Abstract
In February 2000, a radiation incident involving a medical 60Co source occurred in a metal scrapyard in Thailand. Several individuals were suspected to have received chronic or fractionated exposures ranging from a few mGy to a several Gy. Using fluorescence in situ hybridization to paint chromosomes, we determined the frequencies of chromosome aberrations in peripheral blood lymphocytes of 13 people who entered the scrapyard, 3 people who involved in recovering the source, and 9 nearby residents. Aberration frequencies greater than controls were observed in 13 of the donors at 3 months postexposure. The predominant form of aberration observed was simple, complete, symmetrical translocations. An approximate 50% decrease in these aberrations and in total color junctions was observed in 7 donors resampled at 16 months postexposure. Although high, acute exposures are known to have detrimental effects, the biological consequences of chronic, low dose-rate radiation exposures are unclear. Thirteen of the donors had elevated aberration frequencies, and 6 also had symptoms of acute radiation syndrome. If there are any long-term health consequences of this incident, it will most likely occur among this group of individuals. The consequences for the remaining donors, who presumably received lower total doses delivered at lower dose rates, are less clear.
Keywords: chromosome aberrations, chronic, FISH
Introduction
In February 2000, 4 metal salvagers acquired a teletherapy unit near Bangkok, Thailand, and transported it to a local scrapyard. The unit was dismantled on February 1, 2000, exposing an unshielded 60Co source, later determined to be 15.7 TBq.1 In this accident situation, there was uncertainty in the received doses and dose rates to different individuals. Efforts to geographically model dose-rate patterns to individuals were complicated by the movement of individuals into and out of the scrapyard and by confounding factors such as shielding from materials in the scrapyard. Acute radiation syndrome (ARS) from high doses was evident shortly after the exposures, with symptoms such as nausea, vomiting, hair loss, depressed lymphocyte counts, and acute skin necrosis (Table 1). Overall, there have been 3 fatalities, and 6 individuals were hospitalized for the treatment of ARS related to this incident.
Table 1.
Age, Sex, Involvement, Estimated Doses, and Clinical Symptoms of Lymphocyte Donors.a
| Donorb | Age | Sex | Involvement | Estimated Dose, Gy | Clinical Symptoms |
|---|---|---|---|---|---|
| 1 | 45 | F | Scrapyard owner | >6 | Nausea, vomiting, epilation, epistaxis, lymphopenia |
| 2 | 75 | F | Donor 1’s mother | 2-6 | Nausea, vomiting, lymphopenia |
| 3 | 33 | F | Donor 1’s maid | 2-6 | Nausea, vomiting, headache, epilation, lymphopenia |
| 4 | 3 | F | Donor 1’s daughter | <0.032 | None |
| 5 | 25 | M | Associate of donor 23 | 2 | Burns, nausea, vomiting, epilation, skin necrosis |
| 6 | 35 | F | Temporary scrapyard worker | <0.032 | None |
| 7 | 23 | M | Donor 23’s brother in law | 1 | Burns, mild nausea, vomiting, skin necrosis |
| 8 | 22 | F | Temporary scrapyard worker | <0.032 | None |
| 9 | 25 | M | Temporary scrapyard worker | <0.032 | None |
| 10 | 57 | M | Scrapyard office employee | <0.032 | None |
| 11 | 45 | F | Nearby resident—control | <0.032 | None |
| 12 | 44 | F | Nearby resident—control | <0.032 | None |
| 13 | 20 | F | Nearby resident (sister of donor 14 and daughter of donor 15) | <0.032 | None |
| 14 | 19 | F | Nearby resident (sister of donor 13 and daughter of donor 15) | <0.032 | None |
| 15 | 46 | M | Nearby resident (father of donors 13 and 14) | <0.032 | None |
| 16/28 | 37 | M | Scrapyard worker | <0.032 | None |
| 17/25 | 33 | F | Nearby resident, hairdresser | <0.032 | None |
| 18 | 62 | M | Nearby resident—control | <0.032 | None |
| 19 | 66 | F | Nearby resident—control | <0.032 | None |
| 22/27 | 43 | F | Nearby resident—control | <0.032 | None |
| 23 | 40 | M | Scrap collector—acquired source | 2 | Burns, nausea, vomiting, epilation, lymphopenia, skin necrosis |
| 24 | 19 | M | Associate of donor 23 | 2 | Burns, nausea, vomiting, epilation, lymphopenia, skin necrosis |
| 29 | 45 | M | OAEP worker—control | <0.032 | None |
| 30 | ? | M | OAEP worker—control | <0.032 | None |
| 31 | ? | M | OAEP worker—control | <0.032 | None |
Abbreviations: F, female; M, male; OAEP, Office of Atomic Energy for Peace; ?, ages unknown for donors 30 and 31.
a Doses were estimated based on clinical symptoms.1
b Donor 1 in this study is referred to as patient P7/JJ in the study by International Atomic Energy Agency,1 donor 2 as patient P10/TJ, donor 3 as patient P9/SY, donor 5 as patient P2/SS, donor 7 as patient P4/VS, donor 23 as patient P1/JC, and donor 24 as patient P3/BS.
An important need in responding to an incident involving potential exposure to ionizing radiation is the identification of individuals who are at greatest risk of short- and long-term radiation effects, with the identification of the latter being more difficult. Physical factors such as dose and dose rate, biological factors such as genetic predisposition and age, and environmental factors all play an important role in determining the level of biological consequences of an accidental radiation exposure. The purpose of this research was to determine the level of radiation damage in the lymphocytes collected from donors exposed to unknown levels of chronic γ radiation in order to estimate the potential health impacts.
