Abstract
The establishment of planar cell polarity (PCP) in epithelial and mesenchymal cells is a critical, evolutionarily conserved process during development and organogenesis. Analyses in Drosophila and several vertebrate model organisms have contributed a wealth of information on the regulation of PCP. A key conserved pathway regulating PCP, the so-called core Wnt-Frizzled PCP (Fz/PCP) signaling pathway, was initially identified through genetic studies of Drosophila. PCP studies in vertebrates, most notably mouse and zebrafish, have identified novel factors in PCP signaling and have also defined cellular features requiring PCP signaling input. These studies have shifted focus to the role of Van Gogh (Vang)/Vangl genes in this molecular system. This review focuses on new insights into the core Fz/Vangl/PCP pathway and recent advances in Drosophila and vertebrate PCP studies. We attempt to integrate these within the existing core Fz/Vangl/PCP signaling framework.
Keywords: PCP, Wnt, Frizzled, Vangl, Dishevelled, morphogenesis
INTRODUCTION
Cell signaling is essential to coordinate growth and patterning, two key events that govern the morphogenesis of a complex multicellular organism. During growth and patterning, cells are instructed by both quantitative and directional information. Quantitative information regulates certain biological processes in a dose-dependent manner. For example, cell fate induction along morphogen gradients provides positional information (Neumann & Cohen 1997, Schwank & Basler 2010). Directional information is employed to break symmetry and to generate the organized complex patterns, shapes, and cellular architecture required by many tissues and organs to function. Directional information instructs cells to form/localize certain structures or to perform certain functions selectively in one direction. Examples of such polarization include oriented cell division, cell migration, differential adhesion across cells, orientation of cytoskeletal elements, and positioning of cell extensions, such as cilia and axons. Directed cellular polarization is a key feature of organismal development and organogenesis and is also critical for organ function/homeostasis. Despite the critical function of directional information in cellular polarization, how it is provided and regulated in morphogenesis is still not well understood. Directional information is provided both locally, to orient a cell relative to its neighbors, and globally, to orient cells in a larger field along a specific axis. An emerging mechanism critical for the generation of both global and local directional information is planar cell polarity (PCP). This type of polarization is observed in both epithelial cells and associated organs (here, it is orthogonal to epithelial apical-basal polarity) as well as in mesenchymal cells that undergo morphological changes in certain directions (see below; Adler 2012, Devenport 2014, Goodrich & Strutt 2011, Klein & Mlodzik 2005, Lawrence et al. 2007, Seifert & Mlodzik 2007, Vladar et al. 2009).
PCP was originally identified in Drosophila, in which adult cuticular structures display striking PCP phenotypes (Adler 2012, Goodrich & Strutt 2011, Mlodzik 2002, Singh & Mlodzik 2012). Processes requiring PCP signaling in vertebrates are constantly being discovered and include skin development, body hair orientation, polarization of sensory hair cells in the inner ear, polarization of cells in the oviduct and respiratory tract, polarized localization of cilia in many tissues, directed cell movement and intercalation of mesenchymal cell populations during gastrulation and neurulation [generally called convergent extension (CE)], and long bone cartilage elongation (e.g., Devenport 2014, Gao et al. 2011, Gray et al. 2011, Guo et al. 2004, Keller 2002, Montcouquiol et al. 2003, Tissir & Goffinet 2013, Wallingford et al. 2000). The underlying signaling pathway, controlled by core PCP signaling proteins, is an evolutionarily conserved phenomenon, and its cellular and molecular regulation is conserved from Drosophila to mammals. The aforementioned developmental processes in different organisms are largely controlled by the same set of core PCP proteins, which were originally identified in Drosophila (Devenport 2014, Goodrich & Strutt 2011, Gray et al. 2011, McNeill 2010, Seifert & Mlodzik 2007, Tissir & Goffinet 2013, Vladar et al. 2009, Wang & Nathans 2007). PCP establishment also employs a second molecular signaling cassette, the Dachsous/Fat (Ds/Ft) system, which is well studied in Drosophila but less well analyzed in vertebrates, in which it appears conserved as well (Saburi et al. 2012, Sharma & McNeill 2013). The core PCP system [centered around Wnt-Frizzled (Fz) signaling in Drosophila; see below] and the Ds/Ft system are thought to act in parallel to each other and even redundantly in some tissues (Donoughe & DiNardo 2011, Lawrence et al. 2007). The two PCP systems are able to polarize cells independently and share similarities in regulatory schemes. For instance, intercellular interactions mediated by key polarity regulatory proteins are required by both systems: In the Ds/Ft system, Ds and Ft form heterodimers, whereas in the Fz/Van Gogh (Vang)/Starry Night (Stan) system, there are Fz-Vang heterodimers and Stan-Stan homodimers. As PCP in both systems relies on primary long-range gradients of secreted morphogens to drive secondary gradients that polarize cells more directly (Lawrence & Casal 2013), both PCP systems can be used to interpret long-range global cues and to coordinate short-range polarity during morphogenesis. The Fz-Vang core PCP system, the vertebrate counterpart of the Fz/PCP system in Drosophila, is functionally conserved. However, the Ds/Ft PCP system is less understood in vertebrates, and it can regulate processes independently of PCP (Saburi et al. 2012).
Historically, the study of PCP originates from work in insects, primarily the fruit fly, Drosophila, in which it was initially referred to as tissue polarity. Pioneering work by Peter Lawrence (Lawrence & Shelton 1975) and Paul Adler (Vinson & Adler 1987) put the problem of PCP regulation on the map more than 30 years ago. Their work was followed by genetic analyses of several PCP genes (reviewed in Adler 2002, Mlodzik 2002, Strutt 2003) and the first molecular cloning of a Drosophila PCP gene (Vinson et al. 1989). Systematic genetic screens in Drosophila and subsequent molecular analyses of the identified PCP factors have significantly advanced our understanding of PCP pathways (Table 1) (Adler 2002, Mlodzik 2002, Strutt 2003). Whereas PCP in Drosophila appears restricted to epithelial organs/tissues, in vertebrates, PCP is also required for the polarized cell behavior of mesenchyme cells. Studies in Xenopus, zebrafish, chick, and mouse have independently established that processes such as CE during gastrulation, muscle cell alignment, and limb bud elongation represent essential and critical roles for PCP in mesenchymal cells (Gao et al. 2011, Hopyan et al. 2011, Jessen et al. 2002, Keller 2002, Kibar et al. 2001, Marlow et al. 2002, Murdoch et al. 2001, Myers et al. 2002, Wallingford 2004, Yin et al. 2009). Strikingly, several mammalian epithelial features and organs are now also firmly established as PCP models, highlighting the regulatory similarities between the PCP of the Drosophila cuticle and that of mammalian epithelia (Adler 2012, Devenport 2014, Goodrich & Strutt 2011, Gray et al. 2011, Singh & Mlodzik 2012, Gao & Yang 2013) (Table 1). More recently, mutations in PCP genes have been identified in human diseases, such as spina bifida and Robinow syndrome (RS) (Afzal et al. 2000; Doudney et al. 2005; Kibar et al. 2007, 2009; Lei et al. 2013, 2014; Person et al. 2010; Robinson et al. 2012; van Bokhoven et al. 2000; Wang et al. 2006). As such, evolutionarily conserved protein families regulate PCP from flies to humans, and the defects observed are virtually identical in the respective mutants/diseases, indicating similar principles at work in all contexts.
Table 1.
Core PCP genes and new additions to the core group in Drosophila and vertebrates
| Drosophila genes | Vertebrate genes | Molecular features | Tissues/processes affected, studied | |
|---|---|---|---|---|
| Drosophila | Vertebratesa | |||
| Core Fz/PCP components | ||||
| frizzled ( fz) |
Fzd1 Fzd2 Fzd3 Fzd6 Fzd7 and others (best analyzed in mice) |
Seven-pass transmembrane receptors, bind Wnt ligands; intracellular binding to Dsh; recruit Dsh and Dgo to membrane; co-IP with Fmi/Celsr | All adult tissues | CE, inner ear, skin/dermis, and more |
| dishevelled (dsh) |
Dvl1 Dvl2 Dvl3 DVL1 DVL2 DVL3 XDsh (Xenopus) |
Cytoplasmic protein containing DIX, PDZ, DEP domains; recruited to membrane by Fz; binds Fz, Pk, Vang, and Dgo; undergoes extensive phosphorylation | All adult tissues | CE, inner ear, heart development, and more |
| Van Gogh [Vang; also known as strabismus (stbm)] |
Vangl2 Vangl1 trilobite (tri/Vangl, zebrafish) xStbm (Xenopus) |
Novel four-pass transmembrane protein; binds Pk, Dsh, and Dgo; recruits Pk to membrane; co-IPs with Fmi/Celsr | All adult tissues | CE, inner ear, neural tube defects, limb elongation/bud, skin/dermis, and more |
| flamingo [ fmi; also known as starry night (stan)] |
Celsr1 Celsr2 Celsr3 |
Atypical cadherin with seven-pass transmembrane receptor features; homophilic cell adhesion; co-IPs with Fzs and Vang(l)s | All adult tissues | CE, inner ear, neural tube defects, skin/dermis, and more |
| prickle ( pk; also known as prickle-spiny legs) |
Pk1 Pk2 xPk (Xenopus) |
Cytoplasmic protein with three LIM domains and PET domain; recruited to membrane by Vang; physically interacts with Vang, Dsh, and Dgo; competes with Dgo for Dsh binding | All adult tissues | CE, limb elongation, airway tracts, cleft palate, heart development, kidney, neural tube defects, and more |
| diego (dgo) |
Diversin (ankyrin repeat domain 6) Inversin (invs) |
Cytoplasmic ankyrin repeat proteins; recruited to membrane by Fz; bind Dsh, Vang, and Pk; compete with Pk for Dsh binding | All adult tissues | CE, left-right asymmetry, inner ear, and more |
| New or vertebrate-specific core Fz/PCP-associated factors | ||||
| NA | mRor2 (mouse), XRor2 (Xenopus) | Receptor tyrosine kinase; contains extracellular Frizzled-like CRDs and Kringle domain; acts as a coreceptor for Wnt5a to mediate noncanonical Wnt signaling | NA | CE, inner ear, neural tube defects, limb elongation/bud, and more |
| Derailed | Ryk (mouse) | Receptor tyrosine kinase; contains extracellular Wnt-binding WIP domain; acts as coreceptor for Wnt5a | None | CE, limb elongation, and more |
| furrowed ( fw) | ND | Selectin family cell adhesion molecule; promotes homophilic adhesion; co-IPs with Fz | All tissues tested to different degrees (eye, wing, thorax) | ND |
| VhaPRR | ND | Subunit of proton pump V-ATPase; colocalizes and co-IPs with Fmi | All tissues tested (eye, wing) | ND |
| ND | Ptk7 | Receptor tyrosine kinase–like membrane protein with IgG domain | NA | CE, inner ear, neural tube defects, limb elongation, etc. |
Only tested tissues mentioned; combination of analyses in Xenopus, zebrafish, and mouse.
