Abstract
Objective
To determine the effects of symptoms and presence of confirmed influenza on intention to receive an influenza vaccine, specifically in patients recovering from a medically-attended acute (≤ 7 days’ duration) respiratory illness (ARI).
Methods
During the 2013–2014 influenza season, individuals seeking outpatient care for an ARI that included cough were tested for influenza using reverse transcription polymerase chain reaction assays (PCR) and completed surveys. Children (6 months–18 years) and adults (≥ 18 years) were grouped by their combined current season’s influenza vaccination status (vaccinated/not vaccinated) and their vaccination intentions for next season (intend/do not intend).
Results
Forty-one percent (323/786) were unvaccinated at enrollment, of whom nearly half (151/323) intended to be vaccinated next season. When adjusting for demographic, health and other factors, unvaccinated individuals who intended to be vaccinated next season were approximately 1.5 times more likely to have PCR-confirmed influenza compared with vaccinated individuals who intended to be vaccinated next season.
Conclusion
The combined experience of not being vaccinated against influenza and seeking medical attention for an ARI seemed to influence approximately one-half of unvaccinated participants to consider influenza vaccination for next season.
Keywords: intention to vaccinate, influenza vaccination
INTRODUCTION
Suboptimal influenza vaccination rates among certain population groups including older children, adolescents, and young and middle age adults1 confirm the persistence of barriers to vaccine receipt. Since implementation of the ACIP’s 2008 universal recommendations for annual influenza vaccination,2 some barriers including access, cost, and not knowing that influenza vaccine was recommended, have been mitigated by factors such as broader insurance coverage for influenza vaccination and expanding venues, eg, retail outlets, offering influenza vaccination.3–5
Behavioral factors that may either facilitate or interfere with vaccination have remained, and include individual attitudes, social support, and perceived benefits and risks of vaccination. For example, a study of self-reported and medical record-documented influenza vaccination have found that the likelihood of influenza vaccination among healthcare personnel is associated with an increased perception of emotional benefits including less worry about contracting influenza.6 Among community-dwelling adults, obtaining influenza vaccination was associated with higher perceived susceptibility to influenza infection, perceived benefits of vaccination, and cues to action such as doctor recommendation.7 Influenza vaccination of children was associated with higher parental perception of severity of disease and of benefits of vaccination.7 Children’s vaccine uptake was higher when their parents: 1) wished to prevent influenza and its symptoms; 2) had a history of influenza vaccination (for themselves or their children); or 3) had had to miss work to take care of a child with influenza.8
Behavioral factors are also associated with intention to receive the influenza vaccine. A study among adults identified significant associations between intention to receive the 2009 pandemic H1N1 influenza vaccine and increased positive attitude towards the vaccine, increased benefits of vaccination, increased perceived control, increased susceptibility to infection, increased severity of disease, and increased anticipated regret if they were not vaccinated.9 Another study among older adults found that intention to receive the seasonal influenza vaccine was associated with positive attitudes, social support for vaccination, past vaccination behavior and anticipated regret if they were not vaccinated.10
Despite this evidence that influenza vaccination behavior is in part, shaped by avoidance of influenza illness and anticipated regret if not vaccinated, previous studies have included individuals without regard to their history of influenza or influenza-like illness. The present study examined intention to receive influenza vaccine in the season after recovering from an acute respiratory infection (ARI) for which outpatient medical care was sought. The purpose was to compare the characteristics of those who were not currently vaccinated but intended to receive influenza vaccine next season with those who expressed no change in their intention to receive/not receive influenza vaccine next season.
METHODS
This study was approved by the University of Pittsburgh Institutional Review Board.
Participants
Participants provided informed consent and were enrolled in the University of Pittsburgh’s center for the US Flu VE Network study, described previously.11 Eligibility criteria included age ≥ 6 months as of 9/1/2013, presentation at one of the participating primary care or urgent care centers for treatment of an upper respiratory illness with cough, of ≤ 7 days duration, and no history of taking an influenza antiviral medication (oseltamivir or zanamivir) for this illness.
