Abstract
Humans and chimpanzees are the natural hosts for HIV. Non-human primate models of SIV/SHIV infection in rhesus, cynomologus and pigtail macaques have been used extensively as excellent model systems for pathogenesis and vaccine studies. However, owing to the variability of disease progression in infected macaques, a phenomenon identical to humans, coupled with their prohibitive costs, there exists a critical need for the development of small-animal models in which to study the untoward effects of HIV-1 infection. Owing to the fact that rodents are not the natural permissive hosts for lentiviral infection, development of small animal models for studying virus infection has used strategies that circumvent the steps of viral entry and infection. Such strategies involve overexpression of toxic viral proteins, SCID mice engrafted with the human PBLs or macrophages, and EcoHIV chimeric virus wherein the gp120 of HIV-1 was replaced with the gp80 of the ecotropic murine leukemia virus. Additional strategy that is often used by investigators to study the toxic effect of viral proteins involves direct stereotactic injection of the viral protein(s) into specific brain regions. The present report is a compilation of the applications of direct administration of Tat into the striatum to mimic the effects of the viral neurotoxin in the CNS. Added advantage of this model is that it is also amenable to repeated intraperitoneal cocaine injections, thereby allowing the study of the additive/synergistic effects of both the viral protein and cocaine. Such a model system recapitulates aspects of HAND in the context of drug abuse.
Keywords: HIV, Animal model, Cocaine
Introduction
Intravenous drug use (IVDU) and HIV-1 infections are two linked global health crises since needle sharing is a well-recognized mode of HIV-1 transmission. Drugs of abuse accelerate the incidence and progression of HIV-1-associated neurological disorders (HAND). Drug-abusing HIV-1 positive individuals exhibit more severe cognitive impairment compared with the non drug-abusing HIV positive counterparts. Cocaine, often abused by HIV-infected patients, has been suggested to worsen HIV-associated CNS disease. Although antiretroviral therapy (ART) has diminished the incidence of HIV-1-associated dementia (HAD), milder forms of neurological disease do persist. Increased survival rates resulting from ART therapy usage have led to an increase in the prevalence of HAND. Despite the recognized impact of the abuse of cocaine on the clinical course of HIV-1-associated brain pathology, mechanisms underlying the ability of cocaine to enhance the pathological effects of HIV-1 in the brain remain elusive.
It has been demonstrated earlier that use of crack cocaine is a risk factor for acquisition of HIV-infection and is also independently associated with progression to AIDS (Larrat and Zierler 1993; Fiala et al. 1998; Webber et al. 1999). In recent years there has been an emergence of a cohort of HIV-infected individuals that are cocaine-abusers. It is thus likely that interplay of HIV-1 and cocaine in HIV-infected cocaine abusers plays a role in progression of clinical AIDS. Cocaine impairs the functions of macrophages and lymphocytes (Klein et al. 1993; Mao et al. 1996; Baldwin et al. 1997; Eisenstein and Hilburger 1998; Friedman et al. 2003) and enhances HIV-1 expression in these cells (Peterson et al. 1990; Bagasra and Pomerantz 1993; Nair et al. 2000; Roth et al. 2002; Steele et al. 2003). It has been postulated that cocaine acts as a catalyst in the susceptibility and progression of HAND (Larrat and Zierler 1993; Fiala et al. 1998; Webber et al. 1999). Epidemiological studies on drug abusers with AIDS link abuse of cocaine (by different routes), even more than other drugs, to increased incidence of HIV seroprevalence and progression to AIDS (Chaisson et al. 1989; Anthony et al. 1991; Chiasson et al. 1991; Baldwin et al. 1998; Doherty et al. 2000).
HAND comprises of varying degrees of cognitive impairments categorized either as asymptomatic neurocognitive impairment (ANI), minor cognitive disorder (MCD) or HAD. While a number of excellent non-human primate models of HAD are available, recapitulation of HAND in an animal model remains problematic in the field. While various rodent models have been developed and while none of these truly represent HAND, they serve the purpose of mimicking some specific aspects of HAND. These models have been useful in evaluating HIV-1 gene function and pathologic effects by HIV-1 genes and gene products. There are a number of modified mouse strains and models available in which HIV infection, pathogenesis and anti-HIV drug pharmacology can be studied. Some of the models have been utilized to evaluate vaccine-induced HIV-1 immunity, usually limited to short-term vaccine or therapeutic protocols. These models include: 1) Direct injection of HIV proteins either into the CNS or blood via intravenous (IV) route in rats or mice (Hayman et al., 1993; Philippon et al., 1994; Jones et al., 1998; Bansal et al., 2000; Aksenov et al., 2001); 2) Severe combined immunodeficient (SCID) model involving direct stereotactic injection of HIV-infected human monocytes into the CNS of immunodeficient mice (Tyor et al., 1993; Persidsky et al., 1996; Persidsky and Gendelman, 2002); 3) Transgenic (Tg) mice with constitutive or inducible expression of HIV proteins (Kim et al., 2003). Additionally, there is also the HIV Tg rat model expressing all of the HIV proteins except gag and pol (Reid et al., 2001; Vigorito et al., 2007). Models involving direct injection of viral proteins/infected cells into the CNS have the inherent advantage: 1) rapid induction of neurotoxicity; 2) dissect the role of specific proteins in the intact CNS milieu; 3) allows short term testing of therapeutic targets such as platelet derived growth factor (PDGF). The present report will test the hypothesis that microinjection of HIV proteins into specific brain regions coupled with administration of drugs of abuse such as cocaine can serve as valuable animal models to test the toxicity and reciprocally, to test the efficacy of therapeutic interventions. Such model systems can be valuable in recapitulating aspects of HAND in the context of drug abuse while also providing a platform for preclinical testing of therapeutic interventions.