It was suggested 53 years ago that the incidence of radiation-induced chromosome aberrations in human lymphocytes could be used to determine the relative magnitude of an unknown accidental exposure.2 Measuring the level of chromosome damage and estimating the approximate dose received usually involve in vitro experimental construction of dose–response curves for chromosomal aberrations, using exposures of the same radiation quality and dose rate, and subsequent comparison of in vivo aberration frequencies of exposed individuals.3 Although specific chromosome rearrangements are involved in many human cancers,4 long-term health effects estimation based on chromosome aberration frequency remains speculative. However, it is assumed that there is an increased health risk when large numbers of aberrations are observed after a large acute exposure. Observed health effects from low dose, low dose-rate radiation exposures include less constitutive damage,5and increased resistance to damage from subsequent radiation exposures through an adaptive response.6–10 Therefore, predicting health risk from measurements of chromosome aberration frequencies is a complicated task. Furthermore, measuring chromosome aberrations gives an estimate of the biological effect of the radiation exposure to the individual but may not extrapolate directly to total physical dose, especially when radiation quality, dose rate, and fractionation are unknown. However, recent advances in molecular cytogenetics combined with retrospective health risk measurements afford the possibility that molecular markers, such as specific chromosome rearrangements, may be useful tools for radiation risk assessment.
Conventional and molecular cytogenetic techniques are used to detect chromosome damage and quantify human exposure to ionizing radiation.11 Fluorescence in situ hybridization (FISH) with whole chromosome paints has greatly facilitated the identification of chromosome aberrations, such as translocations, and is now widely used in the field of human radiation cytogenetics.12–16 It is particularly relevant to use the FISH method following chronic or past exposure, since FISH offers the possibility to detect and use stable translocations for accurate biological dosimetry.3,17,18
Materials and Methods
Lymphocyte Culture and Harvest
Blood samples from 25 potentially exposed and control individuals were obtained approximately 3 months after the accident. The samples originated from 13 individuals who on occasion visited or worked in the scrapyard (donors 1-10, 16/28, 23, and 24), 9 nearby residents (donors 11-15, 17-19, and 22), and 3 individuals who involved in the recovery of the 60Co source (donors 29-31). A second round of sampling was conducted about 16 months after the accident for 7 of the donors showing the highest aberration frequencies in the first round of sampling. All donors signed informed consent with the Thailand Ministry of Public Health.
Approximately 25 to 35 mL of whole blood was collected from each donor into sodium heparinized vacutainers and confirmed to be HIV negative. Culturing of blood samples was conducted at Chulalongkorn University in Bangkok, Thailand. Lymphocytes were separated from whole blood by centrifuging on a Ficoll (Sigma, St Louis, Missouri) gradient. The cells were then washed twice by resuspension in room-temperature phosphate-buffered saline solution and centrifuged at 200g. Cultures were established at a cell concentration of 1 × 106 cells/mL of medium. In the cultures used for FISH analysis, the lymphocytes were incubated in RPMI 1640 medium, containing sodium bicarbonate (final concentration: 24 mmol/L), l-glutamine (2 mmol/L), gentamicin (4 mg/L), phytohemagglutinin (1%), and 20% vol/vol fetal bovine serum. All tissue culture reagents were supplied by Gibco (Grand Island, New York). Cells were cultured in an incubator at 37°C, 5% CO2, for 72 hours to maximize the number of metaphase cells.19 In some cases, this will result in cells at in the second poststimulation division, however, this would be the same for all donors as all the cultures were treated identically. Colcemide (6 μg/mL) was added 45 minutes prior to harvesting cells for metaphase spread preparation. Lymphocytes were separated from culture media by centrifugation at 200g and then placed in 0.075 mol/L KCl at room temperature (RT) for approximately 10 minutes. Cells were again centrifuged and resuspended in 10 mL of 5% acetic acid for 5 minutes. Finally, cells were centrifuged and resuspended in Carnoy fixative (3 parts methanol and 1 part acetic acid) to preserve the cells.
Irradiation and culturing of whole blood samples for the construction of a dose–response curve were conducted at McMaster University. Blood samples from 3 healthy donors (2 males and 1 female) were irradiated with 0, 0.25, 0.5, 1, and 2 Gy 60Co γ rays (Taylor source, McMaster University, dose rate 0.1 Gy/min) at RT. Immediately after irradiation, whole blood cultures were initiated with RPMI 1640 medium. Cultures were incubated at 37°C, 5% CO2, for 44 hours. Colcemide (1 μg/mL) was added 4 hours prior to harvesting cells for metaphase spreads. Cells were separated from culture media by centrifugation at 200g and then placed in 0.075 mol/L KCl at 37°C for 15 minutes. Cells were again centrifuged and fixed 3 times in 10 mL of cold Carnoy fixative for 15 minutes. The cell suspensions were kept at −20°C.
Fluorescence In Situ Hybridization
Glass microscope slides were cleaned by soaking in 70% methanol overnight. Metaphase spreads were prepared and those suitable for FISH (a high density of intact metaphases, well-spread chromosomes) were identified under phase contrast using 40× air objective and brightfield illumination. Chromosome painting was performed using Chromoprobe-124 kits supplied by Cytocell (Oxon, United Kingdom), which directly labeled chromosome 1 with Texas Red, chromosome 2 with fluorescein isothiocyanate (FITC), and chromosome 4 with diethyl aminomethyl coumarin (DEAC). Chromosomes were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Approximate respective excitation and emission wavelengths for the microscope filter sets used were 571 and 627 nm for Texas Red, 495 and 531 nm for FITC, 436 and 480 nm for DEAC, and 402 and 462 nm for DAPI. The hybridization procedure was performed as directed by the manufacturer. Hybridization mix consisting of dextran sulfate, formamide, and saline–sodium citrate (SSC) was prewarmed to 37°C. Slides were then washed in 2× SSC (0.3 mol/L NaCl and 0.03 mol/L sodium citrate) for 2 minutes at RT and then quenched in an ethanol series (70%, 85%, and 100% ethanol in water) for 2 minutes each at RT. Slides and coverslips coated with the whole chromosome probes were prewarmed to 37°C for approximately 15 minutes. The hybridization mix and coverslips were then placed on the slides, and the edges were sealed with rubber cement. Slides were hybridized for 16 hours in a sealed, humid chamber maintained at 37°C. The rubber cement and coverslips were removed, and the slides were washed in 0.4× SSC for 2 minutes and then in RT 2× SSC and 0.05% Tween 20 at RT for approximately 25 seconds. Slides were then counterstained with approximately 17 μL DAPI in antifade (Cytocell), covered with a 20 mm2 glass coverslip, and the edges of the coverslip were sealed with clear nail polish.
