Abstract
Diabetes mellitus (DM) frequency is a growing problem worldwide, because of long life expectancy and life style modifications. In old age (≥60–65 years old), DM is becoming an alarming public health problem in developed and even in developing countries as for some authors one from two old persons are diabetic or prediabetic and for others 8 from 10 old persons have some dysglycemia. DM complications and co-morbidities are more frequent in old diabetics compared to their young counterparts. The most frequent are cardiovascular diseases due to old age and to precocious atherosclerosis specific to DM and the most bothersome are visual and cognitive impairments, especially Alzheimer disease and other kind of dementia. Alzheimer disease seems to share the same risk factors as DM, which means insulin resistance due to lack of physical activity and eating disorders. Visual and physical handicaps, depression, and memory troubles are a barrier to care for DM treatment. For this, old diabetics are now classified into two main categories as fit and independent old people able to take any available medication, exactly as their young or middle age counterparts, and fragile or frail persons for whom physical activity, healthy diet, and medical treatment should be individualized according to the presence or lack of cognitive impairment and other co-morbidities. In the last category, the fundamental rule is “go slowly and individualize” to avoid interaction with poly medicated elder persons and fatal iatrogenic hypoglycemias in those treated with sulfonylureas or insulin.
Keywords: Cognitive impairment, complications, diabetes mellitus, elderly, insulin resistance, micro-nutriments, treatment individualization
INTRODUCTION
Diabetes mellitus (DM) is a chronic metabolic disease characterized by hyperglycemia and high glycated hemoglobin[1,2,3] with or without glycosuria. Glucose metabolism disorder (GMD) results from a defect in insulin secretion by the pancreas, insulin action on the target tissues (or insulin resistance), or both.[4] Chronic hyperglycemia leads to damage and failure of various organs, especially the heart, blood vessels, eyes, kidneys, and nerves.[5] Those macro and micro angiopathies, which can be observed even in newly diagnosed patients[6] are due to GMD long-term duration.
DM prevalence, in general, is growing worldwide,[7,8] and is becoming an epidemic and endemic problem with the social and economic burden.[9,10] However, its prevalence and its co-morbidities and mortality are higher in elderly than in young people.[11] According to Caspersen et al. diagnosed and/or undiagnosed DM affects 10.9 million US adults aged 65 years and older, and this number is projected to reach 26.7 million by 2050, which means 55% of all diabetes cases.[12] For the same authors, almost 8 from 10 old people have some form of dysglycemia according to different tests.[12] This allows epidemiologists to classify DM with its complications as the most alarming health problem of the current century in middle age people and elderly.
The elderly is a heterogeneous group with different physiological profiles and varying functional capabilities and life expectancy.[13]
DM definition in old people is similar to the one of other people, which means fasting glycaemia ≥1.26 g/l (7.0 mmol/L) or glycemia after glucose loading (75 g) ≥2 g/l (11.11 mmol/L). People with postprandial or postloading glycemia between 1.40 and 1.99 g/l (7.78–11.06 mmol/L) suffer from a reduction in glucose tolerance.
The definition of elderly is a subject of controversies. In general, a person is considered as old if her civil age is ≥ 60 or 65 years old.[14] However, on the scientific point a person is supposed to be old if her age is superior or equal to 75.[15] Nevertheless, everyone agrees that it is more important to consider the physiological or vascular age. That one varies according to genetic background, environmental factors and presence or lack of morbidities such as DM, high blood pressure, arthritis or other rheumatologic diseases, obesity, cognitive dysfunction, renal insufficiency, and heart failure. For this, the international diabetes federation (IDF) divides old patients into three functional groups.[16]
The first category includes patients who are functionally independent and rely on their own. In this group, DM may be the only medical problem or be associated with some diseases which are not life-threatening
-
The second category is composed of patients who are not autonomous which means they are functionally dependent on someone else. This group is subdivided into two subcategories: Frail patients and patients with cognitive impairment.
- Frail or fragile patients are characterized by a combination of fatigue, weight loss, and severe restriction in their mobility and/or strength, which increases the risk of falls and institutionalization
- The second subcategory includes patients with dementia, which means they have cognitive impairment and are unable to self-care. This category is at increased risk for both hypoglycemia and hyperglycemia poor control
The third group includes patients at the end of life care. These persons have a significant medical illness or malignancy. Consequently, they have a short life expectancy.
The aim of our study was to review relevant and recent full articles about DM in old people in order to focus on epidemiology, physiopathology of type 2 DM in old people, clinical manifestations, complications, and therapeutic aspects. Our research was based on English literature published in PubMed and Google Scholar.
EPIDEMIOLOGY OF DIABETES MELLITUS IN ELDERLY
The aging population is growing worldwide and the proportion of people above 60 years old accounts for 15% of the whole population which is estimated to 7.5 billion.[17]
In general, 20% of old people have DM, and a similar proportion have undiagnosed DM.[18] Reported frequencies vary from 18% to 33%.[19,20] This range may reflect differences in the age, life style, and genetic background of the analyzed populations. On another hand, 30% of old people have impaired glucose regulation which means an increased risk for DM.[21]
Actually, DM in elderly includes two groups: “survivors” of young or middle age onset of diabetes, and incident diabetes in older age or type 2 DM.
Type 1 DM is exceptional in elderly as auto immune diseases affect young populations. So old people with type 1 DM are practically at the end stage of their disease and are multi complicated.
Most people over than 60 years old suffer from type 2 DM due to insulin resistance. However, insulin secretion may be severely reduced at the end stage of type 2 DM.