Method and materials
Animals
Pregnant female Sprague Dawley rats and C57BL/6 N wild type (WT) mice were purchased from (Charles River Laboratories, Inc., Wilmington, MA). CCR2 knockout (KO) mice (Taconic, Hudson, NY) have been backcrossed 10 generations to a C57BL/6 N inbred background. All of animals were housed under conditions of constant temperature and humidity on a 12-h light, 12-h dark cycle, with lights on at 0700 h. Food and water were available ad libitum. All animal procedures were performed according to the protocols approved by the Institutional Animal Care and Use Committee of the University Of Nebraska Medical Center.
Surgery and microinjection
Ten week old C57BL/6 N and CCR2 KO mice (n=3/group) were anesthetized with 2.5 % isofluorane and placed in a stereotaxic apparatus for cannula implantation. Using the stereotaxic coordinates, AP +0.86 mm posterior, ML-1.8 mm lateral to midline, and DV −3.5 mm to bregma according to Franklin and Paxinos mouse brain atlas. A permanent 26-gauge stainless steel guide cannula (C315G; Plastics One, Roanoke, VA) was implanted into the right striatum. The guide cannula was secured in place using gel adhesive and dental cement applied sequentially to the skull. A 33-gauge stainless steel dummy cannula was used to seal the guide cannula when not in use. After surgery the animals were housed individually to avoid damage to the dummy guide cannula.
Following striatum cannulation animals were allowed to recover for 7 d. For CCL2+Tat group, 2 μl of CCL2 (400 ng/μl) was microinjected into the striatum once daily for 2 days followed by microinjection of Tat (500 ng/μl) for another 2 days. Control and Tat alone group of mice were injected with the same volume of Tat or sterile saline. All microinjections were performed using a 33-gauge stainless steel injector connected to a 10 μl syringe which was operated by an infusion pump set at the rate of 0.4 μl/min. An additional minute was allowed for diffusion and prevention of backflow through the needle track before the injector was withdrawn. Histological verification of the striatum cannula was performed at the end of each experiment.
For the neuroprotection study of PDGF, following striatal cannulation animals were allowed to recover for 7 d and were randomly divided into six groups (n=5): Saline, Tat, PDGF alone, PDGF plus Tat, and SKF96365 plus PDGF plus Tat (using two doses of the SKF96365 -see below) groups. Tat and PDGF each were injected at a concentration of 1 μg per 2 μl/mouse/day for 2 days. Saline control mice were injected with the same volume of sterile saline (2 μl). In the PDGF plus Tat group of mice, PDGF was first micro-injected for 2 days followed by HIV-1 Tat microinjection for subsequent 2 days. To determine the optimal dose of the TRP channel blocker in mice, SKF 96365 freshly dissolved in 0.9 % sterile saline, was injected at two different concentrations, (0.02 μmol & 0.2 μmol/2 μl/mouse; concentrations determined from our in vitro study) for 2 consecutive days, followed by PDGF and Tat microinjections. All microinjections were performed using a 33-gauge stainless steel injector connected to a 10 μl syringe, which was operated by an infusion pump set at the rate of 0.4 μl/min. An additional minute was allowed for diffusion and prevention of back-flow through the needle track before the injector was withdrawn. Histological verification of the striatum cannula was performed at the end of each experiment.
Immunohistochemistry
For immunohistochemistry mice were perfused by transcardial perfusion using chilled 4 % paraformaldehyde. Free-floating sections encompassing the entire midbrain were sectioned at 40 μm on a cryostat. For tyrosine hydroxylase (TH) immunostaining, tissue sections were incubated with primary antibodies overnight at 4 °C. Primary antibodies used in this study were as follows: rabbit anti-TH (1:4000, Sigma, USA). Immunostaining was visualized by using 3, 3′-diaminobenzidine as the substrate. Quantitative (neuronal number) estimates of TH positive cell bodies were performed ipsilaterally in the areas of interest using stereology method.
Stereology
Quantitative (neuronal number) estimates of TH positive cell bodies were performed ipsilaterally in the areas of interest. This brain area was defined anatomically by atlas and the agreement of three neuroscientists. Selecting every third section of the SNc, from a random initial sort, insured random sampling. Counts utilized the optical fractionator method and the Stereologer software package. The microscope used was a Nikon Eclipse 80i, linked to a Sony 3CCD Color Digital Video Camera, operating an Advanced Scientific Instrumentation MS-2000 motorized Stage input into a Dell Precision 650 Server and a high resolution plasma monitor. The area of interest was defined with 4×/1.3 aperture dry lenses and the stereology was performed at high magnification with 100×/1.4 aperture oil immersion lenses (yielding 3,600×). This allowed for clear visualization of the nucleolus and precise definition of the cell walls. When the areas of interest were identified, the area was precisely outlined and checked against an atlas. The inclusion grid was randomly applied by the software and high resolution microscopy was used to count TH positive neurons. Descriptive data was presented for numbers of TH positive neurons per animal. Independent samples tests (t-test for equality of means) are presented between groups and within groups for cell counts of TH positive cells. Univariate Analysis of Variance (ANOVA) is performed for cell counts.
