SUMMARY
Asrij/OCIAD1 is an endosomal protein expressed in stem cells and cardiovascular lineages and aberrantly expressed in several cancers. We show that dose-dependent modulation of cytokine-dependent JAK/STAT signaling by Asrij regulates mouse embryonic stem cell pluripotency as well as Drosophila hematopoietic stem cell maintenance. Furthermore, mouse asrij can substitute for Drosophila asrij, indicating that they are true homologs. We identify a conserved region of Asrij that is necessary and sufficient for vesicular localization and function. We also show that Asrij and STAT3 colocalize in endosomes and interact biochemically. We propose that Asrij provides an endosomal scaffold for STAT3 interaction and activation, and may similarly control other circuits that maintain stemness. Thus, Asrij provides a key point of control for spatial and kinetic regulation of stem cell signals.
INTRODUCTION
The molecular complexity and spatiotemporal control required to maintain stem cells suggest that several components involved in regulating key signaling pathways remain unidentified. Although the focus has been on signaling molecules, receptors, and target gene activation by transcription factors, transduction of the signal through the cytosol is an important phase that provides an opportunity for signal amplification and regulation (Dobrowolski and De Robertis, 2012). Recent evidence suggests that signal transduction is not limited to the soluble cytosol, and that endosomes and endosome-associated proteins may play a greater role in the process than was previously thought (Sehgal, 2008). In addition to its canonical role in intracellular trafficking, the “endocytic matrix” is integrated into cellular signaling circuits, allowing rapid spatial and temporal control of key cell signaling and transport processes (Scita and Di Fiore, 2010; Sorkin and Von Zastrow, 2002). Understanding how these circuits function in stem cell biology is important for enabling control of stem cell fate and cell reprogramming. Here, we used two divergent model systems to study conserved regulation of signals by the endosomal protein Asrij.
Asrij is a member of the ovarian carcinoma immunoreactive antigen (OCIA) domain family of conserved endocytic proteins of unknown function that are expressed in mouse embryonic stem cells (mESCs) and cardiovascular lineages (Mukhopadhyay et al., 2003). OCIAD1 (human Asrij) is important for integrin-mediated cancer cell adhesion and secondary colony formation (Sengupta et al., 2008; Wang et al., 2010). The high level of asrij expression seen in mESCs is rapidly downregulated upon induction of differentiation, suggesting that Asrij may function in pluripotency. Asrij is also a hematopoietic stem cell (HSC) marker (Phillips et al., 2000). In Drosophila, Asrij is a blood cell marker (Inamdar, 2003) and maintains the HSC niche (Kulkarni et al., 2011). The trafficking function of Asrij is required to regulate hemocyte differentiation. However, the role of asrij in maintaining stem cells is not known. We show that Asrij has a conserved role in maintaining stemness and can modulate signals by controlling effector activation. We propose that in endosomes, Asrij promotes the interaction of signaling components and aids in signal transduction to the nucleus, thereby controlling circuits that maintain stem cell potency.
RESULTS
Asrij Affects ESC Proliferation, Clonogenicity, and Pluripotency
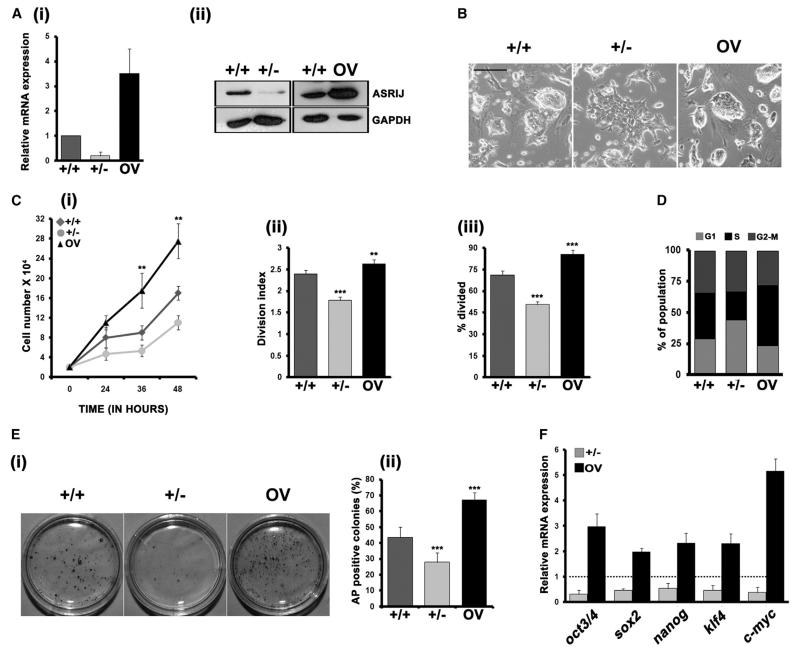
In mESC cultures that differentiated in the absence of Leukemia Inhibitory Factor (LIF), we found that asrij messenger RNA (mRNA) expression was rapidly downregulated as differentiation proceeded (Figures S1A–S1C). This led us to investigate the role of Asrij in ESCs. We used stable ESC lines to modulate Asrij expression (Figure 1A) and analyzed the phenotype. When cultured on mouse embryonic fibroblasts (mEFs), Asrij-depleted (+/−) and -overexpressing (OV) ESCs showed a wild-type (+/+) morphology (Figure S1D). However, in feeder-free culture with LIF, +/− cells formed flat colonies, whereas OV formed refractile compact colonies comparable to those of +/+ cells (Figure 1B). In a growth-curve analysis, +/− cells showed less proliferation and increased doubling time with a larger proportion of cells in G1 phase, whereas OV cells had a significantly higher growth rate, lower doubling time, and an increased S phase as compared with +/+ ESCs (Figures 1C and 1D). These data indicate that Asrij affects ESC proliferation, probably by affecting the cell cycle. We next checked the influence of Asrij levels on the ability of mESCs to remain pluripotent and self-renew. Clonal analysis of Asrij-modulated ESC lines showed that the Asrij level is proportional to the self-renewal capacity (Figure 1E). This was reflected in the expression of the core pluripotency factors oct3/4, sox2, nanog, and klf4, with a lower level of expression in +/− ESCs and a higher level in OV compared with controls (Figure 1F). c-myc expression was also changed in correlation with the altered proliferation capacity. All three mESC lines could generate teratomas in vivo with primary germ layer derivatives; however, OV teratomas showed incomplete differentiation and high total OCT4 expression (Figures S1E and S1F). Taken together, these observations indicate that Asrij overexpression more effectively maintains ESC self-renewal.