Consequently, complications, and management of DM in elderly vary according to hyperglycemia duration, personal background, and co-morbidities. Some old people do not have any complication and are easy to manage; others are multi complicated and have additional severe diseases difficult to treat even in highly specialized centers. The last group is encountered among survivors of young onset DM. The main troublesome co-morbidities in elderly are heart and kidney insufficiencies leading to limitation in medicine prescription.
TYPE 2 DIABETES MELLITUS MECHANISM IN ELDERLY
Elderly type 2 DM is apparently due to several mechanisms among which one can cite genetic background, long life expectancy leading to a decrease in insulin secretion, and the modification of some environmental factors responsible for central obesity. The last one is responsible for insulin resistance,[22] which is the main cause of metabolic syndrome and type 2 DM in adults and old people. The lack of physical activity added to eating disorders characterizing modern life style is the most incriminated factors. However, some recent studies have also demonstrated the role of other factors such as arginine vasopressin (AVP) or its c-terminal fragment, called Copeptin, in the mechanism of DM in older people via lower insulin sensitivity.[23] AVP affects liver glycogenolysis and glucagon secretion too.[24]
In old people, Vitamin D deficiency seems to be an additional factor, as some authors think Vitamin D deficiency is a link between osteoporosis, insulin resistance,[25] obesity, DM,[26,27] and cognitive impairment, especially Alzheimer disease.[28,29] However, for others deficit in Vitamin D may be a consequence of obesity and chronic diseases such as GMDs.[27] On another hand, according to a review done by Cândido and Bressan,[27] there are experimental evidences that Vitamin D inhibits fat accumulation, preserves pancreatic islet cells, increases insulin synthesis, decreases insulin resistance, and reduces hunger. If those actions are real, Vitamin D substitution should prevent and control GMDs. However, nowadays, there are not enough scientific evidences to support Vitamin D use in prevention and/or treatment of DM, obesity, and Alzheimer disease. Deficiency in other micro nutriments such as magnesium[30] and potassium have been incriminated in type 2 DM development and GMD poor control. Actually, magnesium, a cofactor of various enzymes in carbohydrate oxidation, has an important role in glucose transporting mechanism. It has also been involved in insulin secretion and activity, so its deficit may induce insulin resistance. In elderly hypomagnesemia may result from various causes, including deficient intake, gastrointestinal troubles, and renal loss.[31] In general, hypomagnesemia is associated with poor glycemic control and increased risk for complications such as retinopathy, nephropathy, and foot ulcers.[32] However, to date there is no scientific proof demonstrating that one can prevent type 2 DM development and avoid complications by systematic intake of various micro nutriments.
SYMPTOMS AND POSITIVE DIAGNOSIS OF DIABETES MELLITUS IN ELDERLY
Since the renal threshold for glucose increases with age and thirst mechanisms are impaired in elderly, DM typical semiology (i.e., polyuria and polydipsia) is usually lacking in old people.[33] Consequently, common symptoms leading to DM diagnosis are complications such as neuropathy or nephropathy, heart and vascular problems and/or recurrent urinary infections or skin problems. Fatigue, hypotension, incontinence, cognitive impairment or functional decline, depression, and dementia that might be the first manifestations of the disease are usually wrongly attributed to aging.
Signs of advanced dehydration such as dry mouth, dry eyes, and dry skin should attract the attention, but usually, old people with DM are diagnosed at the late stage of dehydration with confusion, agitation, delirium, or hyperosmolar coma.
On another hand, high blood pressure and dyslipidemia, cerebrovascular, and chronic pulmonary diseases generally coexist with DM in elderly, which increases the risk of poly medication.
Old people usually have severe osteoporosis due to gonadal and/or vitamin deficiencies. Under nutrition due to isolation, depression, dental and/or socioeconomic problems contribute to bone demineralization too. Vitamin D deficiency is one of the most frequent deficiencies in the elderly. It promotes diabetes through insulin resistance and insulin deficiency. It also predisposes to other metabolic, cardiovascular, and cancerous diseases. Furthermore, Vitamin D deficiency is a strong factor for proximal muscle weakness, falls, and fractures. Old people are particularly at risk for low Vitamin D levels. Solar exposure is usually limited because of less outdoor activity and diet is less varied with a lower natural Vitamin D content. The cutaneous production of Vitamin D decreases with age because of skin changes, with a reduced amount of Vitamin D precursors.[34]
Elderly may also experience subclinical hypo or hyperthyroidism or another endocrine disease responsible for osteoporosis.[35] Dizziness and orthostatic hypotension are responsible for old people falls. Sarcopenia or a strong decline in skeletal muscle tissue due to hormonal imbalance and body composition in elderly is one of the most important causes of loss of independence in old and frail people.[36,37,38] Muscles weakness obliges old people to use a cane or a wheel chair and exaggerates osteoporosis, which put old people at a high-risk of frequent falls and high incidence of hip, spine, and distal forearm fractures.[39,40]
Regarding positive diagnosis, there is not any specific one for old people. However, if we want to achieve an early diagnosis, we should take into account postprandial glycemia or glycemia after glucose loading especially in obese people or those with DM background.[41]
In elderly A1C is not accurate for diagnosis and DM control, because of frequent situations that affect red blood cell life span. Those are anemia (independently of its causes), recent transfusions or erythropoietin infusions, acute illenesses and/or hospitalizations, and chronic liver diseases.
