Abstract
Several studies over the past decade have now consistently indicated that the serum anti-Mullerian hormone (AMH) levels are at least 2–3-fold higher in the patients with polycystic ovary syndrome (PCOS), which also corresponds to the increased number of AMH producing preantral and small antral follicles. Moreover, AMH levels have been found to be associated in direct proportion to the follicle numbers per ovary or antral follicular count, assessed by the transvaginal ultrasound (TVS). Furthermore, AMH correlates directly with the rising serum testosterone and luteinizing hormone levels in PCOS. Hence, serum AMH in women with oligo-anovulation and/or hyperandrogenemia could indicate the presence of underlying PCOS, when reliable TVS is not feasible, or not acceptable, either due to the virginal status or psycho-social issue. In addition, the imaging quality of abdominal ultrasound is often impaired by obesity, which typically occurs in PCOS women. Indeed, PCOS occurs most commonly in young females who cannot be subjected to invasive TVS for various reasons; therefore, a desirable alternative to TVS is urgently required to diagnose the most prevalent endocrine abnormality of young women. This review will analyze the currently available evidence regarding the role of AMH in the diagnosis of PCOS.
Keywords: Anti-Mullerian hormone, hyperandrogenemia, insulin resistance, oligo-ovulation, polycystic ovary disease
INTRODUCTION
Polycystic ovary syndrome (PCOS) is the most common cause of anovulatory infertility and the commonest endocrine abnormalities in women of reproductive age.[1] As the name suggests it is a syndrome, therefore no single test would be diagnostic and hence, several criteria over the time have been laid down for its diagnosis. Truly, its diagnosis remains a challenge in the reproductive medicine.
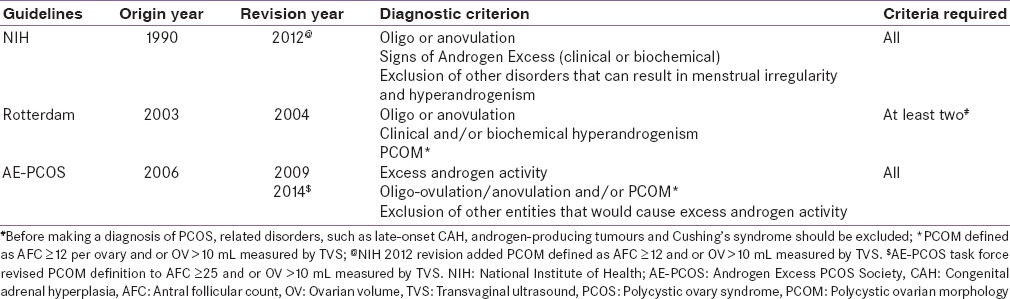
Currently, three diagnostic criteria exist in the literature to diagnose PCOS, which essentially appears similar in principle; although they are not uniform [Table 1]. While the National Institutes of Health (NIH) 1990 criteria, included oligo/anovulation and hyperandrogenism, the Rotterdam 2003 consensus criteria included polycystic ovarian morphology (PCOM) on transvaginal ultrasonography (TVS).[2,3] PCOM on TVS was not essential in 1990 NIH criteria. The presence of clinical or biochemical hyperandrogenemia (HA) is mandatory in both NIH and the Androgen Excess/PCOS Society (AE-PCOS) 2006 guidelines.[4,5] In contrast to the NIH and AE-PCOS criteria, the Rotterdam criteria allowed the diagnosis of PCOS to be made in women with PCOM and chronic oligo/anovulation even without the evidence of HA. While this created some controversies, 20–40% of PCOS may not exhibit biochemical HA as seen in some study.[6] Exclusion of other causes of AE was common in all three guidelines. Although, the 1990 NIH guideline did not include evidence of PCOM by TVS in their diagnostic criteria, the latest recommendation of NIH 2012 have now included PCOM.[7] Taken together, it is apparent that diagnosis of PCOS is still a challenge in the clinical practice, primarily due to ever changing criteria and definitions as PCOS women have a heterogeneous spectrum of presentation.
Table 1.
Definition of PCOS by different guidelines
Consequently, reported the prevalence of PCOS varied from 2.2% to 26% of premenopausal women because of lack of uniformity and depending upon the diagnostic criteria used in the studies. While, its prevalence in women of fertile age ranged from 6% to 10% using the NIH criteria, it varied from 14% to 17% using the Rotterdam criteria.[8,9] There are also enough reasons to believe that many women in the general population are either over-diagnosed or could be undiagnosed, because of the extensive heterogeneity in the clinical presentation of PCOS. Furthermore, with the rising epidemic of obesity, there could be increased the prevalence of PCOS, as obesity potentially worsens the endocrine and metabolic profile of PCOS.[10] These entire factors underline the importance of identifying PCOS with a uniform standardized diagnostic tool with the minimal inter-observer variation.
WHY AN ALTERNATIVE TO ULTRASOUND SEEMS NECESSITY CURRENTLY?
Ultrasonographic (USG) diagnosis of PCOM is an area which kept changing over the time. Adams et al. in 1985, arbitrarily described PCOM as an ovary containing ≥10 follicles (measuring 2–8 mm in diameter) in one cross-section of the ovary, examined by trans-abdominal USG and this threshold became the most widely adopted criteria until Rotterdam 2004 revised criteria changed this to ≥12 follicle.[11] Moreover, follicular number per ovary (FNPO) rather than follicle number per se ction (FNPS) as suggested earlier by Adams et al. got importance. This difference of FNPO versus FNPS was another important distinction that has also created confusion in clinical practice. Meanwhile, with the development of highly sensitive ultrasound transducer, transvaginal approaches have replaced the transabdominal USG on account of better resolution and a greater likelihood of detecting the cystic ovaries.
Recent 2004 revised Rotterdam consensus criteria defines PCOM as FNPO threshold of ≥12 follicles measuring 2–9 mm in diameter (mean of both ovaries) with or without ovarian volume (OV) of ≥10 mL. OV is calculated by the formula as V = π/6 × length × width × thickness or 0.526 × lengths × width × thickness. It is interesting to note that most commonly used ultrasound definition of FNPO employed to date in Rotterdam PCOM criteria as well as in 2009 revised Androgen Excess Society (AES) guideline, was primarily based on expert agreement and on the findings of a single study by Jonard et al.
In 2003 Jonard et al. conducted the first study using receiver operator characteristics (ROC) curve analyzes for defining PCOM and suggested that FNPO threshold of ≥12 follicles (2–9 mm in diameter) by trans-vaginal USG holds a 75% sensitivity and 99% specificity in distinguishing PCOS from non-PCOS controls.[12] Indeed, several studies conducted thereafter in women of child-bearing age found FNPO ≥12 threshold resulted in a larger prevalence of PCOM. Some recent studies found a median FNPO of 11–13 in women with regular menstrual cycles, who had no evidence of HA. Overall, it appears that 50% of the controls (aged 20–35 years) have characteristics of PCOM when the threshold of FNPO ≥12 is used to define PCOM.[13,14,15] Additionally, FNPO ≥12 to diagnose PCOS leaves little room for non-PCOS-related causes of normo-gonadotrophic anovulation like functional hypothalamic amenorrhea.[16]
All these emerging data pointed that the current threshold of ≥12 follicles to diagnose PCOM, remains no longer adequate, and this led many to question the entity PCOM to have no pathological significance; although others recommended increasing the diagnostic threshold for FNPO.[13,17] It appears that this discrepancy primarily occurred due to the technical advances in imaging with the identification of more follicles which led to the artificial increase in the prevalence of PCOM. Dewailly et al. in an ROC curve analysis proposed a higher threshold of FNPO ≥19 with 81% sensitivity and 92% specificity for the diagnosis of PCOS, although control were excluded in this analysis.[18] Recently, Luzan et al. in an ROC curve analysis finds, FNPO ≥26 having the highest sensitivity and specificity in differentiating PCOS with control.[15]
Most recently, the 2014 AES-PCOS task force have recently proposed a cut-off FNPO ≥25 per the whole ovary to diagnose PCOM, while using a transducer frequency of ≥8 MHz.[19] This recommendation was based on the basis of a systematic review comparing normative data on FNPO from a total of 1127 women of reproductive age and found FNPO ≥25 diagnostic of PCOM. It should be noted, however, that these data were primarily obtained from Caucasian and Europeans and may not apply to all populations. Interestingly, Chen et al. in an ROC curve analysis confirmed FNPO threshold ≥12 for diagnosing PCOM in the Chinese population, while Kösüs et al. proposed an FNPO threshold of 8 follicles per ovary for Turkish women.[20,21] While these threshold cut-offs in Asians appeared to be far below compared to the newly proposed values of ≥25 for the Western population, it is not yet clear whether such a difference appeared due to ethnic variation or due to the use of lower frequency transducers in Asian population. Arguably, the proposition to increase in FNPO threshold currently appears to be due to the advances in ultrasound technology rather than a true biological discrimination. However, the future implications of these changes are still unknown.