Bone Marrow Macrophage (BMM) isolation and cultivation
Both male C57 BL/6 mice (Charles River Laboratory) and CCR2 KO mice (4–5 week) were used as bone marrow-derived macrophage (BMMs) donors. Briefly, the femur was removed, bone marrow cells were dissociated into single-cell suspensions, and were cultured for 10 days supplemented with 1,000 U/ml macrophage colony stimulating factor (MCSF, a generous gift from Wyeth-Pfizer, Cambridge, MA). Cultured BMMs were 98 % CD11b+ as evidenced by flow cytometry. For tracking experiments, cells were labeled with the membrane dye PKH26 according to the manufacturer's instructions (Sigma-Aldrich, St. Louis, MO).
In vivo monocyte transmigration assay
Assay of monocyte transmigration into the brain was performed in C57BL/6 mice. Animals were divided into 3 groups (n=6). These were saline, cocaine, and BD1047 and cocaine. Cocaine and BD1047 each were injected intraperitoneally (i.p.) at a doses of 20 mg/kg once daily. In the BD1047 plus cocaine group of mice, BD1047 was first injected for 2 days followed by cocaine injection for subsequent 7 days. For CCR2 KO mice, cocaine was injected as described above. On the 8th day, animals were injected with PKH 26-labeled BMM at a concentration at 107/100 μl through tail vein. Twenty four hrs following cell infusion, the animals were sacrificed and subjected to transcardial perfusion with saline to remove labeled monocytes from tissue blood vessels. Brain tissues were then removed and frozen at −80 °C until cryosection. Perivascular and parenchyma was counted in the entire area of three coronal brain sections: 1.94, 1.34 and 0.14 mm to bragma according to the previous report (Schilling et al. 2009).
Assessment of the Blood Brain Barrier (BBB) integrity
Assay of BBB integrity was performed in C57BL/6 and Egr-1−/−mice. C57BL/6 mice were divided into 5 groups (n=6): (a) Saline, (b) Cocaine, (c) Cocaine plus anti-PDGF-BB neutralizing antibody, (d) Cocaine plus isotype control antibody and (e) Mannitol. Egr-1−/− mice were treated with either saline or cocaine. Cocaine was injected at a dose of 20 mg/kg once daily on day 1 for 7 days intraperitoneally (i.p.). In the antibody plus cocaine group, cocaine was first injected for 2 days followed by antibody injection (i.p.) on days 3 and 5 at a concentration of 25 μg/mouse/injection (anti-PDGF-BB neutralizing Ab; AF-220-NA, or isotype control goat IgG1; AB-108-C, R&D systems). On the 8th day, animals were injected in the tail vein with 200 μl sodium fluorescein (6 mg/ml; Sigma) in PBS that was allowed to circulate for 15 min. As a positive control, 200 μl of mannitol (2 M) was injected through tail vein to circulate for 1 h followed by sodium fluorescein injection. The mice were then anesthetized with isoflurane in oxygen and perfused with 30 ml heparinized saline through the left ventricle. The brains were then harvested and homogenized in PBS (1:10 g/v). The homogenates were precipitated in 15 % trichloroacetic acid (1:1 v/v) and centrifuged at 1,000 g for 10 min. The pH was adjusted by adding 125 μl of 5 M sodium hydroxide to 500 μl supernatant aliquots, and fluorescence was detected with a fluorescence plate reader with excitation at 485 nm and emission at 530 nm, followed by quantification according to the previously described report (Chen et al. 2009).
In vivo monocyte transmigration assay
Assay of monocyte transmigration into the brain was performed in C57BL/6 mice. Animals were divided into 4 groups (n=6): (a) Saline, (b) Cocaine, (c) Cocaine plus anti-ALCAM and (d) Cocaine plus isotope control antibody. Cocaine was injected at a dose of 20 mg/kg i.p for 7 d. For the ALCAM neutralizing antibody study mice were injected with cocaine for 7 d as described above; additionally on days 3, 5 and 7 of cocaine injection, co-administration of either anti-ALCAM or isotope control antibody (250 μg each i.p; monoclonal MAB 656 or mouse IgG1, MAB 002, R&D Systems, Minneapolis, MN) was also performed. Antibody concentrations and treatment regimens were based on previously published report (Cayrol et al. 2008). On the 8th day post-cocaine injection, animals were injected with PKH 26-labeled BMM at a concentration of 107 cells/100 μl through tail vein. Twenty four hrs following cell infusion, the animals were sacrificed and subjected to transcardial perfusion with saline to remove labeled monocytes from tissue blood vessels. Brain tissues were then removed and frozen at −80 °C until cryosection. In all the six animals per group perivascular and parenchyma were counted in the entire area of the three coronal brain sections: 1.94, 1.34 and 0.14 mm to bregma according to the previously described report (Schilling et al. 2009).
Microvessel isolation and immunofluorescence staining
Brain microvessels were isolated as described previously (Huang et al. 2010). Under anesthesia, animals were perfused with saline; the brains were removed and immediately immersed in ice-cold isolation buffer A [103 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, and 15 mM HEPES; pH 7.4 with Complete Protease Inhibitor (Roche)]. The choroid plexus, meninges, cerebellum, and brain stem, were removed followed by homogenization of the brains in 5 ml isolation buffer B [Buffer A with 25 mM NaHCO3, 10 mM glucose, 1 mM Na pyruvate, and dextran (molecular weight 64kD; 10 g/l); pH 7.4] with Complete Protease Inhibitor. 26 % dextran (was then added to the homogenates, followed by centrifugation at 5,800×g for 20 min. Cell pellets were resuspended in isolation buffer B and filtered through a 70 μm mesh filter (Becton Dickinson). Filtered homogenates were repelleted by centrifugation and smeared on slides.