COMPLICATIONS AND COMORDIDITIES OF DIABETES MELLITUS IN OLD POPULATION
Old people with DM are at similar risk for macro and microvascular complications as their younger counterparts, but they have a much higher absolute risk for cardiovascular diseases and a higher rate of morbidity and mortality than old people without DM. They are also at high-risk for physical and functional disabilities, co-morbidities, and rheumatic pain. Geriatric syndromes such as cognitive impairment, depression, and especially Alzheimer disease are more frequent in elderly. Actually old age, lack of physical and mental activity, DM, high blood pressure, sleep apnea, smoking, deficit in some nutriments are the most important modifiable risk factors for memory deficits, dementia, and Alzheimer.[42] Insulin resistance and deficit in some vitamins seem to be the link between DM and brain deficiency in older adults. Vitamin D substitution and DM good control seem to improve mental activity; however, it has never been proved that Vitamin D and/other micro nutriments substitution cure Alzheimer or another cerebral decline.
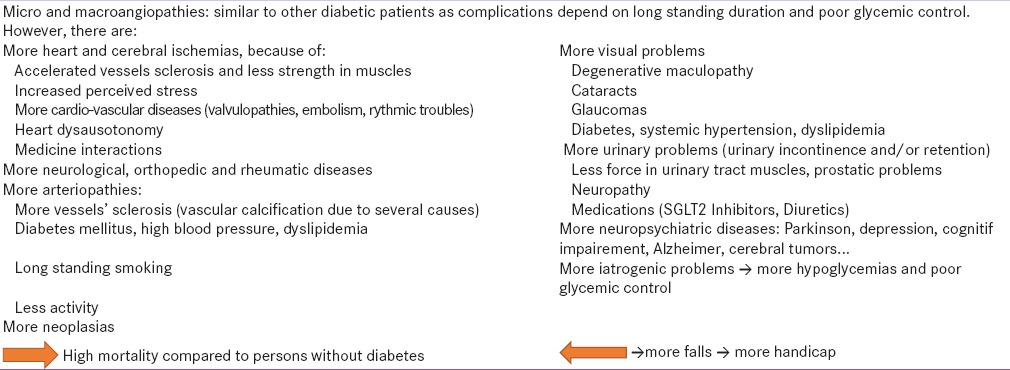
Cardiovascular diseases are the most common complications because of accelerated atherosclerosis.[43] Urinary and fecal incontinence are higher in old diabetics compared to nondiabetic population.[44,45] Reduced vision is also much higher in old people with DM[46] because of degenerative maculopathy, hypertensive retinopathy, cataracts, and glaucoma. Table 1 summarizes the main complications and co-morbidities in old age diabetics and their interactions on falls and premature death.
Table 1.
Summary of complications and comorbidities in elderly with DM
TREATMENT OF OLD DIABETIC PEOPLE
Prevention of DM is the best way to reduce the burden of disability in elderly people.[22] Physical activity and reduction in fat and sugary food are the only way to prevent obesity and insulin resistance in adulthood and in elderly too. In obese persons and people with DM background, postprandial blood glucose checking may help to diagnose DM at an early stage before complications
Curative treatment:
DM treatment in old people should obey to the fundamental rule “Go slowly and individualize.”[13,47]
Glycemic targets
Blood glucose targets vary according to patient's health status and life expectancy. According to IDF recommendations,[16] old people who are physically fit or independent and cognitively intact should have a hemoglobin A1c (HbA1c) below 7% which means a rate similar to the one of younger diabetics, because of their long life expectancy. For other categories, glycemic control should be less stringent with individual criteria to avoid hypoglycemia that is dangerous for the brain and heart. Consequently, an A1c target between 7% and 7.5% is recommended for old people without major co-morbidities, but for fragile patients, A1c should be between 7.5% and 8.5%.
Lifestyle modification based on physical activity to strengthen muscle force, weight reduction in obese patients, and diet limiting sugary and fatty food is as important as in young population to improve glycemic control. Even sugar-sweetened beverages should be avoided in elderly as they are correlated with predisposition for developing type 2 DM in people with reduced glucose tolerance.[48] Sugar sweetened products seem to influence body weight, insulin resistance, and the ability of the pancreatic beta cells to compensate for insulin resistance.[48]
Modification in life style is necessary at any stage of the disease. However, for physical activity rheumatic diseases and fear of fall may limit its practice. For weight reduction, it is important to consider that only obese patients may benefit from caloric restriction and an important increase in physical activity. The weight loss goal should be inferior or equal to 5% of body mass in old population because an important weight loss increases the risk of morbidity and mortality due to under nutrition.[49]
Therefore, a less stringent diet is a good alternative for DM control in old people, but financial problems may lead to a limitation in diet rules, as poor people cannot afford appropriate things. Social isolation is another problem as it may lead to the lack of interest in preparing and varying meals. Due to memory troubles, old people from the second category forget eating and drinking. Their dental problems and change in their taste may also act negatively on their diet.[50]
Medications
All types of oral hypoglycemic drugs and insulin are safe in older patients if the treatment is well conducted, although each medication has some limitations due to hypoglycemic risk or to co-morbidities.
Metformin
Metformin reduces glucose levels by increasing insulin sensitivity, reducing hepatic glucose release and increasing muscle uptake.[51] Due to its low-risk of hypoglycemia, metformin is an attractive agent to use in older adults. However, it should be avoided in the patients with risk of lactic acidosis such as people with stroke, pneumonia, myocardial infarction, heart failure, and renal insufficiency.[52,53] The safe level for renal failure is a glomerular filtration rate (GFR) = 30 ml/min. Other limiting factors are weight loss and gastrointestinal problems. Old people are advised to take a lot of water to avoid dehydration and renal impairment especially in hot summer or when fasting for a long time.
Metformin should be stopped before any surgery or if an old person has to undergo an exploration requiring iodinated contrast, because of the risk for renal insufficiency whose risk is greater in elderly.
