Abstract
Sequential fractionation has helped improving our understanding of the lability and bioavailability of P in soil. Nevertheless, there have been no reports on how manipulation of the different fractions prior to analyses affects the total P (TP) concentrations measured. This study investigated the effects of sample digestion, filtration, and acidification on the TP concentrations determined by ICP-OES in 20 soil samples. Total P in extracts were either determined without digestion by ICP-OES, or ICP-OES following block digestion, or autoclave digestion. The effects of sample filtration, and acidification on undigested alkaline extracts prior to ICP-OES were also evaluated. Results showed that, TP concentrations were greatest in the block-digested extracts, though the variability introduced by the block-digestion was the highest. Acidification of NaHCO3 extracts resulted in lower TP concentrations, while acidification of NaOH randomly increased or decreased TP concentrations. The precision observed with ICP-OES of undigested extracts suggests this should be the preferred method for TP determination in sequentially extracted samples. Thus, observations reported in this work would be helpful in appropriate sample handling for P determination, thereby improving the precision of P determination. The results are also useful for literature data comparison and discussion when there are differences in sample treatments.
Three decades ago, Hedley et al.1 developed a sequential extraction procedure as a tool to investigate changes in individual soil phosphorus (P) pools as affected by various management strategies. Since then, more than 1300 peer-reviewed studies have cited this sequential extraction procedure, and many others have adapted it for soils, manures, and other biological samples2,3,4,5. In brief, the original sequential extraction procedure consists of 0.5 g (dry weight; DM) of soil being extracted with resin in a given amount of solution (30 mL), followed by extraction of the soil residue with 30 mL of 0.5 M NaHCO3, 0.1 M NaOH, 0.1 M NaOH after sonication, and 1.0 M HCl. The remaining residue is then digested with a mixture of sulfuric acid (H2SO4) and hydrogen peroxide (H2O2) to determine residual P1. The fractions were functionally defined as follows: soluble (labile) inorganic P (Pi) is extracted in the resin and NaHCO3 fractions; Pi associated with Al and Fe is extracted with NaOH; Pi occluded in the interiors of amorphous Al/Fe hydrous oxides is extracted with NaOH and sonication; Pi associated with Ca and primary minerals is extracted with HCl; and the most stable organic P (Po) and occluded Pi are estimated after the H2SO4/H2O2 digestion of the residue1,6. Organic P (Po) concentrations are then estimated for each fraction as the difference in Pi detected by colorimetric procedures, such as the Murphy and Riley7 molybdate blue method, before and after digestion of Po to Pi1.
The final calculated concentration of Po in sequentially fractionated samples is, in most instances, affected by several factors, including the pore size of filters used to remove suspended materials in the extract and degree of oxidation of organophosphates during the digestion procedure. Although the original method as described by Hedley et al.1 does not include filtration, many have adapted the method to incorporate the use of a 0.45 μm pore-size nitrocellulose acetate filter for soil extracts5,8,9 and biological samples8,10,11. For the digestion of several different extracts, some have used potassium persulfate (K2S2O8) and H2SO412,13,14,15,16, or concentrated H2SO4 in combination with H2O2 for soil samples5,12 and for biological samples3,8,12. Other researchers have used perchloric acid (HClO4) for digestion13,17. However, Pierzynski18 indicated that laboratory digestion procedures were not capable of completely oxidizing soil organic compounds and liberating Pi into solution, thereby underestimating Po concentrations in a sample. Indeed, total P (TP) determined after digestion was even lower than Pi determined without digestion in some cases14,19. In addition, the detection of Po can be influenced by the method of Pi determination due to hydrolysis of Po or solubilization of condensed Pi by the reagents used during color development in molybdate blue colorimetric methods20.
The most important criteria for recommending digestion and quantification methods for P determination in soils are accuracy, simplicity, and rapidity of determination21. The use of inductively coupled plasma optical emission spectroscopy (ICP-OES) is becoming increasingly prevalent for determination of TP in aqueous samples, including soil extracts. The inductively coupled plasma from ICP-OES causes atom and ion excitation with emission of electromagnetic radiation at wavelengths characteristic of specific elements22. As all compounds in a sample solution are decomposed to elemental plasma statuses, ICP-OES determines TP (i.e., both Pi and Po) in a sample without the need for prior digestion22,23,24. The calculated difference in P content of a sample evaluated by both ICP-OES and a colorimetric procedure to determine Pi is generally considered to represent Po. Numerous papers have been published to compare the difference in soil test P levels determined by colorimetric and ICP methods21,23,25,26. In general, the P concentrations measured with ICP-OES are greater than those obtained following colorimetric procedures, reflecting the amount of Po in the samples21,23,25,26. However, to our knowledge, there have been no studies that reported or compared the concentrations of TP in sequentially extracted soil samples as determined by ICP-OES following different digestion methods as well as in undigested extracts.
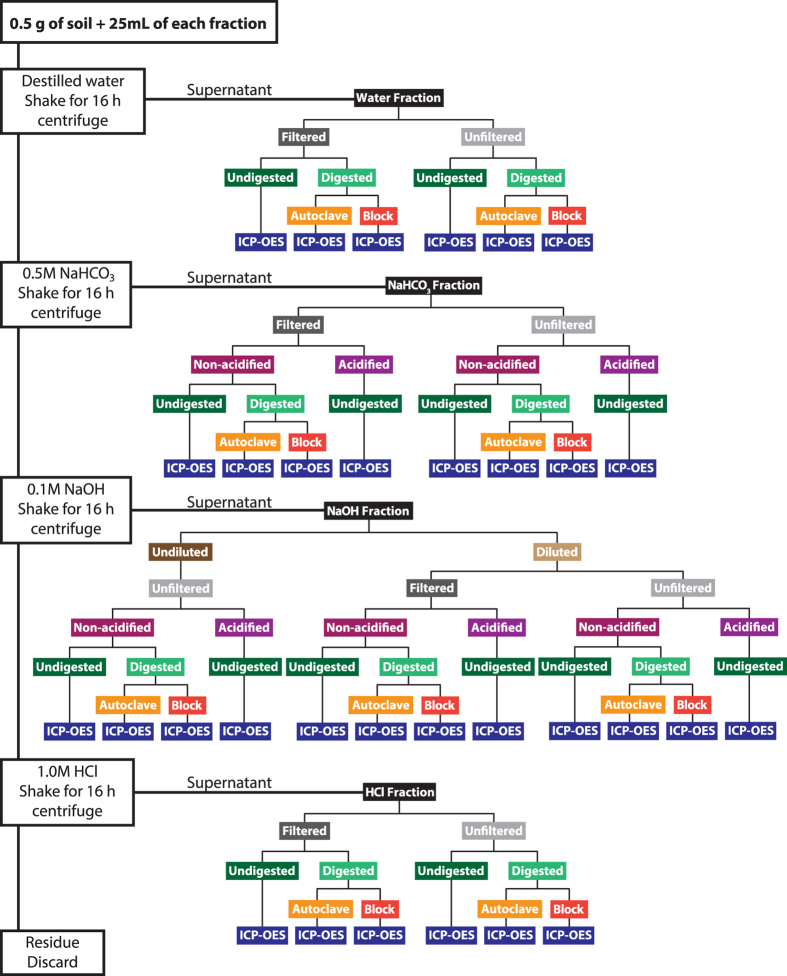
This study was conducted to compare P concentrations in sequentially extracted solutions following digestion by autoclaving with H2SO4 and K2S2O8, heated digestion with H2SO4 and H2O2, and direct determination of undigested extracts with ICP-OES. In addition, the effects of sample filtration (all fractions) and acidification (NaHCO3 and NaOH fractions) were evaluated. Acidification of sequential extracts has been performed for two reasons: (1) the phosphatase hydrolysis method of He and Honeycutt (2001) for functional identification of specific organic P forms requires a standardized pH, and (2) ICP-OES analyses of alkaline samples could lead to metal precipitation in the aerosol chamber of the ICP instrument, which is avoided by using acidified samples27. The current study was conducted with 20 soil series collected from Brazil and four states across the U.S.: Minnesota, Iowa, Wisconsin, and Texas. Figure 1 shows a detailed description of the sequential fractionation and sample manipulation used in this study. Our hypothesis was that no significant differences would be found in TP concentration determined in extracts between the digestion methods and undigested analysis using ICP-OES.