It should be worthwhile to note that OV of ≥10 mL to diagnose PCOM remains unchanged even with the advancement in the imaging technique. Nevertheless, FNPO is still recommended to diagnose PCOS over OV, because of higher predictive power and lesser variability.[19]
With this background knowledge, it is apparently clear that obtaining a good data on ovarian morphology demands time and resource consuming ultrasound examination apart from the judgmental and inter-observer variability. It is also obvious that the lack of standardization in diagnosing PCOM could also lead to differences in diagnosis. Definition of PCOM may not be applicable to an adolescent cohort, where it is believed that the ovaries normally have an enlarged multi-follicular appearance. Moreover, PCOM changes through the menstrual cycle and with the concomitant oral contraceptive (OCP) use, making a reliable and standardized assessment of PCOM even more difficult.[22]
The larger concern in reality is the application of transvaginal USG based diagnosis of FNPO as recommended in Rotterdam criteria and AES-PCOS Society guideline. Indeed, the majority of PCOS population are teenagers and women of reproductive age for whom trans-vaginal ultrasound could be unacceptable or unethical, because of their virginal status, while concomitant obesity may also yield poor results on transabdominal USG. These compound hindrance of heterogenic phenotypes, nonuniform diagnostic criteria, ever-changing PCOM definition, and unacceptability of trans-vaginal ultrasound in the target population, clearly demands to find a suitable alternative, in particular, to replace invasive TVS.
WHY ANTI-MULLERIAN HORMONE COULD BE A POTENTIAL ALTERNATIVE?
Anti-Mullerian hormone (AMH) is produced from small antral follicles and measuring it as a stable product, which is not subjective to ongoing technical advances or operator dependence, would be an attractive option. The correlation of AMH and antral follicular count (AFC) is also well known and strong. AMH correlates strongly with biochemical hyperandrogenism (serum testosterone and androstenedione), oligomenorrhea and mean OV. Thus, AMH could be a potential biological marker and its application could avoid the need for invasive ultrasound examinations. Consequently, it is tempting and desirable to find a single diagnostic threshold of AMH to diagnose PCOS, keeping in mind the limitations of its sensitivity and specificity. Moreover, as no single value of AMH may be capable of defining the entire spectrum of PCOS, at least it appears to be a strong predictor of PCOM.
This review will analyze the recent progress with AMH in PCOS and PCOM.
REVIEW METHOD
The PubMed/MEDLINE search was conducted to identify relevant studies, those published in The English language, since January 2000 to March 2015, which evaluated the role of AMH in PCOS women compared to normal control. Search also included the studies which evaluated the relation of AMH with the AFC and PCOM.
ANTI-MULLERIAN HORMONE
AMH initially referred to as Mullerian-inhibiting substance wasfirst described in 1947 by Jost as a gonadal factor produced by Sertoli cells of male embryo causing regression of the Mullerian ducts and allowing Wolffian ducts to develop into the male reproductive tract under the influence of testosterone.[23] Testicular production of AMH has been described as early as 8 weeks gestation.[24] Expression of AMH in the granulosa cell of the chicken ovary wasfirst reported by Hutson et al. in 1981.[25] In the human fetus, ovarian AMH production starts around birth.[26] AMH secreted from antral follicle is predominantly secreted into the intra-follicular compartment, sufficiently large enough to permit the detection of AMH in the circulation.[27]
The ovary contains a limited number of primordial follicles and this pool of primordial follicle is called ovarian reserve. Primordial follicles leave the pool, enters into the growing pool and sequentially converted into primary, secondary, preantral, small antral, and dominant/preovulatory follicle, although the vast majority intended to undergo atresia. Only at the onset of puberty, one follicle a month escapes atresia and proceeds to ovulation.
AMH is secreted mainly by the small-antral follicles (4–8 mm size), and its level is proportionate to the follicular fluid level of small preantral and antral follicular pool. Its level gradually declines with the increase in the size of the follicles and a sharp decline in the serum/follicular AMH level takes place once the size of the follicle reaches 8 mm or dominant follicle is selected.[28] Preovulatory follicles > mm fail to produce AMH. Although the physiological pattern of rise of AMH prior to the age of 25 years yielded conflicting results (some suggested biphasic pattern with peaks and troughs, while other finds a gradual rise from birth until 15 years of age then decline) literature appears to agree that the values decline yearly at a fairly consistent rate after 25 years of age (peak) until below the detection limit by age 50. In view of the biphasic effect of age on serum AMH values, some authors have suggested adapting different thresholds according to the patients’ age in especially in young adults, in whom AMH levels are rising.[29] Nevertheless, this information also allows the clinicians to be more cautious in interpreting AMH value in younger PCOS.
FACTORS WHICH CAN INFLUENCE ANTI-MULLERIAN HORMONE LEVEL
The most attractive aspects about AMH value, unlike serum follicle stimulating hormone (FSH), luteinizing hormone (LH) and inhibin B testing, is the lack of large variation in the average values at the given population level. However, with the recent recognition of the significant variability in a specific situation, interpretation of its value warrants some caution. Various factors can alter the AMH value which should be kept in mind while interpreting its result [Table 2].
Table 2.
