Abstract
Background
Jumonji C (JmjC) domain-containing proteins are a group of functionally conserved histone lysine demethylases in Eukaryotes. Growing evidences have shown that JmjCs epigenetically regulate various biological processes in plants. However, their roles in plant biotic stress, especially in rice bacterial blight resistance have been barely studied so far.
Results
In this study, we found that the global di- and tri-methylation levels on multiple lysine sites of histone three were dramatically altered after being infected by bacterial blight pathogen Xanthomonas oryzae pv. oryzae (Xoo). Xoo infection induced the transcription of 15 JmjCs, suggesting these JmjCs are involved in rice bacterial blight defense. Further functional characterization of JmjC mutants revealed that JMJ704 is a positive regulator of rice bacterial blight resistance as the jmj704 became more susceptible to Xoo than the wild-type. In jmj704, the H3K4me2/3 levels were significantly increased; suggesting JMJ704 may be involved in H3K4me2/3 demethylation. Moreover, JMJ704 suppressed the transcription of the rice defense negative regulator genes, such as NRR, OsWRKY62 and Os-11N3, by reducing the activation marks H3K4me2/3 on them.
Conclusions
JMJ704 may be a universal switch controlling multiple genes of the bacterial blight resistance pathway. JMJ704 positively regulates rice defense by epigenetically suppressing master negative defense regulators, presenting a novel mechanism distinct from its homolog JMJ705 which also positively regulates rice defense but via activating positive defense regulators.
Electronic supplementary material
The online version of this article (doi:10.1186/s12870-015-0674-3) contains supplementary material, which is available to authorized users.
Keywords: Rice (Oryza sativa L.), Xanthomonas oryzae pv. oryzae, JmjC domain-containing demethylase, Histone modification
Background
Histone methylation is a very important post-translational modification and plays an essential role in chromatin remodeling, gene transcription and genome stability in eukaryotic cells [1–3]. Mono-, di- or tri-methylation for histone H3 at lysine 4, 9, 27, 36(H3K4me1/me2/me3,H3K9me1/me2/me3,H3K27me1/me2/me3,H3K36me1/me2/me3) has been implicated in epigenetic gene regulation [4]. Generally, H3K4 and H3K36 methylations are associated with actively transcribed genes, whereas methylations of H3K9 and H3K27 have the transcriptional repressing function [4, 5]. Histone lysine methylation can be reversed by histone lysine demethylases (KDMs) [6]. KDMs contain two known evolutionarily conserved types: lysine specific demethylase1 (LSD1) [7] and histone demethylases featured with the jumonji C (JmjC) domain [8, 9]. LSD1 has been demonstrated to be responsible for H3K4 demethylation [7]. In Arabidopsis, the three homologues LSD1-like 1 (LDL1), LSD1-like 2 (LDL2) and FLOWERING LOCUS D (FLD) were shown to repress FLOWERING LOCUS C (FLC) expression via demethylating mono- and di-methylated H3K4 [10]. FLD is also required to systemic acquired resistance [11, 12]. The JmjC domain-containing histone demethylases are generally conserved in yeast, animal and plant [8, 13]. JmjC proteins preferentially remove di- and tri-methylations in histone lysines through ferrous ion and α-ketoglutaric acid-dependent oxidative reactions [8]. For examples, JHDM1 specifically demethylates H3K36me2 in human and yeast [8], while Arabidopsis JMJ14, JMJ15 and JMJ18 are H3K4me2/me3 demethylases [14–16]. In rice, there are totally 20 JmjC domain-containing proteins named JMJ701-JMJ720 [17–20]. JMJ701-JMJ720 are classified into five different groups on the basis of the JmjC domain and the overall protein domain architecture, including JmjC domain-containing histone demethylase 2 (JHDM2), JmjC domain-containing 2 (JMJD2), JmjC protein containing AT-rich interaction domain (JARID), JmjC domain only and N-terminal FY-rich_C-terminal FY-rich ( FYRN_FYRC) [18].
Recently, emerging evidence has shown that JmjCs participate in various aspects of rice developmental processes and response to stresses. In FYRN_FYRC group, JMJ703 was reported to be a H3K4 demethylase. The jmj703 mutant displayed pleiotropic phenotypes such as dwarf, erected leaves, less secondary panicles and smaller grain size. In addition, JMJ703 could repress the retrotransposon activity by demethylating the lysine 4 site of histone 3, which is the main mechanism to maintain the rice genome stability [21, 22]. JMJ706, a JMJD2 group member, was identified as a H3K9 demethylase and involved in the regulation of floral development [18]. Recently, it was found that JMJ705, a H3K27 di- and tri-methylation demethylase was involved in plant defense response to the bacterial blight (BB) disease pathogen (Xanthomonas oryzae pv. oryzae, Xoo) infection. Mutation of JMJ705 reduced rice resistance to Xoo, while overexpression of JMJ705 enhanced rice resistance to Xoo. It was suggested that JMJ705 demethylase activity is subject to the methyl jasmonate induction during the pathogen infection, and the induced JMJ705 may remove H3K27me3 from marked defense-related genes and enhance the rice disease resistance [23]. Interestingly, JMJ705 is not the sole case in which plant immunity to pathogens is subjected to epigenetic regulation. Previous studies have demonstrated that the Arabidopsis (Arabidopsis thaliana) histone H3K36 methyltransferase SET DOMAIN GROUP8 (SDG8) and H3K4 methyltransferase TRITHORAX1 (ATX1) play crucial roles in biotic stress as well. Mutation of SDG8 reduced resistance to the necrotrophic fungal pathogens Alternaria brassicicola and Botrytis cinerea [24, 25], and down-regulated the expression of resistance (R) gene against Pst DC3000 [25, 26]. In atx1 mutant, the salicylic acid (SA)-responsive pathway was suppressed, while the ethylene (ET)/ jasmonic acid (JA) responsive pathway was elevated to against Pst DC3000 infection [25, 27, 28]. The research on JMJ705, SDG8 and ATX1 fully supported the hypothesis that histone demethylation/methylation are involved in plant defense to pathogens.
Bacterial blight (BB) of rice caused by Xoo infection is one of the most devastating diseases for rice production as the yield loss can be up to 50 % [29]. Though many BB resistance genes, such as Xa4, xa5, xa13 and Xa21, have been identified and applied in breeding by single gene introduction or gene pyramiding [30], the acquired resistance could be soon lost as the pathogen evolves very quickly to overcome the resistance. Therefore, discovery of novel BB resistant genes, especially epigenetic genes controlling the reprogramming of gene transcription, would be of great importance to obtain sustainable BB resistance in rice breeding. In this work, we examined the global level of various histone methylation modifications under Xoo infection. The Xoo infection induced expression patterns indicated that JmjC demethylase genes are involved in BB resistance. Knock-down of JMJ704, a potential H3K4me2/3 demethylase, significantly increased the plant susceptibility to Xoo infection when compared with the wild-type. Meanwhile, several negative master regulators of rice disease resistance, including NRR, OsWRKY62 and Os-11N3, were up-regulated in jmj704, suggesting that JMJ704 positively regulates rice BB resistance via epigenetically suppressing the transcription of negative regulators during the pathogen infection.