Figure 1. Visual representation of sequential fractionation and post-extraction manipulation and treatments of extracts.
Results and Discussion
Effects of digestion method and filtration on measured water-extractable P
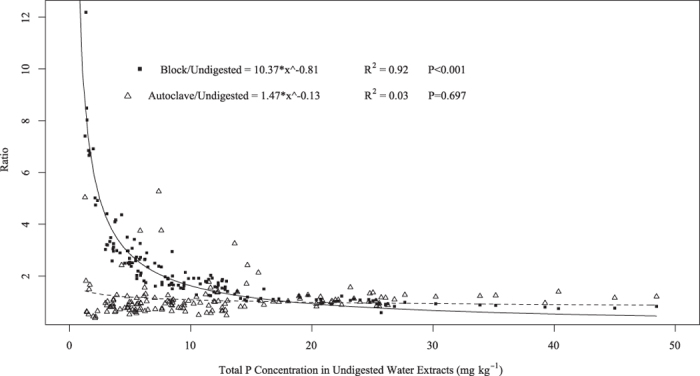
Total water-extractable P concentrations of soils measured with ICP-OES varied greatly depending on which digestion method was used, the specific soil series, and whether the samples were filtered or not prior to digestion (Table1). The block digestion method resulted in the highest measured TP concentrations in most water extracts, while most of the undigested water-extracts had lower measured TP concentrations. The general trend was that when the P concentration in the undigested sample was greater than 14.0 mg kg−1 there were no significant (P > 0.05) differences in TP concentrations among digestion methods, with the exception of the Clarion soil (Table 1). The ratio of TP concentration determined with the block and autoclave methods, with respect to the undigested method, was calculated by dividing the block P concentration and the autoclave P concentration by the undigested P concentration determined by ICP-OES (Fig. 2). There is a clear non-linear relationship between the P concentrations measured following block digestion and those measured in undigested water extracts (Fig. 2). The non-linear regression shows that the ratio between measured P concentrations in block digested and undigested extracts become one at 17.9 mg kg−1 (value calculated by solving the equation 1.0 = 10.4 * X ^ - 0.8 reported in Fig. 2). This non-linear relationship was not observed for the autoclave digested extracts when compared to undigested extracts, likely due to high variability when the measured TP concentrations in undigested extracts were below 17.9 mg kg−1. Similar results were found by Matula28 while investigating the effect of digestion (block digestion with K2S2O8) on water-soluble P from soil samples. The authors reported that at low water-soluble P concentrations, the digestion method overestimated the amount of P in solution and the relationship followed an exponential function28. Vadas and Kleinman29 also reported that digested water extracts of manure samples consistently had higher TP concentration compared with undigested samples. Although no direct cause has been determined for the fact that TP concentration in digested samples containing low P concentrations, it is possible that differences in the sample matrix could be involved. For example, de Boer et al.30 reported that the presence of K, Mg, and Na could interfere with P determination when using ICP for determination of total P water samples.
Table 1. Average total P concentration in sequentially extracted soil samples as determined in undigested, block digestion, and autoclave digestion methods in the water fraction.
| Soil Series | Filtered | Extract |
Block Digestion |
Auto Clave |
|||
|---|---|---|---|---|---|---|---|
| mg kg−1 | |||||||
| Amarillo | No | 3.8 | c† | 11.4 | a | 6.2 | b |
| Yes | 3.7 | c | 11.1 | a | 3.5 | b | |
| Barnes | No | 0.7 | b | 14.0 | a | 3.7 | b |
| Yes | 1.4 | b | 12.1 | a | 1.1 | b | |
| Brazil | No | 6.3 | b | 18.3 | a | 1.7 | c |
| Yes | 6.1 | b | 17.2 | a | 5.1 | b | |
| Clarion | No | 14.6 | bc | 16.6 | ab | 6.5 | d |
| Yes | 12.5 | c | 19.1 | a | 12.6 | c | |
| Cordova | No | 11.3 | a | 15.9 | a | 9.1 | a |
| Yes | 9.7 | a | 16.0 | a | 10.3 | a | |
| Estherville | No | 8.3 | c | 13.6 | a | 8.4 | bc |
| Yes | 6.0 | c | 11.3 | ab | 5.2 | c | |
| Fargo | No | 22.9 | a | 20.8 | a | 24.1 | a |
| Yes | 24.2 | a | 25.1 | a | 19.0 | a | |
| Formdale | No | 14.3 | ab | 19.6 | a | 18.1 | a |
| Yes | 12.4 | b | 20.0 | a | 9.1 | b | |
| Hubbard | No | 10.8 | c | 20.9 | a | 18.6 | a |
| Yes | 8.8 | d | 16.2 | b | 7.7 | d | |
| Lester | No | 12.4 | b | 21.7 | a | 18.8 | a |
| Yes | 9.8 | c | 20.4 | a | 10.4 | bc | |
| MtCarrol | No | 2.2 | bc | 10.7 | a | 5.1 | b |
| Yes | 1.3 | c | 10.8 | a | 2.1 | bc | |
| Nicollet | No | 5.5 | bc | 16.3 | a | 7.9 | b |
| Yes | 2.2 | c | 14.5 | a | 5.1 | bc | |
| Normania | No | 4.6 | c | 12.7 | a | 6.4 | b |
| Yes | 3.5 | c | 12.5 | a | 4.8 | c | |
| Pella | No | 21.2 | a | 22.2 | a | 22.1 | a |
| Yes | 17.0 | a | 18.5 | a | 16.7 | a | |
| Pullman | No | 43.3 | a | 33.8 | bc | 37.3 | ab |
| Yes | 31.7 | bc | 28.9 | bc | 25.8 | c | |
| Randall | No | 24.0 | a | 27.0 | a | 23.9 | a |
| Yes | 19.6 | a | 23.3 | a | 16.3 | a | |
| Seaton | No | 6.6 | b | 14.6 | a | 12.1 | a |
| Yes | 5.1 | b | 12.2 | a | 5.4 | b | |
| Storden | No | 5.1 | b | 13.2 | a | 8.3 | ab |
| Yes | 3.2 | b | 9.5 | a | 3.5 | b | |
| Walter | No | 25.7 | ab | 23.7 | b | 29.5 | a |
| Yes | 21.2 | b | 19.7 | b | 21.1 | b | |
| Zimmerman | No | 7.9 | bc | 12.9 | a | 10.8 | ab |
| Yes | 5.0 | bc | 11.6 | a | 6.1 | c | |
†Means for a soil series followed by the different letter are significantly different (P < 0.05).
Figure 2. Ratio between total P concentration determined by the block method (black squares) and autoclave method (open triangle) in relation to the total P concentration determined by the undigested method.
Filtration was found to be a bigger issue with the autoclave method than the block method or undigested water extracts (Table 1). For samples digested using the autoclave method, 50% of the soils showed a significant (P < 0.05) filtration effect, while only one and three of the soils were affected by filtration for the block digested and undigested samples, respectively (Table 1). Filtering the samples prior to digestion, in most cases, caused a significant decrease in the TP concentration, with the exception of the Brazil and Clarion soils. Although the results are not well understood, it is possible that filtration through a pore size of 0.45 μm prohibited the inclusion of large organophosphates, other organic-P complexes, and some mineral-associated P colloids31. Correlation analyses were performed to try and identify if any of the soil properties presented in Table 2 could be affecting filtration for the soils that showed a significant filtration effect, in particular regarding the clay distribution of the soils. We observed no significant correlations between the ratio of block digestion divided by undigested samples and autoclave digestion divided by undigested samples. However, the lack of consistency on the effect of filtration among the digestion methods makes it difficult to draw final conclusions regarding the effect of filtration on the water fraction as in some cases there was an increase in the TP measured.
