Abstract
Octopamine is an important neuromodulator of neural function in invertebrates. Octopamine increases male moth sensitivity to female sex pheromones, however, relatively little is known as to the role of octopamine in the female olfactory system, nor its possible effects on the reception of non-pheromone odorants. The purpose of this study was to determine relative effects of octopamine on the sensitivity of the peripheral olfactory system in male and female Heliothis virescens. Single sensillum recording was conducted in both sexes following injection with octopamine or Ringer solution, and during odorant stimulation with conspecific female sex pheromone or host plant volatiles. Results indicate that octopamine plays a significant modulatory role in female sex pheromone detection in female moths; and that male and female pheromone detection neurons share distinct pharmacological and physiological similarities in H. virescens despite sexual dimorphism at the antennal level.
Introduction
The olfactory system is important for sensory detection and is particularly important for invertebrates [1]. Insects use olfaction to engage in critical behaviors, including selection of food, choice of oviposition sites, identification of predators, and detection and selection of conspecific mates [1]. In general, there are two main classifications of odors important to phytophagous insects: host plant volatiles, and pheromones, being important for oviposition/feeding and mating, respectively [2].
Insect antennae are primary organs for detection of olfactory cues. Antennae typically bear large numbers of sensilla, which house dendrites of a single or multiple olfactory receptor neuron(s) [1][3]. Sexual dimorphism is also evident in many species of insects, having differential antennal structure, sensillar distributions and sensillar morphologies. For example, Almaas and Mustaparta [4][5] reported that male tobacco budworm moth, Heliothis virescens F. (Lepidoptera: Noctuidae), possess long trichoid sensilla specialized for the detection of sex pheromones and short trichoid sensilla specialized for the detection of host plant odors; whereas females only have short, ‘generally tuned’, trichoid sensilla.
Dimorphism is also evident in the antennal lobe–the first synaptic output of ORNs from the antenna. The insect antennal lobe is a portion of the deutocerebrum of the insect brain and is usually demarcated by numerous spherical glomeruli (neuropil subcompartments) spread throughout which are the first synaptic neuropil of the olfactory system [6]. In many insects, there are a similar number of glomeruli in male and female insects, however, in most male moths pheromone-sensitive ORNs project axons to a sexually dimorphic macroglomerular complex (MGC)[6][7][8][9]. The MGC consists of several large glomeruli located closest to the base of the antennal nerve [10]. A varying number of satellite glomeruli surround a primary large glomerulus; H. virescens has four glomeruli in the MGC [6][7]. A similar, smaller, structure has been observed in female H. virescens at the base of the antennal nerve and is known as the medial and central large female glomeruli (mLFG and cLFG, respectively) [8]. We recently found that the cLFG receives significant innervation from olfactory receptor neurons which detect cis-9-tetradecenal (Z9-14:Ald), a primary component of H. virescens female sex pheromone [11][12].
In early studies, it was reported that male and female moths were both equally tuned to plant odors, but male moths were exclusively able to detect female pheromone components in the vast majority of cases [13]. However, Seabrook et al. [14] reported that some species of Lepidoptera do not show marked morphological sexual dimorphism, such as Choristoneura spp. Clemens (Lepidoptera: Tortricidae) and Trichoplusia ni Hübner, (Lepidoptera: Noctuidae) but can detect sex pheromone in relatively small concentrations. Ochieng et al. [15] found that Spodoptera littoralis Boisduval (Lepidoptera: Noctuidae) shows comparable ‘auto-detection’ of female sex pheromone relative to males. Electroantennograms (EAGs) of female H. virescens also show significant responses to components of conspecific female sex pheromone [11]. A large concentration of 'male' type pheromone binding proteins have been found in the antenna of female H. virescens; and female pheromone receptor gene expression is also present in female H. virescens antenna [16][17]. A study by Skiri et al. [18] using calcium imaging was unable to detect female pheromone activation in the antennal lobe of female H. virescens, however, it was reported that the cLFG was not in focus during the study. Conversely, Hillier et al. [11] reported that female H. virescens do indeed detect and respond to their own sex pheromone despite morphological sexual dimorphism of their trichoid sensilla; and that female pheromone-detecting ORNs do exclusively target the female cLFG analogous to the MGC in males. Despite this, the evolutionary and behavioral importance of the ability of female H. virescens to detect female pheromone is not yet known.
Biogenic amines are of particular importance in insect olfaction and are expressed in relatively few antennal lobe neurons [19][20]. Octopamine (4-(2-amino-1-hydroxy-ethyl) phenol; hereafter ‘OA’) is a neuromodulator of insect olfaction as well as a neurohormone and neurotransmitter throughout the nervous system [19][20]. The most widely, and arguably evolutionarily important, observation of OA is the apparent similarity with the noradrenergic system of vertebrates. In the periphery, OA functions as a regulator of activity and stress hormone, enhancing muscle contraction and increasing metabolism of the fat body [21][22].
All major areas of the insect brain (optic lobes, central body, and mushroom bodies) are innervated with octopamine containing neurons [22]. Octopaminergic neurons, as clusters of cell bodies and perikarya, are classified into ten groups (I-X) [23]. Of particular interest are the unpaired, Group VIII and IX, neurons situated in the ventral midline or the dorsal midline of the subesophageal, thoracic, and abdominal ganglia. These referred to as VUM (ventral unpaired median) and DUM (dorsal unpaired median) nerves and innervate most of the insect brain [23]. In the locust the DUMETi neuron (a DUM neuron) innervates the extensor tibiae muscle and modulates neuromuscular transmission; octopamine injections have been shown to increase the contractile force of this muscle [24]. In Apis mellifera L the VUMmx1 neuron originates in the suboesophageal ganglion and projects into the brain innervating the glomeruli of the antennal lobes, lateral protocerebrum and mushroom body calyces [25]. The VUMmx1 neuron has been proposed as being octopaminergic, possibly mediating olfactory learning, by providing reinforcement similar to that of sugar in olfactory learning trials [25][26].
