Abstract
Peripheral challenge with a viral mimetic, polyinosinic-polycytidylic acid (PIC) induces hippocampal hyperexcitability in mice. Here, we characterized this hippocampal response through a whole genome transcriptome analysis. Intraperitoneal injection of PIC resulted in temporal dysregulation of 625 genes in the hippocampus, indicating an extensive genetic reprogramming. The bioinformatics analysis of these genes revealed the complement pathway to be the most significantly activated. The gene encoding complement factor B (CfB) exhibited the highest response, and its upregulation was commensurate with the development of hyperexcitability. Collectively, these results suggest that the induction of hippocampal hyperexcitability may be mediated by the alternative complement cascades.
Keywords: polyinosinic-polycytidylic acid, inflammation, hippocampus, complement system, transcriptome, immunofluorescence
1. Introduction
It has been well established that peripheral inflammation exerts profound effects on the brain. A classic example is the induction of sickness behavior, an assembly of behavioral traits that alter the priorities of the affected individual to promote recovery (Dantzer, 2006; Dantzer and Kelley, 2007; Quan and Banks, 2007). The underlying mechanisms involve the activation of local innate immune cells that mount a fulminant inflammatory response characterized by the production of a slew of cytokines, chemokines and other factors. Subsequently, these peripheral inflammatory signals are relayed to the brain whereby they induce a “mirror” inflammatory response. The cerebrally-generated inflammatory factors, as well as the peripherally-generated factors that gain access to the brain parenchyma, interact with specific neuronal circuits and change their activities, leading to behavioral changes such as fever, depression, anhedonia, malaise, anorexia, adipsia, lethargy and fatigue. Although sickness behavior has evolved as a protective mechanism, the activation of cerebral inflammatory pathways carries a risk for exacerbating other neuropathologies. For example, systemic administration of a viral mimic, polyinosinicpolycytidylic acid (PIC), heightens prion-related neurodegeneration (Field et al., 2010), and autoimmune retinal damage (Ren et al., 2011). The administration of a bacterial mimic, lipopolysaccharide (LPS) increases the formation of neurofibrillary tangles in a transgenic model of Alzheimer's disease (Kitazawa et al., 2005), degeneration of the nigrostriatal dopaminergic system in a model of Parkinson's disease (Machado et al., 2011) and degeneration of motor axons in a model of amyotrophic lateral sclerosis (Nguyen et al., 2004). LPS challenge also reactivates focal autoimmune lesions in a model of multiple sclerosis (Serres et al., 2009), increases post-stroke mortality (Denes et al., 2011) and hampers post-stroke regeneration (Yousuf et al., 2013). These effects are congruent with clinical evidence that the burden of peripheral infections aggravates dementia in Alzheimer's disease (Holmes et al., 2003), increases the severity of functional detriments in Parkinson's disease (Ferrari and Tarelli, 2011) and in amyotrophic lateral sclerosis (Zhang et al., 2009), and exacerbates relapses in multiple sclerosis (Buljevac et al., 2002; Edwards et al., 1998). However, the underlying mechanisms remain elusive.
We have recently demonstrated that intraperitoneal injection of PIC renders the brain hyperexcitable as seen from a profound increase in the susceptibility of C57BL/6 mice to kainic acid (KA)-induced seizures (Kirschman et al., 2011; Michalovicz and Konat, 2014). Hyperexcitability is a critical component and a putative mediator in various neuropathologies, including epilepsy (McNamara, 1999), Alzheimer's disease (Khedr et al., 2011), Parkinson's disease (Ikoma et al., 1994), amyotrophic lateral sclerosis (Bae et al., 2013), multiple sclerosis (Caramia et al., 2004; Rossi et al., 2012), stroke (Carmichael, 2003; Huynh et al., 2013) and traumatic brain injury (Nardone et al., 2011). Consequently, hyperexcitability is likely to provide a mechanistic link between peripheral inflammation and the progression of neurodegeneration. In our studies (Kirschman et al., 2011; Michalovicz and Konat, 2014), PIC-induced hyperexcitability manifested as a several-fold increase in the intensity and duration of KA-induced status epilepticus that persisted for three days. Because the hippocampus is the ictal onset region for KA-induced seizures (Ben-Ari and Cossart, 2000), we evaluated changes in the expression of selected hippocampal genes induced by PIC challenge (Michalovicz and Konat, 2014). In concordance with the previous studies in the whole brain (Fil et al., 2011; Konat et al., 2009), we found the upregulation of a battery of genes encoding cytokines, chemokines and chemokine receptors in the hippocampus. Moreover, PIC challenge altered the expression of several genes related to glutamatergic and GABAergic neurotransmission, as well as several microRNAs associated with seizure pathology and/or with modulation of neuro-immune functions. This polygenic response warranted further, more comprehensive genetic studies to delineate molecular/cellular pathways that govern the development of hippocampal hyperexcitability.