Abbreviations: CE, convergent extension during gastrulation; co-IP, coimmunoprecipitate; CRD, cysteine-rich domain; NA, not applicable; ND, not yet determined; PCP, planar cell polarity.
The mechanisms of PCP establishment remain poorly understood and represent an exciting frontier in developmental biology. How individual cells, hundreds of cell diameters apart, acquire the same orientation within the plane of an epithelium and how thousands of mesenchymal cells establish uniform polarization and coordinate their behaviors, such as migration and intercalation, are fascinating developmental and cell biological problems. Although progress has been made in recent years, the more we know, the more questions arise, and, as such, the molecular and cellular features of PCP establishment are far from being solved.
Here, we briefly outline our understanding of Wnt-Fz/Vangl/PCP signaling in Drosophila and vertebrates, integrating current data and recently identified players and working models. Along these lines, vertebrate limb bud patterning and elongation and mammalian skin development have emerged as excellent models for detailed studies of PCP in vertebrates. We compare and contrast these with Drosophila PCP studies. We apologize for research areas and viewpoints that we could not include here owing to space limitations.
CONSERVATION OF THE FRIZZLED/PLANAR CELL POLARITY CORE SYSTEM
The so-called Fz/PCP core proteins (also called the Fz-Vangl/PCP module in vertebrates) are historically composed of six proteins that interact with each other inter- and intracellularly. These interactions separate two PCP complexes to opposing sides of each cell, which provides the cell with a planar orientation axis. Whereas in Drosophila only one protein acts in these complexes (Table 1), the vertebrate situation is more complex and is complicated by redundancy, with multiple members per core component (Table 1). Of the six core factors, three are transmembrane components: Fz itself (also known as the 7-TM protein), the 4-TM protein Vang [also known as Strabismus (Stbm); Vang-like (Vangl) in vertebrates], and the atypical cadherin Flamingo (Fmi, also known as Stan; Celsr in vertebrates) (reviewed in Adler 2012, Goodrich & Strutt 2011, Gray et al. 2011, Singh & Mlodzik 2012). The complement of six is completed by three cytoplasmic factors: Dishevelled [Dsh; Dishevelled-like (Dvl) in vertebrates], Prickle (Pk), and Diego (Dgo; Inversin and Diversin in vertebrates) (reviewed in Adler 2012, Goodrich & Strutt 2011, Gray et al. 2011, Seifert & Mlodzik 2007, Singh & Mlodzik 2012, Wang & Nathans 2007).
In general, these core PCP signaling molecules interact with each other both across cell membranes and intracellularly to segregate two complexes to opposing sides of each cell, leading to the formation of an Fz-Fmi-Dsh-Dgo complex on one side and a Vang-Fmi-Pk complex on the other (Figure 1). The interactions are thought to be inhibitory intracellularly, as, for example, Vang and Pk inhibit the formation of the Fz-Dsh complex by directly binding to and affecting Dsh levels/stability (Das et al. 2004; Jenny et al. 2003, 2005; Narimatsu et al. 2009; Tree et al. 2002). Dgo antagonizes the effect of Pk on Dsh and thus protects and stabilizes the Fz-Dsh complex. Specifically, Pk can bind Dsh and antagonize its Fz-mediated membrane recruitment (Tree et al. 2002). This Pk function is supported by Vang, which recruits Pk to the membrane and binds and possibly inhibits Dsh function (Bastock et al. 2003, Jenny et al. 2003). Dgo binding to Dsh competes with Pk binding to Dsh and thus antagonizes the inhibitory effect of Pk. As such, the two complexes Fz-Dsh-Dgo and Vang-Pk are resolved to mutually exclusive regions at opposite sides of each cell (Figure 1). Similar observations of mutually exclusive localization of the Fz-Dsh and Vang-Pk complexes have been made in vertebrate PCP models, most notably in the mouse inner ear, skin, and limb and in the zebrafish presomitic mesoderm (Devenport & Fuchs 2008, Gao et al. 2011, Guo et al. 2004, Montcouquiol et al. 2003, Wang et al. 2006, Yin et al. 2008) (Figure 3).
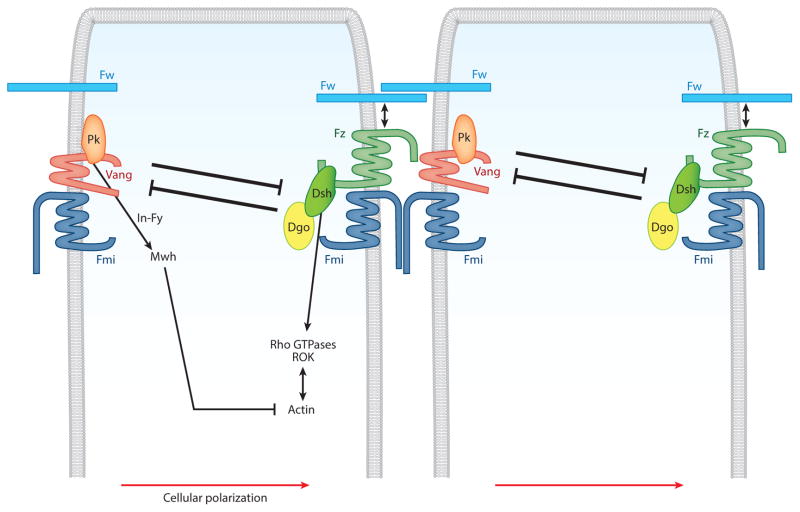
Figure 1.
Schematic depiction of Wnt-Frizzled/planar cell polarity (Fz/PCP) core component interactions across a two-cell border in Drosophila. Fz and Van Gogh (Vang) act in the interpretation of both long-range signal(s)—likely Wg/dWnt4 gradients—and short-range/local cell-cell transmembrane interactions. The latter is also mediated by the homophilic interactions of Flamingo (Fmi) and Furrowed (Fw), which are thought to physically interact with Fz and Vang and just Fz, respectively. The cytoplasmic components Dishevelled (Dsh), Diego (Dgo), and Prickle (Pk) are essential for negative intracellular feedback loops within individual cells, as they antagonize each other, and for activating intracellular pathways; for example, Dsh promotes actin polymerization by activating/localizing Rho-family GTPases and downstream effectors such as dROK (Rho-associated kinase). Inturned (In) and Fuzzy (Fy) serve to recruit Multiple wing hairs (Mwh), which is thought to inhibit actin polymerization. For more details and for Fz-Dsh downstream intracellular signaling pathways, readers are referred to Adler (2002), Mlodzik (2002), Strutt (2003), and Veeman et al. (2003).
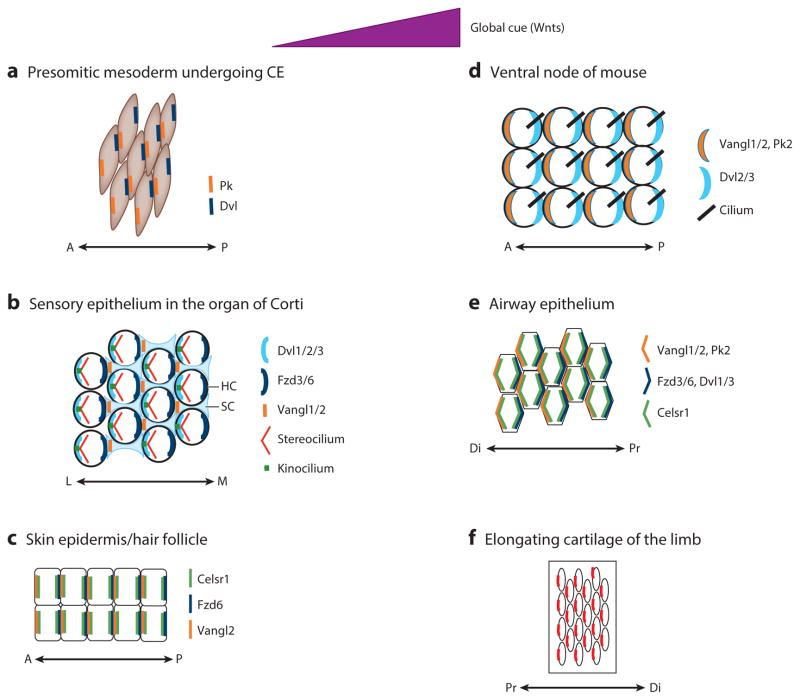
Figure 3.
Conserved asymmetrical localization patterns of core PCP components in six distinct epithelial and mesenchymal tissues (a–f ) in developing vertebrate embryos. In response to global cues (likely provided by Wnts), PCP is established. Molecularly, PCP establishment leads to coordinated, asymmetrical localization of core PCP components uniformly across all cells in any given tissue. It is interesting that the pattern of asymmetrical localization is conserved among different tissues. Moreover, the spatial relationships between global cue direction and the resulting asymmetrical pattern of polarity protein localization are also conserved. Abbreviations: A, anterior; CE, convergent extension; Di, distal; Dvl, Dishevelled; FzD, Frizzled; HC, hair cell; L, lateral; M, medial; P, posterior; PCP, planar cell polarity; Pk, Prickle; Pr, proximal; SC, supporting cell; Vangl, Van Gogh–like.
In contrast to their intracellular antagonism, intercellularly, the two complexes stabilize each other and thus serve a positive-feedback function. Fmi serves a homophilic adhesion function and colocalizes and coimmunoprecipitates with both Fz and Vang. Intercellular interactions between Fmi-Fmi and Fz-Vang have been documented (Strutt & Strutt 2008, Usui et al. 1999, Wu & Mlodzik 2008). As Fmi interacts with both Fz and Vang, it has been suggested that homophilic Fmi bridges span cell membranes, facilitating intercellular Fz-Vang interaction. This is thought to stabilize the complexes across membranes and also to be essential for propagating PCP from cell to cell (Chen et al. 2008, Lawrence et al. 2008, Struhl et al. 2012, Strutt & Strutt 2008, Wu & Mlodzik 2008).
A hallmark of the core PCP factors is that prior to their interactions/PCP signaling, they are located uniformly around subapical cell membranes, largely overlapping with adherens junctions/E-cadherin, and their asymmetrical localization is the first detectable sign of PCP. Moreover, loss or overexpression of any core PCP genes leads to random distribution of other core PCP factors, as the system requires physiological levels of all of them to work. As such, too much of a core PCP factor is as bad as too little, largely resulting in the same defects, such as random PCP complex localization and random polarity. In other words, disturbing the localization of one component [via either loss of function (LOF) or gain of function (GOF)] affects the localization of all others, even those from the opposite complex because of feedback loops (Seifert & Mlodzik 2007, Strutt 2003). This phenomenon is distinct from components becoming asymmetrically localized in response to core PCP factor localization; these proteins are thought to act downstream of the core PCP signaling cassette. Inturned, for example, is initially localized in a uniform apical ring in wing cells and subsequently becomes localized exclusively to proximal cell membranes, but the loss of inturned does not affect the localization of the core PCP gene products (Adler et al. 2004).
