Abstract
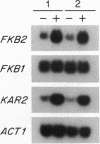
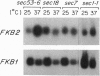
The FKB2 gene of Saccharomyces cerevisiae encodes a homolog of mammalian FKBP-13, an FK506/rapamycin-binding protein that localizes to the lumen of the endoplasmic reticulum (ER). We have found that FKB2 mRNA levels increase in response to the accumulation of unfolded precursor proteins in the ER. FKB2 mRNA levels are elevated in cells blocked in N-glycosylation--i.e., in wild-type cells treated with tunicamycin and in the sec53-6 mutant grown at the nonpermissive temperature. Mutations that block other steps in secretion have no effect on FKB2 mRNA levels, indicating that increases in FKB2 mRNA are not the consequence of a general block in secretion. The increase in FKB2 mRNA in response to unfolded proteins in the ER is mediated through a 21-bp unfolded-protein response (UPR) element located in the 5' noncoding region of FKB2. UPR elements present in other ER chaperone genes, such as yeast KAR2 (BiP), mammalian GRP78 (BiP), and GRP94, function in an analogous manner to that in FKB2. As with KAR2, FKB2 mRNA levels are also elevated by heat shock. The similarities in the regulation of FKB2 and other ER chaperone genes suggest that FKBP-13 may play a role in protein trafficking in the ER.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber S., Krause K. H., Caroni P. s-cyclophilin is retained intracellularly via a unique COOH-terminal sequence and colocalizes with the calcium storage protein calreticulin. J Cell Biol. 1992 Jan;116(1):113–125. doi: 10.1083/jcb.116.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein M., Hoffmann W., Ammerer G., Schekman R. Characterization of a gene product (Sec53p) required for protein assembly in the yeast endoplasmic reticulum. J Cell Biol. 1985 Dec;101(6):2374–2382. doi: 10.1083/jcb.101.6.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. C., Erwin A. E., Lee A. S. Glucose-regulated protein (GRP94 and GRP78) genes share common regulatory domains and are coordinately regulated by common trans-acting factors. Mol Cell Biol. 1989 May;9(5):2153–2162. doi: 10.1128/mcb.9.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J., Kuo C. J., Crabtree G. R., Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992 Jun 26;69(7):1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- Colley N. J., Baker E. K., Stamnes M. A., Zuker C. S. The cyclophilin homolog ninaA is required in the secretory pathway. Cell. 1991 Oct 18;67(2):255–263. doi: 10.1016/0092-8674(91)90177-z. [DOI] [PubMed] [Google Scholar]
- Davis J. M., Boswell B. A., Bächinger H. P. Thermal stability and folding of type IV procollagen and effect of peptidyl-prolyl cis-trans-isomerase on the folding of the triple helix. J Biol Chem. 1989 May 25;264(15):8956–8962. [PubMed] [Google Scholar]
- Deshaies R. J., Schekman R. A yeast mutant defective at an early stage in import of secretory protein precursors into the endoplasmic reticulum. J Cell Biol. 1987 Aug;105(2):633–645. doi: 10.1083/jcb.105.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R. J., Schekman R. SEC62 encodes a putative membrane protein required for protein translocation into the yeast endoplasmic reticulum. J Cell Biol. 1989 Dec;109(6 Pt 1):2653–2664. doi: 10.1083/jcb.109.6.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- Esmon B., Novick P., Schekman R. Compartmentalized assembly of oligosaccharides on exported glycoproteins in yeast. Cell. 1981 Aug;25(2):451–460. doi: 10.1016/0092-8674(81)90063-5. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fischer G., Schmid F. X. The mechanism of protein folding. Implications of in vitro refolding models for de novo protein folding and translocation in the cell. Biochemistry. 1990 Mar 6;29(9):2205–2212. doi: 10.1021/bi00461a001. [DOI] [PubMed] [Google Scholar]
