Abstract
Numerous epidemiological studies suggest that individuals who exercise have decreased cardiac morbidity and mortality. Pre-clinical studies in animal models also find clear cardioprotective phenotypes in animals that exercise, specifically characterized by lower myocardial infarction and arrhythmia. Despite the clear benefits, the underlying cellular and molecular mechanisms that are responsible for exercise preconditioning are not fully understood. In particular, the adaptive signaling events that occur during exercise to “trigger” cardioprotection represent emerging paradigms. In this review, we discuss recent studies that have identified several different factors that appear to initiate exercise preconditioning. We summarize the evidence for and against specific cellular factors in triggering exercise adaptations and identify areas for future study.
Keywords: Preconditioning, cardiac, mitochondria, exercise, heart
INTRODUCTION
The beneficial effects of exercise on the cardiovascular system have been well characterized over the last several decades and it is now accepted that exercise can be used as primary prevention for cardiovascular disease (Oberman 1985). Manifestations of cardiovascular disease are blunted with exercise in experimental animal models, and epidemiological data in humans further support these findings (Wang et al. 1993; Hamalainen et al. 1995). Exercise-induced protection against acute coronary syndromes encompasses a reduction in myocardial infarction (Brown et al. 2005b; Lee et al. 2012; Frasier et al. 2013), arrhythmia (Frasier et al. 2011b; Frasier et al. 2013), and stunning (Bowles et al. 1992; Taylor et al. 1999; Lennon et al. 2004; Taylor et al. 2007). While there is an abundance of literature on proposed mechanisms that seek to explain the protective effects of exercise (Starnes and Taylor 2007; Frasier et al. 2011a; Lee et al. 2012), a large portion of this research focuses on end points of protection as well as the downstream signaling events that protect the myocardium.
During exercise, an increase in cardiac output is warranted so that the heart can meet the demands of exercising muscles. Aside from matching cardiac output with peripheral demand, exercise also induces preconditioning whereby the heart is more resistant to injury even long after the exercise has ceased. The proverbial “triggers” that induce cardioprotective signaling are clearly multi-factorial, and include neural, endocrine, and paracrine factors, as well as autocrine signaling and adaptations that arise from within the heart itself.
Exercise can be thought of as eustress; positive stress that a cell responds to in a way that allows it to better cope with that stressor. The adaptive mechanisms associated with exercise ultimately induce a cardioprotective phenotype, resulting in increased tolerance to metabolic stressors (i.e. ischemia). Proposed triggers of exercise cardioprotection include: adenosine, opioids, adenosine monophosphate-activated protein kinase (AMPK), cytokines, mitochondrial and cytosolic derived reactive oxygen species (ROS), nitric oxide (NO), and adrenergic signaling. This review will focus on studies investigating cardioprotection induced by acute aerobic exercise regimens (i.e. days, weeks, and months of training) at moderate to high intensity. The windows of protection include an early window that occurs within the first hour after exercise, and a late window that typically lasts from 24 to 72 hours (Yamashita et al. 1999; Brown et al. 2003). Studies that utilize different exercise regimens or include protection outside of these time points will be described in detail. We will start by briefly discussing epidemiological findings in humans pertaining to exercise duration and disease risk prevention, and then shift the focus to the various biological compounds that are responsible for cardioprotection. The main objectives are: 1. To review the literature addressing the different factors that induce cardioprotective adaptive responses during/after exercise, and 2. To shed light on gaps in the literature where future studies will advance the field. The first half of our review will focus on circulating factors released during exercise that converge on the heart, with the latter portion focusing on adaptations that occur within the heart during exercise.
What dose of exercise is needed for cardioprotection?
Although there are benefits of exercise across intensities, both epidemiological and animal studies suggest that moderate to high-intensity exercise is best for the heart. The dose-response aspect relating the quantity of exercise that results in a reduction in cardiovascular risk has been extensively investigated across a number of human epidemiological studies. In a longitudinal study Lee et al. (2000) tracked physical activity in 482 males (average 66 years of age) over a five year period and showed that energy expenditure was the key variable in reducing coronary heart disease risk. They found shorter intervals of exercise at a higher intensity provides the same protective benefit as longer intervals of exercise at a lower intensity, as long as the overall energy expenditures were equal. The study also supports the idea that exercise intensity is an important determinant of cardioprotection following an acute exercise regimen (e.g. days to weeks), and that multiple small bouts of intense exercise may have the same net result as one extended bout of exercise. Mora et al. (2007) investigated differing levels of physical activity in a group of 27,055 healthy women, determined by kcal/wk expended. They showed a dose-dependent relationship with 200-599, 600-1499 and >1500 kcal/wk groups having a 27%, 32% and 41% reduction in cardiovascular disease risk, respectively, compared to the baseline group which expended less than 200 kcal/wk. Although the authors acknowledged more research was necessary to determine the exact biological mechanisms that resulted in this protection, they found that the reduction in risk seen with increasing levels of physical activity can be explained in large part by a reduction in inflammatory/hemostatic biomarkers.
In animal studies, cardioprotection from I/R injury has been shown to occur after only a single bout of exercise and is sustainable if the exercise continues for many months (reviewed in (Frasier et al. 2011a; Quindry and Hamilton 2013)). The majority of our focus herein is on factors released during exercise itself. Long-term chronic exercise is likely a combination of acute factors (reaping the benefits of each individual exercise session) and additional adaptations that include shifted autonomic nervous system tone, heightened levels of cardioprotective proteins (described below), and beneficial hypertrophy. In terms of acute exercise, cardioprotection (reductions in myocardial infarction) is observed after moderately high-intensity exercise (>70% VO2 max) (Yamashita et al. 1999; Hoshida et al. 2002; Brown et al. 2003; French et al. 2008; Quindry et al. 2010b), consistent with the notion that higher intensity appears to be the most beneficial for the heart. In the following sections, we will describe the different factors released during exercise that initiate the protective phenotypic shift.