Results
The global histone lysine methylation dynamics in response to Xoo infection
Though some examples indicating that histone lysine methylation mediates plant disease resistance have been reported, the dynamics behind the global histone methylation level in response to Xoo infection remain unclear. To address this question, we investigated the histone di- and tri-methylation levels of various lysine sites at different time points after Xoo infection. The nuclear-rich proteins were isolated from the leaves of four-week-old Nipponbare plants inoculated with Xoo at 0, 4, 8, 12, 24, and 72 h, and Western blot analysis was performed using antibodies against di- and tri-methylated H3K4, H3K9, H3K27, and H3K36 respectively. An antibody against unmodified histone H3 was used as the loading control. As shown in Fig. 1, the global methylation level at various lysine sites changed with the time of Xoo inoculation. For histone H3 lysine 4, the di-methylation level remained unchanged at 4 h (P > 0.05), but became significantly decreased from 8 to 24 h (P < 0.05). The lowest H3K4me2 intensity was detected at 8 h. After this, the signal intensity gradually returned back to 0.91 at the time point of 72 h. We observed that the H3K4me3 intensity was significantly increased at 8 and 12 h, but reduced to 0.9 and 0.81 at 24 and 72 h respectively, when compared with the 0 h (P < 0.05). For H3K9, the di-methylation and tri-methylation exhibited similar inclinations. The H3K9me2/3 started to decrease at 12 h, reached the lowest at 24 h, and remained a low level till 72 h (P < 0.05), suggesting that H3K9me2/3 are functional in the late response to Xoo infection. In the lysine site 27 of histone H3, we found that the di-methylation level showed significant reduction to 0.68 at 72 h. Interestingly, H3K27me3, H3K36me2 and H3K36me3 displayed very similar tendency, although their final effects in gene regulation are distinct. The three modification reached the highest level at 8 h, then gradually decrease to their lowest level at 24 h (P < 0.05). The results above suggested that plant disease resistance is a complex event with methylation of multiple histone lysine sites being involved, while different histone methylation modifications may play distinct roles in this process.
Fig. 1.
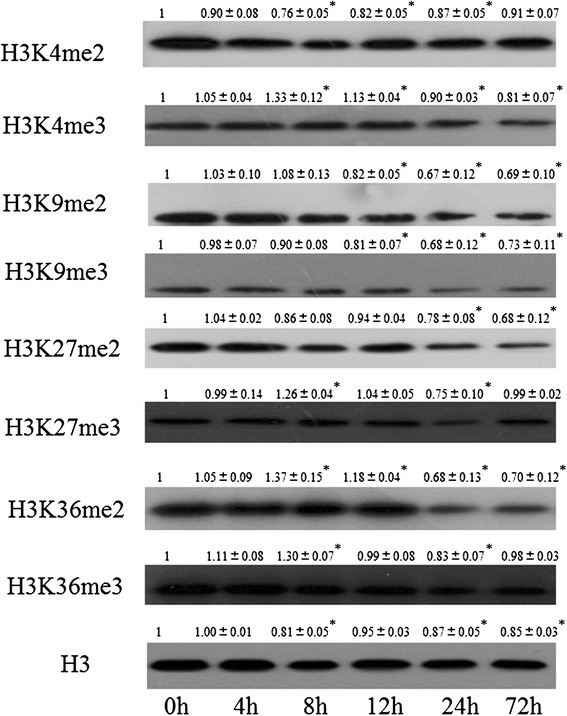
Time-course analysis of histone methylation levels on lysine residues of histone H3 in rice. Histone proteins were isolated from four-week-old rice leaves at 0, 4, 8, 12, 24, 72 h after Xoo inoculation and analyzed by Western blot using antibodies against histone methylation marks as indicated. The mean signal intensities ± standard deviation (from three biological replicates) of various methylation modifications are shown as numbers normalized to the rice plant inoculated at 0 h level. The intensity of plant inoculated at 0 h was set to 1. Histone H3 was used as a loading control. Significance of differences between the plants inoculated at 0 h and 4, 8, 12, 24, 72 h was determined by Student’s t tests. *P < 0.05
The Xoo infection induced expression profile of rice JmjC genes
Previous studies have revealed a total of 20 JmjC genes in the rice genome (Table 1). Prior to the investigation of the Xoo infection induced expression profile, we examined the tissue-specific expression of the JmjC genes among callus, root, leaf, sheath, flower and young/medium /old developing seed of rice by qRT-PCR. As shown in Additional file 1: Figure S1, most of the JmjC genes were constitutively expressed. Then, qRT-PCR was conducted using total RNA isolated from the leaves of four-week-old wild-type rice cv Nipponbare plants inoculated with Xoo at the time points of 0, 6, 12, 24, and 72 h. After Xoo infection, the expression levels of four genes (JMJ701, JMJ717, JMJ719 and JMJ720) remained unchanged, while all the remaining genes could be induced in various patterns by Xoo infection. As shown in Fig. 2, 24 h after inoculation seemed to be a key time point for most of the JmjC genes reacting to Xoo infection. The expressions of 11 JmjC genes (JMJ702, JMJ703, JMJ704, JMJ705, JMJ706, JMJ708, JMJ709, JMJ711, JMJ713, JMJ715 and JMJ716) were up-regulated with the peak being reached at 24 h. Among these 11 JmjC genes, JMJ702, JMJ705 and JMJ716 reacted most vigorously to Xoo attack as their expression levels were over 10 folds up-regulated at 24 h. Our result of JMJ705 is consistent with the previous report [23]. The JMJ710 and JMJ714 were around 3.5 folds up-regulated at 6 h, suggesting their roles in the early response. For JMJ712, the gene expression remained almost at the same level as the control at 6 h. However, a very sharp increase (32-fold) was observed at 12 h. After this, the JMJ712 went back to the control level. In contrast to the up-regulated JmjC genes, we also found that JMJ707 was down-regulated by the Xoo infection.
Table 1.
The characteristics of JmjC genes identified in rice
| Gene | Loc. No. | Group | Length (aa) | Known histone substrate | Function | Reference |
|---|---|---|---|---|---|---|
| JMJ701 | LOC_Os03g05680 | JMJD2 | 1488 | ND | ||
| JMJ702 | LOC_Os12g18150 | JMJD2 | 1367 | ND | ||
| JMJ703 | LOC_Os05g10770 | FYRN_FYRC | 1239 | H3K4me | Stem elongation transposon silencing | [21, 22] |
| JMJ704 | LOC_Os05g23670 | FYRN_FYRC | 972 | ND | ||
| JMJ705 | LOC_Os01g67970 | JMJD2 | 1287 | H3K27 | Disease resistance | [23] |
| JMJ706 | LOC_Os10g42690 | JMJD2 | 859 | H3K9 | Floral development | [18] |
| JMJ707 | LOC_Os02g46930 | JMJD2 | 808 | ND | ||
| JMJ708 | LOC_Os06g51490 | JARID | 1417 | ND | ||
| JMJ709 | LOC_Os01g36630 | JmjC domain only | 396 | ND | ||
| JMJ710 | LOC_Os11g36450 | JmjC domain only | 522 | ND | ||
| JMJ711 | LOC_Os03g27250 | JmjC domain only | 954 | ND | ||
| JMJ712 | LOC_Os09g31380 | JmjC domain only | 399 | ND | ||
| JMJ713 | LOC_Os01g56640 | JmjC domain only | 552 | ND | ||
| JMJ714 | LOC_Os09g31050 | JmjC domain only | 360 | ND | ||
| JMJ715 | LOC_Os03g31594 | JHDM2 | 1057 | ND | ||
| JMJ716 | LOC_Os03g22540 | JHDM2 | 928 | ND | ||
| JMJ717 | LOC_Os08g39810 | JmjC domain only | 377 | ND | ||
| JMJ718 | LOC_Os09g22540 | JHDM2 | 380 | ND | ||
| JMJ719 | LOC_Os02g01940 | JHDM2 | 998 | ND | ||
| JMJ720 | LOC_Os02g58210 | JHDM2 | 996 | ND |
ND not determined
Fig. 2.
