician and not by the patient, there is the potential for misclassification of symptoms. For example, in our dataset, 10% of patients with KCCQ scores <25 were rated as NYHA class I–II by their physician while 67% of patients with KCCQ scores ≥75 were classified as NYHA class III-IV (Supplemental Table 3). Prior studies in patients with coronary artery disease have also shown substantial discordance between patient-reported and physician-assessed symptoms.29 While additional studies are needed to further define the discrepancy between physician- and patient-assessed health status in patients undergoing TAVR, we hypothesize that using a validated instrument to assess symptoms and functional status directly from the patient provides much greater reliability, and thus greater predictive ability, as compared with more indirect assessments.
Patient-reported outcomes are increasingly a focus of measurement in the healthcare system, which is likely only to increase as healthcare reform evolves. The KCCQ has established value in the setting of TAVR (a procedure designed not only to improve survival but also improve quality of life)—quantifying the patient’s response to therapy, enabling the comparison of new devices as technologies advance, and ensuring appropriate patient selection (through responder analyses). However, our data demonstrate that the KCCQ also has value as a correlate of subsequent outcomes at the time of TAVR—improving risk adjustment for mortality beyond a model that included 39 demographic, clinical, and echocardiographic variables. These results highlight the value of measuring the KCCQ in these patients, both pre-procedurally (to risk stratify) and through follow-up (to monitor response to therapy).
Limitations
This study has a number of potential limitations that warrant further discussion. First, a substantial proportion of patients were missing baseline health status data. Prior to TAVR, these patients tended to be sicker and at higher risk for morbidity and mortality. They were also more likely to die in the year following TAVR, as compared with patients who completed the KCCQ. We attempted to address this limitation by performing a sensitivity analysis with imputation of missing baseline KCCQ data. As expected, after imputation, the proportion of patients with low and very low KCCQ scores at baseline increased. However, the relationship between the KCCQ and mortality was not materially altered by inclusion of these patients with missing data. Second, as part of the TVT registry, the KCCQ is also collected in follow-up after TAVR. However, at this time, follow-up health status data are missing in a large proportion of patients, precluding meaningful examination of this important additional endpoint. Given the importance of improved health status as a goal of TAVR, future studies are needed to explore this outcome. Finally, as with any observational analysis, there is a possibility for residual confounding. We attempted to mitigate this risk through extensive adjustment for demographic and clinical characteristics. Furthermore, our results are consistent with prior studies demonstrating the predictive value of patient health status in other populations, thereby increasing our confidence in the validity of our results.
Conclusions
Patient-reported health status, as assessed by the KCCQ, is substantially impaired among US patients undergoing TAVR. Moreover, worse pre-procedure health status is associated with an increased risk of in-hospital and 1-year mortality after TAVR, even after adjusting for an extensive list of demographic and clinical characteristics. These findings demonstrate the importance of reliably assessing patient-reported symptoms and functional capacity prior to TAVR in order to best risk stratify patients and improve prediction and communication of long-term risks. Since the KCCQ is reliable, patient-centered, and easily collected in routine clinical practice, we believe that these findings should encourage the continued collection of the KCCQ as a part of the routine evaluation of TAVR patients. Future work is needed to examine the value of the KCCQ for predicting outcomes that integrate survival and quality of life.
Supplementary Material
What is Known
1-year mortality after transcatheter aortic valve replacement (TAVR) remains high and challenging to predict.
The Kansas City Cardiomyopathy Questionnaire (KCCQ) is a health status measure that integrates patients’ symptoms, functional status, and quality of life into a single measure—factors that could be associated with mortality after TAVR.
What the Study Adds
Among 7769 patients from 286 sites in the STS-ACC TVT Registry, we found that the majority of patients who underwent commercially available TAVR in the US between 2011–2014 had poor or very poor pre-procedure disease-specific health status.
Worse health status at baseline was associated with a greater risk of in-hospital and 1-year mortality, although event rates among those with even the worst pre-TAVR health status were not high enough to consider poor health status, in isolation, a contraindication for TAVR.
Since the KCCQ is reliable, patient-centered, and easily collected in routine clinical practice, we believe that these findings support the measurement and integration of the KCCQ into mortality risk assessments for TAVR and should encourage the continued collection of these data as a part of the TVT Registry and as a part of the routine evaluation of TAVR.
Acknowledgments
SOURCES OF FUNDING: The STS/ACC TVT Registry™ is an initiative of the Society of Thoracic Surgeons and the American College of Cardiology. This research was supported by the American College of Cardiology’s National Cardiovascular Data Registry (NCDR). The views expressed in this manuscript represent those of the authors, and do not necessarily represent the official views of the NCDR or its associated professional societies identified at CVQuality.ACC.org/NCDR. The study sponsors were not involved in the design and conduct of the study; analysis and interpretation of the data; preparation of the manuscript; or decision to submit the manuscript for publication. Dr. Arnold is supported by a Career Development Grant Award (K23 HL116799) from the National Heart, Lung, and Blood Institute. Dr Green is supported by a Career Development Grant Award (K23 HL12114) from the National Heart, Lung, and Blood Institute.