For immunofluorescence staining, brain microvessels smeared on slides were fixed for 10 min at 95 °C, followed by incubation with 3 % formaldehyde in PBS for 10 min at 25 °C. The slides were washed five times with PBS, permeabilized with 0.1 % Triton X-100 for 30 min, rewashed five times in PBS, and blocked in 1 % BSA in PBS for 30 min at 25 °C. Samples were then incubated with rabbit ALCAM (polyclonal rabbit, 1:100 dilution; sc-25624 Santa Cruz Biotechnology) and Cav-1 (monoclonal mouse, 1:100 dilution; sc-70516 Santa Cruz Biotechnology) antibodies (1:100) overnight at 4 °C. The slides were washed with PBS and incubated with Alexa Fluor 594-conjugated anti-rabbit or AlexaFluor 488-conjugated anti-mouse IgG for 1 h at RT. After a final washing with PBS, the slides were mounted with ProLong Gold Antifade reagent to visualize the nuclei as previously reported (Huang et al. 2010). The immunofluorescent images were captured using confocal microscopy.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance with a post hoc Student t-test. Results were judged statistically significant if p<0.05 by analysis of variance.
Results
Protective role of CCL2/CCR2 axis against Tat-induced dopaminergic neuronal loss in the substantia nigra
One of the hallmark neuropathological features of HAD is the loss of nigrostriatal neurons (Itoh et al. 2000). In order to determine the protective effect of CCL2 on dopaminergic neurons, WT and CCR2 KO mice were injected with either saline or CCL2 in the striatum followed by HIV-1 Tat microinjection and examined 7 days later for TH positive neurons in the susbtantia nigra region of the brain. As shown in Fig. 1a and, as expected, there was increased neuronal loss in the Tat-injected WT mice, as evidenced by decreased TH staining. Interestingly, there was a greater reduction of TH positive neurons in the substantia nigra of Tat-injected CCR2 KO mice. While CCL2 mediated protection in the WT mice (as evidenced by increased TH positive neurons), there was no protection against Tat in CCR2 KO mice as quantified in Fig. 1b.
Fig. 1.

Protective role of CCL2/CCR2 axis against Tat-induced dopaminergic neuronal loss in the substantia nigra. (a) Representative mesencephalic sections from WT and CCR2 KO mice microinjected with either saline or Tat in the presence and absence of CCL2 were examined 7 days later for TH positive neurons. (b) Fewer SNc TH-positive neurons survived in Tat treated CCR2 KO mice compared to WT littermates. CCL2 exerted neuroprotection in WT but not CCR2 KO mice. SNc neurons (means ± S.E.M. from 4 mice per group) were counted by stereology. **p<0.01 vs saline WT mice; ˆˆp<0.01 vs saline KO mice; #p<0.05 vs Tat injected WT mice; +p<0.05 vs Tat injected WT mice. Scale bar: 300 μm
PDGF-mediated protection of dopaminergic neurons against HIV Tat in vivo
To investigate the relevance of PDGF-mediated protection in vivo, the neuroprotective effect of PDGF on neuronal survival was determined in adult mice injected intrastriatally with either saline or PDGF followed by microinjection with HIV-1 Tat. Seven days later mice brains were examined for TH positive neurons in the substantia nigra. As shown in Fig. 2a, there was increased neuronal loss in Tat-injected mice as evidenced by decreased TH staining. This effect was ameliorated in mice pre-treated with PDGF as quantified in Fig. 2b, indicating that PDGF protected neurons against HIV Tat. Similar to saline injected mice, exposure to PDGF alone had no significant effect on neuronal survival. Furthermore, to confirm the role of transient receptor potential (TRP) channels in PDGF-mediated neuroprotection against Tat, mice were pretreated the TRP channels blocker SKF96365 prior to treatment with PDGF and HIV Tat. Pre-treatment of mice with SKF96365 (0.2 μmol) followed by exposure to PDGF and Tat resulted in attenuation of PDGF-mediated neuroprotection. SKF96365 alone did not exert any effect on dopaminergic neurons.
Fig. 2.

PDGF protects dopaminergic neurons against Tat-induced neurotoxicity in the substantia nigra. (a) Representative mesencephalic sections from different groups of mice treated with PDGF and/or HIV Tat in the presence or absence of TRPC blocker SKF96365 were examined for TH positive neurons counted by stereology (b). There was increased loss of TH-positive neurons in the substantia nigra of Tat alone treated group of mice compared with the saline controls. Pre-treatment with PDGF resulted in amelioration of Tat toxicity in the substantia nigra neurons, and this effect was significantly attenuated in mice pre-treated with SKF 96365 (0.2 μmol). *p<0.05 vs saline group; #p<0.05 vs Tat; +p<0.05 vs PDGF + Tat. SN: Substantia Nigra. Scale bar: 300 μm. SKF: SKF 96365 (0.2 μmol)
Role of MCP-1 in cocaine-mediated monocyte transmigration in vivo
To validate the role of cocaine/σ-1R axis in MCP-1-mediated monocyte transmigration, PKH26-labeled bone marrow-derived mouse monocytes were transplanted by IV injection into mice treated with or without cocaine. Of particular note, the distribution of these PKH26 labeled monocytes was mostly within the perivascular cuff, although some of the monocytes also entered the parenchyma as indicated by arrows in Fig. 3a. Histological examination of mouse brain sections revealed increased transmgiration of PKH26-labeled monocytes into the CNS of cocaine-treated mice compared with mice not injected with cocaine, and this effect was ameliorated by pre-treatment of mice with the σ-1R antagonist (Fig. 3b). These findings were also corroborated by a genetic approach using the CCR2 KO mice. Unlike the WT mice that demonstrated enhanced monocyte transmigration in response to cocaine compared with the saline controls (Fig. 3b), CCR2 KO mice exposed to cocaine failed to show any difference in monocyte transmigration compared with the saline injected animals (Fig. 3c).