The modulatory effect of OA on the olfactory system of insects is of particular interest. Within the Lepidoptera, octopaminergic neurons and OA receptors have been cloned from multiple species, with extensive evidence for its role in sensory processing and behavioral modulation [27][28][29]. In studies on oriental fruit moths (Grapholita molesta Busck), OA treatments cause a marked increase in sensitivity to pheromone signals resulting in increased numbers of males taking flight and orienting to the odor source in a wind tunnel [30]. Linn & Roelofs [31] also found male flight response to pheromone signals modulated by circadian rhythm, temperature and photoperiodic cues are linked to endogenous neuromodulators. Subsequent work with cabbage looper moths (T. ni) showed that not only were male moths sensitized to pheromone after OA injection but that their behavioral response was similar to response of untreated moths to much higher pheromone concentrations, and that OA antagonists reduced male response rates to female pheromone dramatically [32]. However, in studies with male gypsy moths (Lymantria dispar L. (Lepidoptera: Erebidae)) it was determined that the behavioral effect of OA injections did not occur at all times in the light cycle. Injections at the start of scotophase had no affect on pheromone response whereas injections only an hour earlier showed marked increase in pheromone response [33]. As well, in Agrotis ispilon Hufnagel (Lepidoptera: Noctuidae), it has been shown that OA treatment enhances flight of virgin males to female sex pheromone, it does not restore mating behavior in post-mated moths, despite restoring response characteristics within the antennal lobe [34]. These studies show that context is critical for the activity of OA. OA release, and its neuromodulatory action, is under circadian control and that light level detection is key in its regulation, and that mechanisms under OA control may be part of more complex regulatory pathways controlling behavioral outputs.
Centrally, increased intracellular cyclic adenosine monophosphate (cAMP) activity in the antennal lobe has been purported as a likely pathway for increased sensitivity to pheromone [35]. On the antenna, Pophof [36] also investigated transepithelial potential changes to observe the modulatory effects of OA on peripheral olfactory receptor neurons in the silkworm moth (Antheraea polyphemus Busck (Lepidoptera: Saturiniidae)). OA injections increased peak nerve impulse frequency in a dose dependent fashion in response to pheromone stimulation whereas the antagonist epinastine decreased sensitivity in a dose-dependent fashion [36]. It was concluded that the likely mechanism was modulation of the Na/K membrane pump of the ORNs via cAMP induced PKA action. These results were later confirmed in Mamestra brassicae L (Lepidoptera: Noctuidae) [37]. In addition they showed that chlorpromazine, an OA antagonist, decreases ORN pheromone sensitivity and may decrease long term adaptation of ORNs keeping them in a sensitized state despite long exposure to pheromone cascade [37]. OA injections also increase ORN responses to pheromones in male Bombyx mori L. (Lepidoptera: Bombycidae) but did not increase ORN responses to general odorants in females [38]. This led to the conclusion that despite the olfactory transduction pathway being the same for pheromone-sensitive and general odorant-sensitive ORNs a modulatory pathway exists in male pheromone sensitive sensilla that is absent or less sensitive in females.
Recent evidence found that a large portion of female H. virescens short trichoid sensilla do detect their conspecific female sex pheromone, which lends the question as to whether these sensilla are modulated by OA [11]. OA is expressed on the antennae of H. virescens, with reactive cells present at the base of sensilla [17]. Given this knowledge, does OA also increase the sensitivity of these sensilla, making them similar to the long pheromone sensitive trichoid sensilla of male H. virescens? Or is their sensitivity not increased by OA, making them pharmacologically similar to other short trichoid sensilla tuned to non-pheromone odorants? Based on the evidence of octopaminergic neurons in the brain and receptors in the antenna of female H. virescens it is hypothesized that OA will increase the sensitivity of female ORNs to their conspecific female sex pheromone. In effect, this would indicate a significant degree of similarity in neuropharmacology between male and female pheromone detecting systems, despite morphological differences. The objective of this study is to utilize single sensillum recordings (SSR) to compare any modulatory effect of OA injections on male and female H. virescens response to pheromones and host plant volatiles.
Materials and Methods
Insects
Three to four day old H. virescens were selected from an established colony at Acadia University (Wolfville, Nova Scotia). Larvae were reared on Tobacco Budworm Diet purchased from Southland Products, Inc. (Southland™ Products Inc., Lake Village, Arkansas). Pupae were then separated by sex and placed into an environmentally controlled room (24°C, 60% relative humidity) on a reversed light schedule (14D:10L) until eclosion and testing.
Chemicals
Female pheromone components were selected based on their ability to elicit single sensillum responses in female H. virescens moths [11]. The female sex pheromone components were as follows: (Z)-9-tetradecenal (Z9-14:Ald), (Z)-11-hexadecenal (Z11-16:Ald) and (Z)-11-hexadecenyl acetate (Z11-16:OAc) and were obtained from Bedoukian Research, Inc. (Danbury, Connecticut, USA). Putative host volatiles were selected from compounds that have shown physiological or behavioral effects on H. virescens in previous studies [11][12][39][40]: 2-phenyl ethanol, Z3-hexenol, along with racemic linalool and β-caryophyllene were obtained from Sigma Aldrich (St. Louis, MO, USA). All chemicals were diluted in hexane at decade steps from 10ng/μl-100μg/μl, and stored at -20°C. OA-hydrochloride was obtained from Sigma Aldrich (St. Louis, MO, USA) and stored at room temperature. OA was diluted to 50μg/μl in hemolymph Ringer solution [41] and stored in a fridge at 4°C.
Odorant Stimulation
Z11-hexadecenal (Z11-16:Ald), Z9-tetradecenal (Z9-14:Ald) and Z11-hexadecenyl acetate (Z11-16:OAc) were used as pheromone stimuli, whilst 2-phenyl ethanol, Z3-hexenol, (+/-) linalool and β-caryophyllene were selected as host volatiles for testing. All stimuli were diluted to 10−4 to 102 μg/μl, applied to filter paper at 10−3 to 103 μg loads and inserted into glass pipettes for stimulations. Hexane was used as the solvent blank for control stimulations. Stimuli were introduced as a 100ms pulse to a continuous stream of humidified, charcoal filtered air at a flow rate of 1 L/min. When a sensillum was contacted and a stable recording achieved, each stimulus was presented in random order at a 1μg stimulus load, along with the hexane blank to determine relative sensitivity to all compounds. After an observed response to an individual odor stimulus, a full concentration series was tested.