Table 2. Summary of selected soil properties.
| Soil Series | Olsen P | Bray-1 P | Ca† | Mg | Fe | Al | Sand | Clay | OM | pH |
|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | % | |||||||||
| Amarillo | 3 | 8 | 1538 | 248 | 38 | 491 | 72.2 | 13.5 | 1.6 | 7.6 |
| Barnes | 7 | 16 | 3815 | 526 | 48 | 32 | 22.7 | 22.4 | 5.5 | 8.0 |
| Brazil | 2 | 8 | 451 | 137 | 43 | 702 | 78.2 | 20.1 | 2.8 | 5.3 |
| Clarion | 44 | 75 | 2770 | 593 | 201 | 586 | 36.2 | 38.4 | 4.4 | 6.2 |
| Cordova | 35 | 46 | 4899 | 566 | 82 | 11 | 17.4 | 29.6 | 8.9 | 7.5 |
| Estherville | 16 | 44 | 3084 | 536 | 118 | 259 | 60.1 | 22.7 | 3.9 | 7.0 |
| Fargo | 42 | 87 | 3606 | 1410 | 47 | 17 | 5.2 | 6.7 | 6.8 | 7.7 |
| Formdale | 31 | 51 | 3480 | 816 | 46 | 10 | 29.4 | 21.0 | 6.9 | 7.6 |
| Hubbard | 20 | 82 | 580 | 95 | 95 | 716 | 82.5 | 11.8 | 1.3 | 6.6 |
| Lester | 21 | 25 | 2645 | 425 | 106 | 278 | 40.2 | 32.1 | 5.1 | 6.6 |
| Mt Carroll | 13 | 22 | 2829 | 321 | 83 | 134 | 15.3 | 16.0 | 4.5 | 7.9 |
| Nicollet | 17 | 9 | 2757 | 647 | 120 | 347 | 36.1 | 35.1 | 4.4 | 6.7 |
| Normania | 23 | 44 | 2414 | 608 | 231 | 703 | 37.0 | 30.9 | 4.4 | 5.5 |
| Pella | 37 | 96 | 691 | 166 | 185 | 633 | 26.4 | 22.6 | 5.6 | 7.7 |
| Pullman | 52 | 68 | 2953 | 414 | 40 | 144 | 31.4 | 34.3 | 2.7 | 7.2 |
| Randall | 40 | 74 | 4725 | 569 | 115 | 362 | 30.1 | 40.3 | 3.4 | 7.8 |
| Seaton | 34 | 39 | 2046 | 425 | 149 | 442 | 7.4 | 25.9 | 3.4 | 6.7 |
| Storden | 25 | 42 | 1995 | 349 | 162 | 656 | 43.3 | 30.9 | 4.8 | 5.5 |
| Wheatville | 22 | 24 | 3062 | 925 | 20 | 0 | 19.3 | 11.5 | 4.7 | 8.3 |
| Zimmerman | 15 | 38 | 1561 | 60 | 211 | 368 | 81.0 | 14.3 | 1.9 | 8.0 |
†Ca, Mehlich-3 extractable calcium; Mg, Mehlich-3 extractable magnesium; Fe, Mehlich-3 extractable iron; Al, Mehlich-3 extractable aluminum.
Overall, it appears that both the block and autoclave digestion methods tended to overestimate the amount of water-extractable TP in a sample, particularly at low P concentrations. Analyses of water extractable P with ICP-OES, without digestion or filtration, decreased variability as compared to block and autoclave digestion or on extracts that had undergone filtration.
Effects of digestion method and filtration on measured NaHCO3-extractable P
The concentrations of TP in the NaHCO3 fraction changed significantly (P < 0.05) based on specific soil series, digestion method, filtration, and acidification (Tables 3 and 4). In contrast to results observed for water extracts, measured TP concentrations were generally lowest with block digestion and highest following autoclave digestion. Filtering the NaHCO3 extracts caused a significant (P < 0.05) reduction in TP soluble in NaHCO3 in 15 of the 20 soils following digestion with the autoclave method and in three soils following digestion with the block method. In contrast, as compared to undigested NaHCO3 extracts, filtration caused an increase in measured total NaHCO3-soluble P for the Estherville soil, autoclave digested Hubbard soil, and four soils digested with the block method (Table 3). As observed for water extracts, filtration likely retained large P-containing organic and inorganic compounds. Turner and Haygarth32 reported that from 21% to 46% of TP in leachate was retained by filtration at 0.45 μm. In the present study, the amount of NaHCO3-P that was presumably retained by filtration ranged between 15% and 57% for the autoclave and block methods. As observed with water extracts, filtration did not cause significant (P > 0.05) changes in TP measured in undigested samples, with the exception of the Estherville soil. The lack of other studies that compared filtration and digestion methods makes it difficult to draw conclusions or hypothesize the rationale for increased TP concentration measured after filtration.
Table 3. Total P concentration in the sequentially extracted NaHCO3 fraction of soil samples determined by ICP-OES after undigested, block digestion, and autoclave digestion treatments.
| Soil Series | Filtered | Undigested |
Block digestion |
Autoclave digestion |
|||
|---|---|---|---|---|---|---|---|
| mg kg−1 | |||||||
| Amarillo | No | 8.8 | b† | 9.5 | b | 17.0 | a |
| Yes | 10.0 | b | 9.8 | b | 7.3 | b | |
| Barnes | No | 16.8 | b | 20.5 | b | 17.8 | b |
| Yes | 18.0 | b | 28.3 | a | 15.8 | b | |
| Brazil | No | 11.5 | c | 17.8 | a | 15.3 | b |
| Yes | 11.8 | c | 17.8 | a | 11.8 | c | |
| Clarion | No | 71.0 | b | 69.5 | b | 79.8 | a |
| Yes | 70.0 | b | 58.0 | d | 63.8 | c | |
| Cordova | No | 61.3 | b | 60.3 | b | 72.5 | a |
| Yes | 61.0 | b | 51.5 | c | 50.3 | c | |
| Estherville | No | 43.5 | c | 33.5 | d | 50.3 | a |
| Yes | 48.0 | b | 41.5 | c | 42.8 | c | |
| Fargo | No | 74.3 | ab | 62.0 | b | 82.8 | a |
| Yes | 74.0 | ab | 65.5 | ab | 61.8 | b | |
| Formdale | No | 45.5 | b | 39.3 | c | 45.8 | b |
| Yes | 45.0 | b | 51.0 | a | 43.5 | bc | |
| Hubbard | No | 39.3 | b | 23.0 | d | 36.0 | c |
| Yes | 39.0 | b | 39.8 | b | 42.8 | a | |
| Lester | No | 42.8 | a | 38.3 | b | 38.0 | b |
| Yes | 43.0 | a | 40.5 | ab | 40.5 | ab | |
| MtCarrol | No | 35.3 | bc | 32.5 | c | 39.5 | a |
| Yes | 36.8 | ab | 34.8 | bc | 31.8 | c | |
| Nicollet | No | 36.8 | a | 34.5 | a | 36.3 | a |
| Yes | 37.5 | a | 35.0 | a | 34.0 | a | |
| Normania | No | 42.8 | b | 39.3 | c | 48.0 | a |
| Yes | 42.8 | b | 38.5 | c | 39.3 | c | |
| Pella | No | 64.5 | b | 49.3 | c | 74.0 | a |
| Yes | 67.8 | b | 49.3 | c | 48.8 | c | |
| Pullman | No | 108.3 | b | 58.3 | d | 142.0 | a |
| Yes | 110.5 | b | 24.5 | e | 95.8 | c | |
| Randall | No | 81.8 | b | 51.8 | c | 95.8 | a |
| Yes | 88.5 | ab | 49.5 | c | 78.3 | b | |
| Seaton | No | 49.8 | ab | 47.0 | bc | 52.5 | a |
| Yes | 50.8 | ab | 43.0 | c | 44.8 | c | |
| Storden | No | 28.8 | bc | 23.0 | c | 41.8 | a |
| Yes | 32.0 | b | 20.5 | c | 25.3 | c | |
| Walter | No | 85.0 | b | 59.5 | d | 92.8 | a |
| Yes | 83.8 | b | 63.5 | d | 77.3 | c | |
| Zimmerman | No | 32.0 | ab | 29.8 | bc | 36.0 | a |
| Yes | 31.5 | bc | 28.0 | bc | 27.0 | c | |
†Means for a soil series followed by the different letter are significantly different (P < 0.05).
