Abstract
Objective:
Growth hormone through insulin-like growth factor 1 (IGF-1) plays an important role in both bone growth and mineralization. This cross-sectional study was carried out to evaluate the relationship between serum IGF-1 concentrations and dual energy X-ray (DXA) measured whole body less head bone area (BA), lean body mass (LBM), and bone mineral content (BMC).
Methods:
One hundred and nineteen children (boys = 70, age = 7.3–15.6 years) were studied for their anthropometric parameters by standard methods and bone and body composition by DXA. Their fasting serum IGF-1 concentrations were assessed by enzyme-linked immunosorbent assay and Z-scores were calculated using available reference data. Bone and body composition parameter Z-scores were calculated using ethnic reference data.
Results:
Mean age of the boys and girls was similar (11.5 ± 1.8 years). The mean serum IGF-1concentrations and IGF-1 Z-scores were similar (P > 0.1) between boys and girls and were of the order of (302.3 ± 140.0 and − 1.4 ± 1.1, respectively). The LBM for age and BA for age Z-score was greater in children with IGF-1 Z-score > median than children with IGF-1 Z-score < median. The mean BMC for age Z-scores were 0.4 ± 0.9 and − 0.2 ± 0.8 in children with above and below the median of IGF-1 Z-score (P > 0.1).
Conclusion:
Serum IGF-1 levels were more strongly associated with BA and LBM, suggesting that its effect on bone is greater with respect to periosteal bone acquisition and through its effect on muscle mass.
Keywords: Bone mineral content, Indian children, insulin-like growth factor 1
INTRODUCTION
Growth hormone (GH) and its physiological mediator, insulin-like growth factor-1(IGF-1), have a major role in linear bone growth and accrual of bone mass during childhood and adolescence. In general, linear growth-promoting effects of GH appear to depend upon the production of IGF-1 and perhaps other IGF peptides.[1] IGF-1 acts on the cartilage, stimulating cell proliferation, and the synthesis of DNA, RNA, protein, and proteoglycans. In muscles, IGF-1 stimulates amino acid, glucose transport and glycogen, and protein synthesis.[2] IGF-1 is a major component of the organic skeletal matrix and is the most important differentiative factor for osteoblasts. Studies carried out in animal models have shown that IGF-1 can enhance longitudinal growth, periosteal circumference, and bone mineral density.[3] The relationship of IGF-1 with bone mass or bone density has been studied in adults as well as elderly population. Some researchers have found a positive association of IGF-1 concentrations with bone density[4,5,6,7] and some have not found any relation.[8,9,10] In children and adolescents, few researchers have studied the association of IGF-1 and serum bone markers and suggested that IGF-1 plays a role in regulating bone turnover.[11,12,13] In addition, IGF-1 exerts a greater effect on height and bone mass during pubertal years in children.[11,14,15] However, studies investigating the relationship of IGF-1 and bone status in children and adolescents are scarce.
Variation in IGF concentrations between various ethnic populations has been reported by several researchers.[16,17,18] Genetic factors are believed to determine 38% of the variability in serum IGF-1 concentrations.[19] However, IGF-1 concentrations are also regulated by nutritional factors. Both the amount and type of protein in diet have an effect on IGF-1 concentrations. High protein diets with adequate calcium intakes are associated with greater bone mass and fewer fractures.[20] In a study on normal Indian children, from birth to 20 years, Dehiya et al.[21] reported that IGF-1 concentrations were lower in the study population compared with their Western counterparts, at all ages and in both sexes. Various reports have shown that Indian children's diets are majorly based on cereal-pulse combinations, hence, the type and amount of protein, as well as calcium intake, are suboptimal.
With this background, the objective of this study was to evaluate the relationship between serum IGF-1 concentrations and dual energy X-ray (DXA) measured bone and body composition parameters in 7–15-year-old apparently, healthy children.