An interval of at least 60 seconds was allowed to pass between stimulation to prevent adaptation, in addition a constant vacuum flow (30 cm/s) was provided to clear residual odor away from the preparation between stimulation. Stimulations were carried out for 100ms and recordings were initiated 2 seconds before stimulation and continued 4 seconds after stimulation for a 6 second total recording. All recording were made using Spikehound software (Physiology Recording and Identification of Multiple Events; MATLAB®, The Mathworks, Inc.)[42], a freely-available Windows based program for digital recording and analyses of event/spikes.
OA Injections
All moths were individually restrained in cut disposable 1ml micropipette tips with dental wax. Injections of OA (1 μl volume) were made using a 50 μl syringe at a 1mm depth to the vertex (posterior head, ‘neck region’), corresponding to 0.1 μg OA/mg of body weight per moth [38]. SSR were conducted 30 minutes after injection to coincide with previous reports of the optimal window of activity of OA on ORNs [38][39]. Control moths were handled in a similar manner, but injected with Ringer only.
Single Sensillum Recordings
Restrained, injected moths were placed horizontally on a microscope slide with their antennae fastened to a small plastic cube with water-soluble correction fluid. Correction fluid was also applied to the base of the antenna to ensure stability. The reference electrode was inserted in the ipsilateral eye. The preparation was viewed at 200x magnification under a Nikon Eclipse Fixed Stage Microscope.
Glass electrodes were produced using borosilicate capillary glass in a Sutter Instrument Co P-97 Flaming/Brown Micropipette Puller (Sutter Instruments, Novato, CA). Individual sensilla were cut using fine glass capillary mounted on a piezoelectric crystal controlled by a function generator (INSTEK GFG-8215A). The glass capillary was made to resonate at high speed by altering the frequency (square wave and 100K signal amplification setting) of the signal from the function generator to the sound emitting piezo crystal [11][43]. Advancement of the resonating glass capillary with the micromanipulator allowed the distal 25% of a given sensillum selected from the proximal ventral portion of the antenna to be cut.
Once a sensillum was cut, a glass-recording electrode (chloridized silver wire in a Ringer -filled glass capillary) was micromanipulated to the tip of the cut sensillum. ORN activity was filtered and amplified using an EX-1 amplifier (Dagan Corporation, Danbury, CT, USA; Low Cut Filter:10, High Cut Filter: 3K, Gain: 50, Notch Filtered), monitored using a Oscilloscope (GOS-620FG, Instek, New Taipei City, Taiwan) and converted to audio using a AM Systems, Inc. 3300 Audio Monitor for ease of ORN activity detection. Signals digitally acquired and passed to a computer via a DAQ card (PCI-6251; National Instruments, Austin, TX, USA). As mentioned previously odor stimulations and subsequent responses were controlled and digitally recorded using Spikehound® software.
Data Analysis
Recordings were filtered (300 high cut, 3000 low cut) to remove any background electrical noise. Spikehound was used to extract, analyze and save the nerve impulse (i.e. spike) data. Individual ORNS were sorted by spike amplitude. Stimulus-induced spike frequencies were calculated using a one second window following stimulus onset. Pre-stimulus spontaneous impulse frequencies were calculated from a 1 second window during the recording (time points 0–1 second before stimulus delivery. Pre-stimulus and post-stimulus data were exported as text files.
The mean change in impulse frequency (post-stimulus frequency–pre-stimulus frequency) for all odors was calculated for the control and OA injected groups across the concentration series, and standardized versus a solvent (hexane) control stimulus. Data was segregated by sex, and a multi-way ANOVA was performed to testing injection treatment, stimulus and concentration as factors [44]. Mean pre-stimulus spike frequency was also calculated to compare spontaneous firing prior to stimulus onset. A two-way ANOVA was conducted comparing pooled spontaneous activity of pheromone-sensitive versus host volatile sensitive sensilla, and injection of OA or control factors. Tukey's HSD (honest significant difference; α = 0.05) test at was used for post-hoc comparisons. All statistical analyses were conducted in in JMP® 10 [45].