Table 4. Effects of filtration and acidification on total P concentration in the undigested NaHCO3 fraction of soil samples determined by ICP-OES.
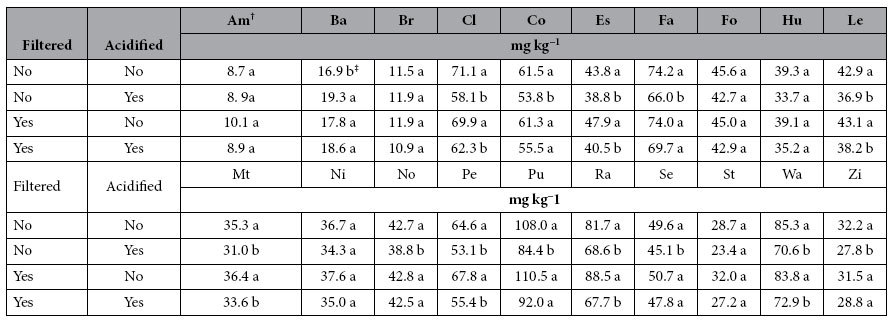
†Soil series are: Am: Amarillo, Ba: Barnes, Br: Brazil, Cl: Clarion, Co: Cordova, Es: Esthervile, Fa: Fargo, Fo: Formdale, Hu: Hubbard, Le: Lester, Mt: Mt. Carroll, Ni: Nicollet, No: Normania, Pe: Pella, Pu: Pullman, Ra: Randall, Se: Seaton, St: Storden, Wa: Walter, Zi: Zimmerman.
‡Means for a soil series followed by the different letters within filtration and dilution are significantly different (P < 0.05).
Acidification of the NaHCO3 extracts, in most cases, caused a significant (P < 0.05) reduction in TP concentrations (Table 4). For the unfiltered samples, acidification caused a TP concentration reduction in 13 of the 20 soils tested and increased TP concentration in the Barnes soil. For the filtered samples, there was a decrease in TP concentration in 7 of the 20 NaHCO3 extracts. It is possible that acidification of this fraction caused precipitation of Po and/or Al/Fe-associated P, which led to reduced concentrations of NaHCO3-P. As an example, soil humic acids are typically purified by acidification, where high molecular weight humic acids are precipitated from solution with 1.0 M HCl33.
Effects of digestion method, dilution and filtration on measured NaOH-extractable P
High concentrations of OM in NaOH extracts made it necessary to dilute this fraction prior to filtration in order to avoid clogging of membrane filter pores. Therefore, the treatments for this fraction were: unfiltered/undiluted, unfiltered/diluted, and filtered/diluted NaOH extracts. As observed for the other fractions, most of the significant differences (P < 0.05) were observed with the block and autoclave digestion methods; while the least amount of variability was observed in the undigested samples. Figure 3 presents the distribution of the NaOH fraction for unfiltered/undiluted, unfiltered/diluted, and filtered/diluted samples analyzed using all three methods: undigested extracts analyzed directly with ICP-OES (Fig. 3A), block digestion (Fig. 3B), and autoclave digestion (Fig. 3C). It is clear from the R2 values that analyzing the NaOH extract directly with ICP-OES (no digestion) produced the least variable results (R2 = 0.99, unfiltered; R2 = 0.97, filtered) (Fig. 3A–C). Diluting samples prior to analyses caused a significant (P < 0.05) change in total NaOH-P concentrations in 6 out of 20 (block digestion) and 15 out of 20 (autoclave digestion) soils, with no significant changes in P concentration for undigested NaOH extracts. In 5 of the 6 block digested NaOH extracted soils, there were decreased measured TP concentrations after dilution, decreases which ranged between 49.9 to 105.7 mg P kg−1. However, one soil showed an increase (P < 0.05) in measured P concentration of 19.5 mg P kg−1 (Fig. 3B). Whereas, for the autoclave method, TP significantly (P < 0.05) increased after dilution in 6 soils and decreased in 9 soils (Fig. 3C). There were no significant (P > 0.05) differences due to dilution in undigested NaOH extracts (Fig. 3A).
Figure 3. Relationship between total P concentrations in NaOH extracts for the undiluted unfiltered samples plotted against the diluted unfiltered (full square) and diluted filtered (plus sign) for the undigested (A), block (B), and autoclave (C) methods. Solid line in all panels is the 1:1 relationship.
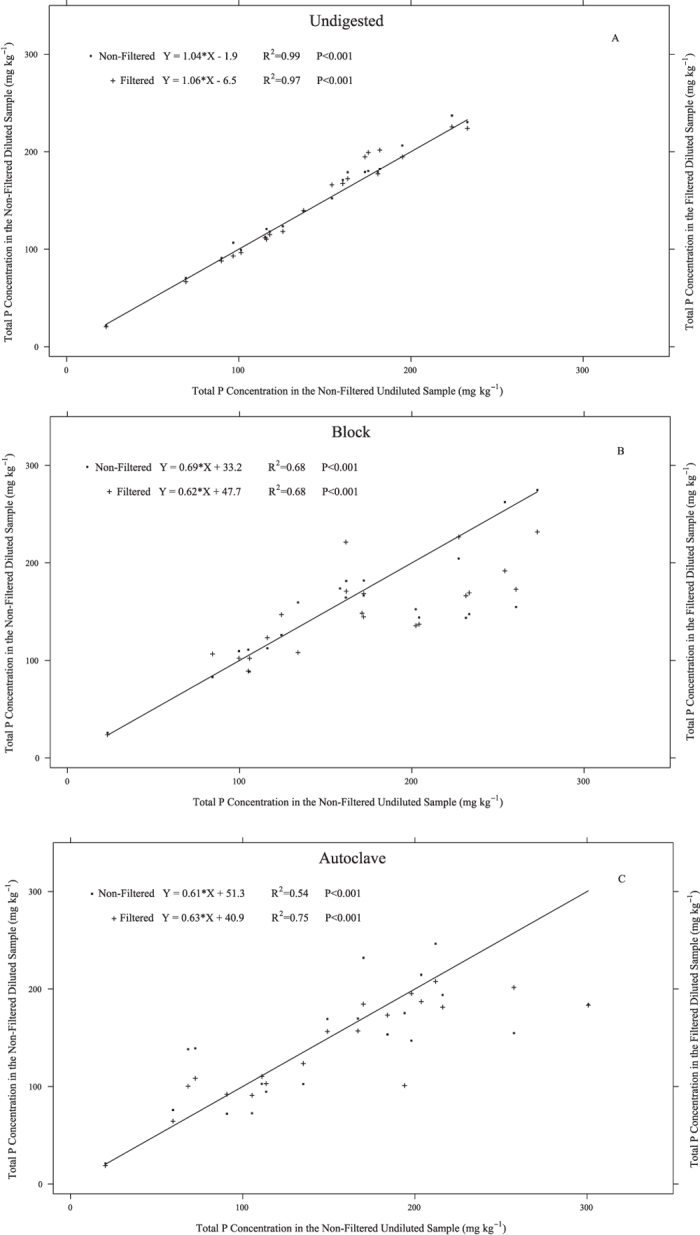
The effects of filtration on measured NaOH-P were conflicting and depended on the digestion method. In some cases, filtration caused a decrease in measured NaOH-P concentrations, but in others it caused an increase for both block and autoclave digested extracts (Fig. 3A–C). As observed for the water and NaHCO3 fractions, both digestion methods introduced a good deal of variability in measured NaOH-P as indicated by the number of significant differences due to filtration (27 out of 40 samples for both digestion methods combined), while undigested extracts showed very little variation. For the undigested method, only the Mt. Carroll NaOH extract had a significant (P < 0.05) increase of 13.8 mg P kg−1 in TP concentration after filtration. Filtration played a more important role in block and autoclave digested extracts. Block digested NaOH extracts from 7 soils showed an increase in measured TP after filtration, and 6 soils had decreased NaOH-P levels after filtration. For the autoclave method, filtration caused an increase in total NaOH-P levels in 6 soils and a reduction in 8 soils. As observed for NaHCO3-P, the reduction in measured P following filtration could be attributed to retention of large organic or mineral compounds that contain P, or clogging of pores could have kept small organic compounds from passing through the filter. However, the rationale for the observed increase in TP concentration in some filtered samples cannot be readily elucidated. The variation added by filtration in some cases exceeded 50%. For example, the Barnes soil that was unfiltered and undiluted had a measured NaOH-P concentration of 68.5 mg kg−1 following autoclave digestion, while the diluted, unfiltered NaOH extract contained 138.3 mg P kg−1.