Chromosome aberrations were scored according to the Protocol for Aberration Identification and Nomenclature Terminology (PAINT) nomenclature system,20 with minor modification. Apparently, simple interchange aberrations were defined as those in which both of the chromosomes involved were bicolored and had only 1 color junction each (2 such chromosomes were considered 1 complete exchange). Bicolored chromosomes containing more than 1 color junction were defined as complex.21 A complete translocation was defined as one in which 2 bicolored chromosomes were visible in the cell. If only 1 bicolored chromosome was visible, the translocation was defined as incomplete even though some of these could have been hidden complete translocations which appeared incomplete because of the resolution of the detection system.22–24 Finally, symmetrical translocations were defined as those in which each bicolored chromosome had exactly 1 centromere, whereas asymmetrical translocations were defined as those in which a bicolored chromosome had 2 centromeres and was accompanied by a bicolored acentric fragment for complete translocations or by a painted acentric fragment or no visible fragment for incomplete translocations.
Results
White Blood Cell Counts
White blood cell (WBC) counts were taken soon after the accident from donors 1, 2, 3, 23, and 24 during the course of their treatment for ARS. The results showed that donors 1, 2, and 3 initially experienced acute lymphopenia following the accident and then returned to normal, although the count for donor 1 may have been persistently elevated during the testing period (Figure 1A). Abnormal WBC counts were also observed in donors 23 and 24 (Figure 1B). White blood cell counts may have been slightly depressed in donor 23 from February 26 to March 4, 2000 (25-31 days after initial exposure), but then both donors showed an elevation in WBC counts, possibly indicating persistent infection following recovery.
Figure 1.
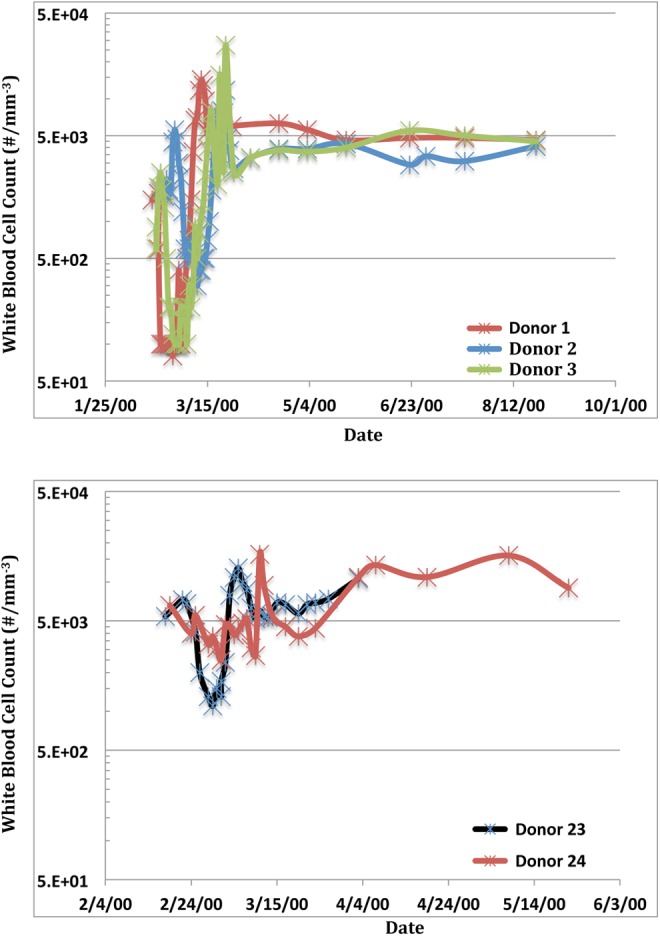
White blood cell counts of individuals showing acute radiation syndrome following exposure to 60 Co source (Top panel: Donors 1, 2, and 3. Bottom panel: Donors 23 and 24).
Fluorescence In Situ Hybridization
Table 2 summarizes the different types of aberrations observed for 25 donors sampled approximately 3 months postexposure. Analysis of the 7 of the donors with the highest aberration frequencies was repeated 16 months postirradiation and is also shown in Table 2. Chromosome aberrations were observed in 13 individuals 3 months after the accident. The remaining 12 samples had no detectable aberrations after scoring at least 200 cells, indicating that the background frequency of chromosomal aberrations from other sources was low in control populations.
Table 2.
Translocation and Color Junction Frequencies in Peripheral Blood Lymphocytes.a
| Donor | N | Total Color Junctionsb | Symmetrical Translocations | Asymmetrical Translocations | ||
|---|---|---|---|---|---|---|
| Complete | Incomplete | Complete | Incomplete | |||
| 3 months postexposure | ||||||
| 1 | 137 | 55 (40.1) | 24 (17.5) | 2 (1.5) | 2 (1.5) | 1 (0.7) |
| 3 | 206 | 57 (27.7) | 25 (12.1) | 4 (1.9) | 0 (0.0) | 0 (0.0) |
| 4 | 226 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 358 | 52 (14.5) | 23 (6.4) | 6 (1.7) | 0 (0.0) | 0 (0.0) |
| 7 | 200 | 6 (3.0) | 3 (1.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 8 | 318 | 27 (8.5) | 8 (2.5) | 1 (0.3) | 5 (1.6) | 0 (0.0) |
| 9 | 271 | 6 (2.2) | 1 (0.4) | 3 (1.1) | 0 (0.0) | 1 (0.4) |
| 10 | 350 | 11 (3.1) | 4 (1.1) | 3 (0.9) | 0 (0.0) | 0 (0.0) |
| 11 | 224 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 12 | 220 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 13 | 220 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 14 | 233 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 15 | 281 | 6 (2.1) | 2 (0.7) | 2 (0.7) | 0 (0.0) | 0 (0.0) |
| 16 | 213 | 1 (0.5) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) |
| 17 | 300 | 2 (0.7) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 22 | 478 | 7 (1.5) | 2 (0.4) | 0 (0.0) | 1 (0.2) | 1 (0.2) |
| 23 | 224 | 24 (10.7) | 8 (3.6) | 4 (1.8) | 1 (0.4) | 2 (0.9) |
| 24 | 270 | 18 (6.7) | 8 (3.0) | 1 (0.4) | 0 (0.0) | 1 (0.4) |
| 29 | 232 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 30 | 221 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 31 | 225 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 16 months postexposure | ||||||
| 1 | 209 | 38 (18.2) | 17 (8.1) | 4 (1.9) | 0 (0.0) | 0 (0.0) |
| 2 | 159 | 12 (7.5) | 6 (3.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 205 | 28 (13.7) | 13 (6.3) | 2 (1.0) | 0 (0.0) | 0 (0.0) |
| 5 | 205 | 19 (9.3) | 8 (3.9) | 3 (1.5) | 0 (0.0) | 0 (0.0) |
| 6 | 203 | 5 (2.5) | 2 (1.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) |
| 7 | 220 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 9 | 213 | 3 (1.4) | 1 (0.5) | 1 (0.5) | 0 (0.0) | 0 (0.0) |
a Samples were collected at 3 months postexposure, and for a subset of donors, at 16 months postexposure. Numbers in parentheses are percentages (100 × [no. aberrations/no. cells scored]).