Metformin induces Vitamin B12 deficiency in 18.7%[53] to 30%[54] of patients with DM. The deficit in cyanocobalamin is apparently correlated to old age, metformin dose, and treatment duration.[53] Vitamin B12 status should be systematically checked in people responding to above mentioned situations. The deficit in Vitamin B12 induces peripheral neuropathy with or without anemia[54] and leads or worsens cognitive dysfunction in old population.[55]
Insulin secretagogues: Sulfonylureas and meglitinides
Due to their efficacy, long experience use, and low cost, sulfonylureas are widely used in the general population. They are usually well-tolerated drugs even in elderly. However, hypoglycemia is the most common and the most dangerous side effect.
Hypoglycemia is more common with long-acting sulfonylurea drugs such as chlorpropamide, glyburide, and glimepiride. Those medications should be avoided in elderly especially if they have diarrhea, they are addicted to alcohol and have memory troubles; situations that increase the risk for hypoglycemia and weight gain.
Short action-acting sulfonylureas such as meglitinides are preferable in elderly because of their low rate of hypoglycemia, but they have the same risk for weight gain.
Thiazolidinediones
The thiazolidinediones such as rosiglitazone and especially pioglitazone improve insulin resistance and may improve insulin secretion in response to glucose in people with reduced glucose tolerance.
Actually, pioglitazone is now the only marketed product that can be used as a good alternative for older people due to its low-risk for hypoglycemia. It can be used alone or in association with metformin. Its main side effect is fluid retention. Therefore, it is not suitable for patients with congestive heart failure.
Glitazones require 2–4 weeks to exert their full anti-hyperglycemic effect. Therefore, they can be used in a patient with lower initial HbA1c and those who are allergic to sulfonylureas, or those not willing to use insulin.
Although they are well-tolerated in old people, and can be given in renal insufficiency, their high cost and problems regarding fluid retention, increased incidence of fractures (because of bone loss), and bladder cancer limit their usefulness.
Alpha-glucosidase inhibitors
Alpha-glucosidase inhibitors (AGI) (acarbose and miglitol) are products that inhibit the gastrointestinal alpha-glucosidases which are enzymes converting carbohydrates into monosaccharides. Consequently, they reduce rise in postprandial blood glucose after meals.
AGI can be used alone or in combination with metformin, sulfonylurea or insulin. However, they are not widely tested in older diabetic patients, although they are likely to be safe and efficient. The main side effects are gastrointestinal troubles such as flatulence and diarrhea, which are a limitation of their use.
Incretin-based therapies
Dipeptidylpeptidases-4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists seem to be very interesting drugs for elderly as they are associated to low hypoglycemic risk when used alone or as add-on therapy to metformin.[18]
DPP-4 inhibitors are deemed to be relatively weak agents lowering blood glucose through several mechanisms when used alone or at add-on therapy with metformin, sulfonylurea, and thiazolidinediones. They have no risk of hypoglycemia and are weight-neutral. Therefore, they may be attractive agents to use in older adults, but a long-term safety is not proved yet, and their price is relatively high. Furthermore, their dose should be adjusted in kidney insufficiency, although, Halimi et al.'s study showed efficacy and safety of DPP-4 inhibitors in the elderly even in the presence of renal impairment.[56] On another hand Shankar et al. have recently demonstrated that in elderly and compared to sulfonylureas Sitagliptin has the same efficacy, but a lower risk for hypoglycemia and was accompanied with weight loss.[57]
GLP-1 agonists that include exenatide, liraglutide and recently lixisenatide may also be advantageous, as they do not increase hypoglycemic risk unless they are associated with sulfonylureas. They also cause weight loss and are interesting in over-weight or obese old patients. A pooled analysis of 06 randomized trials showed that liraglutide is effective and well-tolerated in old persons. However, liraglutide dosage needs to be adjusted according to kidney function.[58] These agents require a period of 2–4 weeks for dose titration to reach their maximal effect. GLP-1 agonists may also have neuro-protective properties and may be useful in old patients with neurodegenerative diseases.[59]
Sodium-glucose co-transporter type 2 inhibitors
The Inhibitors of sodium-glucose co-transporter type 2 (SGLT2) such as canagliflozin and dapagliflozin represent a novel class of glucose-lowering agents that lower plasma glucose levels through pharmacological inhibition of glucose reuptake from the kidney.[60] Compared to placebo, SGLT2 lower HbA1c by 0.5–0.8% when used as monotherapy or add-on therapy.[61]
SGLT2 inhibitors prescription is limited due to the high frequency of urinary and genital mycotic infections and because of other adverse effects including hypotension, dizziness, and renal function worsening. Serious adverse effects including severe hypoglycemia due to depletion of hepatic glycogen storage, acceleration of diabetes-associated sarcopenia, and ketoacidosis have been reported in some rare cases.[60] Therefore, SGLT2 inhibitors should be used with caution in old people of the first category. However, they should be totally avoided in other categories, especially in people with chronic kidney diseases, sarcopenia, and risk of dehydration.[61]
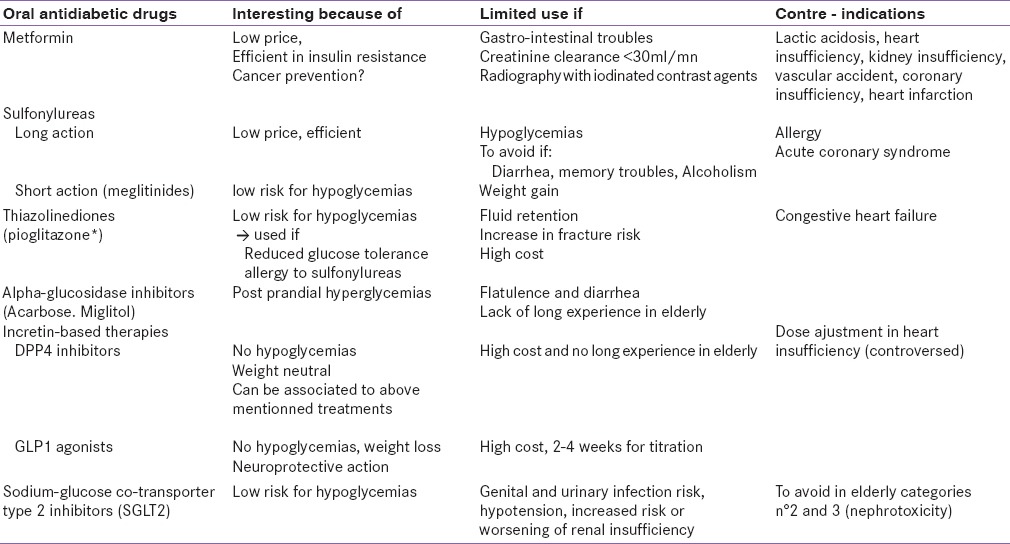
Table 2 summarizes the oral medications, which can be used in old diabetic people.