In the present study, we use a transcriptome-based microarray approach to identify putative regulatory pathways in the hippocampus that may underlie development of the hyperexcitable phenotype instigated by PIC-induced peripheral inflammation. We found the complement pathway to be the primary pathway upregulated by PIC challenge. Because the cerebral complement system emerges as a potent regulator of neuronal excitability (Schafer et al., 2012), PIC-induced complement upregulation may provide a mechanistic link between peripheral inflammation and hippocampal hyperexcitability.
2. Materials and Methods
2.1. Animals
Eight-week old C57BL/6 mice were procured from Charles River Laboratories (Wilmington, MA) and housed under 12-h light/dark conditions (lights on at 6 am) with unrestricted access to food and water. The animals were intraperitoneally (i.p.) injected with 12 mg/kg of high molecular weight PIC (Invivogen, San Diego, CA) in 100 μl of saline. Control mice were injected with saline only. After 3 h, the development of sickness behavior was assessed by the rearing test (Michalovicz and Konat, 2014) to confirm successful i.p. injection. All procedures were approved by the West Virginia University Animal Care and Use Committee and conducted in compliance with the guidelines published in the NIH Guide for the Care and Use of Laboratory Animals.
2.2. Microarray analysis
At 6, 24 and 48 h after PIC challenge, mice were deeply anesthetized with 65 mg/kg of pentobarbital (Fatal Plus, Vortech Pharmaceutical, Dearborn, MI) administered i.p., sacrificed by pneumothorax, and transaortically perfused with saline. The hippocampi were dissected out, homogenized in TRI Reagent (Molecular Research Center, Inc., Cincinnati, OH), and total RNA was isolated per manufacturer's protocol. RNA integrity was verified by the Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA). The microarray analysis was performed using the Illumina BeadChip mouse WG-6 format (Illumina, Inc., San Diego, CA). The BeadChips were scanned using the Illumina iSCAN system and analyzed by Illumina's GenomeStudio 2011.1 Gene Expression Analysis Module 1.9.0 (Illumina, Inc, San Diego, CA). The RNA integrity and microarray analysis work for this publication was performed in the Genomics Research Core at the University of Pittsburgh. Data analysis was performed using the caGEDA web application (Patel and Lyons-Weiler, 2004). Briefly, fluorescence intensities were median normalized and log2 transformed. Subsequently, differential gene expression between time points (0, 6, 24 and 48 h) was evaluated using the J5 test (Patel and Lyons-Weiler, 2004) with a threshold cutoff of 6.0. The J5 test identified DEGs based on the average difference between controls and 6, 24 and 48 h for a particular gene over the average difference for all genes on the array. In addition to individual time point comparisons, a gene expression profile was created for a comparison of 0 h to all other time points (0-ALL) which comprised only those genes whose average expression across 6, 24 and 48 h was above threshold. Each individual time point comparison, i.e. 0-6, 0-24 and 0-48, was tested for overlap with the 0-ALL comparison, using the Overlap4 tool (http://bioinformatics.pitt.edu/GE2/Overlap4.html) and only the overlapping genes were included in later analysis. Gene ontology analysis was performed using the Database for Annotation, Visualization and Integrated Discovery (DAVID) (Dennis, Jr. et al., 2003). Pathways analysis was performed using the Pathway Express application (Draghici et al., 2007).
2.3. qRT-PCR
Total RNA was isolated as above. cDNA was synthesized using SuperScript III First-strand Synthesis kit (Invitrogen, Carlsbad, CA) and quantified using RT2 SYBRGreen (Qiagen, Valencia, CA). qRT-PCR was performed in an ABI7500 Real-Time PCR system (Applied Biosystems, Foster City, CA). Glyceraldehyde phosphate dehydrogenase (GAPDH) mRNA was used as an internal control. The ΔΔCt method was used for quantitation. Primer sequences are available upon request.
2.4. Immunofluorescence
Mice were sacrificed as described above and transaortically perfused with saline followed by 4% paraformaldehyde. Brains were removed from the skull and infused in 4% paraformaldehyde for a minimum of 24 h at 4°C. The brains were cryoprotected in 30% sucrose for 24 h at 4°C before sectioning. 35 μm slices were cut on a freezing microtome and stored in 4% paraformaldehyde. For immunofluorescent staining, free-floating sections were blocked in PBS with 5% FBS (Atlanta Biologicals, Lawrenceville, GA) and 0.5% Triton-X 100 (Fisher Scientific, Waltham, MA) for 1 h at room temperature and then incubated in primary antibody overnight at 4°C. After washing in PBS, sections were incubated in secondary antibody for 1 h at room temperature. Sections were then washed and mounted on slides using Vectashield hard set (Vector Laboratories, Burlingame, CA). Confocal imaging was performed at the WVU Microscope Imaging Facility with a Zeiss LSM 510 laser scanning confocal on a LSM Axio Imager upright microscope (Zeiss, Jena, Germany). Primary antibodies were: goat-anti-Cfb (N-14; Santa Cruz Biotechnology, Dallas, TX) and mouse-anti-NeuN (Millipore, Billerica, MA), rabbit-anti-GFAP (Dako; Carpinteria, CA), rabbit-anti-Iba1 (Wako; Richmond, VA). Secondary antibodies were anti-goat, anti-mouse and anti-rabbit conjugated to Alexa Fluor 488 or Alexa Fluor 555 (Invitrogen, Carlsbad, CA), respectively. Images were analyzed using the ZEN 2012 image analysis software (Zeiss, Jena, Germany).