b Two color junctions in donor 3 were associated with the insertion of an unpainted fragment into chromosome 1. All other color junctions were associated with apparently simple symmetrical or asymmetrical translocations.
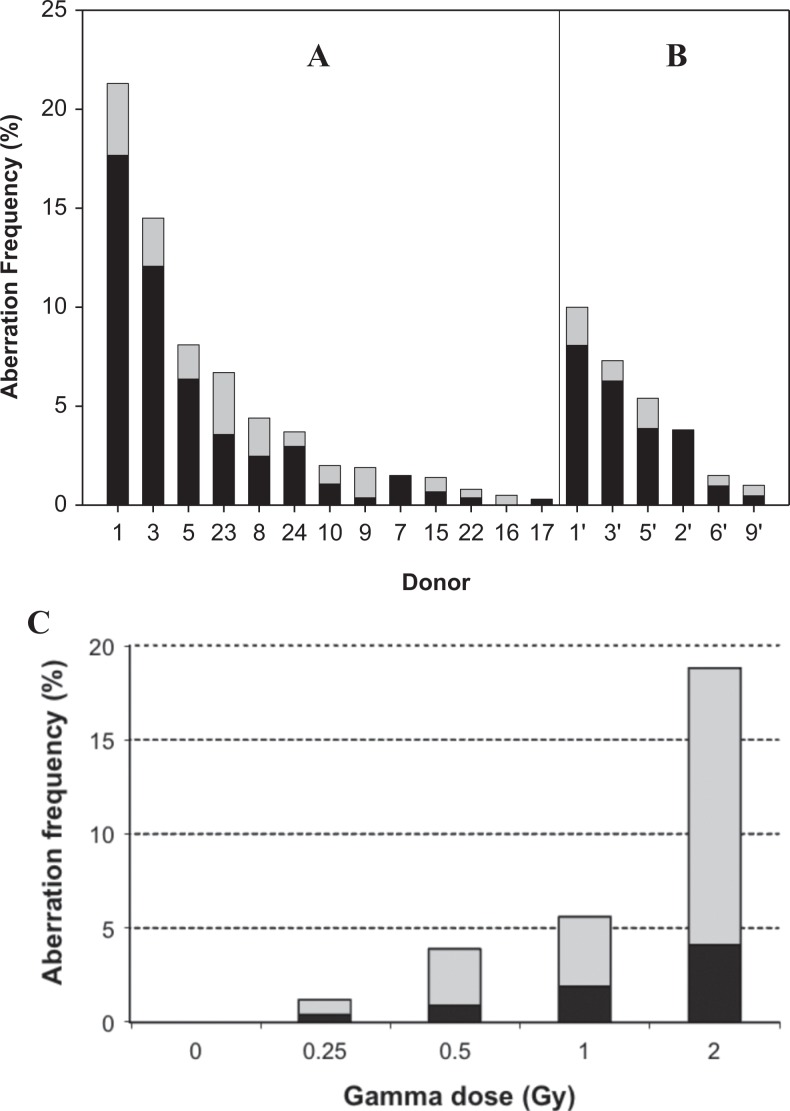
The predominant form of observed aberrations was apparently simple, complete, symmetrical translocations. Much lower frequencies of unstable chromosome exchanges (incomplete symmetrical and complete and incomplete asymmetrical exchanges) were also observed. The highest frequencies of chromosome aberrations were observed in donors who displayed ARS symptoms. Therefore, this result is consistent with the clinical symptoms of accidental, high-dose radiation exposure (ie, lymphopenia, skin necrosis, nausea, hair loss, etc). The exception to this is donor 8 who had slightly higher aberration frequencies than donor 24, in spite of the fact that donor 24 showed ARS symptoms, whereas donor 8 did not. The frequencies of complete symmetrical translocations [100 × (no. aberrations)/(no. cells scored)] ranged from 0% to 17.5% but exceeded 5% in only 3 of the samples examined (Figure 2A). Chromosome 1 was involved in 41.6%, chromosome 2 in 42.6%, and chromosome 4 in 15.8% of the aberrations at 3 months postexposure (data not shown).
Figure 2.
Exchange aberration frequencies (100 × [no. aberrations]/[no. cells scored]) in chromosomes 1, 2, and 4 in peripheral blood lymphocytes at (A) 3 and (B) 16 months postexposure. Dark bars indicate frequencies of stable aberrations (complete, symmetrical translocations), and light bars indicate frequencies of unstable aberrations (incomplete symmetrical translocations and asymmetrical translocations). No aberrations were detected in donors 4, 11, 12, 13, 14, 18, 19, 29, 30, and 31 in the 3-month samples or in donor 7 in the 16-month sample. C, Total aberration frequencies in chromosomes 1, 2, and 4 in peripheral blood lymphocytes after in vitro exposure to different doses of γ radiation (60Co source).