Intercellular PCP communication is also evident in the nonautonomous effects of fz and Vang mutant clones, which affect the orientation of neighboring wild-type cells, often referred to as domineering nonautonomy (e.g., Vinson & Adler 1987). Not only do mutant clones lacking Fz show PCP defects, they also reorient adjacent cells toward the clone, whereas cells lacking Vang reorient neighboring cells to point away (Taylor et al. 1998, Vinson & Adler 1987). Strikingly, fz, Vang double mutant clones reorient hairs to point toward the clone, very much like single fz mutant clones (Wu & Mlodzik 2008). These data suggest that Fz is required for sending a polarity signal and Vang for its reception; Fmi is needed on both sides. In the absence of Fz or Vang, the ability of cells to communicate with Fmi-Fmi bridges alone is highly reduced (Wu & Mlodzik 2008, 2009; Struhl et al. 2012), suggesting that instructive input from the Fz-Vang interaction is essential for intercellular PCP signaling. It should be interesting to establish whether new PCP regulators/modulators can directly modify Fmi-Fmi interactions or bias their directionality. Importantly, dsh, pk, and dgo null mutations appear to affect PCP only within mutant clones and not within adjacent cells (Feiguin et al. 2001, Strutt & Strutt 2002, Tree et al. 2002), suggesting that Dsh, Pk, and Dgo are primarily involved in intracellular interactions and cell-autonomous PCP signal transduction within cells (Strutt 2003, Strutt & Strutt 2007, Veeman et al. 2003, Wu & Mlodzik 2009).
What acts downstream of these core PCP interactions? The Fz-Dsh side/complex has been linked to activation of the so-called Fz-Dsh PCP pathway (a rather uninformative term largely coined to differentiate it from canonical Wnt-Fz signaling). The downstream readouts of Fz-Dsh PCP signaling are thought to differ depending on context (e.g., cytoskeletal reorganization in fly wings versus a transcriptional response in fly eyes) and have been discussed in detail previously (reviewed in Mlodzik 2002, Veeman et al. 2003). Briefly, a combination of genetic and biochemical studies in Drosophila and vertebrates established that the Fz-Dsh PCP pathway (downstream of Dsh) consists of small GTPases of the Rho subfamily (Rho, Rac, and cdc42), the Rho-associated kinase (ROK), the STE20-like kinase Misshapen (Msn in flies), and the JNK-type mitogen-activated protein kinase (MAPK) cascade. The importance and contribution of each of these factors can vary between tissues, with significant redundancy between some of the GTPases, even in Drosophila (e.g., Fanto et al. 2000, Hakeda-Suzuki et al. 2002, Munoz-Descalzo et al. 2007). ROK affects cytoskeletal aspects of PCP and links Fz-Dsh signaling to myosin regulation (Marlow et al. 2002, Winter et al. 2001), whereas the JNK/p38-type MAPK modules and Jun-Fos (AP-1) transcription factors act downstream of Dsh in the transcriptional response in the fly eye (Boutros et al. 1998; Paricio et al. 1999; Weber et al. 2000, 2008). JNK signaling is also likely required in the context of CE in vertebrates (Yamanaka et al. 2002), suggesting a general JNK requirement in PCP, albeit a redundant one, as phenotypic effects become apparent only when more than one related kinase is affected (Paricio et al. 1999). Consistent with this hypothesis, Dsh is a potent JNK activator in biochemical assays (Boutros et al. 1998, Yamanaka et al. 2002).
An important aspect of Fz/Dsh-PCP signaling is the juxtamembrane subcellular localization of Dsh (Axelrod 2001, Boutros et al. 2000, Wong et al. 2003), but it remains unclear how Dsh relays a signal to downstream effectors. In Xenopus, the formin homology domain protein Daam1 has been proposed as such a bridging factor between Dsh and Rho GTPases/ROK (Habas et al. 2001), but an exact sequence of events involving Dsh/Daam1/RhoA remains to be resolved, as a C-terminal Daam1 fragment that lacks the RhoA binding region behaves like activated Daam1 (Habas et al. 2001). A Daam1 homolog is present in Drosophila, but its role in PCP establishment must be redundant, as PCP defects are not detected in dDaam1 LOF mutants, whereas GOF experiments display classical PCP defects (Matusek et al. 2006). Similarly, mouse Daam1 mutants do not show PCP defects (Li et al. 2011), suggesting that in both Drosophila and mouse, Daam1 is redundant with formin homology domain factors.
NEW/ADDITIONAL MEMBERS OF CORE PLANAR CELL POLARITY SIGNALING
There are several recent additions to and complications of the core PCP factor network and signaling regulation. First, Drosophila genetic studies have suggested the presence of additional factors, and second, still other, vertebrate-specific core PCP signaling factors are known, such as the Wnt coreceptor Ror2 (see below).
Recent work in Drosophila suggests that in addition to the six historical core PCP components (Table 1), there may be others that could also be considered members of the core group but have been overlooked, as they are not solely dedicated to PCP. These components include Furrowed (Fw), a Drosophila selectin family member (Chin & Mlodzik 2013), and the VhaPRR accessory subunit of the proton pump V-ATPase (Buechling et al. 2010, Hermle et al. 2010). Both affect PCP in a similar manner as the core components in the tissues in which they were analyzed (wing, eye, and thorax; they have not yet been studied in the abdomen), and, importantly, they colocalize with core factors and can also be coimmunoprecipitated with Fz and Fmi (in the case of VhaPRR) or specifically with Fz (in the case of Fw). The function of Fw appears to partly overlap with that of Fmi, as it is required to stabilize Fz in plasma membrane complexes and also to promote homophilic cell adhesion (Chin & Mlodzik 2013, Usui et al. 1999). Fw also mediates intercellular binding/interactions between Fz and Vang, again similar to Fmi (Chin & Mlodzik 2013, Strutt & Strutt 2008). Fw and Fmi could even be partially redundant in mediating Fz-Vang interactions or Fz membrane localization, as the tissues in which fmi mutants have weaker phenotypes (e.g., the thorax) are those in which fw LOF is most severe, whereas in the eye the opposite occurs. The main functional difference between Fw and Fmi is that Fw only associates with Fz and affects its membrane localization/stability (Chin & Mlodzik 2013), whereas Fmi associates with Fz and Vang (Figure 1). Importantly, the PCP defects in fw, fmi double mutants are not more severe than those in fz null flies, suggesting that both proteins require Fz to function.
The role of VhaPRR is less clear, but functional studies suggest that it affects the trafficking or membrane stability of Fmi and possibly Fz. VhaPRR can be coimmunoprecipitated with both Fz and Fmi but only directly physically associates with Fmi (Hermle et al. 2013). Although the function of Fw in epithelial/cuticular patterning is largely restricted to PCP (it also can affect growth in the eye and has a potential role in cytoskeletal regulation), VhaPRR appears to be generally required for trafficking, as it also affects Fz2 (canonical Wingless/Wg signaling), Notch, and E-cad levels in the membrane (Hermle et al. 2010, 2013).
The vertebrate complications of the six-core-factor scheme are at least twofold. First, there are several equivalent proteins for each core factor (if not many; e.g., many Frizzleds), with significant redundancy in at least some tissues. Second, the transmembrane factors also include Ror2, which serves as a Wnt coreceptor with Fzs. Although the functional details are not completely worked out, Ror2 appears to be a Wnt5a coreceptor, helping to relay the signal to Vangl2 and induce Vangl2 phosphorylation (Gao et al. 2011). Wnt5a also induces Dvl phosphorylation through Ror1 and Ror2 (Ho et al. 2012). However, the function of Dsh/Dvl phosphorylation in PCP signaling, although often used as a readout, remains unclear (Yanfeng et al. 2011). In addition, Ryk, which mediates the function of Wnt5 in axon guidance in Drosophila (Yoshikawa et al. 2003), also acts as a Wnt5a coreceptor in mice (Andre et al. 2012, Macheda et al. 2012). Like Ror2, Ryk mediates Wnt signaling in PCP regulation. As such, parallels exist between the Wnt receptor complex for canonical Wnt signaling and Fz and LRP5/6 working as coreceptors for canonical Wnts, whereas in Wnt-PCP signaling in vertebrates, Fz, Vangl, and Ror2/Ryk can serve as coreceptors (Grumolato et al. 2010). The role of Ror2 in PCP is addressed in detail below. The Wnt/PCP signaling in vertebrates is further complicated by the finding of PTK7, a transmembrane protein required in mouse PCP signaling (Lu et al. 2004). However, it is still not clear whether it is regulated by Wnts.
LONG-RANGE REGULATION OF FRIZZLED/PLANAR CELL POLARITY CORE POLARIZATION IN DROSOPHILA
What about long-range regulation? Until recently, it had been suggested that Wnts were not involved in Drosophila PCP, largely owing to a lack of LOF phenotypes. However, in the wing and eye, PCP axes are oriented toward the prospective margins, the source of Wnt expression and, in particular, Wg and dWnt4 expression (Aigouy et al. 2010, Sagner et al. 2012, Wu et al. 2013). Moreover, the cysteine-rich domain (CRD) of Fz, which is also the Wnt-binding domain (Bhanot et al. 1996), is critical for the nonautonomous aspect of Fz/PCP signaling, suggesting that Wnts may contribute. And then, of course, evidence from vertebrates shows that Wnt5a and Wnt11 (and possibly more Wnts) are largely dedicated to PCP (see below).
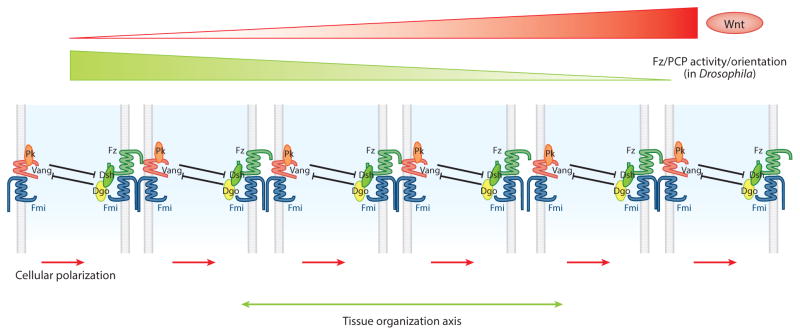
Recent Drosophila wing experiments clearly demonstrate an instructive role for dWnt4 and Wg in orienting PCP axes (Wu et al. 2013), which is an overdue and timely discovery. GOF experiments with Wg and dWnt4 in the Drosophila wing show that these proteins serve an instructive role, as their misexpression alters Fz/PCP core complex protein localization and polarity axis orientation, largely orthogonal to the expression domain of the Wnts, and orients cells to face the wind (Wnt) so to speak. Complicated genetic assays confirmed that Wg and dWnt4 are required for PCP establishment but act redundantly; hence, this function was missed in earlier studies (Wu et al. 2013). As Wg is required for many patterning steps, from early embryogenesis to the formation of (almost) all adult structures, studying it was a complicated task requiring the use of temperature-sensitive wg alleles combined with dWnt4 null mutants (Wu et al. 2013). Taken together, these experiments establish an instructive and necessary function for Wnts in defining PCP axes in Drosophila. In agreement with the proposed Fz activity gradient thought to direct PCP orientation (Adler et al. 1997, Lawrence et al. 2004, Wu & Mlodzik 2008), Wg and Wnt4 modulate intercellular Fz-Vang interactions. Specifically, they antagonize Fz-Vang binding across cell membranes. Co-overexpression of Fz and Wnt4 allows them to buffer each other’s effects in vivo, suggesting that Wnts inhibit Fz-Vang interaction (Wu et al. 2013). Thus, current models for long-range core PCP axis establishment include Wg/dWnt4 as instructive regulators of Fz-Vang polarization, at least in development of the Drosophila wing and eye imaginal discs (with other tissues being more complex).