Factors influencing AMH level
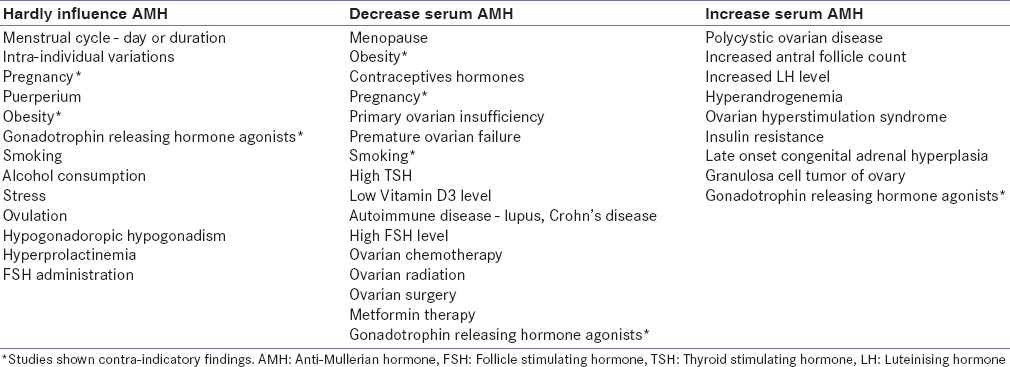
Broadly, there are three recognized sources for this variability which includes, biological fluctuation within certain individuals; exposure to medications and surgical procedure; and laboratory specific (types of the assay).[30,31,32,33,34,35,36,37,38,39,40,41,42,43]
Studies are contra indicatory regarding intra- and inter-cycle variability of AMH levels. While van Disseldorp et al. found no variation; Hehenkamp et al. found mild fluctuation which could be possibly related to the gradual changes in antral follicles number in each menstrual cycle.[31,32] In contrast, Wunder et al. found substantial fluctuations and Overbeek et al. reported extensive fluctuation in AMH during the menstrual cycle.[33,34] The latter finding argues measuring AMH levels in the early follicular phase only. Furthermore, some studies found AMH levels remain fairly constant with exogenous sex steroids used either for cycle regulation or contraception, other studies including a recent large (n = 2000) cohort study by Dólleman et al. found decrease in AMH with current use of OCPs which reversed after stopping OCP.[35,36] These findings suggest a reversible suppressive subtle effect of OCP on AMH. Moreover, some studies found exogenous GnRH agonist administration could also influence AMH levels significantly while other suggested no changes.[37,38]
Besides, several other factors can also influence AMH concentrations including smoking, bodyweight, ethnicity, Vitamin D status, polymorphisms of AMH receptor, and genetic variants across the genome.[39,40,41,42,43] The clinical relevance of these findings remains to be elucidated in future. Ethnicity can show a large variation in the number of antral-follicles present at the same age. African, Americans, and Hispanic women have lower serum AMH values as compared to their Caucasians counterpart.[39]
However, even with these variations, AMH is still the best available serum marker of quantitative aspects of ovarian reserve currently, surpassing LH, FSH and inhibin B measurement.[30]
ANTI-MULLERIAN HORMONE ASSAY
Two type of assay are currently available, one Diagnostic Systems Laboratories (DSL assay) from Diagnostic Systems Laboratories Inc., Webster, Texas, USA and another Immunotech (IOT assay) from Immunotech-Beckman Coulter, Marseille, France. Both are convenient microplate-based two-site ELISA using peroxidase labels. Serum AMH values were standardized to give AMH measurements in nanograms per milliliter using the following conversion formula: 1 ng/mL = 7.143 pmol/L. A high correlation between values obtained with the two assays is observed, although absolute values in the DSL assay are approximately half of those for the same samples with the IOT assay.[44]
There is probably no great significance in this difference, which arises because the two assays use different preparations of recombinant human AMH. Use of conversion factors from one assay to the other is also available; however, its accuracy is still controversial. With the availability of newer AMH Gen II assay by Beckman Coulter Inc., Brea, CA (USA), which is a combination of DSL and IOT assay, there is finally a single commercially available assay. AMH Gen II assay retains the cross-species specificity of the DSL assay and is calibrated to the IOT standards. Therefore, the values generated by AMH Gen II are similar to the original IOT assay and 40% higher than the previous DSL version as suggested by Wallace et al.[45] In other words, AMH Gen II value is 1.4 times higher than DSL assay value.
However, currently, there is no international reference standard for AMH. Li et al. find a good correlation between the new (AMH Gen II) and old AMH assay kits by IOT and DSL (r2 = 0.971 and 0.930, respectively). The regression equations to convert AMH value by AMH Gen II from IOT and DSL assay are AMH Gen II = 1.353 × AMH (IOT) +0.051 and AMH (Gen II) = 1.223 × AMH (DSL) −1.270, respectively.[46] However, Rustamov et al. did not find good correlation and warns against using conversion formula.[47] It should be noted that false-low AMH value (hook effect) can also happen at extremely high analyte concentrations, and false-high results may also happen due to heterophilic antibody interferences, if not blocked by the assay's blocking regents.
From the Indian perspective, although AMH Gen II assay is now available in few standard laboratories, ELISA by IOT or DSL is widely available throughout the country. AMH Gen II assay by Beckman Coulter suggest reference ranges of 1–7 ng/mL while it is 2.0–6.8 ng/mL by IOT ELISA assay.
Currently, the major issue for the clinician trying to apply serum AMH values in clinical practice is primarily its estimation. Each laboratory provides their own value ranges depending upon the methodologies used, which could be significantly different and noninterchangeable. Universally accepted methods and assays to measure AMH is clearly the need of the hour.
RATIONAL FOR THE USE OF ANTI-MULLERIAN HORMONE IN THE DIAGNOSIS OF POLYCYSTIC OVARY DISEASE AND POLYCYSTIC OVARIAN MORPHOLOGY
Serum AMH value is expected to be increased in PCOS women because their ovaries exhibit an increased number of AMH-producing preantral and small antral follicles and also because granulosa cell production of AMH is greatly increased. Moreover, AMH significantly correlates with the other criteria of PCOS including oligomenorrhea and HA.[48,49,50,51,52,53,54,55] Furthermore; Serum AMH is significantly related to the serum LH and to the ratio of LH/FSH. AMH concentrations were independently predicted with LH, testosterone, and AFC.[12] Indeed, increased LH levels could be the most significant independent link to AMH rise in PCOS.[51] It was proposed that there could be an earlier LH receptor gain in anovulatory patients with PCOS. Premature LH action on the granulosa cell could additionally contribute to the follicular arrest and the observed increase in AMH produced from granulosa cell.[53] Some study also found a significant increase of serum AMH in women with PCOS having higher insulin resistance.[56]
Anti-Mullerian hormone level in polycystic ovary syndrome
Fallat et al. in 1997 conducted a prospective study of infertile women (n = 66) and found significantly higher mean AMH level in both follicular fluid (7.01 vs. 1.65 ng/mL) as well as serum (2.97 vs. 0.92 ng/mL) of infertile patients with PCOS (n = 17) compared to infertile women with tubal factor.[57]
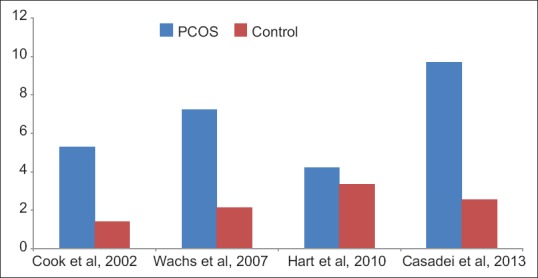
Since then, several observational (both prospective and retrospective) comparative studies have been conducted in past decade which finds significantly higher AMH level in PCOS cohort, compared to control [Table 3, Figures 1 and 2].[18,50,52,54,55,56,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78] Cook et al., found significantly higher mean serum AMH level (5.3 vs. 1.4 ng/mL, P < 0.0001) in women with PCOS (n = 27) diagnosed by NIH criteria, compared to normal women (n = 20). In addition, AMH level were not statistically different in the patients with PCOS according to body mass index (BMI) (≥30 kg/m2 vs. ≤30 kg/m2).[58] It is worthwhile to note that relation of AMH with BMI have conflicting results. While Freeman et al. reported AMH level 65% lower in obese women compared to normal-weight women, which was further supported by another study by Piouka et al.; however, Siow et al. and Cook et al. reported no significant association between AMH levels with BMI.[51,58,62,79] Interestingly, Dolfing et al. found significantly higher AMH level (11.1 ± 3.0 ng/mL vs. 3.3 ± 1.8 ng/mL, P < 0.01) in lean PCOS (n = 20) over lean control, although no differences in metabolic parameters and insulin resistance existed between lean PCOS versus controls.[80]
Table 3.