Figure 1. Asrij Maintains mESC Self-Renewal and Pluripotency.
Analysis of asrij-modulated ESC lines.
(A) Expression of mRNA (i) and protein (ii).
(B) Morphology.
(C) Quantitative analysis of proliferation over 48 hr (i), and population doubling time analysis (i and ii). Panel (ii) shows increased doubling time for +/− cells and faster doubling for OV cells, and panel (iii) shows reduced and increased percentages of dividing cells for +/− and OV cells, respectively.
(D) Cell-cycle profiles analyzed by flow cytometry. Bars represent % distribution of cells in G1, S, and G2-M.
(E) Image (i) and graph (ii) showing AP+ colonies in a clonal assay.
(F) Quantitative RT-PCR (qRT-PCR) analysis for pluripotency markers.
Statistical significance is indicated by **p < 0.05, ***p < 0.001. Error bars show SD of the mean. Scale bar, 100 μM. See also Figure S1.
Asrij OV mESCs Remain Pluripotent upon Withdrawal of LIF
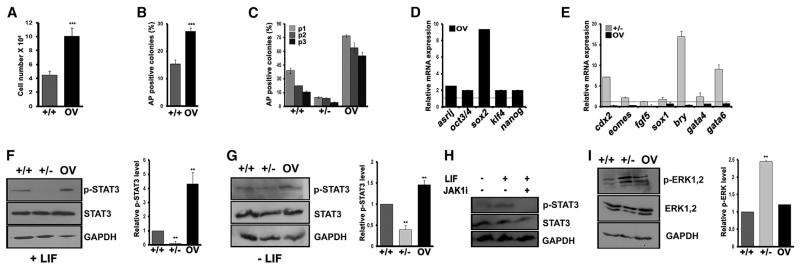
Because mESCs require LIF to maintain the undifferentiated state, we analyzed the ability of +/− and OV cells to maintain pluripotency in a LIF withdrawal assay (see Experimental Procedures). OV cells retained the capacity for increased proliferation, clonogenicity, and pluripotency gene expression compared with controls, even after 4 days of LIF withdrawal (Figures 2A, 2B, and 2D) and over multiple passages of the cultures in the absence of LIF, in contrast to +/+ and +/− cells, which differentiated rapidly. Whereas OV cells showed a higher proportion of alkaline phosphatase (AP)-positive clones compared with controls, +/− cells generated very few undifferentiated clones and could not be cultured beyond three passages (Figure 2C). The ability of OV cells to grow in the absence of LIF was also maintained in serum-free culture (Figure S2A), ruling out the possible contribution of extraneous serum-derived factors. Whereas LIF withdrawal induced the expression of differentiation markers in +/− and +/+ cells within 4 days, OV cells expressed a significantly higher level of pluripotency markers compared with +/+ cells (Figures 2D and 2E). Thus, Asrij expression reduces the LIF dependence of mESCs and hinders their differentiation.
Figure 2. Asrij Reduces the LIF Dependence of ESCs and Promotes STAT3 Phosphorylation.
mESC lines were cultured on 0.1% gelatin in media without LIF for 48 hr unless indicated otherwise. In all cases, values for +/− and OV were compared with those for +/+ cells.
(A–C) Graph representing (A) cell proliferation over 4 days, (B) clonogenicity, and (C) the effect of serial subculture at clonal density for three passages (p).
(D and E) qRT-PCR analysis of (D) pluripotency marker and (E) lineage differentiation marker gene expression.
(F–H) Western blot and graphical representation showing (F–H) pSTAT3 level in culture (F) with LIF or (G) after 4 days of LIF withdrawal or (H) upon treatment with a JAK inhibitor.
(I) pERK level.
Statistical significance is indicated by **p < 0.01, ***p < 0.001. Error bars show SD of the mean. See also Figure S2.
Asrij Promotes STAT3 Phosphorylation and Checks ERK Phosphorylation
LIF is an interleukin-6 (IL-6)-type cytokine that signals by binding to its cognate receptors LIFr and gp130 (Ernst et al., 1996). In mESCs, LIF binding results in the activation of JAK kinases, leading to phosphorylation of cytoplasmic STAT3 by JAKs (Narazaki et al., 1994). STAT3 is the primary effector of the LIF-JAK-STAT signaling axis. Phosphorylated STAT3 (pSTAT3) dimers translocate to the nucleus (Watanabe et al., 2004) to bring about expression of core pluripotency markers, including oct3/4, sox2, and nanog (van Oosten et al., 2012). Since OV cells do not require external LIF, we sought to determine whether Asrij affects JAK-STAT signaling. OV cells showed high levels of pSTAT3 compared with controls, whereas +/− cells had low levels of pSTAT3 activation in both the presence and absence of LIF, although the total STAT3 level was unaffected (Figures 2F and 2G). JAK1 is reported to phosphorylate STAT3 in a LIF-dependent manner (Kunisada et al., 1996). Culturing OV cells with a JAK1 inhibitor abrogated STAT3 phosphorylation (Figure 2H), indicating that Asrij affects JAK1-mediated STAT3 phosphorylation. We also used a known STAT3 phosphorylation inhibitor, JSI-124/cucurbitacin (Blaskovich et al., 2003), which showed a dose-dependent decrease in STAT3 phosphorylation (Figures S3A and S3B). When cultured in the presence of JSI-124, +/+ and OV cells showed a drastic reduction in the expression of pluripotency markers as well as stem cell properties (Figures S3C–S3G). This indicates that STAT3 phosphorylation is indeed required for Asrij-mediated maintenance of ESC properties.
