Abstract
Granulosa cell tumors are representative of estrogenic ovarian tumors, and some Sertoli-stromal cell tumors are also estrogenic. The exact cells that are responsible for estrogenesis, however, have yet to be identified. In the present study, 25 sex cord-stromal tumors (20 granulosa cell tumors, 4 Sertoli-Leydig cell tumors, and a Sertoli cell tumor) were immunohistochemically examined for expression of P450 aromatase, which is critical for estrogenesis. All of the tumors had been evaluated for estrogenic function, including contemporaneous endometrial hyperplasia and/or elevation of serum estradiol. Eleven of 14 estrogenic granulosa cell tumors showed sparse or aggregated immunoreactivity for aromatase, whereas 5 of 6 nonestrogenic tumors did not. Aromatase was selectively expressed by plump granulosa cells with eosinophilic or vacuolated cytoplasm, resembling luteinized granulosa cells. Such a localization of aromatase is analogous to that in normal ovaries. Aromatase expression in primary tumors was recapitulated by recurrent tumors. In Sertoli-stromal cell tumors, either undifferentiated plump cells or well-differentiated Sertoli cells expressed aromatase. In conclusion, the expression of P450 aromatase corresponds to specific cell morphology in sex cord-stromal tumors, including recurrent tumors. Aromatase status in granulosa cell tumors provides helpful information on whether serum estradiol could be a marker for recurrence.
Key Words: Granulosa cell tumors, Sertoli-stromal cell tumors, Ovary, Estrogens, P450 aromatase, Recurrence
Sex cord-stromal tumors are often functioning ovarian tumors that may be associated with endocrine manifestations. Among them, granulosa cell tumors frequently show an estrogenic function, including thickening of the endometrium or abnormal maturation of cervical smears, particularly in postmenopausal women. Some Sertoli-stromal cell tumors are also estrogenic, although Sertoli-stromal cell tumors are usually androgenic 1. As the removal of these tumors results in the normalization of serum estrogen levels, it has been suggested that these tumors themselves synthesize excessive estrogen. However, there is little in situ evidence that sex cord-stromal tumors are responsible for the synthesis of estrogen, and the type of cells involved in estrogenesis is unknown.
In the present study, a total of 25 sex cord-stromal tumors were immunohistochemically examined for the expression of P450 aromatase, which is critical for estrogenesis.
MATERIALS AND METHODS
Cases
Twenty-five sex cord-stromal tumors of the ovary were analyzed. Details of the tumor histology are: adult granulosa cell tumor (n=18), juvenile granulosa cell tumor (n=2), Sertoli-Leydig cell tumor (n=4; 1 showing intermediate differentiation and 3 showing poor differentiation), and a Sertoli cell tumor (Tables 1 and 2). All of the tumors were surgically resected, and fixed in formalin and embedded in paraffin. Three patients with adult granulosa cell tumor (cases 16–18) showed peritoneal recurrences and they were treated with surgery or chemotherapy; cases 16 and 18 had repeated recurrences over 6 and 22 yr, respectively, and case 17 showed a recurrence 3 yr after surgery. The recurrent tumors were also analyzed.
TABLE 1.