AMH value in PCOS versus control
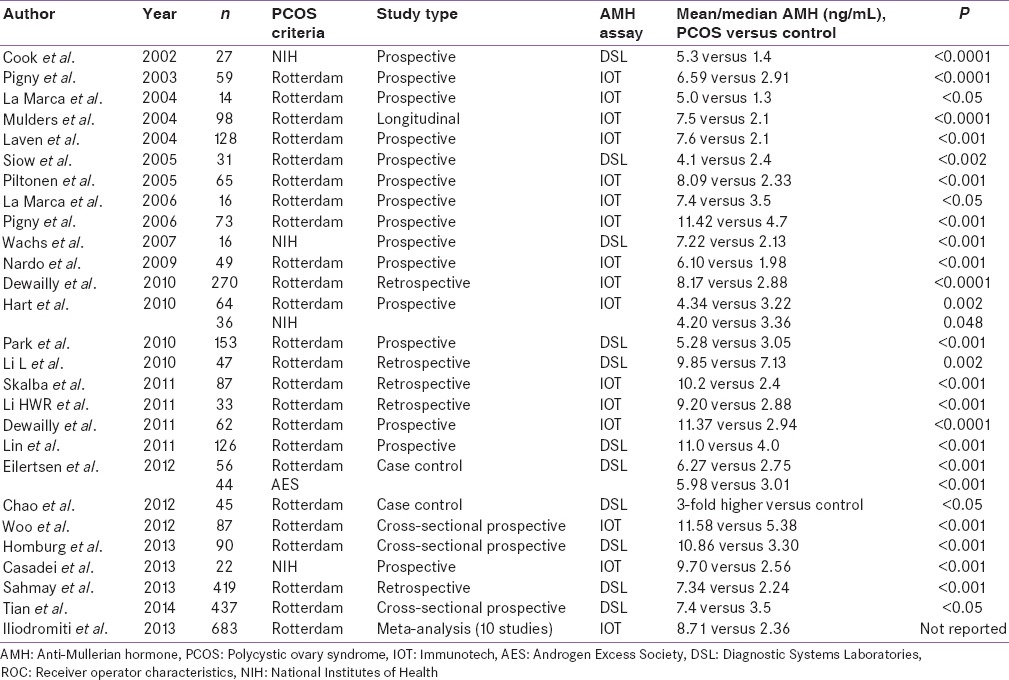
Figure 1.
Anti-Mullerian hormone value (ng/mL) in polycystic ovary syndrome versus control using National Institutes of Health criteria
Figure 2.
Anti-Mullerian hormone value (ng/mL) in polycystic ovary syndrome versus control using Rotterdam criteria
Pigny et al. also found significantly higher AMH value (6.59 vs. 2.91 ng/mL, P < 0.0001) in PCOS women (n = 59) diagnosed by Rotterdam criteria, compared to controls (n = 45). Moreover, AMH positively correlated to the serum testosterone (P < 0.0005) and androstenedione (P < 0.002) levels in PCOS.[59] Laven et al. in a larger cohort of PCOS (n = 128) women also replicated the same findings and suggested significantly higher AMH value (7.6 vs. 2.1 ng/mL, P < 0.001) compared to control (n = 41). While a significant negative correlation between age and AMH levels were observed in both control (P < 0.002) and PCOS (P < 0.001) women, the decrease in AMH levels with increasing age was significantly (P < 0.001) higher in controls, compared to PCOS women.[50] Dewailly et al. concluded that both excessive follicle number (FN) assessed by TVS and/or serum AMH may be used as surrogates for HA, as serum AMH were significantly higher (8.17 vs. 2.88 ng/mL, P < 0.001) in PCOS women (n = 270), compared to non-PCOS infertile control (n = 217). In addition, AMH correlated well with the clinical and biochemical HA.[66] Homburg et al. measured AMH in women with PCOS (n = 90), PCOM (n = 35), and with normal ovaries (controls, n = 90). Mean serum AMH were significantly higher in PCOS and PCOM patient compared to control (PCOS vs. PCOM vs. control; 10.86 vs. 7.31 vs. 3.3 ng/mL, s respectively, P < 0.001). Moreover, the combination of AMH >.72 ng/mL and LH > IU/l could diagnose 82.6% of women with PCOS.[75]
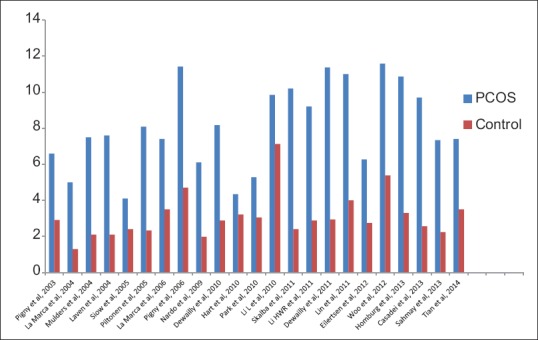
Two recent and largest studies conducted so far, compared AMH level in PCOS women to controls, found the same result. Sahmay et al. evaluated PCOS women (n = 419) and finds significantly higher serum AMH value (7.34 vs. 2.24 ng/mL, P < 0.001) to non-PCOS control (n = 151).[77] Tian et al. compared AMH level in PCOS women (n = 437) to normal women (n = 150) in China and finds significantly (2–3-fold) higher AMH in PCOS women (7.4 vs. 3.5 ng/mL, P < 0.05).[56] Only meta-analysis currently available in literature by Iliodromiti et al. derived from 10 studies, finds similarly higher AMH level in PCOS women (8.71 vs. 2.36 ng/mL) compared to control [Figure 3].[78]
Figure 3.
Meta-analysis of 10 studies
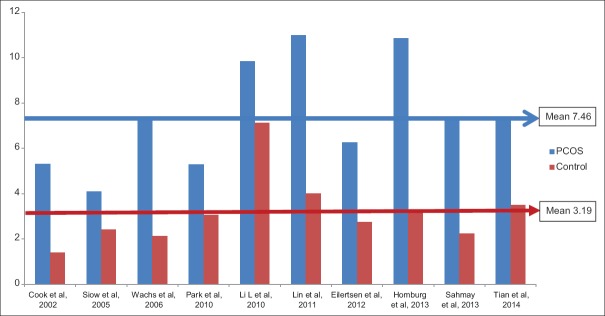
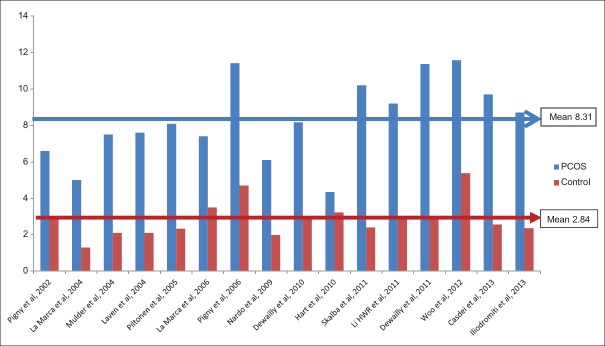
To summarize, although all these studies clearly pointed to a significant 2–3-fold higher AMH value in PCOS women compared to control, no uniform single value of AMH could be derived across the studies. This could perhaps have happened due to the sample size, different criteria used to define PCOS and assay of measuring AMH used. However, when these studies were divided on the basis of AMH assay used, a mean value of 7.46 versus 3.19 ng/mL and 8.31 versus 2.84 ng/mL were observed in PCOS versus control, while using DSL and IOT assay, respectively [Figures 4 and 5].
Figure 4.
Anti-Mullerian hormone value (ng/mL) in polycystic ovary syndrome versus control using Diagnostic Systems Laboratories assay
Figure 5.
Anti-Mullerian hormone value (ng/mL) in polycystic ovary syndrome versus control using Immunotech assay
Cut-off value of anti-Mullerian hormone to diagnose polycystic ovary syndrome
Statistically, an ideal screening test should have zero or near-zero false positives. The relationship between false positivity rate and specificity can be established by an equation. False-positive rate = 100 − specificity. Hence, it can be concluded that a test cut-off with a high specificity approaching 100% would achieve the objective of zero false positives. Nevertheless, choosing a high specificity could compromise the sensitivity and therefore, looking at a test cut-off with a near 100% specificity may not be enough. On the other hand, the screening test should be equally balanced with an acceptable level of sensitivity, in order to have a minimally acceptable number of false negatives.
Although several threshold cut-off values of AMH have been proposed, they exhibit varying sensitivity and specificity [Table 4 and Figure 6]. Pigny et al. in an ROC curve analysis, found AMH cut-off value of 8.4 ng/mL with 67% sensitivity and 92% specificity, for PCOS diagnosis using IOT assay.[64] Dewailly et al., using ROC curve analysis concluded that a serum AMH level of cut-off 4.9 ng/mL could replace the finding of PCOM in the definition of PCOS with 92% sensitivity and 97% specificity.[18] Sahmay et al. using ROC curve analysis found a threshold AMH cut-off value of 3.94 mg/mL to diagnose PCOS, with 80% sensitivity and 90% specificity by DSL assay.[77] A meta-analysis by Iliodromiti et al. using ROC analysis found a threshold AMH of 4.7 ng/mL to have 83% sensitivity and 79% specificity.[78]
Table 4.