ERK phosphorylation is a mark of mESC differentiation, and suppression of ERK signaling promotes ground-state pluripotency (Nichols et al., 2009). The +/− mESCs showed increased pERK, which correlates with their reduced proliferation and propensity to differentiate (Figure 2I; for further analysis, see Extended Results and Discussion; Figures S2A–S2L). Taken together, these results indicate that Asrij promotes a pluripotent state in ESCs by increasing STAT3 phosphorylation and controlling ERK phosphorylation.
Drosophila Asrij Regulates STAT Activation for HSC Maintenance
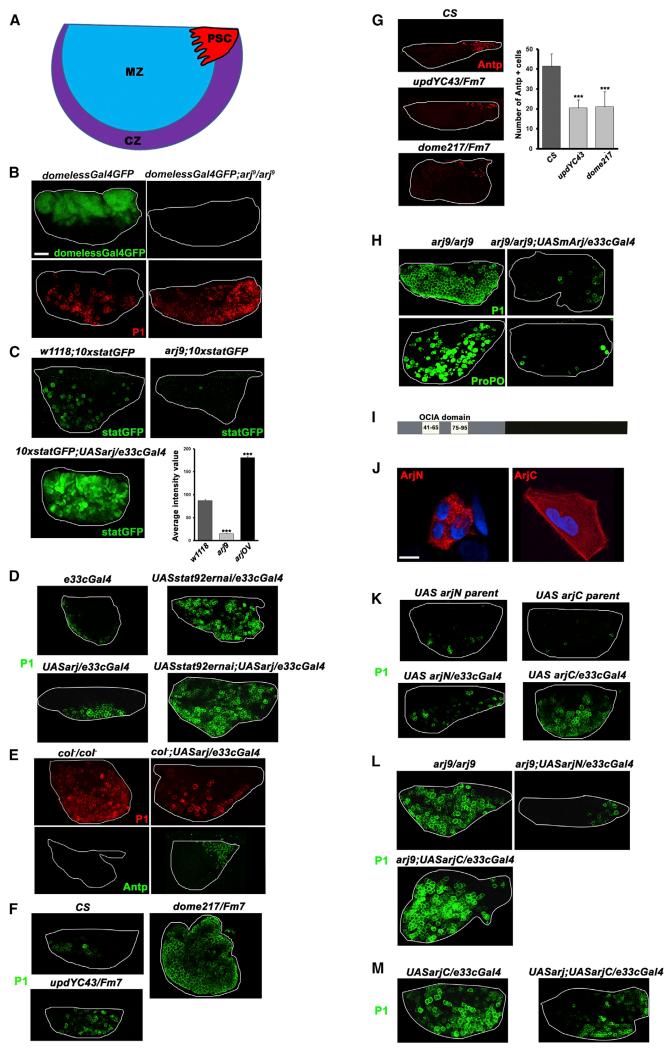
JAK-STAT signaling controls a wide range of cellular functions, including stem cell potency and hematopoiesis. Vertebrates have several IL-6-type cytokines and multiple JAK and STAT family members that often show functional complementation (Liongue et al., 2012; Rawlings et al., 2004). On the other hand, Drosophila has only one LIF receptor (domeless) that binds to the lymph-gland-specific ligand Unpaired3 (upd3), one JAK (hopscotch), one STAT (STAT92e), and one OCIAD1 family member (Asrij). Hence, Drosophila hematopoiesis provides an excellent model to elucidate the relation between Asrij and the JAK-STAT pathway. The main site of Drosophila hematopoiesis is the primary lymph gland lobe, which consists of three developmental zones (Figure 3A). We depleted or overexpressed asrij in the larval lymph gland and assayed for the effect on hematopoiesis and JAK-STAT signaling. The HSC niche (posterior signaling center [PSC]) secretes Upd3, which binds and activates Domeless in multipotent HSCs that reside in the lymph gland medullary zone (MZ) (Jung et al., 2005; Krzemień et al., 2007). Using a DomelessGal4-driven reporter GFP, we found that asrij depletion abolishes Dome expression (Figure 3B), thereby prohibiting JAK-STAT signaling. This is reflected in the inability to maintain stem cells and increased differentiation as reported earlier (Kulkarni et al., 2011). STAT activation is a key outcome of JAK-STAT signaling and is essential for maintenance of hemocyte precursors. Using a 10xStatGFP reporter for STAT activation, we found that Asrij depletion caused a severe reduction in STAT activity, whereas overexpression increased STAT activity compared with controls (Figure 3C). Stat knockdown resulted in reduced STAT activation and hence increased differentiation (Figure 3D). Asrij overexpression in Stat92e knockdown also showed similarly increased differentiation. This indicates that the phenotype resulting from Asrij overexpression is suppressed, supporting the observation that Asrij functions through STAT.
Figure 3. Conserved Role for Asrij in Regulating JAK/STAT Activity and Maintaining Stemness.
(A) Schematic representation of the Drosophila primary lymph gland lobe.
(B–M) The primary lobes of control and mutant/modulated larval lymph glands were assessed and compared. Genotypes are as indicated.
(B) Domeless expression marked by DomelessGFP is lost in the asrij null mutant with precocious differentiation into P1+ plasmatocytes.
(C) A JAK/STAT pathway activation reporter assay shows highly increased stat-GFP activation upon asrij overexpression and decreased statGFP activity in the asrij null mutant. The graph shows the average statGFP intensity values for each genotype (n = 10).
(D) Increased hemocyte differentiation is seen upon stat92e knockdown in Asrij overexpression larvae.
(E) Asrij overexpression in the collier mutant larval lymph gland can rescue premature differentiation into P1+ plasmatocytes and restore a fully functional Antennapedia+ niche.