Fig. 3.
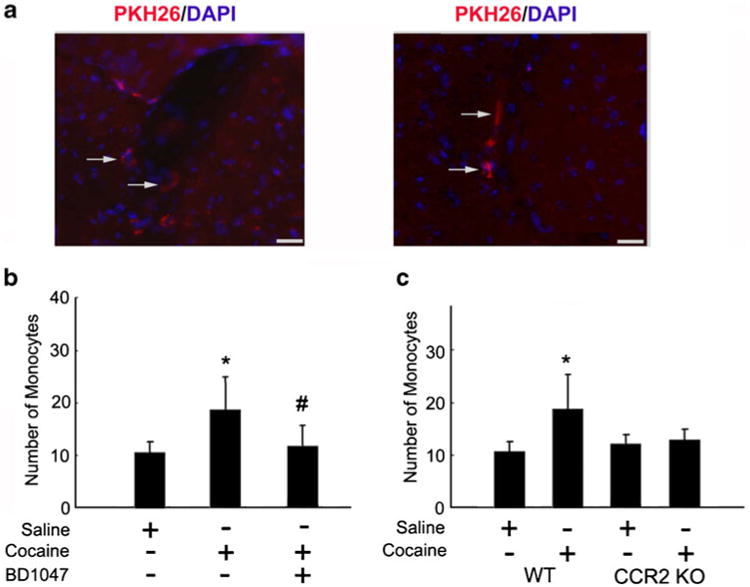
Cocaine-mediated induction of MCP-1 enhances monocyte transmigration in vivo. (a) Detection of PKH26-labeled monocytes in the brains of mice treated with cocaine. Fluorescence micrographs show monocytes in the perivascular cuff (left panel; see arrows) and the parenchymal (right panel; see arrows) areas of the brain. (b) Increased monoctye transmigration observed in the cocaine treated group was ameliorated by pre-treatment of mice with BD1047. (c) Increased monocyte transmigration in the cocaine treated WT but not CCR2 KO mice. *p<0.05 vs saline group; #p<0.05 vs cocaine group counted from the parenchyma. Scale bar: 20μm
Cocaine-mediated permeability in vivo involves PDGF-BB
To validate cocaine-mediated induction of PDGF-BB in vivo, brain microvessels isolated from cocaine versus saline-treated mice were assessed for expression of PDGF-BB. Cocaine administration resulted in increased expression of PDGF-BB in isolated microvessels compared with saline injected mice as demonstrated by both immunostaining of freshly isolated brain microvessels (Fig. 4a) and by western blots (Fig. 4b) of the microvessel homogenates. Intriguingly, cocaine mediated upregulation of PDGF-BB was ameliorated in Egr-1−/− mice compared with wild-type controls (Fig. 4c).
Fig. 4.

Cocaine-mediated induction of PDGF-BB in vivo. (a) Cocaine administration resulted in increased expression of PDGF-BB (red-lower panels) in isolated microvesssels (Caveolin positive - green) compared with saline-injected animals (upper panels). PDGF-BB: red; Cav-1: green; nuclei: blue; n=4 per group; Scale bar: 20 μm. (b) Western blot analysis of lysates from isolated microvessels from cocaine administered mice demonstrated increased expression of PDGF-BB compared with saline-injected animals. (c) Egr-1−/− mice treated with cocaine failed to upregulate PDGF-BB in the isolated micro-vessels. (d) Pretreatment of mice with PDGF-BB neutralizing antibody ameliorated cocaine-mediated increase in BBB permeability. Mannitol treated group as a positive control. (e) Cocaine administration resulted in increased BBB permeability in WT but not in the Egr-1 −/− mice. All the data are presented as mean ± SD, n=6 per group. *p<0.05 vs saline group; #p<0.05 vs cocaine group
To validate the role of PDGF-BB in impairment of barrier integrity, we examined the permeability of low-molecular-weight sodium fluorescein into the brain in cocaine-administered and untreated mice. Briefly, mice injected with cocaine were administered either anti-PDGF-BB neutralizing antibody or the isotype control for 7 days, followed by tail vein injection of sodium fluorescein. As demonstrated in Fig. 4d, and as expected, cocaine injection disrupted the BBB, and this effect was ameliorated by anti-PDGF-BB antibody, but not the isotype control, lending support to the suggestion that PDGF-BB played a critical role in BBB damage. Since cocaine induced Egr-1 which in turn, was critical for cocaine-induced expression of PDGF-BB both in vitro and in vivo systems, we next examined the effect of knocking down the expression of Egr-1 on cocaine mediated change in BBB permeability (Fig.4e). It is for this reason that we used Egr-1 −/− mice.