- Forsburg S. L., Guarente L. Mutational analysis of upstream activation sequence 2 of the CYC1 gene of Saccharomyces cerevisiae: a HAP2-HAP3-responsive site. Mol Cell Biol. 1988 Feb;8(2):647–654. doi: 10.1128/mcb.8.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R. B. Protein disulfide isomerase: multiple roles in the modification of nascent secretory proteins. Cell. 1989 Jun 30;57(7):1069–1072. doi: 10.1016/0092-8674(89)90043-3. [DOI] [PubMed] [Google Scholar]
- Freskgård P. O., Bergenhem N., Jonsson B. H., Svensson M., Carlsson U. Isomerase and chaperone activity of prolyl isomerase in the folding of carbonic anhydrase. Science. 1992 Oct 16;258(5081):466–468. doi: 10.1126/science.1357751. [DOI] [PubMed] [Google Scholar]
- Galat A., Lane W. S., Standaert R. F., Schreiber S. L. A rapamycin-selective 25-kDa immunophilin. Biochemistry. 1992 Mar 3;31(8):2427–2434. doi: 10.1021/bi00123a031. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Guarente L., Mason T. Heme regulates transcription of the CYC1 gene of S. cerevisiae via an upstream activation site. Cell. 1983 Apr;32(4):1279–1286. doi: 10.1016/0092-8674(83)90309-4. [DOI] [PubMed] [Google Scholar]
- Guarente L. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 1983;101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- Jayaraman T., Brillantes A. M., Timerman A. P., Fleischer S., Erdjument-Bromage H., Tempst P., Marks A. R. FK506 binding protein associated with the calcium release channel (ryanodine receptor). J Biol Chem. 1992 May 15;267(14):9474–9477. [PubMed] [Google Scholar]
- Jin Y. J., Albers M. W., Lane W. S., Bierer B. E., Schreiber S. L., Burakoff S. J. Molecular cloning of a membrane-associated human FK506- and rapamycin-binding protein, FKBP-13. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6677–6681. doi: 10.1073/pnas.88.15.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepes F., Schekman R. The yeast SEC53 gene encodes phosphomannomutase. J Biol Chem. 1988 Jul 5;263(19):9155–9161. [PubMed] [Google Scholar]
- Koltin Y., Faucette L., Bergsma D. J., Levy M. A., Cafferkey R., Koser P. L., Johnson R. K., Livi G. P. Rapamycin sensitivity in Saccharomyces cerevisiae is mediated by a peptidyl-prolyl cis-trans isomerase related to human FK506-binding protein. Mol Cell Biol. 1991 Mar;11(3):1718–1723. doi: 10.1128/mcb.11.3.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozutsumi Y., Segal M., Normington K., Gething M. J., Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988 Mar 31;332(6163):462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- Lang K., Schmid F. X., Fischer G. Catalysis of protein folding by prolyl isomerase. Nature. 1987 Sep 17;329(6136):268–270. doi: 10.1038/329268a0. [DOI] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Melnick J., Aviel S., Argon Y. The endoplasmic reticulum stress protein GRP94, in addition to BiP, associates with unassembled immunoglobulin chains. J Biol Chem. 1992 Oct 25;267(30):21303–21306. [PubMed] [Google Scholar]
- Mori K., Sant A., Kohno K., Normington K., Gething M. J., Sambrook J. F. A 22 bp cis-acting element is necessary and sufficient for the induction of the yeast KAR2 (BiP) gene by unfolded proteins. EMBO J. 1992 Jul;11(7):2583–2593. doi: 10.1002/j.1460-2075.1992.tb05323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss M. L., Palmer R. E., Kuzmic P., Dunlap B. E., Henzel W., Kofron J. L., Mellon W. S., Royer C. A., Rich D. H. Identification of actin and HSP 70 as cyclosporin A binding proteins by photoaffinity labeling and fluorescence displacement assays. J Biol Chem. 1992 Nov 5;267(31):22054–22059. [PubMed] [Google Scholar]