(F and G) Primary lymph gland lobes of unpaired and domeless hypomorphs phenocopy asrij null mutants as seen by P1 expression (F), and have fewer Antennapedia+ niche cells as indicated in the graph (G).
(H) Precocious differentiation seen in the asrij null mutant is repressed upon expression of mouse asrij as seen by P1+ plasmatocytes and ProPO+ crystal cells.
(I) Schematic representation of Asrij full-length protein showing the N-terminal fragment containing the OCIA domain (ArjN, gray bar) and the C-terminal fragment lacking the domain (ArjC, black bar). Putative hydrophobic stretches are indicated (white bars).
(J) HEK293 cells bearing FLAG-tagged Asrij fragments and immunostained to visualize localization of the fragment ArjN or ArjC.
(K) Lymph gland lobes of wild-type larvae additionally expressing ArjN or ArjC and stained for P1+ plasmatocytes show premature differentiation in ArjC lymph glands.
(L) Premature differentiation in the asrij null mutant can be rescued by forced expression of ArjN, but not ArjC.
(M) Reduced dominant-negative effect of ArjC in Asrij overexpressing lobes.
Statistical significance is indicated by ***p < 0.001. Nuclei were viewed with DAPI staining and the image was used to draw the lymph gland boundary (white line).
Scale bars, 50 μm (B–H and K–M) and 12.5 μm (J).
The early B cell factor (EBF) ortholog Collier is expressed in the PSC and activates JAK-STAT signaling to maintain prohemocytes. col mutants (amorphs) have no prohemocytes (Krzemień et al., 2007). Asrij overexpression in the col mutant lymph glands could rescue the col phenotype (Figure 3E), suggesting that Asrij can maintain niche function in the absence of Col. JAK-STAT pathway mutants, such as hypomorphs of the ligand unpaired (updYC43) and the receptor Domeless (dome217), show premature differentiation to P1+ plasmatocytes (Figure 3F), phenocopying the asrij null mutant. In addition, the Antennapedia-positive (Antp+) niche is reduced (Figure 3G). This confirms the developmental role of Asrij in regulating JAK-STAT signaling and indicates a direct correlation between Asrij levels and STAT activation in maintaining stem cell self-renewal.
Mouse Asrij Can Rescue the Drosophila Null Mutant Phenotype
Because JAK/STAT signaling is highly conserved and Asrij shows significant sequence conservation, especially in the OCIA domain, we tested whether Asrij function is also conserved. Transgenic flies that expressed mouse asrij in the Drosophila null mutant showed complete rescue of premature differentiation (Figure 3H). This indicates that the mouse and fly gene are homologs and could regulate similar signaling networks to maintain stem cells.
The OCIA Domain Is Necessary and Sufficient for Endosomal Localization
Other than the OCIA domain containing two hydrophobic stretches, no remarkable features are predicted for Asrij and the nondomain region is intrinsically unstructured (http://elm.eu.org) (Figure 3I). FLAG-tagged reporter constructs expressing a mouse Asrij-N-terminal fragment (arjN, aa 1–132) that encompasses the OCIA domain or a C-terminal fragment lacking the OCIA domain (arjC, aa 133–257) in human embryonic kidney 293 (HEK293) cells showed vesicular or cytoplasmic localization, respectively (Figure 3J). The same was seen with Drosophila Asrij fragments in Drosophila hemocytes (described below). Thus, the OCIA domain is sufficient to target proteins to endocytic vesicles.
Dominant-Negative Effect of Asrij-C-Terminal Fragment on mESC Pluripotency and Drosophila Hematopoiesis
To test whether vesicular targeting of Asrij is essential for its function, and to analyze the biological significance of the OCIA domain, we generated transgenic Drosophila carrying Asrij fragments (arjN or arjC) downstream of upstream activating sequences (UAS) and expressed them in the lymph gland to look for a dominant-negative effect in the wild-type or a functional rescue in the null mutant (arj9). ArjN expression did not hinder development or hematopoiesis, whereas arjC gave a phenotype similar to that of the null mutant (Figure 3K). Forced expression of arjN, but not arjC, could rescue the premature differentiation in asrij null mutant (Figure 3L). Also, in the presence of excess expression of full-length Asrij, the dominant-negative phenotype of arjC overexpression was milder (Figure 3M). Analysis of fragment localization in hemocytes showed that in transgenic Drosophila too, the arjN fragment that contains the OCIA domain was necessary and sufficient for vesicular localization. Importantly, the unstructured arjC could interfere with Asrij function.
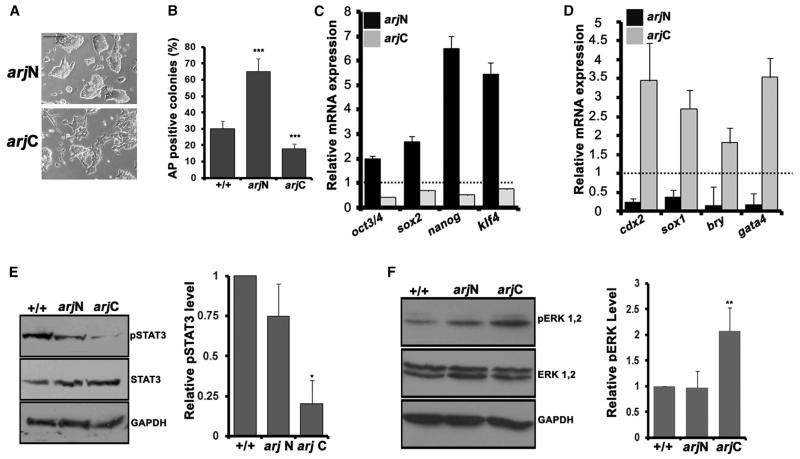
We also generated stable ESC lines additionally expressing either arjN or arjC of mouse Asrij (see Experimental Procedures; Figure S3H). Whereas the arjN ESC colonies resembled OV colonies, the arjC colonies looked flat and differentiated (Figure 4A). This was reflected in the expression of pluripotency marker genes, which was increased in arjN and reduced in arjC compared with controls (Figure 4B). Further, upon LIF withdrawal, differentiation was suppressed and clonogenicity improved in arjN, with the opposite effect in arjC cells (Figures 4C and 4D). arjC also showed reduced pSTAT3 levels (Figure 4E), a phenotype similar to +/− cells, indicating a dominant-negative effect as seen in Drosophila. However, we did not see an increase in STAT3 phosphorylation in the arjN line, suggesting that the full-length protein may be required for this. As for the +/− cells, we observed an increase in ERK phosphorylation in the arjC line (Figure 4F) indicating that full-length Asrij is required to keep ERK phosphorylation in check.