Results
Successful recordings (complete recordings in which responses to all stimuli were obtained) were made from 239/400 sensillar contacts from females, and from 275/365 contacts in males in the control group (the greater success rate in males is attributed to ease in identifying pheromone-sensitive long sensilla trichodea; Table 1). In the OA-injected group, 119/212 sensillar contacts yielded useful recordings from females, along with 140/189 contacts in males.
Table 1. Number of successful single sensillum recordings from male and female Heliothis virescens injected with ringer or octopamine which responded to selected stimuli.
| Female | Male | |||
|---|---|---|---|---|
| Successful recordings | Ringer | Octopamine | Ringer | Octopamine |
| 2-Phenyl ethanol | 15 | 15 | 15 | 15 |
| Beta-caryophyllene | 25 | 20 | 21 | 20 |
| Linalool | 20 | 24 | 20 | 24 |
| Z3-hexenol | 15 | 15 | 15 | 15 |
| Z9-14:Ald | 15 | 15 | 23 | 22 |
| Z11-16:Ald | 15 | 15 | 21 | 20 |
| Z11-16:OAc | 15 | 15 | 20 | 24 |
| Total | 120 | 119 | 135 | 140 |
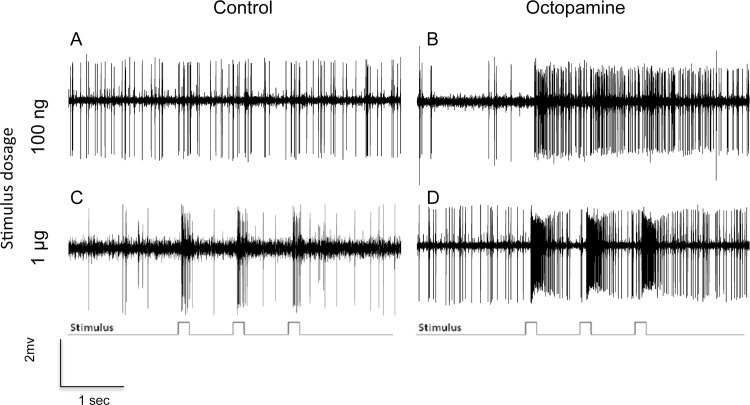
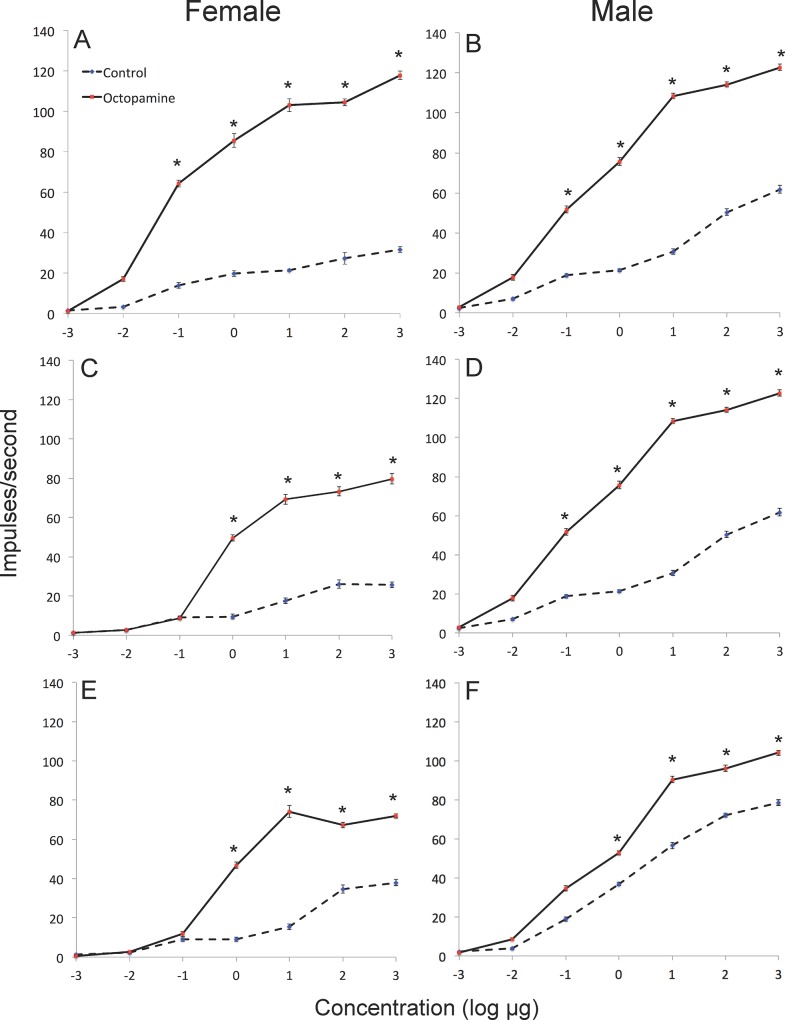
Mean spontaneous impulse frequency of ORNs within pheromone-sensitive sensilla was significantly greater in the OA-injected group than in the control group for both males (F3, 155 = 107.2; p<0.001) and females (F3, 194 = 97.5; p<0.001). In males, responses by ORNs housed in long sensilla trichodea were represented by Type A, responding to Z11-16:Ald, Type B, responding to Z9-14:Ald, and Type C responding to Z11-16:OAc, and other components. In females, responses to selected pheromone components were localized to short sensilla trichodea which have been previously shown to respond to key pheromone components. Pheromone component-sensillar types were as follows: Z11-16:Ald (Type 5), Z9-14:Ald (Type 3), Z11-16:OAc (Type 1). Nomenclature for sensillar types for males and females is based on numerous previous studies (4)(5)(11)(34). ORNs from OA-injected female (F1,90 = 4853.2; p<0.001) and male (F1,130 = 5757.4; p<0.001) moths were significantly more sensitive to all female sex pheromone compounds than control moths (Figs 1 and 2). This was observed in recordings from ORNs housed in long sensilla trichodea in males and from those within short sensilla trichodea in females, which responded to these stimuli. This effect was most pronounced at stimulus concentrations above 100ng/μl-1μg/μl.
Fig 1. Exemplar responses of female type 3 sensilla to stimulation of three 100ms pulses of Z9-14:Ald at concentrations of 100ng and 1μg following injection of Ringer control or octopamine.
Fig 2. Dose-response curves showing impulses/second from sensillar recordings of male and female Heliothis virescens, following injection of Ringer control or octopamine, and stimulation with: (A, B) Z9-14:Ald; (C, D) Z11-16:Ald; and (E, F) Z11-16:OAc.
Asterisks indicate significant differences between responses in control and octopamine treatments to the same compound/concentration of stimulus (Tukey’s HSD, p<0.05).
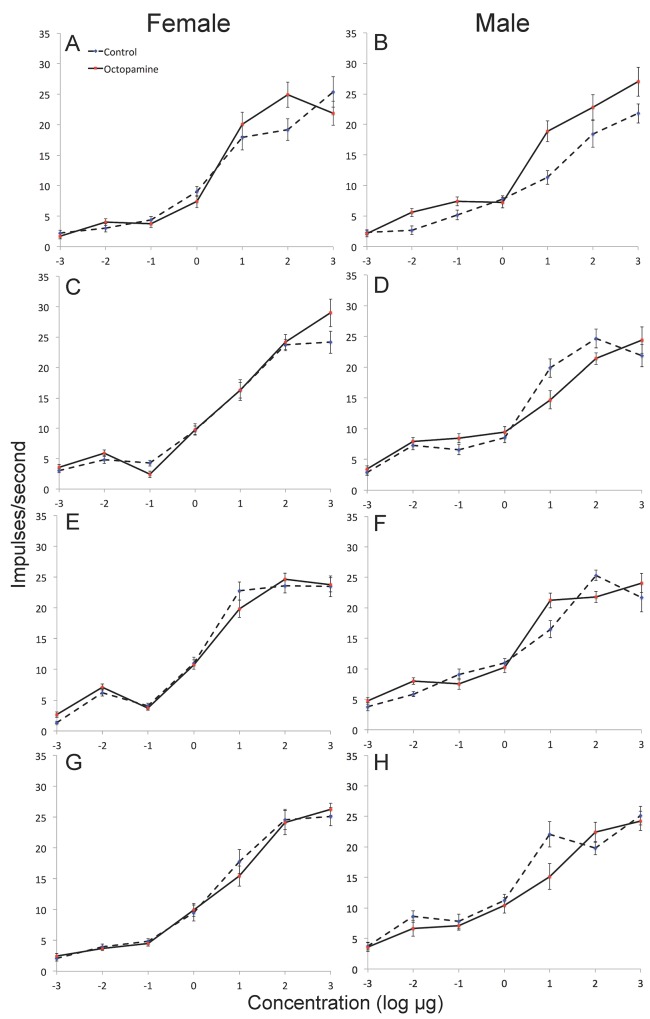
By comparison, no significant differences were observed in ORN responses within short sensilla trichodea between control and OA-injected groups of both sexes when stimulated with selected host plant stimuli (females—F3,149 = 0.5727; p = 0.45; males—F3,145 = 2.5; p = 0.11; Type 7–13 sensilla in both males and females; Fig 3). Increasing stimulus concentrations within both sexes and experimental groups yielded very similar stimulus profiles (Fig 3). Furthermore, the spontaneous impulse frequency of host volatile-sensitive neurons in both sexes was not significantly different following injection of OA (F2,294 = 0.49; p = 0.65).