Studies finding cut-off value of AMH to diagnose PCOS through ROC analysis
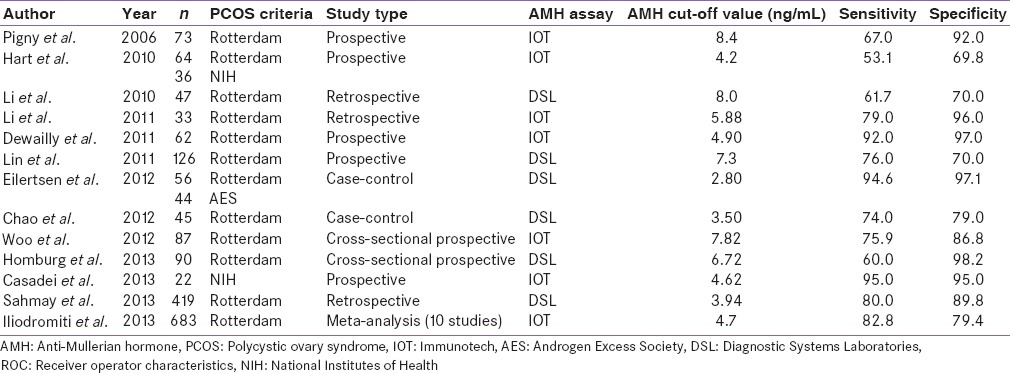
Figure 6.
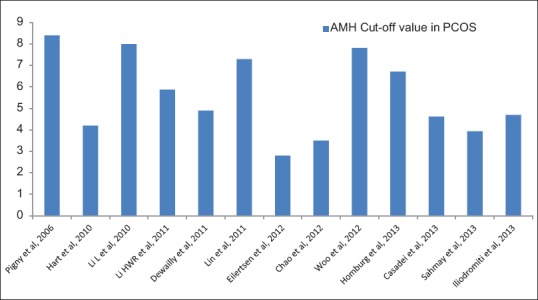
Anti-Mullerian hormone cut-off value (ng/mL) for polycystic ovary syndrome in receiver operator characteristics curve analysis
Correlation of anti-Mullerian hormone with antral follicular count
There exist a good correlation between AMH and antral follicle count. van Rooij et al. in a study of 119 patients found serum AMH levels highly correlating (r = 0.77; P < 0.01) with the number of antral follicles.[48] Fanchin et al. also found AMH levels (n = 75) more robustly correlated with the number of early antral follicles compared to inhibin B, E2, FSH, and LH.[49] Pigny et al. found AMH level positively related to the follicles of 2–5 mm size (P < 0.03) but not in the 6–9 mm size.[59] Laven et al. in a study of PCOS women (n = 128), also found AMH levels to significantly correlate with the mean FN (r = 0.308; P < 0.001) and the mean OV (r = 0.421; P < 0.001). Interestingly, when PCOS women were categorized into those with and without PCOM (FNPO/AFC ≥12 follicles of size 2–9 mm and/or OV > 10 mL), AMH levels were significantly higher in PCOS with PCOM compared to PCOS without PCOM (9.3 vs. 6.4 ng/mL, P < 0.001).[50] Piltonen et al. found serum AMH levels correlated positively with the follicle count (P < 0.012) and negatively with the age (P < 0.014) in PCOS (n = 65) women. Additionally, AMH was positively correlated with testosterone level (P < 0.011).[52] Dewailly et al. concluded that a serum AMH level of >.9 ng/mL could be able to replace the finding of PCOM.[18] Eilertsen et al. using the DSL assay suggested a cut-off value of 2.8 ng/mL for PCOM, with 80% sensitivity and 72% specificity.[72] Homburg et al. by pooling the results from DSL to Gen II assay using a conversion factor of 1.4, found that a serum AMH threshold of 6.72 ng/mL had an excellent (98%) specificity while using FNPO ≥12 threshold for PCOM diagnosis. However, this excellent (98%) specificity appeared at the expense of a poor (60%) sensitivity.[75] Casadei et al. using ROC analysis yielded a threshold cut-off of AMH >.64 ng/mL for PCOM diagnosis, with 95% sensitivity and 95% specificity using IOT assay.[76]
The only contradictory study of AMH cut-off value to diagnose PCOM emerged in a study by Hart et al., where an ROC threshold to predict PCOM of 4.2 ng/mL could miss 45% of PCOM and 48% of PCOS. While the authors acknowledge the limitation of this study, as the control group included normal girls with irregular cycles, who would have otherwise diagnosed as PCOS by Rotterdam criteria, they found PCOM had significantly elevated serum AMH concentration (4.5 vs. 3.0 ng/mL, P < 0.001).[67] Villarroel et al. found AMH value of 8.40 ng/mL could be used to diagnose PCOM in normally menstruating adolescents with 64% sensitivity and 90% specificity, using IOT assay.[81]
It was not surprising to find lower sensitivity and/or specificity obtained with the ROC curve analysis, because in several studies, the serum AMH level were tested against the PCOM which was predefined by an FNPO ≥12. In addition, the normative data for serum AMH concentrations were also difficult to compare, as the supposedly “normal” controls may not have necessarily be representative of the normal population. This discrepancy could perhaps be minimized with the recently proposed definition of PCOM by an FNPO ≥25.
Correlation of anti-Mullerian hormone with ovarian volume
Although the Rotterdam consensus statement suggested an OV threshold of 10 mL based on expert opinion, other researchers proposed much lower cut-off values.[82] A lower cut-off OV threshold of 6.4, 6.7, 7.0, and 7.5 mL was proposed by Kösüs et al., Chen et al., Dewailly et al., and Carmina et al., respectively.[18,20,21,83] These different OV threshold values could have happened due to the difference in ethnicity, BMI and other metabolic characteristics.
However, the AES-PCOS Task Force 2014 recommends using the former threshold OV of 10 mL, since this is closest to the 95th percentile of the largest pooled control (n = 1021) data. Moreover, OV has less diagnostic potential than FNPO to discriminate between PCOS and controls. The Task Force recommends using OV for the diagnosis of PCOM when the image quality does not allow a reliable estimate of FNPO, especially when the transvaginal route is not feasible.[19]
In a regression analysis, Piouka et al. found AMH level positively related to the mean OV (r = 0.178, P = 0.007).[51] Dolfing et al. also suggested AMH level positively related to mean OV (r = 0.75, P < 0.0001) in lean PCOS.[80]
CONCLUSION
To sum up, all the studies till date which evaluated serum AMH in PCOS women have reported a significant increase in mean serum AMH levels compared to the controls. Although, both mean serum AMH levels and ROC curve analysis generated threshold cut-off values for AMH, varied among the studies, no single value of AMH has universally emerged. This could have happened, perhaps due to the differences in the sample size, sample selection criteria, PCOS phenotypes, ethnic difference and the type of AMH assays used in these studies. Due to these uncertainties around AMH assays, the recent 2014 AES-PCOS Society Task Force does not recommend increased serum AMH concentration as a surrogate marker of PCOM yet.
However, with the recent availability of AMH Gen II assay, which appears to produce reliable and reproducible results, AMH may revisit as an emerging biochemical marker of PCOS and PCOM in future. It is also reasonably apparent, that no single AMH value may be capable of diagnosing a syndrome like PCOS, due to heterogeneous nature of its presentation; however, AMH could be an exciting alternative to the transvaginal USG evaluation. Nevertheless, even newer generation AMH assay needs to be internationally standardized.
Moreover, as the AMH concentration correlates significantly well with the oligomenorrhea and HA, this would further suggest that a diagnostic cut-off of AMH could be achievable with an acceptable threshold of the sensitivity and specificity.