Demographic and Other Variables
Participants completed a survey at enrollment from which age, race, health insurance type, employment status (adults), personal smoking status and household smoking (someone in the household smokes), household composition, asthma diagnosis, exercise frequency, influenza vaccination status, subjective social status (measured using a 10-point scale comparing one’s overall life situation with others), symptoms of ARI, overall health rating before ARI, and severity of illness on day of enrollment (measured using a 100-point visual analog scale) were recorded. Body mass index (BMI) was calculated from self-reported height and weight. Influenza vaccination status was assessed using both the electronic medical record (EMR) data and self-report. Time to recovery, loss of productivity and intention to receive influenza vaccine next season were assessed on the follow-up survey that was completed by participants at least 7 days post enrollment. Influenza infection was detected using the Centers for Disease Control and Prevention’s real-time reverse transcription polymerase chain reaction (PCR) method described previously.12 Because of the lag time between specimen collection, PCR analysis for influenza, reporting back to the physician’s office, and optional reporting of results by physician to patients, participants were unlikely to have been aware of their influenza status at the time of survey completion that was ≥ 7 days post enrollment.
Statistical Analyses
Study data were collected during the 2013–2014 influenza season and managed using REDCap electronic data capture tools.13 Data were analyzed with SAS v9.2 (SAS Institute, Inc., Cary, NC). The outcome variable was created by classifying participants into 3 groups: 1) did not receive influenza vaccine in the current season and does not intend to receive it in the following season (Unvaccinated-no intention); 2) did not receive influenza vaccine in the current season and intends to receive it in the following season (Unvaccinated-vaccination intention); and 3) received influenza vaccine this season and intends to receive it next season (Vaccinated-vaccination intention) (Figure). Participants who received influenza vaccine this season and do not intend to receive it next season were excluded due to a small cell size for both children and adults. Analyses were performed separately on children (age < 18 years) and adults (age ≥ 18 years) because of different follow-up outcomes (ie, school vs. work absenteeism for children and adults, respectively).
Figure.
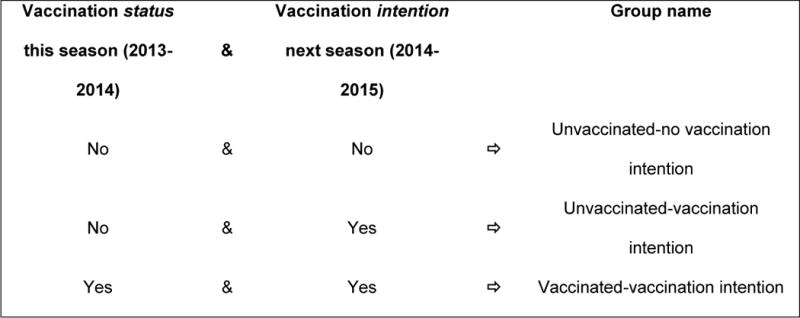
Nomenclature for Analysis Groups Based on Receipt of Influenza Vaccine During the Study Season (2013–14) and Intention to Receive the Vaccine Next Season (2014–2015)
Summary statistics of baseline demographics, social and health measures, symptoms and severity are presented as means and standard deviations for continuous variables (baseline severity) and percentages for discrete variables (race). One-way analysis of variance (ANOVA) was used to compare continuous variables across the 3 groups using the F statistic and chi-square tests were used to compare the discrete variables across the 3 groups. If significant differences were detected, post-hoc, pairwise comparisons were made with Bonferroni corrections (p value < .05/3 for 3 comparisons to indicate statistical significance). Multinomial regression models were used to assess the association of intention to receive influenza vaccine in the 3 groups, adjusted for the significant factors from univariate comparisons, with unadjusted and adjusted odds ratios (OR) and 95% confidence intervals (CI) reported. Overall, an alpha level of 0.05 was used to indicate statistical significance.
RESULTS
Of the total 1207 participants enrolled in the study, 800 returned completed surveys, for a total return rate of 66%. The excluded cases were 3 children and 11 adults, who were vaccinated and did not intend to receive the influenza vaccine in the following season. The final numbers for this analysis were 173 children and 613 adults. The participants were predominantly white, non-Hispanic, privately insured, nonsmokers, employed adults, and reported very good general health and moderately-good subjective social status (Table 1).
Table 1.