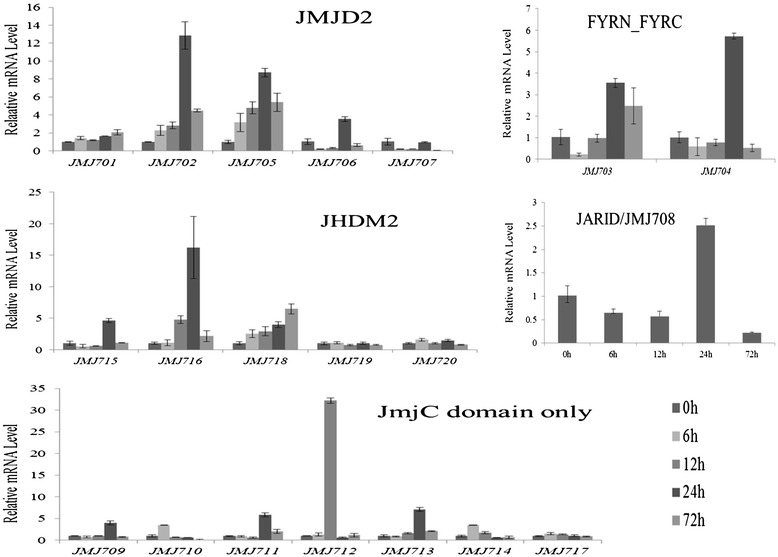
Time-course expression analysis of JmjC genes in rice after Xoo inoculation. The expression levels of 20 JmjC genes were detected at 0, 6, 12, 24, 72 h after Xoo inoculation by qRT-PCR. Total RNA was extracted from the infected four-week-old rice leaves. Ubiquitin gene was used as the internal control and error bars indicate the SD from three technical replicates
Knock-down of JMJ704 reduced the rice resistance to Xoo infection
The global histone methyaltion dynamics results and Xoo-inducible expression pattern of JmjCs intrigued us to investigate the possible function of JmjC genes in BB resistance. T-DNA insertional mutant lines of JmjCs were ordered from RMD rice mutant database (http://rmd.ncpgr.cn/) [31] and Postech rice mutant database [32] for Xoo inoculation assay. Among these mutants, jmj704 was further studied as the mutants exhibited an interesting phenotype. Two allelic jmj704 mutants were ordered and studied in this research; jmj704-1 is derived from Zhonghua 11 (ZH11) background which harbors a T-DNA insertion in the 4th intron of the gene, while jmj704-2 has a T-DNA insertion in the 4th exon in Hwayoung background (HY) (Fig. 3a). The PCR reactions by using the primer set of the gene specific primers (F1 + R1, F2 + R2 ) together with the T-DNA left border primers NTLB5 and 2707 L enables us to easily identify the T-DNA homozygous lines (Fig. 3b). In the mRNA level, qRT-PCR analysis also confirmed that the expression level of JMJ704 in both jmj704-1 and jmj704-2 were significantly knocked down (Fig. 3c). Subsequently, Xoo inoculation assay was conducted on 3 progeny lines for each allelic mutant along with the wild-type control at the booting stage. Fifteen days after the inoculation, the necrotic areas were shown and lesion areas were surveyed. Interestingly, both jmj704-1 and jmj704-2 lines became more susceptible to the Xoo infection when compared with the wild-type (Fig. 3d). In the wild-type (ZH11 and HY), the lesion areas on leaf were about 34 % and 26 % respectively. In contrast, the lesion areas were significantly increased in the six tested jmj704 lines with the lesion area ranging from 43 to 53 % (P < 0.05) (Fig. 3e). We also found that the Xoo growth rates in wild-type were significantly slower than in jmj704 lines at 3 DPI and 7 DPI (P < 0.05) (Fig. 3f). The almost identical phenotype in jmj704-1 and jmj704-2 strongly indicated that the reduced bacterial blight resistance is attributed to the loss-of-function of JMJ704. Meanwhile, these results suggested that JMJ704 might be a positive regulator in response to bacterial blight.
Fig. 3.
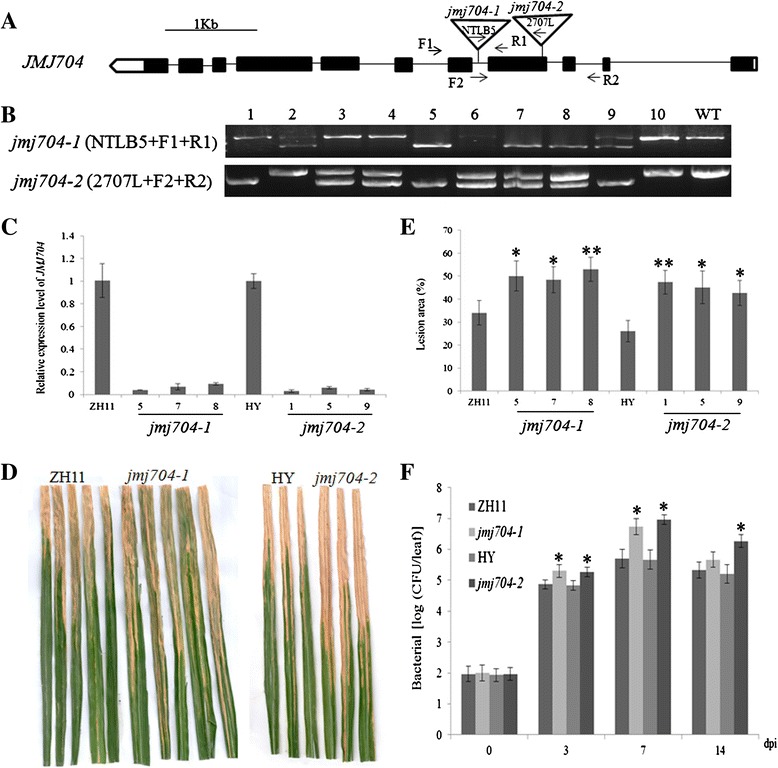
Characterization of mutation of JMJ704. a Schematic representation of T-DNA insertion mutations jmj704-1 and jmj704-2 (open triangle). The positions of the primers used for genotyping are indicated. b Genotyping of jmj704-1 and jmj704-2 segregates and the wild-type (WT) using the primer sets as indicated. c qRT-PCR detection of JMJ704 transcripts in jmj704-1 and jmj704-2 lines. d Leaf phenotypes. The booting stage plants (jmj704-1 /ZH11 and jmj704-2/HY) were inoculated Xoo by clipping method respectively. Leaf phenotype was observed at 15 days after inoculation. e Leaf lesion area (%) in the plants (jmj704-1 /ZH11 and jmj704-2/HY) at 15 days after inoculation with the Xoo. f Bacterial growth rate (Log [Colony-Forming Units/leaf]) measured at 0 (2 h post inoculation) and 3 to 14 days post inoculation (dpi) on mutants jmj704-1 and jmj704-2 leaves compared with the wild-type ZH11 and HY, respectively. Bar indicates the SD from three biological replicates. Significance of differences between the wild-type and mutants was determined by Student’s t tests. *P < 0.05, **P < 0.01
Di- and tri-methylation levels of H3K4 were increased in jmj704
Previous studies have demonstrated that JmjCs are demethylase removing di- and/or tri-methylations of various lysine sites in histone 3. It has been clear that JMJ703 is involved in H3K4 demethylation [21, 22], JMJ705 specifically demethylates H3K27me2/3 [23], while JMJ706 is an H3K9me2/me3 demethylase [18]. However, the substrate for JMJ704 remains uncertain so far. Though a conference abstract claimed that JMJ704 may be a H3K4 demethylase, but no formal publication with experimental evidence support is publically available [19]. To specify the JMJ704 substrates, histone proteins of ZH11 and jmj704-1 were extracted for immune-blot against the anti-H3k4me2 and anti-H3K4me3. In Fig. 4, our immune-blot results clearly showed that the global methylation levels of H3K4me2 and H3K4me3 increased 30 and 60 % in jmj704-1 respectively (P < 0.05), when an equal amount of histones were loaded for the analysis as indicated by the H3 antibody. Given the fact that most of the reported JmjC members are functionally conserved histone demethylases, our result hinted that JMJ704 may be a potential H3K4me2/3 demethylase in rice, although this still needs to be further confirmed by histone demethylase activity assay in our future study. According to the phylogenetic analysis of JmjC genes by Lu et al. (2008), JMJ703, JMJ704 and Arabidopsis JMJ14, JMJ18 belong to the same KDM5/JARIDI category [17]. Interestingly, all these reported members of KDM5/JARIDI are H3K4 demethylases, indicating that the substrate of this subgroup is highly conserved among plant species.