Along these lines, in vertebrates, the PCP involvement of Wnt5a and Wnt11 is well documented (e.g., Andre et al. 2015, Gros et al. 2009, Heisenberg et al. 2000, Tada & Smith 2000), but the mechanism of action for PCP establishment has just started to be worked out (see below) (Gao et al. 2011). Strikingly, Wnt5a appears to use a receptor complex that likely contains Fz, Vangl2, Ror2, and Ryk in PCP signaling (Andre et al. 2012, Gao et al. 2011, Grumolato et al. 2010). The mechanistic mix is likely to be more complex in vertebrates, as Wnt5a and Wnt11 may employ distinct mechanisms in exerting their functions. Nonetheless, an instructive role for Wnt11 has been suggested (Gros et al. 2009) and is likely for Wnt5a as well (Gao et al. 2011) (see below).
WNT/PLANAR CELL POLARITY PROVIDES DIRECTIONAL INFORMATION IN VERTEBRATE MORPHOGENESIS
When vertebrates evolved from invertebrates, new signaling components and pathways, as well as new regulatory schemes and networks, emerged to regulate the development of embryos with ever-increasing complexity. Among these, the Wnt/PCP signaling pathway stands out, as its functions and regulatory modes have greatly expanded in vertebrate development. PCP became a fundamentally important mechanism, controlling many processes in embryonic morphogenesis. When an embryo forms from a single, fertilized egg through morphogenesis, directional information needs to be provided both globally and locally to break the initial symmetry in many developmental contexts. Establishing PCP in a field of hundreds of cells in response to global directional cues is an important symmetry-breaking mechanism. As vertebrate embryos grow and form more complex tissues and organs, the role of Wnt/PCP becomes very prominent. However, the cellular and molecular mechanisms of Wnt/PCP signaling in vertebrates have only begun to be dissected in recent years.
PCP was first found to be essential for regulating anterior-posterior (A-P) body axis elongation during vertebrate gastrulation through CE, in which mesenchymal cells intercalate with each other laterally and move toward the midline. In this way, cells converge mediolaterally, forcing the body axis to extend along the A-P axis (Heisenberg et al. 2000, Keller 2002, Tada & Smith 2000, Topczewski et al. 2001, Wallingford et al. 2000). Interestingly, PCP can also control oriented cell division in this process (Gong et al. 2004, Quesada-Hernandez et al. 2010). PCP-regulated CE is also essential for neurulation. In frogs and fish, deficiency in PCP results in widening of the floor plate and in neural tube malformations (Ciruna et al. 2006, Tawk et al. 2007, Wallingford & Harland 2002). In mouse, mutants for core PCP components, such as Vangl2, exhibit open neural tubes (craniorachischisis) (Kibar et al. 2001, Murdoch et al. 2001). Importantly, human mutations in both VANGL1 and VANGL2 are associated with spina bifida, a severe condition caused by incomplete neural tube closure (Kibar et al. 2007, Lei et al. 2010). Neural tube closure in higher vertebrates is a complicated morphogenetic process in which PCP may regulate additional polarized cellular events, such as apical constriction (Nishimura et al. 2012). Nevertheless, CE, like hair polarity in Drosophila wings, is an evolutionarily conserved, PCP-controlled event in vertebrates, and CE defects and open neural tubes are hallmarks of impaired vertebrate PCP.
In mammals, the known roles of PCP have recently been expanded: PCP is a fundamentally important mechanism governing many morphogenetic events. Apart from the initial studies on A-P axis elongation and neural tube closure, PCP is critically required to orient sensory hair cells in the inner ear (Curtin et al. 2003, Ma & Moses 1995, Montcouquiol et al. 2003), hair shafts and follicles in mice (Devenport & Fuchs 2008, Guo et al. 2004), and cilia in node cells and airway epithelium (Antic et al. 2010, Borovina et al. 2010, Hashimoto et al. 2010, Sepich et al. 2011, Song et al. 2010, Vladar et al. 2012), as well as to control limb elongation (Gao et al. 2011, Gros et al. 2010) and axon guidance (Fenstermaker et al. 2010, Goodrich 2008).
An evolutionarily conserved event in PCP establishment is the asymmetrical localization of core PCP components uniformly across planar polarized epithelia and mesenchymal cells (Figures 1–3). This localization also serves as a definitive molecular readout of PCP. Consistent with asymmetrical localization of core PCP components along the proximal-distal (Pr-Di) axis of fly wing discs, with distal Fz and Dsh localization versus proximal Vang and Pk localization in wing disc cells (Figure 2), Pk and Dvl proteins in presomitic zebrafish cells undergoing CE are localized to the anterior and posterior cell edges, respectively (Yin et al. 2008) (Figure 3). This localization also parallels (a) anterior localization of Pk2, Vangl1, and Vangl2 (Antic et al. 2010, Song et al. 2010) and posterior localization of Dvl2 and Dvl3 in node cells (Hashimoto et al. 2010) (Figure 3) during gastrulation and (b) proximal localization of the Vangl2 protein in chondrocytes in the elongating limb (Gao et al. 2011) (Figure 4). In the mouse epidermis, Vangl2 and Fzd6 proteins are asymmetrically localized to the anterior and posterior sides of the cells, respectively (Devenport et al. 2012) (Figure 3). In the airway epithelial cells, Vangl1, Vangl2, and Pk1 are localized distally, whereas Fzd3, Dvl1, and Dvl3 are localized proximally (Song et al. 2010, Vladar et al. 2012) (Figure 3). In inner ear sensory cells, the polarity of stereocilia and core PCP proteins aligns with the lateral-medial axis of the organ of Corti (Figure 3). Dvl1, Dvl2, and Dvl3 are asymmetrically localized to the lateral side of the hair cell (Wang et al. 2005), whereas Fzd3, Fzd6, and Vangl2 are all localized to the medial side of the hair cell (Montcouquiol et al. 2006, Wang et al. 2006). However, a recent, closer look at the localization of Vangl1 and Vangl2 revealed that they are not colocalized with Fzd in the same hair cells. Instead, Vangl1 and Vangl2 are asymmetrically localized to the lateral side of juxtaposed supporting cells (Ezan & Montcouquiol 2013). These studies indicate that the pattern of asymmetrical localization of core PCP components is largely conserved from Drosophila to mouse in multiple developmental contexts. In addition, the parallel localization patterns mentioned above are associated with the appearance of Wnt gradients in many developmental contexts, underscoring evolutionary conservation of regulatory mechanisms in PCP establishment.
Figure 2.
Schematic presentation of Frizzled/planar cell polarity (Fz/PCP) core component interactions across an epithelial layer (apical view) relative to a Wnt (Wg/dWnt4) concentration gradient. An initial asymmetry along the Wg/Wnt4 gradient is subsequently amplified via negative intracellular and positive intercellular interactions among the core PCP factors. At least in the eye and wing of Drosophila, the PCP orientation axis is along a Wnt concentration slope. It is likely that in other tissues in which PCP alignment is more complicated relative to Wnt gradients, such as the Drosophila leg, a more complex morphogen alignment is in place. Abbreviations: Dgo, Diego; Dsh, Dishevelled; Fmi, Flamingo; Pk, Prickle; Vang, Van Gogh.
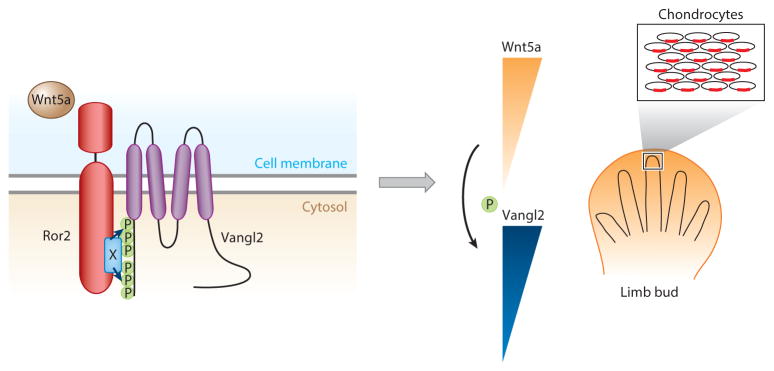
Figure 4.
The Wnt5a gradient controls directional limb elongation by regulating planar cell polarity (PCP). The Wnt5a signal induces Ror2-Vangl2 (Van Gogh–like 2) complex formation; it is likely that a Frizzled family member also serves a key function as a Wnt coreceptor in this complex. As a result, Vangl2 is phosphorylated at conserved Ser and Thr residues in a Wnt5a dose-dependent manner. Therefore, a Wnt5a gradient is translated into a Vangl2 phosphorylation gradient. As Vangl2 activity appears to be regulated by phosphorylation (Gao et al. 2011), the Wnt5a gradient is translated into a Vangl2 activity gradient in the limb. A chondrocyte in the middle of the newly formed cartilage can sense its positional information by comparing Vangl2 activity with that of its immediate neighbors through cell-cell interactions. Such cell-cell interactions eventually lead to asymmetrical Vangl2 protein localization through feedback loops, laying the groundwork for further asymmetric cellular behavior, such as directional cartilage elongation.
Indeed, similar to Drosophila, mouse PCP mutations disrupt asymmetrical localization of PCP components as well as corresponding morphogenetic events. In addition, the phenomenon of domineering nonautonomy has been conserved in vertebrate PCP signaling. For instance, wild-type cells transplanted into trilobite (tri )/Vangl2 mutant embryos fail to undergo CE in zebrafish ( Jessen et al. 2002), and ciliary orientation is reversed in cells anterior to tissues that overexpress Vangl2 (Mitchell et al. 2009). Furthermore, PCP control of the planar polarized hair follicle can propagate through the adjacent epidermis (Devenport & Fuchs 2008). Wild-type hair follicle explants can be repolarized by a wild-type epidermis, but they remain unpolarized when flanked by a homozygous Vangl2 mutant epidermis. In addition, intercellular Celsr1 homodimers are required to recruit Vangl2 or Fzd6 to the membrane, similar to the function of Fmi in PCP signaling in Drosophila (Bastock et al. 2003, Chen et al. 2008, Lawrence et al. 2004).
