Abstract
Epidemiologic studies show that the cardiovascular diseases are associated with multiple factors such as raised serum total cholesterol, increased LDL, increased platelet aggregation, hypertension and smoking. In-vitro studies have confirmed the ability of some plants of Allium species to reduce these parameters. Therefore, we evaluated anti-platelet aggregation effect of some Allium species (Allium ampeloprasum, A. hirtifolium, A. haemanthoides, A. vavillovi, A. atroviolaceum, A. jesdianum, A. shelkovnikovii) using arachidonic acid (AA) and adenosine diphosphate (ADP) as platelet aggregation inducers. The screening results for methanolic extract of Allium species showed that the maximum effect of anti-platelet aggregation was related to A. atroviolaceum. This extract inhibited the in-vitro platelet aggregation induced by AA and ADP with IC50 values of 0.4881 (0.4826-0.4937) mg/ml and 0.4945 (0.4137-0.5911) mg/ml respectively. These results support the hypothesis that the dietary intake of Allium could be beneficial for prevention of cardiovascular diseases.
Key Words: Allium species, Alliaceae, Cardiovascular disease, Anti-platelet, Platelet aggregation
Introduction
Cardiovascular diseases are the most common reason of morbidity and mortality worldwide (1). In the western countries they account for almost 17 million deaths; in developing countries their occurrence is becoming important along with industrialization, urbanization and changing life styles (2). Cardiovascular disease is associated with multiple factors including the increased ability of platelets to aggregate (3). Increased platelet aggregation has an important role in the etiology of cardiovascular diseases, which might be the result of multiple different mechanisms. In addition, in the atherosclerotic states, platelets adhere to the damaged vessel surface and aggregate themselves in response to agonists such as adenosine-5-diphosphate (ADP), thrombin, collagen and thromboxane A2 (4)
Interfering with the process of platelet aggregation is one of the therapeutic strategies for the treatment of platelet-related cardiovascular diseases.
In recent years, increasing attention has been drawn to health benefits of food, beverages, spices and seasonings beyond their nutritional, tasting and flavoring significance. Especially, plant-originating foodstuff such as green tea, soy bean, grape and their products have been recognized to exhibit the preventive and/or therapeutic effects on various diseases (5). Plant materials have long been used as traditional medicines for the treatment of a number of ailments and diseases (2). Scientific search for new therapeutic agents is strongly supported by epidemiological studies on medicinal plants. One would assert that it is feasible to make detailed studies so as to identify active ingredients of plant aiming to develop new drugs in the case the beneficial aspects are realized (6).
Since ancient time, a deep knowledge has supported the use of Allium species as food and/or medicine. In-vitro studies have confirmed the ability of some plants of Allium species to reduce the parameters which are taken as risk factors in cardiovascular diseases such as raised serum total cholesterol, increased LDL, increased platelet aggregation, hypertension and smoking (7). Allium is the largest and most important representative genus of alliaceae family. Alliaceae is a family of flowering plants, includes about 30 genera and more than 670 species, and is widely distributed in the northern hemisphere (8).
It is believed that Allium is a very variable and taxonomically difficult genus. The most recent taxonomy proposal for this genus, based on morphological characters and as also molecular data, accepted about 780 species belonging to fifteen subgenera and 56 sections. Recently, more taxa were newly described raising this number to more than 800 species. Currently, about 115 species (including more than 40 endemic ones) are known in the territory of Iran. These numbers indicate that Iran belongs to main center of Allium diversity in southwest and central Asia (9).
A. atroviolaceum has been used as foods, spices, and herbal remedies (10). The initial report of scientific research on Allium began in second half of the 19th century, coined by the work of Louis Pasteur who highlighted the antibacterial properties of garlic in 1858. Garlic has also been used for several epidemic diseases. In recent years, much attention has focused on various aspects of the pharmacological effects of extracts and isolated compounds from these plants. Several biological activities have been reported for Allium species such as antibacterial, antiviral, antiparasitic, antifungal, anti-protozoa and anti-diabetic, effects on the circulatory and cardiovascular system, antioxidant, anticarcinogenic effects and treatment of common cold (8).