ADENOSINE
Adenosine is a purine nucleoside molecule that has been identified as a trigger of exercise-induced adaptations within the myocardium. Signaling occurs through four cell-surface receptors distributed heterogeneously throughout regions of the myocardium: adenosine A1, A2A, A2B, and A3 receptors (Fredholm et al. 2011). Adenosine receptor activation signals through G-protein coupled receptors (Gi, Gs, Go, and Gq) leading to the targeting of various downstream effectors and divergent regulation of cardiac function (Chandrasekera et al. 2010). During exercise cardiac adenosine levels rise proportional to increasing heart rate (Watkinson et al. 1979). A potential interplay between heart rate and adenosine release in exercise cardioprotection was demonstrated in dogs where the infarct salvage observed following intermittent bouts of tachycardia was abolished with administration of an adenosine receptor blocker (Domenech et al. 1998). Support for the cardioprotective effect of adenosine is also provided in non-exercise, non-I/R studies whereby treatment with adenosine leads to the activation of endogenous antioxidant defense systems, and the adenosine receptor antagonist theophylline abolishes this effect (Maggirwar et al. 1994; Husain and Somani 2005). Similarly, adenosine receptor blockade during exercise exacerbates post-exercise oxidative stress biomarkers (Husain and Somani 2005). Taken together, these findings indicate that the increase in heart rate during exercise leads to a transient oxidative stress which is blunted through adenosine-induced upregulation of the antioxidant defense system. However, the intermediate signaling of adenosine that may be responsible for triggering exercise cardioprotection is less well defined. Non-exercise studies suggests that A1 receptor activation reduces infarct size by priming the opening of mitochondrial potassium adenosine triphosphate (mitoKATP) channels, presumably through a PKC mediated mechanism (Sato et al. 2000). One study demonstrated that opening of mitoKATP channels may play a role in the early phase of exercise cardioprotection, as the early window of protection was abolished with channel blockade during exercise (Domenech et al. 2002). However, exercise-induced mitoKATP channel activity has not been linked to adenosine signaling and merits further research before conclusions can be drawn. Therefore, these results suggest that transient increases in adenosine levels are important for ROS buffering during acute exercise, but whether or not this is due to opening of mitoKATP channels is not known. While cardiac adenosine signaling following exercise seems to be important for the activation of redox networks, adenosine has not been established as being solely responsible for the increase in antioxidant capacity. In addition, it is also unknown if adenosine receptor blockade during consecutive exercise bouts would mitigate the upregulation in antioxidant defense systems.
One of the limitations in our understanding of adenosine as a trigger for exercise cardioprotection is the lack of knowledge pertaining to the specificity of adenosine receptor activation following exercise. As mentioned previously, there are four different adenosine receptors, and the specific subtypes activated following exercise has not been well characterized. For example, pharmacological blockade of adenosine receptors with theophylline is thought to inhibit signaling through A1 and A2A receptors (Jacobson et al. 2012). Theophylline is commonly used as an adenosine receptor blocker (Domenech et al. 1998; Husain and Somani 2005), but the specificity of its action and the downstream signaling events has not been tested in exercise-preconditioning studies. The use of non-specific pharmacological compounds is problematic from a mechanistic standpoint because adenosine receptor activation elicits divergent effects depending on the subtype of receptors activated. Further, adenosine receptors possess the ability to dimerize with other subtypes (Fredholm et al. 2011), leading to greater complexity in the biological actions of adenosine. Nonetheless, adenosine appears to exert a substantial effect on cardiac physiology and pathophysiology, but more research is needed to solidify adenosine as a required trigger for exercise cardioprotection.
OPIOIDS
Opioids are another cell-surface signaling molecule that can trigger a protective phenotype. Endorphins, enkephalins, and dynorphins predominately signal through μ-, δ-, and κ-opioid receptors respectively, each with various subtypes distributed centrally and peripherally (van den Brink et al. 2003). Pharmacological activation of κ- and δ-opioid receptors reduces infarct size, with a ‘second window of preconditioning’ similar to what is seen with exercise (Schultz et al. 1998; Fryer et al. 1999). Opioid-mediated signaling occurs throughout the nervous system, and there is evidence that striated muscle can produce preproenkephalin mRNA and peptide products (Springhorn and Claycomb 1989; Weil et al. 2006).
Several studies have examined opioids following exercise. A ten-fold increase in overall serum opioid activity immediately following exhaustive exercise has been observed in human (Rahkila et al. 1988; Schwarz and Kindermann 1992) and rodent models (Debruille et al. 1999), with release of various endorphins being most prominent following high-intensity exercise (>90% VO2max). These data are particularly interesting from a dose-response standpoint, as the opioid release occurred following near-maximal exercise, and many studies find benefit after sub-maximal exercise regimens (Brown et al. 2005a; Brown and Moore 2007; Calvert et al. 2011; Frasier et al. 2011a; Taylor and Starnes 2012; Quindry and Hamilton 2013; Powers et al. 2014).
Further support for the role of opioids in exercise-induced cardioprotection comes from studies examining blood-borne factors. Michelsen et al. (2012) recently observed infarct size reductions in isolated rabbit hearts that were perfused with human plasma dialysates conditioned by acute high-intensity exercise. Co-perfusion with a non-specific opioid antagonist reduced the infarct sparing effect (Michelsen et al. 2012), which is consistent with other studies where pre-exercise administration of the non-specific opioid antagonist naloxone/naltrexone abolished protection afforded by a 12-week exercise regimen (Dickson et al. 2008; Galvao et al. 2011).
Like adenosine mediated protection, there is evidence that opioid signaling also acts through the mitoKATP channel. In non-exercise studies, protection observed following opioid receptor activation is abolished with the mitoKATP blocker 5-HD (Fryer et al. 1999). Administration of 5-HD prior to I/R also abolishes the anti-arrhythmic effect of exercise, but opioid levels were not measured (Quindry et al. 2010b). However, unlike adenosine-mediated protection, opioid signaling may not exert its protective effects through the upregulation of antioxidant defense systems. Twenty-four hours after a five-day exercise regimen, mRNA levels of opioid precursors and receptors increased in unstressed hearts while there was no change in superoxide dismutase, HSPs, or catalase (Dickson et al. 2008). Even though these specific antioxidant gene transcripts did not change, enhanced ROS buffering cannot be ruled out because antioxidant capacity was not comprehensively analyzed. Although we are still early in our understanding of how opioids are influencing exercise cardioprotection, these preliminary studies provide rationale for their release and biological activity following exercise.
CYTOKINES
Cytokine production during exercise is another putative triggering mechanism of exercise cardioprotection that has received less attention from the scientific community. During exercise contracting muscle acts as an endocrine organ by secreting various cytokines that can facilitate downstream biological actions (Donges et al. 2014). In non-exercise studies, early work demonstrated a cardioprotective role for cytokines in I/R injury that involved lower oxidative stress during the reperfusion period (Brown et al. 1990; Eddy et al. 1992). Subsequent studies by Yamashita et al. (1999) sought to determine how exercise-induced cytokine production influenced infarct salvage. They demonstrated that administration of TNF-α and IL-1 antibodies prior to a single exercise bout abolished the early- and late-windows of cardioprotection. However, aside from the cardioprotective effects that cytokines can exert on the myocardium, there are deleterious effects as well. The discrepant findings in the literature regarding adverse and cardioprotective actions of cytokines on I/R has been reviewed (Saini et al. 2005). The cardioprotective action of cytokines appears to occur at lower concentrations, whereas higher concentrations may exert harmful effects. Moving forward more research is needed to uncover the divergent roles of cytokines on myocardial physiology before they can be implemented as therapeutic agents for I/R injury.