METHODS
Sample selection
We studied 119 apparently, healthy school going children (boys = 70, girls = 49) in the range of 7–15 years of age from a school, randomly selected from a list of 20 schools from Pune, Western Maharashtra, catering to middle socioeconomic population. This cross-sectional study was approved by the Institutional Ethics Committee. Children with a history of fracture or prolonged immobilization within the past 12 months and any major systemic disease were excluded. A written informed consent from the parents and an assent from the children were obtained before the study.
Anthropometric parameters
Standing height was measured to the nearest millimeter using a Portable Stadiometer (Leicester Height Meter, Child Growth Foundation, UK), and weight was measured using an Electronic scale to the nearest 100 g. The height for age Z-score (HAZ), weight for age Z-score (WAZ), and body mass index for age Z-score (BAZ) were calculated using ethnic reference database.[22] Pubertal staging was performed by a trained pediatric endocrinologist using Tanner method.[23,24]
Insulin-like growth factor 1 analysis
A fasting blood sample (5 ml) was collected between 7 am and 9 am in a vacutainer by a trained pediatric nurse. The serum IGF-1 concentrations were analyzed by a solid phase enzyme-linked immunosorbent assay with an intra-assay coefficient of variation (CV) of 4.7% and inter-assay CV of 7.2%. The IGF-1 concentrations were then converted into Z-scores using available contemporary reference data.[25]
Bone and body composition parameter assessment
The GE-Lunar DPX PRO (GE Healthcare, Waukesha, WI, USA) Pencil Beam DXA scanner (software encore 2005 version 9.30.044) was used to measure total body (TB) bone mineral content (BMC [g]), bone area (BA [cm2]), lean body mass (LBM [g]), and fat mass (FM [g]). The manufacturer's appointed service engineer was requested to review the calibration data and provide a scanner maintenance check to ensure the system's performance before the first child was scanned, and to confirm that no instrumentation drift occurred. The machine calibration was performed daily using the phantom provided by the manufacturer. All scans were taken and their analysis were performed by the same operator. The precision of repeat measurements in children for the DPX PRO was estimated to be 0.98% for TB BMC, 1.13% for TB BA, 0.74% for TB LBM, and 1.1% for TB FM from two repeat measurements on 31 children.
Statistical methods
All statistical analyses were performed using SPSS software (version 16.0. 2007, SPSS Inc., Chicago, IL, USA). Normality of the data was checked using Kolmogorov–smirnov test. Differences in means were tested using Student's t-test.
RESULTS
Anthropometric and biochemical parameters for 119 children and adolescents of age ranging from 7 to 15 years (boys: 70, girls: 49) are given in Table 1. Differences in anthropometric parameters of boys and girls were not significant. Growth parameters of the majority of children were within the reference range with only 9% and 4% of children being below −2 for their HAZ, WAZ respectively. Only 2% of children had BAZ below −2.
Table 1.
Anthropometric and biochemical characteristics
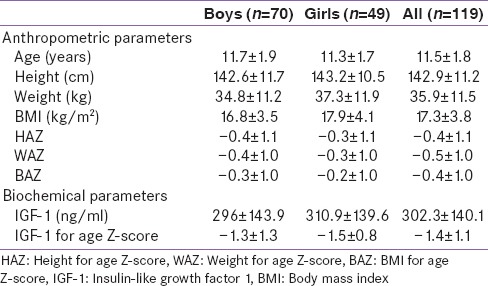
Eighteen percent of children were from the age group of 7–9 years, 46% were in 10–12 years, and 36% were in 13–15 years. However, for further analysis of data, Z-scores of IGF-1 and bone parameters were used, hence, adjusting for age and gender. The mean IGF-1 for age Z-scores were similar in boys and girls [Table 1]. Since there were no significant differences in the anthropometric and biochemical parameters between boys and girls, further analysis was carried out on the entire group of children. To study the relationship of IGF-1 and bone and body composition parameters, children were classified according to their IGF-1 for age Z-scores above (Group A) and below median (Group B) (the median IGF-1 Z-score was −1.5). The IGF-1 Z-scores were also calculated using Indian reference data.[21] However, the results remain unchanged. Children from both the groups were in similar pubertal stages (P > 0.01).