DISCLOSURES: Dr. Kirtane has received institutional research grants from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, St. Jude Medical, Vascular Dynamics, Eli Lilly, and Glaxo Smithkline. Dr. Mack is a member of the Executive Committee of the PARTNER Trial of Edwards Lifesciences (uncompensated). Dr. Reynolds is a consultant to Medtronic and has received research grant support from Edwards Lifesciences and Medtronic. Dr. Cohen has received research grant support from Edwards Lifesciences, Medtronic, and Boston Scientific and consulting fees from Medtronic.
REFERENCES
- 1.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 2.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 3.Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J, Jr, Kleiman NS, Chetcuti S, Heiser J, Merhi W, Zorn G, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte J, Maini B, Mumtaz M, Chenoweth S, Oh JK. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–1798. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 4.Gilard M, Eltchaninoff H, Iung B, Donzeau-Gouge P, Chevreul K, Fajadet J, Leprince P, Leguerrier A, Lievre M, Prat A, Teiger E, Lefevre T, Himbert D, Tchetche D, Carrie D, Albat B, Cribier A, Rioufol G, Sudre A, Blanchard D, Collet F, Dos Santos P, Meneveau N, Tirouvanziam A, Caussin C, Guyon P, Boschat J, Le Breton H, Collart F, Houel R, Delpine S, Souteyrand G, Favereau X, Ohlmann P, Doisy V, Grollier G, Gommeaux A, Claudel JP, Bourlon F, Bertrand B, Van Belle E, Laskar M. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med. 2012;366:1705–1715. doi: 10.1056/NEJMoa1114705. [DOI] [PubMed] [Google Scholar]
- 5.Moat NE, Ludman P, de Belder MA, Bridgewater B, Cunningham AD, Young CP, Thomas M, Kovac J, Spyt T, MacCarthy PA, Wendler O, Hildick-Smith D, Davies SW, Trivedi U, Blackman DJ, Levy RD, Brecker SJ, Baumbach A, Daniel T, Gray H, Mullen MJ. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol. 2011;58:2130–2138. doi: 10.1016/j.jacc.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 6.Rodes-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Osten M, Feindel CM, Natarajan MK, Velianou JL, Martucci G, Devarennes B, Chisholm R, Peterson M, Thompson CR, Wood D, Toggweiler S, Gurvitch R, Lichtenstein SV, Doyle D, Delarochelliere R, Teoh K, Chu V, Bainey K, Lachapelle K, Cheema A, Latter D, Dumesnil JG, Pibarot P, Horlick E. Long-term outcomes after transcatheter aortic valve implantation: insights on prognostic factors and valve durability from the canadian multicenter experience. J Am Coll Cardiol. 2012;60:1864–1875. doi: 10.1016/j.jacc.2012.08.960. [DOI] [PubMed] [Google Scholar]
- 7.Mok M, Nombela-Franco L, Urena M, Delarochelliere R, Doyle D, Ribeiro HB, Cote M, Pibarot P, Delarochelliere H, Laflamme L, Poirier P, Dumont E, Rodes-Cabau J. Prognostic value of exercise capacity as evaluated by the 6-minute walk test in patients undergoing transcatheter aortic valve implantation. J Am Coll Cardiol. 2013;61:897–898. doi: 10.1016/j.jacc.2012.10.050. [DOI] [PubMed] [Google Scholar]
- 8.Green P, Cohen DJ, Genereux P, McAndrew T, Arnold SV, Alu M, Beohar N, Rihal CS, Mack MJ, Kapadia S, Dvir D, Maurer MS, Williams MR, Kodali S, Leon MB, Kirtane AJ. Relation between six-minute walk test performance and outcomes after transcatheter aortic valve implantation (from the PARTNER trial) Am J Cardiol. 2013;112:700–706. doi: 10.1016/j.amjcard.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seiffert M, Schnabel R, Conradi L, Diemert P, Schirmer J, Koschyk D, Linder M, Kersten JF, Grosser A, Wilde S, Blankenberg S, Reichenspurner H, Baldus S, Treede H. Predictors and outcomes after transcatheter aortic valve implantation using different approaches according to the valve academic research consortium definitions. Catheter Cardiovasc Interv. 2013;82:640–652. doi: 10.1002/ccd.24751. [DOI] [PubMed] [Google Scholar]
- 10.Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–715. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Di Giulio P. Should patients perception of health status be integrated in the prognostic assessment of heart failure patients? A prospective study. Qual Life Res. 2014;23:49–56. doi: 10.1007/s11136-013-0468-8. [DOI] [PubMed] [Google Scholar]