By 16 months postexposure, aberrations were observed in 6 of the 7 resampled donors (Figure 2B). Similar to the samples collected at 3 months postexposure, the predominant form of aberrations was apparently simple, complete, symmetrical translocations. Again, the donors with the highest frequencies of aberrations were those who had displayed ARS after the accident. For the 5 donors where a sample was available for both periods following the accident (3 and 16 months), the frequency of total color junctions and complete symmetrical translocations observed from samples collected at 16 months had declined to approximately 50% of that observed in samples collected at 3 months (Figure 2). The frequency of aberrations in chromosome 1 (48.4%) was still more than expected, however, chromosome 2 (22.6%) had become less than expected and chromosome 4 (29.0%) almost matched expectations based on chromosome length (data not shown). This represents a significant deviation from length proportionality (χ2 test) at both 3 (P < .001) and 16 (P = .05) months postexposure.
Discussion
The frequency of chromosome aberrations measured by FISH was higher than background in the donors who presented with ARS following the accidental exposure. Therefore, it is not unreasonable to assume that at least some of the damage observed in the chromosomes of these donors was caused by the radiation exposure they received from the 60Co source. It is important to note that, as with most accidental exposure scenarios, no preexposure samples were available. Therefore, it cannot be unequivocally stated that all of the aberrations observed were due to radiation exposure from the 60Co source. However, as the data for the unexposed individuals indicate, background frequencies of chromosome aberrations appear to be low in this population.
With the exception of donor 8, blood samples from donors who were possibly exposed, but did not show any ARS, did not have aberration frequencies above those of the control donors. It is known that the number of chromosome aberrations in the cells of individuals increases with age25–29 and by exposure to environmental factors including smoking tobacco,30–33 however, the background frequency of chromosome aberrations in this genetically similar population was low (Table 2), although the donors ranged in age from 3 to 75 years.
Stability of Symmetrical and Asymmetrical Translocations
We observed few asymmetrical translocations compared to the number of symmetrical translocations in all samples we examined. Conventional theory holds that these aberrations should occur in equal number in the first mitosis following radiation exposure,34 and this has been observed by a number of investigators.35–41 However, some studies have observed a higher frequency of symmetrical translocations.42–47 We observed a similar frequency at the first mitosis of symmetrical translocations compared to asymmetrical translocations following in vitro exposures (data not shown).
It has been reported that in the absence of a pancentromeric probe, some asymmetrical translocations can be misclassified as symmetrical,48 leading to an apparent excess of symmetrical (and corresponding deficit of asymmetrical) translocations. To verify that this was not the source of the excess of symmetrical translocations observed in this study, approximately 50 complete DAPI G-banded metaphases from donor 5 (3 months postexposure) were scored (data not shown), and no asymmetrical translocations were observed.
This result is not surprising, as the equality between symmetrical and asymmetrical translocations is postulated only for the first postirradiation mitosis. It is well known that unstable aberrations, such as asymmetrical translocations, disappear in successive cell generations.49,50 In our work, the predominance of complete symmetrical translocations in the individuals who were exposed to high doses was most likely due to fact that the samples were collected approximately 3 months after the accident. The donors who exhibited elevated frequencies of chromosome aberrations also had ARS (Tables 1 and 2). The peripheral blood lymphocyte pool at the time of sampling certainly had to have been repopulated from stem cells following ARS-related lymphopenia (Figure 1). Therefore, many of the circulating peripheral blood lymphocytes present at the time of irradiation were likely gone, and the stem cells had undergone numerous rounds of cell division prior to sampling, which probably resulted in the depletion of asymmetrical translocations and other unstable aberrations in lymphocytes and bone marrow.
A comparison of the samples collected 3 months postexposure with samples from the same individuals 16 months after exposure revealed a decline in the frequency of complete symmetrical translocations. The evidence on the stability of these aberrations is mixed, with long-term stability reported by some investigators,41,50–52 but declines in translocation frequency over time reported by others.13,52–55 It has been suggested that especially at high acute doses, a fraction of the initial symmetrical translocations is lost due to coincident occurrence of these aberrations with unstable aberrations56 and/or due to the loss of genetic material in fragments too small to be detected by FISH. Following the loss of this initial fraction, long-term stability is observed in the remaining fraction after high acute exposures.28,57,58 However, the individuals from this accident were exposed to protracted, fractionated exposures, and the possibility exists that long-term stability may not be observed under these irradiation conditions. It has been shown that following protracted whole-body exposures, there is a temporal decline in both unstable dicentrics (half-time of about 14 months) and stable reciprocal translocations (half-time of about 3-4 years).54 Therefore, the utility of translocation frequencies for retrospective dose reconstruction has been suggested to be limited to about 11 years following high-level protracted exposures.59 Sevan’kaev et al12 attempted a retrospective dosimetry using FISH translocations on victims of the Chernobyl accident. From 10 to 13 years postaccident, they showed that this technique works well at acute doses up to about 3 Gy.12 Camparoto et al60 similarly used FISH translocation to estimate doses in victims of the Goiânia accident.60 In our experimental protocol, we stimulated the lymphocytes in the samples from the exposed individuals for 72 hours, so it is possible that we observed some cells in their second poststimulation mitosis. This also occurred after a period of several months following radiation exposure, during which the lymphocyte pool was being repopulated, at least in the most highly exposed individuals. This means it is likely that several cell divisions had already occurred following irradiation. Therefore, it is not surprising that we observed more symmetrical than asymmetrical translocations.
This research has shown a significant decline in stable rearrangements after 16 months and affords the opportunity to test the long-term stability of these aberrations after protracted, fractionated exposures. It is not possible to determine whether the frequency of complete, symmetrical translocations will stabilize at elevated levels in these victims without additional sampling over time.