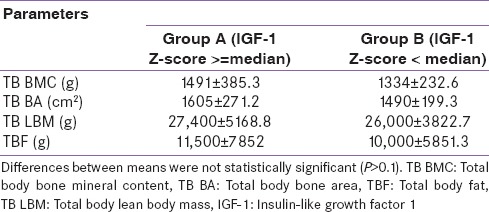
Table 2 presents the bone and body composition parameters in children. The TB BMC, BA, fat, and LBM were greater in children from Group A than Group B children. However, this difference was nonsignificant (P > 0.1).
Table 2.
Bone and body composition parameters in children above and below median IGF-1 Z-scores
Further, to adjust for the variation in the bone and body composition parameters due to age and gender, Z-scores were calculated using ethnic reference database.[26]
Thus, TB BMC for age, TB BA for age, and TB LBM for age Z-scores were calculated. In addition, bone and body size approach suggested by Mølgaard et al.[27] was used to understand whether the child had low BMC due to low Ht Z-score for age (short bones; TB BMC for Ht Z-score), or whether the child had adequate BA for height (narrow bones; TB BA for Ht Z-score), and/or adequate BMC for the BA (light bones; TB BMC for TB BA Z-score). Further, as suggested by Crabtree et al.,[28] to understand whether LBM (surrogate for muscle mass) was appropriate for height, and if the child's bone mass was appropriate for his or her LBM, TB LBM for height and TB BMC for TB LBM Z-score was calculated. The bone Z-scores were then tested for significance of the difference between Group A and B. The mean TB BA and TB LBM for age Z-scores were significantly (P < 0.05) greater in children from Group A (IGF-1 Z-scores > median) than children from Group B (IGF-1 Z-scores < median). Although the mean TB BMC was greater in Group A children, the difference was marginally significant (P < 0.1) [Figure 1]. When children were grouped in Group A (≥median) and B (< median) with the use of IGF-1 Z-scores calculated with Indian reference data,[21] similar trends in bone Z-scores were found (TB BMC for age: 0.3, −0.1; TB BA for age: 0.5, −0.2; TB LBM for age: 0.7, −0.02; TB BA for Ht: −0.1, 0.02; TB BMC for BA: −0.6, 0.2; TB LBM for Ht: 0.1, 0.2; TB BMC for TB LBM: −0.4, −0.05, respectively, for Group A and B).
Figure 1.
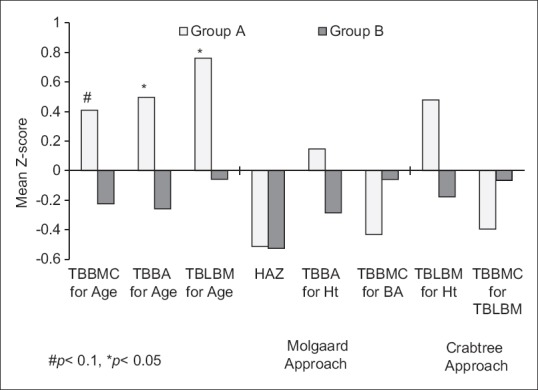
Mean bone parameter Z-scores in Group A (IGF Z-score ≥median) and Group B (IGF Z-score <median) children
Further, children from both the groups had their HAZ above −2, indicating that none of them had short bones from either of the groups. When the mean TB BA for height Z-scores were assessed, Group A children showed better BA for height than Group B children. However, the BMC for BA (TB BMC for TB BA Z-score) was lower in children from Group A (IGF-1 Z-scores above median), indicating that the increase in BA precedes the increase in BMC with the increase in IGF-1 concentrations during growth. Similarly, in children from Group A, lower BMC for their LBM (TB BMC for TB LBM Z-score) was found as compared to Group B children, indicating the role of IGF-1 in accumulation of LBM [Figure 1].
