Objective:
Patients with peripheral vestibular dysfunction because of gravitational receptor asymmetries display signs of cognitive dysfunction and are assumed to have neurobehavioral sequelae. This was tested with pre- and postoperatively quantitative measurements in three cohort groups with superior semicircular canal dehiscence syndrome (SSCDS) symptoms with: 1) superior canal dehiscence (SCD) repaired via a middle cranial fossa craniotomy and canal plugging only; 2) otic capsule defects not visualized with imaging (no-iOCD) repaired with round window reinforcement (RWR) only; or 3) both SCD plugging and subsequent development of no-iOCD followed by RWR.
Study Design:
Prospective patient series.
Setting:
Tertiary referral center.
Patients:
There were 13 adult and 4 pediatric patients with SSCDS who had completion of neuropsychology test batteries pre- and every 3 months postoperatively. Eight patients had no-iOCD and RWR exclusively, 5 had SCD and plugging exclusively, and 4 had both SCD plugging and then development of no-iOCD with RWR. These cohorts included SSCDS with 2 different dehiscence locations.
Interventions:
Completion of a neuropsychology test battery preoperatively and at 3, 6, 9, and 12 months postoperatively that included: Beck Depression Inventory-II (BDI); Wide Range Intelligence Test (WRIT FSIQ) including average verbal (crystallized intelligence) and visual (fluid intelligence); Wide Range Assessment of Memory and Learning (WRAML), including the four domains of verbal memory, visual memory, attention/concentration, and working memory; and Delis–Kaplan Executive Function System (D-KEFS). The Dizziness Handicap Inventory (DHI) and the Headache Impact Test (HIT-6) were also completed to assess the impact of their disease on activities pre- and postoperatively.
Main Outcome Measures:
Quantitative and statistical analysis of their cognitive and neurobehavioral function.
Results:
The pattern of differences between the SCD group and the no-iOCD group from WRAML verbal, visual, and attention test performance indicate different postoperative clinical trajectories. For the WRAML, there was a statistically significant improvement for visual memory and verbal memory for the no-iOCD only and both (SCD and subsequent no-iOCD) groups, but no mean improvement for the SCD only group. By contrast, the no-iOCD group had significantly lower scores on the WRAML attention test preoperatively, but they recovered postoperatively to match the other groups. The preoperative findings and postoperative outcomes did not differ significantly among patient groups on the WRAML working memory test, D-KEFS motor scores, D-KEFS number and letter scores, or Wide Range Intelligence Test scores. There was a significant decrease in the BDI for all groups. The IQ scores were unchanged. There was a statistically significant improvement in the DHI and HIT-6 scores postoperatively in all groups.
Conclusions:
There was a marked overall improvement in cognitive and neurobehavioral function postoperatively. Variability may result from duration of underlying disease before intervention. The initial decrement or delay in performance improvement measured in several patients may represent brain reorganization. Greater longitudinal data and greater subject numbers are necessary to better understand and optimize cognitive recovery.
Keywords: Cognitive dysfunction, Depression, Memory, Migraine, Otic capsule dehiscence syndrome, Perilymph fistula, Superior canal dehiscence syndrome, Traumatic brain injury
Clinicians managing patients with peripheral vestibular disorders are challenged with signs and symptoms of altered cognitive function, which often introduce challenges when trying to elicit a cogent history. Cognitive alterations seem to be associated with many vestibular asymmetries (1) and with otic capsule defects. Nearly a quarter century ago, Black et al. (2) reported that the majority of patients with perilymph fistula (PLF) experience altered cognitive status. Similar cognitive changes have recently been described in patients with superior semicircular canal dehiscence syndrome (SSCDS) symptoms (3). Video recordings of consenting patients before and after intervention help to further document these obvious alterations in ways that complement standardized neuropsychology testing (4–12).
Recently, a prospective cohort of 12 patients with long-term follow-up and with SSCDS, 6 with radiographic evidence of superior canal dehiscence (SCD) treated with a middle fossa approach and plugging; and 6 with no imaging visible otic capsule dehiscence (no-iOCD) treated with round window reinforcement (RWR) was reported (3). It has been suggested that the term SSCDS be replaced with otic capsule dehiscence syndrome (OCDS) because the same SSCDS symptoms and diagnostic findings can occur with lateral and posterior semicircular canal dehiscence, internal carotid artery-cochlea dehiscence, posterior semicircular canal-jugular bulb dehiscence, posttraumatic hypermobile stapes footplate (Dr. Arun Gadre, personal communication, August 1, 2015) and in patients with no-iOCD (3,6,13–17).
This study used a battery of neuropsychological tests to provide the first quantitative characterization of the preoperative and postoperative cognitive function changes in patients undergoing surgical management of their OCDS. A comprehensive neuropsychology test battery was administered preoperatively and at 3, 6, 9, and when possible 12 months postoperatively. This battery included: the Beck Depression Inventory-II; the Wide Range Intelligence Test including average verbal (crystallized intelligence) and visual (fluid intelligence); the Wide Range Assessment of Memory and Learning, including the four domains of verbal memory, visual memory, attention/concentration, and working memory; and the Delis–Kaplan Executive Function System (for an in-depth description of these neuropsychology tests, see Supplemental Digital Content) (18–28). These neuropsychological tests showed distinctive patterns that provide greater insight into the nature of the cognitive dysfunction these patients experience and suggest that additional interventions may maximize and/or accelerate their cognitive recovery. These OCDS patients, with two different dehiscence locations and resolved surgically, may provide a novel opportunity to gain deeper insight into cognitive neuroscience.
METHODS
Subjects
The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration. Our Institutional Review Board approved these studies.
Seventeen healthy subjects with SSCDS/OCDS who had SCD, no-Iocd, or both agreed to participate in and completed the study. There were 13 adults and 4 children. The entire cohort had a mean age of 39.5 years (range, 12.99–60.29 yr) at first surgery, with 5 males and 12 females. Group 1 (no-iOCD only) (n = 8) had a mean age at first surgery of 39.4 years (range, 12.99–60.29 yr), with two males and six females. Group 2 (both SCD and subsequent no-iOCD) (n = 4) had a mean age at first surgery of 43.42 years (range, 33.46–53.79 yr), with one male and three females. It should be noted that in Group 2 (SCD and no-iOCD) all RWR operations occurred subsequent to SCD plugging of a radiographically identified SCD. Group 3 (SCD only) (n = 5) had a mean age at first surgery of 36.36 years (range, 14.48–56.30 yr), with two males and three females. At the time of manuscript submission, the entire cohort had a mean age of 41.58 years (range, 14.82–62.67 yr). Group 1 (no-iOCD only) had a mean age at the time of manuscript submission of 41.65 years (range, 14.82–62.67 yr, n = 8). Group 2 (SCD and subsequent no-iOCD) had a mean age at the time of manuscript submission of 46.11 years (range, 36.93–57.10 yr, n = 4). Group 3 (SCD only) had a mean age at the time of manuscript submission of 37.86 years (range, 15.63–58.72 yr, n = 5). The patient demographics and clinical features for each subject are summarized in Tables 1 and 2. The methods for the diagnostic studies performed can be found in the Supplemental Digital Content (2,29–31).
TABLE 1.
Patient demographics, surgical procedures, and neuropsychology test battery intervals
| Patient | Sex | Age at First Surgery | Current Age* | Diagnosis at Initial Referral | Surgery 1 | Surgery 2 | Surgery 3 | Postoperative Complicating Factors | Last Surgery to First Assessment | Last Surgery to Most Recent Assessment | First Surgery to Most Recent Assessment | |
| Group 1 Patients with otic capsule dehiscence syndrome and no imaging visible otic capsule dehiscence only | ||||||||||||
| 1 | M | 12.99 | 14.82 | TBI, migraine | R RWR | L RWR | ELH, resolved | 1 month | 6 months | 8 months | ||
| 2 | F | 17.19 | 18.86 | Conversion disorder, migraine | R RWR | None | 6 months | 8 months | 8 months | |||
| 3 | F | 35.32 | 37.31 | Migraine | R RWR | None | 2 months | 12 months | 12 months | |||
| 4 | F | 43.03 | 45.43 | Migraine, ELH | L RWR | ELH, resolved | 4 months | 12 months | 12 months | |||
| 5 | F | 46.82 | 48.96 | Migraine, hemiplegic migraine | L RWR | L RWR | Appendicitis, severe vomiting | 3 months | 10 months | 11 months | ||
| 6 | F | 49.29 | 52.60 | Migraine, ELH | R RWR | ELH | 3 months | 6 months | 6 months | |||
| 7 | F | 50.32 | 52.51 | Migraine, Menière disease | R RWR | ELH | 2 months | 9 months | 9 months | |||
| 8 | M | 60.29 | 62.67 | Autophony | L RWR | ELH | 2 months | 12 months | 12 months | |||
| Group 2 Patients with otic capsule dehiscence syndrome and having superior canal dehiscence and subsequently another otic capsule dehiscence not visualized with imaging | ||||||||||||
| 9 | F | 33.46 | 36.93 | Migraine | R SCD | R RWR | Migraine | 3 months | 17 months | 29 months | ||
| 10 | F | 35.47 | 37.45 | Migraine, fall while rock climbing | R SCD | L SCD | L RWR | ELH, intermittent | 2 months | 2 months | 13 months | |
| 11 | M | 50.94 | 52.95 | SCD, migraine | R SCD | R RWR | Chronic EtOH abuse | 2 months | 4 months | 11 months | ||
| 12 | F | 53.79 | 57.10 | MVA, concussion, migraine | R SCD | R RWR | ELH, Irlen syndrome | 3 months | 13 months | 22 months | ||
| Group 3 Patients with otic capsule dehiscence syndrome and having superior canal dehiscence only | ||||||||||||
| 13 | M | 14.48 | 15.63 | Migraine, concussion | R SCD | Withdrew from study | 3 months | 9 months | 9 months | |||
| 14 | F | 16.38 | 17.28 | Migraine | R SCD | None | 4 months | 9 months | 6 months | |||
| 15 | F | 39.98 | 41.38 | Migraine | R SCD | None | 3 months | 9 months | 6 months | |||
| 16 | M | 54.66 | 56.30 | Otolithic crisis of Tumarkin, ELH | L SCD | ELH | 3 months | 6 months | 6 months | |||
| 17 | F | 56.30 | 58.72 | SCD, migraine | L SCD | R SCD | ELH, slowly resolved | 9 months | 18 months | 21 months | ||
*As of 4/1/2015 (age in years); ELH indicates endolymphatic hydrops; EtOH, ethanol; RWR, round window reinforcement; SCD, superior canal dehiscence.