Fig. 4.
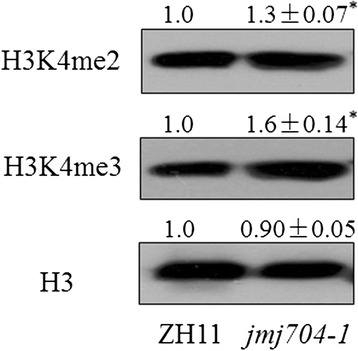
H3K4me2/me3 difference revealed by Western blot in the wild-type (ZH11) and jmj704-1 plants. Rabbit ployclonal antibody to Histone H3, or H3K4me2/me3 was used in Western blot. The mean signal intensity ± SD (from three biological replicates) of H3K4me2/me3 is shown as numbers normalized to the ZH11 level. The intensity of ZH11 was set to 1. Histone H3 was used as a loading control. Significance of differences between ZH11 and jmj704-1 plants was determined by Student’s t tests. *P < 0.05
JMJ704 regulates the expression of rice BB defense-related genes
To evaluate the effects of JMJ704 mutation on rice gene expression, RNA-seq experiment was performed for the ZH11 and jmj704-1 mutant young seedlings at two weeks after germination using Illumina HiSeqTM 2500 platform. A total of 446 genes were found to be differentially expressed between the mutant and wild-type, including 271 genes which showed over 2 fold up-regulation and 175 genes were down-regulated in jmj704 (|log 2Ratio| ≥1; FDR <0.001) (Additional file 2: Table S1). Among these DEGs (Differentially Expressed Genes), several have been known to be plant defense-related. For examples, NRR (LOC_Os01g03940), OsWRKY62 (LOC_Os09g25070) and Os-11N3 (LOC_Os11g31190) were reported to be negative regulators for Xoo resistance in rice [33–35]. In jmj704, all these three genes were significantly up-regulated which is in accordance to the phenotype that jmj704 became more susceptible to BB.
Gene ontology (GO) analysis of DEGs revealed 8 categories of enriched genes. In particular, category of “response to stress” was significantly enriched for the up-regulated genes (20 of 271; P < 0.05) and down-regulated genes (54 of 175; P < 0.05) (Additional file 3: Table S2). These results suggested that JMJ704 might be a regulator of stress-responsive gene expression. Moreover, pathway analysis also found that DEGs were preferentially involved in metabolic pathway, plant-pathogen interaction and biosynthesis of secondary metabolites (Additional file 4: Figure S2).
To validate the RNA-seq data, 12 DEGs were randomly selected for qRT-PCR verification. As shown in Table 2, the qRT-PCR result significantly correlated with the RNA-seq experiments (r = 0.863, P < 0.01) before Xoo infection, suggesting that the RNA-seq data is highly reliable in this study. Moreover, we also examined the mRNA abundance of the 12 DEGs in jmj704 mutant and wild-type at 24 h after Xoo infection. The DEGs exhibited similar differential expression pattern (up-regulated or down-regulated) under either Xoo infection or normal growth condition, though the extent may vary from gene to gene (Table 2), which further confirmed that these DEGs are subject to the regulation of JMJ704.
Table 2.
qRT-PCR validations of 12 randomly selected genes from the differentially expressed genes in the RNA-seq results
| Gene ID | Gene annotation | Ratio in RNA-seq (before inoculation) | Fold-change in qRT-PCR (before inoculation) | Fold-change in qRT-PCR (24 h after inoculation) |
|---|---|---|---|---|
| LOC_Os01g03940 | Negative regulator of disease resistance , expressed protein | 4.02 | 4.36 | 5.39 |
| LOC_Os01g12160 | OsGH3.3-Probable indole-3-acetic acid-amido synthetase, expressed | 3.90 | 3.06 | 4.64 |
| LOC_Os01g36070 | nodulin MtN3 family protein, putative, expressed | 4.93 | 3.30 | 1.23 |
| LOC_Os02g39660 | receptor kinase, putative, expressed | 0.003 | 0.09 | 0.25 |
| LOC_Os02g40784 | WAX2, putative, expressed | 0.50 | 0.45 | 0.28 |
| LOC_Os05g25770 | Superfamily of TFs having WRKY and zinc finger domains, expressed | 15.34 | 10.94 | 3.6 |
| LOC_Os06g38830 | receptor-like protein kinase precursor, putative, expressed | 0.11 | 0.25 | 0.73 |
| LOC_Os09g25070 | OsWRKY62 - Superfamily of TFs having WRKY and zinc finger domains, expressed | 22.75 | 8.89 | 6.43 |
| LOC_Os11g12050 | NBS-LRR type disease resistance protein, putative, expressed | 0.17 | 0.37 | 0.62 |
| LOC_Os11g12320 | disease resistance protein RPM1, putative | 0.026 | 0.35 | 0.81 |
| LOC_Os11g31190 | nodulin MtN3 family protein, putative, expressed | 2.78 | 6.48 | 8.01 |
| LOC_Os12g36880 | pathogenesis-related Bet v I family protein, putative, expressed | 3.13 | 2.14 | 0.77 |
H3K4me2/3 on NRR, OsWRKY62 and Os-11N3 were increased in jmj704
To study the relationship between JMJ704-mediated H3K4me2/3 and the expression of the three negative defense regulators, we checked the H3K4me2 and H3K4me3 levels of the three genes in jmj704-1 and ZH11 background by using ChIP-PCR (Chromatin immuno-precipitation followed by PCR) method. The ratio of abundance of each gene in ChIP and Input (chromatin before immunoprecipitation) DNA were used to evaluate the enrichment of the corresponding histone modification on the genes. As we expected, the H3K4me2 associated with NRR, OsWRKY62 and Os-11N3 were significantly enriched in jmj704-1 when compared with the ZH11 (Fig. 5). For example, the ChIP/Input percentage of Os-11N3 was 0.086 in ZH11, while the number reached 0.381 in jmj704-1, which was enriched 4 times in the mutant. The H3K4me2 enrichment of the other two genes in jmj704-1 ranged from 0.8 to 1.5. A similar tendency was also observed in the H3K4me3 ChIP-PCR analysis (Fig. 5). This observation is in accordance with our results that knock-down of JMJ704 could increase the H3K4me2/3 level. On the other hand, given the H3K4me2/3 are activation marks, the elevated H3K4me2/3 on NRR, OsWRKY62 and Os-11N3 also well explained the fact that these three genes are up-regulated in jmj704 mutant. It’s notable that this ChIP-PCR result was achieved from plant tissues under normal growth condition, which is essentially the stage of 0 h after Xoo infection, while the H3K4me2/3 abundance on NRR, OsWRKY62 and Os-11N3 at different time points after Xoo infection remains unknown. Investigating the dynamics of H3K4me2/3 on the three specific loci in jmj704-1 and ZH11 background will be of great interests to elucidate the mechanism underlying the jmj704 mutant susceptibility to Xoo infection.
Fig. 5.