Demographic Characteristics of Participants at Enrollment
| Characteristics | Total N = 786 |
Child < 18 years N = 173 (22%) |
Adult ≥ 18 years N = 613 (78%) |
p value |
|---|---|---|---|---|
| Age Group, % | — | |||
| 6 months–4 years | 10.3 | 46.8 | – | |
| 5–17 years | 11.7 | 53.2 | – | |
| 18–49 years | 48.1 | – | 61.7 | |
| ≥ 50 years | 29.9 | – | 38.3 | |
| Sex, % | .001 | |||
| Male participants | 43.0 | 54.3 | 39.8 | |
| Female participants | 57.0 | 45.7 | 60.2 | |
| Race, % | < .001 | |||
| White | 90.8 | 82.9 | 93.0 | |
| Black | 7.9 | 15.3 | 5.9 | |
| Others | 1.3 | 1.8 | 1.1 | |
| Not Hispanic, % | 98.6 | 95.9 | 99.3 | .001 |
| Insurance Status, % | < .001 | |||
| Public | 22.2 | 49.1 | 14.7 | |
| Private | 71.2 | 47.3 | 77.9 | |
| Both | 4.8 | 3.0 | 5.3 | |
| None | 1.8 | 0.6 | 2.1 | |
| Child attends school outside home | – | 65.3 | – | |
| Currently employed | – | – | 71.3 | – |
| Subjective Social Status, %; range = 1 (low) to 9 = (high) |
.02 | |||
| 1–4 | 15.6 | 12.9 | 16.4 | |
| 5 | 31.2 | 41.2 | 28.4 | |
| 6 | 25.0 | 22.4 | 25.8 | |
| 7–9 | 28.2 | 23.5 | 29.4 | |
| Self-reported health status, % | < .001 | |||
| Fair/Poor | 6.9 | 1.2 | 8.5 | |
| Good | 24.7 | 9.8 | 28.9 | |
| Very Good | 42.0 | 37.6 | 43.2 | |
| Excellent | 26.4 | 51.4 | 19.4 | |
| Smoker, % | – | – | 13.6 | |
| Household smoking, % | 13.5 | 16.8 | 12.6 | .15 |
| Asthma diagnosis, % | 21.2 | 19.1 | 21.8 | .44 |
| Any high risk condition, % | 28.1 | 22.5 | 29.7 | .06 |
| Vaccination Status for 2013–14/2014–15, % | < .001 | |||
| No current/No intention | 22.1 | 12.7 | 24.8 | |
| No current/Yes intention | 19.2 | 16.2 | 20.1 | |
| Yes current/Yes intention | 58.7 | 71.1 | 55.1 |
Demographic Characteristics
Children and adults differed on several measures. The adult sample had a greater proportion of female participants, whites, and privately insured individuals, and reported higher subjective social status scores and fewer numbers in the consistently vaccinated group. Among children, 13% were in the Unvaccinated-no intention group, 16% were in the Unvaccinated-vaccination intention group, and 71% were in the Vaccinated-vaccination intention group. Among adults, 25% were in the Unvaccinated-no intention group, 20% were in the Unvaccinated-vaccination intention group and 55% were in the Vaccinated-vaccination intention group.
Characteristics by Current Vaccination-vaccination Intention Group
For children, few differences among the 3 vaccination groups were evident (Table 2). In the Unvaccinated-vaccination intention group there was a significantly smaller percentage of boys (42.9%) than girls (57.1%; p < .04). Moreover, in this group, the illness itself seemed to have been related to parents’ decision to have their children vaccinated next season; 36% had confirmed influenza compared with 11% in the Vaccinated-vaccination intention group and 13% in the Unvaccinated-no intention group (p = .01). Those who were in the Unvaccinated-vaccination intention group were more likely than those in the Vaccinated-vaccination intention group to report experiencing fatigue and having lower ability to perform regular activities while sick (p < .016).
Table 2.