- 12.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 13.Heidenreich PA, Spertus JA, Jones PG, Weintraub WS, Rumsfeld JS, Rathore SS, Peterson ED, Masoudi FA, Krumholz HM, Havranek EP, Conard MW, Williams RE. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol. 2006;47:752–756. doi: 10.1016/j.jacc.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 14.Dunlay SM, Gheorghiade M, Reid KJ, Allen LA, Chan PS, Hauptman PJ, Zannad F, Maggioni AP, Swedberg K, Konstam MA, Spertus JA. Critical elements of clinical follow-up after hospital discharge for heart failure: insights from the EVEREST trial. Eur J Heart Fail. 2010;12:367–374. doi: 10.1093/eurjhf/hfq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation. 2004;110:546–551. doi: 10.1161/01.CIR.0000136991.85540.A9. [DOI] [PubMed] [Google Scholar]
- 16.Arnold SV, Spertus JA, Lei Y, Allen KB, Chhatriwalla AK, Leon MB, Smith CR, Reynolds MR, Webb JG, Svensson LG, Cohen DJ. Use of the Kansas City Cardiomyopathy Questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6:61–67. doi: 10.1161/CIRCHEARTFAILURE.112.970053. [DOI] [PubMed] [Google Scholar]
- 17.Carroll JD, Edwards FH, Marinac-Dabic D, Brindis RG, Grover FL, Peterson ED, Tuzcu EM, Shahian DM, Rumsfeld JS, Shewan CM, Hewitt K, Holmes DR, Jr, Mack MJ. The STS-ACC transcatheter valve therapy national registry: a new partnership and infrastructure for the introduction and surveillance of medical devices and therapies. J Am Coll Cardiol. 2013;62:1026–1034. doi: 10.1016/j.jacc.2013.03.060. [DOI] [PubMed] [Google Scholar]
- 18.Mack MJ, Brennan JM, Brindis R, Carroll J, Edwards F, Grover F, Shahian D, Tuzcu EM, Peterson ED, Rumsfeld JS, Hewitt K, Shewan C, Michaels J, Christensen B, Christian A, O'Brien S, Holmes D. Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310:2069–2077. doi: 10.1001/jama.2013.282043. [DOI] [PubMed] [Google Scholar]
- 19.Holmes DR, Jr, Brennan JM, Rumsfeld JS, Dai D, O'Brien SM, Vemulapalli S, Edwards FH, Carroll J, Shahian D, Grover F, Tuzcu EM, Peterson ED, Brindis RG, Mack MJ. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313:1019–1028. doi: 10.1001/jama.2015.1474. [DOI] [PubMed] [Google Scholar]
- 20.Pettersen KI, Reikvam A, Rollag A, Stavem K. Reliability and validity of the Kansas City cardiomyopathy questionnaire in patients with previous myocardial infarction. Eur J Heart Fail. 2005;7:235–242. doi: 10.1016/j.ejheart.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Jones PG, Gosch KL, Li Y, Reid KJ, Tang F, Chan PS, Spertus JA. The KCCQ-12: A Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes. 2013;6:A248. doi: 10.1161/CIRCOUTCOMES.115.001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg. 2008;135:180–187. doi: 10.1016/j.jtcvs.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Raghunathan te, Lepkowski jm, Van Hoewyk j, Solenberger p. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodology. 2001;27:85–95. [Google Scholar]
- 24.Sullivan MD, Levy WC, Russo JE, Crane B, Spertus JA. Summary health status measures in advanced heart failure: relationship to clinical variables and outcome. J Card Fail. 2007;13:560–568. doi: 10.1016/j.cardfail.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Flint KM, Matlock DD, Sundareswaran KS, Lindenfeld J, Spertus JA, Farrar DJ, Allen LA. Pre-operative health status and outcomes after continuous-flow left ventricular assist device implantation. J Heart Lung Transplant. 2013;32:1249–1254. doi: 10.1016/j.healun.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flint KM, Tang F, Jones PG, Fendler TJ, Spertus JA, Allen LA. Health Status and Outcomes Following Left Ventricular Assist Device Placement. Circ Cardiovasc Qual Outcomes. 2015;8:A8. doi: 10.1161/CIRCOUTCOMES.115.001856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genovese EA, Dew MA, Teuteberg JJ, Simon MA, Bhama JK, Bermudez CA, Lockard KL, Winowich S, Kormos RL. Early adverse events as predictors of 1-year mortality during mechanical circulatory support. J Heart Lung Transplant. 2010;29:981–988. doi: 10.1016/j.healun.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnold SV, Reynolds MR, Lei Y, Magnuson EA, Kirtane AJ, Kodali SK, Zajarias A, Thourani VH, Green P, Rodes-Cabau J, Beohar N, Mack MJ, Leon MB, Cohen DJ. Predictors of poor outcomes after transcatheter aortic valve replacement: results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation. 2014;129:2682–2690. doi: 10.1161/CIRCULATIONAHA.113.007477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shafiq A, Kureshi F, Arnold SV, Gosch K, Breeding T, Jones PG, Spertus JA. Patient and Physician Discordance in Reporting Symptoms of Angina: Insights from the Angina Prevalence and Provider Evaluation of Angina Relief (APPEAR) Study. Circ Cardiovasc Qual Outcomes. 2015;8:A7. doi: 10.1016/j.ahj.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.