Despite this important conservation in regulation and function, Wnt/PCP signaling in vertebrates possesses new signaling components and regulatory schemes to cope with its significantly expanded functional spectrum. Therefore, it is imperative to extend our understanding of PCP signaling and its global cues through studies of vertebrates. Such studies will both significantly advance our knowledge about Wnt signaling complexity and provide new insights into identifying the cellular and molecular nature of generation, amplification, perception, maintenance, and plasticity of directional information, processes critical for embryonic development, adult physiology, and tissue regeneration. The expanded number of vertebrate core PCP homologs, however, complicates deciphering PCP signaling in vertebrates.
Nineteen different Wnt proteins exist in mammalian genomes, most of which transduce their signals by stabilizing β-catenin via canonical Wnt/β-catenin signaling, which is fundamentally important and evolutionarily conserved. This pathway primarily regulates cell fate determination, proliferation, and survival and has been extensively studied in development and tumorigenesis (McDonald & Silver 2009). Wnt5a and Wnt11 are quite special in that they do not stabilize β-catenin in vivo in most cases. They have been proposed to transduce their signals through multiple distinct pathways instead, including the Wnt/PCP pathway (Andre et al. 2015, Veeman et al. 2003). Although it is complicated and challenging to prove in Drosophila that Wnts do indeed act as global cues to regulate PCP (Wu et al. 2013), strong genetic evidence implicates Wnts in PCP regulation in vertebrates (Heisenberg et al. 2000, Jessen et al. 2002, Rauch et al. 1997). In zebrafish, the Vangl2 mutant tri exhibits a shortened and broadened A-P axis, owing to defects in CE movements. The Wnt5b mutant pipetail ( ppt) and the Wnt11 mutant silberblick (slb) also exhibit a broadened and shortened A-P body axis with defective CE movement, suggesting that Wnt signaling is required to regulate CE through PCP. Furthermore, in mice, Wnt5a and one of its coreceptors, Ror2, genetically interact with the core PCP protein Vangl2 (Gao et al. 2011, Qian et al. 2007). Because context-dependent combinations of Wnt receptors determine activities of Wnts and the pathway(s) they activate, other Wnts have also been implicated in regulating PCP (van Amerongen et al. 2008). Therefore, the term Wnt/PCP has been widely used in vertebrates.
To investigate whether and how Wnts act as global cues to establish PCP, one must start by uncovering the molecular mechanisms whereby Wnt signaling is transduced in the PCP context. However, despite the important roles of Fzd proteins in PCP, it would be a difficult task to dissect their highly redundant functions in PCP and the canonical Wnt pathway. Most mouse Fzd mutants exhibit PCP defects that are less severe and tissue specific than in Vangl2 mutants (e.g., Yu et al. 2010, Wang et al. 2010). As 10 different Fzd genes exist in the mammalian genome, and most cells express multiple Fzd genes, different Fzd receptors share functional redundancy (e.g., Wang et al. 2006), such that regulation of PCP by Fzd receptors is dose dependent. Because only one PCP-dedicated Fzd exists in Drosophila (the original Fzd gene that gave the whole family its name), and Fzd mutants display PCP defects as strong as for any other PCP core factor, it is safe to assume significant redundancy among the vertebrate Fzd proteins. By contrast, we can use genetic approaches to address the functions and signaling mechanisms of Wnts and PCP more easily for Vangl proteins, which have fewer family members (just two) and are dedicated to PCP.
DECIPHERING GLOBAL CUES IN THE DEVELOPING LIMB BUD
Analogous to what occurs along the A-P body axis, the developing limb bud undergoes directional elongation along the Pr–Di axis. PCP has been suggested to regulate cartilage elongation in the developing limb (Li & Dudley 2009). Wnt5a is expressed in a distal-to-proximal gradient in the developing limb bud and is the only Wnt expressed in the limb mesenchyme. This expression is required for limb and cartilage elongation through a pathway independent of β-catenin activation (Topol et al. 2003, Yamaguchi et al. 1999, Yang et al. 2003, Zhu et al. 2012). Therefore, Wnt5a is an attractive candidate for a global cue required to regulate limb elongation along the Pr–Di axis. The limb bud offers a unique system for deciphering how global cues regulate PCP. First, a specific Wnt ligand essential for PCP establishment has not been found in other tissues in which PCP plays critical roles, such as the sensory epithelium in the inner ear, the presomitic endoderm undergoing CE, and the skin epidermis. In the developing limb, in which PCP plays a critical morphogenetic role, a specific Wnt ligand(s) is essential for PCP establishment. Second, clear morphological landmarks allow easy, quantitative detection of even small changes in Pr–Di limb elongation. Third, signaling centers of the limb bud that direct three-dimensional growth and patterning have been thoroughly characterized, and a comprehensive set of genetic tools has been developed to dissect gene function in limb bud and cartilage development.
The first definitive and rigorous demonstration of PCP in the developing limb was the identification of asymmetrical Vangl2 localization in newly formed chondrocytes along the Pr–Di limb axis (Figure 4) (Gao et al. 2011). Asymmetrical Vangl2 localization is not detected in interdigital mesenchyme or in early limb mesenchyme cells prior to cartilage formation. As cartilage elongation is the major driving force of limb elongation, it is not surprising that asymmetrical Vangl2 localization is most prominent in chondrocytes. Furthermore, in the Vangl2−/− and Vangl2 dominant negative Loop-tail mutant (Vangl2Lp), limbs are shortened (Gao et al. 2011, Song et al. 2010, Wang et al. 2011). If Vangl1, the second mammalian Vang homolog, is also deleted in the Vangl2 mutant background, the limb shortening defects are even more severe (Song et al. 2010). These studies demonstrate that PCP signaling is required for limb elongation. Importantly, the observation that both Wnt5a and Ror2 null mouse mutants exhibit loss of Vangl2 asymmetrical localization and a shorter and broader cartilage phenotype connects Wnt5a/Ror2 signaling to PCP, suggesting that Wnt5a signals through Vangl2 and Ror2 to regulate PCP and limb elongation (DeChiara et al. 2000, Gao et al. 2011, Takeuchi et al. 2000, Yamaguchi et al. 1999). Indeed, the dominant Vangl2Lp mutant limb exhibits digit and cartilage defects resembling the phenotypes in human brachydactyly types B and RS (Wang et al. 2011), which are caused by WNT5A or ROR2 mutations (Afzal et al. 2000, DeChiara et al. 2000, Person et al. 2010, Schwabe et al. 2000, van Bokhoven et al. 2000). In addition, both Wnt5a and Ror2 genetically interact with Vangl2, and Vangl2Lp enhances the severity of Wnt5a and Ror2 mutant embryos, indicating that they may function in the same pathway (Gao et al. 2011, Qian et al. 2007). Furthermore, a Ror2−/−, Vangl2−/−double mutant phenocopies Wnt5a−/− mutants in the limb, exhibiting failure of digit outgrowth and long bone elongation (Gao et al. 2011). These studies provide strong genetic evidence that Wnt5a signals through Ror2, Vangl1, and Vangl2 to regulate PCP in the developing limb and likely other tissues. Biochemically, Wnt5a may act via a Vangl-Ror-Fzd coreceptor complex and Dvl to regulate PCP (Gao et al. 2011, Grumolato et al. 2010), but it will be difficult to define which Fzd proteins are critical in developing limbs, primarily owing to the presumed redundancy among the many family members.
To determine how a Wnt5a signal instructs limb elongation by regulating PCP, one must understand at the cellular level how the signal is transduced and interpreted by Ror2, Vangl2, and other PCP components. Interestingly, Wnt5a induces Ror2-Vangl2 receptor complex formation, supporting the genetic evidence that a Ror2−/−, Vangl2−/− double mutant phenocopies Wnt5a−/−in the limb (Gao et al. 2011). More importantly, this receptor complex formation leads to the phosphorylation of Vangl2 at two clusters of conserved Thr/Ser residues (Gao et al. 2011). Vangl2 phosphorylation is induced by Wnt5a in a dose-dependent manner and is functionally important (Figure 4). Phosphorylation of more Vangl2 Thr/Ser residues correlates with stronger Vangl2 activity in zebrafish assays (Gao et al. 2011). Therefore, one can imagine that the levels or numbers of Thr/Ser residue phosphorylation in Vangl2 serve as a digital readout of Wnt5a dosage, which can be further amplified or translated into distinct downstream signaling events. Thus, newly formed chondrocytes may orient themselves along the Pr–Di axis by sensing Wnt5a dosage and intercellular interactions. This forms the basis for PCP establishment in the limb bud (Figure 4).
ESTABLISHMENT OF PLANAR CELL POLARITY IN LIMB BUD MESENCHYME CELLS
How and when PCP is first established in developing limbs are fundamentally important questions. Functionally, Wnt5a regulates asymmetrical cell behavior during both limb bud initiation and early limb bud growth (Gros et al. 2010, Wyngaarden et al. 2010). It is likely that PCP signaling is already induced at early stages but may not be stabilized, precluding detectable asymmetrical localization of Vangl2. If the appearance of biased Vangl2 localization is the first sign of stable and strong PCP, PCP is strongest in chondrocytes formed between embryonic day (E)11.5 and E12.5, as the Vangl2 protein appears to be distributed randomly in the plasma membrane in mesenchymal cells until E11.5 (Gao et al. 2011). At E12.5, Vangl2 asymmetrical localization can be clearly observed in Sox9-positive chondrocytes but not in nonchondrogenic mesenchymal cells, likely because stabilization of PCP proteins requires close cell-cell contacts, which are weaker in loose mesenchymal cells and stronger in the condensed mesenchymal cells that are differentiating into chondrocytes. However, the role of PCP in polarized cell behavior in limb buds prior to E11.5 cannot be excluded for several reasons. First, Wnt5a is critical in directional cell migration and division in earlier limb bud developmental stages (Gros et al. 2010, Wyngaarden et al. 2010). It will be interesting to directly test whether PCP signaling affects these processes in the early limb bud. Second, subtle biased PCP protein localization, which may occur earlier, is difficult to detect. Interestingly, in Drosophila wings, PCP was long thought to be established at the mid-pupa stage, but more sensitive imaging and detection methods have recently revealed that PCP is already established in wing imaginal discs at late larval stages (Aigouy et al. 2010, Sagner et al. 2012). Third, and most importantly, the asymmetrical localization of core PCP proteins is a readout of PCP but not the only indicator of active PCP signaling.
PCP proteins coordinate a field of cells, orienting them uniformly by regulating intracellular cytoskeletal arrangements and intercellular interactions between neighboring cells. Such cell-cell interactions are reinforced and amplified by positive feedback loops, leading to asymmetric localization of core PCP proteins and hence PCP establishment. Therefore, core proteins must receive polarizing information prior to visible, asymmetrical localization of core PCP proteins. In this regard, it is important to determine whether polarizing information needs to be continuously provided to maintain PCP or whether PCP itself is required later in limb development.