ADRENERGIC SIGNALING
The role of adrenergic receptor stimulation during exercise has become recognized as a part of exercise-induced cardioprotection. In response to systemic demand, β-adrenergic stimulation increases cardiac chronotropy, inotropy and lusitropy (reviewed in (Steinberg 1999)). These effects are mainly attributed to the β1-adrenergic receptor which is the predominant isoform in the heart, but β3-adrenergic receptors appear to play a contradictory role, as stimulation leads to a negative inotropic response (Tavernier et al. 2003; Napp et al. 2009). The negative inotropic effect is mediated through downstream activation of eNOS (Gauthier et al. 1998). However, the existence of a functional β3-adrenergic receptor in the human heart has recently been called into question due to the lack of selectivity of pharmacological tools used to study its function and expression (reviewed in (Michel et al. 2011)). Nonetheless, it has been postulated that β-adrenergic stimulation may trigger exercise cardioprotection by increasing NO bioavailability. β-adrenergic stimulation of cardiac tissue via the sympathetic nervous system has been shown to be important in triggering the protective phenotype, as ablation of the cardiac sympathetic nerve with topical application of phenol abolishes the infarct salvage afforded by seven days of exercise in mice (Akita et al. 2007). The authors attributed these effects to a decrease in eNOS activity because an increase in eNOS phosphorylation was not observed in mice with cardiac sympathetic nerve ablation, but was increased with exercise alone. Interestingly, the transient oxidative stress observed with exercise was also absent with cardiac sympathetic nerve ablation, indicating interplay between adrenergic stimulation, NO, and ROS in exercise cardioprotection. In another study Calvert et al. (2011) also demonstrated that adrenergic receptors play an important role in exercise cardioprotection via interaction with the NOS isoforms. Plasma catecholamine and β3-adrenergic receptor density increased following four weeks of voluntary wheel running, with no changes in the β1 and β2 isoforms. The cardioprotection against myocardial infarction observed in the voluntary wheel running mice was abolished in β3-adrenergic receptor deficient mice. Similar to the previous study that linked adrenergic signaling to increased eNOS phosphorylation, eNOS phosphorylation as well as cardiac NO metabolites were depressed in β3-adrenergic receptor deficient mice exposed to voluntary wheel running. These findings implicate adrenergic signaling as a triggering mechanism during exercise. In this regard, Calvert et al. (2011) demonstrated that a single epinephrine bolus increased eNOS phosphorylation and heart NO metabolites. Importantly, infarct salvage following voluntary wheel running was lost when NO metabolites returned to normal levels after four weeks of exercise cessation. Taken together, there is strong evidence for a role of adrenergic signaling in the triggering phase of exercise cardioprotection and the subsequent upregulation of NO bioavailability. However, more research is needed to fully characterize the specific role of β3-adrenergic receptor stimulation in NOS activation, especially in light of the fact that β2-adrenergic receptor activation has also been shown to be cardioprotective and can increase eNOS activity and NO metabolites (Bhushan et al. 2012).
NITRIC OXIDE
NO was initially thought to act only through local mediation of vasodilation due to its short half-life and high reactivity with biological substrate (Liu et al. 1998). However, more recent work implicates NO in downstream mechanisms distant from the site of production (Calvert et al. 2011; Chouchani et al. 2013; Farah et al. 2013), as well as in cardiac myocytes themselves (reviewed in (Bloch et al. 2001)). During exercise, blood flow and vascular shear stress are elevated in tissue beds with high metabolic activity, which leads to the activation of endothelial nitric oxide synthase (eNOS) and heightened release of NO (Wang et al. 1993; Sessa et al. 1994; Zhang et al. 2009; Barbosa et al. 2013). NO metabolites such as nitrite, nitrate, and nitrosothiols were once thought of as inert, but are now widely accepted as storage forms of NO that undergo inter-conversion to exert biological effects (Zweier et al. 1995; Webb et al. 2004; Bryan et al. 2005; Calvert et al. 2011). In non-exercise studies, the molecular reduction of nitrite to NO and nitrosothiols during I/R is cardioprotective (Webb et al. 2004; Bryan et al. 2007; Chouchani et al. 2013), which indicates that an increase in NO bioavailability may be an important determinant of exercise-induced cardioprotection.
Following exercise, there is an increase in eNOS activation and NO metabolites (Akita et al. 2007; Calvert et al. 2011), and when eNOS is genetically knocked out, the infarct sparing effects after seven days of exercise is abolished (Akita et al. 2007). The study also demonstrated that upregulation of eNOS during exercise was necessary for the subsequent increased activity of inducible NOS (iNOS) and the downstream infarct sparing effect of exercise (Akita et al. 2007). Others have observed an increase in iNOS activity following an acute bout of exercise, and when an iNOS inhibitor was administered prior to I/R the antiarrhythmic effect of exercise was abolished (Babai et al. 2002). However, the role of iNOS in exercise cardioprotection has been called into question due to the interspecies variability in expression patterns and a lack of increase following various exercise regimens (Quindry et al. 2010a; Calvert et al. 2011). More recently Farah et al. (2013) demonstrated a role for eNOS in exercise cardioprotection in rats after five weeks of training. Following the exercise regimen, phosphorylation of eNOS and levels of s-nitrosylated proteins and nitrite were increased in the exercise group. Perfusion with a global NOS inhibitor prior to and immediately after I/R abolished the infarct sparing and mechanical recovery observed with exercise. They also demonstrated that eNOS uncoupling during the reperfusion period was required for the cardioprotection. However, not all groups demonstrate an essential role for NO in exercise cardioprotection. Taylor et al. (2007) administered a global NOS inhibitor prior to two days of exercise with the idea that cardioprotection would be lost. However, the beneficial effects of exercise on mechanical recovery and LDH release after I/R in rats were not different than with exercise alone (Taylor et al. 2007). The main difference in these studies is the timing of NOS inhibition (before exercise vs before I/R), and the duration of the exercise regimen. The study by Taylor et al. (2007) provides evidence against a role for NO production during exercise as a triggering mechanism for cardioprotection. However, NO production during exercise may not be responsible for cardioprotection per se, rather the increase in NO bioavailability and increase in eNOS activation (phosphorylation) seems to be more important in the cardioprotective phenotype. A mechanism whereby NO production can increase after exercise has been demonstrated. Following acute exercise, circulating bradykinin levels increase (Blais et al. 1999), stimulating the production of NO and NO metabolites (Zhou et al. 2010). Furthermore, bradykinin has been demonstrated to mediate its anti-arrhythmic effects through liberation of NO during I/R (Vegh and Parratt 2005). Given the discrepant findings, a few questions are left that need to be addressed moving forward: What is the locus of NO production that leads to an increase in NO metabolites (endothelium vs cardiac myocytes)? What are the temporal characteristics of NO production during and/or following exercise? When precisely does the cardioprotective phenotype become evident? In response to the latter, most studies indicate that storage forms of NO precipitate their cardioprotective effects during reperfusion. Clearly more work is needed to definitively determine the role of NO production during/after exercise and how this affects NO metabolite accumulation en route to cardioprotection.