Fig 3. Dose-response curves showing impulses/second from sensillar recordings of male and female Heliothis virescens, following injection of Ringer control or octopamine, and stimulation with: (A, B) 2-Phenyl ethanol; (C, D) β-caryophyllene (E, F) linalool; and (G, H) Z3-hexenol.
No significant differences were noted between responses in control and octopamine treatments to the same compound/concentration of stimulus (Tukey’s HSD, p<0.05).
Discussion
Single sensillum recordings compared the response of Heliothis virescens pheromone-sensitive sensilla (long sensilla trichodea in males and short sensilla trichodea in females), and those detecting a selection of host plant volatiles (short sensilla trichodea in both sexes). OA injections selectively increased sensitivity of male and female H. virescens olfactory receptor neurons to pheromone components versus other compounds tested. This study further confirms previous work by Hillier et al. [11] that H. virescens females can detect components of their own sex pheromone.
Following OA injection, threshold to response by males and females to pheromone components was reduced by two-to-three orders of magnitude versus control group. In other words, OA treatment confers a sensitivity and response to a 100ng/μl stimulus which is as great as the response to a 10μg/μl simulus in a control moth (three orders of magnitude greater). In addition, an increase in spontaneous firing was observed in pheromone-sensitive ORNs from both sexes, but not to those receptive to the host volatiles.
The mechanism of OA action on neurons is proposed via the G-coupling of OA receptors to adenylate cyclase [35]. Although the underlying mechanisms of transduction are not understood, it has been proposed that OA binding induces the production of cAMP, and OA-dependent CAMP levels putatively regulate pheromone receptor ion channel complexes, modifying sensitivity to key odorants [46]. Subsequent increases of subthreshold potentials (and sensitivity) have been proposed through increased leaking of the olfactory receptor/coreceptor ion channel complex [47][48], and/or increased opening of PKC-inhibited L-type Ca 2+ and CNG-channels [47][49]. Overall, the current work suggests biogenic amine susceptibility, and thus OA receptors, may be linked to olfactory receptor protein types which are differentially expressed in both long and short sensilla trichodea in male and female moths.
As discussed, until relatively recently, much insect olfactory research has been based on the presumption that many species of female moths may not effectively detect female sex pheromone. The combination of morphological and physiological data to date has therefore suggested that dimorphism in the detection and processing of pheromones maintains exclusivity based upon sex. Female H. virescens do, however, have sensilla that detect, and a cluster of glomeruli at the base of the antennal lobe which process, female sex pheromone components [11]. This study further validates that females both detect and modulate pheromone responses in a manner similar to conspecific males.
Modulation of ORN sensitivity in male moths is not surprising. It is logical that increasing sensitivity to female sex pheromone is extremely important to male moths. Indeed, Roelofs and Linn [50] demonstrated that injections of OA cause male moths to detect, take flight, and orient to smaller concentrations of female pheromone in wind tunnel assays. However, minimal behavioral research has been conducted as to why females detect their own sex pheromone. Indeed, few reliable behavioral paradigms have been devised to examine if females respond behaviorally to female sex pheromone, while many exist exquisitely showing that males orient and fly upwind to female pheromone sources [51][52]. As a consequence, more research is required to determine if increased peripheral sensitivity to pheromone in females will affect behavioral response to pheromones.
While sensitivity of female ORNs to pheromone components has been previously documented [15], the selective modulation of such ORNs in a manner consistent with male pheromone-sensitive ORNs is novel. Such modulation is proposed to impact yet undescribed pheromone-mediated behaviors in females for which modulation of sensitivity is required (similar to males [30][31][32][33]). As in males, this may influence circadian rhythms of response, orientation, or calling behavior directly [53][54][55][56]. Without a clear understanding of the role of such ‘autodetection’, it remains to be determined how OA modulation of peripheral sensitivity influences olfactory-driven behaviors or physiological changes.
Three primary hypotheses exist for female ‘autodetection’: 1) First, female moths may detect the pheromone mixture produced by other conspecific females, and thus, can position themselves to reduce intraspecific competition for mates [53]. Second, pheromone emission may trigger females to join together to form choruses of joint pheromone-emission [54]. Third, the detection of conspecific female pheromone by immature female moths triggers a biochemical process (i.e. priming) by which immature female moths initiate production and emission of their own sex pheromone [55][56]. It is clear, however, more research is required in this area, and no previous evidence is known to support either hypothesis specifically for female autodetection in H. virescens.
Recent electrophysiological evidence has shown that females of two Noctuid moth species, S. littoralis and H. virescens, detect their own female sex pheromone [11][15] despite having only morphologically ‘short’ trichoid sensilla. Additionally, olfactory receptor neurons of these female sex pheromone-detecting sensilla target a large glomerulus (central large female glomerulus, aka. cLFG) at the base of the antennal nerve analogous to the MGC in female H. virescens [11]. As well, female pheromone receptor gene expression and 'male' type olfactory binding proteins have been previously found on H. virescens antenna [16][17]. From an evolutionary perspective, our study demonstrates octopaminergic effects on ORNs within female H. virescens pheromone-sensitive sensilla that suggests similarity in their physiological function as ORNs in male pheromone-sensitive sensilla. This suggests that pharmacological specialization of pheromone-sensitive ORNs via octopaminergic modulation predates divergence of sexually dimorphic morphological differences seen in this species (i.e. differences in sensillar length and a relatively large MGC).
Pophof [38] reported that OA injections in males increased their sensitivity to female sex pheromone, but similar injections in females did not increase sensitivity to general odorants. This suggests that an OA-dependent modulatory pathway is either absent, or less sensitive, in female ORNs. It should be noted that to date, conspecific female pheromone-detecting ORNs in female A. polyphemus have not been reported [38].