Activated leukocyte cell adhesion molecule (ALCAM) promotes monocyte adhesion and transmigration in mice treated with cocaine
To test whether cocaine-mediated induction of ALCAM in vivo, we examined the expression level of ALCAM in brain capillaries isolated from cocaine versus saline-treated mice. Following administration of cocaine, there was an increase in the expression level of ALCAM in isolated microvessels from cocaine-exposed mice compared with controls (Fig. 5a). Since ALCAM expression was increased in cocaine-treated mice, to validate the role of ALCAM in monocyte transmigration in vivo, mice were treated with cocaine, followed by tail vein injection of labeled mouse BMM and their detection in the brain. Of particular note, the distribution of labeled monocytes was primarily within the perivascular cuffs (See arrowheads in Fig. 5b), with some localization in the parenchyma (See arrows in Fig. 5b). Quantification of the brain sections revealed increased transmigration in the CNS of cocaine-treated mice compared with controls. Specificity of ALCAM action was further demonstrated in mice pre-treated with the neutralizing antibody to ALCAM. Monocyte transmigration in these mice was ameliorated lending further support to the role of ALCAM (Fig. 5c).
Fig. 5.
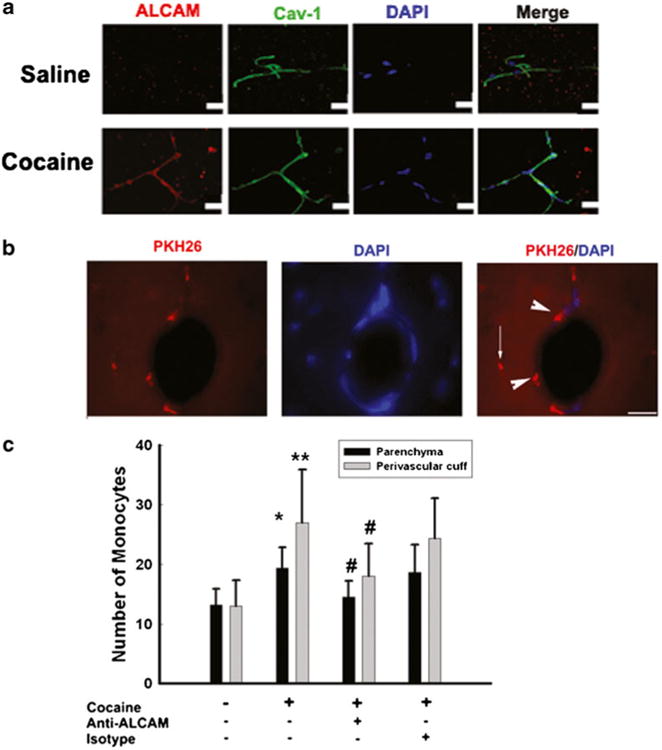
Cocaine-mediated induction of ACLAM enhances monocyte adhesion and transmigration in vivo. (a) Cocaine induced increased expression of ALCAM in isolated microvesssels compared with those from saline-injected animals (upper panels). ALCAM: red; Cav-1: green; nuclei: blue. n=4 per group. (b) Representative fluorescence micrographs demonstrating cocaine-mediated adhesion of PKH26-labeled monocytes in the perivascular cuff (see arrowheads) and the parenchymal (see arrows) areas of the mice brain. (c) Cocaine-mediated increased monocyte transmigration was ameliorated in mice pre-treated with ALCAM neutralizing antibody. *p<0.05 vs saline group; #p<0.05 vs cocaine group counted from the perivascular cuff and parenchyma
Discussion
A number of excellent primate and rodent models for HAND are available. Each of these models has advantages and limitations. Although various small animal models comprising of a) overexpressed viral protein transgenes, b) SCID mice involving either direct stereotactic injection of HIV-infected human monocytes into the CNS or engraftment of human peripheral blood leucocytes, and c) direct injection of HIV proteins into the CNS or vial IV route have been reported, each of these has been exploited to answer specific aspects of HAND.
The present report describes the advantage of employing direct administration of viral protein(s) and/or therapeutic targets within specific brain regions such as the striatum.
For example having identified previously the neuroprotective roles of both CCL2 and PDGF-B against HIV Tat toxicity, we exploited the direct injection model system to test the efficacy of both of these neuroprotective agents in vivo against toxicity mediated by viral protein(s). Although CCL2 has primarily been linked to the deleterious effects associated with the pathology of HAND (E. A. Eugenin et al., 2006), similar to the paradoxical roles of other chemokines (O. Meucci et al., 1998; O. Meucci et al., 2000), beneficial roles of this chemokine have also been increasingly documented. Our in vivo findings validated the results obtained in the in vitro culture system, thereby underpinning the role of microinjected rodent models systems as a next best system for proof-of-concept studies pertaining to HAND. Such model systems can be used to test various therapeutic targets against a range of viral and cellular factors, thereby mimicking aspects of HAND. It must be pointed out that direct administration does cause local inflammation at the injection site; however, having saline injected controls overcomes this concern.
In addition to recapitulating aspects of HAND, added advantage of the direct administration model is that it can also be used to examine the concurrent or individual effects of drugs of abuse in the animals. Our findings indicate the role of cocaine as a multifaceted agent that impairs the functioning of cells critical for HIV-associated neurological disorders (HAND). Both HIV and cocaine mediate CNS toxicity by impairing the functioning of endothelial and glial cells. For example, similar to the HIV Tat-induced expression of the vascular permeant PDGF-BB in astrocytes, cocaine exposure of the endothelial cells also results in exacerbated expression of PDGF-BB via multiple signaling pathways, thus contributing to increased pool of mediators critical for blood -brain barrier disruption. Complementing this mechanism of barrier compromise is the cocaine-mediated release by endothelial cells of yet another mediator, adhesion molecule ALCAM, which also functions to increase monocyte migration in the CNS, an effect similar to that manifested by HIV/HIV proteins.