ADENOSINE MONOPHOSPHATE-ACTIVATED PROTEIN KINASE
Cardiac myocytes are densely packed with mitochondria in order to support cellular energetic requirements. In the healthy heart, the heightened rate of ATP hydrolysis during exercise increases mitochondrial respiration, ultimately allowing healthy myocytes to efficiently match ATP generation to cardiac workload. While cellular ATP:ADP ratios remain constant, AMP levels are thought to rise with increasing exercise intensity, leading to the activation of AMPK in cardiac muscle (Frederich and Balschi 2002; Coven et al. 2003). In this context, the activation of AMPK stimulates catabolic processes and down regulates anabolism allowing the cell to regulate metabolism for the production of ATP (Coven et al. 2003). AMPK has been deemed as one of the energy sensors of the cell and its activity increases by phosphorylation within 10 minutes of the onset of moderate and high intensity exercise (Coven et al. 2003). AMPK has also been shown to be important in post-ischemic cardiac injury, with exacerbated injury in transgenic mice expressing a dominant negative kinase dead α subunit of AMPK (Russell et al. 2004). Canonical AMPK signaling increases glucose and lipid oxidation, which is essential for replenishing ATP following an ischemic period.
In addition to increasing catabolism, AMPK has been shown to play a role in ischemic preconditioning by regulating sarcolemmal KATP channel trafficking and activity (Sukhodub et al. 2007). These studies suggest an important role for AMPK activity following ischemia/reperfusion (I/R), but the extent to which AMPK influences exercise cardioprotection has received less attention. Although studies have consistently shown that exercise increases the phosphorylation of AMPK (Coven et al. 2003; Ogura et al. 2011), AMPK has not been shown to be crucial for exercise adaptations. Similar levels of exercise can be attained in transgenic mice expressing a cardiac-specific dominant-negative AMPKα2 subunit (Musi et al. 2005). Following 30 minutes of exercise, transgenic mice had similar cardiac glycogen and ATP levels as wild-type controls. A similar metabolic profile between the wild type and transgenic mice indicates that AMPK may not be crucial for enhanced cardiac metabolism, and that other overlapping pathways can help meet energy requirements during increased demand. Although AMPK is an attractive target for the cell to regulate its energy needs during metabolic stress, there is a gap in the literature linking exercise-induced AMPK activation with cardioprotection. More research is required to definitively determine if/how exercise influences AMPK activity in the heart, and whether or not these changes modify cardioprotection.
REACTIVE OXYGEN SPECIES
Cardiac ROS are another potential candidate involved in exercise cardioprotection, as well as other preconditioning stimuli such as ischemic and pharmacological preconditioning (Garlid et al. 2013). A large body of literature suggests that exercise induces a transient oxidative stress that leads to upregulation in antioxidant defense systems; however the locus of ROS production and downstream effectors during exercise remains unclear. In this section we will focus on evidence for the role of mitochondrial ROS in exercise cardioprotection, and cytosolic ROS in the following section.
ROS have received considerable attention in the cardiac literature due to their role in pathologies like I/R injury, heart failure, and cardiomyopathies. However, a growing body of literature suggests that ROS exert hormesis, where transient bursts of ROS leads to favorable adaptive redox signaling. Cellular ROS can act as second messengers in downstream signaling by altering the activity of redox sensitive enzymes throughout the cytosol and/or mitochondria of cardiac myocytes (Sadoshima 2006). Similar mechanisms may occur when transient bursts of ROS are generated during exercise (Bolli 1988; Hamilton et al. 2004; Dabkowski et al. 2008). Following acute exercise there is an alteration in cellular redox status towards a more oxidized environment which may act as a signal to activate endogenous protective mechanisms (Nelson et al. 2011; Frasier et al. 2013).
There is general consensus that an increase in antioxidant enzymes is responsible for a large portion of exercise cardioprotection, and transient oxidative stress with exercise may play a role in this adaptation. Evidence for this has been provided by several groups who have observed increases in key antioxidant enzymes following exercise (Yamashita et al. 1999; Demirel et al. 2001; Hamilton et al. 2001; Quindry et al. 2010b; Frasier et al. 2011b; Lee et al. 2012; Frasier et al. 2013). Studies in favor of this hypothesis have shown that administration of antioxidants prior to exercise abolishes infarct salvage (Yamashita et al. 1999; Akita et al. 2007) and prevents exercise-induced improvements in cardiac performance (Nelson et al. 2011). However, another study indicated that ROS generated during exercise were not required for functional recovery following I/R (Taylor and Starnes 2012). The antioxidant frequently used in these studies was N-(2-mercaptopropionyl)glycine) (MPG), which was administered intraperitoneally 10-30 minutes prior to exercise. An important note to consider is that MPG has been shown to have higher specificity for hydroxyl radicals rather than hydrogen peroxide (H2O2) and superoxide (Bolli et al. 1989), indicating that not all ROS signaling is abolished with treatment. In addition, MPG has a plasma half-life of approximately 7 minutes (Horwitz et al. 1994), making it difficult to interpret how effective the treatment was at scavenging ROS during hour-long exercise bouts. These methodological differences make it difficult to directly compare their results (i.e. differences in species, duration of I/R, duration of exercise, measurement of injury, the timing of the administration of antioxidants, and in vivo versus ex vivo experiments).
Mitochondrial ROS
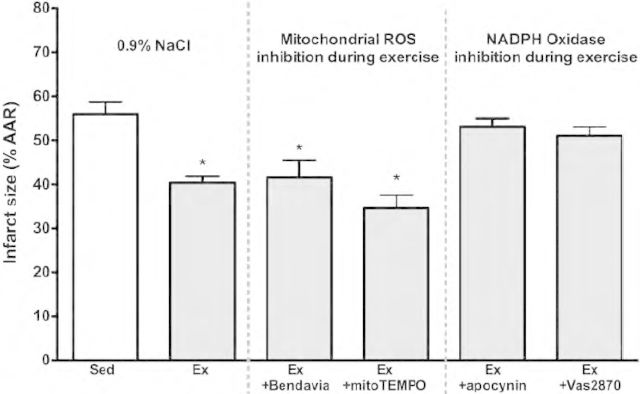
ROS signaling is a highly regulated and localized process, implying that the origin of ROS generated during exercise may be extremely important. Although mitochondrial ROS are thought to play a central role in ischemic preconditioning (Baines et al. 1997; Pain et al. 2000; Kloner and Jennings 2001), there is a paucity of evidence suggesting a role in exercise preconditioning. Frasier et al. (2013) recently found that the locus of ROS production during exercise is not mitochondrial in origin. As shown in Figure!1, exercise cardioprotection was not lost when administering agents that reduce mitochondrial ROS prior to exercise (mito TEMPO and Bendavia). This indicates that extramitochondrial-derived ROS may be responsible for redox signaling following exercise.
FIGURE 1.
Reductions in infarct size are abolished by inhibiting NADPH Oxidase (with pre-exercise treatment of apocynin or Vas2870) during exercise. Inhibition of mitochondrial ROS during exercise (with pre-exercise administration of TEMPO or the mitochondria-targeting peptide Bendavia) had no effect on exercise cardioprotection. Figure reproduced from Frasier et al., Cardiovascular Research 2013, with permission (pending).