2.5. Statistical Analysis
Data were analyzed by ANOVA and expressed as means ± SD. Statistical comparisons between groups were performed using Student's t test. Differences between groups were considered significant at P ≤ 0.05.
3. Results
3.1. Genome-wide expression analysis
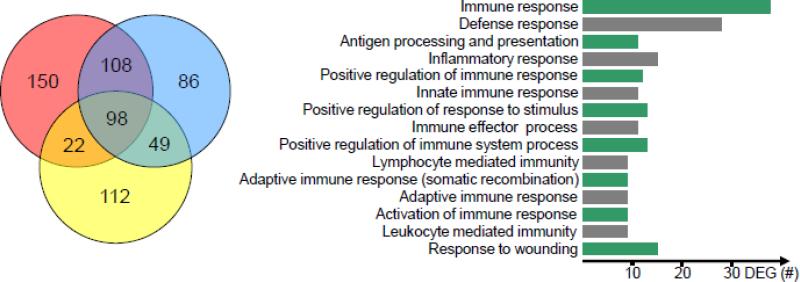
Previously, we have demonstrated that peripheral challenge with PIC results in the alteration of a plethora of inflammatory, neurotransmission-related and miRNA genes in the mouse hippocampus (Michalovicz and Konat, 2014). To gather a more global perspective of this genomic response, we performed a genome-wide array analysis of the hippocampi at 0, 6, 24 and 48 h following PIC challenge. As shown in Fig. 1 (left panel), a total of 625 differentially expressed genes (DEGs) were identified across all time points when compared to control (0 h). While the expression of many DEGs was restricted to particular intervals after PIC challenge, 98 DEGs were altered at all of the time points.
Figure 1.
The response of hippocampal transcriptome to peripheral inflammation triggered by PIC challenge. Mice were i.p. injected with 12 mg/kg of PIC. After 0, 6, 24 and 48 h, hippocampal gene expression was profiled and analyzed as described in Materials and Methods. Left panel; Dynamic gene dysregulation. The Venn diagram shows differentially expressed genes (DEGs) identified by the average difference between 0 and 6 h (left red circle), between 0 and 24 h (right blue circle) and between 0 and 48 h (lower yellow circle) after PIC injection. The numbers denote the number of genes. The data for each time point is from three animals (n=3). Right panel; Gene ontology. Fifteen top biological processes affected by the set of 179 dynamically expressed DEGs (see 3.1.) as determined by the DAVID ontology analysis are presented. The processes are arranged by increasing P values (top to bottom) that ranged from 8×10−28 to 4×10−7.
Taking advantage of our microarray time course, we interrogated the dataset for genes that showed dynamic (changing) expression over all three time windows, i.e., from 0 to 6 h, from 6 to 24 h, and from 24 to 48 h. Briefly, the DEGs from the 0-6 h set were tested for overlap with the 0-ALL set (see Materials and Methods). This set of 256 overlapping genes was then used to interrogate the 6-24 h and 24-48 h sets for common genes, yielding 108 and 142 DEGs, respectively. To include genes that may have remained stable from 24 to 48 h, the sets of 108 and 142 DEGs were combined to create a dynamic set of 179 unique genes. These genes were subjected to bioinformatics analysis by the DAVID ontology tools to reveal their functional clustering. Immune- and inflammation-related processes were the most affected by PIC challenge. The top 15 of these are presented in Fig. 1 (right panel). Subsequently, the genes were analyzed by Pathway Express to identify their biological functions. Five pathways were found to be significantly affected (Table 1). The “Complement and coagulation pathway” was the primary pathway upregulated by PIC challenge. Four other pathways related to immune and/or pathological processes were: “Toll-like receptor signaling”, “Systemic lupus erythematous”, “Proteosome” and “Epithelial cell signaling in H. pylori infection” pathways. Because the complement system has been shown to control synaptic function (Schafer et al., 2012; Stephan et al., 2013; Stevens et al., 2007), and thus, the excitability of neuronal networks, we focused on the characterization of the genes encoding complement proteins.
Table 1.