DISCUSSION
Results from the present study illustrate the relationship of IGF-1 concentrations with bone and body composition parameters in children and adolescents from 7 to 15 years of age. Our study suggests that children with higher IGF-1 concentrations showed greater muscle mass, BA, and BMC for age. In addition, it was observed that the BMC for LBM and BA in children with IGF-1 Z-score above the median were low as compared to children with IGF-1 Z-score below the median. Since there was no difference in the pubertal status of children, these results suggest an independent association of IGF-1 with the LBM accumulation and increase in BA but not with the BMC.
Studies examining the relationship of IGF-1 and DXA-measured bone and body composition parameters in children are scarce. Researchers have studied the relationship between IGF-1 status and serum bone turnover markers in children of similar age groups.[11,12,13] In line with our results, Mora et al. have reported the effect of IGF-1 on bone mass through an increase in the cross-sectional and cortical BA in the appendicular skeleton.[13] Léger et al. have demonstrated an association between IGF-1 and serum bone turnover markers in children independent of the pubertal stage of the children.[12] Similarly, Kanbur et al. have speculated that the increase in the bone formation during puberty may rather be a function of increase in IGF-1/IGFBP3 ratio than the pubertal stage.[11] As seen in our children, greater LBM reported in children with greater IGF-1 concentrations was also reported by Alderete et al.[29]
Further, protein undernutrition reduces the IGF-1 production and in turn decreases skeletal acquisition during growth. The stimulation of bone formation in response to IGF-I is impaired in the presence of an inadequately low intake of proteins. At the skeletal level, IGF-I exerts a positive effect on the bone mass by a direct action on osteogenic cells. At the kidney level, IGF-I enhances the renal reabsorption of inorganic phosphate (Pi) and the production of calcitriol, the hormonal form of Vitamin D that stimulates the intestinal absorption of calcium and Pi, the two main bone mineral elements.[30] Both the level and type of protein in the diet may have an effect on IGF-1 levels. Studies have found that meat as a protein source is associated with higher serum levels of IGF-1, which is in turn associated with increased bone mineralization.[20]
Although the sample size of this study was small, and hence data could not be analyzed as per children's tanner stages and dietary assessments could not be performed, the results presented are of importance for understanding linear growth in children. In summary, our study results suggest that serum IGF-1 concentrations play an important role in the accumulation of LBM and BA in children and adolescents but not in the accrual of BMC to the same extent. However, further validation of the results with large sample size is warranted. It is also necessary to study the modifiable factors affecting the IGF-1 concentrations such as nutrition, especially amount and type of protein and consequently formulate strategies to improve the IGF-1 concentrations in Indian children.
CONCLUSION
Serum IGF-1 concentrations were more strongly associated with the lean body mass accumulation and increase in bone area than bone mineral content in 7 to 15 years old school going children.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Acknowledgment
Our sincere thanks to all the children who participated in this study and also to the school teachers and principal for their support. We are grateful to Dr. U. P. Divate, MD (Director, Hirabai Cowasji Jehangir Medical Research Institute [HCJMRI]), for giving permission for carrying out this project. Finally, we thank the staff of HCJMRI, Ms. Ashwini Mohite, and Ms. Shamim Momin for their help with the study.