Monoamine oxidase-A (MAO-A) is another potential site for mitochondrial ROS production. MAO-A is located on the outer mitochondrial membrane and catalyzes the oxidative deamination of neurotransmitters such as norepinephrine and serotonin while generating H2O2 as a byproduct in the reaction. A recent review highlights the importance of MAO-A in pathological states such as heart failure and I/R (Kaludercic et al. 2011). Accumulation of serotonin released by platelets during I/R can lead to the production of H2O2 and subsequent apoptotic signaling cascades (Bianchi et al. 2005). Recent findings indicate that exercise leads to down-regulation of MAO-A (Kavazis et al. 2009), which may play a role in the attenuation of I/R damage associated with exercise cardioprotection. Moreover, these findings indicate that there is likely a reduction in mitochondrial ROS production with exercise, given the decrease in MAO-A expression and increases in the activity of key antioxidants such as MnSOD and glutathione reductase (GR) (Quindry et al. 2010b; Frasier et al. 2013). A decrease in cardiac mitochondrial MAO-A would theoretically dampen the oxidative burden imposed on the cell, not only during exercise, but also during thrombus formation and subsequent I/R injury. A mechanism for the decrease in MAO-A expression following exercise has not been investigated and therefore the triggering event for this adaptation is purely speculative. Perhaps acute increases in cardiac sympathetic nerve stimulation and increasing norepinephrine levels during exercise play a role in downstream silencing of MAO-A through non-canonical adrenergic pathways (Vidal et al. 2012). Cardiac sympathetic stimulation increases contractility and myocardial stretch during exercise, which in and of itself may trigger a cardioprotective phenotype through elevated cytosolic ROS production (Frasier et al. 2013; Ward et al. 2014). Furthermore, cardiac sympathetic nerve ablation has been shown to abolish the infarct sparing effect of exercise, but this was not linked to silencing of MAO-A expression (Akita et al. 2007). A hypothetical adrenergic/MAO-A axis scenario opens up an exciting area of research to explore mechanisms controlling MAO-A expression in cardiac tissue during normal physiological as well as pathophysiological states. We will further expand on the topic of stretch-induced activation of cardioprotection in the next section.
Cytosolic ROS
Free-radical generating enzyme systems outside of the mitochondria have also received considerable interest in normal physiology as well as in pathological states such as I/R injury (McCord 1985; Bolli 1988; Misra et al. 2009) and heart failure (Tsutsui et al. 2011; Harzand et al. 2012). Sources of extramitochondrial-derived ROS in cardiac myocytes include xanthine oxidase, NADPH oxidase, and uncoupled nitric oxide synthase. Of these, the NADPH oxidase (NOX2 in particular) complex generates ROS in a highly localized manner in the sarcolemmal and t-tubule membranes during physiological stretch (Sanchez et al. 2008; Prosser et al. 2011). Myocardial contraction and wall stress increases during exercise as a function of heart rate and adrenergic signaling. The increased inotropic and chronotropic state is an autoregulatory mechanism that allows for tight regulation of blood pressure and delivery of nutrients to metabolically active tissue. Recent work indicates that the sarcolemmal NOX2-generated ROS system plays a central role in this phenomenon. NOX2-generated ROS imposes redox signaling through ryanodine receptors leading to increased calcium release and subsequent contractile activity (Sanchez et al. 2008; Donoso et al. 2014). Stretch induction through the microtubule network and NOX2 activation has been termed X-ROS signaling (Prosser et al. 2011). X-ROS signaling describes the transfer of a mechanical to a chemical signal throughout the heart via the microtubule system, leading to assembly of the NOX2 ROS generating complex. Recently, several independent groups have established a role for NOX2 as a potential trigger for the cardioprotective phenotype associated with exercise (Sanchez et al. 2008; Frasier et al. 2013).
As mentioned previously, a critical threshold of exercise intensity appears to be important for cardioprotection, and at higher exercise intensities myocardial contraction increases in conjunction. In line with the X-ROS signaling hypothesis, increased inotropy and myocardial stretch during exercise may lead to activation of NOX2 and perhaps downstream adaptations. We and others have demonstrated that inhibition of NOX2 prior to exercise abolishes the infarct salvage of early and late phases of exercise cardioprotection (Sanchez et al. 2008; Frasier et al. 2013). Furthermore, the upregulation of GR activity that is typically observed following exercise (Venditti and Di Meo 1996; Ramires and Ji 2001; Kakarla et al. 2005; Frasier et al. 2011b) is also abolished immediately and 24 hours after the exercise bout when NOX2 is inhibited during exercise (Frasier et al. 2013). GR is a central enzyme involved in cellular redox control by utilizing NADPH to convert oxidized glutathione to the reduced form. Therefore, increasing GR activity allows the cell to maintain the glutathione pool in the reduced state, thus providing a greater buffering power during oxidative insults. During an exercise bout mechanical stretch of the myocardium increases, leading to NOX2-generated ROS and activation of GR (Frasier et al. 2013). ROS signaling through GR may be a mechanism where GR acts as a sensor during oxidative shifts of the redox environment, leading to upregulation of endogenous defense systems. Future studies examining the time frame of GR activation and sustainability of protection will shed light on signaling between NOX2 and GR during the cardioprotective window of exercise. Also, studies that determine the importance of GR compartmentalization are warranted, particularly with regard to cytosolic and/or mitochondrial GR pools involved in this adaptive signaling network (Kang et al. 2014). While it seems apparent that exercise upregulates redox buffering capacity, more research is needed to definitively determine if transient bursts of ROS during exercise act as a signal to trigger downstream cardioprotection.
CONCLUSIONS
We have described a number of circulating and intrinsic factors postulated to induce cardioprotective signaling with exercise. These factors converge on the myocardium, and result in downstream adaptations that characterize the protective phenotype. Subsequent investigation into these downstream effects using novel approaches will greatly advance the field. For example, ROS production during exercise is an intriguing factor that leads to both post-translational modifications to existing proteins in the short-term, as well as altered protein expression on a longer time-scale. Given that 21,000 to 42,000 thiols in the proteome can contribute to the integration of metabolic function through redox signaling (Jones 2008), further exploration of the redox hypothesis in the context of exercise adaptations is warranted. The convergent effects of cellular ROS production and elevated levels of cell-signaling molecules such as adenosine, NO, cytokines, and catecholamines during elevated workloads transduce the exercise stimulus that culminates into a hormetic cardiac response. Inhibition of any one of these putative triggers can dampen the cardioprotective phenotypic switch observed with exercise, but ultimately these adaptations lead to tolerance to I/R injury characterized by lower arrhythmia and decreased myocardial infarction. Given that exercise is known to confer protection in humans, future studies that continue to advance our understanding of the intrinsic factors responsible for evoking this protective phenotype may ultimately pave the way for novel therapies to reduce the burden of acute coronary syndromes.