Biological pathways significantly affected by PIC challenge.
| Rank | Pathway name | Number of DEGs | p-value |
|---|---|---|---|
| 1 | Complement and coagulation | 9 | 9.27×10−5 |
| 2 | Toll-like receptor signaling | 10 | 4.40×10−4 |
| 3 | Systemic lupus erythematous | 9 | 3.26×10−3 |
| 4 | Proteosome | 4 | 2.79×10−2 |
| 5 | Epithelial cell signaling in H. pylori infection | 5 | 3.43×10−2 |
3.2. Temporal expression of the complement genes
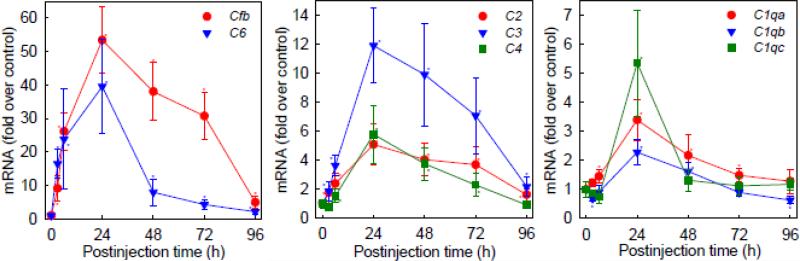
Expanding upon the results of the microarray study, we used qRT-PCR to evaluate temporal expression of the genes encoding the complement proteins over the time frame encompassing the hyperexcitability, i.e., from 0 to 96 h after the administration of PIC (Michalovicz and Konat, 2014). Eight genes, i.e., the C1qa, C1qb, C1qc, C2, C3, C4, C6 and Cfb genes, displayed upregulation in the hippocampus by PIC challenge reaching a peak at 24 h (Fig. 2). The CfB, C6 and C3 genes featured the highest upregulation of 53-, 40-, and 12-fold, respectively. With the exception of the C1qc gene that showed a rapid decline by 48 h, expression of the other genes featured a protracted upregulation up to 72 h and a decline to approximately control levels by 96 h post PIC challenge. Of note, this protracted upregulation closely followed temporal progression of seizure hypersusceptibility that also lasted 3 days after PIC challenge (Michalovicz and Konat, 2014). The genes encoding C5, C7, C8 and C9 components displayed very low and highly variable expression with no significant dysregulation by PIC challenge (not shown).
Figure 2.
Peripheral inflammation triggered by PIC challenge upregulates expression of the complement genes in the hippocampus. Mice were i.p. injected with 12 mg/kg of PIC and levels of the complement mRNAs were determined in the hippocampi by qRT-PCR at different time points as indicated. Data represent means ± S.D. from 3-8 animals. Asterisks indicate values significantly (p ≤ 0.05) different from the respective controls.
3.3. Hippocampal generation of CfB
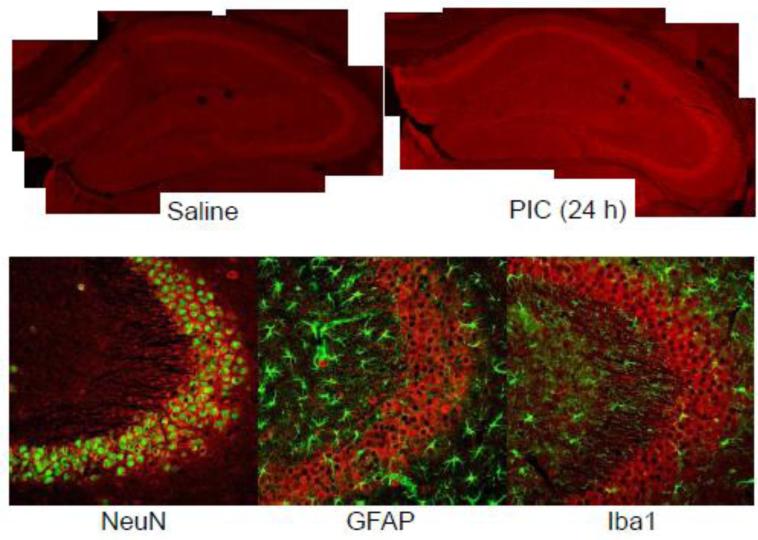
The robust upregulation of the Cfb mRNA (Fig. 2) commensurate with the course of hyperexcitability (Michalovicz and Konat, 2014) warranted further characterization of this gene's expression at the protein level. As shown in Fig. 3, detectable amounts of CfB were present throughout the control hippocampus as revealed by immunofluorescence. The most intense staining was present within the pyramidal cell layers, while the rest of the tissue featured a diffused staining. This result is in concordance with a previous study showing neuronal expression of CfB in the human brain (Strohmeyer et al., 2000). The signal intensity was profoundly augmented by PIC challenge indicating increased production of the protein over the basal level. All hippocampal regions appeared to be equally affected. Like the Cfb mRNA, CfB protein staining gradually decreased to the basal level by 96 h (not shown), closely matching temporal evolution of the hyperexcitable phenotype (Michalovicz and Konat, 2014). Double immunostaining revealed a predominant localization of CfB within the perikarya and dendritic arbors of pyramidal neurons. No significant co-localization of CfB within astrocytes and microglia was detectable.