Demographic, Social, Health and Other Characteristics of Children and Adults by Current and Intended Influenza Vaccination Behavior (Unvaccinated-no Intention, Unvaccinated-vaccination intention, and Vaccinated-vaccination Intention)
|
|
Children <18 years
|
p value | Adults ≥ 18 years
|
p value | ||||
|---|---|---|---|---|---|---|---|---|
|
|
Vaccination status in 2013–2014 and vaccination intent in 2014–2015 |
Vaccination status in 2013–2014 and vaccination intent in 2014–2015 |
||||||
| Unvaccinated -no intention |
Unvaccinated -vaccination intention |
Vaccinated- vaccination intention |
Unvaccinated -no intention |
Unvaccinated -vaccination intention |
Vaccinated -vaccination intention |
|||
|
|
|
|||||||
| Characteristics | N = 22, 12.7% | N = 28, 16.2% | N = 123, 71% | N = 152, 24.8% | N = 123, 20.0% | N = 338, 55% | ||
| Age Group, % | .11 | < .001 | ||||||
| 6 mo-4 years | 27.3 | 42.9 | 51.2 | – | – | – | ||
| 5–17 years | 72.7 | 57.1 | 48.8 | – | – | – | ||
| 18–49 years | – | – | – | 73.7‡ | 74.0* | 51.8‡* | ||
| ≥ 50 years | – | – | – | 26.3 | 26.0 | 48.2 | ||
| Sex, % | .04 | .005 | ||||||
| Male participants | 77.3† | 42.9† | 52.8 | 44.1 | 49.6* | 34.3* | ||
| Female participants | 22.7 | 57.1 | 47.2 | 55.9 | 50.4 | 65.7 | ||
| Race, % | .39 | .71 | ||||||
| White | 86.4 | 75.0 | 84.2 | 93.3 | 90.3 | 93.8 | ||
| Black | 9.1 | 25.0 | 14.2 | 6.0 | 8.1 | 5.0 | ||
| Other | 4.5 | 0.0 | 1.6 | 0.7 | 1.6 | 1.2 | ||
| Not Hispanic, % | 100.0 | 100.0 | 94.2 | — | 100.0 | 100.0 | 98.8 | — |
| Insurance Status, % | .22 | .03 | ||||||
| Public | 27.3 | 57.1 | 51.3 | 11.3‡ | 11.6 | 17.3‡ | ||
| Private | 63.6 | 42.9 | 45.4 | 82.0 | 82.6 | 74.3 | ||
| Both | 9.1 | 0.0 | 2.5 | 2.7 | 3.3 | 7.2 | ||
| None | 0.0 | 0.0 | 0.8 | 4.0 | 2.5 | 1.2 | ||
| Child attends school outside home, % | 66.7 | 60.7 | 66.1 | .85 | – | – | – | |
| Adult currently employed, % | – | – | – | 75.5 | 77.5 | 67.2 | .05 | |
| Social Status, %; range = 1 (low) to 9 (high) |
.91 | .05 | ||||||
| 1–4 | 4.5 | 17.9 | 13.3 | 21.5 | 18.7 | 12.3 | ||
| 5 | 45.5 | 39.3 | 40.8 | 32.2 | 23.6 | 28.5 | ||
| 6 | 22.7 | 21.4 | 22.5 | 24.8 | 29.3 | 24.9 | ||
| 7–9 | 27.3 | 21.4 | 23.3 | 21.5 | 28.4 | 33.3 | ||
| Self-reported health status, % | .79 | .03 | ||||||
| Fair/Poor | 0.0 | 3.6 | 0.8 | 7.9 | 4.1* | 10.4* | ||
| Good | 4.5 | 7.1 | 11.4 | 26.3 | 25.2 | 31.3 | ||
| Very Good | 40.9 | 39.3 | 36.6 | 42.8 | 43.9 | 43.2 | ||
| Excellent | 54.6 | 50.0 | 51.2 | 23.0 | 26.8 | 15.1 | ||
| Smoker, % | – | – | – | 20.4‡ | 13.5 | 10.5‡ | .01 | |
| Smoker in household, % | 9.1 | 17.9 | 17.9 | .59 | 15.8 | 15.5 | 10.1 | .12 |
| Asthma diagnosis, % | 13.6 | 25.0 | 18.7 | .58 | 21.7 | 17.1 | 23.5 | .33 |
| Any high risk condition, % | 13.6 | 17.9 | 25.2 | .39 | 16.5‡ | 15.5* | 40.8‡* | < .001 |
| At enrollment: | ||||||||
| Symptoms in addition to cough, % | ||||||||
| Fever | 68.2 | 67.9 | 68.3 | .99 | 48.0† | 66.7†* | 43.2* | < .001 |
| Fatigue | 81.8 | 85.7* | 56.9* | .003 | 77.0 | 86.0 | 78.7 | .08 |
| Wheezing | 13.6 | 32.1 | 31.7 | .22 | 34.9 | 43.1 | 39.1 | .38 |
| Sore throat | 50.0 | 64.3 | 48.8 | .33 | 79.6 | 74.0 | 72.2 | .22 |
| Nasal Congestion | 77.3 | 96.4 | 87.0 | .13 | 77.6 | 79.7 | 82.0 | .52 |
| Shortness of breath | 27.3 | 17.9 | 32.5 | .30 | 51.3 | 50.4 | 53.0 | .87 |
| Any other symptom | 100.0 | 100.0 | 98.4 | — | 99.3 | 99.2 | 98.5 | .68 |
| Ability perform usual activities, % | .04 | .005 | ||||||
| Not at all (0–5) | 45.4 | 57.2* | 30.3* | 51.3 | 63.4* | 45.4* | ||