Many of the biological effects of Allium species are related to thiosulfinate, volatile sulfur compounds, typical of these plants, which are also responsible for their pungent aroma and taste properties. However, these compounds are unstable and give rise to transformed products. For this reason, recent attention has been focused on polar compounds which are more stable for cooking and storage. Among these compounds, sapogenins, saponins, and flavonoids are the main classes found (8, 11).
The present research was aimed at investigating the effect of total extracts of selected group of Allium species on platelet aggregation induced by AA and ADP. No previous anti-platelet aggregation study has been conducted on the plants which are selected for the present research.
Experimental
Chemicals and Instruments
All the chemicals were purchased from Sigma-Aldrich Chemie Gmbh (Germany) or Merck (Germany) companies. The chemicals were of analytical grade. ADP and AA were purchased from Bio/Data, Corp (Germany). Anti-platelet aggregation activity of Allium species extracts was determined, using APACT-4004 aggregometer (LABiTec, Ahrensburg, Germany).
Plant material
The herbs were selected based on two factors: Iranian traditional source of medicine and scientific papers. Having surveyed the flora of Iran and the diversity of Allium species, different regions were chosen as target. After collecting specimens, voucher specimens were confirmed and deposited at the herbarium (Table 1).
Table 1.
Scientific names, part of herb, place of collection, voucher number and place of deposition of plants which have been used in the present study
| Scientific Name | Part of herb | Place of collection | Voucher number | Place of specimen deposition |
|---|---|---|---|---|
| Allium ampeloprasum L. | Aerial part | Mount Rig, lordegan- Shahr-e-kord | 8012 | 1 |
| Allium hirtifolium Boiss. | Bulb | Mount Sabz-e-koh, Shahr-e-kord | 8010 | 1 |
| Allium hirtifolium Boiss. | Aerial part | Mount Sabz-e-koh, Shahr-e-kord | 8010 | 1 |
| Allium haemanthoides Boiss. & Reut. | Aerial part | Izeh, Ahvaz | 8008 | 1 |
| Allium vavillovi M.Pop & Vved. | Aerial part | Shahr-e-reza, Isfehan | 8009 | 1 |
| Allium atroviolaceum Boiss | Aerial part | Mount Rig, lordegan- Shahr-e-kord | 8013 | 1 |
| Allium jesdianum Boiss. & Buhse | Bulb | Tezerjan-Yazd | 2252 | 2 |
| Allium shelkovnikovii Grossh. | Bulb | Tabriz | 3033 | 3 |
1Herbarium of department of Pharmacognosy of faculty of Pharmacy of Shahid Beheshti University of medical sciences
2 Herbarium of Yazd Research Center for Agriculture and Natural Resources
3 Herbarium of East Azarbaijan Research Center for Agriculture and Natural Resources
Extraction
Aerial parts of herbs were carefully washed under tap water and left to dry in controlled temperature (22˚C) without exposure to the light and moisture. They were chopped and then passed through sieve size 60 (25/0mm). The bulbous onions of plants, after collecting and cleaning up, were kept in nitrogen tanks in order to preserve the volatile compounds. They were then transferred to Freeze Dryer and dried. In each case, 500g of powdered plant was extracted with 1500ml methanol by maceration method. Extraction was conducted for 48h and repeated four times under stirring at 80r/ minute rate. The extracts were then filtered and concentrated under vacuum at 40˚C using a rotary evaporator.