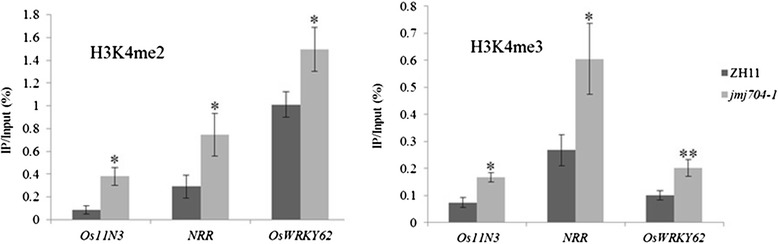
H3K4me2/3 from NRR, OsWRKY62 and Os-11N3 were increased in jmj704-1. The obtained DNA by anti- H3K4me2/3 ChIP assay was performed for quantitative PCR with primer sets corresponding to transcriptional start site regions of NRR, OsWRKY62 and Os-11N3 (primer sequences seen in Additional file 5: Table S3) in the wild-type (ZH11) and jmj704-1 plants. The genomic region of the genes for ChIP-PCR assay is shown in Additional file 6: Figure S3. Error bars indicate the SD from three biological replicates. Significance of differences was determined by Student’s t tests. *P < 0.05, **P < 0.01
Discussion
Histone modifications are extensively involved in the plant disease resistance
Recently, emerging evidences have shown that histone modifications such as H3K4me3, H3K9me2, H3K9ac, H3K23ac, H3K27ac, H3K27me3, and H4ac, may be an important mechanism controlling various biological processes. For instance, the acetylation levels of histone H3K18, H3K27, and H4K5 were found to be significantly elevated in rice when drought stress was applied, while the H3K9 acetylation level remained unchanged [36]. In this study, a survey of the global methylation levels of various lysine sites on histone 3 revealed that the di-and tri-methylation levels of H3K4, H3K9, H3K27 and H3K36 were obviously altered at different time points after Xoo infection, indicating that histone modification play a vital role in plant disease resistance. Indeed, previous reports have demonstrated that H3K4, H3K27 and H3K36 methylations were involved in defense response upon pathogen attack in Arabidopsis and rice [23–28, 37–39]. Loss-of-function of histone methyltransferase SDG8 reduced the Arabidopsis resistance to necrotic fungi pathogen infection. Evidences also showed that SDG8 is involved in the JA/ET signaling pathway [24]. Histone deacetylase HDA19 plays a positive role in Arabidopsis basal defense to pathogens by repressing the transcription factors WRKY38 and WRKY62 [40]. Meanwhile, as a master regulator of disease resistance in Arabidopsis, HDA19 represses the SA biosynthesis and SA-mediated defense to prevent overreaction of the plant when under unnecessary circumstances, thus to assure successful growth and development [41]. In rice, HDT701, a histone H4 deacetylase plays a negative role in plant defense to Magnaporthe oryzae and Xoo. In the rice HDT701 overexpression lines, the global histone H4 acetylation level was reduced and plants became more susceptible to the rice pathogens M. oryzae and Xoo. Further evidence suggested that HDT701 physically binds to and modulates the levels of histone H4 acetylation of pattern recognition receptor (PRR) and defense-related genes, such as MAPK6 and WRKY53 [42].
Roles of JmjC genes in rice BB resistance
A stress-inducible expression pattern of a gene usually indicates its function in the stress. In this work, we provided the expression profiles of 20 JmjC demethylase genes in response to Xoo infection in rice. Interestingly, 15 JmjC genes could be induced by Xoo, which strongly hinted that they may be involved in plant defense response to BB. On the other hand, it has been clear that JmjC domain-containing demethylases preferentially remove di-methylation and tri-methylation of histone lysines through ferrous ion [Fe [26]] andα-ketoglutaric acid-dependent oxidative reactions [8]. Given the JmjC gene induced expression profiles and the histone methylation dynamics in the process of Xoo infection, it is rational for us to hypothesize that JmjCs-mediated histone modification plays important roles in rice BB resistance. In 2013, a literature already reported that JMJ705 encoding a JmjC demethylase regulates rice defense response to Xoo by removing histone H3K27 tri-methylation of JA-induced genes, which well supported our speculation. In this study, we, for the first time, reported that JMJ704 positively regulates the rice resistance to Xoo infection, as indicated by the Xoo inoculation assay results that multiple jmj704 lines exhibited increased susceptibility to Xoo infection than the wild-type. Moreover, we found that the global level of H3K4me2/3 in jmj704 was increased when compared with the wild-type, implying that JMJ704 is involved in H3K4me2/3 demethylation. Even though JMJ704 and JMJ705 both play the same positive role in plant defense response, the mechanisms underlying their roles are distinct. JMJ705 activates positive defense related genes by removing the suppressing modification H3K27me3 on them, which finally enhances the plant resistance. Nevertheless, JMJ704 suppresses the transcription of the negative regulator genes by reducing the activation marks H3K4me2/3 on them, but reaches the same goal as JMJ705. Therefore, the JmjCs regulate BB resistance via a dual pathway including up-regulation of positive regulators as well as the down-regulation of negative regulators. Considering the strong Xoo inducible expression pattern that was detected on many other rice JmjCs such as JMJ702, JMJ712, and JMJ716, we believe that these genes may also be potentially involved in the rice BB resistance, which will be explored in our future study. It is also noteworthy that the majority of the Xoo inducible JmjCs were maximally induced at 24 h after induction, this stage would be a key time point for the histone modification regulation in plant disease resistance.
JMJ704-regulated bacterial blight defense pathway in rice
Our RNA-seq experiment identified 446 DEGs between jmj704 and ZH11, among which several have been known as defense-related. KEGG pathway analysis found that DEGs preferentially occurred in the plant-pathogen interaction pathway, which supported our conclusion that JMJ704 is involved in BB resistance. In particular, three negative BB resistance regulators NRR, OsWRKY62 and Os-11N3 were up-regulated in the jmj704 lines. Further ChIP-PCR analysis showed that the JMJ704-dependent H3K4me2/3 were significantly enriched on NRR, OsWRKY62 and Os-11N3 in jmj704, indicating JMJ704 could epigenetically suppress the transcription of negative defense regulators possibly via reducing the activation modifications H3K4me2/3 on them. NRR (Negative Resistance Regulator) is an up-regulated gene in jmj704. NRR could interact with rice NH1 and Arabidopsis NPR1, which are key regulators of systemic acquired resistance. It was reported that when NRR was constitutively over-expressed, rice reduced basal resistance, age-related resistance as well as the Xa21-mediated resistance, which eventually resulted in enhanced susceptibility to Xoo [33]. OsWRKY62, another negative regulator of plant innate immunity, encodes two splice variants (OsWRKY62.1 and OsWRKY62.2). Transgenic plants over-expressing OsWRKY62.1 are compromised in basal defense and Xa21-mediated resistance to Xoo. Furthermore, over-expression of OsWRKY62.1 suppresses the activation of defense-related genes PR1a, Betv1, PR10 and PBZ1 [34]. OsWRKY62 could block the Xa21 function possibly through direct protein-protein interaction. A study by Park et al. (2012) revealed that, in the defense reaction, an intracellular kinase domain of Xa21 was the cleaved and translocated to the nucleus, where the domain interacted with OsWRKY62 to trigger the Xa21-mediated immune response [43]. Os-11N3 is a close homolog of rice BB resistance recessive gene xa13. Os-11N3 and xa13 could be induced by the same TAL effectors AvrXa7 or PthXo3. It was reported that the silencing of Os-11N3 resulted in plants with loss of susceptibility specifically to strains of Xoo dependent on AvrXa7 or PthXo3 for virulence. AvrXa7 drives expression of Os-11N3, and AvrXa7 interacts and binds specifically to an effector binding element within the Os-11N3 promoter, supporting the predictive models for TAL (transcription activator-like) effector binding specificity [35]. Figure 6 presents a deduced BB responsive pathway that was regulated by JMJ704 based on the results of this study. In addition to the Os-11N3 pathway, JMJ704 coordinates other two key genes in Xa21-related BB defense pathway in rice, suggesting that JMJ704 is a universal switch controlling multiple sites of the BB resistance.