Figure 3.
Peripheral inflammation triggered by PIC challenge increases hippocampal production of complement factor B (CfB). Mice were i.p. injected with 12 mg/kg of PIC or saline. After 24 h, the brains were perfusion fixed and sectioned (35 μm). Sections were stained with specific antibodies and imaged by confocal microscopy. Upper panel; Composites of 10X images of hippocampi from saline and PIC-challenged mice stained with anti-CfB antibody. Lower panel; Cellular localization of CfB in the CA3 region of the hippocampus. Cerebral sections were double stained with anti-CfB and anti-NeuN (neuronal marker), anti-GFAP (astrocytic marker) or anti-Iba1 (microglial marker) antibodies and imaged at 20X.
Moreover, we detected a substantial C3 staining and a weak C1q staining in the normal hippocampus that also featured predilection for the pyramidal cell layers (not shown). This is congruent with neuronal expression of the complement components reported previously (Terai et al., 1997). However, the intensity of the staining of both C3 and C1q proteins was apparently unaffected by PIC challenge at any time point.
4. Discussion
The present study has demonstrated a rapid and robust genomic response of the hippocampus to peripheral inflammation instigated by PIC challenge as seen from the dysregulation of 625 genes at some time points within the 48 h period (Fig. 1). Moreover, 179 genes featured dynamic dysregulation during the entire period. This resonates well with very potent immune-to-brain signaling mechanisms. As expected, the majority of the 625 hippocampal DEGs were related to immune and inflammatory processes pertaining to both the innate and acquired immunity. Our previous studies have shown that this genomic response is robust as the dysregulation of several genes can reach several thousand-fold over the baseline (Fil et al., 2011; Konat et al., 2009; Michalovicz and Konat, 2014). Altogether, these results indicate a profound genomic reprogramming of the hippocampal cells in response to PIC challenge.
Recently, cerebral response to peripheral challenge with LPS, a ubiquitous component of the cell wall of gram negative bacteria was evaluated by microarray analysis (Thomson et al., 2014). Although the authors used the whole brain while we used the hippocampus, the results can be compared because there is no drastic variation between brain regions regarding the response to PIC challenge (Fil et al., 2011; Konat and Borysiewicz, 2009). Thus, Thompson's group observed a differential expression of 85 entities containing 71 known genes in the whole brain, 48 h after LPS administration. We detected 281 entities including 260 known genes, 48 h after PIC challenge (Fig. 1). Like in the LPS study, the DEGs dysregulated by PIC were related predominantly to immune and inflammatory processes. However, only 23 genes were common between LPS and PIC datasets. Moreover, 48 genes were exclusively dysregulated by LPS, while 237 were dysregulated only by PIC. This comparison indicates that these two inflammagens instigate different neuroinflammatory response in the brain. The difference is likely related to disparate pharmacodynamics and pharmacokinetics of LPS and PIC. For example, bacterial LPS activates Toll-like receptor 4 (TLR4) while PIC, a viral mimetic, acts chiefly through TLR3. These two receptors employ variant signaling pathways that result in cytokine profiles tailored to antibacterial and antiviral defenses, respectively. These cytokine profiles may in turn elicit different inflammatory responses in the brain. Furthermore, LPS is a stable molecule that rapidly (within minutes) passes from the peritoneal cavity into the bloodstream (Lenczowski et al., 1997; Romanovsky et al., 2000), and therefore, can activate various populations of peripheral immune cells throughout the body for extended periods of time. It can also directly activate cerebral endothelial and circumventricular organ cells (Murray et al., 2011). Thus, in the LPS paradigm, the brain is exposed to a combination of LPS itself and to LPS-induced peripheral mediators that evolve over time. In contrast, PIC is a very labile molecule subjected to rapid degradation by omnipresent RNases in body fluids (Krasowska-Zoladek et al., 2007). We have also demonstrated that intraperitoneally injected PIC does not reach the circulation, and elicits a cerebral response through blood-borne inflammatory mediators (Fil et al., 2011). Consequently, PIC acts as a bolus activator of peritoneal macrophages and mesothelial cells.
The major finding of the present study is the upregulation of the complement genes that may provide a mechanistic link between peripheral PIC challenge and cerebral hyperexcitability. The complement system has emerged as a major modulator of neuronal activity (Schafer et al., 2012; Stephan et al., 2013; Stevens et al., 2007). This multi-step process entails tagging of specific synapses with C1q, the recruitment of the C3 component, formation of an active complex on the tagged synapses and ultimately, microglia-mediated modification of the synapses. Synaptic modification per se can occur via several potential mechanisms (Ji et al., 2013; Kettenmann et al., 2013). Thus, “synaptic stripping” entails the removal and phagocytosis of the presynaptic terminals by microglia. Microglia can secrete proteases that digest adhesion molecules and alter synaptic stability, or can secrete microvesicles that act at the presynaptic site and enhance excitatory transmission. Moreover, in a process called “synaptic scaling” microglia can release TNFα that stimulates perisynaptic astrocyte processes, resulting in the release of glutamate that augments synaptic transmission. The net effect on the neuronal networks depends on the type of synapses and on the modifying mechanism. For example, stripping inhibitory synapses leads to hyperexcitability, while the removal/impairment of excitatory synapses leads to hypoexcitability.