Preparation of samples for Platelet aggregation test
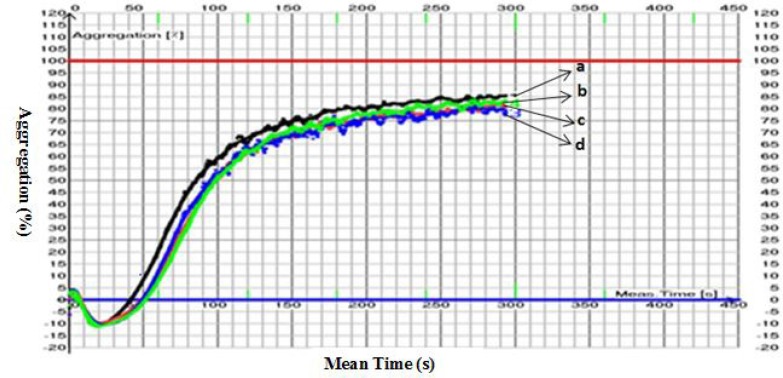
The extracts were dissolved in DMSO at the concentration of 250mg/ml. In fact, the initial stock solution contained the maximum amount of the extract which was soluble in 1 ml. The entire stock was passed through a Syringe Filter PVDF 0.22µm which is compatible with the organic solvent DMSO. One microliter of the solution of extract in DMSO was added to 200µl of plasma so that, the final concentrations of extracts in plasma were from 1.25 to 0.3125mg/ml. Inspecting the filtrate for particles showed that despite the filtration, some particulate matters formed in the solution a few minutes after filtration. Therefore, in order to overcome the problem, polyvinyl alcohol (PVA) was added to the solutions at the concentration of 1% prior to the filtration process. No particulate matter formed in the solution even after one hour of the addition of PVA. A blank mixture of DMSO and PVA in plasma showed no effect on platelet aggregation process (Figure 1). Therefore a series of solutions of the plant extract was prepared in DMSO/PVA 1%.
Figure 1.
Aggregograms obtained for DMSO (a), DMSO/PVA 1% (b, c, d).
Blood collection
Blood was obtained from healthy volunteers who did not take any medication for 14 days and were fasting overnight prior to the study. Blood was collected in falcon tube containing 0.1 volume of 2.2% sodium citrate. Platelet rich plasma (PRP) was prepared by the centrifugation of citrated blood at 100g for 10min. The residual blood was centrifuged at speed of 1500g for 15 min to give platelet poor plasma (PPP). Platelets were counted under microscope and the platelet count was adjusted to (250 ± 25) ×109/L. Supernatant PRP was diluted with PPP.
Platelet aggregation studies
Platelet aggregation responses were monitored with a turbidometric method using an optical aggregometer. Platelet aggregation was expressed as an increase in light transmission. The levels of transmission were calibrated by PPP and PRP. Aliquots of 200μl of PRP were distributed in the test cuvettes and placed in incubation chamber of aggregometer at 37°C. Platelet aggregation was measured using PRP after activation by the addition of ADP or AA according to Born method (12). 1µl of the prepared solution of methanolic extracts in DMSO/PVA was added to the PRP, 5 min prior to the activation with ADP or AA. The extent of aggregation was quantified by determining the maximum height of the curve. The platelet aggregation inhibitory activity was expressed as percent inhibition compared with that measured for the vehicle (DMSO/PVA1%) alone (13). Finally, percent inhibition for extract is calculated according to the following formula:
100 × ((1 - (D/S)) = percent inhibition
Aggregation in the sample = D
Aggregation in the presence of solvent (DMSO/PVA1%) = S
Statistical analysis
The anti-aggregation value of each extract was expressed as either % inhibition or IC50 values (the concentration of the compound causing 50% inhibitory effects). The IC50 values were estimated by non linear curve-fitting and presented as their respective 95% confidence limits. One-way analysis of variance (ANOVA) followed by Tukey’s post test was used to assess the significant differences (p < 0.05) between the responses caused by the extracts. All the statistical analyses were accomplished using the computer software GraphPad Prism 3.02 for Windows (GraphPad Software, USA).
Results and discussion
Aerial parts of the plant species Allium were extracted by maceration method with methanol. After concentrating under the vacuum at 40˚C with rotary evaporator, the yield of extraction was calculated (Table 2). The highest % yield is related to leaves of A. atroviolaceum. While the bulb of A. jesdianum Boiss. & Buhse has lowest % yield of extraction.
Table 2.
Yields of methanolic extraction by maceration method
| Yield of extraction | Scientific name |
|---|---|
| 7.3% | Allium ampeloprasum L. |
| 5% | Allium hirtifolium Boiss. |
| 3% | Allium hirtifolium Boiss. |
| 6% | Allium haemanthoides Boiss. & Reut |
| 7% | Allium vavillovi M.Pop & Vved . |
| 8% | Allium atroviolaceum Boiss. |
| 2.5% | Allium jesdianum Boiss. & Buhse |
| 2.7% | Allium shelkovnikovii Grossh. |
PVA (polyvinyl alcohol) was used to prevent the formation of particulate matters in the plant extract solutions in DMSO. Investigating the effect of blank DMSO/PVA solution on platelet aggregation confirmed that the presence of PVA at 1% concentration has no effect on platelet behavior. To the best of our knowledge, this is the first report for the application of PVA in determining anti-platelet aggregation activity of solutions which tend to form insoluble particles in DMSO.