Summary of clinicopathologic findings in 20 granulosa cell tumors of the ovary
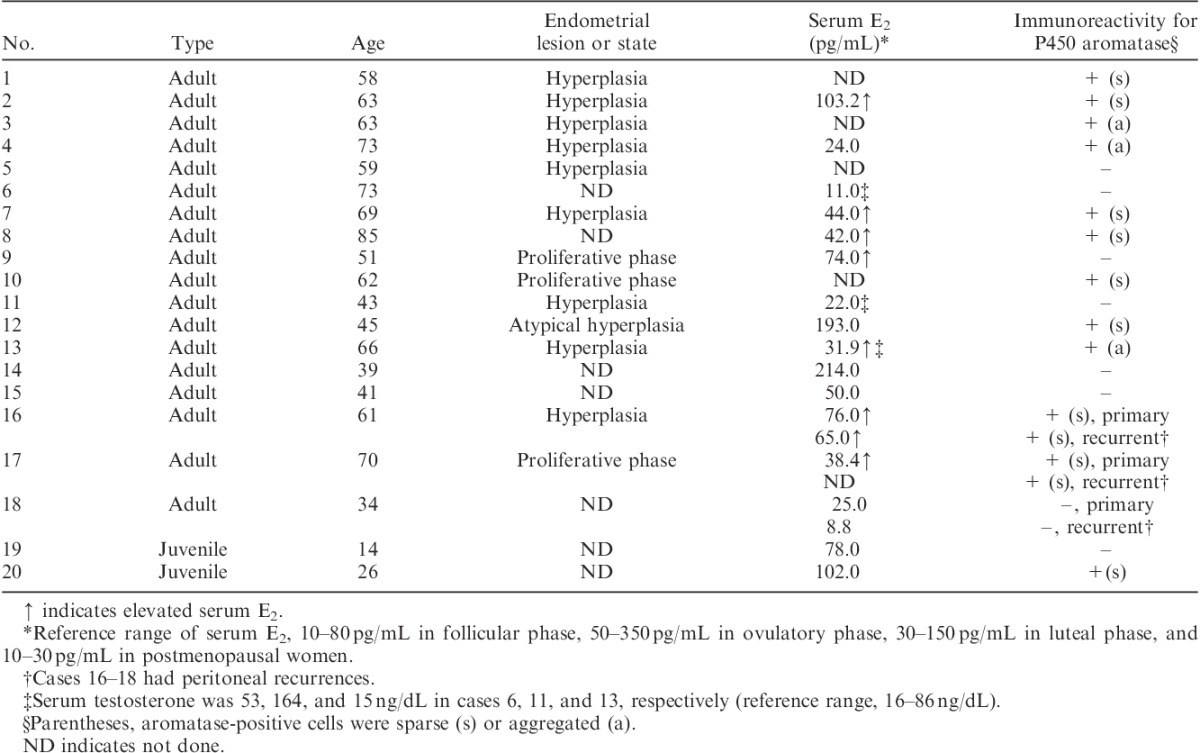
TABLE 2.
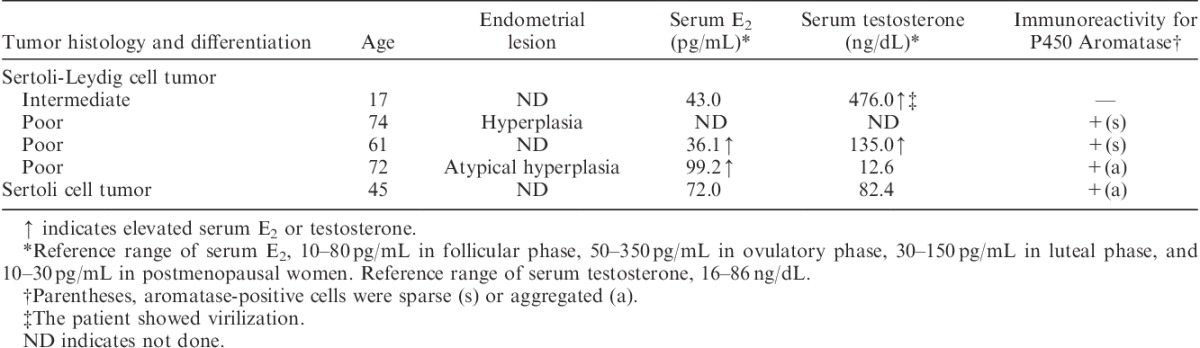
Summary of clinicopathologic findings in 4 Sertoli-Leydig cell tumors and a Sertoli cell tumor of the ovary
Immunohistochemistry
One to 3 representative sections of each tumor were used for immunohistochemistry for P450 aromatase, which catalyzes the conversion of androgens to estrogens. For antigen retrieval, the sections were pretreated by microwave heating at 95°C for 21 min in sodium citrate buffer (10 mM sodium-citrate monohydrate, pH 6.0). The slides were incubated with anti-P450 aromatase monoclonal antibody at 1:50 (Serotec, Oxford, UK) at 4°C overnight. The corresponding sections of Sertoli-stromal cell tumors were also immunostained with anticytokeratin monoclonal antibody CAM5.2 (Becton-Dickinson, San Jose, CA) to recognize Sertoli cell components. All of the slides were visualized using the Histofine SAB-PO kit. Immunoreactivity for aromatase was evaluated either sparse or aggregated: immunoreactive cells were sparsely distributed without forming clusters in the former, and immunoreactive cells were aggregated and there were multiple clusters of >10 tumor cells in the latter.