Longitudinal growth of long bones primarily occurs through continual elongation of chondrocyte columns in the growth plate and subsequent chondrocyte hypertrophy in a process called endochondral ossification (Olsen et al. 2000). Columnar chondrocytes divide laterally, and then daughter cells intercalate back into the original column of the parental cell, leading to longitudinal elongation of the growth plate. This CE-like process may be regulated by PCP (Li & Dudley 2009). Li & Dudley (2009) have found that retrovirally expressed Vangl2 or dominant-negative Fzd7 in the chick growth plate affects the division plane of proliferative chondrocytes, suggesting a role for PCP in the columnar organization of chondrocytes during endochondral ossification. Interestingly, Randall et al. (2012) have shown that they can promote columnar organization of dissociated growth plate chondrocytes when they form cell pellets in vitro by activating Wnt/PCP pathway components. The best columns form with a combination of Ror2, Fzd7, and Wnt5a. However, it is also possible that the severe long bone phenotype of the Wnt5a−/−, Ror2−/− and Vangl1−/−, Vangl2−/− mutants may result from defective cartilaginous anlage formation caused by impaired PCP at earlier stages of limb development and not from the direct function of these proteins in the growth plate. Therefore, rigorous genetic experiments are required to test these hypotheses.
As mentioned above, uniform planar polarity across a large field of cells requires a global cue with respect to the body axis. In the developing limb, Wnt5a is the only Wnt morphogen that forms a distal-to-proximal gradient (Parr et al. 1993) and regulates PCP (Gao et al. 2011). Thus, it is possible that mesenchymal cells in developing limb buds gain directional information by interpreting Wnt5a dosage changes, with cartilage extending distally in the direction of the Wnt5a gradient. However, a permissive role of Wnt5a signaling in establishing PCP cannot be excluded prior to further rigorous genetic investigations. Collectively, the evidence suggests that Wnt5a/PCP signaling plays a key role in limb morphogenesis. Its exact functional mechanism at different developmental stages warrants further study.
Although Wnt5a null mutants exhibit severe Pr–Di elongation defects, the Pr–Di axis per se is still established to some extent. In the early limb bud of the Wnt5a mutant, prior to cartilage formation, distal limb mesenchymal cells located adjacent to the apical ectodermal ridge (AER) still move toward the overlying ectoderm, albeit at reduced velocity and efficiency compared with those in the wild-type limb. In addition, the division orientation of these distal mutant cells is normal (Gros et al. 2010). This has been explained by AER-derived fibroblast growth factor (FGF) signaling, which controls the velocity of (randomly) moving cells. Cells influenced by FGFs move faster and eventually move closer to the AER through mass action (Gros et al. 2010). A similar model has been proposed for posterior elongation of the tail bud (Benazeraf et al. 2010). Such evidence raises an important question: Is Wnt5a the only morphogen providing an instructive cue to PCP in limb development? As planar polarity of limb mesenchymal cells is always perpendicular to the AER, the AER or FGFs may interact with PCP either directly, as an alternative global cue, or indirectly, by maintaining normal Pr–Di limb patterning. Nonetheless, Wnt5a seems to be absolutely required for PCP establishment in developing limbs, because Wnt5a null mutants show no sign of planar polarity (Gao et al. 2011). Thus, the future challenges surrounding PCP in limb morphogenesis will be to dissect PCP regulatory mechanisms at different stages of development, to distinguish the role of Wnt5a as an instructive cue or a permissive signal in PCP, and to understand the cross talk between Wnt5a and other signaling pathways in regulating PCP. In conclusion, recent advances in identifying the molecular mechanisms underlying Wnt regulation of PCP have shed new light on how limb morphogenesis is achieved at the cellular and molecular levels. The identified signal transduction pathway has opened a door to further unraveling the global cues in PCP signaling and general mechanisms during the regulation of other morphogenetic processes that require PCP signaling, including tail bud elongation, neurulation, neuronal pathfinding, and craniofacial morphogenesis.
CONCLUDING REMARKS
Drosophila genetics has provided a functional framework for the study of PCP and continues to identify new members associated with the core PCP group, as well as their regulators and modulators. Although this wealth of information provides a solid foundation for understanding PCP in other organisms, most notably in vertebrates and specifically in mammals, and recent studies link PCP signaling to human disease, many areas in the PCP field need to be addressed in a vertebrate system. The similarities between fly and vertebrate PCP establishment are overwhelming, but the vertebrate-specific features of PCP require rigorous genetic studies of their own. In particular, PCP-regulated mesenchymal cell polarity, movement, and cellular alignment during vertebrate-specific developmental processes such as gastrulation (CE) and limb elongation require analyses in the mouse and zebrafish. These model systems provide a striking link to congenital diseases that involve defects in PCP. Moreover, the recent link of PCP signaling to ciliopathies and the specific role of cilia positioning as an important downstream morphogenetic event or readout of PCP (Borovina et al. 2010, Hashimoto et al. 2010, Simons & Mlodzik 2008, Song et al. 2010) show that disruption of PCP signaling has significant consequences that go beyond cellular polarity.
In general terms, PCP regulation in vertebrates is more complex than in Drosophila, as it plays very diverse roles during development (including the polarization of epithelial and mesenchymal cells and the regulation of ciliary positions/functions in different tissues). Moreover, the analysis of PCP regulation in vertebrates suffers from problems of redundancy. Thus, genetic studies in Drosophila will continue to identify new regulators and provide insights, enhancing our understanding of the molecular mechanisms and framework of PCP establishment in development and disease.
In addition, a critical issue that we do not discuss here because of space restrictions is the not-yet-resolved relationship between the core Fzd/PCP pathway and the Fat/Ds PCP system. How are they coordinated or linked at the cellular and molecular levels, and how might they converge on cellular effector pathways (if at all)? Moreover, we certainly lack mechanistic insight into the long-range global regulation of PCP orientation in Drosophila and/or vertebrates. Thus, the coordination of cellular polarization across whole tissues and organs remains largely unresolved.
Acknowledgments
Y.Y. is supported by the Intramural Research Program of the National Human Genome Institute of the National Institutes of Health and the Harvard School of Dental Medicine, and M.M. is supported by grants from the NIGMS, NICHD, and NEI of the National Institutes of Health. We wish to thank our lab members and many colleagues for continued stimulating discussions and critical input. We apologize to colleagues whose work could not be cited directly owing to space restrictions.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Yingzi Yang, Email: yingzi_yang@hsdm.harvard.edu.
Marek Mlodzik, Email: Marek.Mlodzik@mssm.edu.
LITERATURE CITED
- Adler PN. Planar signaling and morphogenesis in Drosophila. Dev Cell. 2002;2:525–35. doi: 10.1016/s1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- Adler PN. The frizzled/stan pathway and planar cell polarity in the Drosophila wing. Curr Top Dev Biol. 2012;101:1–31. doi: 10.1016/B978-0-12-394592-1.00001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler PN, Krasnow RE, Liu J. Tissue polarity points from cells that have higher Frizzled levels towards cells that have lower Frizzled levels. Curr Biol. 1997;7:940–49. doi: 10.1016/s0960-9822(06)00413-1. [DOI] [PubMed] [Google Scholar]
- Adler PN, Zhu C, Stone D. Inturned localizes to the proximal side of wing cells under the instruction of upstream planar polarity proteins. Curr Biol. 2004;14:2046–51. doi: 10.1016/j.cub.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Afzal AR, Rajab A, Fenske CD, Oldridge M, Elanko N, et al. Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nat Genet. 2000;25:419–22. doi: 10.1038/78107. [DOI] [PubMed] [Google Scholar]
- Aigouy B, Farhadifar R, Staple DB, Sagner A, Roper JC, et al. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 2010;142:773–86. doi: 10.1016/j.cell.2010.07.042. [DOI] [PubMed] [Google Scholar]
- Andre P, Song H, Kim W, Kispert A, Yang Y. Wnt5a and Wnt11 regulate mammalian anterior-posterior axis elongation. Development. 2015;142:1516–27. doi: 10.1242/dev.119065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre P, Wang Q, Wang N, Gao B, Schilit A, et al. The Wnt coreceptor Ryk regulates Wnt/planar cell polarity by modulating the degradation of the core planar cell polarity component Vangl2. J Biol Chem. 2012;287:44518–25. doi: 10.1074/jbc.M112.414441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antic D, Stubbs JL, Suyama K, Kintner C, Scott MP, Axelrod JD. Planar cell polarity enables posterior localization of nodal cilia and left-right axis determination during mouse and Xenopus embryogenesis. PLOS ONE. 2010;5:e8999. doi: 10.1371/journal.pone.0008999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod JD. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 2001;15:1182–87. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–14. doi: 10.1242/dev.00526. [DOI] [PubMed] [Google Scholar]
- Benazeraf B, Francois P, Baker RE, Denans N, Little CD, Pourquie O. A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature. 2010;466:248–52. doi: 10.1038/nature09151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanot P, Brink M, Samos CH, Hsieh J-C, Wang Y, et al. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–30. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Borovina A, Superina S, Voskas D, Ciruna B. Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat Cell Biol. 2010;12:407–12. doi: 10.1038/ncb2042. [DOI] [PubMed] [Google Scholar]
- Boutros M, Mihaly J, Bouwmeester T, Mlodzik M. Signaling specificity by Frizzled receptors in Drosophila. Science. 2000;288:1825–28. doi: 10.1126/science.288.5472.1825. [DOI] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–18. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- Buechling T, Bartscherer K, Ohkawara B, Chaudhary V, Spirohn K, et al. Wnt/Frizzled signaling requires dPRR, the Drosophila homolog of the prorenin receptor. Curr Biol. 2010;20:1263–68. doi: 10.1016/j.cub.2010.05.028. [DOI] [PubMed] [Google Scholar]
- Chen WS, Antic D, Matis M, Logan CY, Povelones M, et al. Asymmetric homotypic interactions of the atypical cadherin Flamingo mediate intercellular polarity signaling. Cell. 2008;133:1093–105. doi: 10.1016/j.cell.2008.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin ML, Mlodzik M. The Drosophila selectin Furrowed mediates intercellular planar cell polarity interactions via Frizzled stabilization. Dev Cell. 2013;26:455–68. doi: 10.1016/j.devcel.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature. 2006;439:220–24. doi: 10.1038/nature04375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–33. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- Das G, Jenny A, Klein TJ, Eaton S, Mlodzik M. Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes. Development. 2004;131:4467–76. doi: 10.1242/dev.01317. [DOI] [PubMed] [Google Scholar]