Table 2.
Oral medications that can be used in old people with DM
Pramlintide
Pramlintide is an amylin synthetic product resembling human amylin used in type 1 and types 2 DM. It is administered subcutaneously at meal times with insulin.
Pramlintide acts on glucose regulation by stimulating glucose absorption by peripheral tissues and slowing gastric emptying. It also promotes satiety via hypothalamic receptors and inhibits inappropriate secretion of glucagon. Pramlintide seems to stimulate the acute first-phase insulin response threshold following food intake.
According to Herrmann et al.,[62] pramlintide therapy improves A1c, decreases body weight, and is associated to low rates of severe hypoglycemia among the patients with type 2 DM, regardless of baseline insulin use. However, the multiple injections limit its use in the management of DM in elderly.
Insulin
All over the world, insulin is under-utilized in older adults because of fear of hypoglycemia by the patient[63] and his family,[64,65] but also by the clinician.[66] They all think multi injections are too complicated and dangerous for an old person.
Nowadays, availability of long-acting insulin with new pens and glucometers lead to easier use of insulin analogs in older patients. Newer insulin and other new technologies will certainly improve patients’ acceptance and quality of life in diabetic patients.[67] Noninvasive insulin delivery systems will probably overcome the most pressing problem regarding therapy compliance. To overcome the problem, noninvasive routes such as oral, buccal, pulmonary, nasal, and or transdermal insulins have been proposed.[68]
Actually, the quality of life has already improved considerably in patients taking one or two daily doses of intermediate insulin. However, before beginning insulin therapy, it is important to assess whether or not the patient is physically and especially cognitively able to use insulin. If a patient is capable of drawing up his insulin, knows to use an insulin pen, is able to decide for an appropriate insulin dose, knows how to monitor properly his blood glucose, and recognizes and treats his hypoglycemia, insulin is a very good alternative. However, for older patients taking a fixed daily dose of insulin, capable of giving the insulin shot, but not drawing it up because of visual problems or another cause, a family member or a pharmacist may prepare a week's supply of insulin in syringes and leave them in the refrigerator.[50] Such a plan may allow an older patient to remain living independently at home,[50] especially in-developed countries where people are used to live on their own. This problem is not a major one in developing countries as most old people live with their family. For example in North Africa, a survey showed only 2.6% of old people live on their own.[69]
As, insulin metabolism is altered in patients with chronic renal failure; less insulin is needed when the GFR is below 50 ml/min.
In older patients who require more than one agent, electronic programmable pill-dosing dispensers[70] may help to improve adherence, but family members may be required too.
Some other medications can be added to specific treatment of DM, especially systematic Vitamin D supplementation and cures of magnesium and Vitamins E and B. Those products might strengthen physical and mental activity in elderly with or without DM.
However, to all useful treatments in elderly, there are some barriers to care. Actually old people may skip medication doses because of memory troubles or lack of interest in their life when depressed. On another hand, their dexterity is limited and their eyesight is generally poor which affect their ability to monitor blood glucose levels and insulin doses.[50]
The ideal care of old diabetic is a family help and a multidisciplinary approach in order to reduce cardiovascular risk factors and increase life expectancy with a high quality of life.