It is worthwhile noting that other studies provide evidence of OA-modulation of peripheral olfactory responses to non-pheromonal odors. In Tortricid moths Choristoneura rosaceana Harris and Arygrotaenia velutinana Walker, injection of OA enhances electroantennogram responses to several host plant volatiles, mimicking sensitization observed by pre-exposure to complex host mixtures [57]. As well, cockroaches, Periplaneta americana L, have differential modulation of olfactory receptor neurons. Specifically, application of OA to male cockroaches increased ORN responses to the non-pheromone volatile (hexan-1-ol) [58]. This study further found that different sensilla responsive to non-pheromone volatiles were influenced differently, with firing rates of short, but not long-alcohol-sensitive sensilla being increased. Differential results between results found in cockroaches and other studies on Lepidoptera remain to be reconciled, however, each of these studies used differing concentrations of OA injection, and results garnered from other studies on Lepidoptera using electroantennograms may include responses from many different types of sensilla (comprising both pheromone and host-plant sensitive sensilla).
Recently octopamine/tyramine receptors have been cloned from a Mamestra brassicae L., with extensive expression noted within both long and short sensilla trichodea of both sexes [59], also suggesting that OA modulates allelochemical and pheromone detection. This varies from previous Digoxigenin (DIG)-labeling results which found OA expression in H. virescens antennae was isolated to cells at the base of sensilla, rather than within sensilla themselves [29]. Therefore, behavioral and evolutionary significance of the set of odorants tested in each species, along with the context of presentation and endogenous OA concentrations may also influence observed responses.
Evidence that OA enhancement of ORN sensitivity in H. virescens is exclusive to pheromone-sensitive neurons (at least based on the set of odorants tested) provides new directions for investigation of OA on non-pheromone based behaviors. OA can replace the sugar stimulus in honey bee proboscis extension reflex (PER) conditioning trials, and concurrent OA injection can greatly increases PER responsiveness to sucrose [60][61]. More recently, work on Manduca sexta L. has also demonstrated a marked effect of OA injection on conditioning to floral volatile organic compounds, displaying PER responsiveness to OA, and multichannel neural recordings which were similar to those obtained from moths which had learned with a standard PER protocol [62]. Future work in H. virescens might therefore investigate if OA exhibits effects on olfactory conditioning using odorants documented in the current study, and if these may be modulated through higher order activity in the brain, rather than via direct enhancement of peripheral sensitivity.
In conclusion, this study demonstrates that OA dramatically increases the sensitivity of female insect olfactory receptor neurons, specifically those detecting female-produced sex pheromone components. This provides new avenues to examine the connection between the modulatory role of OA on pheromone-detecting ORNs and female moth behavior. Moreover, these results provide new insights regarding the divergence and evolution of sexual dimorphism.
Acknowledgments
The authors thank Christopher Ogbuah for assistance with rearing moths. Funding for this project was provided by an NSERC Discovery Grant to N.K.H. Infrastructure support was also provided by the Canadian Foundation for Innovation, Atlantic Canada Opportunities Agency–Atlantic Innovation Fund, the Nova Scotia Research Innovation Trust, and Acadia University.
Abbreviations
- cLFG
Central large female glomerulus
- cAMP
cyclic adenosine monophosphate
- mLFG
medial large female glomerulus
- OA
Octopamine: OA
- ORN
olfactory receptor neuron
- MGC
macroglomerular complex
- SSR
single sensillum recording
- PKA
protein kinase A
- PER
proboscis extension reflex
- Z9-14:Ald
cis-9- tetradecenal
- Z11-16:Ald
cis-11-hexadecenal
- Z11-16:OAc.
cis-11-hexadecenyl acetate: Z11-16:OAc
Data Availability
All relevant data are available via Dryad (DOI: 10.5061/dryad.67n14).
Funding Statement
Funded by an NSERC Discovery grant (356109-2008 RGPIN) to NKH, Canada Foundation for Innovation (22087) to NKH, Atlantic Canada Opportunities Agency – Atlantic Innovation Fund (197853) to NKH, the Nova Scotia Research Innovation Trust, Acadia University funds to NKH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hansson BS (2002) A bug's smell—research into insect olfaction. Trends Neurosci 25: 270–274. [DOI] [PubMed] [Google Scholar]
- 2. Harrewijn P, Minks AK, Mollema C (1994) Evolution of plant volatile production in insect-plant relationships. Chemoecol 5: 55–73. [Google Scholar]
- 3. Keil TA (1999) Morphology and development of the peripheral olfactory organs In: Insect olfaction. Hansson BS, editor. Springer-Verlag; 6–47 p. [Google Scholar]
- 4. Baker TC, Cossé AA, Lee SG, Todd JL, Quero C, Vickers NJ (2004). A comparison of responses from olfactory receptor neurons of Heliothis subflexa and Heliothis virescens to components of their sex pheromone. J Comp Physiol A 190: 155–165. [DOI] [PubMed] [Google Scholar]
- 5. Almaas TJ, Mustaparta H (1991) Heliothis virescens: Response characteristics of receptor neurons in sensilla trichodea type 1 and type 2. J Chem Ecol 17: 953–972. 10.1007/BF01395602 [DOI] [PubMed] [Google Scholar]
- 6. Vickers NJ, Christensen TA, Hildebrand JG (1998) Combinatorial odor discrimination in the brain: attractive and antagonist odor blends are represented in distinct combinations of uniquely identifiable glomeruli. J Comp Neurol 400: 35–56. [PubMed] [Google Scholar]
- 7. Berg BG, Almaas TJ, Bjaalie JG, Mustaparta H (1998) The macroglomerular complex of the antennal lobe in the tobacco budworm moth Heliothis virescens: specified subdivision in four compartments according to information about biologically significant compounds. J Comp Physiol A 183: 669–682. [Google Scholar]
- 8. Berg BG, Almaas TJ, Bjaalie JG, Mustaparta H (2002) Digital atlases of the antennal lobe in two species of tobacco budworm moths the oriental Helicoverpa assulta (male) and the american Heliothis virescens (male and female). J Comp Neurol 446: 123–134. [DOI] [PubMed] [Google Scholar]
- 9. Vickers NJ, Christensen TA (2003). Functional divergence of spatially conserved olfactory glomeruli in two related moth species. Chem Senses 28: 325–338. [DOI] [PubMed] [Google Scholar]
- 10. Hansson BS, Christensen TA, Hildebrand JG (1991) Functionally distinct subdivisions of the macroglomerular complex in the antennal lobe of the sphinx moth Manduca sexta . J Comp Neurol 312: 264–278. [DOI] [PubMed] [Google Scholar]