Fig. 6.
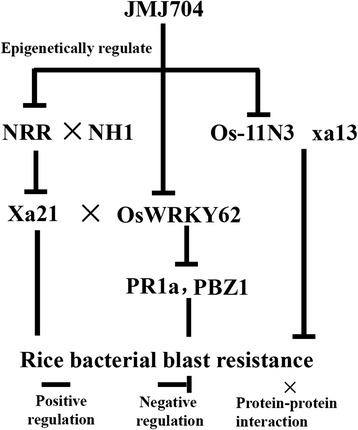
A hypothesized BB defense pathway regulated by JMJ704 in rice
Conclusion
JMJ704 positively regulates rice defense by epigenetically suppressing master negative defense regulators, presenting a novel mechanism distinct from its homolog JMJ705 which also positively regulates rice defense but via activating positive defense regulators. All this data indicates that chromatin remodeling accomplished through histone modifications is a key process in the orchestration of plant biotic stress responses, and histone-modifying enzymes are critical regulators to plant defense to pathogen attack. On the other hand, to figure out the direct target genes of JMJ704 from the DEGs would be of great importance in elucidating the regulatory network in plant disease resistance. High through-put techniques such as ChIP-sequencing will be employed for this purpose in our near future work.
Methods
Plant materials
Rice cultivars (Oryza sativa spp japonica) Nipponbare, Zhonghua11 and Hwayoung were used in this study. The T-DNA insertion mutants jmj704-1 (03Z11EQ18) and jmj704-2 (1C-14923) were obtained from the RMD rice mutant database (http://rmd.ncpgr.cn/) [31, 44] and Postech rice mutant database respectively [32]. The insertions in two jmj704 mutants were confirmed by PCR using the primers (F1+ R1+ NTLB5; F2 + R2 + 2707 L) (seen in Additional file 5: Table S3). All the plants were grown in the greenhouse of China National Rice Research Institute. Four-week-old Nipponbare plants were subjected to tissue expression and stress analysis. T-DNA mutants, ZH11 and HY plants in booting-stage were used for Xoo inoculation assay.
Rice bacterial blight inoculation
Chinese Xoo strain (Zhe173) was used for the inoculation assay. Briefly, plants in four-week-old and booting-stage were inoculated with Zhe173 (3 × 108 /ml) by the leaf clipping method in growth chambers (90 % relative humidity, 30/28 °C, 14 h light/10 h dark cycle) [45]. Plant tissues were harvested in the proper time after inoculation, and immediately kept in liquid nitrogen until use. Disease was scored (3 to 5 leaves for each plant) as the percent lesion area (lesion length/leaf length) at 15 days after inoculation. The bacterial growth rate for Zhe173 strain was also analyzed by counting colony-forming units as the previous study [23].
RNA isolation and quantitative RT-PCR (qRT-PCR)
Total RNA of various tissues was isolated using Trizol (Invitrogen) according to the manufacturer’s manual. Four micrograms of total RNA was reverse transcribed using first strand cDNA synthesis Kit (Toyobo). For real-time quantitative RT-PCR, all the primers used are listed in Additional file 5: Table S3, and an ubiquitin gene was used as an internal control. Quantitative RT-PCR was performed in a total reaction volume of ten microliter (5 μL THUNDERBIRD SYBR® qPCR Mix [Toyobo], 0.5μLcDNA, 0.5μLprimers, and 4μLwater) on the LightCycler 4.80 real-time PCR detection system (Roche). Expression was assessed by evaluating threshold cycle (CT) values. The relative expression level of tested genes was normalized to ubiquitin gene and calculated by the 2-ΔΔCT method [46]. The experiment was performed in technical triplicates.
Histone extraction
Histone-enriched proteins were extracted from rice leaves using the sulfuric acid–extraction method as described previously [47]. Briefly, nuclei were isolated from 1 g of rice leaf tissue with buffer containing 50 mM Tris pH8.0, 60 mM KCl, 5 mM MgCl2, 15 mM NaCl, 1 mM CaCl2, 0.25 M sucrose, 0.8 % triton X-100, 2 mM dithiothreitol (DTT) and 2 mM phenylmethylsulfonyl fluoride (PMSF). Then, isolates were incubated in 6 N H2SO4 for 4–6 h at 4 °C and precipitated in 100 % acetone overnight. Lastly, the pellets were washed in acetone and re-suspended in 1X SDS loading buffer.
Western blot analysis
The methylation modification status of histones was analyzed by Western blot. The extracted histone proteins were resolved on 15 % SDS-polyacrylamide gels, and subsequently transferred onto polyvinylidene fluoride fluoropolymer (PVDF) membrane using an electrophoretic blotting system (Bio-Rad). Membrane was blocked with 5 % (w/v) bovine serum albumin followed by incubation with the rabbit polyclonal primary antibodies against histone H3 (ab1791, Abcam), H3K4me2 (07–030, Millipore), H3K4me3 (07–473, Millipore), H3K9me2 (07–441, Millipore), H3K9me3 (07–442, Millipore), H3K27me2 (07–452, Millipore), H3K27me3 (07–449, Millipore), H3K36me2 (ab9049, Abcam), H3K36me3 (ab9050, Abcam) and then a goat anti-rabbit IgG secondary antibody conjugated to horseradish peroxidase (IH-0011, Dingguo). The antibody complexes on the membrane were detected by the enhanced chemilluminescence (Pierce) method. Quantification of the band intensities on the immunoblots was performed using the ImageJ software according to the instructions (http://rsb.info.nih.gov/ij/docs/menus/analyze.html#gels). All the sample intensities were first normalized to the loading control histone 3, and then calculated based on the ratio to set the intensity level of 0 h (Fig. 1) or ZH11 (Fig. 4) samples into 1.
RNA-seq analysis
For RNA-seq analysis, 14-day-old seedlings of JMJ704 T-DNA mutant and wild-type plants ZH11 under normal growth conditions were harvested for RNA-seq analysis. RNA samples were extracted using TRIzol according to the manufacturer’s instructions (Invitrogen). cDNA library that was constructed as previously described and sequenced by using Illumina HiSeqTM 2500 platform [48]. Gene expression changes between the samples were analyzed by SOAP aligner/SOAP2 software. For GO analysis of RNA-seq data, we used the GO::TermFinder software to find different expression gene enrichment [49], and choose P < 0.05 as the cutoff for significant GO terms.
Chromatin immuno-precipitation (ChIP) and ChIP-PCR
ChIP was performed as described previously [50]. Briefly, chromatin was isolated from 2 g of leaves of JMJ704 T-DNA mutant and wild-type plants respectively. After fragment sonication, the DNA/protein complex was immune-precipitated with ChIP-grade antibody against H3K4me2 (07–030, Millipore) and H3K4me3 (07–473, Millipore). After reverse cross-linking and proteinase K treatment, the immunoprecipitated DNA was extracted with phenol/chloroform. The immunoprecipitated and input DNA was performed for quantitative PCR using gene-specific primers (Additional file 5: Table S3) as described above. The quantitative PCR results were analyzed by following a method reported in the manual of Magna ChIP™ HiSens kit (Millipore). All the quantitative ChIP-PCR was performed in three biological replicates.
Availability of supporting data
The RNA-seq data described in this article have been deposited into the NCBI’s GEO database (GSE74670). Nucleic acid sequence data can be found in the Rice Genome Annotation Project website (http://rice.plantbiology.msu.edu/). The accession numbers: JMJ704 (LOC_Os05g23670), NRR (LOC_Os01g03940), OsWRKY62 (LOC_Os09g25070) and Os-11N3 (LOC_Os11g31190).