REFERENCES
- 1.Parks JS, Felner EI. Hormones of the hypothalamus and pituitary. In: Kliegman RM, Behrman RE, Jenson HB, editors. Kliegman: Nelson Textbook of Pediatrics. 18th ed. Philadelphia: Saunders Elsevier; 2007. [Google Scholar]
- 2.Tirapegui J. Effect of insulin-like growth factor-1 (IGF-1) on muscle and bone growth in experimental models. Int J Food Sci Nutr. 1999;50:231–6. doi: 10.1080/096374899101102. [DOI] [PubMed] [Google Scholar]
- 3.Yakar S, Rosen CJ. From mouse to man: Redefining the role of insulin-like growth factor-I in the acquisition of bone mass. Exp Biol Med (Maywood) 2003;228:245–52. doi: 10.1177/153537020322800302. [DOI] [PubMed] [Google Scholar]
- 4.Rucker D, Ezzat S, Diamandi A, Khosravi J, Hanley DA. IGF-I and testosterone levels as predictors of bone mineral density in healthy, community-dwelling men. Clin Endocrinol (Oxf) 2004;60:491–9. doi: 10.1111/j.1365-2265.2004.02006.x. [DOI] [PubMed] [Google Scholar]
- 5.Langlois JA, Rosen CJ, Visser M, Hannan MT, Harris T, Wilson PW, et al. Association between insulin-like growth factor I and bone mineral density in older women and men: The Framingham Heart Study. J Clin Endocrinol Metab. 1998;83:4257–62. doi: 10.1210/jcem.83.12.5308. [DOI] [PubMed] [Google Scholar]
- 6.Zhao HY, Liu JM, Ning G, Zhao YJ, Chen Y, Sun LH, et al. Relationships between insulin-like growth factor-I (IGF-I) and OPG, RANKL, bone mineral density in healthy Chinese women. Osteoporos Int. 2008;19:221–6. doi: 10.1007/s00198-007-0440-y. [DOI] [PubMed] [Google Scholar]
- 7.Szulc P, Joly-Pharaboz MO, Marchand F, Delmas PD. Insulin-like growth factor I is a determinant of hip bone mineral density in men less than 60 years of age: MINOS study. Calcif Tissue Int. 2004;74:322–9. doi: 10.1007/s00223-003-0090-9. [DOI] [PubMed] [Google Scholar]
- 8.Bennett AE, Wahner HW, Riggs BL, Hintz RL. Insulin-like growth factors I and II: Aging and bone density in women. J Clin Endocrinol Metab. 1984;59:701–4. doi: 10.1210/jcem-59-4-701. [DOI] [PubMed] [Google Scholar]
- 9.Rudman D, Drinka PJ, Wilson CR, Mattson DE, Scherman F, Cuisinier MC, et al. Relations of endogenous anabolic hormones and physical activity to bone mineral density and lean body mass in elderly men. Clin Endocrinol (Oxf) 1994;40:653–61. doi: 10.1111/j.1365-2265.1994.tb03018.x. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd ME, Hart DJ, Nandra D, McAlindon TE, Wheeler M, Doyle DV, et al. Relation between insulin-like growth factor-I concentrations, osteoarthritis, bone density, and fractures in the general population: The Chingford study. Ann Rheum Dis. 1996;55:870–4. doi: 10.1136/ard.55.12.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanbur NO, Derman O, Kinik E. The relationships between pubertal development, IGF-1 axis, and bone formation in healthy adolescents. J Bone Miner Metab. 2005;23:76–83. doi: 10.1007/s00774-004-0544-9. [DOI] [PubMed] [Google Scholar]
- 12.Léger J, Mercat I, Alberti C, Chevenne D, Armoogum P, Tichet J, et al. The relationship between the GH/IGF-I axis and serum markers of bone turnover metabolism in healthy children. Eur J Endocrinol. 2007;157:685–92. doi: 10.1530/EJE-07-0402. [DOI] [PubMed] [Google Scholar]
- 13.Mora S, Pitukcheewanont P, Nelson JC, Gilsanz V. Serum levels of insulin-like growth factor I and the density, volume, and cross-sectional area of cortical bone in children. J Clin Endocrinol Metab. 1999;84:2780–3. doi: 10.1210/jcem.84.8.5874. [DOI] [PubMed] [Google Scholar]
- 14.Johansen JS, Giwercman A, Hartwell D, Nielsen CT, Price PA, Christiansen C, et al. Serum bone Gla-protein as a marker of bone growth in children and adolescents: Correlation with age, height, serum insulin-like growth factor I, and serum testosterone. J Clin Endocrinol Metab. 1988;67:273–8. doi: 10.1210/jcem-67-2-273. [DOI] [PubMed] [Google Scholar]