- 11. Hillier NK, Kleineidam C, Vickers NJ (2006) Physiology and glomerular projections of olfactory receptor neurons on the antenna of female Heliothis virescens (Lepidoptera: Noctuidae) responsive to behaviorally relevant odors. J Comp Physiol A 192: 199–219. [DOI] [PubMed] [Google Scholar]
- 12. Hillier NK, Vickers NJ (2007) Physiology and antennal lobe projections of olfactory receptor neurons from sexually isomorphic sensilla on male Heliothis virescens . J Comp Physiol A 193: 649–663. [DOI] [PubMed] [Google Scholar]
- 13. Hansson BS, Van Der Pers JNC, Lofqvist J (1989) Comparison of male and female olfactory cell response to pheromone compounds and plant volatiles in the turnip moth, Argotis segetum . Physiol Entomol 14: 147–155. [Google Scholar]
- 14. Seabrook WD, Linn CE, Dyer LJ, Shorey HH (1987) Comparison of electroantennograms from female and male cabbage looper moths (Trichoplusia ni) of different ages and for various pheromone concentrations. J Chem Ecol 13: 1443–1453. 10.1007/BF01012290 [DOI] [PubMed] [Google Scholar]
- 15. Ochieng SA, Anderson P, Hansson BS (1995) Antennal lobe projection patterns of olfactory receptor neurons involved in sex pheromone detection in Spodoptera littoralis (Lepidoptera: Noctuidae). Tissue and Cell 27: 221–232. [DOI] [PubMed] [Google Scholar]
- 16. Callahan FE, Vogt RG, Tucker ML, Dickens JC, Mattoo AK (2000) High level expression of "male specific" pheromone binding proteins (PBPs) in the antennae of female noctuid moths. Insect Biochem Mol Biology 30: 507–514. [DOI] [PubMed] [Google Scholar]
- 17. Krieger J, Grosse-Wilde E, Gohl T, Dewer YME, Raming K, Breer H (2004) Genes encoding candidate pheromone receptors in a moth (Heliothis virescens). PNAS 101: 11845–11850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Skiri HT, Galizia CG, Mustaparta H (2004) Representation of primary plant odorants in the antennal lobe of the moth Heliothis virescens using calcium imaging. Chem Senses 29: 253–267. [DOI] [PubMed] [Google Scholar]
- 19. Homberg U (1994) Distribution of neurotransmitters in the insect brain. Prog Zool 40. [Google Scholar]
- 20. Homberg U, Muller U (1999) Neuroactive substances in the antennal lobe In: Insect olfaction. Hansson BS, editor. Springer-Verlag; 182–206 p. [Google Scholar]
- 21. Orchard I, Lange AB (1985) Evidence for Octopaminergic modulation of an insect visceral muscle. J Neurobiol 16: 171–181. [DOI] [PubMed] [Google Scholar]
- 22. Orchard I, Lange AB (1984) Cyclic AMP in locust fat body: Correlation with octopamine and adipokinetic hormones during flight. J Insect Physiol 30: 901–904. [Google Scholar]
- 23.Dacks AM, Christensen TA, Agricola H-J, Wollweber L, Hildebrand JG (2005) Octopamine-immunoreactive neurons in the brain and subesophageal ganglion of the Hawkmoth Manduca sexta. 488: 255–268. [DOI] [PMC free article] [PubMed]
- 24. Evans PD, Siegler MVS (1982) Octopamine mediated relaxation of maintained and catch tension in locust skeletal muscle. J Physiol 324:93–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hammer M (1993) An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honeybees. Nature 266:59–63. [DOI] [PubMed] [Google Scholar]
- 26. Hammer M, Menzel R (1998) Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learning Memory 5:146–156. [PMC free article] [PubMed] [Google Scholar]
- 27. Dacks AM, Dacks JB, Christensen TA, Nighorn AJ (2006) The cloning of one putative octopamine receptor and two putative serotonin receptors from the tobacco hawkmoth, Manduca sexta . Insect Biochem Molec Biol 36: 741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lam F, McNeil JN, Donly C (2013) Octopamine receptor gene expression in three lepidopteran species of insect. Peptides 41: 66–73. 10.1016/j.peptides.2012.03.034 [DOI] [PubMed] [Google Scholar]
- 29. Von Nickisch-Rosenegk E, Krieger J, Kubrick S, Laage R, Strobel J, Strotmann J, et al. (1996) Cloning of biogenic amine receptors from moths (Bombyx mori and Heliothis virescens). Insect Biochem Molec Biol 26: 817–827. [DOI] [PubMed] [Google Scholar]
- 30. Linn CE, Roelofs WL (1984) Sublethal effects of neuroactive compounds on pheromone response thresholds in male oriental fruit moths. Arch Insect Biochem Physiol 1: 331–344. [Google Scholar]
- 31. Linn CE, Roelofs WL (1986) Modulatory effects of octopamine and serotonin on male sensitivity and periodicity of response to sex pheromone in the cabbage looper moth Trichoplusia ni . Arch Insect Biochem Physiol 3: 161–172. [Google Scholar]
- 32. Linn CE, Roelofs W (1992a) Role of photoperiod cues in regulating the modulatory action of octopamine on pheromone-response thresholds in the cabbage looper moth. Arch Insect Biochem Physiol 20(4):285–302. [Google Scholar]
- 33. Linn CE, Roelofs WL (1992b) Modulatory effects of octopamine and 5-hydroxytryptamine on locomotor and pheromone response in male gypsy moths, Lymantria dispar . Arch Insect Biochem Physiol 20: 265–284. [Google Scholar]
- 34. Barrozo RB, Jarriault D, Simeone X, Gaertner C, Gadenne C, Anton S (2010) Mating-induced transient inhibition of responses to sex pheromone in a male moth is not mediated by octopamine or serotonin. J Exp Biol 213: 1100–1106. 10.1242/jeb.040139 [DOI] [PubMed] [Google Scholar]
- 35. Farooqui T (2007) Octopamine -mediated neuromodulation of insect senses. Neurochem Res 32: 1511–1529. [DOI] [PubMed] [Google Scholar]
- 36. Pophof B (2000) Octopamine modulates the sensitvity of silkmoth pheromone receptor neurons. J Comp Physiol A 186: 307–313. [DOI] [PubMed] [Google Scholar]
- 37. Grosmaitre X, Marion-Poll F, Renou M (2001) Biogenic amines modulate olfactory receptor neurons firing activity in Mamestra brassicae . Chem Senses 26: 653–661. [DOI] [PubMed] [Google Scholar]
- 38. Pophof B (2002) Octopamine enhances moth olfactory responses to pheromones, but not those to general odorants. J Comp Physiol A 188: 659–662. [DOI] [PubMed] [Google Scholar]
- 39. Cunningham JP, Moore CJ, Zalucki MP, West SA (2004) Learning, odour preference and flower foraging in moths. J Exp Biol 207:87–94. [DOI] [PubMed] [Google Scholar]
- 40. De Moraes CM, Mescher MC, Tumlinson JH (2001) Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410: 557–580 [DOI] [PubMed] [Google Scholar]
- 41. Kaissling K-E (1995) Single unit and electroantennogram recordings in insect olfactory organs In: Spielman AI, Brand JG (eds). Experimental cell biology of taste and olfaction. Current techniques and protocols CRC Press, Boca Raton, pp 361–377. [Google Scholar]
- 42.Lott JK (2007) Hybridizing cellular and behavioral neurobiology with modern engineering tools: Microelectronics, microfabricated devices, and software solutions for physiology. Doctoral thesis, Cornell University, NY, USA.