RESULTS
Endocrine Features
Preoperative serum levels of estradiol (E2) were measured in 20 cases (16 granulosa cell tumors, 3 Sertoli-Leydig cell tumors and a Sertoli cell tumor). Among them, 9 (7 granulosa cell tumors and 2 Sertoli-Leydig cell tumors) were associated with significant elevation of serum E2 levels. One case of granulosa cell tumor (case 16) also showed elevation of serum E2 at recurrence (Tables 1 and 2).
Fifteen patients received hysterectomy along with resection of ovarian tumors. Histologic examination of the resected uterus revealed that 12 had endometrial hyperplasia (10 nonatypical type and 2 atypical type) and 3 had proliferative endometrium despite a postmenopausal state. Among the 12 cases with endometrial hyperplasia, 10 had granulosa cell tumors and 2 had Sertoli-Leydig cell tumors in the ovary. All of the 3 cases with proliferative endometrium had granulosa cell tumors in the ovary (Tables 1 and 2).
Preoperative serum levels of testosterone were measured in 7 cases (3 granulosa cell tumors, 3 Sertoli-Leydig cell tumors and a Sertoli cell tumor). Among them, 3 (1 granulosa cell tumor and 2 Sertoli-Leydig cell tumors) were associated with elevation of serum testosterone and 1 case of Sertoli-Leydig cell tumor showed virilization (Tables 1 and 2).
Immunohistochemistry for P450 Aromatase
Among the 20 granulosa cell tumors, 12 (60%) showed immunoreactivity for P450 aromatase, either sparse (9 tumors) or aggregated (3 tumors). It was exclusively detected in the granulosa cell component. Aromatase-positive cells had moderate to large amounts of eosinophilic or vacuolated cytoplasm, resembling luteinized granulosa cells to varying extent. In 3 recurrent cases (cases 16–18), the aromatase status of recurrent tumors was the same as that of primary tumors; both primary and recurrent tumors were immunoreactive for P450 aromatase in cases 16 and 17, whereas neither was immunoreactive in case 18 (Table 1, Fig. 1).