| Somewhat (6–8) | 27.3 | 35.7 | 41.0 | 38.2 | 29.3 | 38.3 | ||
| Able to perform (9) | 27.3 | 7.1 | 28.7 | 10.5 | 7.3 | 16.3 | ||
| Sleep Quality, % | .45 | .48 | ||||||
| Worst (0–4) | 50.0 | 60.7 | 49.5 | 62.3 | 67.5 | 57.7 | ||
| Mild (5–6) | 27.3 | 21.4 | 16.3 | 19.2 | 17.9 | 23.1 | ||
| Moderate (7–8) | 18.2 | 10.7 | 17.1 | 11.9 | 9.8 | 14.5 | ||
| Normal (9) | 4.5 | 7.2 | 17.1 | 6.6 | 4.8 | 4.7 | ||
| Illness rating, mean (SD); range = 0 (worst) to 100 (best) | 73.7 (14.4) | 60.8 (21.0) | 66.6 (20.4) | .07 | 58.3 (18.9) | 54.2 (20.4)* | 62.0 (18.5)* | < .001 |
| At follow-up: | ||||||||
| Child missed school, % | 92.9 | 94.1 | 86.3 | .56 | – | – | – | |
| Days child missed school, mean (SD) | 2.5 (2.1) | 2.4 (1.6) | 2.0 (1.8) | .62 | – | – | – | |
| Adult missed work due to child’s illness, % | 46.7 | 26.1 | 34.4 | .43 | – | – | – | |
| Days adult missed work due to child’s illness, mean (SD) | 2.7 (2.0) | 2.3 (1.4) | 2.1 (1.5) | .59 | – | – | – | |
| Productivity at work, % | .13 | |||||||
| No effect (0–3) | – | – | – | 30.5 | 19.8 | 34.1 | ||
| Moderate effect (4–7) | – | – | – | 45.7 | 46.5 | 41.7 | ||
| Worst effect (8–10) | – | – | – | 23.8 | 33.7 | 24.2 | ||
| Work hours missed due to illness mean (SD) | – | – | – | 12.8 (11.9)† | 18.0 (13.9)† | 13.6 (16.7) | .04 | |
| Another adult missed work due to adult’s illness, % | – | – | – | 9.9 | 17.8 | 10.0 | .12 | |
| PCR-confirmed influenza | 13.6 | 35.7* | 11.4* | .01 | 22.4† | 42.8†* | 20.7* | < .001 |
Significant difference between Unvaccinated-vaccination intention group and Vaccinated-vaccination intention group at p < .016 (0.05/3, Bonferroni correction)
Significant difference between Unvaccinated-no intention group and Unvaccinated-vaccination intention group at p < .016 (0.05/3, Bonferroni correction)
Significant difference between Unvaccinated-no intention group and Vaccinated-vaccination intention group at p < .016 (0.05/3, Bonferroni correction)
Demographic and illness differences were more common across adult vaccination groups (Table 2). In contrast to Unvaccinated-no intention adults, those who were vaccinated and planned to be vaccinated next season were older (≥ 50 years), not employed, publicly insured, with more high risk conditions and generally scored higher on the subjective social status scale. Compared with Vaccinated-vaccination intention adults, those in the Unvaccinated-vaccination intention group were younger, reported better self-rated health and fewer high risk conditions, presented with fever more frequently, were less able to perform their regular activities while sick and felt worse at enrollment. They also more frequently tested positive for influenza. The Unvaccinated-vaccination intention group also more often presented with fever, tested positive for influenza and missed more work time due to illness (all p < .016) compared with the Unvaccinated-no intention group.
Predictors of Vaccination Intention
In multinomial logistic regression analyses (adjusting for significant variables identified in the univariate analyses), the likelihood of being in the Unvaccinated-no intention group of children was positively associated with only one variable: reported fatigue at enrollment. That is, Unvaccinated-no intention children were 1.93 times more likely to report fatigue than Vaccinated-vaccination intention children. Children in the Unvaccinated-vaccination intention group were nearly twice as likely as the Vaccinated-vaccination intention group to have confirmed influenza (OR = 1.70; 95% CI = 1.02–2.82).