Acidification of the NaOH extracts caused a significant (P < 0.05) change in TP concentrations measured in almost all soils; however the effect of acidification was confounded with the filtration and dilution effects (Table 5). Although there was a significant acidification effect, there was no consistent trend of whether measured NaOH-P concentrations increased or decreased after acidification. There were no significant (P > 0.05) changes in measured TP concentration in the Barnes soil. However, there were contradicting results observed for some soils. For example, acidification effects were not significant for undiluted Estherville NaOH-P, but acidification increased (P < 0.05) measured TP in diluted/unfiltered extracts by 70.5 mg P kg−1, and decreased (P < 0.05) TP in filtered/diluted extracts by 15.7 mg P kg−1. Similar results where the effects of acidification were dependent on filtration and dilution were also observed for the Normania and Storden soils. There were no soil properties measured among the soils studied that would suggest a possible explanation for the observed results, as no correlation were detected. For the remaining soils, acidification would either significantly (P < 0.05) increase or decrease TP concentrations determined in the extracts. However, none of the soil properties measured were useful in trying to understand the nature of the behavior observed for the effect of acidification. As previously mentioned for NaHCO3 extracts, it could be due to flocculation or precipitation of acid-insoluble inorganic and organic P-containing compounds. However, it is not known why acidification would increase TP determined in the samples under some conditions but not others.
Table 5. Effects of dilution and acidification on total P concentration in the undigested NaOH fraction of soil samples determined by ICP-OES.
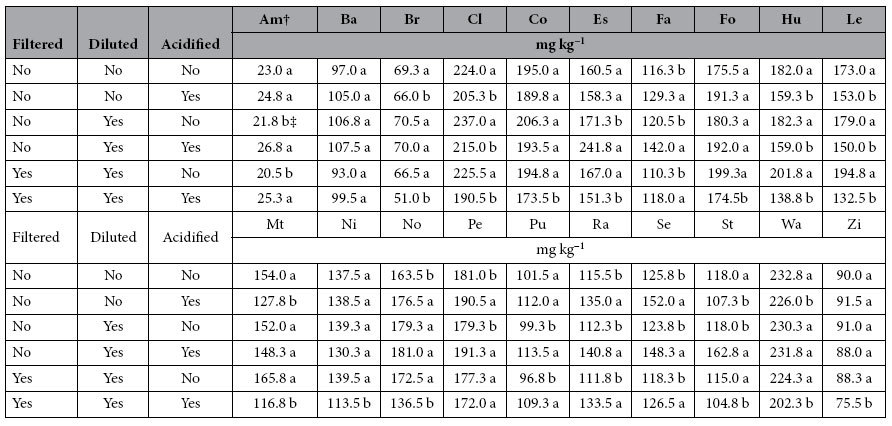
†Soil series are: Am: Amarillo, Ba: Barnes, Br: Brazil, Cl: Clarion, Co: Cordova, Es: Esthervile, Fa: Fargo, Fo: Formdale, Hu: Hubbard, Le: Lester, Mt: Mt. Carroll, Ni: Nicollet, No: Normania, Pe: Pella, Pu: Pullman, Ra: Randall, Se: Seaton, St: Storden, Wa: Walter, Zi: Zimmerman.
‡Means for a soil series followed by the different letters within filtration and dilution are significantly different (P < 0.05).
Effects of digestion method and filtration on measured HCl-extractable P
The amount of P measured in the HCl fraction was significantly (P < 0.05) affected by the digestion method, filtration, and their interaction (Table 6). In many cases (9 out of 20 extracts), unfiltered/block digestion treatment resulted in the highest measured HCl-P concentrations, followed by unfiltered/autoclave digested and unfiltered/undigested HCl extracts. However, there were cases where either the undigested or autoclave unfiltered samples were the highest, showing an inconsistent trend (Table 6).
Table 6. Total P concentration in the sequentially extracted HCl fraction of soil samples determined by ICP-OES after undigested, block digestion, and autoclave digestion treatments.
| Soil Series | Filtered | Undigested |
Block digestion |
Autoclave digestion |
|||
|---|---|---|---|---|---|---|---|
| mg kg−1 | |||||||
| Amarillo | No | 22.3 | a† | 23.1 | a | 14.8 | c |
| Yes | 19.3 | b | 18.1 | b | 14.8 | c | |
| Barnes | No | 95.3 | b | 112.3 | a | 94.5 | b |
| Yes | 98.0 | b | 60.0 | c | 102.3 | b | |
| Brazil‡ | No | . | . | . | |||
| Yes | . | . | . | ||||
| Clarion | No | 54.5 | cd | 77.8 | a | 57.3 | c |
| Yes | 56.3 | c | 52.3 | d | 60.3 | b | |
| Cordova | No | 185.8 | c | 235.3 | a | 187.0 | c |
| Yes | 189.8 | c | 161.5 | d | 217.8 | b | |
| Estherville | No | 96.5 | b | 79.3 | c | 105.5 | a |
| Yes | 98.3 | b | 68.0 | d | 98.3 | b | |
| Fargo | No | 363.0 | c | 415.0 | b | 459.3 | a |
| Yes | 362.0 | c | 281.3 | d | 409.3 | b | |
| Formdale | No | 107.3 | c | 136.5 | a | 117.0 | b |
| Yes | 110.3 | c | 94.5 | d | 97.5 | d | |
| Hubbard | No | 38.8 | c | 55.3 | a | 43.3 | b |
| Yes | 39.5 | c | 34.8 | d | 34.8 | d | |
| Lester | No | 48.8 | c | 67.8 | a | 59.3 | b |
| Yes | 49.3 | c | 42.0 | d | 47.5 | c | |
| MtCarrol | No | 46.3 | c | 61.0 | a | 53.0 | b |
| Yes | 44.0 | c | 37.5 | d | 45.0 | c | |
| Nicollet | No | 25.0 | c | 35.3 | a | 30.0 | b |
| Yes | 21.5 | c | 16.0 | d | 21.3 | c | |
| Normania | No | 51.3 | b | 48.0 | c | 47.5 | c |
| Yes | 51.5 | b | 6.8 | d | 62.3 | a | |
| Pella | No | 33.5 | ab | 28.0 | c | 29.3 | bc |
| Yes | 35.0 | a | 5.0 | d | 35.8 | a | |
| Pullman | No | 130.8 | b | 114.5 | c | 146.5 | a |
| Yes | 118.5 | c | 91.0 | d | 135.3 | b | |
| Randall | No | 254.3 | b | 209.8 | d | 265.0 | ab |
| Yes | 235.0 | c | 258.0 | b | 272.8 | a | |
| Seaton | No | 81.5 | b | 78.8 | b | 66.8 | c |
| Yes | 81.3 | b | 9.8 | d | 95.5 | a | |
| Storden | No | 35.3 | bc | 37.0 | b | 37.8 | b |
| Yes | 30.0 | c | 44.3 | a | 31.0 | c | |
| Walter | No | 92.3 | ab | 86.8 | bc | 80.3 | d |
| Yes | 96.8 | a | 66.0 | e | 82.5 | cd | |
| Zimmerman | No | 81.5 | a | 79.8 | a | 67.8 | b |
| Yes | 83.8 | a | 57.5 | c | 83.5 | a | |
†Means for a soil series followed by the different letter are significantly different (P < 0.05).
‡No measurable HCl P was detected in this soil.