FIG. 1.
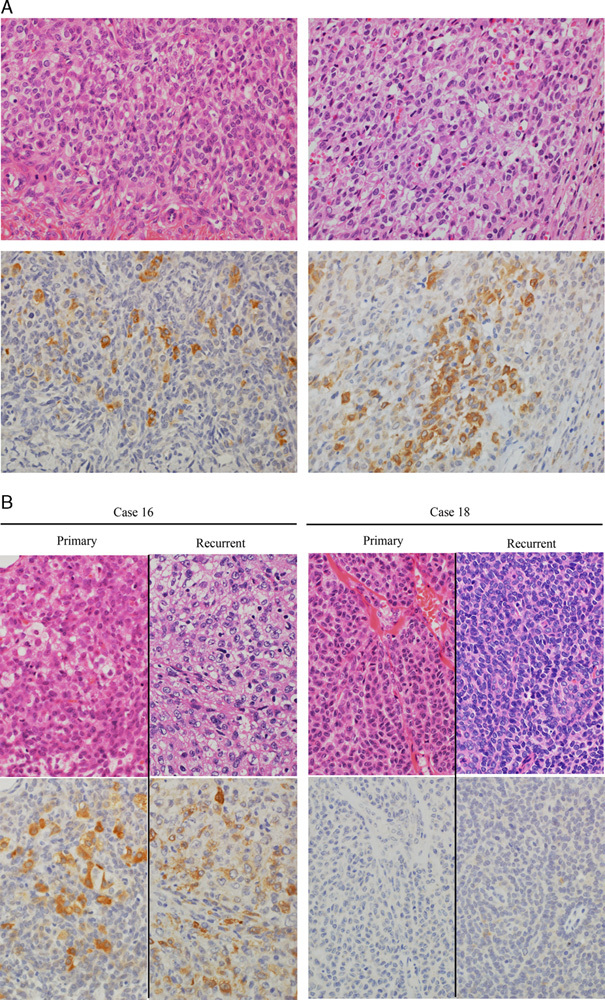
Immunohistochemistry for P450 aromatase in adult granulosa cell tumors of the ovary. (A) P450 aromatase was localized in the granulosa cell component, either sparse (left, case 2) or aggregated (right, case 13). Aromatase was expressed by granulosa cells with a moderate to large amount of eosinophilic cytoplasm, resembling luteinized granulosa cells. (B) In case 16, primary and recurrent tumors contained plump or vacuolated granulosa cells that expressed aromatase. In contrast, both primary and recurrent tumors were composed of granulosa cells with scant cytoplasm, and they were negative for aromatase in case 18. Upper, hematoxylin and eosin; lower, P450 aromatase immunostain. Original magnification, 40×.
Three poorly differentiated Sertoli-Leydig cell tumors and a Sertoli cell tumor showed immunoreactivity for P450 aromatase. In the former, aromatase was detected in the cytokeratin-negative undifferentiated cells with plump cytoplasm. Cytokeratin-positive plump cells did not express aromatase. In the Sertoli cell tumor, aromatase was expressed in the well-differentiated Sertoli cells forming hollow tubules. No Leydig cells showed immunoreactivity for aromatase (Table 2, Fig. 2).
FIG. 2.
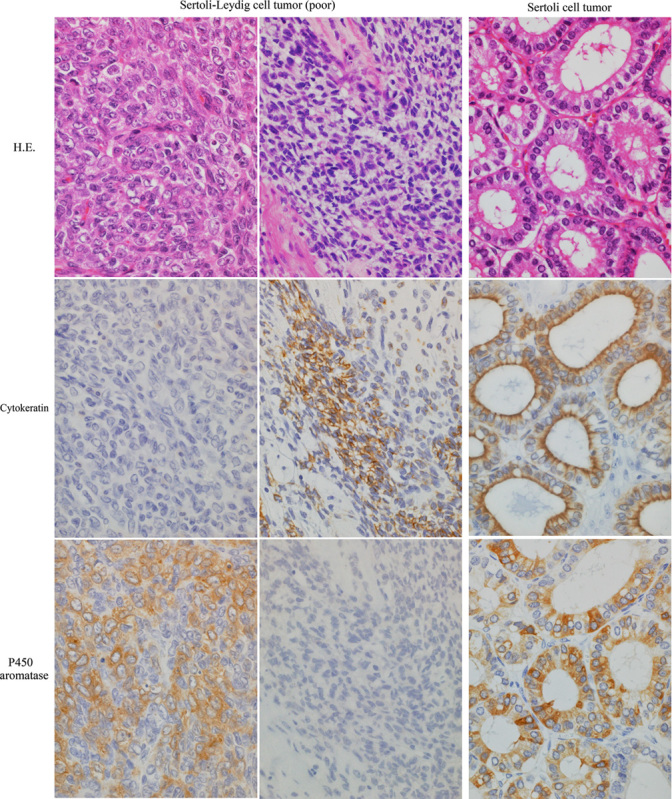
Immunohistochemistry for P450 aromatase in poorly differentiated Sertoli-Leydig cell tumors and a Sertoli cell tumor of the ovary. In poorly differentiated Sertoli-Leydig cell tumors, cytokeratin-negative undifferentiated cells with plump cytoplasm were immunoreactive for P450 aromatase, whereas cytokeratin-positive plump cells, suggestive of poorly differentiated Sertoli cells, were not immunoreactive for aromatase. In a Sertoli cell tumor, tall columnar Sertoli cells expressed P450 aromatase. Upper, hematoxylin and eosin (H.E.); middle, cytokeratin (CAM5.2) immunostain; lower, P450 aromatase immunostain. Original magnification, 40×.