TABLE 2.
Patient history, symptoms, physical findings and results of diagnostic studies before surgical intervention
| Patient | Sound-induced | Hearing Internal Sounds | 256 Hz Tuning Fork | Cognitive Dysfunction | Spatial Disorientation | Anxiety | Nausea | Migraine Character | Trauma | Pseudoconductive Hearing Loss | Endolymphatic Hydrops | cVEMP | Moving Platform Pressure Test | High-Resolution CT | |
| Group 1 Patients with otic capsule dehiscence syndrome and no imaging visible otic capsule dehiscence only | |||||||||||||||
| 1* | HA, dizziness, pain | Eyes blinking | Positive | Yes | Yes | No | Yes | 24/7, light sensitivity | Snowboarding accident, LOC | Bilateral | Left | Positive, bilateral | Positive, bilateral | Normal | |
| 2* | Increased HA | Eyes blinking, autophony | Positive | Yes | Yes | No | Mild | 24/7, severe | Concussion after falling down stairs, later flu and vomiting | Right | No | Positive, right | Positive, right | Normal | |
| 3 | Dizziness, nausea | Autophony, eyes moving, chewing | Positive | Yes | Yes | No | Yes | 24/7, light sensitive, vestibular migraine with episodic rotational vertigo | No | Right | Right poor morphology, left no | Normal | Positive, right | Normal | |
| 4 | Dizziness, nausea, vibration left head | No | Negative | Yes | Yes | No | Mild | Frequent, light sensitivity | No | Bilateral | Left | Positive, left | Positive, left | Normal | |
| 5* | Dizziness, nausea, agitated | Eyes blinking, heartbeat, swallowing, autophony | Positive | Yes | Yes | Yes | Yes | 24/7, light sensitive, left hemiplegic migraine, ocular migraine, rare vestibular migraine | Not before 1st surgery, recurrence after intractable vomiting and appendicitis | Left | Left | Positive, left | Positive, left | Normal | |
| 6 | Dizziness, nausea, HA | Heartbeat | Positive | Yes | Yes | No | Mild | Frequent | Airplane flight descent | Bilateral | Right | Absent | Positive, right much greater than left | Normal | |
| 7 | Dizziness, nausea | Eyes blinking, heartbeat | Not performed | Yes | Yes | No | Mild | Frequent, light sensitive | No | Bilateral | Bilateral | Positive right | Positive, right | Normal | |
| 8 | Ear pressure, increased tinnitus | Autophony | Positive | Yes | Yes | No | No | None | No | Bilateral | No | Positive, left | Negative | Normal | |
| Group 2 Patients with otic capsule dehiscence syndrome and having superior canal dehiscence and subsequently another otic capsule dehiscence not visualized with imaging | |||||||||||||||
| 9 | Tilting, nausea | Breathing, chewing, heel strike | Positive | Yes | Yes | No | Yes, vomiting | Migraine | No | Right | Right | Absent | Positive, right | SCD | |
| 10 | Dizziness, nausea, HA | TMJ movement | Positive | Yes | Yes | Yes | Yes | Migraine, ocular migraine | Rock climbing fall | Bilateral | No | Positive, bilateral | Positive, left | SCD, bilateral | |
| 11 | Legs buckle, nausea | Eyes moving, autophony | Positive | Yes | Yes | No | Mild | Daily “sinus HA” with normal CT | No | Bilateral | Right | Positive, right | Positive, right | SCD | |
| 12 | HA | None | Positive | Yes | Yes | No | No | Daily migraine | MVA, concussion | Right | Right | Absent | Positive, right | SCD | |
| Group 3 Patients with otic capsule dehiscence syndrome and having superior canal dehiscence only | |||||||||||||||
| 13 | Dizziness, HA | Autophony, joints moving | Positive | Yes | Yes | Yes | Yes | Daily migraine, light sensitivity | Multiple concussions | Right | No | Positive, right | Negative | SCD | |
| 14 | Dizziness, HA | Joints moving, chewing, heartbeat | Positive | Yes | Yes | Yes | Yes | 24/7, light sensitivity | No | Right | No | Positive, right | Negative | SCD | |
| 15 | Dizziness, nausea, pain | Eyes moving, heel strike, breathing, autophony | Positive | Yes | Yes | No | Mild | Daily migraine, light sensitivity | No | Right | Bilateral | Normal, but reduced amplitude in SCD ear relative to normal ear | Negative | SCD | |
| 16 | Sound distortion | Thumping | Positive | Yes | Yes | No | Occasional | None | Sudden hearing loss with rotational vertigo, left | Bilateral | Bilateral | Positive, right | Negative | SCD | |
| 17 | Titling, nausea, nystagmus | Eyes moving, heel strike, joint movement, blood flowing, autophony | Positive | Yes | Yes | Yes | Mild | Daily migraine, light sensitivity | Symptomatic with flying and driving over mountains | Bilateral | Bilateral | Absent | Not performed | SCD, bilateral | |
*See video links in (4–6); 24/7 indicates migraine headache present constantly, 24 h per day and 7 days per week; 256 Hz, ability to hear or feel the vibration of the head of the tuning fork when applied to knees and elbows; cVEMP positive, increased amplitude response and decreased threshold; Dizziness, gravitational receptor asymmetry type of vertigo (e.g., as if on a boat, rocky, wavy, tilting, being pushed, tilting, or sense of floor falling our from under them); Endolymphatic hydrops, abnormal summating potential/action potential ratio with electrocochleography; HA, headache; MVA, motor vehicle accident; SCD, superior canal dehiscence; TMJ, temporomandibular joint.
Dizziness Handicap Inventory
The methods for the Dizziness Handicap Inventory (DHI) studies performed can be found in the Supplemental Digital Content (32).
Headache Impact Test
The methods for the Headache Impact Test (HIT-6) studies performed can be found in the Supplemental Digital Content (33,34).
Computerized Dynamic Posturography
The methods for the computerized dynamic posturography studies performed can be found in the Supplemental Digital Content (35–37).
Neuropsychology Test Battery
Completion of a neuropsychology test battery preoperatively and at 3, 6, 9, and 12 months postoperatively that included: Beck Depression Inventory-II (BDI); Wide Range Intelligence Test (WRIT FSIQ) including average verbal (crystallized intelligence) and visual (fluid intelligence); Wide Range Assessment of Memory and Learning (WRAML-2), including the four domains of verbal memory, visual memory, attention/concentration, and working memory; and Delis–Kaplan Executive Function System. Detailed information regarding each of these neuropsychology tests can be found in the Supplemental Digital Content.
Statistical Analysis
The scores from each test were analyzed by mixed design analysis of variance (ANOVA), with patient group (SCD, RWR, or both surgeries) as a between subjects factor and the test time (preoperative, early postoperative, and late postoperative times) as the repeated measures factor (SYSTAT 11 software). Least significant differences post-hoc tests were used for paired comparisons in cases where significant main or interaction effects were shown by ANOVA. A criterion of p < 0.05 was regarded as significant.
RESULTS
Although not the focus of the present study, once each patient completed their final surgical procedure and medical management resolved any of the factors complicating their postoperative recovery, their presenting symptoms and signs were returned near their baseline before developing SSCDS/OCDS. Additional information regarding the limitations of reporting the hearing and cVEMP outcomes can be found in the Supplemental Digital Content (30). We completed quantitative comparisons of postural control using computerized dynamic posturography to better understand the changes in vestibular function as a consequence of surgical intervention using this more system-based approach.
The MRI with CISS sequences that demonstrated plugging of the superior semicircular canal in the 4 patients (patients 9–12) who subsequently developed no-iOCD and treated with RWR can be observed in Figure 1.
FIG. 1.