Figure 4. Overexpression of ArjC Reduces STAT3 Phosphorylation in ESCs.
Analysis of arjN and arjC ESC lines.
(A) Morphology.
(B) Graph showing AP+ colonies in a clonal assay.
(C and D) qRT-PCR analysis showing expression of pluripotency markers (C) and differentiation markers (D).
(E and F) Western blot and graphical representation showing the (E) pSTAT3 level and (F) pERK level. GAPDH was used as a normalizing control.
Statistical significance is indicated by *p < 0.02, **p < 0.05, ***p < 0.001. Error bars show SD of the mean. Scale bar, 10 μM. See also Figure S3.
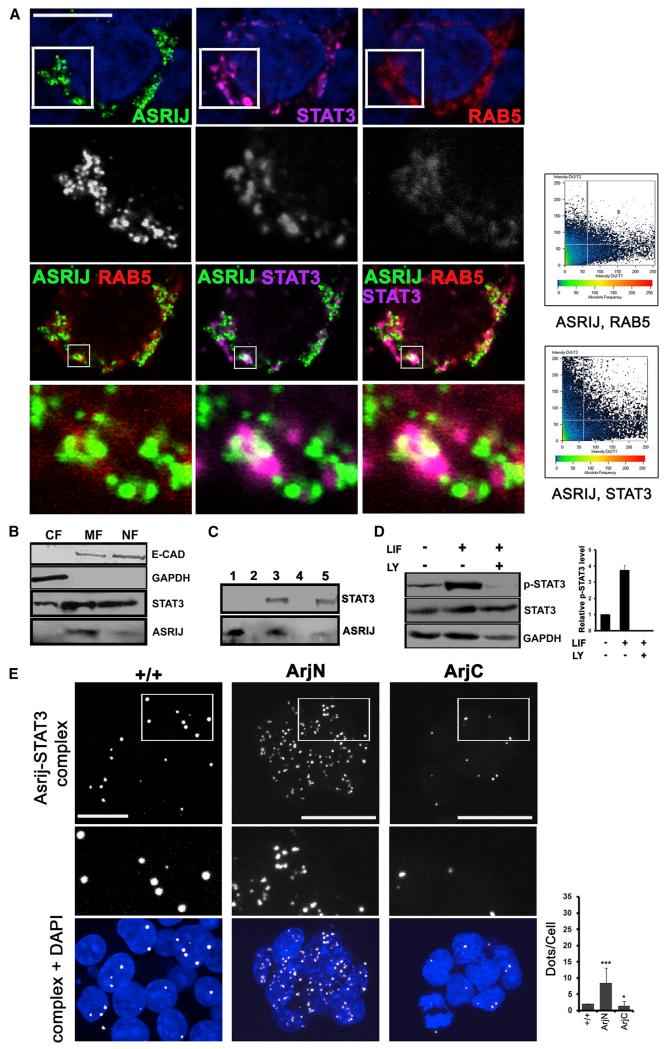
Asrij Colocalizes and Interacts with pSTAT3
Our results show that in mESCs and Drosophila, an endogenous level of Asrij is required for STAT3 activation. Previous reports have suggested that STAT3 could be activated on endosomes (Shah et al., 2006). To determine whether Asrij and STAT3 reside on the same endosomes, we cotransfected asrij ORF and FLAG-STAT3 in HEK293 cells and, using immunolocalization, found that Asrij and STAT3 colocalize on Rab5+ endosomes (Figure 5A). Cellular fractionation showed that Asrij resides primarily in the membrane compartment and to a small extent in the nuclear fraction (Figure 5B). Further, Asrij coimmunoprecipitates with STAT3 upon pull-down with FLAG antibody (Figure 5C). Western blot analysis showed that a significant amount of Asrij protein interacts with STAT3. LY294002 is a known phosphatidylinositol 3-kinase (PI3K) inhibitor that blocks endocytosis by blocking the fusion of clathrin or nonclathrin vesicles with early endosomes (Naslavsky et al., 2003). OV cells cultured in LY294002 showed abrogation of pSTAT3 even after LIF stimulation (Figure 5D). These experiments indicate that endosomal recruitment is essential for STAT3 activation, which could be mediated by Asrij.
Figure 5. Asrij Interacts with STAT3.
(A) HEK293 cells bearing plasmids expressing Asrij, Rab5-RFP, and STAT-FLAG were analyzed by coimmunolocalization of Rab5, STAT3 (FLAG), and Asrij. Graphs show colocalizing pixels.
(B) Western blot of mESC lysate subjected to differential fractionation shows Asrij primarily in the membrane fraction. CF, cytoplasmic fraction; MF, membrane fraction; NF, nuclear fraction.
(C) Western blot analysis of lysate from cells expressing Asrij alone or with FLAG-STAT3 as indicated, subjected to coimmunoprecipitation by FLAG antibody and probed with STAT3 and Asrij antibodies to assess interaction. Lanes 1 and 3: input control; lane 4: beads alone; lanes 2 and 5: FLAG immunoprecipitation, showing coimmunoprecipitation of Asrij only in the presence of STAT3.
(D) Blocking endocytosis abolishes STAT3 phosphorylation. Western blot analysis of the relative pSTAT3 level in +/+ cells treated with the PI3K inhibitor LY290042.