Acknowledgments
We thank Dr. Hana Mujahid of Mississippi State University, U.S.A. for the critical reviewing of the manuscript. This work was supported by Agricultural Sciences and Technologies Innovation Program, Chinese Academy of Agricultural Sciences to Shiwen Huang (Rice Pests Management Research Group) and Jian Zhang (Rice Reproductive Developmental Biology Group), and the Special Transgenic Program of the Chinese Ministry of Agriculture (2013ZX08010005).
Abbreviations
- Xoo
Xanthomonas oryzae pv. oryzae
- BB
Bacterial blight
- JmjC
Jumonji C
- H3K4me2/3
Di- or tri-methylation for histone H3 at lysine 4
- KDMs
Histone lysine demethylases
- JHDM2
JmjC domain-containing histone demethylase 2
- JMJD2
JmjC domain-containing 2
- JARID
JmjC protein containing AT-rich interaction domain
- FYRN_FYRC
N-terminal FY-rich _ terminal FY-rich
- DEGs
Differentially Expressed Genes
- ChIP
Chromatin immuno-precipitation
Additional files
Tissue-specific expression analysis of JmjC genes in rice by qRT-PCR. Ubiquitin gene was used as the internal control and Error bars indicate the SD from three technical replicates. (TIF 7231 kb)
The differenced expressed genes in jmj704 mutant plants compared with the wild type. (XLS 108 kb)
Gene ontology analysis of up- and down-regulated genes in JMJ704 T-DNA mutant detected with RNA-seq analysis. (XLSX 10 kb)
A statistic pathway enrichment analysis of differentially expressed genes between jmj704 and ZH11. The top 20 enriched pathway are selected based on the Q value. (TIF 12146 kb)
Nucleotide sequences of primers used in this study. (XLSX 12 kb)
A schematic representation showing the genomic regions of the three genes for ChIP-PCR assay. White box indicates untranslated region, black box indicates coding sequence, line through the box indicates intron region of the genes, lines and numbers above the gene indicate the regions and positions used for ChIP-PCR assay. (TIF 2795 kb)
Footnotes
Competing interests
The authors declare no competing interests.
Authors’ contributions
HY, WLY, WL, LLM, LL, SL and RQ performed the experiments and analyzed the data, HS and ZJ conceived of the project, participated in its design and coordination, and wrote the manuscript. All the authors read and approved the final manuscript.
Contributor Information
Yuxuan Hou, Email: houyuxuan@caas.cn.
Liyuan Wang, Email: wly0121@qq.com.
Ling Wang, Email: wangling03@caas.cn.
Lianmeng Liu, Email: liulianmeng@caas.cn.
Lu Li, Email: lulu9003@hotmail.com.
Lei Sun, Email: 925458987@qq.com.
Qiong Rao, Email: qiong.rao@163.com.
Jian Zhang, Email: zhangjian@caas.cn.
Shiwen Huang, Email: huangshiwen@caas.cn.
References
- 1.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447(7143):407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 2.Pfluger J, Wagner D. Histone modifications and dynamic regulation of genome accessibility in plants. Curr Opin Plant Biol. 2007;10(6):645–652. doi: 10.1016/j.pbi.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Margueron R, Trojer P, Reinberg D. The key to development: interpreting the histone code? Curr Opin Genet Dev. 2005;15(2):163–176. doi: 10.1016/j.gde.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol. 2005;6(11):838–849. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 5.Liu C, Lu F, Cui X, Cao X. Histone methylation in higher plants. Annu Rev Plant Biol. 2010;61:395–420. doi: 10.1146/annurev.arplant.043008.091939. [DOI] [PubMed] [Google Scholar]
- 6.Lan F, Nottke AC, Shi Y. Mechanisms involved in the regulation of histone lysine demethylases. Curr Opin Cell Biol. 2008;20(3):316–325. doi: 10.1016/j.ceb.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119(7):941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439(7078):811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- 9.Klose RJ, Kallin EM, Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet. 2006;7(9):715–727. doi: 10.1038/nrg1945. [DOI] [PubMed] [Google Scholar]
- 10.Jiang D, Yang W, He Y, Amasino RM. Arabidopsis relatives of the human lysine-specific Demethylase1 repress the expression of FWA and FLOWERING LOCUS C and thus promote the floral transition. Plant Cell. 2007;19(10):2975–2987. doi: 10.1105/tpc.107.052373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh V, Roy S, Giri MK, Chaturvedi R, Chowdhury Z, Shah J, et al. Arabidopsis thaliana FLOWERING LOCUS D is required for systemic acquired resistance. Mol Plant Microbe Interact. 2013;26(9):1079–1088. doi: 10.1094/MPMI-04-13-0096-R. [DOI] [PubMed] [Google Scholar]
- 12.Singh V, Roy S, Singh D, Nandi AK. Arabidopsis flowering locus D influences systemic-acquired-resistance- induced expression and histone modifications of WRKY genes. J Biosci. 2014;39(1):119–126. doi: 10.1007/s12038-013-9407-7. [DOI] [PubMed] [Google Scholar]
- 13.Luo M, Hung F-Y, Yang S, Liu X, Wu K. Histone lysine demethylases and their functions in plants. Plant Mol Biol Rep. 2013;32(2):558–565. doi: 10.1007/s11105-013-0673-1. [DOI] [Google Scholar]
- 14.Lu F, Cui X, Zhang S, Liu C, Cao X. JMJ14 is an H3K4 demethylase regulating flowering time in Arabidopsis. Cell Res. 2010;20(3):387–390. doi: 10.1038/cr.2010.27. [DOI] [PubMed] [Google Scholar]
- 15.Yang H, Han Z, Cao Y, Fan D, Li H, Mo H, et al. A companion cell-dominant and developmentally regulated H3K4 demethylase controls flowering time in Arabidopsis via the repression of FLC expression. PLoS Genet. 2012;8(4):e1002664. doi: 10.1371/journal.pgen.1002664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang H, Mo H, Fan D, Cao Y, Cui S, Ma L. Overexpression of a histone H3K4 demethylase, JMJ15, accelerates flowering time in Arabidopsis. Plant Cell Rep. 2012;31(7):1297–1308. doi: 10.1007/s00299-012-1249-5. [DOI] [PubMed] [Google Scholar]
- 17.Lu F, Li G, Cui X, Liu C, Wang XJ, Cao X. Comparative analysis of JmjC domain-containing proteins reveals the potential histone demethylases in Arabidopsis and rice. J Integr Plant Biol. 2008;50(7):886–896. doi: 10.1111/j.1744-7909.2008.00692.x. [DOI] [PubMed] [Google Scholar]
- 18.Sun Q, Zhou DX. Rice jmjC domain-containing gene JMJ706 encodes H3K9 demethylase required for floral organ development. Proc Natl Acad Sci U S A. 2008;105(36):13679–13684. doi: 10.1073/pnas.0805901105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou D, Hu Y. Regulatory function of histone modifications in controlling rice gene expression and plant growth. Rice. 2010;3:103–111. doi: 10.1007/s12284-010-9045-8. [DOI] [Google Scholar]