When adjusting for other variables, Unvaccinated-no intention adults were more likely to be men (OR = 1.41; 95% CI = 1.08–1.84) and smokers (OR = 1.72; 95% CI = 1.17–2.54), and less likely to have another high risk condition (OR = 0.50; 95%CI = 0.36–0.73) than Vaccinated-vaccination intention adults. Unvaccinated-vaccination intention adults were less likely to have a high risk condition (OR = 0.53 95%CI = 0.36–0.80), and more likely to have confirmed influenza (OR = 1.41; 95%CI = 1.03–1.93) than Vaccinated-vaccination intention adults.
DISCUSSION
Among approximately 800 individuals seeking outpatient medical care for an acute respiratory infection during the 2013–2014 influenza season who completed a follow-up survey, the percentages of participants vaccinated against influenza were higher than national vaccination rates for both children (approximately 70% vs. 57% nationally) and adults (approximately 55% vs. 42% nationally).1 These higher rates may be partially explained by the fact that the study was conducted in primary care and urgent care centers where enrollees were more likely to have received care in the past that included opportunities for influenza vaccination.
In this study, the unvaccinated participants who intended to receive influenza vaccine next season more often reported fever, fatigue, less ability to perform usual activities, feeling worse at enrollment and missing more hours of work because of the illness. Previous research using the same methodology has shown that confirmed influenza compared with other respiratory viruses is more often associated with fever,16 and is associated with a longer time to return to normal activities.17 In adjusted multivariate regression analyses, the presence of unrevealed but PCR-confirmed influenza was the only significant correlate of influenza vaccination intention among currently unvaccinated children. Presence of confirmed influenza and lack of a high risk condition were significant correlates of influenza vaccination intention among unvaccinated adults.
The combined experience of not being vaccinated and being sick enough with an ARI to seek medical attention seemed to influence approximately one half of unvaccinated participants to consider vaccination next season. This finding aligns with adults’ reported anticipated regret for not receiving influenza vaccine,9,10 perhaps because of their previous experience with actual influenza infection.
Previous research has determined that habit is best predictor for future influenza vaccination; those who have received an influenza vaccine in the past are significantly more likely to report intention to receive influenza vaccine in the future.6,8,10,14 Attitudes, social support, perceived susceptibility, severity, and benefits have been shown to relate to both intention to vaccinate and actual vaccine uptake.6,9,10,15 The reasons for vaccination initiation may be more complex.
This study was not designed using a particular behavioral construct because only one behavioral question was asked; thus, the behavioral factors to which the influenza vaccination intention can be attributed are unknown. Those who changed their minds about vaccination may have also altered their perceived susceptibility to influenza, perceived severity of influenza and perceived benefits of influenza vaccination (all constructs of the Health Belief Model), as a result of their illness.
Strengths and Limitations
We did not assess whether being unvaccinated during the influenza season of this study year was a single aberration from a usual pattern of annual vaccination or confirmation of usual practice. If the former were true, inclusion of usual vaccine recipients who missed vaccination this season among the unvaccinated who intended to be vaccinated group, the reported odds ratios would underestimate the actual association with confirmed influenza infection. This study was conducted in a single region where the demographic and behavioral characteristics may not reflect a broader population. However, it is the first study to examine vaccination intention change behavior among those recovering from an acute respiratory illness.
Conclusion
Among individuals who sought outpatient medical care for an acute respiratory infection and were unvaccinated against influenza, those with confirmed influenza were more likely to report their intention to receive influenza vaccination next season. The severity of symptoms associated with influenza may have contributed to this decision.
Table 3.