- 43. Gödde J (1989) Vibrating glass stylets: tools for precise microsurgery on cuticular structures. J Neurosci Methods 29: 77–83 [DOI] [PubMed] [Google Scholar]
- 44. Sokal RR, Rohlf FJ (1995) Biometry, 3rd Edition WH Freeman and Company. [Google Scholar]
- 45. SAS Institute Inc. (2012) New Features in JMP® 10. Cary, NC: SAS Institute Inc. [Google Scholar]
- 46. Flecke C, Stengl M (2009) Octopamine and tyramine modulate pheromone-sensitive olfactory sensilla of the hawkmoth Manduca sexta in a time-dependent manner. J Comp Physiol A 195: 529–545. [DOI] [PubMed] [Google Scholar]
- 47. Stengl M (2010) Pheromone transduction in moths. Frontiers Cell Neuro 4: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wicher D, Schäfer R, Baurenfeind R, Stensmyr MC, Heller R, Heinemann SH, et al. (2008) Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature 452: 1007–1011. 10.1038/nature06861 [DOI] [PubMed] [Google Scholar]
- 49. Krannich S, Stengl M (2008) Cyclic nucleotide-activated currents in cultured olfactory receptor neurons of hawkmoth Manduca sexta . J Neurophysiol 100: 2866–2877. 10.1152/jn.01400.2007 [DOI] [PubMed] [Google Scholar]
- 50. Roelofs WL, Linn CE (1987) Neuroactive substances as probes of central thresholds in sex pheromone perception. Mol Entomol 10: 67–77. [Google Scholar]
- 51. Kennedy JS, Marsh D (1974) Pheromone-regulated anemotaxis in flying moths. Science 184: 999–1001. [DOI] [PubMed] [Google Scholar]
- 52. Lelito JP, Myrick AJ (2008) Interspecific pheromone plume interference among sympatric heliothine moths: A wind tunnel test using live, calling females. J Chem Ecol 34: 725–733. 10.1007/s10886-008-9475-6 [DOI] [PubMed] [Google Scholar]
- 53. Schneider D, Schulz S, Priesner E, Ziesmann J, and Francke W (1998) Autodetection and chemistry of female and male pheromone in both sexes of the tiger moth Panaxia quadripunctaria . J Comp Physiol A 182: 153–161. [Google Scholar]
- 54. Lim H, Greenfield M (2008) Female Arctiid moths, Utethesia ornatrix, orient towards and join pheromonal choruses. Animal Behavior 75: 673–680. [Google Scholar]
- 55. Anton S, Hansson BS (1994) Central processing of sex pheromone, host odor, and oviposition deterrent information by interneurons in the antennal lobe of female Spodoptera littoralis (Lepidoptera: Noctuidae). J Comp Neurol 350: 199–214. [DOI] [PubMed] [Google Scholar]
- 56. Lim H, Park KC, Baker TC, Greenfield M (2007) Perception of conspecific female pheromone stimulates female calling in an Arctiid moth, Utethesia ornatrix . J Chem Ecol 33: 1257–1271. [DOI] [PubMed] [Google Scholar]
- 57. Stelinski LL, Miller JR, Ressa NE, Gut LJ (2003) Increased EAG responses of tortricid moths after prolonged exposure to plant volatiles: evidence for octopamine-mediated sensitization. J Insect Physiol 49: 845–856. [DOI] [PubMed] [Google Scholar]
- 58. Zhukovskaya MI (2012). Modulation by octopamine of olfactory responses to nonpheromone odorants in the cockroach, Periplaneta americana L. Chem Senses 37: 421–429. 10.1093/chemse/bjr121 [DOI] [PubMed] [Google Scholar]
- 59. Brigaud I, Grosmaître X, François MC, Jacquin-Joly E (2009) Cloning and expression pattern of a putative octopamine/tyramine receptor in antennae of the noctuid moth Mamestra brassicae . Cell Tiss Res 335: 455–463. [DOI] [PubMed] [Google Scholar]
- 60. Hammer M, Menzel R (1998) Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learning and Memory 5: 146–156. [PMC free article] [PubMed] [Google Scholar]
- 61. Menzel R, Heyne A, Kinzel C (1999) Pharmacological dissociation between the reinforcing, sensitizing, and response-releasing functions of reward in honeybee classical conditioning. Behav Neurosci 113: 744–754. [PubMed] [Google Scholar]
- 62. Riffell JA, Lei H, Abrell L, Hildebrand JG (2013) Neural basis of a pollinator’s buffet: olfactory specialization and learning in Manduca sexta . Science 339: 200–204. 10.1126/science.1225483 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are available via Dryad (DOI: 10.5061/dryad.67n14).