(E) In situ PLA for Asrij and STAT3 in ESCs. Interaction of STAT3 with Asrij or fragments in control cells (+/+) or those overexpressing ArjN or ArjC (as indicated) is seen as white complexes. The graph represents the PLA complex dots/cell.
Nuclei in (A) and (E) were viewed with DAPI staining (blue). Boxed regions in (A) and (E) are shown as a magnified inset below the respective panel, with the blue channel off. Statistical significance is indicated by *p < 0.01, ***p < 0.0001. Error bars show SD of the mean. Scale bar, 12.5 μm.
The interaction of Asrij with STAT3 and the difference seen between arjN and arjC fragments in terms of cellular localization and function raise the interesting question as to which region of Asrij interacts with STAT3. To address this, we used the in situ proximity ligation assay (PLA), which has been used successfully in mESCs to demonstrate protein-protein interactions (Johansson et al., 2010). To assess the interaction of Asrij with STAT3 in situ and correlate it with the pSTAT3 status, we performed a PLA on +/+, ArjN, and ArjC lines. As expected, we saw that Asrij and STAT3 interacted in vivo in mESCs (Figure 5E). Interestingly, ArjN cells showed a significantly increased number of Asrij-STAT3 complexes, indicating that this region can interact with STAT3. On the other hand, ArjC overexpression caused a significant reduction in Asrij-STAT3 complexes compared with +/+, indicating that the dominant-negative phenotype of ArjC results from its ability to block Asrij-STAT3 interaction. This correlates well with the pSTAT3 levels observed in each cell line (Figure 4E).
DISCUSSION
Recent studies have shown that signaling networks are not controlled solely by soluble cytosolic proteins and transcription factors, and that components of the transport machinery can exert rapid spatial and temporal control over cell signaling. However, whether endosomal control can achieve specific regulation of a stem cell phenotype in a dynamic environment remains unclear. Our data suggest that Asrij provides a master regulatory switch to control stem cell signaling. Importantly, we show that this mechanism is conserved in evolution and across stem cell types. Our results support the concept that endosomal proteins can provide precise control of the stem cell state and herald a conceptual advance in stem cell biology.
asrij is a conserved developmental gene that exhibits a dynamic and tightly regulated expression pattern from the earliest stages of development. Loss of this regulation leads to carcinogenesis. We found that in diverse systems, such as mESCs and fly hematopoiesis, Asrij levels affect stemness. In the context of mESCs, Asrij reduced LIF dependence and this was irrespective of autocrine stimulation by the ESCs themselves, as seen with hLIF05 blocking.
LIF/UPD-mediated JAK-STAT signaling also governs HSC maintenance in Drosophila. The Drosophila asrij null mutant has fewer Col+ cells, and we found that JAK/STAT pathway activation is compromised as expression of the receptor is lost. Thus, the reduced niche is incapable of maintaining HSCs, leading to a loss of Dome+ prohemocytes, which are required for receiving and transmitting signals of the pathway. Lack of Asrij inactivates the pathway, and the positive feedback loop that controls Dome expression might be deregulated. However, Asrij does not play a downstream role in the JAK-STAT pathway in maintaining the HSC population, as stat92e depletion in lymph glands along with forced expression of Asrij leads to precocious hemocyte differentiation, thereby disrupting hemocyte homeostasis. The same effect is seen with overexpression of the Asrij C-terminal part in ESCs, indicating conservation of structural domains and function. The col mutant phenotype is rescued by forced expression of Asrij, indicating that Asrij promotes niche and HSC maintenance and can do so in a Col-independent manner. Interestingly, mouse Asrij overexpression also renders the mouse ESCs LIF (JAK/STAT pathway activator) independent, making them resistant to differentiation cues.
Sequence conservation is a good indication of functional homology in proteins. Interestingly, we found that the OCIA domain is necessary and sufficient for vesicular localization, whereas absence of this domain renders the protein soluble, regardless of the species of origin. This is also reflected in Asrij’s function. arjN functionally complements the asrij null mutant, indicating that the OCIA domain is the functional domain. These results prove that endosomal localization of Asrij is essential for its function. In contrast, the C-terminal half lacking the OCIA domain is not essential for stem cell maintenance. However, this region could impart an essential function of protein-protein interaction, as indicated by the dominant-negative effect in flies or ESCs overexpressing arjC. The functional interactions of Asrij may be homotypic or heterotypic. This led us to speculate that Asrij may function in the endosome by interacting with or aiding the interaction of proteins that require endosomal activation, such as STAT3. Asrij and STAT3 interact biochemically, supporting this idea. Protein-protein interaction assays demonstrate that the Asrij N-terminal OCIA domain is essential for its interaction with STAT3, whereas the free C-terminal domain hinders this interaction, suggesting that arjC could interact with Asrij and/or STAT3, thereby sequestering/masking relevant sites/motifs and resulting in a dominant-negative effect. Asrij-STAT3 interaction is essential for STAT3 phosphorylation, because arjC also has a dominant-negative effect on pSTAT3 levels. Alternatively, ArjC may interact with other molecule(s) to block STAT3 phosphorylation; however, this is unlikely given the direct correlation among PLA complexes, pSTAT3 levels, and cellular phenotype. Further, this mechanism is also conserved in Drosophila, where arjN is functionally adequate and arjC has a dominant-negative effect, supporting a direct dependence of STAT3 phosphorylation on the arjN region. However, it should be noted that in mESCs, arjN overexpression does not drastically increase pSTAT3 levels or significantly change pERK levels, suggesting that the full-length protein is essential for efficient pSTAT3 phosphorylation.