Although C1q, the initiator of the classical complement pathway, plays a critical role in the complement-mediated synaptic modification described above, we found no effect of PIC challenge on hippocampal C1q. However, we found a robust upregulation of CfB, the initiator of the alternative complement pathway. CfB upregulation was commensurate with the hyperexcitability of the hippocampus (Michalovicz and Konat, 2014) – both processes peaked at 24 h and gradually decreased to the control level at 96 h after PIC challenge. This finding presents an intriguing possibility that the neuromodulatory effects of the complement might be mediated by activation of the alternative pathway. Cerebral upregulation of CfB and activation of the alternative pathway has been shown in neuropathological conditions such as AD (Strohmeyer et al., 2000) and in ischemic stroke (Elvington et al., 2012). These neuropathologies also feature hyperexcitability as an underscoring/comorbid factor (Carmichael, 2003; Huynh et al., 2013; Khedr et al., 2011). However, the causal relationship between the alternative pathway activation and hyperexcitability has not been defined. The alternative pathway converges with the classical pathway at the C5 convertase step. Thus, both pathways lead to the formation of active complement complexes that can tag synapses as well as C3a and C5a anaphylatoxins that activate microglia via the complement receptor 3 (CR3) (Ilschner et al., 1996; Schafer et al., 2012; Zhang et al., 2014). However, it stands to reason that CfB-mediated neuromodulation entails similar, yet distinctly different, mechanisms than those employed by C1q-initiated cascades. For example, CfB may bind to different synaptic domains than C1q and alter synaptic activity in a unique way. The role of CfB in the induction of hippocampal hyperexcitability and elucidation of the underlying mechanism will be addressed in follow-up studies.
In conclusion, the present study has shown that peripheral PIC challenge induces extensive genomic reprogramming in the hippocampus as seen from the dysregulated expression of hundreds of genes. This genomic response entails robust upregulation of CfB, the initiator of the alternative complement pathway, indicating that activation of this pathway may underscore the development of hippocampal hyperexcitability.
Highlights.
i.p. injection of a viral mimetic (PIC) induces hippocampal hyperexcitability
PIC challenge leads to extensive genomic reprogramming in the hippocampus
The complement pathway is significantly upregulated
The expression of complement factor B is commensurate with hyperexcitability
The alternative pathway may mediate hippocampal hyperexcitability
Acknowledgements
This study was supported by the National Institutes of Health/National Institute of General Medical Sciences, U54GM104942. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Bae JS, Simon NG, Menon P, Vucic S, Kiernan MC. The puzzling case of hyperexcitability in amyotrophic lateral sclerosis. J. Clin. Neurol. 2013;9:65–74. doi: 10.3988/jcn.2013.9.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23:580–587. doi: 10.1016/s0166-2236(00)01659-3. [DOI] [PubMed] [Google Scholar]
- Buljevac D, Flach HZ, Hop WC, Hijdra D, Laman JD, Savelkoul HF, van Der Meche FG, van Doorn PA, Hintzen RQ. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain. 2002;125:952–960. doi: 10.1093/brain/awf098. [DOI] [PubMed] [Google Scholar]
- Caramia MD, Palmieri MG, Desiato MT, Boffa L, Galizia P, Rossini PM, Centonze D, Bernardi G. Brain excitability changes in the relapsing and remitting phases of multiple sclerosis: a study with transcranial magnetic stimulation. Clin. Neurophysiol. 2004;115:956–965. doi: 10.1016/j.clinph.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. Plasticity of cortical projections after stroke. Neuroscientist. 2003;9:64–75. doi: 10.1177/1073858402239592. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Neurol. Clin. 2006;24:441–460. doi: 10.1016/j.ncl.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denes A, Ferenczi S, Kovacs KJ. Systemic inflammatory challenges compromise survival after experimental stroke via augmenting brain inflammation, blood-brain barrier damage and brain oedema independently of infarct size. J. Neuroinflammation. 2011;8:164. doi: 10.1186/1742-2094-8-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis G, Jr., Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:3. [PubMed] [Google Scholar]