CONCLUSION
Increasing life expectancy in conjunction with increasing rate of obesity and sedentary lifestyle will lead to a higher prevalence of diabetes among old persons. DM is frequently unnoticed in old patients as it is either asymptomatic or symptoms are nonspecific. Consequently, systematic screening of postprandial GMD is the best way to get an early diagnosis and prevent diabetes-related complications. Furthermore, aging is characterized by high prevalence of associated co-morbidities and risk of frailty. Therefore, it is important to provide high quality and specific care for old diabetic patients. Any treatment should be based on elderly classification and individualization to avoid iatrogenic complications, especially dehydration and hypoglycemias.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Alqahtani N, Khan WA, Alhumaidi MH, Ahmed YA. Use of glycated hemoglobin in the diagnosis of diabetes mellitus and pre-diabetes and role of fasting plasma glucose, oral glucose tolerance test. Int J Prev Med. 2013;4:1025–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Hu Y, Liu W, Chen Y, Zhang M, Wang L, Zhou H, et al. Combined use of fasting plasma glucose and glycated hemoglobin A1c in the screening of diabetes and impaired glucose tolerance. Acta Diabetol. 2010;47:231–6. doi: 10.1007/s00592-009-0143-2. [DOI] [PubMed] [Google Scholar]
- 3.d'Emden MC, Shaw JE, Jones GR, Cheung NW. Guidance concerning the use of glycated haemoglobin (HbA1c) for the diagnosis of diabetes mellitus. Med J Aust. 2015;203:89–90. doi: 10.5694/mja15.00041. [DOI] [PubMed] [Google Scholar]
- 4.ADA. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31(Suppl 1):S62–7. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26:77–82. [Google Scholar]
- 6.Heydari I, Radi V, Razmjou S, Amiri A. Chronic complications of diabetes mellitus in newly diagnosed patients. Int J Diabetes Mellitus. 2010;2:61–3. [Google Scholar]
- 7.Borissova AM, Shinkov A, Kovatcheva R, Vlahov J, Dakovska L, Todorov T. Changes in the prevalence of diabetes mellitus in Bulgaria (2006-2012) Clin Med Insights Endocrinol Diabetes. 2015;8:41–5. doi: 10.4137/CMED.S24742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen TH, Nguyen TN, Fischer T, Ha W, Tran TV. Type 2 diabetes among Asian Americans: Prevalence and prevention. World J Diabetes. 2015;6:543–7. doi: 10.4239/wjd.v6.i4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam DW, LeRoith D. The worldwide diabetes epidemic. Curr Opin Endocrinol Diabetes Obes. 2012;19:93–6. doi: 10.1097/MED.0b013e328350583a. [DOI] [PubMed] [Google Scholar]
- 10.Kalra S, Kumar A, Jarhyan P, Unnikrishnan AG. Endemic or epidemic. Measuring the endemicity index of diabetes? Indian J Endocrinol Metab. 2015;19:5–7. doi: 10.4103/2230-8210.144633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sloan FA, Bethel MA, Ruiz D, Jr, Shea AM, Feinglos MN. The growing burden of diabetes mellitus in the US elderly population. Arch Intern Med. 2008;168:192–9. doi: 10.1001/archinternmed.2007.35. [DOI] [PubMed] [Google Scholar]
- 12.Caspersen CJ, Thomas GD, Boseman LA, Beckles GL, Albright AL. Aging, diabetes, and the public health system in the United States. Am J Public Health. 2012;102:1482–97. doi: 10.2105/AJPH.2011.300616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornick T, Aron DC. Managing diabetes in the elderly: Go easy, individualize. Cleve Clin J Med. 2008;75:70–8. doi: 10.3949/ccjm.75.1.70. [DOI] [PubMed] [Google Scholar]
- 14.WHO definition of an older or elderly person. [Last accessed on 2015 Sep 04]. Available from: http://www.who.int/healthinfo/survey/ageingdefnolder/en/
- 15.Orimo H, Ito H, Suzuki T, Araki A, Hosoi T, Sawabe M. Reviewing the definition of “elderly”. Geriatr Gerontol Int. 2006;6:149–58. [Google Scholar]
- 16.Sinclair A, Dunning T, Colagiuris S. Managing older people with type 2 diabetes: Global guideline IDF 2013. [Last accessed on 2015 Sep 29]. Available from: http://www.idf.org/guidelines/managing-older-people-type-2-diabetes .
- 17. [Last access on 2015 Sep 05]. Available from: http://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2013.pdf .
- 18.Sinclair A, Morley JE, Rodriguez-Mañas L, Paolisso G, Bayer T, Zeyfang A, et al. Diabetes mellitus in older people: Position statement on behalf of the International Association of Gerontology and Geriatrics (IAGG), the European Diabetes Working Party for Older People (EDWPOP), and the International Task Force of Experts in Diabetes. J Am Med Dir Assoc. 2012;13:497–502. doi: 10.1016/j.jamda.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 19.International Diabetes Federation. IDF Diabetes Atlas. 5th ed. Brussels, Belgium: International Diabetes Federation; 2011. [PubMed] [Google Scholar]
- 20.Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650–64. doi: 10.2337/dc12-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crandel J. Pharmacotherapy in older adults. ADA. 2014. [Last access on 2015 Sep 04]. Available from: http://www.professional.diabetes.org/admin/UserFiles/CE/PG/Crandall%20.%20ADA%20post%20grad%202014.pdf .
- 22.Tyrovolas S, Koyanagi A, Garin N, Olaya B, Ayuso-Mateos JL, Miret M, et al. Diabetes mellitus and its association with central obesity and disability among older adults: A global perspective. Exp Gerontol. 2015;64:70–7. doi: 10.1016/j.exger.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Wannamethee SG, Welsh P, Papacosta O, Lennon L, Whincup PH, Sattar N. Copeptin, insulin resistance and risk of incident diabetes in older men. J Clin Endocrinol Metab. 2015;100:3332–9. doi: 10.1210/JC.2015-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enhörning S, Struck J, Wirfält E, Hedblad B, Morgenthaler NG, Melander O. Plasma copeptin, a unifying factor behind the metabolic syndrome. J Clin Endocrinol Metab. 2011;96:E1065–72. doi: 10.1210/jc.2010-2981. [DOI] [PubMed] [Google Scholar]