Correlation Between Estrogenic Function and Expression of P450 Aromatase
Among the 20 granulosa cell tumors examined, 14 were associated with estrogenic function, including endometrial hyperplasia and/or significant elevation of serum E2, whereas 6 were not. Aromatase was detected in 11 of the 14 estrogenic cases, whereas it was not detected in 5 of the 6 nonestrogenic cases. In aromatase-positive estrogenic cases, there was no correlation between the pattern of aromatase-positive cells (sparse or aggregated) and estrogenic function. At recurrence, case 16 showed both elevation of serum E2 and expression of aromatase in the recurrent tumors, whereas case 18 showed neither (Table 1).
Three cases of Sertoli-Leydig cell tumors were associated with estrogenic function including endometrial hyperplasia and/or elevation of serum E2. Aromatase was detected in 3 of the 3 estrogenic cases. One case with aggregated aromatase-positive cells showed a relatively high level of serum E2, as well as atypical endometrial hyperplasia. Although a case of Sertoli cell tumor showed immunoreactivity for aromatase, it was not associated with significant elevation of serum E2 (Table 2).
DISCUSSION
The exact cells responsible for estrogenesis in sex cord-stromal tumors have not yet been identified. It is difficult to detect estrogens immunohistochemically, and, even if possible, interpretation of the results, either synthesis or storage of estrogens by immunoreactive cells, would be controversial. Alternatively, the expression of P450 aromatase indicates that tumor cells themselves have a capacity for estrogenesis, because P450 aromatase is a critical enzyme that catalyzes the conversion of androgen to estrogen 2. Previous immunohistochemical studies, however, barely detected aromatase-positive tumor cells in sex cord-stromal tumors of the ovary 3,4. Because several factors, including tissue fixation, antigen retrieval, or amplification of immunoreaction, are likely to influence the results of immunohistochemistry, additional studies in different conditions had been needed to verify aromatase expression in sex-cord stromal tumors.
In the present study, aromatase expression and estrogenic function showed a positive correlation in 16 of 20 (80%) cases of granulosa cell tumors. Aromatase was selectively expressed in the granulosa cell component. The stromal component, even if it was luteinized, did not express aromatase. This is distinct from aromatase expression in ovarian tumors with functioning stroma, in which the stromal cells, both theca-like and lutein-like cells, express aromatase 5. In granulosa cell tumors, the morphology of aromatase-positive granulosa cells was different from that of aromatase-negative granulosa cells: aromatase-positive cells had moderate to large amounts of eosinophilic cytoplasm resembling luteinized granulosa cells to varying extent, whereas aromatase-negative cells had scant pale cytoplasm. Such a localization of aromatase is analogous to that in normal ovaries: P450 aromatase is expressed by plump granulosa cells of mature preovulatory follicles and the corpus luteum, but not expressed by lean granulosa cells of early follicles 6. These findings indicate that granulosa cell tumors recapitulate the cell morphology and estrogenic function of granulosa cells in normal ovaries.
Among the 3 recurrent granulosa cell tumors, 2 had repeated recurrences over 6 and 22 yr. It is interesting that aromatase expression and the estrogenic function in recurrent tumors were the same as those in primary tumors. Every time tumors recurred, the serum level of E2 was elevated and the tumors expressed aromatase in one case, whereas serum E2 remained normal and aromatase was negative in the other case. Serum E2 was a useful marker for recurrence in the former, whereas periodic radiologic examinations were necessary in the latter. These findings suggest that the aromatase status, once it is programmed, is maintained by recurrent tumors. In granulosa cell tumors, recurrence is commonly detected >5 yr postoperatively, and sometimes after intervals of decades 1. Examination of aromatase expression in primary tumors, as well as of serum E2, is likely to provide helpful information on how to follow up patients long-term. Further study involving a larger series is necessary to compare aromatase expression between primary and recurrent granulosa cell tumors.