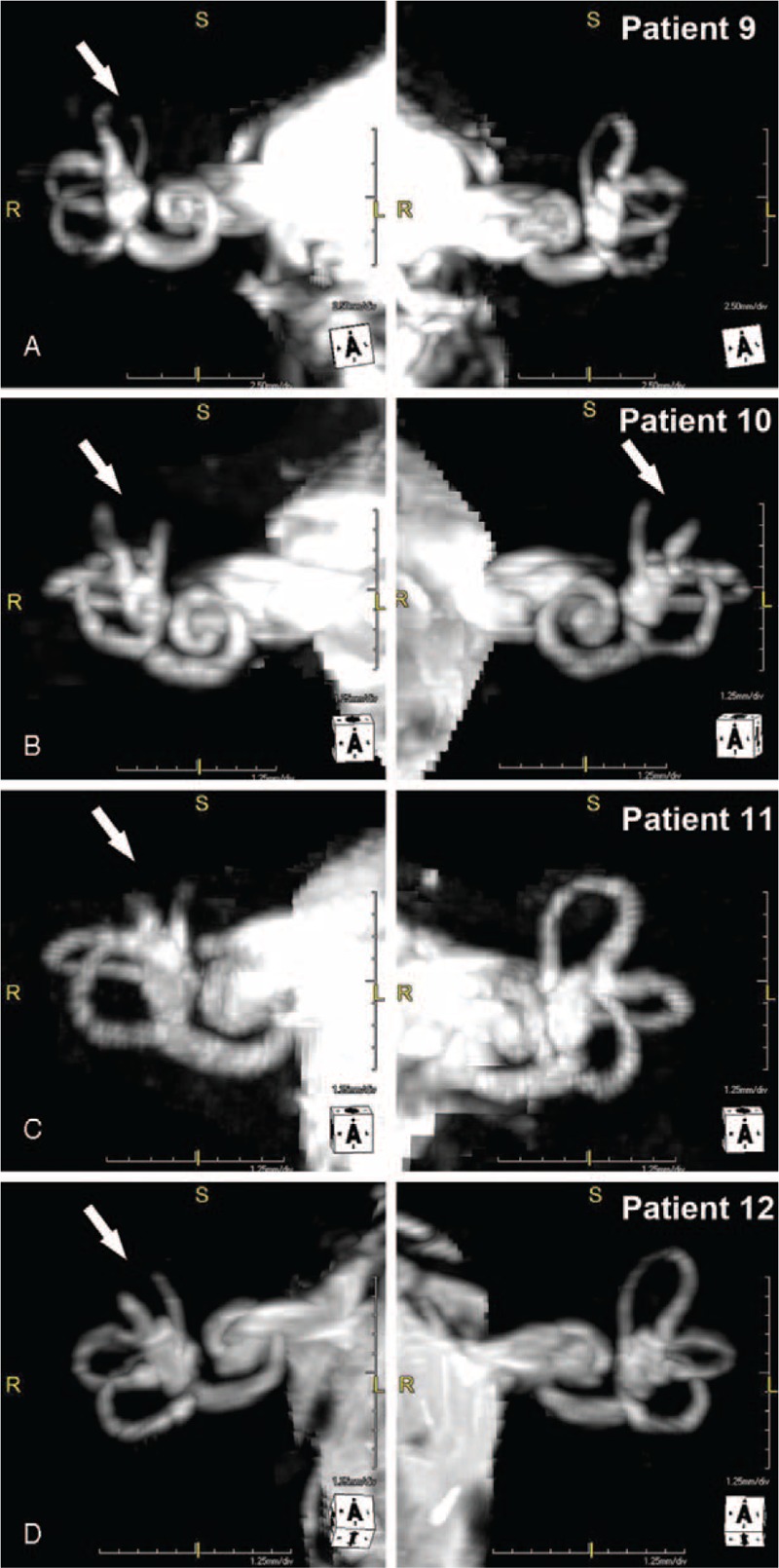
MRI with CISS sequences after the recurrence of otic capsule dehiscence syndrome after surgical plugging of the superior canal dehiscence(s) shows that the surgically managed superior canals (SC) remain plugged (arrows). (A), Patient 9 with right SC plugged (arrow). (B), Patient 10 with right and left SC plugged (arrows). (C), Patient 11 with right SC plugged (arrow). (D) Patient 12 with right SC plugged (arrow). Copyright © Ear and Skull Base Center, used with permission.
The neurobehavioral features of the patients attributed to neurological and psychiatric disorders (Tables 1 and 2) resolved over time (for example, see patients 1 and 2 (4,6)).
Dizziness Handicap Inventory
The DHI data revealed that there was a highly significant improvement pre- versus postoperatively (repeated measures ANOVA, F(1,11) = 254.6, p < 0.001) overall and for each group (Fig. 2), but no significant difference between patient groups (repeated measures ANOVA, F(2,11) = 1.8, p > 0.2). For the no-iOCD patients, the mean DHI score was 74 (range, 48–98, SD ± 17.48) preoperatively and 1.33 (range, 0–4, SD ± 1.63) postoperatively. This improvement was statistically significant (p < 0.001). For the both SCD and no-iOCD patients, the mean DHI score was 78.5 (range, 64–88, SD ± 11.0 preoperatively and 19.5 (range, 0–42, SD ± 19.1) postoperatively. This improvement was statistically significant (p < 0.001). For the SCD only patients, the mean DHI score was 72.5 (range, 68–76, SD ± 4.12 preoperatively and 8.5 (range, 4–18, SD ± 6.4) postoperatively. This improvement was statistically significant (p < 0.001).
FIG. 2.
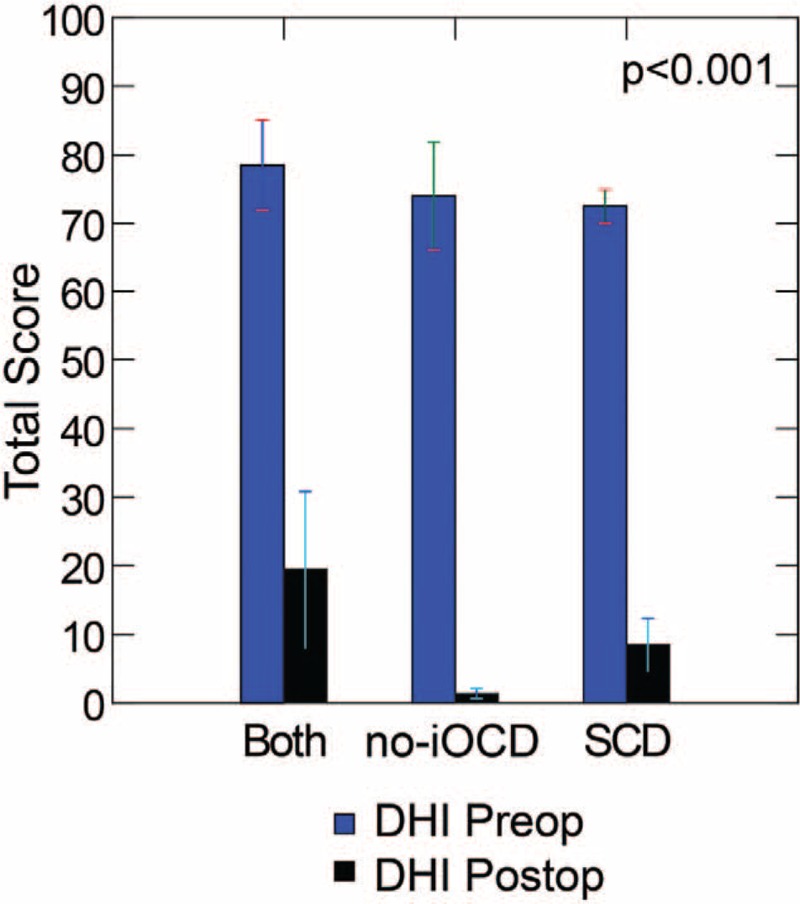
Dizziness Handicap Inventory (DHI). The DHI data revealed that there was a highly statistically significant improvement pre- versus postoperatively (repeated measures ANOVA, F(1,11) = 254.6, p < 0.001) overall and between groups (Fig. 2), but no significant difference between patient groups (repeated measures ANOVA, F(2,11) = 1.8, p > 0.2). Both indicates SCD plugging, subsequent development of no-iOCD managed with RWR; no-iOCD, no imaging visible otic capsule dehiscence only managed with RWR; SCD, superior semicircular canal dehiscence only managed with middle cranial fossa approach and plugging. Copyright © Ear and Skull Base Center, used with permission.
The DHI physical subscores differed significantly across patient groups (repeated measures ANOVA, F(2,11) = 7.5, p < 0.01), despite the fact that all groups showed a significant reduction after surgery (repeated measures ANOVA, F(1,11) = 168.1, p < 0.001). This difference was because of a significantly higher postoperative subscore (least significant differences test) in the group given operations for both SCD plugging and subsequent no-iOCD RWR (7.00 ± 5.29 (SD)) than for either the SCD plugging alone (2.00 ± 1.63 (SD), p < 0.05) or no-iOCD with RWR alone (0.33 ± 0.82 (SD), p < 0.01) groups.