Filtration of HCl extracts was a major issue for the block and autoclave digestion methods, but less so for undigested extracts (Table 6). Nineteen of the extracts analyzed by ICP-OES after block digestion had decreased HCl-P if filtered prior to digestion. One exception was the Storden soil, which showed a significant (P < 0.05) increase P concentration after filtration. The reduction in TP after filtration of the block digested samples ranged between 8% and 80%. For the autoclave method, 9 soils showed decreased TP after filtration, decreases which ranged between 6.8% and 28%, and 6 soils showed an increase in TP after filtration, ranging between 5% and 43%. For the undigested HCl extracts, there were decreased TP concentrations for only three soils: the reduction ranged between 8% and 14%. The significant (P < 0.05) filtration effect for both block and autoclave digested HCl-P suggests that the Po extracted in this fraction was likely associated with large organic moieties. However, the lack of a similar trend in the undigested HCl extracts precludes a thorough understanding of the mechanisms behind decreased measured TP in filtered, undigested HCl extracts. Until recently the HCl fraction was believed to predominantly contain inorganic-P forms of P (e.g., Ca-phosphates). However, He et al.20 showed that a significant amount of Po is present in HCl extracts of soils and manures: in some cases, HCl-Po concentrations were higher than HCl-Pi.
Implications of this work’s findings
During sequential fractionation studies it is mostly common to partition the P extracted from each fractions into Pi and Po. The amount of Po in each extract is estimated as the difference between the TP determined in a sample minus the Pi determined in the same sample. Therefore, when the TP portion is wrongly determined, the Po in that samples will automatically be wrongly calculated.
In this study, the significant effect of digestion and filtration on measured TP in the sequential extracts, led to significant effects of digestion and filtration in the total Po determined in the soils studied (Table 7). The effect of filtration was significant (P < 0.05) and the magnitude of the changes in total Po determined varied by digestion method used. For the undigested samples, filtration caused a reduction in total Po determined in 4 samples and the decrease ranged between 9 to 16%; while there was an increase of 11% in total Po measured after filtration for the Hubbard soil (Table 7). For the block digested samples, filtration caused a reduction in calculated Po concentration of 10 soils, which ranged between 19 and 71%, and increased the calculated Po concentration in three soils, increase which ranged between 11 and 112%. For autoclave digested extracts, filtration caused a reduction in calculated Po that ranged between 11 and 70% in 10 soils, and increases of 26 and 34% for the Seaton and Normania soils, respectively. As observed for the other fractions, filtration would be expected to decrease concentrations of calculated Po; however, the rationale behind increased Po after filtration is difficult to explain. Other researchers have also reported increased TP concentration determined in digested filtered solutions. For example, McDowell and Sharpley34 and Vadas et al.35 reported 13% increase in TP concentration in filtered digested samples and Sharpley and Moyer36 reported up to 24% increases in TP concentration in digested filtered samples.
Table 7. Average Effects of treatments on total organic P (Po) concentration in the four sequentially extracted fractions of soil samples calculated from the difference in P concentration in undigested, block digested and autoclave digested extracts determined by ICP-OES and blue colorimetry.
| Soil Series | Filtered | Undigested |
Block digestion |
Autoclave digestion |
|||
|---|---|---|---|---|---|---|---|
| mg kg−1 | |||||||
| Amarillo | No | 30.0 | b | 55.3 | ab | 32.3 | b |
| Yes | 26.3 | b | 79.3 | a | 17.3 | b | |
| Barnes | No | 101.0 | bc | 188.5 | a | 135.5 | b |
| Yes | 85.3 | c | 83.0 | c | 94.5 | bc | |
| Brazil | No | 68.5 | cd | 127.5 | a | 73.3 | c |
| Yes | 58.0 | de | 97.5 | b | 54.8 | e | |
| Clarion | No | 220.5 | b | 282.3 | a | 233.3 | b |
| Yes | 195.8 | c | 192.5 | c | 176.0 | d | |
| Cordova | No | 239.0 | c | 348.3 | a | 274.8 | b |
| Yes | 216.5 | d | 182.0 | e | 224.0 | cd | |
| Estherville | No | 172.3 | a | 160.8 | a | 170.3 | a |
| Yes | 143.5 | a | 76.5 | b | 168.0 | a | |
| Fargo | No | 221.3 | b | 308.3 | a | 346.0 | a |
| Yes | 209.0 | b | 155.3 | c | 236.8 | b | |
| Formdale | No | 172.3 | ab | 175.0 | ab | 160.5 | b |
| Yes | 188.3 | a | 159.5 | b | 173.0 | ab | |
| Hubbard | No | 186.8 | b | 158.5 | c | 207.5 | a |
| Yes | 207.5 | a | 175.3 | b | 183.5 | b | |
| Lester | No | 168.3 | bc | 160.5 | bc | 216.0 | a |
| Yes | 173.5 | b | 149.0 | c | 162.3 | bc | |
| MtCarroll | No | 151.5 | bc | 161.5 | bc | 183.3 | a |
| Yes | 170.8 | ab | 143.5 | c | 147.5 | c | |
| Nicollet | No | 115.8 | c | 147.8 | a | 152.8 | a |
| Yes | 119.0 | c | 119.5 | c | 135.3 | b | |
| Normania | No | 155.8 | ab | 159.5 | a | 127.8 | c |
| Yes | 139.3 | bc | 97.8 | d | 170.8 | a | |
| Pella | No | 138.3 | ab | 104.0 | b | 140.8 | a |
| Yes | 137.0 | ab | 158.8 | a | 42.0 | c | |
| Pullman | No | 172.3 | a | 100.5 | c | 188.8 | a |
| Yes | 147.5 | b | 36.5 | d | 138.8 | b | |
| Randall | No | 158.3 | a | 84.3 | b | 173.0 | a |
| Yes | 165.8 | a | 60.7 | b | 169.0 | a | |
| Seaton | No | 116.0 | a | 107.0 | ab | 88.5 | c |
| Yes | 97.3 | bc | 30.8 | d | 111.5 | a | |
| Storden | No | 113.0 | b | 125.5 | ab | 108.3 | bc |
| Yes | 114.0 | b | 154.5 | a | 71.0 | c | |
| Walter | No | 215.0 | a | 156.5 | b | 168.8 | b |
| Yes | 204.3 | a | 154.3 | b | 142.8 | b | |
| Zimmerman | No | 109.5 | b | 102.5 | b | 84.8 | b |
| Yes | 104.0 | b | 217.5 | a | 103.3 | b | |
†Means for a soil series followed by the different letter are significantly different (P < 0.05).
The results of this study showed that sample manipulation after sequential fractionation of soil samples can strongly effect TP concentration measured by ICP-OES. Some of the factors contributing to the observed differences in TP measured after digestion are incomplete oxidation, which depends on concentration of oxidant, digestion temperature, sample matrix, soil type and organic matter content, and hydrolysis of organically bound P during analysis37. The least variability in measured TP was obtained by direct ICP-OES analysis without digestion, filtration, or acidification of soil extracts; whereas, either block or autoclave digestion increased data variability. Dilution or filtration of block and autoclave digested extracts were sources of variation, further indicating that direct ICP-OES analysis of undigested extracts is the preferred method to minimize data variability. As a result of the high data variability with digestion, the amount of calculated Po in the extracts also varied greatly. We believe observations reported in this work are useful and suggest appropriate handling of soil and other environmental (e.g. runoff water, leachate, lake water, and so forth) samples for P determination, thus improving the precision of P determination. These results are also helpful for data interpretation and discussion when there are differences in sample treatments among different studies. Therefore, we recommend direct ICP-OES, without extract manipulation, for future research with sequential fractionation of soil or other environmental samples, such as lake sediment extracts, runoff rain water, drainage water, and others.