Likelihood of Self-reporting Unvaccinated-no Intention and Self-reporting Unvaccinated-vaccination Intention Compared to Self-reporting Vaccinated-vaccination Intention, in Multivariate Logistic Regression, Adjusting for PCR-confirmed Influenza, Demographics and Symptoms
| Children < 18 years | Adults ≥ 18 years | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Vaccination status in 2013–2014 and vaccination intent in 2014–2015 | Vaccination status in 2013–2014 and vaccination intent in 2014–2015 | |||||
|
| ||||||
| Unvaccinated-no intention | Unvaccinated-vaccination intention | Unvaccinated-no intention | Unvaccinated-vaccination intention | |||
| Model details: | OR (95% CI) | OR (95% CI) | p value | OR (95% CI) | OR (95% CI) | p value |
| Unadjusted Model | ||||||
|
| ||||||
| PCR-confirmed influenza, ref. = influenza negative | 1.11 (0.57–2.17) | 2.08 (1.29–3.35) | 0.009 | 1.05 (0.83–1.32) | 1.67 (1.34–2.09) | < 0.001 |
|
| ||||||
| Adjusted Model | ||||||
|
| ||||||
| PCR-confirmed influenza, ref. = influenza negative | 0.97 (0.48–1.98) | 1.70 (1.02–2.82) | 0.10 | 0.87 (0.62–1.20) | 1.41 (1.03–1.93) | 0.02 |
| Male sex, ref. = female | 1.78 (1.03–3.06) | 0.77 (0.49–1.20) | 0.03 | 1.41 (1.08–1.84) | 1.31 (0.99–1.74) | 0.02 |
| Age group 18–49 yrs., ref. = ≥ 50 yrs. | – | – | 1.25 (0.95–1.66) | 1.27 (0.94–1.71) | 0.15 | |
| Has health insurance, ref. = no health insurance | – | – | 0.99 (0.56–1.77) | 0.78 (0.41–1.48) | 0.72 | |
| Subjective social status | – | – | ||||
| Low (1–4), ref. = high (7–9) | – | – | 1.60 (0.86–2.98) | 1.33 (0.68–2.59) | 0.19 | |
| Medium (5), ref. = high (7–9) | – | – | 1.36 (0.88–2.10) | 0.97 (0.60–1.59) | ||
| Moderate (6), ref. = high (7–9) | – | – | 0.73 (0.46–1.15) | 1.08 (0.68–1.72) | ||
| Smoker, ref. = nonsmoker | – | – | 1.72 (1.17–2.54) | 1.23 (0.78–1.95) | 0.02 | |
| Self-reported health status | – | – | 0.72 | |||
| Fair/Poor vs. Excellent | – | – | 0.53(0.15–1.85) | 0.88(0.27–2.84) | ||
| Good vs. Excellent | – | – | 0.88(0.50–1.57) | 0.88(0.49–1.58) | ||
| Very Good vs. Excellent | – | – | 1.28(0.75–2.20) | 1.02(0.60–1.75) | ||
| Has a high risk condition, ref. =no high risk condition | – | – | 0.50 (0.35–0.73) | 0.53 (0.36–0.80) | < 0.001 | |
| Reported fatigue at enrollment, ref. = no fatigue | 1.93 (1.03–3.61) | 1.63 (0.89–2.98) | 0.04 | – | – | |
| Reported fever at enrollment, ref. = no fever | – | – | 0.97 (0.74–1.29) | 1.31 (0.97–1.78) | 0.15 | |
| Illness rating at enrollment, range = 0 (worst) to 100 (best) | – | – | 1.00 (0.98–1.02) | 1.00 (0.98–1.01) | ||
| No ability to perform usual activity, ref. = able to perform usual activity | 1.11 (0.56–2.19) | 1.79 (0.87–3.67) | 0.38 | 0.94 (0.60–1.48) | 0.99 (0.60–1.64) | 0.98 |
| Moderate ability to perform usual activity, ref. = able to perform usual activity | 0.64 (0.32–1.29) | 1.19 (0.59–2.40) | 0.92 (0.62–1.35) | 0.99 (0.63–1.54) | 0.84 | |
| Work hours missed due to illness | – | – | 0.99 (0.97–1.02) | 1.01 (0.99–1.03) | 0.46 | |
Reference group is Vaccinated-vaccination intention (vaccinated this season and intends to be vaccinated next season)
Acknowledgments
This investigation was supported by the grant U01 IP000467 from the Centers for Disease Control and Prevention and is subject to the CDC’s Open Access Policy. The views expressed herein are those of those authors and not those of the funding agency. The CTSI infrastructure was also supported by the National Institutes of Health through Grant Numbers UL1 RR024153 and UL1TR000005. This study does not have a clinical trial number because it is an observational study.
Footnotes
Human Subjects Statement
This study was approved by the University of Pittsburgh Institutional Review Board.
Conflict of Interest Statement
Dr. Zimmerman has a research grant from Sanofi Pasteur, Inc. Drs. Zimmerman and Nowalk have a pending research grant from Merck & Co, Inc. and research grant funding from Pfizer, Inc. The other authors have no conflicts to report.
Contributor Information
Mary Patricia Nowalk, Associate Professor, University of Pittsburgh Department of Family Medicine, Pittsburgh PA.
G. K. Balasubramani, Research Assistant Professor, Department of Epidemiology, University of Pittsburgh Graduate School of Public Health, Pittsburgh PA.
Mallory Schaffer, Research Assistant, University of Pittsburgh Department of Family Medicine, Pittsburgh PA.