The generation of small animal models, which preserve their ability for eliciting primary and memory immune responses of the engrafted human immune cells and in which, robust HIV-1 infection can occur, could enable rapid screening, development and evaluation of HIV-1 protective vaccines and adjuvants. Herein we report that direct micro-injecting of viral proteins into specific brain regions such as the striatum of rodents, provides an in vivo system to test both the toxicity and reciprocally, also to test the protective effect of therapeutic agents such as neurotropins. Additionally, administration of cocaine to small animal models also presents another valuable feature to test the efficacy of potential therapeutic targets such as ALCAM or PDGF-BB, for preclinical studies aimed at preserving BBB integrity in the presence of cocaine and/or HIV proteins.
In summary although lentiviruses are not natural hosts for the rodents, these small animals can be exploited to model aspects of HAND and/or drug abuse, specifically with respect to BBB breach, monocyte recruitment, neuronal damage and glial activation. Further ramifications of such models can be also applied to monitoring the effect of viral and/or cellular toxins on neuronal progenitor cells. Effect of other substances of abuse such as opiates, methamphetamine and alcohol can also be tested either with or without the background of viral/cellular toxins. In the light of difficult funding precluding the use of non human primates for research, such model systems offer valuable alternatives for research involving statistically significant data and are excellent for testing efficacy of therapeutic targets for translational research.
Acknowledgments
This work was supported by grants MH-068212, DA020392, DA023397 and DA024442 (SB) and DA030285 (HY) from the National Institutes of Health.
Footnotes
Conflicts of Interest The authors indicate no potential conflicts of interest.
References
- Aksenov MY, Hasselrot U, Bansal AK, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Oxidative damage induced by the injection of HIV-1 Tat protein in the rat striatum. Neurosci Lett. 2001;305:5–8. doi: 10.1016/s0304-3940(01)01786-4. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Vlahov D, Nelson KE, Cohn S, Astemborski J, Solomon L. New evidence on intravenous cocaine use and the risk of infection with human immunodeficiency virus type 1. Am J Epidemiol. 1991;134:1175–1189. doi: 10.1093/oxfordjournals.aje.a116021. [DOI] [PubMed] [Google Scholar]
- Bagasra O, Pomerantz RJ. Human immunodeficiency virus type 1 replication in peripheral blood mononuclear cells in the presence of cocaine. J Infect Dis. 1993;168:1157–1164. doi: 10.1093/infdis/168.5.1157. [DOI] [PubMed] [Google Scholar]
- Baldwin GC, Tashkin DP, Buckley DM, Park AN, Dubinett SM, Roth MD. Marijuana and cocaine impair alveolar macrophage function and cytokine production. Am J Respir Crit Care Med. 1997;156:1606–1613. doi: 10.1164/ajrccm.156.5.9704146. [DOI] [PubMed] [Google Scholar]
- Baldwin GC, Roth MD, Tashkin DP. Acute and chronic effects of cocaine on the immune system and the possible link to AIDS. J Neuroimmunol. 1998;83:133–138. doi: 10.1016/s0165-5728(97)00229-4. [DOI] [PubMed] [Google Scholar]
- Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. 2000;879:42–49. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- Cayrol R, Wosik K, Berard JL, Dodelet-Devillers A, Ifergan I, Kebir H, Haqqani AS, Kreymborg K, Krug S, Moumdjian R, Bouthillier A, Becher B, Arbour N, David S, Stanimirovic D, Prat A. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol. 2008;9:137–145. doi: 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
- Chaisson RE, Bacchetti P, Osmond D, Brodie B, Sande MA, Moss AR. Cocaine use and HIV infection in intravenous drug users in San Francisco. JAMA. 1989;261:561–565. [PubMed] [Google Scholar]
- Chen L, Swartz KR, Toborek M. Vessel microport technique for applications in cerebrovascular research. J Neurosci Res. 2009;87:1718–1727. doi: 10.1002/jnr.21973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson MA, Stoneburner RL, Hildebrandt DS, Ewing WE, Telzak EE, Jaffe HW. Heterosexual transmission of HIV-1 associated with the use of smokable freebase cocaine (crack) AIDS. 1991;5:1121–1126. doi: 10.1097/00002030-199109000-00011. [DOI] [PubMed] [Google Scholar]
- Doherty MC, Garfein RS, Monterroso E, Brown D, Vlahov D. Correlates of HIV infection among young adult short-term injection drug users. AIDS. 2000;14:717–726. doi: 10.1097/00002030-200004140-00011. [DOI] [PubMed] [Google Scholar]
- Eisenstein TK, Hilburger ME. Opioid modulation of immune responses: effects on phagocyte and lymphoid cell populations. J Neuroimmunol. 1998;83:36–44. doi: 10.1016/s0165-5728(97)00219-1. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala M, Gan XH, Zhang L, House SD, Newton T, Graves MC, Shapshak P, Stins M, Kim KS, Witte M, Chang SL. Cocaine enhances monocyte migration across the blood–brain barrier. Cocaine's connection to AIDS dementia and vasculitis? Adv Exp Med Biol. 1998;437:199–205. doi: 10.1007/978-1-4615-5347-2_22. [DOI] [PubMed] [Google Scholar]