- Nadler S. G., Tepper M. A., Schacter B., Mazzucco C. E. Interaction of the immunosuppressant deoxyspergualin with a member of the Hsp70 family of heat shock proteins. Science. 1992 Oct 16;258(5081):484–486. doi: 10.1126/science.1411548. [DOI] [PubMed] [Google Scholar]
- Nielsen J. B., Foor F., Siekierka J. J., Hsu M. J., Ramadan N., Morin N., Shafiee A., Dahl A. M., Brizuela L., Chrebet G. Yeast FKBP-13 is a membrane-associated FK506-binding protein encoded by the nonessential gene FKB2. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7471–7475. doi: 10.1073/pnas.89.16.7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normington K., Kohno K., Kozutsumi Y., Gething M. J., Sambrook J. S. cerevisiae encodes an essential protein homologous in sequence and function to mammalian BiP. Cell. 1989 Jun 30;57(7):1223–1236. doi: 10.1016/0092-8674(89)90059-7. [DOI] [PubMed] [Google Scholar]
- Novick P., Ferro S., Schekman R. Order of events in the yeast secretory pathway. Cell. 1981 Aug;25(2):461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Novick P., Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondek B., Hardy R. W., Baker E. K., Stamnes M. A., Shieh B. H., Zuker C. S. Genetic dissection of cyclophilin function. Saturation mutagenesis of the Drosophila cyclophilin homolog ninaA. J Biol Chem. 1992 Aug 15;267(23):16460–16466. [PubMed] [Google Scholar]
- Park S. T., Aldape R. A., Futer O., DeCenzo M. T., Livingston D. J. PPIase catalysis by human FK506-binding protein proceeds through a conformational twist mechanism. J Biol Chem. 1992 Feb 15;267(5):3316–3324. [PubMed] [Google Scholar]
- Partaledis J. A., Fleming M. A., Harding M. W., Berlin V. Saccharomyces cerevisiae contains a homolog of human FKBP-13, a membrane-associated FK506/rapamycin binding protein. Yeast. 1992 Aug;8(8):673–680. doi: 10.1002/yea.320080812. [DOI] [PubMed] [Google Scholar]
- Partaledis J. A., Mason T. L. Structure and regulation of a nuclear gene in Saccharomyces cerevisiae that specifies MRP13, a protein of the small subunit of the mitochondrial ribosome. Mol Cell Biol. 1988 Sep;8(9):3647–3660. doi: 10.1128/mcb.8.9.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A., Harding M. W., Fleming M. A., DeCenzo M. T., Lippke J. A., Livingston D. J., Benasutti M. Expression and characterization of human FKBP52, an immunophilin that associates with the 90-kDa heat shock protein and is a component of steroid receptor complexes. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10974–10978. doi: 10.1073/pnas.89.22.10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. J., Grove J. R., Calvo V., Avruch J., Bierer B. E. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science. 1992 Aug 14;257(5072):973–977. doi: 10.1126/science.1380182. [DOI] [PubMed] [Google Scholar]
- Renoir J. M., Radanyi C., Faber L. E., Baulieu E. E. The non-DNA-binding heterooligomeric form of mammalian steroid hormone receptors contains a hsp90-bound 59-kilodalton protein. J Biol Chem. 1990 Jun 25;265(18):10740–10745. [PubMed] [Google Scholar]
- Rose M. D., Misra L. M., Vogel J. P. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell. 1989 Jun 30;57(7):1211–1221. doi: 10.1016/0092-8674(89)90058-5. [DOI] [PubMed] [Google Scholar]