Materials and Methods
Soil sample collection and sequential fractionation
Soil samples were collected from Brazil (an Ultisol known as Red-Yellow Argisol, hereinafter Brazil) and across the U.S., including soils from Minnesota Barnes loam, Clarion loam, Cordova clay loam, Fargo silty clay, Formdale clay loam, Hubbard loamy sand, Lester loam, Mount Carroll silt loam, Nicollet clay loam, Normania loam, Seaton silt loam, Storden loam, Walter sandy loam, and Zimmerman sand; the Texas Panhandle Amarillo sandy loam, Pullman clay loam, and Randall clay; Iowa Estherville sandy loam; and Wisconsin Pella clay loam. Most of the soils were from cropped fields under conventional agricultural practices, but had differing histories of cropping system, management, and fertilizer use, with the exception of the Randall series. The Randall clay soil is not typically cropped, as it has high clay content (~38 to 42%), very low permeability, and extensive cracking when dry. The Randall clay used in this study was collected from the bottom of a playa basin in Bushland, Texas. All soils samples were collected from 0 to 15 cm to represent the depth most affected by tillage operations.
After collection, soils were sieved (2 mm), air-dried, and stored at room temperature (22 °C) until analyses. The use of soils encompassing a wide range of origins, textures, and chemical compositions should allow for broader inferences regarding the concentrations of measured P as a function of digestion method, filtration, and acidification. Soil pH was measured in water (1:1 ratio w/w). Organic matter (OM) content was measured by loss on ignition at 360 °C, and particle size analyses were performed using the hydrometer method of Bouyoucos38. Soil test P was extracted with the Bray-1 and Olsen reagents39 and determined by the molybdate blue method of Murphy and Riley7. Calcium (Ca2+), magnesium (Mg2+), iron (Fe3+), and aluminum (Al3+) were extracted using Mehlich-339 and determined by ICP-OES (PerkinElmer, Optima 8 × 00, Norwalk, CT). Selected soil properties are presented in Table 1.
For the sequential fractionation, the original procedure of Hedley et al.1 was used with the modifications of He et al.20: soil mass was increased from 0.5 g to 2.0 g and extraction solution volume was decreased from 25 mL to 20 mL; distilled water was used for extraction of labile P, rather than resin; and the 0.1 M NaOH extraction following ultra-sonication step was omitted. Soil samples (2.0 g dry weight, four replications per sample) were sequentially extracted with deionized water (20 mL) for 16 h at 22 °C on an orbital shaker (250 rpm). Extracts were then centrifuged at 3,400 × g for 15 min at 4 °C and supernatants were carefully decanted into clean 50 mL tubes. Soil residues retained in the tubes were then sequentially extracted with 20 mL each of 0.5 M NaHCO3 (pH 8.5), 0.1 M NaOH, and 1.0 M HCl for 16 h, with extraction conditions and supernatant collection as previously described.
Manipulation of sequential fractions: filtration, digestion, dilution and P determination
After sequential extraction, the water and HCl fractions were separated into two sub-fractions: (1) unfiltered and (2) filtered (0.45-μm nitrocellulose acetate membrane). The NaHCO3 extracts were separated into four sub-fractions: (1) unfiltered and non-acidified, (2) unfiltered and acidified, where NaHCO3 extracts were diluted and pH adjusted with 6 M HCl, (3) filtered and non-acidified, and (4) filtered and acidified. The NaOH extracts were separated into six sub-fractions based on pH and filtration interference by OM. High concentrations of OM were solubilized during NaOH extraction tended to clog filter pores; thus, extracts were diluted 1:10 with deionized water. Some NaOH sub-fractions were acidified with 1 M HCl. As a result, the NaOH extract was separated into six sub-fractions: (1) non-diluted, unfiltered, non-acidified, (2) non-diluted, unfiltered, acidified, (3) diluted, unfiltered, non-acidified, (4) diluted, unfiltered, acidified, (5) diluted, filtered, non-acidified, and (6) diluted, filtered, acidified. For the purpose of this research, acidification of samples was done primarily because ICP-OES analyses are commonly conducted on acidified samples. Samples were acidified to pH ranging between 0.0 and 1.0. There was no observed precipitation of soil minerals, however, there was precipitation of OM complexes following acidification of many of the NaHCO3 and NaOH samples. A schematic diagram of extract handling procedures is presented in Fig. 1. There were a total of 14 treatments conducted on each soil sample.
Two methods were used for sample digestion of all sub-fractions, with the exception of the acidified NaHCO3 and NaOH extracts that were analyzed directly with ICP-OES. The digestion methods were: (1) concentrated H2SO4 + 7.5% K2S2O8, where 2 mL of samples were mixed with 1 mL of H2SO4 and 10 mL of a 7.5% K2S2O8 and autoclaved at 121 °C for 2 h at 1 atm (hereinafter “autoclave” method) (EPA ESS method 310.2); and (2) concentrated H2SO4 + H2O2, where 2 mL of each extract was mixed with 2 mL of concentrated H2SO4 and heated to 225 °C, with additions 1 mL H2O2 at 10 min intervals until the digestate was clear (hereinafter referred to as “block”) (Hedley et al.1). For comparison, all extracts were also directly analyzed with ICP-OES (hereinafter referred to as “undigested”). For quality control, blanks and controls were included in the analyses, where soils were omitted and the extracting solutions were digested to assure the absence of contamination and correct for background. In addition, extraction solutions with known amounts of TP were prepared and treated as samples during digestion and subsequent P determination to assure no loss of P occurred during the digestion and that ICP-OES results were valid.
For P determination, Pi was determined in all undigested samples using the molybdate blue method as described by He and Honeycutt40 using a Biotek Epoch microplate spectrophotometer (Biotek, Winooski, VT). TP was determined using an ICP-OES.
TP in a sample is the sum of Pi and Po and can be described as:
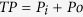 |
however, during ICP-OES analysis both, the Pi and Po are analyzed as Pi as described earlier.
Statistical analyses
The effect of sample filtration, extract acidification, dilution, digestion method, and their interactions (when appropriate, e.g. HCl and water fractions were not acidified and therefore no interaction existed) were evaluated using repeated measures analyses with Proc Glimmix in SAS 9.341,42. The Akaike information criteria (AIC) value was used as the model selection criteria to determine the best covariance model for the repeated variable. Significance of differences among the sample manipulation methods (P < 0.05) were determined by mean separation using Fisher’s least significance difference test (LSD). Regression analysis and correlation analysis were performed using R43. All data analyses were performed on replicate data, while the results are presented as the average of four replicates. The dataset was analyzed for the presence of outliers before any statistical test was conducted.
Additional Information
How to cite this article: do Nascimento, C. A. C. et al. Phosphorus Concentrations in Sequentially Fractionated Soil Samples as Affected by Digestion Methods. Sci. Rep. 5, 17967; doi: 10.1038/srep17967 (2015).
Footnotes
Author Contributions C.N.: Was one of the two PhD. students who helped in the design and execution of all of the analyses needed for this manuscript in addition to performing the statistical analysis. P.P.: Was the main adviser of the PhD. students, he helped with designing the experiment, literature review, and writing of the manuscript. D.S.: Was one of the two PhD. students who helped in the design and execution of all of the analyses needed for this manuscript, this student also helped with the revisions of this manuscript prior to submission for review. Z.H.: Was a collaborator who helped with designing the experiment and writing and revision of the manuscript prior to submission for review. H.W.: Was a collaborator who helped with methods development, results interpretation, editorial reviews, formatting, and reviews prior to submission for review.
References
- Hedley M. J., Stewart J. W. B. & Chauhan B. S. Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 46, 970–976 (1982). [Google Scholar]
- Kuo S. [Phosphorus] Methods of Soil Analysis Part 3 Chemical Methods. [ Sparks D. L. (ed.)] [869–919]. SSSA Book Series, Madison, WI, 1996.