Rhett H. Lieberman, Assistant Professor of Pediatrics, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh PA.
Heather Eng, Senior Data Manager, University of Pittsburgh Department of Epidemiology, Pittsburgh PA.
Shakala Kyle, Research Assistant, University of Pittsburgh Department of Family Medicine, Pittsburgh PA.
Stephen Wisniewski, Professor, Department of Epidemiology and Co-Director, Epidemiology Data Center University of Pittsburgh Graduate School of Public Health, Pittsburgh PA.
Richard K. Zimmerman, Professor, University of Pittsburgh Department of Family Medicine, Pittsburgh PA.
Donald B. Middleton, Professor, University of Pittsburgh Department of Family Medicine, Pittsburgh PA.
References
- 1.Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2012–13 influenza season. 2013 http://www.cdc.gov/flu/fluvaxview/coverage-1213estimates.htm. Accessed March 25, 2014.
- 2.Fiore AE, Shay DK, Broder K, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. Morb Mortal Wkly Rep (MMWR) Aug. 2008;57(RR-7):1–60. [PubMed] [Google Scholar]
- 3.Schuller KA, Probst JC. Factors associated with influenza vaccination among US children in 2008. J Infect Public Health. 2013 Apr;6(2):80–88. doi: 10.1016/j.jiph.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Cohen B, Ferng YH, Wong-McLoughlin J, et al. Predictors of flu vaccination among urban Hispanic children and adults. J Epidemiol Community Health. 2012 Mar;66(3):204–209. doi: 10.1136/jech.2009.099879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takayama M, Wetmore CM, Mokdad AH. Characteristics associated with the uptake of influenza vaccination among adults in the United States. Prev Med. 2012 May;54(5):358–362. doi: 10.1016/j.ypmed.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Thompson MG, Gaglani MJ, Naleway A, et al. The expected emotional benefits of influenza vaccination strongly affect pre-season intentions and subsequent vaccination among healthcare personnel. Vaccine. 2012 May;30(24):3557–3565. doi: 10.1016/j.vaccine.2012.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malosh R, Ohmit SE, Petrie JG, et al. Factors associated with influenza vaccine receipt in community dwelling adults and their children. Vaccine. 2014 Apr;32(16):1841–1847. doi: 10.1016/j.vaccine.2014.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flood EM, Rousculp MD, Ryan KJ, et al. Parents’ decision-making regarding vaccinating their children against influenza: a web-based survey. Clin Ther. 2010 Aug;32(8):1448–1467. doi: 10.1016/j.clinthera.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Myers L, Goodwin R. Determinants of adults’ intention to vaccinate against pandemic swine flu. BMC Public Health. 2011 Jan;11(1):1–8. doi: 10.1186/1471-2458-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallagher S, Povey R. Determinants of older adults’ intentions to vaccinate against influenza: a theoretical application. J Pub Health. 2006 Jun;28(2):139–144. doi: 10.1093/pubmed/fdl008. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Early estimates of seasonal influenza vaccine effectiveness — United States, January 2013. Morb Mortal Wkly Rep (MMWR) 2013;62(02):32–35. [PMC free article] [PubMed] [Google Scholar]
- 12.Ohmit SE, Thompson MG, Petrie JG, et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis. 2014 Feb;58(3):319–327. doi: 10.1093/cid/cit736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nowalk MP, Lin CJ, Zimmerman RK, et al. Establish the habit: influenza vaccination for health care personnel. J Healthc Qual. 2010 Mar-Apr;32(2):35–42. doi: 10.1111/j.1945-1474.2010.00073.x. [DOI] [PubMed] [Google Scholar]
- 15.Nyhan B, Reifler J, Richey S. The role of social networks in influenza vaccine attitudes and intentions among college students in the southeastern United States. J Adolesc Health. 2012 Sep;51(3):302–304. doi: 10.1016/j.jadohealth.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Zimmerman RK, Rinaldo CR, Nowalk MP, et al. Influenza and other respiratory virus infections in outpatients with medically attended acute respiratory infection during the 2011–12 influenza season. Influenza Other Respir Viruses. 2014 Jul;8(4):397–405. doi: 10.1111/irv.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmerman RK, Rinaldo CR, Nowalk MP, et al. Viral infections in outpatients with medically attended acute respiratory illness during the 2012–2013 influenza season. BMC Infect Dis. 2015;15(87) doi: 10.1186/s12879-015-0806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