- Rothblatt J. A., Deshaies R. J., Sanders S. L., Daum G., Schekman R. Multiple genes are required for proper insertion of secretory proteins into the endoplasmic reticulum in yeast. J Cell Biol. 1989 Dec;109(6 Pt 1):2641–2652. doi: 10.1083/jcb.109.6.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez E. R., Faber L. E., Henzel W. J., Pratt W. B. The 56-59-kilodalton protein identified in untransformed steroid receptor complexes is a unique protein that exists in cytosol in a complex with both the 70- and 90-kilodalton heat shock proteins. Biochemistry. 1990 May 29;29(21):5145–5152. doi: 10.1021/bi00473a021. [DOI] [PubMed] [Google Scholar]
- Sanders S. L., Whitfield K. M., Vogel J. P., Rose M. D., Schekman R. W. Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell. 1992 Apr 17;69(2):353–365. doi: 10.1016/0092-8674(92)90415-9. [DOI] [PubMed] [Google Scholar]
- Shortle D., Novick P., Botstein D. Construction and genetic characterization of temperature-sensitive mutant alleles of the yeast actin gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4889–4893. doi: 10.1073/pnas.81.15.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekierka J. J., Wiederrecht G., Greulich H., Boulton D., Hung S. H., Cryan J., Hodges P. J., Sigal N. H. The cytosolic-binding protein for the immunosuppressant FK-506 is both a ubiquitous and highly conserved peptidyl-prolyl cis-trans isomerase. J Biol Chem. 1990 Dec 5;265(34):21011–21015. [PubMed] [Google Scholar]
- Slater M. R., Craig E. A. Transcriptional regulation of an hsp70 heat shock gene in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1987 May;7(5):1906–1916. doi: 10.1128/mcb.7.5.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague G. F., Jr, Jensen R., Herskowitz I. Control of yeast cell type by the mating type locus: positive regulation of the alpha-specific STE3 gene by the MAT alpha 1 product. Cell. 1983 Feb;32(2):409–415. doi: 10.1016/0092-8674(83)90460-9. [DOI] [PubMed] [Google Scholar]
- Stamnes M. A., Rutherford S. L., Zuker C. S. Cyclophilins: a new family of proteins involved in intracellular folding. Trends Cell Biol. 1992 Sep;2(9):272–276. doi: 10.1016/0962-8924(92)90200-7. [DOI] [PubMed] [Google Scholar]
- Stamnes M. A., Shieh B. H., Chuman L., Harris G. L., Zuker C. S. The cyclophilin homolog ninaA is a tissue-specific integral membrane protein required for the proper synthesis of a subset of Drosophila rhodopsins. Cell. 1991 Apr 19;65(2):219–227. doi: 10.1016/0092-8674(91)90156-s. [DOI] [PubMed] [Google Scholar]
- Tachibana C., Stevens T. H. The yeast EUG1 gene encodes an endoplasmic reticulum protein that is functionally related to protein disulfide isomerase. Mol Cell Biol. 1992 Oct;12(10):4601–4611. doi: 10.1128/mcb.12.10.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P. K., Albers M. W., Chang H., Faber L. E., Schreiber S. L. Association of a 59-kilodalton immunophilin with the glucocorticoid receptor complex. Science. 1992 May 29;256(5061):1315–1318. doi: 10.1126/science.1376003. [DOI] [PubMed] [Google Scholar]
- Teyton L., Peterson P. A. Invariant chain--a regulator of antigen presentation. Trends Cell Biol. 1992 Feb;2(2):52–56. doi: 10.1016/0962-8924(92)90163-h. [DOI] [PubMed] [Google Scholar]
- Tuite M. F., Bossier P., Fitch I. T. A highly conserved sequence in yeast heat shock gene promoters. Nucleic Acids Res. 1988 Dec 23;16(24):11845–11845. doi: 10.1093/nar/16.24.11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yem A. W., Tomasselli A. G., Heinrikson R. L., Zurcher-Neely H., Ruff V. A., Johnson R. A., Deibel M. R., Jr The Hsp56 component of steroid receptor complexes binds to immobilized FK506 and shows homology to FKBP-12 and FKBP-13. J Biol Chem. 1992 Feb 15;267(5):2868–2871. [PubMed] [Google Scholar]