Headache Impact Test
Migraine headache was present in 88% (7/8) of subjects with no-iOCD only, 100% (4/4) of subjects with SCD and subsequent no-iOCD, and 80% (4/5) of subjects with SCD only. Interestingly for these patients the migraine headaches by clinical report resolved in all patients (4–6), including those with vestibular migraine, ocular migraine, and hemiplegic migraine (Table 2); however, the HIT-6 data revealed that there was a highly statistically significant improvement pre- versus postoperatively (p < 0.001) overall and between groups (Fig. 3), yet there are two patients who quantitatively became Class II and one patient remained a Class IV. The remaining 11 patients became Class I. For the no-iOCD patients, the mean HIT-6 score was 74 (range, 68–78 [all Class IV], SD ± 4 preoperatively and 45.7 (range, 42–49 [all Class I], SD ± 3.14) postoperatively. This improvement was statistically significant (p < 0.001). For the both SCD and subsequent no-iOCD patients, the mean HIT-6 score was 69.3 (range, 57–78 [one Class III, three Class IV], SD ± 9.7 preoperatively and 46.8 (range, 36–53 [two Class II and two Class I], SD ± 8.10) postoperatively. This improvement was statistically significant (p < 0.001). For the SCD only patients, the mean HIT-6 score was 69.8 (range, 61–76 [all Class IV], SD ± 6.34 preoperatively and 44.5 (range, 36–61 [one Class IV and three Class I], SD ± 11.27) postoperatively. This improvement was statistically significant (p < 0.001).
FIG. 3.
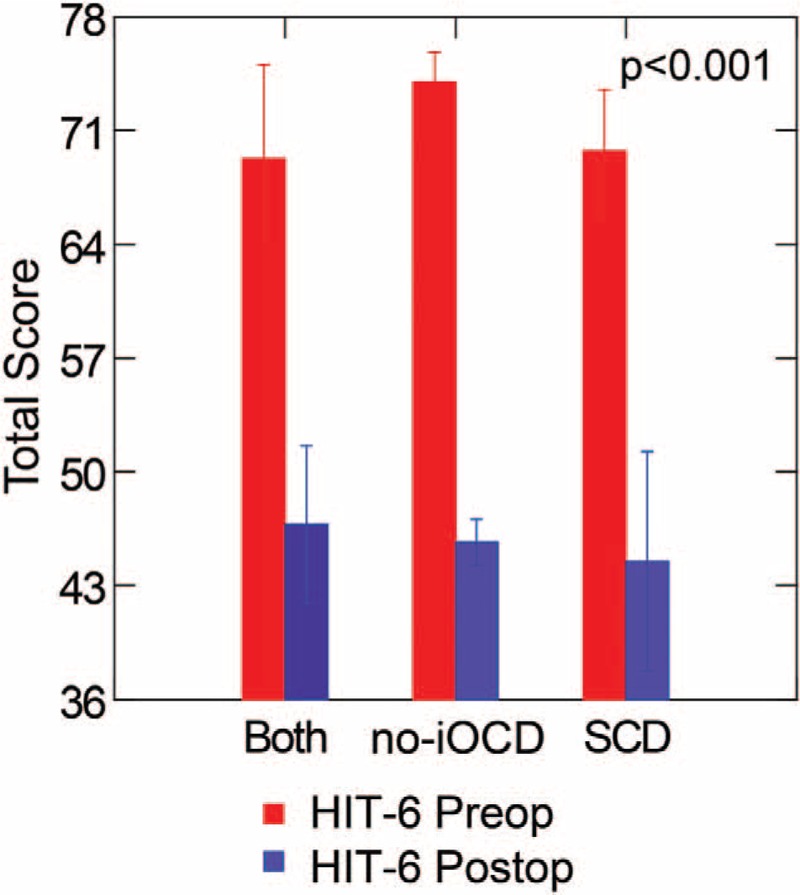
Headache Impact Test (HIT-6). The HIT-6 data revealed that there was a highly statistically significant improvement pre- versus postoperatively (p < 0.001) overall and between groups, yet there are two patients who quantitatively became Class II and one patient remained a Class IV postoperatively. The remaining 11 patients became Class I. For the no-iOCD patients, the mean HIT-6 score was 74 (range, 68–78 [all Class IV], SD ± 4 preoperatively and 45.7 (range, 42–49 [all Class I], SD ± 3.14) postoperatively. This improvement was statistically significant (p < 0.001). For the both SCD and subsequent no-iOCD patients, the mean HIT-6 score was 69.3 (range, 57–78 [one Class III, three Class IV], SD ± 9.7 preoperatively and 46.8 (range, 36–53 [two Class II and two Class I], SD ± 8.10) postoperatively. This improvement was statistically significant (p < 0.001). For the SCD only patients, the mean HIT-6 score was 69.8 (range, 61–76 [all Class IV], SD ± 6.34 preoperatively and 44.5 (range, 36–61 [one Class IV and three Class I], SD ± 11.27) postoperatively. This improvement was statistically significant (p < 0.001). Both indicates SCD plugging, subsequent development of no-iOCD managed with RWR; no-iOCD, no imaging visible otic capsule dehiscence only managed with RWR; SCD, superior semicircular canal dehiscence only managed with middle cranial fossa approach and plugging. Copyright © Ear and Skull Base Center, used with permission.
As shown in Table 1, 15 of 17 patients (88.2%) were diagnosed with migraine and/or migraine variants and managed medically using drugs to prevent migraine from occurring (e.g., topamax, zonegran, verapamil, or tricyclic antidepressants) before undertaking surgical intervention. For the no-iOCD patients (n = 7), the mean duration of treatment was 11.3 months preoperatively (range, 2–19 mo, SD ± 6.8 mo). For the both SCD and subsequent no-iOCD patients (n = 4), the mean duration of treatment was 19.8 months (range, 4–62 mo, SD ± 28.2 mo). For the SCD only patients (n = 4), the mean duration of treatment was 36.5 months (range, 14–60 mo, SD ± 21.2 mo).
Computerized Dynamic Posturography
Figure 4 shows the pre- versus postoperative posture performance for each group using the weighted composite score. Based on the independent samples Kruskal–Wallis test, there was no difference across groups for the preoperative or postoperative continuous EQ (CEQ) scores, either from the overall composite score or from the subscores for conditions 1 to 6. Although there were no significant differences in postoperative performance in each group analyzed separately, there was a significant improvement in postoperative composite score when combining all three groups (p = 0.044, Wilcoxon signed-rank test).
FIG. 4.
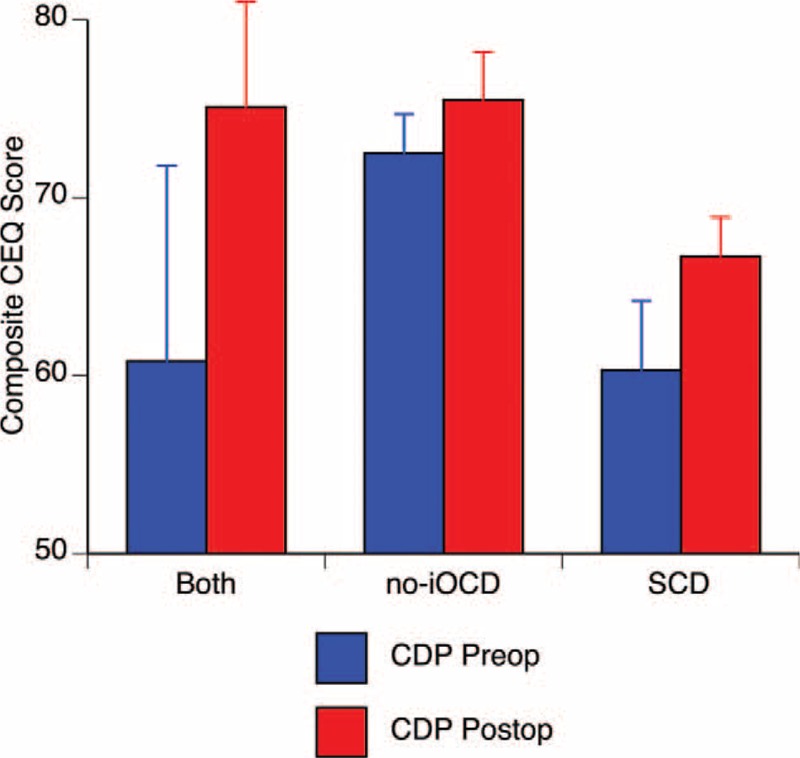
The preoperative versus postoperative composite continuous equilibrium (CEQ) scores during computerized dynamic posturography were not statistically different across the three groups nor between pre- versus postoperative sessions within each group. There was an overall statistically significant postoperative improvement (p = 0.044) in composite CEQ Scores when combining data from all three groups. Both indicates SCD plugging, subsequent development of no-iOCD managed with RWR; no-iOCD, no imaging visible otic capsule dehiscence only managed with RWR; SCD, superior semicircular canal dehiscence only managed with middle cranial fossa approach and plugging. Copyright © Ear and Skull Base Center, used with permission.
Beck Depression Inventory-II
The preoperative scores from the Beck Depression Index-II (BDI) indicated mild depression in all three groups. There was significant and parallel improvement to the minimal depression range after surgery in all three groups (F(1,18) = 9.8, p < 0.01), which appeared on the first postoperative test session (Fig. 5).
FIG. 5.