- 20.Chen X, Hu Y, Zhou DX. Epigenetic gene regulation by plant Jumonji group of histone demethylase. Biochim Biophys Acta. 2011;1809(8):421–426. doi: 10.1016/j.bbagrm.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Cui X, Jin P, Gu L, Lu Z, Xue Y, Wei L, et al. Control of transposon activity by a histone H3K4 demethylase in rice. Proc Natl Acad Sci U S A. 2013;110(5):1953–1958. doi: 10.1073/pnas.1217020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Q, Chen X, Wang Q, Zhang F, Lou Z, Zhang Q, et al. Structural basis of a histone H3 lysine 4 demethylase required for stem elongation in rice. PLoS Genet. 2013;9(1):e1003239. doi: 10.1371/journal.pgen.1003239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li T, Chen X, Zhong X, Zhao Y, Liu X, Zhou S, et al. Jumonji C domain protein JMJ705-mediated removal of histone H3 lysine 27 trimethylation is involved in defense-related gene activation in rice. Plant Cell. 2013;25(11):4725–4736. doi: 10.1105/tpc.113.118802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berr A, McCallum EJ, Alioua A, Heintz D, Heitz T, Shen WH. Arabidopsis histone methyltransferase SET DOMAIN GROUP8 mediates induction of the jasmonate/ethylene pathway genes in plant defense response to necrotrophic fungi. Plant Physiol. 2010;154(3):1403–1414. doi: 10.1104/pp.110.161497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berr A, Menard R, Heitz T, Shen WH. Chromatin modification and remodelling: a regulatory landscape for the control of Arabidopsis defence responses upon pathogen attack. Cell Microbiol. 2012;14(6):829–839. doi: 10.1111/j.1462-5822.2012.01785.x. [DOI] [PubMed] [Google Scholar]
- 26.Palma K, Thorgrimsen S, Malinovsky FG, Fiil BK, Nielsen HB, Brodersen P, et al. Autoimmunity in Arabidopsis acd11 is mediated by epigenetic regulation of an immune receptor. PLoS Pathog. 2010;6(10):e1001137. doi: 10.1371/journal.ppat.1001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarez-Venegas R, Sadder M, Hlavacka A, Baluska F, Xia Y, Lu G, et al. The Arabidopsis homolog of trithorax, ATX1, binds phosphatidylinositol 5-phosphate, and the two regulate a common set of target genes. Proc Natl Acad Sci U S A. 2006;103(15):6049–6054. doi: 10.1073/pnas.0600944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alvarez-Venegas R, Abdallat AA, Guo M, Alfano JR, Avramova Z. Epigenetic control of a transcription factor at the cross section of two antagonistic pathways. Epigenetics. 2007;2(2):106–113. doi: 10.4161/epi.2.2.4404. [DOI] [PubMed] [Google Scholar]
- 29.Verdier V, Vera Cruz C, Leach JE. Controlling rice bacterial blight in Africa: needs and prospects. J Biotechnol. 2012;159(4):320–328. doi: 10.1016/j.jbiotec.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Sundaram R, Chatterjee S, Oliva R, Laha G, Cruz C, Leach J, et al. Update on bacterial blight of rice: Fourth international conference on bacterial blight. Rice. 2014;7:12. doi: 10.1186/s12284-014-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Li C, Wu C, Xiong L, Chen G, Zhang Q, et al. RMD: a rice mutant database for functional analysis of the rice genome. Nucleic Acids Res. 2006;34(Database issue):D745–748. doi: 10.1093/nar/gkj016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeon JS, Lee S, Jung KH, Jun SH, Jeong DH, Lee J, et al. T-DNA insertional mutagenesis for functional genomics in rice. Plant J. 2000;22(6):561–570. doi: 10.1046/j.1365-313x.2000.00767.x. [DOI] [PubMed] [Google Scholar]
- 33.Chern M, Canlas PE, Fitzgerald HA, Ronald PC. Rice NRR, a negative regulator of disease resistance, interacts with Arabidopsis NPR1 and rice NH1. Plant J. 2005;43(5):623–635. doi: 10.1111/j.1365-313X.2005.02485.x. [DOI] [PubMed] [Google Scholar]
- 34.Peng Y, Bartley LE, Chen X, Dardick C, Chern M, Ruan R, et al. OsWRKY62 is a negative regulator of basal and Xa21-mediated defense against Xanthomonas oryzae pv. oryzae in rice. Mol Plant. 2008;1(3):446–458. doi: 10.1093/mp/ssn024. [DOI] [PubMed] [Google Scholar]
- 35.Antony G, Zhou J, Huang S, Li T, Liu B, White F, et al. Rice xa13 recessive resistance to bacterial blight is defeated by induction of the disease susceptibility gene Os-11N3. Plant Cell. 2010;22(11):3864–3876. doi: 10.1105/tpc.110.078964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang H, Liu X, Thorn G, Duan J, Tian L. Expression analysis of histone acetyltransferases in rice under drought stress. Biochem Biophys Res Commun. 2014;443(2):400–405. doi: 10.1016/j.bbrc.2013.11.102. [DOI] [PubMed] [Google Scholar]
- 37.Mosher RA, Durrant WE, Wang D, Song J, Dong X. A comprehensive structure-function analysis of Arabidopsis SNI1 defines essential regions and transcriptional repressor activity. Plant Cell. 2006;18(7):1750–1765. doi: 10.1105/tpc.105.039677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaskiewicz M, Conrath U, Peterhansel C. Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep. 2011;12(1):50–55. doi: 10.1038/embor.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De-La-Pena C, Rangel-Cano A, Alvarez-Venegas R. Regulation of disease-responsive genes mediated by epigenetic factors: interaction of Arabidopsis-Pseudomonas. Mol Plant Pathol. 2012;13(4):388–398. doi: 10.1111/j.1364-3703.2011.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim KC, Lai Z, Fan B, Chen Z. Arabidopsis WRKY38 and WRKY62 transcription factors interact with histone deacetylase 19 in basal defense. Plant Cell. 2008;20(9):2357–2371. doi: 10.1105/tpc.107.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi SM, Song HR, Han SK, Han M, Kim CY, Park J, et al. HDA19 is required for the repression of salicylic acid biosynthesis and salicylic acid-mediated defense responses in Arabidopsis. Plant J. 2012;71(1):135–146. doi: 10.1111/j.1365-313X.2012.04977.x. [DOI] [PubMed] [Google Scholar]
- 42.Ding B, Bellizzi Mdel R, Ning Y, Meyers BC, Wang GL. HDT701, a histone H4 deacetylase, negatively regulates plant innate immunity by modulating histone H4 acetylation of defense-related genes in rice. Plant Cell. 2012;24(9):3783–3794. doi: 10.1105/tpc.112.101972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park CJ, Ronald PC. Cleavage and nuclear localization of the rice XA21 immune receptor. Nat Commun. 2012;3:920. doi: 10.1038/ncomms1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Guo D, Chang Y, You C, Li X, Dai X, et al. Non-random distribution of T-DNA insertions at various levels of the genome hierarchy as revealed by analyzing 13 804 T-DNA flanking sequences from an enhancer-trap mutant library. Plant J. 2007;49(5):947–959. doi: 10.1111/j.1365-313X.2006.03001.x. [DOI] [PubMed] [Google Scholar]
- 45.Chen H, Wang S, Zhang Q. New gene for bacterial blight resistance in rice located on chromosome 12 identified from minghui 63, an elite restorer line. Phytopathology. 2002;92(7):750–754. doi: 10.1094/PHYTO.2002.92.7.750. [DOI] [PubMed] [Google Scholar]
- 46.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 47.Jackson JP, Johnson L, Jasencakova Z, Zhang X, PerezBurgos L, Singh PB, et al. Dimethylation of histone H3 lysine 9 is a critical mark for DNA methylation and gene silencing in Arabidopsis thaliana. Chromosoma. 2004;112(6):308–315. doi: 10.1007/s00412-004-0275-7. [DOI] [PubMed] [Google Scholar]
- 48.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10(1):57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyle EI, Weng S, Gollub J, Jin H, Botstein D, Cherry JM, et al. GO::TermFinder--open source software for accessing Gene Ontology information and finding significantly enriched Gene Ontology terms associated with a list of genes. Bioinformatics. 2004;20(18):3710–3715. doi: 10.1093/bioinformatics/bth456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang J, Nallamilli BR, Mujahid H, Peng Z. OsMADS6 plays an essential role in endosperm nutrient accumulation and is subject to epigenetic regulation in rice (Oryza sativa) Plant J. 2010;64(4):604–617. doi: 10.1111/j.1365-313X.2010.04354.x. [DOI] [PubMed] [Google Scholar]