Anti-platelet aggregation activity is not uncommon from plants extracts. Gady’s et al (2009) have reported a significant platelet aggregation inhibitory activity from the total aqueous extract of leaves of parsley (IC50: 5.6mg/ml) against the aggregation induced by ADP (14). Although there are citations of antiplatelet aggregation activity of some Allium species (15-17), there are no previous reports, at least to our knowledge, on the activity of the genus on in-vitro platelet aggregation induced by AA and ADP. Allium extracts which were added to PRP 5min prior to their stimulation by AA (1.35mM) and ADP (5µM) inhibited platelets aggregation in a concentration-dependent manner. The effects of extracts at different concentrations were measured and compared to each other. The results were expressed as IC50 values with their respective 95% confidence limits (Table 3).
Table 3.
Effect of methanolic extract of Allium species on in-vitro platelet aggregation induced by AA (1.35mM) and ADP (5μM).
| Plant species | IC 50(ADP) (mg/ml) + | IC 50(AA) (mg/ml) + |
|---|---|---|
| A. ampeloprasum (Aerial part) | 0.6982 (0.6089-0.8005)cd | - |
| A. hirtifolium (Bulb) | 0.8693 (0.7806-0.9681)e | 0.8765 (0.8765-0.8766)d |
| A. hirtifolium (Aerial part) | - | 0.6843 (0.5665-0.8266)c |
| A. haemanthoides (Aerial part) | 0.7366 (0.6856-0.7913)d | - |
| A. vavillovi (Aerial part) | 0.5524 (0.4997-0.5525)b | - |
| A. atroviolaceum (Aerial part) | 0.4945 (0.4137-0.5911)a | 0.4881 (0.4826-0.4937)a |
| A. jesdianum (Bulb) | 0.6607 (0.5140-0.8491)c | 0.5228 (0.5057-0.5404)b |
| A. shelkovnikovii (Bulb) | 0.6905 (0.4448-1.072)c | 0.8365 (0.7495-0.9336)d |
Note: The IC50 value of the positive control, quercetin, was measured as 0.1197 (0.1047-0.1362) mg/ml against AA and 0.1982 (0.1405-0.2131) mg/ml against ADP.
The IC50 values are presented with their respective 95% confidence limits (n = 3)
Letters (a-e) denote homogenous subsets at p < 0.05 (Tukey’s post test).
In the present study, among the eight Allium extracts tested, seven extracts (A. ampeloprasum, A. hirtifolium [Bulb], A. haemanthoides, A. vavillovi, A. atroviolaceum, A. jesdianum and A. shelkovnikovii) were found to possess satisfactory inhibitory effect on platelet aggregation against ADP. The IC50 values for A. ampeloprasum, A. hirtifolium (Bulb), A. haemanthoides, A. vavillovi, A. atroviolaceum, A. jesdianum and A. shelkovnikovii extracts are presented in Table 3. According to Tukey’s multiple comparison test, the mentioned extracts showed significant differences in their IC50 values (p < 0.05) except A. jesdianum verses A. haemanthoides, A. jesdianum vs A. shelkovnikovii, A. haemanthoides vs A. ampeloprasum, A. haemanthoides vs A. shelkovnikovii and A. ampeloprasum vs A. shelkovnikovii . The IC50 (ADP) values of extracts increased in the following order: A. atroviolaceum < A. vavillovi < A. jesdianum ≤ A. shelkovnikovii ≤ A. ampeloprasum ≤ A. haemanthoides ≤ A. hirtifolium (Bulb). Based on the data obtained, the A. atroviolaceum extract with IC50 value of 0.4945 (0.4137-0.5911) has maximum potency for inhibition of platelet aggregation induced by ADP.