Taken together, with the available totality of evidence, it may be proposed that AMH could be a suitable substitute at least for the diagnosis of PCOM, if not PCOS as a whole and may replace TVS in future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685–97. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 2.Zawadzki JA, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: Towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic Ovary Syndrome. Boston: Blackwell Scientific; 1992. pp. 377–84. [Google Scholar]
- 3.Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 4.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Positions statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237–45. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 5.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: The complete task force report. Fertil Steril. 2009;91:456–88. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 6.Barber TM, Wass JA, McCarthy MI, Franks S. Metabolic characteristics of women with polycystic ovaries and oligo-amenorrhoea but normal androgen levels: Implications for the management of polycystic ovary syndrome. Clin Endocrinol (Oxf) 2007;66:513–7. doi: 10.1111/j.1365-2265.2007.02764.x. [DOI] [PubMed] [Google Scholar]
- 7.Johnson TR, Kaplan LK, Ouyang P, Rizza RA. Evidence-Based Methodology Workshop on Polycystic Ovary Syndrome. Bethesda, Maryland: National Institutes of Health; 2012. [Google Scholar]
- 8.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: A prospective study. J Clin Endocrinol Metab. 1998;83:3078–82. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 9.Tehrani FR, Simbar M, Tohidi M, Hosseinpanah F, Azizi F. The prevalence of polycystic ovary syndrome in a community sample of Iranian population: Iranian PCOS prevalence study. Reprod Biol Endocrinol. 2011;9:39. doi: 10.1186/1477-7827-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoeger KM. Role of lifestyle modification in the management of polycystic ovary syndrome. Best Pract Res Clin Endocrinol Metab. 2006;20:293–310. doi: 10.1016/j.beem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Adams J, Franks S, Polson DW, Mason HD, Abdulwahid N, Tucker M, et al. Multifollicular ovaries: Clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet. 1985;2:1375–9. doi: 10.1016/s0140-6736(85)92552-8. [DOI] [PubMed] [Google Scholar]
- 12.Jonard S, Robert Y, Cortet-Rudelli C, Pigny P, Decanter C, Dewailly D. Ultrasound examination of polycystic ovaries: Is it worth counting the follicles? Hum Reprod. 2003;18:598–603. doi: 10.1093/humrep/deg115. [DOI] [PubMed] [Google Scholar]
- 13.Bentzen JG, Forman JL, Johannsen TH, Pinborg A, Larsen EC, Andersen AN. Ovarian antral follicle subclasses and anti-Mullerian hormone during normal reproductive aging. J Clin Endocrinol Metab. 2013;98:1602–11. doi: 10.1210/jc.2012-1829. [DOI] [PubMed] [Google Scholar]
- 14.Deb S, Campbell BK, Clewes JS, Pincott-Allen C, Raine-Fenning NJ. Intracycle variation in number of antral follicles stratified by size and in endocrine markers of ovarian reserve in women with normal ovulatory menstrual cycles. Ultrasound Obstet Gynecol. 2013;41:216–22. doi: 10.1002/uog.11226. [DOI] [PubMed] [Google Scholar]
- 15.Lujan ME, Jarrett BY, Brooks ED, Reines JK, Peppin AK, Muhn N, et al. Updated ultrasound criteria for polycystic ovary syndrome: Reliable thresholds for elevated follicle population and ovarian volume. Hum Reprod. 2013;28:1361–8. doi: 10.1093/humrep/det062. [DOI] [PubMed] [Google Scholar]
- 16.Wang JG, Lobo RA. The complex relationship between hypothalamic amenorrhea and polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:1394–7. doi: 10.1210/jc.2007-1716. [DOI] [PubMed] [Google Scholar]
- 17.Kristensen SL, Ramlau-Hansen CH, Ernst E, Olsen SF, Bonde JP, Vested A, et al. A very large proportion of young Danish women have polycystic ovaries: Is a revision of the Rotterdam criteria needed? Hum Reprod. 2010;25:3117–22. doi: 10.1093/humrep/deq273. [DOI] [PubMed] [Google Scholar]
- 18.Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, et al. Diagnosis of polycystic ovary syndrome (PCOS): Revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26:3123–9. doi: 10.1093/humrep/der297. [DOI] [PubMed] [Google Scholar]
- 19.Dewailly D, Lujan ME, Carmina E, Cedars MI, Laven J, Norman RJ, et al. Definition and significance of polycystic ovarian morphology: A task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2014;20:334–52. doi: 10.1093/humupd/dmt061. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Li L, Chen X, Zhang Q, Wang W, Li Y, et al. Ovarian volume and follicle number in the diagnosis of polycystic ovary syndrome in Chinese women. Ultrasound Obstet Gynecol. 2008;32:700–3. doi: 10.1002/uog.5393. [DOI] [PubMed] [Google Scholar]
- 21.Kösüs N, Kösüs A, Turhan NÖ, Kamalak Z. Do threshold values of ovarian volume and follicle number for diagnosing polycystic ovarian syndrome in Turkish women differ from western countries? Eur J Obstet Gynecol Reprod Biol. 2011;154:177–81. doi: 10.1016/j.ejogrb.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Somunkiran A, Yavuz T, Yucel O, Ozdemir I. Anti-Müllerian hormone levels during hormonal contraception in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2007;134:196–201. doi: 10.1016/j.ejogrb.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Jost A. Reserchessur la differenciation de l'embryon de lapin. Arch Anat Microsc Morphol Exp. 1947;36:271–315. [Google Scholar]
- 24.Lee MM, Donahoe PK, Silverman BL, Hasegawa T, Hasegawa Y, Gustafson ML, et al. Measurements of serum müllerian inhibiting substance in the evaluation of children with nonpalpable gonads. N Engl J Med. 1997;336:1480–6. doi: 10.1056/NEJM199705223362102. [DOI] [PubMed] [Google Scholar]
- 25.Hutson J, Ikawa H, Donahoe PK. The ontogeny of Mullerian inhibiting substance in the gonads of the chicken. J Pediatr Surg. 1981;16:822–7. doi: 10.1016/s0022-3468(81)80827-5. [DOI] [PubMed] [Google Scholar]
- 26.Rajpert-De Meyts E, Jørgensen N, Graem N, Müller J, Cate RL, Skakkebaek NE. Expression of anti-müllerian hormone during normal and pathological gonadal development: Association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab. 1999;84:3836–44. doi: 10.1210/jcem.84.10.6047. [DOI] [PubMed] [Google Scholar]
- 27.Jeppesen JV, Anderson RA, Kelsey TW, Christiansen SL, Kristensen SG, Jayaprakasan K, et al. Which follicles make the most anti-Mullerian hormone in humans. Evidence for an abrupt decline in AMH production at the time of follicle selection? Mol Hum Reprod. 2013;19:519–27. doi: 10.1093/molehr/gat024. [DOI] [PubMed] [Google Scholar]
- 28.Grynnerup AG, Lindhard A, Sørensen S. The role of anti-müllerian hormone in female fertility and infertility-An overview. Acta Obstet Gynecol Scand. 2012;91:1252–60. doi: 10.1111/j.1600-0412.2012.01471.x. [DOI] [PubMed] [Google Scholar]
- 29.Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH. A validated model of serum anti-müllerian hormone from conception to menopause. PLoS One. 2011;6:e22024. doi: 10.1371/journal.pone.0022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leader B, Baker VL. Maximizing the clinical utility of antimüllerian hormone testing in women's health. Curr Opin Obstet Gynecol. 2014;26:226–36. doi: 10.1097/GCO.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Disseldorp J, Lambalk CB, Kwee J, Looman CW, Eijkemans MJ, Fauser BC, et al. Comparison of inter- and intra-cycle variability of anti-Mullerian hormone and antral follicle counts. Hum Reprod. 2010;25:221–7. doi: 10.1093/humrep/dep366. [DOI] [PubMed] [Google Scholar]