- Dechiara TM, Kimble RB, Poueymirou WT, Rojas J, Masiakowski P, et al. Ror2, encoding a receptor-like tyrosine kinase, is required for cartilage and growth plate development. Nat Genet. 2000;24:271–74. doi: 10.1038/73488. [DOI] [PubMed] [Google Scholar]
- Devenport D. The cell biology of planar cell polarity. J Cell Biol. 2014;207:171–79. doi: 10.1083/jcb.201408039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat Cell Biol. 2008;10:1257–68. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport D, Oristian D, Heller E, Fuchs E. Mitotic internalization of planar cell polarity proteins preserves tissue polarity. Nat Cell Biol. 2012;13:893–902. doi: 10.1038/ncb2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoughe S, Dinardo S. dachsous and frizzled contribute separately to planar polarity in the Drosophila ventral epidermis. Development. 2011;138:2751–59. doi: 10.1242/dev.063024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudney K, Ybot-Gonzalez P, Paternotte C, Stevenson RE, Greene ND, et al. Analysis of the planar cell polarity gene Vangl2 and its co-expressed paralogue Vangl1 in neural tube defect patients. Am J Med Genet A. 2005;136:90–92. doi: 10.1002/ajmg.a.30766. [DOI] [PubMed] [Google Scholar]
- Ezan J, Montcouquiol M. Revisiting planar cell polarity in the inner ear. Semin Cell Dev Biol. 2013;24:499–506. doi: 10.1016/j.semcdb.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Fanto M, Weber U, Strutt DI, Mlodzik M. Nuclear signaling by Rac and Rho GTPases is required in the establishment of epithelial planar polarity in the Drosophila eye. Curr Biol. 2000;10:979–88. doi: 10.1016/s0960-9822(00)00645-x. [DOI] [PubMed] [Google Scholar]
- Feiguin F, Hannus M, Mlodzik M, Eaton S. The ankyrin repeat protein Diego mediates Frizzled-dependent planar polarization. Dev Cell. 2001;1:93–101. doi: 10.1016/s1534-5807(01)00010-7. [DOI] [PubMed] [Google Scholar]
- Fenstermaker AG, Prasad AA, Bechara A, Adolfs Y, Tissir F, et al. Wnt/planar cell polarity signaling controls the anterior-posterior organization of monoaminergic axons in the brainstem. J Neurosci. 2010;30:16053–64. doi: 10.1523/JNEUROSCI.4508-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Song H, Bishop K, Elliot G, Garrett L, et al. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell. 2011;20:163–76. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Yang Y. Planar cell polarity in vertebrate limb morphogenesis. Curr Opin Genet Dev. 2013;23:438–44. doi: 10.1016/j.gde.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Mo C, Fraser SE. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature. 2004;430:689–93. doi: 10.1038/nature02796. [DOI] [PubMed] [Google Scholar]
- Goodrich LV. The plane facts of PCP in the CNS. Neuron. 2008;60:9–16. doi: 10.1016/j.neuron.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–92. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev Cell. 2011;21:120–33. doi: 10.1016/j.devcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros J, Hu JK, Vinegoni C, Feruglio PF, Weissleder R, Tabin CJ. WNT5A/JNK and FGF/MAPK pathways regulate the cellular events shaping the vertebrate limb bud. Curr Biol. 2010;20:1993–2002. doi: 10.1016/j.cub.2010.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros J, Serralbo O, Marcelle C. WNT11 acts as a directional cue to organize the elongation of early muscle fibres. Nature. 2009;457:589–93. doi: 10.1038/nature07564. [DOI] [PubMed] [Google Scholar]
- Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, et al. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24:2517–30. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo N, Hawkins C, Nathans J. Frizzled6 controls hair patterning in mice. PNAS. 2004;101:9277–81. doi: 10.1073/pnas.0402802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–54. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Hakeda-Suzuki S, Ng J, Tzu J, Dietzl G, Sun Y, et al. Rac function and regulation during Drosophila development. Nature. 2002;416:438–42. doi: 10.1038/416438a. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Shinohara K, Wang J, Ikeuchi S, Yoshiba S, et al. Planar polarization of node cells determines the rotational axis of node cilia. Nat Cell Biol. 2010;12:170–76. doi: 10.1038/ncb2020. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Hermle T, Guida MC, Beck S, Helmstadter S, Simons M. Drosophila ATP6AP2/VhaPRR functions both as a novel planar cell polarity core protein and a regulator of endosomal trafficking. EMBO J. 2013;32:245–59. doi: 10.1038/emboj.2012.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermle T, Saltukoglu D, Grunewald J, Walz G, Simons M. Regulation of Frizzled-dependent planar polarity signaling by a V-ATPase subunit. Curr Biol. 2010;20:1269–76. doi: 10.1016/j.cub.2010.05.057. [DOI] [PubMed] [Google Scholar]
- Ho HY, Susman MW, Bikoff JB, Ryu YK, Jonas AM, et al. Wnt5a-Ror-Dishevelled signaling constitutes a core developmental pathway that controls tissue morphogenesis. PNAS. 2012;109:4044–51. doi: 10.1073/pnas.1200421109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopyan S, Sharpe J, Yang Y. Budding behaviors: growth of the limb as a model of morphogenesis. Dev Dyn. 2011;240:1054–62. doi: 10.1002/dvdy.22601. [DOI] [PubMed] [Google Scholar]
- Jenny A, Darken RS, Wilson PA, Mlodzik M. prickle and strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. EMBO J. 2003;22:4409–20. doi: 10.1093/emboj/cdg424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A, Reynolds-Kenneally J, Das G, Burnett M, Mlodzik M. Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding. Nat Cell Biol. 2005;7:691–97. doi: 10.1038/ncb1271. [DOI] [PubMed] [Google Scholar]
- Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, et al. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4:610–15. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–54. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- Kibar Z, Bosoi CM, Kooistra M, Salem S, Finnell RH, et al. Novel mutations in VANGL1 in neural tube defects. Hum Mutat. 2009;30:E706–15. doi: 10.1002/humu.21026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibar Z, Torban E, McDearmid JR, Reynolds A, Berghout J, et al. Mutations in VANGL1 associated with neural-tube defects. N Engl J Med. 2007;356:1432–37. doi: 10.1056/NEJMoa060651. [DOI] [PubMed] [Google Scholar]
- Kibar Z, Vogan K, Groulx N, Justice MJ, Underhill DA, Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet. 2001;28:251–52. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- Klein TJ, Mlodzik M. Planar cell polarization: An emerging model points in the right direction. Annu Rev Cell Dev Biol. 2005;21:155–76. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Casal J. The mechanisms of planar cell polarity, growth and the Hippo pathway: some known unknowns. Dev Biol. 2013;377:1–8. doi: 10.1016/j.ydbio.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence PA, Casal J, Struhl G. Cell interactions and planar polarity in the abdominal epidermis of Drosophila. Development. 2004;131:4651–64. doi: 10.1242/dev.01351. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Shelton PMJ. The determination of polarity in the developing insect retina. J Embryol Exp Morphol. 1975;33:471–86. [PubMed] [Google Scholar]
- Lawrence PA, Struhl G, Casal J. Planar cell polarity: one or two pathways? Nat Rev Genet. 2007;8:555–63. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G, Casal J. Planar cell polarity: a bridge too far? Curr Biol. 2008;18:R959–61. doi: 10.1016/j.cub.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Zhang T, Li H, Wu BL, Jin L, Wang HY. VANGL2 mutations in human cranial neural-tube defects. N Engl J Med. 2010;362:2232–35. doi: 10.1056/NEJMc0910820. [DOI] [PubMed] [Google Scholar]
- Lei Y, Zhu H, Duhon C, Yang W, Ross ME, et al. Mutations in planar cell polarity gene SCRIB are associated with spina bifida. PLOS ONE. 2013;8:e69262. doi: 10.1371/journal.pone.0069262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Zhu H, Yang W, Ross ME, Shaw GM, Finnell RH. Identification of novel CELSR1 mutations in spina bifida. PLOS ONE. 2014;9:e92207. doi: 10.1371/journal.pone.0092207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Hallett MA, Zhu W, Rubart M, Liu Y, et al. Dishevelled-associated activator of morphogenesis 1 (Daam1) is required for heart morphogenesis. Development. 2011;138:303–15. doi: 10.1242/dev.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Dudley AT. Noncanonical frizzled signaling regulates cell polarity of growth plate chondrocytes. Development. 2009;136:1083–92. doi: 10.1242/dev.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- Ma C, Moses K. wingless and patched are negative regulators of the morphogenetic furrow and can affect tissue polarity in the developing Drosophila compound eye. Development. 1995;121:2279–89. doi: 10.1242/dev.121.8.2279. [DOI] [PubMed] [Google Scholar]
- Macheda ML, Sun WW, Kugathasan K, Hogan BM, Bower NI, et al. The Wnt receptor Ryk plays a role in mammalian planar cell polarity signaling. J Biol Chem. 2012;287:29312–23. doi: 10.1074/jbc.M112.362681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow F, Topczewski J, Sepich D, Solnica-Krezel L. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol. 2002;12:876–84. doi: 10.1016/s0960-9822(02)00864-3. [DOI] [PubMed] [Google Scholar]
- Matusek T, Djiane A, Jankovics F, Brunner D, Mlodzik M, Mihaly J. The Drosophila formin DAAM regulates the tracheal cuticle pattern through organizing the actin cytoskeleton. Development. 2006;133:957–66. doi: 10.1242/dev.02266. [DOI] [PubMed] [Google Scholar]
- McDonald SL, Silver A. The opposing roles of Wnt-5a in cancer. Br J Cancer. 2009;101:209–14. doi: 10.1038/sj.bjc.6605174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill H. Planar cell polarity: Keeping hairs straight is not so simple. Cold Spring Harb Perspect Biol. 2010;2:a003376. doi: 10.1101/cshperspect.a003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KA, Szabo G, Otero AdS. Methods for the isolation of sensory and primary cilia—an overview. Methods Cell Biol. 2009;94:87–101. doi: 10.1016/S0091-679X(08)94004-8. [DOI] [PubMed] [Google Scholar]
- Mlodzik M. Planar cell polarization: Do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet. 2002;18:564–71. doi: 10.1016/s0168-9525(02)02770-1. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–77. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Sans N, Huss D, Kach J, Dickman JD, et al. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci. 2006;26:5265–75. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Descalzo S, Gomez-Cabrero A, Mlodzik M, Paricio N. Analysis of the role of the Rac/Cdc42 GTPases during planar cell polarity generation in Drosophila. Int J Dev Biol. 2007;51:379–87. doi: 10.1387/ijdb.062250sm. [DOI] [PubMed] [Google Scholar]
- Murdoch JN, Doudney K, Paternotte C, Copp AJ, Stanier P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum Mol Genet. 2001;10:2593–601. doi: 10.1093/hmg/10.22.2593. [DOI] [PubMed] [Google Scholar]
- Myers DC, Sepich DS, Solnica-Krezel L. Convergence and extension in vertebrate gastrulae: cell movements according to or in search of identity? Trends Genet. 2002;18:447–55. doi: 10.1016/s0168-9525(02)02725-7. [DOI] [PubMed] [Google Scholar]
- Narimatsu M, Bose R, Pye M, Zhang L, Miller B, et al. Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell. 2009;137:295–307. doi: 10.1016/j.cell.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Neumann C, Cohen S. Morphogens and pattern formation. Bioessays. 1997;19:721–29. doi: 10.1002/bies.950190813. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Honda H, Takeichi M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell. 2012;149:1084–97. doi: 10.1016/j.cell.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- Paricio N, Feiguin F, Boutros M, Eaton S, Mlodzik M. The Drosophila STE20-like kinase Misshapen is required downstream of the Frizzled receptor in planar polarity signaling. EMBO J. 1999;18:4669–78. doi: 10.1093/emboj/18.17.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr BA, Shea MJ, Vassileva G, McMahon AP. Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development. 1993;119:247–61. doi: 10.1242/dev.119.1.247. [DOI] [PubMed] [Google Scholar]