Furthermore, among the eight Allium extracts tested, five extracts (A. hirtifolium [Bulb], A. hirtifolium [Aerial part], A. atroviolaceum, A. jesdianum and A. shelkovnikovii) were found to have satisfactory inhibitory effect on platelet aggregation against AA. The IC50 (AA) values for A. hirtifolium (Bulb), A. hirtifolium (Aerial part), A. atroviolaceum, A. jesdianum and A. shelkovnikovii extracts are presented in Table 3. According to Tukey’s multiple comparison test, the mentioned extracts showed significant differences in their IC50 values (p < 0.05) except A. shelkovnikovii verses A. hirtifolium (Bulb). The IC50 values of extracts increased in the following order: A. atroviolaceum < A. jesdianum < A. hirtifolium (Aerial part) < A. shelkovnikovii ≤ A. hirtifolium (Bulb). Based on the data obtained the A. atroviolaceum extract with IC50 value of 0.4881 mg/ml (0.4826-0.4937) has maximum antiplatelet aggregation capacity induced by ADP.
However, A. hirtifolium (Aerial part) extract produced a weak activity on in-vitro platelet aggregation induced by ADP (5μM) while A. ampeloprasum (Aerial part), A. haemanthoides (Aerial part) and A. vavillovi (Aerial part) extracts caused a weak effect on in-vitro platelet aggregation induced by AA (1.35mM). They did not achieve ≥ 50% inhibition of platelet aggregation activity. The maximum inhibition (%) was 41.32% ± 3.15 for A. hirtifolium (Aerial part), 37.01% ± 4.34 for A. ampeloprasum (Aerial part), 31.85% ± 5.21 for A. haemanthoides (Aerial part) and 41% ± 3.67 for A. vavillovi (Aerial part) at maximum concentration of 1.25 mg/ml.
Blood platelets, beside their physiological function, play a critical role in the pathogenesis of some cardiovascular diseases such as arterial hypertension, atherosclerosis and subsequent ischemic events. This study highlights Allium anti-platelet properties. This finding is in agreement with other previous published data (15-17). We have shown that in-vitro, methanolic Allium extract inhibited in concentration-dependent manner, AA and ADP-induced platelet aggregations. Our results indicate that the maximum effect of anti-platelet aggregation was related to A. atroviolaceum. Therefore, A. atroviolaceum with an IC50 value 0.4881 (0.4826-0.4937) mg/ml inhibits platelet aggregation induced by AA and to a lesser extent aggregation induced by ADP (p value < 0.05). However Hiyasat et al (2009) have reported that A. ursinum and A. sativum inhibit platelet aggregation induced via the ADP pathway and to a lesser extent the aggregation induced by epinephrine, whereas Aggregate Resources Act (ARA)-, collagen- and A23187-induced aggregations were not affected (15).
Medicinal plants are potential sources of lead compounds which can be used for further study and optimization as new drugs (18). Phytochemical analysis of Allium species has revealed the presence of polar compounds such as sulfur compounds and flavonoids, saponins and sapogenins (8). It is very unlikely that only one compound in the extract has been responsible for the observed anti platelet activity and it is more likely that a combination of compounds are involved in the exerted inhibitory effect, such as polyphenolic compounds that could prevent platelet functions. Additionally numerous in-vitro and in-vivo studies have shown that flavonoids inhibited primary homeostasis and many pathways associated with platelet activation and aggregation (14).
In conclusion, the present work demonstrated that Allium extracts could inhibit in-vitro platelet aggregation induced by AA and ADP. These results support the hypothesis that the dietary intake of Allium may be beneficial in normalizing platelet hyperactivation, in nutritional prevention of cardiovascular diseases which are potentially interesting in the development of new prevention strategies. Therefore, they are good candidates for further in-vitro and in-vivo studies to find potential lead compound for antiplatelet aggregation.