The individuals from this accident were exposed to protracted, fractionated exposures. The high frequency of stable translocations detected in the peripheral blood lymphocytes of individuals (especially donors 1, 3, and 5) approximately 3 months postexposure is interpreted to suggest that the bone marrow tissue was exposed to a very high cumulative dose. Comparison with a dose–response curve generated in our laboratory (Figure 2C) using a high dose rate (0.1 Gy/min) indicates that the frequencies of chromosome aberrations observed in donors 1, 3, and 5 at 3 months postexposure exceed the frequencies observed immediately after a 2 Gy acute exposure. Considering the observed decline in aberration frequency with time observed in this study, and the fractionated and protracted dose rates experienced by the donors, total doses most likely significantly exceeded 2 Gy at least for these 3 donors and possibly for donors 8, 23, and 24 as well.
Summary
It is not possible, at the present time, to relate high frequencies of chromosome exchange aberrations to long-term biological effects. It is clear that high acute radiation exposures cause clinical effects, increase the frequency of chromosome aberrations, and increase long-term health risk of cancer in some individuals.61 The individuals exposed in this accident received different whole-body radiation doses (related to exposure time) of low linear energy transfer γ radiation at different dose rates (related to the distance from the radiation source when they were exposed and shielding material between the source and the donor). The biological consequences of these types of radiation exposures are unknown. Thirteen of the donors had elevated aberration frequencies, and 6 also had symptoms of ARS. If there are any long-term health consequences of this incident, it will most likely occur among this group of individuals. The consequences for the remaining donors, who presumably received lower total doses delivered at lower dose rates, are less clear.
Acknowledgments
The authors would like to acknowledge the expert technical assistance of Kathy Gale and Lisa Bernas and the contributions of V. Teodorovych, T. Sueblinvong, N. Siwarungsun, W. Lausoontornsiri, and V. Jinaratana.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by generous contributions from Atomic Energy of Canada Limited, CANDU Owners Group, Ontario Power Generation, and McMaster University. B.A.U. was supported by the H. G. Thode Postdoctoral Fellowship at McMaster University.
References
- 1. International Atomic Energy Agency. The Radiological Accident in Samut Prakarn. STI/PUB/1024. Vienna, Austria: International Atomic Energy Agency; 2002. [Google Scholar]
- 2. Bender MA, Gooch PC. Types and rates of x-ray-induced chromosome aberrations in human blood irradiated in vitro. Proc Natl Acad Sci U S A. 1962;48:522–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crespo RH, Domene MM, Rodriguez MJ. Biodosimetry and assessment of radiation dose. Rep Pract Oncol Radiother. 2011;16(4):131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rowley JD. Molecular cytogenetics: Rosetta stone for understanding cancer—twenty-ninth G. H. A. Clowes memorial award lecture. Cancer Res. 1990;50(13):3816–3825. [PubMed] [Google Scholar]
- 5. Stephan G, Pressl S, Koshpessova G, Gusev BI. Analysis of FISH-painted chromosomes in individuals living near the Semipalatinsk nuclear test site. Radiat Res. 2001;155(6):796–800. [DOI] [PubMed] [Google Scholar]
- 6. Olivieri G, Bodycote J, Wolff S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science. 1984;223(4636):594–597. [DOI] [PubMed] [Google Scholar]
- 7. Mitchel REJ, Azzam EI, De Toledo SM. Adaption to ionizing radiation in mammalian cells In: Koval TM, ed. Stress-Inducible Processes in Higher Eukaryotic Cells. New York, NY: Plenum Press; 1997:221–243. [Google Scholar]
- 8. Wolff S. Chromosomes in the assessment of the effects of low levels of genotoxic agents. Leonard Sagan BELLE Award. Biological Effects Low Level Exposure. Hum Exp Toxicol. 1998;17(11):625–632. [DOI] [PubMed] [Google Scholar]
- 9. Raaphorst GP, Boyden S. Adaptive response and its variation in human normal and tumour cells. Int J Radiat Biol. 1999;75(7):865–873. [DOI] [PubMed] [Google Scholar]
- 10. Ghiassi-nejad M, Mortazavi SM, Cameron JR, Niroomand-rad A, Karam PA. Very high background radiation areas of Ramsar, Iran: preliminary biological studies. Health Phys. 2002;82(1):87–93. [DOI] [PubMed] [Google Scholar]
- 11. Tucker J. Radiation cytogenetics: from chromosomes to single nucleotides and from metaphase cells to tissues. Cancer Metastasis Rev. 2004;23(3-4):341–349. [DOI] [PubMed] [Google Scholar]
- 12. Sevan’kaev AV, Lloyd DC, Edwards AA, et al. A cytogenetic follow-up of some highly irradiated victims of the Chernobyl accident. Radiat Prot Dosimetry. 2005;113(2):152–161. [DOI] [PubMed] [Google Scholar]
- 13. Tucker JD, Cofield J, Matsumoto K, Ramsey MJ, Freeman DC. Persistence of chromosome aberrations following acute radiation: I, PAINT translocations, dicentrics, rings, fragments, and insertions. Environ Mol Mutagen. 2005;45(2-3):229–248. [DOI] [PubMed] [Google Scholar]
- 14. Tucker JD, Cofield J, Matsumoto K, Ramsey MJ, Freeman DC. Persistence of chromosome aberrations following acute radiation: II, does it matter how translocations are scored? Environ Mol Mutagen. 2005;45(2-3):249–257. [DOI] [PubMed] [Google Scholar]