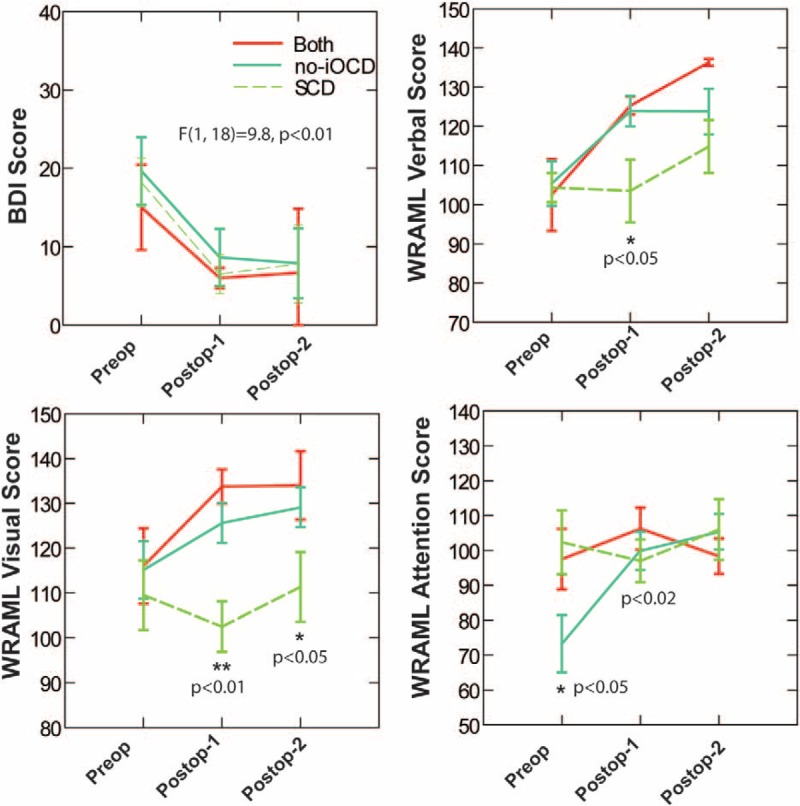
Top left, the preoperative scores from the Beck Depression Index-II (BDI) indicated mild depression in all three groups. There was significant and parallel improvement to the minimal depression range after surgery in all three groups (F(1,18) = 9.8, p < 0.01), which appeared on the first postoperative test session. Note that this recovery is rapid and significantly better, even a few months after surgical intervention. Copyright © Ear and Skull Base Center, used with permission. Top right, for the Wide Range Assessment of Memory and Learning-2 (WRAML) verbal subtest, the SCD only group treated with SCD plugging showed a delayed improvement on the WRAML verbal subtest; it was significantly lower than the no-iOCD only group treated with RWR and the both SCD and no-iOCD group treated with RWR and SCD plugging for the first postoperative test (ANOVA and then least significant differences tests). All three groups showed statistically significant improvement in the verbal subtest by the most recent neuropsychology test battery assessment. (∗ means p < 0.05 by least significant differences tests. Only the between groups differences are indicated). Bottom left, for the WRAML visual subtest, unlike patients with no-iOCD only treated with RWR or both SCD and no-iOCD treated with SCD plugging and RWR surgeries, the SCD only group treated with SCD plugging did not show statistically significant improvement at either the initial or most recent postoperative testing session, and remained significantly lower than either of the other groups (analysis of variance with repeated measures on test times and a between groups factor of operative history, and then least significant difference tests). There was a statistically significant improvement in the visual subtest for the no-iOCD only group treated with RWR and the both no-iOCD and SCD group treated with RWR and SCD plugging, respectively at both the initial postoperative assessment as well as at the most recent assessment. (∗ means p < 0.05 and ∗∗p < 0.01 by least significant differences tests. Only the between groups differences are indicated). Bottom right, for the WRAML attention concentration subtest, preoperatively, the no-iOCD group treated with RWR only showed abnormally low scores on the WRAML attention/concentration subtest (Fig. 1, 95% confidence interval of 55.271 to 91.229 re: normal of 100); however, the performance normalized after surgery. There were significant test time effects overall (improvement in all groups), initially (preoperative) worse in the no-iOCD only than the SCD only and the both SCD and no-iOCD patients (p < 0.02, Fisher's Least Significant Difference [LSD] test), but the same afterward. (∗ means p < 0.05 by least significant differences tests. Only the between groups differences are indicated).
Wide Range Intelligence Test
No significant differences were found in Wide Range Intelligence Test (WRIT FSIQ) scores; including average verbal (crystallized intelligence) and visual (fluid intelligence) when comparing pre- and postoperative performance and also between the three groups.
Wide Range Assessment of Memory and Learning
The Wide Range Assessment of Memory and Learning-2 (WRAML), including the four subtests of verbal memory, visual memory, attention/concentration, and working memory, revealed differences in both the preoperative status and postoperative recovery among the patients with the no-iOCD only group treated with RWR, the SCD only group treated with plugging, and those with SCD treated with plugging who subsequently developed no-iOCD treated with RWR (Fig. 5).
For the verbal subtest, the SCD only group (plugging) showed a delayed improvement on the WRAML verbal subtest; it was significantly lower than the no-iOCD only group treated with RWR and the both SCD (plugging) and subsequently developed no-iOCD (RWR) group for the first postoperative test (ANOVA and then least significant differences tests). All three groups showed statistically significant improvement in the verbal subtest by the most recent neuropsychology test battery assessment (Fig. 5).
For the visual subtest, unlike patients with no-iOCD only (RWR) or both SCD (plugging) and subsequently developed no-iOCD (RWR) (Fig. 5), the SCD only (plugging) group did not show statistically significant improvement at either the initial or most recent postoperative testing session. They remained significantly lower than either of the other groups (analysis of variance with repeated measures on test times and a between groups factor of operative history, and then least significant difference tests). By contrast, there was a statistically significant improvement in the visual subtest scores for the no-iOCD only (RWR) group and the both SCD (plugging) and subsequently developed no-iOCD (RWR) group at both the initial postoperative assessment and the most recent assessment.
Preoperatively, the no-iOCD only (RWR) group showed abnormally low scores on the WRAML attention/concentration subtest (Fig. 5, 95% confidence interval of 55.271 to 91.229 re: normal of 100); however, the performance normalized after surgery. There were significant test time effects overall (improvement in all groups), initially (preoperative) worse in the SCD only (plugging) than the no-iOCD only (RWR) patients (p < 0.02, Fisher's Least Significant Difference [LSD] test), but the same afterward.
Analysis of WRAML of the Working Memory subtest revealed no significant differences preoperatively compared with the first and the most recent neuropsychology test battery assessments across all three groups.
Delis–Kaplan Executive Function System
Analysis of variance showed that there was significant postoperative improvement in both the Delis–Kaplan Executive Function System (D-KEFS) motor score (F(2,28) = 10.31, p < 0.01) and the number and letter score (F(2,28) = 6.04, p < 0.05). There were no significant differences between the treatment group responses (Fig. 6).
FIG. 6.
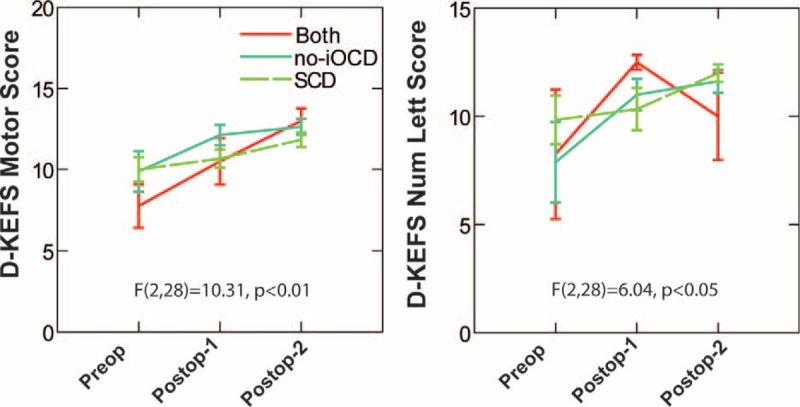
Analysis of variance showed that there was significant postoperative improvement in both the Delis–Kaplan Executive Function System (D-KEFS) motor score (F(2,28) = 10.31, p < 0.01) and the number and letter score (F(2,28) = 6.04, p < 0.05). There were no significant differences between the treatment responses for all three groups (no-iOCD only treated with RWR, both SCD and no-iOCD treated with SCD plugging and subsequent RWR surgeries, and SCD only treated with SCD plugging only). Copyright © Ear and Skull Base Center, used with permission.
DISCUSSION
Discussion of the current limitations in reporting outcomes of surgical intervention in these patient cohorts can be found in the Supplemental Digital Content (30,38,39).
Since the focus of this work was on understanding the degree of cognitive impairment and recovery after intervention, we elected to focus on two more global measures of vestibular function, the DHI and also the continuous equilibrium scores obtained via computerized dynamic posturography before and after intervention. As shown in Figure 2, the DHI data revealed that all patient groups reported a highly statistically significant perceived improvement for pre- versus postoperative status. On the other hand, dynamic posturography showed no significant differences in postoperative performance when each group was analyzed separately, despite a significant improvement in postoperative composite score when all three groups were combined. Hence, the groups did not differ clinically on these standard response metrics either pre- or postoperatively.