- 25.Sung CC, Liao MT, Lu KC, Wu CC. Role of vitamin D in insulin resistance. J Biomed Biotechnol. 2012;2012:634195. doi: 10.1155/2012/634195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li YX, Zhou L. Vitamin D deficiency, obesity and diabetes. Cell Mol Biol (Noisy-le-grand) 2015;61:35–8. [PubMed] [Google Scholar]
- 27.Cândido FG, Bressan J. Vitamin D: Link between osteoporosis, obesity, and diabetes? Int J Mol Sci. 2014;15:6569–91. doi: 10.3390/ijms15046569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keeney JT, Butterfield DA. Vitamin D deficiency and Alzheimer disease: Common links. Neurobiol Dis. 2015:pii: S0969. doi: 10.1016/j.nbd.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 29.Keeney JT, Förster S, Sultana R, Brewer LD, Latimer CS, Cai J, et al. Dietary vitamin D deficiency in rats from middle to old age leads to elevated tyrosine nitration and proteomics changes in levels of key proteins in brain: Implications for low vitamin D-dependent age-related cognitive decline. Free Radic Biol Med. 2013;65:324–34. doi: 10.1016/j.freeradbiomed.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodríguez-Morán M, Simental Mendía LE, Zambrano Galván G, Guerrero-Romero F. The role of magnesium in type 2 diabetes: A brief based-clinical review. Magnes Res. 2011;24:156–62. doi: 10.1684/mrh.2011.0299. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhary DP, Sharma R, Bansal DD. Implications of magnesium deficiency in type 2 diabetes: A review. Biol Trace Elem Res. 2010;134:119–29. doi: 10.1007/s12011-009-8465-z. [DOI] [PubMed] [Google Scholar]
- 32.Dasgupta A, Sarma D, Saikia UK. Hypomagnesemia in type 2 diabetes mellitus. Indian J Endocrinol Metab. 2012;16:1000–3. doi: 10.4103/2230-8210.103020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chau D, Edelman SV. Clinical management of diabetes in the elderly. Clin Diabetes. 2001;19:172–5. [Google Scholar]
- 34.Hossein-Nezhad A, Holick MF. Vitamin D for health: A global perspective. Mayo Clin Proc. 2013;88:720–55. doi: 10.1016/j.mayocp.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owens D, Kalra S, Sahay R. Geriatric endocrinology. Indian J Endocrinol Metab. 2011;15:71–2. doi: 10.4103/2230-8210.81933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walston JD. Sarcopenia in older adults. Curr Opin Rheumatol. 2012;24:623–7. doi: 10.1097/BOR.0b013e328358d59b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keevil VL, Romero-Ortuno R. Ageing well: A review of sarcopenia and frailty. Proc Nutr Soc. 2015;25:1–11. doi: 10.1017/S0029665115002037. [DOI] [PubMed] [Google Scholar]
- 39.Oliveira A, Vaz C. The role of sarcopenia in the risk of osteoporotic hip fracture. Clin Rheumatol. 2015;34:1673–80. doi: 10.1007/s10067-015-2943-9. [DOI] [PubMed] [Google Scholar]
- 40.Curtis E, Litwic A, Cooper C, Dennison E. Determinants of muscle and bone aging. J Cell Physiol. 2015;230:2618–25. doi: 10.1002/jcp.25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jain A, Paranjape S. Prevalence of type 2 diabetes mellitus in elderly in a primary care facility: An ideal facility. Indian J Endocrinol Metab. 2013;17(Suppl 1):S318–22. doi: 10.4103/2230-8210.119647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen ST, Siddarth P, Ercoli LM, Merrill DA, Torres-Gil F, Small GW. Modifiable risk factors for Alzheimer disease and subjective memory impairment across age groups. PLoS One. 2014;9:e98630. doi: 10.1371/journal.pone.0098630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altabas V. Diabetes, endothelial dysfunction, and vascular repair: What should a diabetologist keep his eye on? Int J Endocrinol. 2015;2015:848272. doi: 10.1155/2015/848272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bani-Issa W, Almomani F, Eldeirawi K. Urinary incontinence among adult women with diabetes in Jordan: Epidemiology, correlates and perceived impact on emotional and social well-being. J Clin Nurs. 2014;23:2451–60. doi: 10.1111/jocn.12392. [DOI] [PubMed] [Google Scholar]
- 45.Coggrave M, Norton C, Cody JD. Management of faecal incontinence and constipation in adults with central neurological diseases. Cochrane Database Syst Rev. 2014;1:CD002115. doi: 10.1002/14651858.CD002115.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taheri Tanjani P, Moradinazar M, Esmail Mottlagh M, Najafi F. The prevalence of diabetes mellitus (DM) type II among Iranian elderly population and its association with other age-related diseases, 2012. Arch Gerontol Geriatr. 2015;60:373–9. doi: 10.1016/j.archger.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 47.Baruah MP, Kalra S, Unnikrishnan AG, Raza SA, Somasundaram N, John M, et al. Management of hyperglycemia in geriatric patients with diabetes mellitus: South Asian consensus guidelines. Indian J Endocrinol Metab. 2011;15:75–90. doi: 10.4103/2230-8210.81935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teshima N, Shimo M, Miyazawa K, Konegawa S, Matsumoto A, Onishi Y, et al. Effects of sugar-sweetened beverage intake on the development of type 2 diabetes mellitus in subjects with impaired glucose tolerance: The mihama diabetes prevention study. J Nutr Sci Vitaminol (Tokyo) 2015;61:14–9. doi: 10.3177/jnsv.61.14. [DOI] [PubMed] [Google Scholar]