- 15. Kleinerman RA, Romanyukha AA, Schauer DA, Tucker JD. Retrospective assessment of radiation exposure using biological dosimetry: chromosome painting, electron paramagnetic resonance and the glycophorin A mutation assay. Radiat Res. 2006;166(1 pt 2):287–302. [DOI] [PubMed] [Google Scholar]
- 16. Tucker JD. Chromosome translocations and assessing human exposure to adverse environmental agents. Environ Mol Mutagen. 2010;51(8-9):815–824. [DOI] [PubMed] [Google Scholar]
- 17. Ainsbury EA, Bakhanova E, Barquinero JF, et al. Review of retrospective dosimetry techniques for external ionising radiation exposures. Radiat Prot Dosim. 2011;147(4):573–592. [DOI] [PubMed] [Google Scholar]
- 18. Fenech M. Current status, new frontiers and challenges in radiation biodosimetry using cytogenetic, transcriptomic and proteomic technologies. Radiat Meas. 2011;46(9):737–741. [Google Scholar]
- 19. Brown MG, Lawce HJ. Peripheral blood cytogenetic methods In: Barch MJ, Knutsen T, Spurbeck JL, eds. The ACT Cytogenetics Laboratory Manual. Piladelphia, PA: Lippincott-Raven Publishers; 1997:77–171. [Google Scholar]
- 20. Tucker JD, Morgan WF, Awa AA, et al. A proposed system for scoring structural aberrations detected by chromosome painting. Cytogenet Cell Genet. 1995;68(3-4):211–221. [DOI] [PubMed] [Google Scholar]
- 21. Savage JR, Simpson PJ. FISH “painting” patterns resulting from complex exchanges. Mutat Res. 1994;312(1):51–60. [DOI] [PubMed] [Google Scholar]
- 22. Kodama Y, Nakano M, Ohtaki K, Delongchamp R, Awa AA, Nakamura N. Estimation of minimal size of translocated chromosome segments detectable by fluorescence in situ hybridization. Int J Radiat Biol. 1997;71(1):35–39. [DOI] [PubMed] [Google Scholar]
- 23. Wu H, George K, Yang TC. Estimate of true incomplete exchanges using fluorescence in situ hybridization with telomere probes. Int J Radiat Biol. 1998;73(5):521–527. [DOI] [PubMed] [Google Scholar]
- 24. Deng W, Lucas JN. Combined FISH with pan-telomeric PNA and whole chromosome-specific DNA probes to detect complete and incomplete chromosomal exchanges in human lymphocytes. Int J Radiat Biol. 1999;75(9):1107–1112. [DOI] [PubMed] [Google Scholar]
- 25. Ramsey MJ, Moore DH, II, Briner JF, et al. The effects of age and lifestyle factors on the accumulation of cytogenetic damage as measured by chromosome painting. Mutat Res. 1995;338(1-6):95–106. [DOI] [PubMed] [Google Scholar]
- 26. Lucas JN, Deng W, Moore D, et al. Background ionizing radiation plays a minor role in the production of chromosome translocations in a control population. Int J Radiat Biol. 1999;75(7):819–827. [DOI] [PubMed] [Google Scholar]
- 27. Tucker JD, Spruill MD, Ramsey MJ, Director AD, Nath J. Frequency of spontaneous chromosome aberrations in mice: effects of age. Mutat Res. 1999;425(1):135–141. [DOI] [PubMed] [Google Scholar]
- 28. Spruill MD, Nelson DO, Ramsey MJ, Nath J, Tucker JD. Lifetime persistence and clonality of chromosome aberrations in the peripheral blood of mice acutely exposed to ionizing radiation. Radiat Res. 2000;153(1):110–121. [DOI] [PubMed] [Google Scholar]
- 29. Edwards AA, Lindholm C, Darroudi F, et al. Review of translocations detected by FISH for retrospective biological dosimetry applications. Radiat Prot Dosimetry. 2005;113(4):396–402. [DOI] [PubMed] [Google Scholar]
- 30. Tucker JD, Tawn EJ, Holdsworth D, et al. Biological dosimetry of radiation workers at the Sellafield nuclear facility. Radiat Res. 1997;148(3):216–226. [PubMed] [Google Scholar]
- 31. Littlefield LG, McFee AF, Salomaa SI, et al. Do recorded doses overestimate true doses received by Chernobyl cleanup workers? Results of cytogenetic analyses of Estonian workers by fluorescence in situ hybridization. Radiat Res. 1998;150(2):237–249. [PubMed] [Google Scholar]
- 32. Moore ID, Tucker JD. Biological dosimetry of Chernobyl cleanup workers: inclusion of data on age and smoking provides improved radiation dose estimates. Radiat Res. 1999;152(6):655–664. [PubMed] [Google Scholar]
- 33. Wang LE, Bondy ML, de Andrade M, et al. Gender difference in smoking effect on chromosome sensitivity to gamma radiation in a healthy population. Radiat Res. 2000;154(1):20–27. [DOI] [PubMed] [Google Scholar]
- 34. Lucas JN, Chen AM, Sachs RK. Theoretical predictions on the equality of radiation-produced dicentrics and translocations detected by chromosome painting. Int J Radiat Biol. 1996;69(2):145–153. [DOI] [PubMed] [Google Scholar]
- 35. Cornforth MN, Bedford JS. A quantitative comparison of potentially lethal damage repair and the rejoining of interphase chromosome breaks in low passage normal human fibroblasts. Radiat Res. 1987;111(3):385–405. [PubMed] [Google Scholar]
- 36. Bahari IB, Bedford JS, Giaccia AJ, Stamato TD. Measurement of the relative proportion of symmetrical and asymmetrical chromosome-type interchanges induced by gamma radiation in human-hamster hybrid cells. Radiat Res. 1990;123(1):105–107. [PubMed] [Google Scholar]
- 37. Fernandez JL, Campos A, Goyanes V, Losada C, Veiras C, Edwards AA. X-ray biological dosimetry performed by selective painting of human chromosomes 1 and 2. Int J Radiat Biol. 1995;67(3):295–302. [DOI] [PubMed] [Google Scholar]
- 38. Finnon P, Lloyd DC, Edwards AA. Fluorescence in situ hybridization detection of chromosomal aberrations in human lymphocytes: applicability to biological dosimetry. Int J Radiat Biol. 1995;68(4):429–435. [DOI] [PubMed] [Google Scholar]
- 39. Kanda R, Hayata I. Comparison of the yields of translocations and dicentrics measured using conventional Giemsa staining and chromosome painting. Int J Radiat Biol. 1996;69(6):701–705. [DOI] [PubMed] [Google Scholar]