Although Sertoli-Leydig cell tumors are prone to cause virilization, it occurs in only about one third of the cases: many are nonfunctioning and some even have estrogenic effects 1. In Sertoli-Leydig cell tumors, Leydig cells have been recognized as sources of excessive androgen. The source of estrogen, however, has yet to be identified. It has been questioned whether estrogens are synthesized by tumor cells themselves, or whether tumor-derived androgens are converted into estrogens in the peripheral tissues, including adipose or muscle tissues, which exhibit high aromatase expression 7. The present study provided in situ evidence that Sertoli-Leydig cell tumors themselves are responsible for estrogenesis: undifferentiated plump cells expressed aromatase in 3 of the 3 estrogenic Sertoli-Leydig cell tumors. Alternatively, tall columnar cells forming hollow tubules expressed aromatase in a Sertoli cell tumor. Sertoli cell tumors are usually nonfunctioning but may be estrogenic: prepubertal patients are associated with isosexual pseudoprecocity 8. Although the tumor examined here was not associated with significant elevation of serum E2, the present finding suggests that Sertoli cell tumors themselves have a potential for estrogenesis.
In summary, the expression of P450 aromatase corresponds to specific cell morphology in sex cord-stromal tumors of the ovary. In particular, granulosa cell tumors recapitulate the cell morphology and aromatase expression of granulosa cells in normal ovaries: aromatase is localized in plump or luteinized granulosa cells. As the aromatase status is maintained by recurrent granulosa cell tumors, examination of aromatase in primary tumors is likely to provide helpful information on whether serum E2 could be a marker for recurrence.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Scully RE, Young RH, Clement PB. Atlas of Tumor Pathology Sex Cord-Stromal Tumors Tumors of the Ovary, Maldeveloped Gonads, Fallopian Tube, and Broad Ligament. Washington, DC: Armed Forces Institute of Pathology; 1998:169–226. [Google Scholar]
- 2.Simpson ER, Davis SR. Minireview: Aromatase and the regulation of estrogen biosynthesis – some new perspectives. Endocrinology. 2001;142:4589–94. [DOI] [PubMed] [Google Scholar]
- 3.Sasano H, Okamoto M, Masaon JI, et al. Immunohistochemical studies of steroidogenic enzymes (aromatase, 17α-hydroxylase and cholesterol side-chain cleavage cytochromes P-450) in sex cord-stromal tumors of the ovary. Hum Pathol. 1989;20:452–7. [DOI] [PubMed] [Google Scholar]
- 4.Costa MJ, Morris R, Sasano H. Sex steroid biosynthesis enzymes in ovarian sex-cord stromal tumors. Int J Gynecol Pathol. 1994;13:109–19. [DOI] [PubMed] [Google Scholar]
- 5.Kato N, Hayasaka T, Takeda J, et al. Ovarian tumors with functioning stroma: A clinicopathological study with special reference to serum estrogen level, stromal morphology, and aromatase expression. Int J Gynecol Pathol. 2013;32:556–61. [DOI] [PubMed] [Google Scholar]
- 6.Sasano H, Okamoto M, Mason JI, et al. Immunolocalization of aromatase, 17α-hydroxylase and side-chain-cleavage cytochromes P-450 in the human ovary. J Reprod Fertil. 1989;85:163–9. [DOI] [PubMed] [Google Scholar]
- 7.Longcope C, Pratt JH, Schneider SN, et al. Aromatization of androgens by muscle and adipose tissue in vivo. J Clin Endocinol Metab. 1978;46:146–52. [DOI] [PubMed] [Google Scholar]
- 8.Oliva E, Alvarez T, Young RH. Sertoli cell tumors of the ovary. A clinicopathologic and immunohistochemical study of 54 cases. Am J Surg Pathol. 2005;29:143–56. [DOI] [PubMed] [Google Scholar]