Most of the symptoms that disrupt the lives of patients with SCD, no-iOCD, and/or PLF are related to the severe, chronic, uncompensated asymmetric sensory deficits (3–7,9–12). The acute and chronic sequelae include direct and indirect sensorimotor processing, interoceptive, and cognitive neuronal networks that contribute to fear, anxiety, and altered cognitive performance. The relationship of these networks to vestibular information processing is described in detail in reviews (40,41).
These symptoms are, in part, a consequence of the fact that visual and vestibular sense-organs are anchored in the head. Sensations of visual motion and inertial motion are interpreted within the assumed context of stable head control. This assumption of head stability provides the basis for interpreting balance-related information from the head-fixed sensors in terms of the outside world. The perceptual assumption of stable motor control has two important implications. First, abnormal dynamic postural control can produce unexpected visual (e.g., optic flow or oscillopsia), head motion, or proprioceptive information. The autonomic and cognitive symptoms associated with these apparent sensory mismatches (including “visual-vestibular mismatch” all fall within the rubric described by the sensory conflict hypothesis for motion sickness, simulator sickness, and cybersickness (42–47)). Degradation of cognitive test performance would be expected.
Second, individuals with vestibular abnormalities may adapt functionally by relying on sensory signals that restore a relatively stable level of control. If balance control becomes more responsive to spatial information in the visual channel, a balance phenomenon referred to as visual balance dependence develops. This can be defined operationally as increased sway in response to full-field motion of the visual surround (“optic flow”), a phenomenon that has been observed in patients with primary vestibular disorders (48). If balance control becomes more responsive to somatosensory (tactile and proprioceptive) information, somatosensory dependence develops. These changes in relative central weighting of multisensory signals may have cognitive consequences. Treatment that resolves the sensorimotor control performance would be expected to normalize the cognitive consequences.
However, balance-related information also affects limbic and cortical networks related to anxiety and threat assessment (41,49). It is an open question whether resolution of the peripheral asymmetry alone is sufficient to reverse chronic, adaptive changes in cognition via these mechanisms.
Cognitive Functional Differences Between SCD and No-iOCD Patients
Further discussion of the cognitive differences between SCD and no-iOCD patients can be found in the Supplemental Digital Content (50–59).
Clinically, cognitive alterations are nearly universal in patients with superior canal dehiscence syndrome, whether because of an actual SCD or a no-iOCD. In contrast to these disorders that result in gravitational receptor dysfunction type of vertigo, it is uncommon in patients with rotational receptor dysfunction type of vertigo such as with benign positional vertigo, vestibular neuronitis, or other disorders producing true rotational vertigo. Patients with a no-iOCD and/or SCD often use the following descriptors when describing their cognitive function: “fuzzy, foggy, spacey, out-of-it; memory and concentration are poor; difficulty reading – as if the words are floating on the page; trouble finding the right words; and forgetting what I wanted to say.”
Gurvich et al. (60) published an excellent review of the role of the vestibular system on cognition and psychiatry. The two key anatomical regions that provide links between the vestibular system and neural networks involved in cognitive and emotional processing are the parabrachial nucleus and the hippocampus (49,61–63); however, many of the neuroanatomical regions that are linked to the vestibular system are also implicated in several psychiatric illnesses. The past decade has observed an increased interest in the relationship between the vestibular system and mood, cognition and psychiatric symptoms with studies demonstrating vestibular stimulation can produce changes in mood, cognition, and psychiatric symptoms (64–66). It is also the case that many individuals with SCDS have been assigned a neurological or psychiatric diagnosis before their vestibular disorder was diagnosed and have experienced resolution of their “psychiatric disorder” after surgical intervention (4,5,9–11) (Table 1). This unfortunately is common with children (4,5). The hippocampus is consistently implicated in cognition and models of psychiatric disorders and there is a large body of evidence supporting vestibular–hippocampal interactions (67–71).
Smith et al. and Zheng et al. reported that modulation of memory, but not spatial memory, occurs with vestibular lesions and can be influenced by galvanic vestibular stimulation (72,73). These findings may lead to additional treatment strategies that may accelerate or maximize recovery after repairing a no-iOCD or SCD.
Further discussion of these issues from the historical perspective can be found in the Supplemental Digital Content (74,75).
Altered Spatial Orientation
A discussion of the relationship between diseases producing otic capsule dehiscence syndrome and altered spatial orientation can be found in the Supplemental Digital Content (76–79).
Migraine Headache
A discussion of the relationship between diseases producing otic capsule dehiscence syndrome and migraine headache can be found in the Supplemental Digital Content (3–7,9–12,80–82) (also see Fig. 3 and Table 2).
Current Cohort
Beck Depression Inventory-II
The preoperative scores from the BDI indicated mild depression in all three groups. There was significant and parallel improvement to the minimal depression range after surgery in all three groups (F(1,18) = 9.8, p < 0.01), which appeared on the first postoperative test session (Fig. 5). There were no significant differences between any of the three groups. These findings are not unexpected, as most of these patients have experienced a delay in diagnosis despite having observed several physicians and completed numerous diagnostic studies. Many have been told “it is all in your head” and their interpersonal relationships and work performance have also been threatened or adversely impacted. Many patients develop severe depression because of their inability to perform their normal tasks. As shown in Figure 5, this recovery is rapid and significantly better, even a few months after surgical intervention.
Wide Range Intelligence Test
The finding that the WRIT showed no change in IQ is not surprising and serves as an internal control for these subjects. It would not be expected that these chronic, uncompensated gravitational receptor asymmetries would alter inherent intelligence.
Wide Range Assessment of Memory and Learning
The WRAML, including the four subtests of verbal memory, visual memory, attention/concentration, and working memory, revealed differences in both the preoperative status and postoperative recovery among the patients with no-iOCD treated with RWR, SCD treated with SCD plugging only, and those with SCD and subsequent development of no-iOCD who required both SCD plugging and subsequent RWR (Fig. 5).
For the verbal and visual subtests, the SCD only (plugging) group recovered function differently than the no-iOCD only (RWR) group and the both the SCD (plugging) and subsequently developed no-iOCD (RWR) group (Fig. 5). This is likely multifactorial. It is not unexpected that the both SCD treated with SCD plugging and subsequent development of no-iOCD treated with RWR group recovered in a similar manner as each patient had time to undergo vestibular compensation after closing the superior semicircular canal dehiscence before determining that they also developed a new no-iOCD and treating that dehiscence with a RWR surgery. Table 1 shows that these SCD (plugging) patients who subsequently developed a no-iOCD and had RWR had a mean time from first SCD plugging surgery to most recent neuropsychology assessment of 18.75 months, whereas the SCD only group treated with SCD plugging was 9.6 months. It is possible that there is a finite amount of brain recovery or reorganization that occurs as a function of time and that the process of vestibular compensation delayed the recovery of memory and learning as reflected by WRAML visual and verbal subtests. It should be noted that the WRAML visual subtest scores worsened at the first postoperative assessment in the SCD only patients. It may be that improvement of vestibulomotor function was necessary via vestibular compensation to eliminate these “cognitive jamming” mechanisms before visual recovery could occur. There were also two children included in the SCD only cohort who had factors potentially impacting their test performance. There was a statistically significant improvement in the verbal and visual subtests for the no-iOCD only group treated with RWR and the both SCD and subsequent development of no-iOCD group treated with SCD plugging and RWR, respectively at both the initial postoperative assessment and the most recent assessment.
Although speculative, there are five additional mechanisms that could explain the differences in recovery that we observed in the SCD only group treated with plugging in both the visual and verbal domains: 1) direct vestibular processing effects; 2) erroneous or ambiguous motion information that creates an increased cognitive load for normal balance and navigation functions; 3) acute prodromal (e.g., Sopite syndrome [relates symptoms of fatigue, drowsiness, and mood changes to prolonged periods of motion]) and autonomic effects; 4) fear, anxiety, and phobic responses; and 5) parabrachial nucleus cells have both superior semicircular canal and linear acceleration sensitivity.
Preoperatively, the no-iOCD only (RWR) group showed abnormally low scores on the WRAML attention/concentration subtest (Fig. 5); however, the performance normalized after surgery. If a no-iOCD is at the modiolus, it would be expected that CSF pulsations would repeatedly stimulate the otolithic end-organs on the affected side whereas with a SCD there is no direct communication with the CSF, so perhaps it is the case that the no-iOCD patients have more ongoing impairment of attention/concentration. Ultimately, there were significant test time effects overall (improvement in all groups) by the most recent neuropsychology assessment.
Delis–Kaplan Executive Function System
Analysis of variance showed that there was significant postoperative improvement in both the D-KEFS motor score and the number and letter score. There were no significant differences between the treatment group responses (Fig. 6). This recovery of executive function was rapid and robust and not impacted by vestibular compensation as the no-iOCD patients experienced nearly immediate improvement anecdotally and our short-term follow-up intervals in the SCD only group treated with SCD plugging (4–7,9–11). These functions measured by the D-KEFS largely depend on prefrontal cortex.
Learning Effects
A discussion of learning effects can be found in the Supplemental Digital Content (83–85).