- 40. Boei JJ, Natarajan AT. Combined use of chromosome painting and telomere detection to analyse radiation-induced chromosomal aberrations in mouse splenocytes. Int J Radiat Biol. 1998;73(2):125–133. [DOI] [PubMed] [Google Scholar]
- 41. Lindholm C, Tekkel M, Veidebaum T, Ilus T, Salomaa S. Persistence of translocations after accidental exposure to ionizing radiation. Int J Radiat Biol. 1998;74(5):565–571. [DOI] [PubMed] [Google Scholar]
- 42. Bauchinger M, Schmid E, Zitzelsberger H, Braselmann H, Nahrstedt U. Radiation-induced chromosome aberrations analysed by two-colour fluorescence in situ hybridization with composite whole chromosome-specific DNA probes and a pancentromeric DNA probe. Int J Radiat Biol. 1993;64(2):179–184. [DOI] [PubMed] [Google Scholar]
- 43. Dominguez I, Boei JJ, Balajee AS, Natarajan AT. Analysis of radiation-induced chromosome aberrations in Chinese hamster cells by FISH using chromosome-specific DNA libraries. Int J Radiat Biol. 1996;70(2):199–208. [DOI] [PubMed] [Google Scholar]
- 44. Knehr S, Zitzelsberger H, Braselmann H, Nahrstedt U, Bauchinger M. Chromosome analysis by fluorescence in situ hybridization: further indications for a non-DNA-proportional involvement of single chromosomes in radiation-induced structural aberrations. Int J Radiat Biol. 1996;70(2):385–392. [DOI] [PubMed] [Google Scholar]
- 45. Barquinero JF, Knehr S, Braselmann H, Figel M, Bauchinger M. DNA-proportional distribution of radiation-induced chromosome aberrations analysed by fluorescence in situ hybridization painting of all chromosomes of a human female karyotype. Int J Radiat Biol. 1998;74(3):315–323. [DOI] [PubMed] [Google Scholar]
- 46. Darroudi F, Fomina J, Meijers M, Natarajan AT. Kinetics of the formation of chromosome aberrations in X-irradiated human lymphocytes, using PCC and FISH. Mutat Res. 1998;404(3):55–65. [DOI] [PubMed] [Google Scholar]
- 47. Knehr S, Huber R, Braselmann H, Schraube H, Bauchinger M. Multicolour FISH painting for the analysis of chromosomal aberrations induced by 220 kV X-rays and fission neutrons. Int J Radiat Biol. 1999;75(1-2):407–418. [DOI] [PubMed] [Google Scholar]
- 48. Straume T, Lucas JN. A comparison of the yields of translocations and dicentrics measured using fluorescence in situ hybridization. Int J Radiat Biol. 1993;64(2):185–187. [DOI] [PubMed] [Google Scholar]
- 49. Bauchinger M, Schmid E, Braselmann H, Willich N, Clemm C. Time-effect relationship of chromosome aberrations in peripheral lymphocytes after radiation therapy for seminoma. Mutat Res. 1989;211(2):265–272. [DOI] [PubMed] [Google Scholar]
- 50. Duran A, Barquinero JF, Caballin MR, Ribas M, Barrios L. Persistence of radiation-induced chromosome aberrations in a long-term cell culture. Radiat Res. 2009;171(4):425–437. [DOI] [PubMed] [Google Scholar]
- 51. Lucas JN, Awa A, Straume T, et al. Rapid translocation frequency analysis in humans decades after exposure to ionizing radiation. Int J Radiat Biol. 1992;62(1):53–63. [DOI] [PubMed] [Google Scholar]
- 52. Salassidis K, Georgiadou-Schumacher V, Braselmann H, Muller P, Peter RU, Bauchinger M. Chromosome painting in highly irradiated Chernobyl victims: a follow-up study to evaluate the stability of symmetrical translocations and the influence of clonal aberrations for retrospective dose estimation. Int J Radiat Biol. 1995;68(3):257–262. [DOI] [PubMed] [Google Scholar]
- 53. Tucker JD, Breneman JW, Briner JF, Eveleth GG, Langlois RG, Moore DH., II Persistence of radiation-induced translocations in rat peripheral blood determined by chromosome painting. Environ Mol Mutagen. 1997;30(3):264–272. [DOI] [PubMed] [Google Scholar]
- 54. Bauchinger M, Schmid E, Braselmann H. Time-course of translocation and dicentric frequencies in a radiation accident case. Int J Radiat Biol. 2001;77(5):553–557. [DOI] [PubMed] [Google Scholar]
- 55. Liu QJ, Lu X, Zhao XT, et al. Assessment of retrospective dose estimation, with fluorescence in situ hybridization (FISH), of six victims previously exposed to accidental ionizing radiation. Mutat Res. 2014;759:1–8. [DOI] [PubMed] [Google Scholar]
- 56. Gardner S, Tucker J. The cellular lethality of radiation-induced chromosome translocations in human lymphocytes. Radiat Res. 2002;157(5):539–552. [DOI] [PubMed] [Google Scholar]
- 57. Kano Y, Little JB. Site-specific chromosomal rearrangements induced in human diploid cells by x-irradiation. Cytogenet Cell Genet. 1986;41(1):22–29. [DOI] [PubMed] [Google Scholar]
- 58. Matsumoto K, Ramsey MJ, Nelson DO, Tucker JD. Persistence of radiation-induced translocations in human peripheral blood determined by chromosome painting. Radiat Res. 1998;149(6):602–613. [PubMed] [Google Scholar]
- 59. Bauchinger M, Braselmann H, Savage JR, et al. Collaborative exercise on the use of FISH chromosome painting for retrospective biodosimetry of Mayak nuclear-industrial personnel. Int J Radiat Biol. 2001;77(3):259–267. [DOI] [PubMed] [Google Scholar]
- 60. Camparoto ML, Ramalho AT, Natarajan AT, Curado MP, Sakamoto-Hojo ET. Translocation analysis by the FISH-painting method for retrospective dose reconstruction in individuals exposed to ionizing radiation 10 years after exposure. Mutat Res. 2003;530(1-2):1–7. [DOI] [PubMed] [Google Scholar]
- 61. Swartz HM, Williams BB, Nicolalde RJ, Demidenko E, Flood AB. Overview of biodosimetry for management of unplanned exposures to ionizing radiation. Radiat Meas. 2011;46(9):742–748. [Google Scholar]