- 32.Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ. Anti-müllerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057–63. doi: 10.1210/jc.2006-0331. [DOI] [PubMed] [Google Scholar]
- 33.Wunder DM, Bersinger NA, Yared M, Kretschmer R, Birkhäuser MH. Statistically significant changes of antimüllerian hormone and inhibin levels during the physiologic menstrual cycle in reproductive age women. Fertil Steril. 2008;89:927–33. doi: 10.1016/j.fertnstert.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 34.Overbeek A, Broekmans FJ, Hehenkamp WJ, Wijdeveld ME, van Disseldorp J, van Dulmen-den Broeder E, et al. Intra-cycle fluctuations of anti-müllerian hormone in normal women with a regular cycle: A re-analysis. Reprod Biomed Online. 2012;24:664–9. doi: 10.1016/j.rbmo.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 35.Deb S, Campbell BK, Pincott-Allen C, Clewes JS, Cumberpatch G, Raine-Fenning NJ. Quantifying effect of combined oral contraceptive pill on functional ovarian reserve as measured by serum anti-müllerian hormone and small antral follicle count using three-dimensional ultrasound. Ultrasound Obstet Gynecol. 2012;39:574–80. doi: 10.1002/uog.10114. [DOI] [PubMed] [Google Scholar]
- 36.Dólleman M, Verschuren WM, Eijkemans MJ, Dollé ME, Jansen EH, Broekmans FJ, et al. Reproductive and lifestyle determinants of anti-müllerian hormone in a large population-based study. J Clin Endocrinol Metab. 2013;98:2106–15. doi: 10.1210/jc.2012-3995. [DOI] [PubMed] [Google Scholar]
- 37.Su HI, Maas K, Sluss PM, Chang RJ, Hall JE, Joffe H. The impact of depot GnRH agonist on AMH levels in healthy reproductive-aged women. J Clin Endocrinol Metab. 2013;98:E1961–6. doi: 10.1210/jc.2013-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buyuk E, Seifer DB, Illions E, Grazi RV, Lieman H. Elevated body mass index is associated with lower serum anti-Mullerian hormone levels in infertile women with diminished ovarian reserve but not with normal ovarian reserve. Fertil Steril. 2011;95:2364–8. doi: 10.1016/j.fertnstert.2011.03.081. [DOI] [PubMed] [Google Scholar]
- 39.Seifer DB, Golub ET, Lambert-Messerlian G, Benning L, Anastos K, Watts DH, et al. Variations in serum Müllerian inhibiting substance between white, black, and Hispanic women. Fertil Steril. 2009;92:1674–8. doi: 10.1016/j.fertnstert.2008.08.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merhi ZO, Seifer DB, Weedon J, Adeyemi O, Holman S, Anastos K, et al. Circulating Vitamin D correlates with serum antimüllerian hormone levels in late-reproductive-aged women: Women's Interagency HIV Study. Fertil Steril. 2012;98:228–34. doi: 10.1016/j.fertnstert.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kevenaar ME, Themmen AP, Rivadeneira F, Uitterlinden AG, Laven JS, van Schoor NM, et al. A polymorphism in the AMH type II receptor gene is associated with age at menopause in interaction with parity. Hum Reprod. 2007;22:2382–8. doi: 10.1093/humrep/dem176. [DOI] [PubMed] [Google Scholar]
- 42.Schuh-Huerta SM, Johnson NA, Rosen MP, Sternfeld B, Cedars MI, Reijo Pera RA. Genetic variants and environmental factors associated with hormonal markers of ovarian reserve in Caucasian and African American women. Hum Reprod. 2012;27:594–608. doi: 10.1093/humrep/der391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tal R, Seifer DB. Potential mechanisms for racial and ethnic differences in antimüllerian hormone and ovarian reserve. Int J Endocrinol. 2013;2013:818912. doi: 10.1155/2013/818912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson SM, La Marca A. The journey from the old to the new AMH assay: How to avoid getting lost in the values. Reprod Biomed Online. 2011;23:411–20. doi: 10.1016/j.rbmo.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 45.Wallace AM, Faye SA, Fleming R, Nelson SM. A multicentre evaluation of the new beckman coulter anti-Mullerian hormone immunoassay (AMH Gen II) Ann Clin Biochem. 2011;48(Pt 4):370–3. doi: 10.1258/acb.2011.010172. [DOI] [PubMed] [Google Scholar]
- 46.Li HW, Ng EH, Wong BP, Anderson RA, Ho PC, Yeung WS. Correlation between three assay systems for anti-müllerian hormone (AMH) determination. J Assist Reprod Genet. 2012;29:1443–6. doi: 10.1007/s10815-012-9880-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rustamov O, Smith A, Roberts SA, Yates AP, Fitzgerald C, Krishnan M, et al. Anti-Mullerian hormone: Poor assay reproducibility in a large cohort of subjects suggests sample instability. Hum Reprod. 2012;27:3085–91. doi: 10.1093/humrep/des260. [DOI] [PubMed] [Google Scholar]
- 48.van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, et al. Serum anti-müllerian hormone levels: A novel measure of ovarian reserve. Hum Reprod. 2002;17:3065–71. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- 49.Fanchin R, Schonäuer LM, Righini C, Guibourdenche J, Frydman R, Taieb J. Serum anti-müllerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18:323–7. doi: 10.1093/humrep/deg042. [DOI] [PubMed] [Google Scholar]
- 50.Laven JS, Mulders AG, Visser JA, Themmen AP, De Jong FH, Fauser BC. Anti-müllerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endocrinol Metab. 2004;89:318–23. doi: 10.1210/jc.2003-030932. [DOI] [PubMed] [Google Scholar]
- 51.Piouka A, Farmakiotis D, Katsikis I, Macut D, Gerou S, Panidis D. Anti-Mullerian hormone levels reflect severity of PCOS but are negatively influenced by obesity: Relationship with increased luteinizing hormone levels. Am J Physiol Endocrinol Metab. 2009;296:E238–43. doi: 10.1152/ajpendo.90684.2008. [DOI] [PubMed] [Google Scholar]
- 52.Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-müllerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod. 2005;20:1820–6. doi: 10.1093/humrep/deh850. [DOI] [PubMed] [Google Scholar]
- 53.Willis DS, Watson H, Mason HD, Galea R, Brincat M, Franks S. Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome: Relevance to mechanism of anovulation. J Clin Endocrinol Metab. 1998;83:3984–91. doi: 10.1210/jcem.83.11.5232. [DOI] [PubMed] [Google Scholar]
- 54.Nardo LG, Yates AP, Roberts SA, Pemberton P, Laing I. The relationships between AMH, androgens, insulin resistance and basal ovarian follicular status in non-obese subfertile women with and without polycystic ovary syndrome. Hum Reprod. 2009;24:2917–23. doi: 10.1093/humrep/dep225. [DOI] [PubMed] [Google Scholar]
- 55.Lin YH, Chiu WC, Wu CH, Tzeng CR, Hsu CS, Hsu MI. Antimüllerian hormone and polycystic ovary syndrome. Fertil Steril. 2011;96:230–5. doi: 10.1016/j.fertnstert.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Tian X, Ruan X, Mueck AO, Wallwiener D, Wang J, Liu S, et al. Serum anti-müllerian hormone and insulin resistance in the main phenotypes of non-obese polycystic ovarian syndrome women in China. Gynecol Endocrinol. 2014;30:836–9. doi: 10.3109/09513590.2014.943719. [DOI] [PubMed] [Google Scholar]
- 57.Fallat ME, Siow Y, Marra M, Cook C, Carrillo A. Müllerian-inhibiting substance in follicular fluid and serum: A comparison of patients with tubal factor infertility, polycystic ovary syndrome, and endometriosis. Fertil Steril. 1997;67:962–5. doi: 10.1016/s0015-0282(97)81417-3. [DOI] [PubMed] [Google Scholar]