- 49.Wedick NM, Barrett-Connor E, Knoke JD, Wingard DL. The relationship between weight loss and all-cause mortality in older men and women with and without diabetes mellitus: The Rancho Bernardo study. J Am Geriatr Soc. 2002;50:1810–5. doi: 10.1046/j.1532-5415.2002.50509.x. [DOI] [PubMed] [Google Scholar]
- 50.Weisenberger J. Elder diabetes patients: Know the signs and symptoms of type 2 diabetes in this population to improve care. [Last accessed on 2015 Sep 30];Today's Dietitian. 2013 15:20. Available from: http://www.todaysdietitian.com/newarchives/030413p20.shtml . [Google Scholar]
- 51.Almaleki N, Ashraf M, Hussein MM, Mohiuddin SA. Metformin-associated lactic acidosis in a peritoneal dialysis patient. Saudi J Kidney Dis Transpl. 2015;26:325–8. doi: 10.4103/1319-2442.152498. [DOI] [PubMed] [Google Scholar]
- 52.Kim MJ, Han JY, Shin JY, Kim SI, Lee JM, Hong S, et al. Metformin-associated lactic acidosis: Predisposing factors and outcome. Endocrinol Metab (Seoul) 2015;30:78–83. doi: 10.3803/EnM.2015.30.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haeusler S, Parry-Strong A, Krebs JD. The prevalence of low vitamin B12 status in people with type 2 diabetes receiving metformin therapy in New Zealand – a clinical audit. N Z Med J. 2014;127:8–16. [PubMed] [Google Scholar]
- 54.Bell DS. Metformin-induced vitamin B12 deficiency presenting as a peripheral neuropathy. South Med J. 2010;103:265–7. doi: 10.1097/SMJ.0b013e3181ce0e4d. [DOI] [PubMed] [Google Scholar]
- 55.Ting RZ, Szeto CC, Chan MH, Ma KK, Chow KM. Risk factors of vitamin B (12) deficiency in patients receiving metformin. Arch Intern Med. 2006;166:1975–9. doi: 10.1001/archinte.166.18.1975. [DOI] [PubMed] [Google Scholar]
- 56.Halimi S, Raccah D, Schweizer A, Dejager S. Role of vildagliptin in managing type 2 diabetes mellitus in the elderly. Curr Med Res Opin. 2010;26:1647–56. doi: 10.1185/03007995.2010.485881. [DOI] [PubMed] [Google Scholar]
- 57.Shankar RR, Xu L, Golm GT, O’Neill EA, Goldstein BJ, Kaufman KD, et al. A comparison of glycaemic effects of sitagliptin and sulfonylureas in elderly patients with type 2 diabetes mellitus. Int J Clin Pract. 2015;69:626–31. doi: 10.1111/ijcp.12607. [DOI] [PubMed] [Google Scholar]
- 58.Bode BW, Brett J, Falahati A, Pratley RE. Comparison of the efficacy and tolerability profile of liraglutide, a once-daily human GLP-1 analog, in patients with type 2 diabetes≥65 and<65 years of age: A pooled analysis from phase III studies. Am J Geriatr Pharmacother. 2011;9:423–33. doi: 10.1016/j.amjopharm.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Duarte AI, Candeias E, Correia SC, Santos RX, Carvalho C, Cardoso S, et al. Crosstalk between diabetes and brain: Glucagon-like peptide-1 mimetics as a promising therapy against neurodegeneration. Biochim Biophys Acta. 2013;1832:527–41. doi: 10.1016/j.bbadis.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 60.Yabe D, Nishikino R, Kaneko M, Iwasaki M, Seino Y. Short-term impacts of sodium/glucose co-transporter 2 inhibitors in Japanese clinical practice: Considerations for their appropriate use to avoid serious adverse events. Expert Opin Drug Saf. 2015;14:795–800. doi: 10.1517/14740338.2015.1034105. [DOI] [PubMed] [Google Scholar]
- 61.Mikhail N. Place of sodium-glucose co-transporter type 2 inhibitors for treatment of type 2 diabetes. World J Diabetes. 2014;5:854–9. doi: 10.4239/wjd.v5.i6.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herrmann K, Shan K, Brunell SC, Chen S. Effects of pramlintide in patients with type 2 diabetes mellitus: An analysis using daily insulin dose tertiles. Endocr Pract. 2014;20:1070–5. doi: 10.4158/EP13477.OR. [DOI] [PubMed] [Google Scholar]
- 63.Benroubi M. Fear, guilt feelings and misconceptions: Barriers to effective insulin treatment in type 2 diabetes. Diabetes Res Clin Pract. 2011;93(Suppl 1):S97–9. doi: 10.1016/S0168-8227(11)70021-3. [DOI] [PubMed] [Google Scholar]
- 64.Haugstvedt A, Wentzel-Larsen T, Graue M, Søvik O, Rokne B. Fear of hypoglycaemia in mothers and fathers of children with Type 1 diabetes is associated with poor glycaemic control and parental emotional distress: A population-based study. Diabet Med. 2010;27:72–8. doi: 10.1111/j.1464-5491.2009.02867.x. [DOI] [PubMed] [Google Scholar]
- 65.Davidson JA. Case study: Lessons learned in the care of a 63-year-old woman with type 2 diabetes. Clin Cornerstone. 2005;7(Suppl 3):S18–24. doi: 10.1016/s1098-3597(05)80085-7. [DOI] [PubMed] [Google Scholar]
- 66.Nam S, Chesla C, Stotts NA, Kroon L, Janson SL. Barriers to diabetes management: Patient and provider factors. Diabetes Res Clin Pract. 2011;93:1–9. doi: 10.1016/j.diabres.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 67.Moser EG, Morris AA, Garg SK. Emerging diabetes therapies and technologies. Diabetes Res Clin Pract. 2012;97:16–26. doi: 10.1016/j.diabres.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 68.Sousa F, Castro P, Fonte P, Sarmento B. How to overcome the limitations of current insulin administration with new non-invasive delivery systems. Ther Deliv. 2015;6:83–94. doi: 10.4155/tde.14.82. [DOI] [PubMed] [Google Scholar]
- 69.Arbouche Z. Diabetes in Eldely: A Maghrebin Study. 9th Congress of Endocrinology and Diabetology; 23-25 November, 2012; Algiers. [Google Scholar]
- 70.Boquete L, Rodriguez-Ascariz JM, Artacho I, Cantos-Frontela J, Peixoto N. Dynamically programmable electronic pill dispenser system. J Med Syst. 2010;34:357–66. doi: 10.1007/s10916-008-9248-3. [DOI] [PubMed] [Google Scholar]