- 15.van Coeverden SC, Netelenbos JC, de Ridder CM, Roos JC, Popp-Snijders C, Delemarre-van de Waal HA. Bone metabolism markers and bone mass in healthy pubertal boys and girls. Clin Endocrinol (Oxf) 2002;57:107–16. doi: 10.1046/j.1365-2265.2002.01573.x. [DOI] [PubMed] [Google Scholar]
- 16.Cruickshank JK, Heald AH, Anderson S, Cade JE, Sampayo J, Riste LK, et al. Epidemiology of the insulin-like growth factor system in three ethnic groups. Am J Epidemiol. 2001;154:504–13. doi: 10.1093/aje/154.6.504. [DOI] [PubMed] [Google Scholar]
- 17.Berrigan D, Potischman N, Dodd KW, Hursting SD, Lavigne J, Barrett JC, et al. Race/ethnic variation in serum levels of IGF-I and IGFBP-3 in US adults. Growth Horm IGF Res. 2009;19:146–55. doi: 10.1016/j.ghir.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLellis K, Rinaldi S, Kaaks RJ, Kolonel LN, Henderson B, Le Marchand L. Dietary and lifestyle correlates of plasma insulin-like growth factor-I (IGF-I) and IGF binding protein-3 (IGFBP-3): The multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2004;13:1444–51. [PubMed] [Google Scholar]
- 19.Harrela M, Koistinen H, Kaprio J, Lehtovirta M, Tuomilehto J, Eriksson J, et al. Genetic and environmental components of interindividual variation in circulating levels of IGF-I, IGF-II, IGFBP-1, and IGFBP-3. J Clin Invest. 1996;98:2612–5. doi: 10.1172/JCI119081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heaney RP, Layman DK. Amount and type of protein influences bone health. Am J Clin Nutr. 2008;87:1567S–70S. doi: 10.1093/ajcn/87.5.1567S. [DOI] [PubMed] [Google Scholar]
- 21.Dehiya RK, Bhartiya D, Kapadia C, Desai MP. Insulin-like growth factor-I, insulin-like growth factor binding protein-3 and acid labile subunit levels in healthy children and adolescents residing in Mumbai suburbs. Indian Pediatr. 2000;37:990–7. [PubMed] [Google Scholar]
- 22.Khadilkar VV, Khadilkar AV, Cole TJ, Sayyad MG. Cross-sectional growth curves for height, weight and body mass index for affluent Indian children, 2007. Indian Pediatr. 2009;46:477–89. [PubMed] [Google Scholar]
- 23.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu S, Gu X, Pan H, Zhu H, Gong F, Li Y, et al. Reference ranges for serum IGF-1 and IGFBP-3 levels in Chinese children during childhood and adolescence. Endocr J. 2010;57:221–8. doi: 10.1507/endocrj.k09e-200. [DOI] [PubMed] [Google Scholar]
- 26.Khadilkar AV, Sanwalka NJ, Chiplonkar SA, Khadilkar VV, Mughal MZ. Normative data and percentile curves for dual energy X-ray absorptiometry in healthy Indian girls and boys aged 5-17 years. Bone. 2011;48:810–9. doi: 10.1016/j.bone.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Mølgaard C, Thomsen BL, Prentice A, Cole TJ, Michaelsen KF. Whole body bone mineral content in healthy children and adolescents. Arch Dis Child. 1997;76:9–15. doi: 10.1136/adc.76.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crabtree NJ, Kibirige MS, Fordham JN, Banks LM, Muntoni F, Chinn D, et al. The relationship between lean body mass and bone mineral content in paediatric health and disease. Bone. 2004;35:965–72. doi: 10.1016/j.bone.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Alderete TL, Byrd-Williams CE, Toledo-Corral CM, Conti DV, Weigensberg MJ, Goran MI. Relationships between IGF-1 and IGFBP-1 and adiposity in obese African-American and Latino adolescents. Obesity (Silver Spring) 2011;19:933–8. doi: 10.1038/oby.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonjour JP, Ammann P, Chevalley T, Rizzoli R. Nutrition and insulin growth factor-I in relation to bone health and disease. In: Houston MS, Holly JM, Feldman FL, editors. IGF and Nutrition in Health and Disease. Totowa, NJ: Humana Press Inc.; 2004. [Google Scholar]