How an endosomal protein can impose fine control over signaling, thereby maintaining the delicate balance between pluripotent and differentiated states, has been an intriguing puzzle. Although endosome-associated proteins were previously thought to be soluble, recent studies have suggested that they may play a role in the activation of JAK-STAT signaling (Sehgal, 2008). Preassociation with endosomal membranes (Rab5 and EEA1 positive) is crucial for STAT3 phosphorylation and activation. Small-molecule inhibitors of this association greatly reduce cellular pSTAT3 levels (Sehgal et al., 2002; Shah et al., 2006). Our studies showed that Asrij and STAT3 can reside in the same endosomal compartment. We speculate that Asrij may act as a scaffold, increasing STAT3 recruitment onto endosomes and thus helping in its phosphorylation. Further, overexpression of Asrij, as in OV cells, may produce a factor(s) that stimulates plating efficiency, which in this case would reflect clonogenicity. This factor is likely to be a cytokine (perhaps LIF) or another activator of pathways that regulate pluripotency or cell survival. It is generally accepted that pluripotency is regulated by a complex interconnected signaling network that is stimulated and regulated by extracellular factors (Dejosez and Zwaka, 2012). Because mouse and Drosophila Asrij are homologs, the same mechanism may operate in both systems.
In summary, we show here that an endosomal protein, Asrij, interacts with STAT3 to aid its activation and thereby determine the state of stem cells. Needless to say, this is such a complex process that Asrij could be just one of the numerous adaptors or scaffold proteins that aid in cellular signaling, and many more such molecular integrators invite identification.
EXPERIMENTAL PROCEDURES
The generation of ESC lines with modulated Asrij expression is described in the Extended Experimental Procedures. ESCs were cultured on primary mouse embryonic fibroblasts (mEFs) or with LIF supplementation on 0.1% gelatincoated dishes as previously described (Mukhopadhyay et al., 2003) or in N2B27+LIF+BMP as previously described (Ying et al., 2003). For cell proliferation assays, 2,000 ESCs/cm2 were plated. One set was trypsinized every 12 hr for cell counts. The average of three independent experiments with three replicates for each time point per line was plotted with the SD. For cell-cycle analysis, 50,000 cells/60 mm dish were grown for 48 hr with LIF supplementation. Cells were trypsinized, pelleted, washed, resuspended in PBS, ethanol fixed overnight at 4°C, and stained with propidium iodide containing RNase (BD) for flow-cytometry analysis on a FACS ARIAII (BD). For cell-doubling analysis, 50,000 cells were stained with 2 × 10−6 M PKH26 dye (Sigma) for 5 min. They were then washed, plated on gelatinized 60 mm dishes, harvested by trypsinization after 48 hr, resuspended in PBS, and analyzed by flow cytometry. Data were analyzed by FlowJo software.
For the clonogenicity assay, 100 cells/cm2 were grown for at least 4–5 days with LIF in mES media until visible colonies appeared. Pluripotent clones were identified by AP staining and counted to score for clonogenicity. For clonal passaging, single-cell suspensions were seeded at clonal density on gelatinized dishes and cultured without LIF for 7–10 days in either complete ESC medium or N2B27, harvested for analysis, or passaged again at clonal density until no colonies appeared in the +/− genotype. One set of dishes in each experiment was scored for AP-positive pluripotent clones by staining. Three independent experiments were averaged and plotted with the SD of the mean. For LIF withdrawal assays, 25,000 ESCs per gelatinized 35 mm dish in media without LIF were allowed to differentiate until day 4.
For coimmunolocalization, HEK293 cells cotransfected with constructs pCAG-Asrij that expressed asrij ORF (aa 1–247), pCMVRab5-RFP, and FLAG-STAT3 (a kind gift from Gautam Sethi) were fixed with 4% paraformaldehyde and stained with Asrij and FLAG antibodies, followed by incubation with the appropriate secondary antibodies. Imaging was done using a Zeiss LSM510meta confocal microscope. Images were processed in LSM software and adjusted uniformly for brightness/contrast using Adobe Photoshop CS3.
For coimmunoprecipitation, HEK293T cells were cotransfected with pCAG-Asrij and FLAG-STAT3 constructs, and after 48 hr the processed lysates were incubated with FLAG-antibody-bound Protein-G-Sepharose beads (Sigma). After overnight binding, the beads were pelleted, washed, mixed with loading dye, electrophoresed on SDS-PAGE, and processed for western blot analysis.
To quantitatively determine the fold change in STAT3 and pSTAT3 in the various Asrij-modulated ESC lines, we measured the density and pixel counts for each band using ImageGauge software and normalized the values to the respective glyceraldehyde 3-phosphate dehydrogenase (GAPDH) values before obtaining the pSTAT3/STAT3 ratio. A similar approach was used to determine the fold change in ERK and pERK.
For the in situ PLA, semiconfluent cultures of ESC lines grown on gelatinized coverslip dishes were fixed in 2% paraformaldehyde, washed, permeabilized with 0.1% triton × (Sigma Chemical), blocked using 3% fetal bovine serum in a humid chamber at 37°C incubator for 1 hr, and then incubated with primary antibodies (anti Asrij 1:25 and anti STAT3 1:50) for 2 hr at 37°. The primary antibody was washed off and Duolink in situ PLA reaction (Olink Biosciences) was carried out according to the manufacturer’s instructions. PLA complexes were detected by imaging under a Zeiss LSM510meta confocal microscope and analyzed by LSM software. Only PLA spots larger than 0.5 μm were counted.
The Drosophila stocks used, transgenic fly generation, and additional details are provided in the Extended Experimental Procedures.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ian Chambers (CRM, Edinburgh), Jyotsna Dhawan and Kouichi Hasegawa (inStem, Bangalore), and M.R.S. Rao (JNCASR) for helpful suggestions. We also thank Arpita Mukhopadhyay, Sandip Khadekar, Deeti Shetty, Ridim Mote, and members of our laboratory for help with experiments and inputs. This work was supported by the Department of Science and Technology and the Department of Biotechnology, Government of India, and the UK India Education and Research Initiative (UKIERI).
Footnotes
Supplemental Information includes Extended Results and Discussion, Extended Experimental Procedures, three figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2013.07.029.