CONCLUSION
These data represent the first demonstration that cognitive dysfunction in patients with otic capsule defects resulting in OCDS, regardless of etiology, exist, can be measured and that improvements in depression and cognitive function can be accomplished with appropriate, targeted, vestibular surgery.
Supplementary Material
Acknowledgments
The authors thank Stuart Gardiner, Ph.D., for initial guidance with the statistical analysis strategy.
Footnotes
P.A.W. and C.D.B. are joint first authors.
The Legacy Good Samaritan Foundation and the Ear and Skull Base Center provided funding to help support this work.
IRB Approval Number: LRI-682.
The authors disclose no conflicts of interest.
Supplemental digital content is available in the text.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (http://journals.lww.com/otology-neurotology).
REFERENCES
- 1.Bilgrei R. The psychology of vestibular disorders part I: Cognitive aspects of vestibular disorders. https://vestibular.org/sites/default/files/page_files/Documents/Cognitive%20Aspects%20of%20Vestibular%20Disorders.pdf (Accessed October 8, 2015). [Google Scholar]
- 2.Black FO, Pesznecker S, Norton T, et al. Surgical management of perilymphatic fistulas: A Portland experience. Am J Otol 1992; 13:254–262. [PubMed] [Google Scholar]
- 3.Wackym PA, Wood SJ, Siker DA, et al. Otic capsule dehiscence syndrome: Superior canal dehiscence syndrome with no radiographically visible dehiscence. Ear Nose Throat J 2015; 94:E8–24. [DOI] [PubMed] [Google Scholar]
- 4.Wackym PA. Traumatic otic capsule dehiscence syndrome after snowboarding accident. Patient 1 describing his symptoms before and after round window reinforcement surgery. https://www.youtube.com/watch?v=7azu9sszZSk Published June 8, 2015. (Accessed October 8, 2015). [Google Scholar]
- 5.Wackym PA. Right perilymph fistula: Dizziness, migraine headaches and cognitive dysfunction. Patient 2 describing her symptoms before and after round window reinforcement surgery. https://www.youtube.com/watch?v=ETjsJocMBYk Published March 30, 2014. (Accessed October 8, 2015). [Google Scholar]
- 6.Wackym PA. Left otic capsule dehiscence syndrome with hemiplegic migraine. Patient 5 describing her symptoms before and after round window reinforcement surgery. https://www.youtube.com/watch?v=9xVNxNGys1w Published August 2, 2015. (Accessed October 8, 2015). [Google Scholar]
- 7.Wackym PA. Cognitive dysfunction due to otic capsule dehiscence syndrome. The second patient describing her cognitive dysfunction and recovery is patient 15. https://www.youtube.com/watch?v=1pNZUX1a04g Published May 18, 2015. (Accessed October 8, 2015). [Google Scholar]
- 8.Wackym PA. Tuning fork testing in otic capsule dehiscence syndrome. https://www.youtube.com/watch?v=Szp_kO8oVos Published April 21, 2015. (Accessed October 8, 2015). [Google Scholar]
- 9.Wackym PA. Otic capsule dehiscence syndrome in one ear after a bicycle accident. https://www.youtube.com/watch?v=fkdFozzQBEc Published April 5, 2015. (Accessed October 8, 2015). [Google Scholar]
- 10.Wackym PA. Traumatic otic capsule dehiscence syndrome after skiing accident. https://www.youtube.com/watch?v=2-kD59ygKrE Published April 5, 2015. (Accessed October 8, 2015). [Google Scholar]
- 11.Wackym PA. Otic capsule dehiscence syndrome in one ear after a car accident. https://www.youtube.com/watch?v=1Nl9T6etxqM Published April 5, 2015. (Accessed October 8, 2015). [Google Scholar]
- 12.Wackym PA. Vestibular migraine. Patient video describing symptoms before and after treatment with Topamax. https://www.youtube.com/watch?v=Zy7YjCDnLYM Published April 12, 2012. (Accessed October 8, 2015). [Google Scholar]
- 13.Young L, Isaacson B. Cochlear and petrous carotid canal erosion secondary to cholesteatoma. Otol Neurotol 2010; 31:697–698. [DOI] [PubMed] [Google Scholar]
- 14.Meiklejohn DA, Corrales CE, Boldt BM, et al. Pediatric semicircular canal dehiscence: Radiographic and histologic prevalence, with clinical correlations. Otol Neurotol 2015; 36:1383–1389. [DOI] [PubMed] [Google Scholar]
- 15.Park JJ, Shen A, Loberg C, et al. The relationship between jugular bulb position and jugular bulb related inner ear dehiscence: A retrospective analysis. Am J Otolaryngol 2015; 36:347–351. [DOI] [PubMed] [Google Scholar]
- 16.Bear ZW, McEvoy TP, Mikulec AA. Quantification of hearing loss in patients with posterior semicircular canal dehiscence. Acta Otolaryngol 2015; 135:974–977. [DOI] [PubMed] [Google Scholar]
- 17.Elmali M, Poltat AV, Kucuk H, et al. Semicircular canal dehiscence: Frequency and distribution on temporal bone CT and its relationship with the clinical outcomes. Eur J Radiol 2013; 82:e606–e609. [DOI] [PubMed] [Google Scholar]
- 18.Diagnostic and Statistical Manual of Mental Disorders. 4th edArlington, VA: American Psychiatric Association; 2000. [Google Scholar]
- 19.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571. [DOI] [PubMed] [Google Scholar]
- 20.Beck AT, Rial WY, Rickets K. Short form of depression inventory: Cross-validation. Psychol Rep 1974; 34:1184–1186. [PubMed] [Google Scholar]
- 21.Beck AT, Steer RA. Internal consistencies of the original and revised Beck Depression Inventory. J Clin Psychol 1984; 40:1365–1367. [DOI] [PubMed] [Google Scholar]
- 22.Sharp LK, Lipsky MS. Screening for depression across the lifespan: A review of measures for use in primary care settings. Am Fam Physician 2002; 66:1001–1008. [PubMed] [Google Scholar]
- 23.Glutting J, Adams W, Sheslow D. Wide Range Intelligence Test. Wilmington, DE: Wide Range; 2000. [Google Scholar]
- 24.Coalson D, Raiford S. WAIS IV Technical and Interpretive Manual. San Antonio, TX: Pearson; 2008. [Google Scholar]
- 25.Sheslow D, Adams W. Wide Range Assessment of Memory and Learning. 2nd edLutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- 26.Delis DC, Kramer JH, Kaplan E, et al. Reliability and validity of the Delis-Kaplan executive function system: An update. J Int Neuropsychol Soc 2004; 10:301–303. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt M. Hit or miss? Insight into executive functions. J Int Neuropsychol Soc 2003; 9:962–964. [Google Scholar]
- 28.Delis D, Kaplan E, Kramer J. Examiner's Manual. San Antonio, TX: Pearson; 2001. [Google Scholar]
- 29.Margolis RH, Rieks D, Fournier EM, et al. Tympanic electrocochleography for diagnosis of Ménière's disease. Arch Otolaryngol Head Neck Surg 1995; 121:44–55. [DOI] [PubMed] [Google Scholar]
- 30.Wackym PA, Ratigan JA, Birck JD, et al. Rapid cVEMP and oVEMP responses elicited by a novel head striker and recording device. Otol Neurotol 2012; 33:1392–1400. [DOI] [PubMed] [Google Scholar]
- 31.Black FO, Lilly DJ, Nashner LM, et al. Quantitative diagnostic test for perilymph fistulas. Otolaryngol Head Neck Surg 1987; 96:125–134. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg 1990; 116:424–427. [DOI] [PubMed] [Google Scholar]
- 33.Yang M, Rendas-Baum R, Varon SF, et al. Validation of the Headache Impact Test (HIT-6() across episodic and chronic migraine. Cephalalgia 2011; 31:357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bayliss M, Batenhorst A. The HIT-6TM: A User's Guide. Lincoln, RI: QualityMetric, Inc, 2002. [Google Scholar]
- 35.Winter DA. Biomechanics and Motor Control of Human Movement. New York: Wiley; 2004. [Google Scholar]
- 36.CDP Protocols. Natus Balance and Mobility Web site. http://resourcesonbalance.com/for-clinicians/computerized-dynamic-posturography/cdp-protocols/ Accessed October 8, 2015. [Google Scholar]
- 37.Wood SJ, Reschke MF, Black FO. Continuous equilibrium scores: Factoring in the time before a fall. Gait Posture 2012; 36:487–489. [DOI] [PubMed] [Google Scholar]
- 38.Vlastarakos PV, Proikas K, Tavoulari E, et al. Efficacy assessment and complications of surgical management for superior semicircular canal dehiscence: A meta-analysis of published interventional studies. Eur Arch Otorhinolaryngol 2009; 266:177–186. [DOI] [PubMed] [Google Scholar]
- 39.Ward BK, Agrawal Y, Nguyen E, et al. Hearing outcomes after surgical plugging of the superior semicircular canal by a middle cranial fossa approach. Otol Neurotol 2012; 33:1386–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balaban CD, Jacob RG, Furman JM. Neurologic bases for comorbidity of balance disorders, anxiety disorders and migraine: Neurotherapeutic implications. Expert Rev Neurother 2011; 11:379–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staab JP, Balaban CD, Furman JM. Threat assessment and locomotion: Clinical applications of an integrated model of anxiety and postural control. Semin Neurol 2013; 33:297–306. [DOI] [PubMed] [Google Scholar]