- Person AD, Beiraghi S, Sieben CM, Hermanson S, Neumann AN, et al. WNT5A mutations in patients with autosomal dominant Robinow syndrome. Dev Dyn. 2010;239:327–37. doi: 10.1002/dvdy.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, et al. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121–33. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada-Hernandez E, Caneparo L, Schneider S, Winkler S, Liebling M, et al. Stereotypical cell division orientation controls neural rod midline formation in zebrafish. Curr Biol. 2010;20:1966–72. doi: 10.1016/j.cub.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Randall RM, Shao YY, Wang L, Ballock RT. Activation of Wnt planar cell polarity (PCP) signaling promotes growth plate column formation in vitro. J Orthop Res. 2012;30:1906–14. doi: 10.1002/jor.22152. [DOI] [PubMed] [Google Scholar]
- Rauch GJ, Hammerschmidt M, Blader P, Schauerte HE, Strahle U, et al. Wnt5 is required for tail formation in the zebrafish embryo. Cold Spring Harb Symp Quant Biol. 1997;62:227–34. [PubMed] [Google Scholar]
- Robinson A, Escuin S, Doudney K, Vekemans M, Stevenson RE, et al. Mutations in the planar cell polarity genes CELSR1 and SCRIB are associated with the severe neural tube defect craniorachischisis. Hum Mutat. 2012;33:440–47. doi: 10.1002/humu.21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saburi S, Hester I, Goodrich L, McNeill H. Functional interactions between Fat family cadherins in tissue morphogenesis and planar polarity. Development. 2012;139:1806–20. doi: 10.1242/dev.077461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagner A, Merkel M, Aigouy B, Gaebel J, Brankatschk M, et al. Establishment of global patterns of planar polarity during growth of the Drosophila wing epithelium. Curr Biol. 2012;22:1296–301. doi: 10.1016/j.cub.2012.04.066. [DOI] [PubMed] [Google Scholar]
- Schwabe GC, Tinschert S, Buschow C, Meinecke P, Wolff G, et al. Distinct mutations in the receptor tyrosine kinase gene ROR2 cause brachydactyly type B. Am J Hum Genet. 2000;67:822–31. doi: 10.1086/303084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwank G, Basler K. Regulation of organ growth by morphogen gradients. Cold Spring Harb Perspect Biol. 2010;2:a001669. doi: 10.1101/cshperspect.a001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–38. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- Sepich DS, Usmani M, Pawlicki S, Solnica-Krezel L. Wnt/PCP signaling controls intracellular position of MTOCs during gastrulation convergence and extension movements. Development. 2011;138:543–52. doi: 10.1242/dev.053959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, McNeill H. Fat and Dachsous cadherins. Prog Mol Biol Transl Sci. 2013;116:215–35. doi: 10.1016/B978-0-12-394311-8.00010-8. [DOI] [PubMed] [Google Scholar]
- Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet. 2008;42:517–40. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Mlodzik M. Planar cell polarity signaling: coordination of cellular orientation across tissues. Wiley Interdiscip Rev Dev Biol. 2012;1:479–99. doi: 10.1002/wdev.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Hu J, Chen W, Elliott G, Andre P, et al. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature. 2010;466:378–82. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G, Casal J, Lawrence PA. Dissecting the molecular bridges that mediate the function of Frizzled in planar cell polarity. Development. 2012;139:3665–74. doi: 10.1242/dev.083550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt D. Frizzled signalling and cell polarisation in Drosophila and vertebrates. Development. 2003;130:4501–13. doi: 10.1242/dev.00695. [DOI] [PubMed] [Google Scholar]
- Strutt D, Strutt H. Differential activities of the core planar polarity proteins during Drosophila wing patterning. Dev Biol. 2007;302:181–94. doi: 10.1016/j.ydbio.2006.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H, Strutt D. Nonautonomous planar polarity patterning in Drosophila: dishevelled-independent functions of frizzled. Dev Cell. 2002;3:851–63. doi: 10.1016/s1534-5807(02)00363-5. [DOI] [PubMed] [Google Scholar]
- Strutt H, Strutt D. Differential stability of Flamingo protein complexes underlies the establishment of planar polarity. Curr Biol. 2008;18:1555–64. doi: 10.1016/j.cub.2008.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–38. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Takeda K, Oishi I, Nomi M, Ikeya M, et al. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes Cells. 2000;5:71–78. doi: 10.1046/j.1365-2443.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- Tawk M, Araya C, Lyons DA, Reugels AM, Girdler GC, et al. A mirror-symmetric cell division that orchestrates neuroepithelial morphogenesis. Nature. 2007;446:797–800. doi: 10.1038/nature05722. [DOI] [PubMed] [Google Scholar]
- Taylor J, Abramova N, Charlton J, Adler PN. Van Gogh: a new Drosophila tissue polarity gene. Genetics. 1998;150:199–210. doi: 10.1093/genetics/150.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Shaping the nervous system: role of the core planar cell polarity genes. Nat Rev Neurosci. 2013;14:525–35. doi: 10.1038/nrn3525. [DOI] [PubMed] [Google Scholar]
- Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, et al. The zebrafish glypican Knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell. 2001;1:251–64. doi: 10.1016/s1534-5807(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent β-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002;109:371–81. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, et al. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–95. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Mikels A, Nusse R. Alternative Wnt signaling is initiated by distinct receptors. Sci Signal. 2008;1:re9. doi: 10.1126/scisignal.135re9. [DOI] [PubMed] [Google Scholar]
- van Bokhoven H, Celli J, Kayserili H, van Beusekom E, Balci S, et al. Mutation of the gene encoding the ROR2 tyrosine kinase causes autosomal recessive Robinow syndrome. Nat Genet. 2000;25:423–26. doi: 10.1038/78113. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon: functions and mechanisms of β-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–77. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Vinson CR, Adler PN. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature. 1987;329:549–51. doi: 10.1038/329549a0. [DOI] [PubMed] [Google Scholar]
- Vinson CR, Conover S, Adler PN. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature. 1989;338:263–64. doi: 10.1038/338263a0. [DOI] [PubMed] [Google Scholar]
- Vladar EK, Antic D, Axelrod JD. Planar cell polarity signaling: the developing cell’s compass. Cold Spring Harb Perspect Biol. 2009;1:a002964. doi: 10.1101/cshperspect.a002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladar EK, Bayly RD, Sangoram AM, Scott MP, Axelrod JD. Microtubules enable the planar cell polarity of airway cilia. Curr Biol. 2012;22:2203–12. doi: 10.1016/j.cub.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB. Closing in on vertebrate planar polarity. Nat Cell Biol. 2004;6:687–89. doi: 10.1038/ncb0804-687. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development. 2002;129:5815–25. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wang B, Sinha T, Jiao K, Serra R, Wang J. Disruption of PCP signaling causes limb morphogenesis and skeletal defects and may underlie Robinow syndrome and brachydactyly type B. Hum Mol Genet. 2011;20:271–85. doi: 10.1093/hmg/ddq462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, Qian D, Yoo SJ, et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–85. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chang H, Nathans J. When whorls collide: the development of hair patterns in frizzled 6 mutant mice. Development. 2010;137:4091–99. doi: 10.1242/dev.057455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26:2147–56. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–58. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- Weber U, Paricio N, Mlodzik M. Jun mediates Frizzled-induced R3/R4 cell fate distinction and planar polarity determination in the Drosophila eye. Development. 2000;127:3619–29. doi: 10.1242/dev.127.16.3619. [DOI] [PubMed] [Google Scholar]
- Weber U, Pataki C, Mihaly J, Mlodzik M. Combinatorial signaling by the Frizzled/PCP and Egfr-pathways during planar cell polarity establishment in the Drosophila eye. Dev Biol. 2008;316:110–23. doi: 10.1016/j.ydbio.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter CG, Wang B, Ballew A, Royou A, Karess R, et al. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- Wong H-C, Bourdelas A, Krauss A, Lee H-J, Shao Y, et al. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell. 2003;12:1251–60. doi: 10.1016/s1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Mlodzik M. The frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. Dev Cell. 2008;15:462–69. doi: 10.1016/j.devcel.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Mlodzik M. A quest for the mechanism regulating global planar cell polarity of tissues. Trends Cell Biol. 2009;19:295–305. doi: 10.1016/j.tcb.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Roman AC, Carvajal-Gonzalez JM, Mlodzik M. Wg and Wnt4 provide long-range directional input to planar cell polarity orientation in Drosophila. Nat Cell Biol. 2013;15:1045–55. doi: 10.1038/ncb2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyngaarden LA, Vogeli KM, Ciruna BG, Wells M, Hadjantonakis AK, Hopyan S. Oriented cell motility and division underlie early limb bud morphogenesis. Development. 2010;137:2551–58. doi: 10.1242/dev.046987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–23. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, et al. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3:69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanfeng WA, Berhane H, Mola M, Singh J, Jenny A, Mlodzik M. Functional dissection of phosphorylation of Disheveled in Drosophila. Dev Biol. 2011;360:132–42. doi: 10.1016/j.ydbio.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Topol L, Lee H, Wu J. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development. 2003;130:1003–15. doi: 10.1242/dev.00324. [DOI] [PubMed] [Google Scholar]
- Yin C, Ciruna B, Solnica-Krezel L. Convergence and extension movements during vertebrate gastrulation. Curr Top Dev Biol. 2009;89:163–92. doi: 10.1016/S0070-2153(09)89007-8. [DOI] [PubMed] [Google Scholar]
- Yin C, Kiskowski M, Pouille PA, Farge E, Solnica-Krezel L. Cooperation of polarized cell intercalations drives convergence and extension of presomitic mesoderm during zebrafish gastrulation. J Cell Biol. 2008;180:221–32. doi: 10.1083/jcb.200704150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa S, McKinnon RD, Kokel M, Thomas JB. Wnt-mediated axon guidance via the Drosophila Derailed receptor. Nature. 2003;422:583–88. doi: 10.1038/nature01522. [DOI] [PubMed] [Google Scholar]
- Yu H, Smallwood PM, Wang Y, Vidaltamayo R, Reed R, Nathans J. frizzled 1 and frizzled 2 genes function in palate, ventricular septum and neural tube closure: general implications for tissue fusion processes. Development. 2010;137:3707–17. doi: 10.1242/dev.052001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Zhu H, Zhang L, Huang S, Cao J, et al. Wls-mediated Wnts differentially regulate distal limb patterning and tissue morphogenesis. Dev Biol. 2012;365:328–38. doi: 10.1016/j.ydbio.2012.02.019. [DOI] [PubMed] [Google Scholar]