- Friedman H, Newton C, Klein TW. Microbial infections, immunomodulation, and drugs of abuse. Clin Microbiol Rev. 2003;16:209–219. doi: 10.1128/CMR.16.2.209-219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman M, Arbuthnott G, Harkiss G, Brace H, Filippi P, Philippon V, Thomson D, Vigne R, Wright A. Neurotoxicity of peptide analogues of the transactivating protein tat from Maedi-Visna virus and human immunodeficiency virus. Neuroscience. 1993;53:1–6. doi: 10.1016/0306-4522(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Huang W, Rha GB, Chen L, Seelbach MJ, Zhang B, Andras IE, Bruemmer D, Hennig B, Toborek M. Inhibition of telomerase activity alters tight junction protein expression and induces transendothelial migration of HIV-1-infected cells. Am J Physiol Heart Circ Physiol. 2010;298:H1136–H1145. doi: 10.1152/ajpheart.01126.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Mehraein P, Weis S. Neuronal damage of the substantia nigra in HIV-1 infected brains. Acta Neuropathol. 2000;99:376–384. doi: 10.1007/s004010051139. [DOI] [PubMed] [Google Scholar]
- Jones M, Olafson K, Del Bigio MR, Peeling J, Nath A. Intra-ventricular injection of human immunodeficiency virus type 1 (HIV-1) tat protein causes inflammation, gliosis, apoptosis, and ventricular enlargement. J Neuropathol Exp Neurol. 1998;57:563–570. doi: 10.1097/00005072-199806000-00004. [DOI] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuro-pathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol. 2003;162:1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TW, Matsui K, Newton CA, Young J, Widen RE, Friedman H. Cocaine suppresses proliferation of phytohemagglutinin-activated human peripheral blood T-cells. Int J Immunopharma. 1993;15:77–86. doi: 10.1016/0192-0561(93)90033-u. [DOI] [PubMed] [Google Scholar]
- Larrat EP, Zierler S. Entangled epidemics: cocaine use and HIV disease. J Psychoactive Drugs. 1993;25:207–221. doi: 10.1080/02791072.1993.10472272. [DOI] [PubMed] [Google Scholar]
- Mao JT, Huang M, Wang J, Sharma S, Tashkin DP, Dubinett SM. Cocaine down-regulates IL-2-induced peripheral blood lymphocyte IL-8 and IFN-gamma production. Cell Immunol. 1996;172:217–223. doi: 10.1006/cimm.1996.0235. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Miller RJ. Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci U S A. 2000;97:8075–8080. doi: 10.1073/pnas.090017497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair MPN, Mahajan S, Hou J, Sweet AM, Schwartz SA. The stress hormone, cortisol, synergizes with HIV-1 gp-120 to induce apoptosis of normal human peripheral blood mononuclear cells. Cell Mol Biol. 2000;46:1227–1238. Noisy-le-grand. [PubMed] [Google Scholar]
- Persidsky Y, Limoges J, McComb R, Bock P, Baldwin T, Tyor W, Patil A, Nottet HS, Epstein L, Gelbard H, Flanagan E, Reinhard J, Pirruccello SJ, Gendelman HE. Human immunodeficiency virus encephalitis in SCID mice. Am J Pathol. 1996;149:1027–1053. [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Gendelman HE. Murine models for human immunodeficiency virus type1-associated dementia: the development of new treatment testing paradigms. J Neurovirol. 2002;8(2):49–52. doi: 10.1080/13550280290167993. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Sharp BM, Gekker G, Portoghese PS, Sannerud K, Balfour HH. Morphine promotes the growth of HIV-1 in human peripheral blood mononuclear cell cocultures. AIDS. 1990;4:869–873. doi: 10.1097/00002030-199009000-00006. [DOI] [PubMed] [Google Scholar]
- Philippon V, Vellutini C, Gambarelli D, Harkiss G, Arbuthnott G, Metzger D, Roubin R, Filippi P. The basic domain of the lentiviral Tat protein is responsible for damages in mouse brain: involvement of cytokines. Virology. 1994;205:519–529. doi: 10.1006/viro.1994.1673. [DOI] [PubMed] [Google Scholar]
- Reid W, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MD, Tashkin DP, Choi R, Jamieson BD, Zack JA, Baldwin GC. Cocaine enhances human immunodeficiency virus replication in a model of severe combined immunodeficient mice implanted with human peripheral blood leukocytes. J Infect Dis. 2002;185:701–705. doi: 10.1086/339012. [DOI] [PubMed] [Google Scholar]
- Schilling M, Strecker JK, Ringelstein EB, Kiefer R, Schabitz WR. Turn-over of meningeal and perivascular macrophages in the brain of MCP-1-, CCR-2- or double knockout mice. Exp Neurol. 2009;219:583–585. doi: 10.1016/j.expneurol.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309:99–107. doi: 10.1016/s0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- Tyor WR, Power C, Gendelman HE, Markham RB. A model of human immunodeficiency virus encephalitis in scid mice. Proc Natl Acad Sci U S A. 1993;90:8658–8662. doi: 10.1073/pnas.90.18.8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigorito M, LaShomb AL, Chang SL. Spatial learning and memory in HIV-1 transgenic rats. J Neuroimmune Pharmacol. 2007;2:319–328. doi: 10.1007/s11481-007-9078-y. [DOI] [PubMed] [Google Scholar]
- Webber MP, Schoenbaum EE, Gourevitch MN, Buono D, Klein RS. A prospective study of HIV disease progression in female and male drug users. AIDS. 1999;13:257–262. doi: 10.1097/00002030-199902040-00014. [DOI] [PubMed] [Google Scholar]