- He Z. & Honeycutt C. W. Enzymatic characterization of organic phosphorus in animal manure. J. Environ. Qual. 30, 1685–1692 (2001). [DOI] [PubMed] [Google Scholar]
- Pagliari P. H. & Laboski C. A. M. Investigation of the inorganic and organic phosphorus forms in animal manure. J. Environ. Qual. 41, 901–910 (2012). [DOI] [PubMed] [Google Scholar]
- Ye D. et al. P accumulation of polygonum hydropiper, soil P fractions and phosphatase activity as affected by swine manure. Applied Soil Ecology 86, 10–18 (2015). [Google Scholar]
- Cross A. F. & Schlesinger W. H. A literature review and evaluation of the. hedley fractionation: Applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 64, 197–214 (1995). [Google Scholar]
- Murphy J. & Riley J. P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 27, 31–36 (1962). [Google Scholar]
- He Z., Griffin T. S. & Honeycutt C. W. Enzymatic hydrolysis of organic phosphorus in swine manure and soil. J. Environ. Qual. 33, 367–372 (2004). [PubMed] [Google Scholar]
- Crews T. E. et al. Changes in soil phosphorus fractions and ecosystem dynamics across a long chronosequence in hawaii. Ecology 76, 1407–1424 (1995). [Google Scholar]
- Dail H. W., He Z., Susan Erich M. & Wayne Honeycutt C. Effect of drying on phosphorus distribution in poultry manure. Commun. Soil Sci. Plant Anal. 38, 1879–1895 (2007). [Google Scholar]
- Pagliari P. & Laboski C. M. Dairy manure treatment effects on manure phosphorus fractionation and changes in soil test phosphorus. Biol. Fertil. Soils 49, 987–999 (2013). [Google Scholar]
- Rowland A. P. & Haygarth P. M. Determination of Total Dissolved Phosphorus in Soil Solutions. J. Environ. Qual. 26, 410–415.. [Google Scholar]
- Martin M., Celi L. & Barberis, E. Determination of low concentrations of organic phosphorus in soil solution. Comm. Soil Sciand Plant Anal 30, 1909–1917 (1999). [Google Scholar]
- He Z., Griffin T. S. & Honeycutt C. W. Soil phosphorus dynamics in response to dairy manure and inorganic fertilizer applications. Soil Sci. 171, 598–609 (2006). [Google Scholar]
- Roboredo M., Fangueiro D., Lage S. & Coutinho J. Phosphorus dynamics in soils amended with acidified pig slurry and derived solid fraction. Geoderma 189–190, 328–333 (2012). [Google Scholar]
- Parent L., Parent S. & Ziadi N. Biogeochemistry of soil inorganic and organic phosphorus: A compositional analysis with balances. J. Geochem. Explor. 141, 52–60 (2014). [Google Scholar]
- Mehmood A. et al. Relationship of Phosphorus Uptake with its fractions in different soil parent materials. Intl J Plant Soil Sci. 4, 45–53 (2015). [Google Scholar]
- Pierzynski G. M. Methods of Phosphorous Analysis for Soils, Sediments, Residuals, and Waters, Southern Cooperative Series Bulletin # 396, U.S. Department of Agriculture and North Carolina State University, Raleigh. (2000) Available at: http://pss.okstate.edu/People/faculty-info/zhang-hailin/research/2004%20and%20earlier%20research/Methods_of_P_Analysis_2000.pdf (Accessed: 20th May 2015).
- He Z., Honeycutt C. W., Cade-Menun B. J., Senwo Z. N. & Tazisong I. A. Phosphorus in poultry litter and soil: enzymatic and nuclear magnetic resonance characterization. Soil Sci. Soc. Am. J. 72, 1425–1433 (2008). [Google Scholar]
- He Z., Fortuna A., Senwo Z. N., Tazisong I. A., Honeycutt C. W. & Griffin T. S. Hydrochloric fractions in Hedley fractionation may contain inorganic and organic phosphates. Soil Sci. Soc. Am. J. 70, 893–899 (2006). [Google Scholar]
- Ivanov K. et al. Comparison of inductively coupled plasma mass spectrometry and colorimetric determination of total and extractable phosphorus in soils. Spectrochimica Acta Part B: Atomic Spectroscopy 71, 117–122 (2012). [Google Scholar]
- Steffánsson A., Gunnarsson I. & Giroud N. New methods for the direct determination of dissolved inorganic, organic and total carbon in natural waters by Reagent-FreeTM Ion Chromatography and inductively coupled plasma atomic emission spectrometry. Anal. Chim. Acta. 582, 69–74 (2007). [DOI] [PubMed] [Google Scholar]
- Pittman J. J., Zhang H., Schroder J. L. & Payton M. E. Differences of phosphorus in mehlich 3 extracts determined by colorimetric and spectroscopic methods. Commun. Soil Sci. Plant Anal. 36, 1641–1659 (2005). [Google Scholar]
- Matula J. Differences in available phosphorus evaluated by soil tests in relation to detection by colorimetric and ICP-AES techniques. Plant Soil Environ. 56, 197–304 (2010). [Google Scholar]
- Zhang M., Wright R., Heaney D. & Vanderwel D. Comparison of different phosphorus extraction and determination methods using manured soils. Can. J. Soil Sci. 84, 469–475 (2004). [Google Scholar]
- He Z., Zhang H., Olanya M. O., Frantz J. M. & Larkin R. P. Differences in modified Morgan phosphorus levels determined by colorimetric and inductively coupled plasma methods. Open J. Soil Sci. 2, 256–262 (2012). [Google Scholar]
- Boss C. & Fredeen K. Concepts, instrumentation and techniques in inductively coupled plasma optical emission spectrometry. 3rd Edition. PerkinElmer, USA, (2004). [Google Scholar]
- Matula J. Determination of dissolved reactive and dissolved total phosphorus in water extract of soils. Plant Soil Environ. 57, 1–6 (2011). [Google Scholar]
- Vadas P. A. & Kleinman P. J. A. Effect of methodology in estimating and interpreting water-extractable phosphorus in animal manures. J. Environ. Qual. 35, 1151–1159 (2006). [DOI] [PubMed] [Google Scholar]
- de Boer J. L. M., Kohlmeyer U., Breugem P. M. & van d. V. Determination of total dissolved phosphorus in water samples by axial inductively coupled plasma atomic emission spectrometry. Fresenius J. Anal. Chem. 360, 132–136 (1998). [Google Scholar]
- Makarov M. I., Haumaier L. & Zech W. Nature of soil organic phosphorus: an assessment of peak assignments in the diester region of 31P NMR spectra. Soil Biol. Biochem. 34, 1467–1477 (2002). [Google Scholar]
- Turner B. L. & Haygarth P. M. Phosphorus forms and concentrations in leachate under four grassland soil types. Soil Sci. Soc. Am. J. 64, 1090–1099 (2000). [Google Scholar]
- Swift R. S. [Organic matter characterization] Methods of Soil Analysis Part 3 Chemical Methods. [ Sparks D. L. (ed.)] [869–919]. SSSA Book Series, Madison, WI, (1996).
- McDowell R. W. & Sharpley J. N. Soil phosphorus fractions in solution: in Ūence of fertilizer and manure, filtration and method of determination. Chemosphere 45, 737–748 (2001). [DOI] [PubMed] [Google Scholar]
- Vadas P. A., Meisinger J. J., Sikora L. J., McMurtry J. P. & Sefton A. E. Effect of poultry diet on phosphorus in runoff from soils amended with poultry manure and compost. J. Environ. Qual. 33, 1845–1854 (2004). [DOI] [PubMed] [Google Scholar]
- Sharpley A. & Moyer B. Phosphorus forms in manure and compost and their release during simulated rainfall. J. Environ. Qual. 29, 1462–1469 (2000). [Google Scholar]
- Jarvie et al. Review of robust measurement of phosphorus in river water: sampling, storage, fractionation, and sensitivity. Hydrology and Earth System Sci. 6, 113–132 (2002). [Google Scholar]
- Bouyoucos G. J. Hydrometer method improved for making particle size analyses of Soils. Agron. J. 54, 464–465 (1962). [Google Scholar]
- Brown J. R. Recommended chemical soil test procedures for the North Central region. North Central Regional Publ. 221 (revised). Missouri Agric. Exp. Stn., Columbia, MO, (1998).
- He Z. & Honeycutt C. W. A modified molybdenum blue method for orthophosphate determination suitable for investigating enzymatic hydrolysis of organic phosphates. Commun. Soil Sci. Plant Anal. 36, 1373–1383 (2005). [Google Scholar]
- SAS Institute, 2010. SAS 9.2. SAS Inst., Cary, NC.
- Littell R. C. et al. SAS for Mixed Models. Second Edition. SAS Institute Inc., Cary, NC. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2007 Available at http://www.R-project.org. (Verified 13th Feb. 2015).