- Draghici S, Khatri P, Tarca AL, Amin K, Done A, Voichita C, Georgescu C, Romero R. A systems biology approach for pathway level analysis. Genome Res. 2007;17:1537–1545. doi: 10.1101/gr.6202607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Zvartau M, Clarke H, Irving W, Blumhardt LD. Clinical relapses and disease activity on magnetic resonance imaging associated with viral upper respiratory tract infections in multiple sclerosis. J Neurol. Neurosurg. Psychiatry. 1998;64:736–741. doi: 10.1136/jnnp.64.6.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvington A, Atkinson C, Zhu H, Yu J, Takahashi K, Stahl GL, Kindy MS, Tomlinson S. The alternative complement pathway propagates inflammation and injury in murine ischemic stroke. J. Immunol. 2012;189:4640–4647. doi: 10.4049/jimmunol.1201904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari CC, Tarelli R. Parkinson's disease and systemic inflammation. Parkinsons. Dis. 2011;2011:436813. doi: 10.4061/2011/436813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field R, Campion S, Warren C, Murray C, Cunningham C. Systemic challenge with the TLR3 agonist poly I:C induces amplified IFNalpha/beta and IL-1beta responses in the diseased brain and exacerbates chronic neurodegeneration. Brain Behav. Immun. 2010;24:996–1007. doi: 10.1016/j.bbi.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fil D, Borysiewicz E, Konat GW. A broad upregulation of cerebral chemokine genes by peripherally-generated inflammatory mediators. Metab Brain Dis. 2011;26:49–59. doi: 10.1007/s11011-010-9231-9. [DOI] [PubMed] [Google Scholar]
- Holmes C, El-Okl M, Williams AL, Cunningham C, Wilcockson D, Perry VH. Systemic infection, interleukin 1beta, and cognitive decline in Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 2003;74:788–789. doi: 10.1136/jnnp.74.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh W, Krishnan AV, Vucic S, Lin CS, Kiernan MC. Motor cortex excitability in acute cerebellar infarct. Cerebellum. 2013;12:826–834. doi: 10.1007/s12311-013-0493-8. [DOI] [PubMed] [Google Scholar]
- Ikoma K, Mano Y, Takayanagi T. Pulsed magnetic stimulation and F waves in Parkinson's disease. Intern. Med. 1994;33:77–81. doi: 10.2169/internalmedicine.33.77. [DOI] [PubMed] [Google Scholar]
- Ilschner S, Nolte C, Kettenmann H. Complement factor C5a and epidermal growth factor trigger the activation of outward potassium currents in cultured murine microglia. Neuroscience. 1996;73:1109–1120. doi: 10.1016/0306-4522(96)00107-8. [DOI] [PubMed] [Google Scholar]
- Ji K, Miyauchi J, Tsirka SE. Microglia: an active player in the regulation of synaptic activity. Neural Plast. 2013;2013:627325. doi: 10.1155/2013/627325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77:10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Ahmed MA, Darwish ES, Ali AM. The relationship between motor cortex excitability and severity of Alzheimer's disease: a transcranial magnetic stimulation study. Neurophysiol. Clin. 2011;41:107–113. doi: 10.1016/j.neucli.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Kirschman LT, Borysiewicz E, Fil D, Konat GW. Peripheral immune challenge with dsRNA enhances kainic acid-induced status epilepticus. Metab Brain Dis. 2011;26:91–93. doi: 10.1007/s11011-011-9236-z. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. J. Neurosci. 2005;25:8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konat GW, Borysiewicz E. Cerebellar expression of inflammatory genes triggered by peripheral challenge with dsRNA. J. Neurochem. 2009;108(Suppl. 1):133. [Google Scholar]
- Konat GW, Borysiewicz E, Fil D, James I. Peripheral challenge with double-stranded RNA elicits global up-regulation of cytokine gene expression in the brain. J Neurosci. Res. 2009;87:1381–1388. doi: 10.1002/jnr.21958. [DOI] [PubMed] [Google Scholar]
- Krasowska-Zoladek A, Banaszewska M, Kraszpulski M, Konat GW. Kinetics of inflammatory response of astrocytes induced by TLR 3 and TLR4 ligation. J. Neurosci. Res. 2007;85:205–212. doi: 10.1002/jnr.21088. [DOI] [PubMed] [Google Scholar]
- Lenczowski MJ, Van Dam AM, Poole S, Larrick JW, Tilders FJ. Role of circulating endotoxin and interleukin-6 in the ACTH and corticosterone response to intraperitoneal LPS. Am. J Physiol. 1997;273:R1870–R1877. doi: 10.1152/ajpregu.1997.273.6.R1870. [DOI] [PubMed] [Google Scholar]
- Machado A, Herrera AJ, Venero JL, Santiago M, de Pablos RM, Villaran RF, Espinosa-Oliva AM, Arguelles S, Sarmiento M, Delgado-Cortes MJ, Maurino R, Cano J. Peripheral inflammation increases the damage in animal models of nigrostriatal dopaminergic neurodegeneration: possible implication in Parkinson's disease incidence. Parkinsons. Dis. 2011;2011:393769. doi: 10.4061/2011/393769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara JO. Emerging insights into the genesis of epilepsy. Nature. 1999;399:A15–A22. doi: 10.1038/399a015. [DOI] [PubMed] [Google Scholar]