Acknowledgements
The authors would like to thank Research Deputy of Shahid Beheshti University of Medical Sciences in Iran for the financial support of this research. This study was a part of PhD thesis of Zahra Lorigooini, proposed and approved in Faculty of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
- 1.Mackay J, Mensah G, editors. Atlas of Heart Disease and Stroke. Geneva: World Health Organization (WHO); 2004. pp. 9–24. [Google Scholar]
- 2.Amrani S, Harnafi H, Gadi D, Mekhfi H, Legssyer A, Aziz M, Martin-Nizard F, Bosca L. Vasorelaxant and anti-platelet aggregation effects of aqueous Ocimum basilicum extract. J. Ethnopharmacol. . 2009;125:157–62. doi: 10.1016/j.jep.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 3.Allison GL, Lowe GM, Rahman K. Aged garlic extract may inhibit aggregation in human platelets by suppressing calcium mobilization. J. Nutr. . 2006;136:789s–792s. doi: 10.1093/jn/136.3.789S. [DOI] [PubMed] [Google Scholar]
- 4.Steinhubl SR, Michelson MDAD, Barry SC. The Verifynow System. Platelets. Burlington: Academic Press; 2007. pp. 509–18. [Google Scholar]
- 5.Furusawa M, Tsuchiya H, Nagayama M, Tanaka T, Nakaya K, Iinuma M. Anti-platelet and membrane-rigidifying flavonoids in brownish scale of onion. J. Health Sci. . 2003;49:475–80. [Google Scholar]
- 6.Scherer C, Jacob C, Dicato M, Diederich M. Potential role of organic sulfur compounds from Allium species in cancer prevention and therapy. Phytochem. Rev. . 2009;8 349-68-68. [Google Scholar]
- 7.Rahman K, Lowe GM. Garlic and cardiovascular disease: A critical review. J. Nutr. . 2006;136:736S–740S. doi: 10.1093/jn/136.3.736S. [DOI] [PubMed] [Google Scholar]
- 8.Lanzotti V. The analysis of onion and garlic. J. Chromatogr. A . 2006;1112:3–22. doi: 10.1016/j.chroma.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Neshati F, Fritsch RM. Seed characters and testa sculptures of some Iranian Allium L. species (Alliaceae) Feddes Repert . 2009;120:322–32. [Google Scholar]
- 10.Jabrane A, Ben Jannet H, Miyamoto T, Mirjolet JF, Duchamp O, Harzallah-Skhiri F, Lacaille-Dubois MA. Spirostane and cholestane glycosides from the bulbs of Allium nigrum L. Food Chem. . 2010;125:447–455. [Google Scholar]
- 11.Nickavar B, Yousefian N. Inhibitory effects of six Allium species on α-Amylase enzyme activity. Iran. J. Pharm. Res. . 2009;8:53–7. [Google Scholar]
- 12.Born GVR, Cross MJ. The aggregation of blood platelets. J. Physiol. . 1963;168:178–95. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amidi S, Kobarfard F, Bayandori Moghaddam A, Tabib K, Soleymani Z. Electrochemical synthesis of novel 1, 3-Indandione derivatives and evaluation of their antiplatelet aggregation activities. Iran. J. Pharm. Res. . 2013;12(Suppl.):91–103. [PMC free article] [PubMed] [Google Scholar]
- 14.Gadi D, Bnouham M, Aziz M, Ziyyat A, Legssyer A, Legrand C, Lafeve FF, Mekhfi H. Parsley extract inhibits in vitro and ex vivo platelet aggregation and prolongs bleeding time in rats. J. Ethnopharmacol. . 2009;125:170–4. doi: 10.1016/j.jep.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Hiyasat B, Sabha D, Grötzinger K, Kempfert J, Rauwald JW, Mohr FW, Dhein S. Antiplatelet activity of Allium ursinum and Allium sativum. Pharmacol. . 2009;83:197–204. doi: 10.1159/000196811. [DOI] [PubMed] [Google Scholar]
- 16.Makheja AN, Bailey JM. Antiplatelet constituents of garlic and onion. Agents Actions . 1990;29:360–363. doi: 10.1007/BF01966468. [DOI] [PubMed] [Google Scholar]
- 17.Goldman IL, Kopelberg M, Debaene JE, Schwartz BS. Antiplatelet activity in onion (Allium cepa) is sulfur dependent. Thromb. Haemostasis . 1996;76:450–452. [PubMed] [Google Scholar]
- 18.Chua TK, Koh HL. Medicinal plants as potential sources of lead compounds with anti-platelet and anti-coagulant activities. Mini-Rev. Med. Chem. . 2006;6:611–24. doi: 10.2174/138955706777435751. [DOI] [PubMed] [Google Scholar]