REFERENCES
- Akita Y, Otani H, Matsuhisa S, Kyoi S, Enoki C, Hattori R, Imamura H, Kamihata H, Kimura Y, Iwasaka T. 2007. Exercise-induced activation of cardiac sympathetic nerve triggers cardioprotection via redox-sensitive activation of eNOS and upregulation of iNOS. Am J Physiol Heart Circ Physiol 292:H2051–9 [DOI] [PubMed] [Google Scholar]
- Babai L, Szigeti Z, Parratt JR, Vegh A. 2002. Delayed cardioprotective effects of exercise in dogs are aminoguanidine sensitive: possible involvement of nitric oxide. Clin Sci (Lond) 102:435–45 [PubMed] [Google Scholar]
- Baines CP, Goto M, Downey JM. 1997. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol 29:207–16 [DOI] [PubMed] [Google Scholar]
- Barbosa VA, Luciano TF, Marques SO, Vitto MF, Souza DR, Silva LA, Santos JP, Moreira JC, Dal-Pizzol F, Lira FS, Pinho RA, De Souza CT. 2013. Acute exercise induce endothelial nitric oxide synthase phosphorylation via Akt and AMP-activated protein kinase in aorta of rats: Role of reactive oxygen species. Int J Cardiol 167:2983–8 [DOI] [PubMed] [Google Scholar]
- Bhushan S, Kondo K, Predmore BL, Zlatopolsky M, King AL, Pearce C, Huang H, Tao YX, Condit ME, Lefer DJ. 2012. Selective beta2-adrenoreceptor stimulation attenuates myocardial cell death and preserves cardiac function after ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol 32:1865–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, Seguelas MH, Nistri S, Colucci W, Leducq N, Parini A. 2005. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation 112:3297–305 [DOI] [PubMed] [Google Scholar]
- Blais C, Jr, Adam A, Massicotte D, Peronnet F. 1999. Increase in blood bradykinin concentration after eccentric weight-training exercise in men. J Appl Physiol (1985) 87:1197–201 [DOI] [PubMed] [Google Scholar]
- Bloch W, Addicks K, Hescheler J, Fleischmann BK. 2001. Nitric oxide synthase expression and function in embryonic and adult cardiomyocytes. Microsc Res Tech 55:259–69 [DOI] [PubMed] [Google Scholar]
- Bolli R. 1988. Oxygen-derived free radicals and postischemic myocardial dysfunction (“stunned myocardium”). J Am Coll Cardiol 12:239–49 [DOI] [PubMed] [Google Scholar]
- Bolli R, Jeroudi MO, Patel BS, Aruoma OI, Halliwell B, Lai EK, McCay PB. 1989. Marked reduction of free radical generation and contractile dysfunction by antioxidant therapy begun at the time of reperfusion. Evidence that myocardial “stunning” is a manifestation of reperfusion injury. Circ Res 65:607–22 [DOI] [PubMed] [Google Scholar]
- Bowles DK, Farrar RP, Starnes JW. 1992. Exercise training improves cardiac function after ischemia in the isolated, working rat heart. Am J Physiol 263:H804–9 [DOI] [PubMed] [Google Scholar]
- Brown DA, Chicco AJ, Jew KN, Johnson MS, Lynch JM, Watson PA, Moore RL. 2005a. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. J Physiol 569:913–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Jew KN, Sparagna GC, Musch TI, Moore RL. 2003. Exercise training preserves coronary flow and reduces infarct size after ischemia-reperfusion in rat heart. J Appl Physiol (1985) 95:2510–8 [DOI] [PubMed] [Google Scholar]
- Brown DA, Lynch JM, Armstrong CJ, Caruso NM, Ehlers LB, Johnson MS, Moore RL. 2005b. Susceptibility of the heart to ischaemia-reperfusion injury and exercise-induced cardioprotection are sex-dependent in the rat. J Physiol 564:619–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Moore RL. 2007. Perspectives in innate and acquired cardioprotection: cardioprotection acquired through exercise. J Appl Physiol (1985) 103:1894–9 [DOI] [PubMed] [Google Scholar]
- Brown JM, White CW, Terada LS, Grosso MA, Shanley PF, Mulvin DW, Banerjee A, Whitman GJ, Harken AH, Repine JE. 1990. Interleukin 1 pretreatment decreases ischemia/reperfusion injury. Proc Natl Acad Sci U S A 87:5026–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. 2007. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A 104:19144–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, Maloney RE, Bharti A, Rodriguez J, Feelisch M. 2005. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol 1:290–7 [DOI] [PubMed] [Google Scholar]
- Calvert JW, Condit ME, Aragon JP, Nicholson CK, Moody BF, Hood RL, Sindler AL, Gundewar S, Seals DR, Barouch LA, Lefer DJ. 2011. Exercise protects against myocardial ischemia-reperfusion injury via stimulation of beta(3)-adrenergic receptors and increased nitric oxide signaling: role of nitrite and nitrosothiols. Circ Res 108:1448–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekera PC, McIntosh VJ, Cao FX, Lasley RD. 2010. Differential effects of adenosine A2a and A2b receptors on cardiac contractility. Am J Physiol Heart Circ Physiol 299:H2082–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, Ding S, James AM, Cocheme HM, Reinhold J, Lilley KS, Partridge L, Fearnley IM, Robinson AJ, Hartley RC, Smith RA, Krieg T, Brookes PS, Murphy MP. 2013. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med 19:753–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coven DL, Hu X, Cong L, Bergeron R, Shulman GI, Hardie DG, Young LH. 2003. Physiological role of AMP-activated protein kinase in the heart: graded activation during exercise. Am J Physiol Endocrinol Metab 285:E629–36 [DOI] [PubMed] [Google Scholar]
- Dabkowski ER, Williamson CL, Hollander JM. 2008. Mitochondria-specific transgenic overexpression of phospholipid hydroperoxide glutathione peroxidase (GPx4) attenuates ischemia/reperfusion-associated cardiac dysfunction. Free Radic Biol Med 45:855–65 [DOI] [PubMed] [Google Scholar]
- Debruille C, Luyckx M, Ballester L, Brunet C, Odou P, Dine T, Gressier B, Cazin M, Cazin JC. 1999. Serum opioid activity after physical exercise in rats. Physiol Res 48:129–33 [PubMed] [Google Scholar]
- Demirel HA, Powers SK, Zergeroglu MA, Shanely RA, Hamilton K, Coombes J, Naito H. 2001. Short-term exercise improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. J Appl Physiol (1985) 91:2205–12 [DOI] [PubMed] [Google Scholar]
- Dickson EW, Hogrefe CP, Ludwig PS, Ackermann LW, Stoll LL, Denning GM. 2008. Exercise enhances myocardial ischemic tolerance via an opioid receptor-dependent mechanism. Am J Physiol Heart Circ Physiol 294:H402–8 [DOI] [PubMed] [Google Scholar]