- Michalovicz LT, Konat GW. Peripherally restricted acute phase response to a viral mimic alters hippocampal gene expression. Metab Brain Dis. 2014;29:75–86. doi: 10.1007/s11011-013-9471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CL, Skelly DT, Cunningham C. Exacerbation of CNS inflammation and neurodegeneration by systemic LPS treatment is independent of circulating IL-1beta and IL-6. J. Neuroinflammation. 2011;8:50. doi: 10.1186/1742-2094-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone R, Bergmann J, Kunz A, Caleri F, Seidl M, Tezzon F, Gerstenbrand F, Trinka E, Golaszewski S. Cortical excitability changes in patients with sleep-wake disturbances after traumatic brain injury. J. Neurotrauma. 2011;28:1165–1171. doi: 10.1089/neu.2010.1748. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, D'Aigle T, Gowing G, Julien JP, Rivest S. Exacerbation of motor neuron disease by chronic stimulation of innate immunity in a mouse model of amyotrophic lateral sclerosis. J. Neurosci. 2004;24:1340–1349. doi: 10.1523/JNEUROSCI.4786-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Lyons-Weiler J. caGEDA: a web application for the integrated analysis of global gene expression patterns in cancer. Appl. Bioinformatics. 2004;3:49–62. doi: 10.2165/00822942-200403010-00007. [DOI] [PubMed] [Google Scholar]
- Quan N, Banks WA. Brain-immune communication pathways. Brain Behav. Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Ren X, Zhou H, Li B, Su SB. Toll-like receptor 3 ligand polyinosinic:polycytidylic acid enhances autoimmune disease in a retinal autoimmunity model. Int. Immunopharmacol. 2011;11:769–773. doi: 10.1016/j.intimp.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Ivanov AI, Lenczowski MJ, Kulchitsky VA, Van Dam AM, Poole S, Homer LD, Tilders FJ. Lipopolysaccharide transport from the peritoneal cavity to the blood: is it controlled by the vagus nerve? Auton. Neurosci. 2000;85:133–140. doi: 10.1016/S1566-0702(00)00232-0. [DOI] [PubMed] [Google Scholar]
- Rossi S, Furlan R, De C,V, Motta C, Studer V, Mori F, Musella A, Bergami A, Muzio L, Bernardi G, Battistini L, Martino G, Centonze D. Interleukin-1beta causes synaptic hyperexcitability in multiple sclerosis. Ann. Neurol. 2012;71:76–83. doi: 10.1002/ana.22512. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serres S, Anthony DC, Jiang Y, Broom KA, Campbell SJ, Tyler DJ, van Kasteren SI, Davis BG, Sibson NR. Systemic inflammatory response reactivates immune-mediated lesions in rat brain. J. Neurosci. 2009;29:4820–4828. doi: 10.1523/JNEUROSCI.0406-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan AH, Madison DV, Mateos JM, Fraser DA, Lovelett EA, Coutellier L, Kim L, Tsai HH, Huang EJ, Rowitch DH, Berns DS, Tenner AJ, Shamloo M, Barres BA. A dramatic increase of C1q protein in the CNS during normal aging. J. Neurosci. 2013;33:13460–13474. doi: 10.1523/JNEUROSCI.1333-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Strohmeyer R, Shen Y, Rogers J. Detection of complement alternative pathway mRNA and proteins in the Alzheimer's disease brain. Brain Res. Mol. Brain Res. 2000;81:7–18. doi: 10.1016/s0169-328x(00)00149-2. [DOI] [PubMed] [Google Scholar]
- Terai K, Walker DG, McGeer EG, McGeer PL. Neurons express proteins of the classical complement pathway in Alzheimer disease. Brain Res. 1997;769:385–390. doi: 10.1016/s0006-8993(97)00849-4. [DOI] [PubMed] [Google Scholar]
- Thomson CA, McColl A, Cavanagh J, Graham GJ. Peripheral inflammation is associated with remote global gene expression changes in the brain. J. Neuroinflammation. 2014;11:73. doi: 10.1186/1742-2094-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf S, Atif F, Sayeed I, Wang J, Stein DG. Post-stroke infections exacerbate ischemic brain injury in middle-aged rats: immunomodulation and neuroprotection by progesterone. Neuroscience. 2013;239:92–102. doi: 10.1016/j.neuroscience.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Malik A, Choi HB, Ko RW, Dissing-Olesen L, MacVicar BA. Microglial CR3 activation triggers long-term synaptic depression in the hippocampus via NADPH oxidase. Neuron. 2014;82:195–207. doi: 10.1016/j.neuron.2014.01.043. [DOI] [PubMed] [Google Scholar]
- Zhang R, Miller RG, Gascon R, Champion S, Katz J, Lancero M, Narvaez A, Honrada R, Ruvalcaba D, McGrath MS. Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS). J. Neuroimmunol. 2009;206:121–124. doi: 10.1016/j.jneuroim.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]