- 42.Guedry FE., Jr. Psychophysics of vestibular sensation. In: Vestibular System Part 2: Psychophysics, Applied Aspects and General Interpretations. Berlin: Springer, 1974;3–154. [Google Scholar]
- 43.Kennedy RS, Berbaum KS, Collyer SC, et al. Spatial requirements for visual simulation of aircraft at real-world distances. Hum Factors 1988; 30:153–161. [DOI] [PubMed] [Google Scholar]
- 44.Kennedy RS, Berbaum KS, Lilienthal MG. Disorientation and postural ataxia following flight simulation. Aviat Space Environ Med 1997; 68:13–17. [PubMed] [Google Scholar]
- 45.Kohl RL. Sensory conflict theory of space motion sickness: An anatomical location for the neuroconflict. Aviat Space Environ Med 1983; 54:464–465. [PubMed] [Google Scholar]
- 46.Oman CM. Motion sickness: A synthesis and evaluation of the sensory conflict theory. Can J Physiol Pharmacol 1990; 68:294–303. [DOI] [PubMed] [Google Scholar]
- 47.Reason J, Brand J. Motion Sickness. London: Academic Press; 1975. [Google Scholar]
- 48.Redfern MS, Furman JM. Postural sway of patients with vestibular disorders during optic flow. J Vestib Res 1994; 4:221–230. [PubMed] [Google Scholar]
- 49.Balaban CD, Thayer JF. Neurological bases for balance-anxiety links. J Anxiety Disord 2001; 15:53–79. [DOI] [PubMed] [Google Scholar]
- 50.Bechterew W. Ergebnisse der Durchscheidung des N. acusticus, nebst Erörterung der Bedeutung der semicirculären Canäle für das Körpergleichgewicht. Pflügers Arch f d ges Physiol 1883;30:312–47. [Google Scholar]
- 51.Bergquist F, Ludwig M, Dutia MB. Role of the commissural inhibitory system in vestibular compensation in the rat. J Physiol 2008; 586 (Pt 18):4441–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith PF, Curthoys IS. Mechanisms of recovery following unilateral labyrinthectomy: A review. Brain Res Rev 1989; 14:155–180. [DOI] [PubMed] [Google Scholar]
- 53.Balaban CD, Hoffer ME, Gottshall KR. Top-down approach to vestibular compensation: Translational lessons from vestibular rehabilitation. Brain Res 2012; 1482:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pashler H. Dual task interference in simple tasks: Data and theory. Psychol Bull 1994; 116:220–244. [DOI] [PubMed] [Google Scholar]
- 55.Andersson G, Yardley L, Luxon L. A dual-task study of interference between mental activity and control of balance. Am J Otol 1998; 19:632–637. [PubMed] [Google Scholar]
- 56.Yardley L, Gardner M, Bronstein AM, et al. Interference between postural control and mental task performance in patients with vestibular disorder and healthy controls. J Neurol Neurosurg Psychiat 2001; 71:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Talkowski ME, Redfern MS, Jennings JR, et al. Cognitive requirements for vestibular and ocular motor processing in healthy adults and patients with unilateral vestibular lesions. J Cognitive Neurosci 2005; 17:1432–1441. [DOI] [PubMed] [Google Scholar]
- 58.Al-Yahya E, Dawes H, Smith L, et al. Cognitive motor interference while walking: A systematic review and meta-analysis. Neurosci Biobehav Rev 2011; 35:715–728. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe K, Funahashi S. Neural mechanisms of dual-task interference and cognitive capacity limitation in the prefrontal cortex. Nat Neurosci 2014; 17:601–611. [DOI] [PubMed] [Google Scholar]
- 60.Gurvich C, Maller JJ, Lithgow B, et al. Vestibular insights into cognition and psychiatry. Brain Res 2013; 1537:244–259. [DOI] [PubMed] [Google Scholar]
- 61.Wackym PA, Balaban CD. Molecules, motion, and man. Otolaryngol Head Neck Surg 1998; 118:S15–S23. [DOI] [PubMed] [Google Scholar]
- 62.Balaban CD, McGee DM, Zhou J, et al. Responses of primate caudal parabrachial nucleus and Köliker-fuse nucleus neurons to whole body rotation. J Neurophysiol 2002; 88:3175–3193. [DOI] [PubMed] [Google Scholar]
- 63.Balaban CD. Projections from the parabrachial nucleus to the vestibular nuclei: Potential substrates for autonomic and limbic influences on vestibular responses. Brain Res 2004; 996:126–137. [DOI] [PubMed] [Google Scholar]
- 64.Dodson MJ. Vestibular stimulation in mania: A case report. J Neurol Neurosurg Psychiatry 2004; 75:168–169. [PMC free article] [PubMed] [Google Scholar]
- 65.Levine J, Toder D, Geller V, et al. Beneficial effects of caloric vestibular stimulation on denial of illness and manic delusions in schizoaffective disorder: A case report. Brain Stimul 2012; 5:267–273. [DOI] [PubMed] [Google Scholar]
- 66.Winter L, Kruger TH, Laurens J, et al. Vestibular stimulation on a motion-simulator impacts on mood states. Front Psychol 2012; 3:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Besnard S, Machado ML, Vignaux G, et al. Influence of vestibular input on spatial and nonspatial memory and on hippocampal NMDA receptors. Hippocampus 2012; 22:814–826. [DOI] [PubMed] [Google Scholar]
- 68.Brandt T, Schautzer F, Hamilton DA, et al. Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain 2005; 128:2732–2741. [DOI] [PubMed] [Google Scholar]
- 69.Hüfner K, Hamilton DA, Kalla R, et al. Spatial memory and hippocampal volume in humans with unilateral vestibular deafferentation. Hippocampus 2007; 17:471–485. [DOI] [PubMed] [Google Scholar]
- 70.Sharp PE, Blair HT, Etkin D, et al. Influences of vestibular and visual motion information on the spatial firing patterns of hippocampal place cells. J Neurosci 1995; 15:173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith PF, Horii A, Russel N, et al. The effects of vestibular lesions on hippocampal function in rats. Prog Neurobiol 2005; 75:391–405. [DOI] [PubMed] [Google Scholar]
- 72.Smith PF, Geddes LH, Baek JH, et al. Modulation of memory by vestibular lesions and galvanic vestibular stimulation. Front Neurol 2010; 1:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zheng Y, Geddes L, Sato G, et al. Galvanic vestibular stimulation impairs cell proliferation and neurogenesis in the rat hippocampus but not spatial memory. Hippocampus 2014; 24:541–552. [DOI] [PubMed] [Google Scholar]
- 74.Grimm RJ, Hemenway WG, Lebray PR, et al. The perilymph fistula syndrome defined in mild head trauma. Acta Otolaryngol Suppl 1989; 464:1–40. [DOI] [PubMed] [Google Scholar]
- 75.Gizzi M, Zlotnick M, Cicerone K, et al. Vestibular disease and cognitive dysfunction: No evidence for a causal connection. J Head Trauma Rehabil 2003; 18:398–407. [DOI] [PubMed] [Google Scholar]
- 76.Baek JH, Zheng Y, Darlington CL, et al. Evidence that spatial memory deficits following bilateral vestibular deafferentation in rats are probably permanent. Neurobiol Learn Mem 2010; 94:402–413. [DOI] [PubMed] [Google Scholar]
- 77.Smith PF, Darlington CL, Zheng Y. Move it or lose it: Is stimulation of the vestibular system necessary for normal spatial memory? Hippocampus 2010; 20:36–43. [DOI] [PubMed] [Google Scholar]
- 78.Deroualle D, Lopez C. Toward a vestibular contribution to social cognition. Front Integr Neurosci 2014; 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith PF, Darlington CL. Personality changes in patients with vestibular dysfunction. Front Hum Neurosci 2013; 7:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Furman JM, Marcus DA, Balaban CD. Vestibular migraine: Clinical aspects and pathophysiology. Lancet Neurol 2013; 12:706–715. [DOI] [PubMed] [Google Scholar]
- 81.Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol 2010; 6:573–582. [DOI] [PubMed] [Google Scholar]
- 82.Wackym PA. Ultrastructural organization of calcitonin gene-related peptide immunoreactive efferent axons and terminals in the rat vestibular periphery. Am J Otol 1993; 14:41–50. [PubMed] [Google Scholar]
- 83.Heilbronner RL, Sweet JJ, Attix DK, et al. Official position of the American Academy of Clinical Neuropsychology on serial neuropsychological assessments: The utility and challenges of repeat test administrations in clinical and forensic contexts. Clin Neuropsychol 2010; 24:1267–1278. [DOI] [PubMed] [Google Scholar]
- 84.Calamia M, Markon K, Tranel D. Scoring higher the second time around: meta-analyses of practice effects in neuropsychological assessment. Clin Neuropsychol 2012; 26:543–570. [DOI] [PubMed] [Google Scholar]
- 85.Jessup AB, Grimley MB, Meyer E, et al. Effects of diabetic ketoacidosis on visual and verbal neurocognitive function in pediatric patients presenting with new onset type 1 diabetes. J Diabetes Metab 2014; 5:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