- Domenech R, Macho P, Schwarze H, Sanchez G. 2002. Exercise induces early and late myocardial preconditioning in dogs. Cardiovasc Res 55:561–6 [DOI] [PubMed] [Google Scholar]
- Domenech RJ, Macho P, Velez D, Sanchez G, Liu X, Dhalla N. 1998. Tachycardia preconditions infarct size in dogs: role of adenosine and protein kinase C. Circulation 97:786–94 [DOI] [PubMed] [Google Scholar]
- Donges CE, Duffield R, Smith GC, Short MJ, Edge JA. 2014. Cytokine mRNA expression responses to resistance, aerobic, and concurrent exercise in sedentary middle-aged men. Appl Physiol Nutr Metab 39:130–7 [DOI] [PubMed] [Google Scholar]
- Donoso P, Finkelstein JP, Montecinos L, Said M, Sanchez G, Vittone L, Bull R. 2014. Stimulation of NOX2 in isolated hearts reversibly sensitizes RyR2 channels to activation by cytoplasmic calcium. J Mol Cell Cardiol 68:38–46 [DOI] [PubMed] [Google Scholar]
- Eddy LJ, Goeddel DV, Wong GH. 1992. Tumor necrosis factor-alpha pretreatment is protective in a rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun 184:1056–9 [DOI] [PubMed] [Google Scholar]
- Farah C, Kleindienst A, Bolea G, Meyer G, Gayrard S, Geny B, Obert P, Cazorla O, Tanguy S, Reboul C. 2013. Exercise-induced cardioprotection: a role for eNOS uncoupling and NO metabolites. Basic Res Cardiol 108:389. [DOI] [PubMed] [Google Scholar]
- Frasier CR, Moore RL, Brown DA. 2011a. Exercise-induced cardiac preconditioning: how exercise protects your achy-breaky heart. J Appl Physiol 111:905–15 [DOI] [PubMed] [Google Scholar]
- Frasier CR, Moukdar F, Patel HD, Sloan RC, Stewart LM, Alleman RJ, La Favor JD, Brown DA. 2013. Redox-dependent increases in glutathione reductase and exercise preconditioning: role of NADPH oxidase and mitochondria. Cardiovasc Res 98:47–55 [DOI] [PubMed] [Google Scholar]
- Frasier CR, Sloan RC, Bostian PA, Gonzon MD, Kurowicki J, Lopresto SJ, Anderson EJ, Brown DA. 2011b. Short-term exercise preserves myocardial glutathione and decreases arrhythmias after thiol oxidation and ischemia in isolated rat hearts. J Appl Physiol 111:1751–9 [DOI] [PubMed] [Google Scholar]
- Frederich M, Balschi JA. 2002. The relationship between AMP-activated protein kinase activity and AMP concentration in the isolated perfused rat heart. J Biol Chem 277:1928–32 [DOI] [PubMed] [Google Scholar]
- Fredholm BB, AP IJ, Jacobson KA, Linden J, Muller CE. 2011. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev 63:1–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JP, Hamilton KL, Quindry JC, Lee Y, Upchurch PA, Powers SK. 2008. Exercise-induced protection against myocardial apoptosis and necrosis: MnSOD, calcium-handling proteins, and calpain. FASEB J 22:2862–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer RM, Hsu AK, Eells JT, Nagase H, Gross GJ. 1999. Opioid-induced second window of cardioprotection: potential role of mitochondrial KATP channels. Circ Res 84:846–51 [DOI] [PubMed] [Google Scholar]
- Galvao TF, Matos KC, Brum PC, Negrao CE, Luz PL, Chagas AC. 2011. Cardioprotection conferred by exercise training is blunted by blockade of the opioid system. Clinics (Sao Paulo) 66:151–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlid AO, Jaburek M, Jacobs JP, Garlid KD. 2013. Mitochondrial reactive oxygen species: which ROS signals cardioprotection? Am J Physiol Heart Circ Physiol 305:H960–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier C, Leblais V, Kobzik L, Trochu JN, Khandoudi N, Bril A, Balligand JL, Le Marec H. 1998. The negative inotropic effect of beta3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J Clin Invest 102:1377–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamalainen H, Luurila OJ, Kallio V, Knuts LR. 1995. Reduction in sudden deaths and coronary mortality in myocardial infarction patients after rehabilitation. 15 year follow-up study. Eur Heart J 16:1839–44 [DOI] [PubMed] [Google Scholar]
- Hamilton KL, Powers SK, Sugiura T, Kim S, Lennon S, Tumer N, Mehta JL. 2001. Short-term exercise training can improve myocardial tolerance to I/R without elevation in heat shock proteins. Am J Physiol Heart Circ Physiol 281:H1346–52 [DOI] [PubMed] [Google Scholar]
- Hamilton KL, Quindry JC, French JP, Staib J, Hughes J, Mehta JL, Powers SK. 2004. MnSOD antisense treatment and exercise-induced protection against arrhythmias. Free Radic Biol Med 37:1360–8 [DOI] [PubMed] [Google Scholar]
- Harzand A, Tamariz L, Hare JM. 2012. Uric acid, heart failure survival, and the impact of xanthine oxidase inhibition. Congest Heart Fail 18:179–82 [DOI] [PubMed] [Google Scholar]
- Horwitz LD, Fennessey PV, Shikes RH, Kong Y. 1994. Marked reduction in myocardial infarct size due to prolonged infusion of an antioxidant during reperfusion. Circulation 89:1792–801 [DOI] [PubMed] [Google Scholar]
- Hoshida S, Yamashita N, Otsu K, Hori M. 2002. Repeated physiologic stresses provide persistent cardioprotection against ischemia-reperfusion injury in rats. J Am Coll Cardiol 40:826–31 [DOI] [PubMed] [Google Scholar]
- Husain K, Somani SM. 2005. Interaction of exercise and adenosine receptor agonist and antagonist on rat heart antioxidant defense system. Mol Cell Biochem 270:209–14 [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Balasubramanian R, Deflorian F, Gao ZG. 2012. G protein-coupled adenosine (P1) and P2Y receptors: ligand design and receptor interactions. Purinergic Signal 8:419–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP. 2008. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol 295:C849–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakarla P, Vadluri G, Reddy KS, Leeuwenburgh C. 2005. Vulnerability of the mid aged rat myocardium to the age-induced oxidative stress: influence of exercise training on antioxidant defense system. Free Radic Res 39:1211–7 [DOI] [PubMed] [Google Scholar]