REFERENCES
- Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–1279. [PubMed] [Google Scholar]
- Dejosez M, Zwaka TP. Pluripotency and nuclear reprogramming. Annu. Rev. Biochem. 2012;81:737–765. doi: 10.1146/annurev-biochem-052709-104948. [DOI] [PubMed] [Google Scholar]
- Dobrowolski R, De Robertis EM. Endocytic control of growth factor signalling: multivesicular bodies as signalling organelles. Nat. Rev. Mol. Cell Biol. 2012;13:53–60. doi: 10.1038/nrm3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Oates A, Dunn AR. Gp130-mediated signal transduction in embryonic stem cells involves activation of Jak and Ras/mitogen-activated protein kinase pathways. J. Biol. Chem. 1996;271:30136–30143. doi: 10.1074/jbc.271.47.30136. [DOI] [PubMed] [Google Scholar]
- Inamdar MS. Drosophila asrij is expressed in pole cells, trachea and hemocytes. Dev. Genes Evol. 2003;213:134–137. doi: 10.1007/s00427-003-0305-0. [DOI] [PubMed] [Google Scholar]
- Johansson H, Vizlin-Hodzic D, Simonsson T, Simonsson S. Translationally controlled tumor protein interacts with nucleophosmin during mitosis in ES cells. Cell Cycle. 2010;9:2160–2169. doi: 10.4161/cc.9.11.11841. [DOI] [PubMed] [Google Scholar]
- Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–2533. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- Krzemień J, Dubois L, Makki R, Meister M, Vincent A, Crozatier M. Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature. 2007;446:325–328. doi: 10.1038/nature05650. [DOI] [PubMed] [Google Scholar]
- Kulkarni V, Khadilkar RJ, Magadi SS, Inamdar MS. Asrij maintains the stem cell niche and controls differentiation during Drosophila lymph gland hematopoiesis. PLoS ONE. 2011;6:e27667. doi: 10.1371/journal.pone.0027667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisada K, Hirota H, Fujio Y, Matsui H, Tani Y, Yamauchi-Takihara K, Kishimoto T. Activation of JAK-STAT and MAP kinases by leukemia inhibitory factor through gp130 in cardiac myocytes. Circulation. 1996;94:2626–2632. doi: 10.1161/01.cir.94.10.2626. [DOI] [PubMed] [Google Scholar]
- Liongue C, O’Sullivan LA, Trengove MC, Ward AC. Evolution of JAK-STAT pathway components: mechanisms and role in immune system development. PLoS ONE. 2012;7:e32777. doi: 10.1371/journal.pone.0032777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay A, Das D, Inamdar MS. Embryonic stem cell and tissue-specific expression of a novel conserved gene, asrij. Dev. Dyn. 2003;227:578–586. doi: 10.1002/dvdy.10332. [DOI] [PubMed] [Google Scholar]
- Narazaki M, Witthuhn BA, Yoshida K, Silvennoinen O, Yasukawa K, Ihle JN, Kishimoto T, Taga T. Activation of JAK2 kinase mediated by the interleukin 6 signal transducer gp130. Proc. Natl. Acad. Sci. USA. 1994;91:2285–2289. doi: 10.1073/pnas.91.6.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky N, Weigert R, Donaldson JG. Convergence of non-clathrin- and clathrin-derived endosomes involves Arf6 inactivation and changes in phosphoinositides. Mol. Biol. Cell. 2003;14:417–431. doi: 10.1091/mbc.02-04-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Silva J, Roode M, Smith A. Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development. 2009;136:3215–3222. doi: 10.1242/dev.038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RL, Ernst RE, Brunk B, Ivanova N, Mahan MA, Deanehan JK, Moore KA, Overton GC, Lemischka IR. The genetic program of hematopoietic stem cells. Science. 2000;288:1635–1640. doi: 10.1126/science.288.5471.1635. [DOI] [PubMed] [Google Scholar]
- Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J. Cell Sci. 2004;117:1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- Scita G, Di Fiore PP. The endocytic matrix. Nature. 2010;463:464–473. doi: 10.1038/nature08910. [DOI] [PubMed] [Google Scholar]
- Sehgal PB. Paradigm shifts in the cell biology of STAT signaling. Semin. Cell Dev. Biol. 2008;19:329–340. doi: 10.1016/j.semcdb.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal PB, Guo GG, Shah M, Kumar V, Patel K. Cytokine signaling: STATS in plasma membrane rafts. J. Biol. Chem. 2002;277:12067–12074. doi: 10.1074/jbc.M200018200. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Michener CM, Escobar P, Belinson J, Ganapathi R. Ovarian cancer immuno-reactive antigen domain containing 1 (OCIAD1), a key player in ovarian cancer cell adhesion. Gynecol. Oncol. 2008;109:226–233. doi: 10.1016/j.ygyno.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Shah M, Patel K, Mukhopadhyay S, Xu F, Guo G, Sehgal PB. Membrane-associated STAT3 and PY-STAT3 in the cytoplasm. J. Biol. Chem. 2006;281:7302–7308. doi: 10.1074/jbc.M508527200. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nat. Rev. Mol. Cell Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- van Oosten AL, Costa Y, Smith A, Silva JC. JAK/STAT3 signalling is sufficient and dominant over antagonistic cues for the establishment of naive pluripotency. Nat. Commun. 2012;3:817. doi: 10.1038/ncomms1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Michener CM, Belinson JL, Vaziri S, Ganapathi R, Sengupta S. Role of the 18:1 lysophosphatidic acid-ovarian cancer immunoreactive antigen domain containing 1 (OCIAD1)-integrin axis in generating late-stage ovarian cancer. Mol. Cancer Ther. 2010;9:1709–1718. doi: 10.1158/1535-7163.MCT-09-1024. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Saito K, Kinjo M, Matsuda T, Tamura M, Kon S, Miyazaki T, Uede T. Molecular dynamics of STAT3 on IL-6 signaling pathway in living cells. Biochem. Biophys. Res. Commun. 2004;324:1264–1273. doi: 10.1016/j.bbrc.2004.09.187. [DOI] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.