- 58.Cook CL, Siow Y, Brenner AG, Fallat ME. Relationship between serum Müllerian-inhibiting substance and other reproductive hormones in untreated women with polycystic ovary syndrome and normal women. Fertil Steril. 2002;77:141–6. doi: 10.1016/s0015-0282(01)02944-2. [DOI] [PubMed] [Google Scholar]
- 59.Pigny P, Merlen E, Robert Y, Cortet-Rudelli C, Decanter C, Jonard S, et al. Elevated serum level of anti-Mullerian hormone in patients with polycystic ovary syndrome: Relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957–62. doi: 10.1210/jc.2003-030727. [DOI] [PubMed] [Google Scholar]
- 60.La Marca A, Orvieto R, Giulini S, Jasonni VM, Volpe A, De Leo V. Mullerian-inhibiting substance in women with polycystic ovary syndrome: Relationship with hormonal and metabolic characteristics. Fertil Steril. 2004;82:970–2. doi: 10.1016/j.fertnstert.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 61.Mulders AG, Laven JS, Eijkemans MJ, de Jong FH, Themmen AP, Fauser BC. Changes in anti-müllerian hormone serum concentrations over time suggest delayed ovarian ageing in normogonadotrophic anovulatory infertility. Hum Reprod. 2004;19:2036–42. doi: 10.1093/humrep/deh373. [DOI] [PubMed] [Google Scholar]
- 62.Siow Y, Kives S, Hertweck P, Perlman S, Fallat ME. Serum Müllerian-inhibiting substance levels in adolescent girls with normal menstrual cycles or with polycystic ovary syndrome. Fertil Steril. 2005;84:938–44. doi: 10.1016/j.fertnstert.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 63.La Marca A, Pati M, Orvieto R, Stabile G, Carducci Artenisio A, Volpe A. Serum anti-müllerian hormone levels in women with secondary amenorrhea. Fertil Steril. 2006;85:1547–9. doi: 10.1016/j.fertnstert.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 64.Pigny P, Jonard S, Robert Y, Dewailly D. Serum anti-Mullerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941–5. doi: 10.1210/jc.2005-2076. [DOI] [PubMed] [Google Scholar]
- 65.Wachs DS, Coffler MS, Malcom PJ, Chang RJ. Serum anti-Mullerian hormone concentrations are not altered by acute administration of follicle stimulating hormone in polycystic ovary syndrome and normal women. J Clin Endocrinol Metab. 2007;92:1871–4. doi: 10.1210/jc.2006-2425. [DOI] [PubMed] [Google Scholar]
- 66.Dewailly D, Pigny P, Soudan B, Catteau-Jonard S, Decanter C, Poncelet E, et al. Reconciling the definitions of polycystic ovary syndrome: The ovarian follicle number and serum anti-müllerian hormone concentrations aggregate with the markers of hyperandrogenism. J Clin Endocrinol Metab. 2010;95:4399–405. doi: 10.1210/jc.2010-0334. [DOI] [PubMed] [Google Scholar]
- 67.Hart R, Doherty DA, Norman RJ, Franks S, Dickinson JE, Hickey M, et al. Serum antimullerian hormone (AMH) levels are elevated in adolescent girls with polycystic ovaries and the polycystic ovarian syndrome (PCOS) Fertil Steril. 2010;94:1118–21. doi: 10.1016/j.fertnstert.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 68.Park AS, Lawson MA, Chuan SS, Oberfield SE, Hoeger KM, Witchel SF, et al. Serum anti-Mullerian hormone concentrations are elevated in oligomenorrheic girls without evidence of hyperandrogenism. J Clin Endocrinol Metab. 2010;95:1786–92. doi: 10.1210/jc.2009-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li L, Chen X, Mo Y, Chen Y, Wenig M, Yang D. Elevated serum anti-Mullerian hormone in adolescent and young adult Chinese patients with polycystic ovary syndrome. Wien Klin Wochenschr. 2010;122:519–24. doi: 10.1007/s00508-010-1426-x. [DOI] [PubMed] [Google Scholar]
- 70.Skalba P, Cygal A, Madej P, Dabkowska-Huc A, Sikora J, Martirosian G, et al. Is the plasma anti-müllerian hormone (AMH) level associated with body weight and metabolic, and hormonal disturbances in women with and without polycystic ovary syndrome? Eur J Obstet Gynecol Reprod Biol. 2011;158:254–9. doi: 10.1016/j.ejogrb.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 71.Li HW, Anderson RA, Yeung WS, Ho PC, Ng EH. Evaluation of serum antimullerian hormone and inhibin B concentrations in the differential diagnosis of secondary oligoamenorrhea. Fertil Steril. 2011;96:774–9. doi: 10.1016/j.fertnstert.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 72.Eilertsen TB, Vanky E, Carlsen SM. Anti-Mullerian hormone in the diagnosis of polycystic ovary syndrome: Can morphologic description be replaced? Hum Reprod. 2012;27:2494–502. doi: 10.1093/humrep/des213. [DOI] [PubMed] [Google Scholar]
- 73.Chao KC, Ho CH, Shyong WY, Huang CY, Tsai SC, Cheng HY, et al. Anti-Mullerian hormone serum level as a predictive marker of ovarian function in Taiwanese women. J Chin Med Assoc. 2012;75:70–4. doi: 10.1016/j.jcma.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 74.Woo HY, Kim KH, Rhee EJ, Park H, Lee MK. Differences of the association of anti-Müllerian hormone with clinical or biochemical characteristics between women with and without polycystic ovary syndrome. Endocr J. 2012;59:781–90. doi: 10.1507/endocrj.ej12-0055. [DOI] [PubMed] [Google Scholar]
- 75.Homburg R, Ray A, Bhide P, Gudi A, Shah A, Timms P, et al. The relationship of serum anti-Mullerian hormone with polycystic ovarian morphology and polycystic ovary syndrome: A prospective cohort study. Hum Reprod. 2013;28:1077–83. doi: 10.1093/humrep/det015. [DOI] [PubMed] [Google Scholar]
- 76.Casadei L, Madrigale A, Puca F, Manicuti C, Emidi E, Piccione E, et al. The role of serum anti-Müllerian hormone (AMH) in the hormonal diagnosis of polycystic ovary syndrome. Gynecol Endocrinol. 2013;29:545–50. doi: 10.3109/09513590.2013.777415. [DOI] [PubMed] [Google Scholar]
- 77.Sahmay S, Atakul N, Aydogan B, Aydin Y, Imamoglu M, Seyisoglu H. Elevated serum levels of anti-Müllerian hormone can be introduced as a new diagnostic marker for polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2013;92:1369–74. doi: 10.1111/aogs.12247. [DOI] [PubMed] [Google Scholar]
- 78.Iliodromiti S, Kelsey TW, Anderson RA, Nelson SM. Can anti-Mullerian hormone predict the diagnosis of polycystic ovary syndrome. A systematic review and meta-analysis of extracted data? J Clin Endocrinol Metab. 2013;98:3332–40. doi: 10.1210/jc.2013-1393. [DOI] [PubMed] [Google Scholar]
- 79.Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LC, Strauss JF., 3rd Association of anti-Mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril. 2007;87:101–6. doi: 10.1016/j.fertnstert.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 80.Dolfing J, van Haard P, Schweitzer D, Wolffenbuttel B. Metabolic and hormonal parameters in lean PCOS women. Endocrine Abstracts 29, Poster-981. Presented at 14th European Congress of Endocrinology. 2012. [Last accessed on 22 Apr 2015]. Available from: http://www.endocrine-abstracts.org/ea/0029/ea0029p981.htm .
- 81.Villarroel C, Merino PM, López P, Eyzaguirre FC, Van Velzen A, Iñiguez G, et al. Polycystic ovarian morphology in adolescents with regular menstrual cycles is associated with elevated anti-Mullerian hormone. Hum Reprod. 2011;26:2861–8. doi: 10.1093/humrep/der223. [DOI] [PubMed] [Google Scholar]
- 82.Balen AH, Laven JS, Tan SL, Dewailly D. Ultrasound assessment of the polycystic ovary: International consensus definitions. Hum Reprod Update. 2003;9:505–14. doi: 10.1093/humupd/dmg044. [DOI] [PubMed] [Google Scholar]
- 83.Carmina E, Orio F, Palomba S, Longo RA, Lombardi G, Lobo RA. Ovarian size and blood flow in women with polycystic ovary syndrome and their correlations with endocrine parameters. Fertil Steril. 2005;84:413–9. doi: 10.1016/j.fertnstert.2004.12.061. [DOI] [PubMed] [Google Scholar]