- Kaludercic N, Carpi A, Menabo R, Di Lisa F, Paolocci N. 2011. Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochim Biophys Acta 1813:1323–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang PT, Chen CL, Zen P, Guarini G, Chen YR. 2014. BCNU-Induced gR2 DEFECT mediates S-glutathionylation OF Complex I and RESPIRATORY uncoupling in myocardium. Biochem Pharmacol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavazis AN, Alvarez S, Talbert E, Lee Y, Powers SK. 2009. Exercise training induces a cardioprotective phenotype and alterations in cardiac subsarcolemmal and intermyofibrillar mitochondrial proteins. Am J Physiol Heart Circ Physiol 297:H144–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloner RA, Jennings RB. 2001. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 2. Circulation 104:3158–67 [DOI] [PubMed] [Google Scholar]
- Lee IM, Sesso HD, Paffenbarger RS., Jr 2000. Physical activity and coronary heart disease risk in men: does the duration of exercise episodes predict risk? Circulation 102:981–6 [DOI] [PubMed] [Google Scholar]
- Lee Y, Min K, Talbert EE, Kavazis AN, Smuder AJ, Willis WT, Powers SK. 2012. Exercise protects cardiac mitochondria against ischemia-reperfusion injury. Med Sci Sports Exerc 44:397–405 [DOI] [PubMed] [Google Scholar]
- Lennon SL, Quindry J, Hamilton KL, French J, Staib J, Mehta JL, Powers SK. 2004. Loss of exercise-induced cardioprotection after cessation of exercise. J Appl Physiol (1985) 96:1299–305 [DOI] [PubMed] [Google Scholar]
- Liu X, Miller MJ, Joshi MS, Sadowska-Krowicka H, Clark DA, Lancaster JR., Jr 1998. Diffusion-limited reaction of free nitric oxide with erythrocytes. J Biol Chem 273:18709–13 [DOI] [PubMed] [Google Scholar]
- Maggirwar SB, Dhanraj DN, Somani SM, Ramkumar V. 1994. Adenosine acts as an endogenous activator of the cellular antioxidant defense system. Biochem Biophys Res Commun 201:508–15 [DOI] [PubMed] [Google Scholar]
- McCord JM. 1985. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 312:159–63 [DOI] [PubMed] [Google Scholar]
- Michel MC, Harding SE, Bond RA. 2011. Are there functional beta(3)-adrenoceptors in the human heart? Br J Pharmacol 162:817–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen MM, Stottrup NB, Schmidt MR, Lofgren B, Jensen RV, Tropak M, St-Michel EJ, Redington AN, Botker HE. 2012. Exercise-induced cardioprotection is mediated by a bloodborne, transferable factor. Basic Res Cardiol 107:260. [DOI] [PubMed] [Google Scholar]
- Misra MK, Sarwat M, Bhakuni P, Tuteja R, Tuteja N. 2009. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit 15:RA209–219 [PubMed] [Google Scholar]
- Mora S, Cook N, Buring JE, Ridker PM, Lee IM. 2007. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation 116:2110–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musi N, Hirshman MF, Arad M, Xing Y, Fujii N, Pomerleau J, Ahmad F, Berul CI, Seidman JG, Tian R, Goodyear LJ. 2005. Functional role of AMP-activated protein kinase in the heart during exercise. FEBS Lett 579:2045–50 [DOI] [PubMed] [Google Scholar]
- Napp A, Brixius K, Pott C, Ziskoven C, Boelck B, Mehlhorn U, Schwinger RH, Bloch W. 2009. Effects of the beta3-adrenergic agonist BRL 37344 on endothelial nitric oxide synthase phosphorylation and force of contraction in human failing myocardium. J Card Fail 15:57–67 [DOI] [PubMed] [Google Scholar]
- Nelson MJ, Harris MB, Boluyt MO, Hwang HS, Starnes JW. 2011. Effect of N-2-mercaptopropionyl glycine on exercise-induced cardiac adaptations. Am J Physiol Regul Integr Comp Physiol 300:R993–R1000 [DOI] [PubMed] [Google Scholar]
- Oberman A. 1985. Exercise and the primary prevention of cardiovascular disease. Am J Cardiol 55:10D–20D [DOI] [PubMed] [Google Scholar]
- Ogura Y, Iemitsu M, Naito H, Kakigi R, Kakehashi C, Maeda S, Akema T. 2011. Single bout of running exercise changes LC3-II expression in rat cardiac muscle. Biochem Biophys Res Commun 414:756–60 [DOI] [PubMed] [Google Scholar]
- Pain T, Yang XM, Critz SD, Yue Y, Nakano A, Liu GS, Heusch G, Cohen MV, Downey JM. 2000. Opening of mitochondrial K(ATP) channels triggers the preconditioned state by generating free radicals. Circ Res 87:460–6 [DOI] [PubMed] [Google Scholar]
- Powers SK, Smuder AJ, Kavazis AN, Quindry JC. 2014. Mechanisms of exercise-induced cardioprotection. Physiology (Bethesda) 29:27–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser BL, Ward CW, Lederer WJ. 2011. X-ROS signaling: rapid mechano-chemo transduction in heart. Science 333:1440–5 [DOI] [PubMed] [Google Scholar]
- Quindry JC, French J, Hamilton KL, Lee Y, Selsby J, Powers S. 2010a. Exercise does not increase cyclooxygenase-2 myocardial levels in young or senescent hearts. J Physiol Sci 60:181–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quindry JC, Hamilton KL. 2013. Exercise and cardiac preconditioning against ischemia reperfusion injury. Curr Cardiol Rev 9:220–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quindry JC, Schreiber L, Hosick P, Wrieden J, Irwin JM, Hoyt E. 2010b. Mitochondrial KATP channel inhibition blunts arrhythmia protection in ischemic exercised hearts. Am J Physiol Heart Circ Physiol 299:H175–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahkila P, Hakala E, Alen M, Salminen K, Laatikainen T. 1988. Beta-endorphin and corticotropin release is dependent on a threshold intensity of running exercise in male endurance athletes. Life Sci 43:551–8 [DOI] [PubMed] [Google Scholar]
- Ramires PR, Ji LL. 2001. Glutathione supplementation and training increases myocardial resistance to ischemia-reperfusion in vivo. Am J Physiol Heart Circ Physiol 281:H679–88 [DOI] [PubMed] [Google Scholar]
- Russell RR, 3rd, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. 2004. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest 114:495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J. 2006. Redox regulation of growth and death in cardiac myocytes. Antioxid Redox Signal 8:1621–4 [DOI] [PubMed] [Google Scholar]
- Saini HK, Xu YJ, Zhang M, Liu PP, Kirshenbaum LA, Dhalla NS. 2005. Role of tumour necrosis factor-alpha and other cytokines in ischemia-reperfusion-induced injury in the heart. Exp Clin Cardiol 10:213–22 [PMC free article] [PubMed] [Google Scholar]
- Sanchez G, Escobar M, Pedrozo Z, Macho P, Domenech R, Hartel S, Hidalgo C, Donoso P. 2008. Exercise and tachycardia increase NADPH oxidase and ryanodine receptor-2 activity: possible role in cardioprotection. Cardiovasc Res 77:380–6 [DOI] [PubMed] [Google Scholar]
- Sato T, Sasaki N, O’Rourke B, Marban E. 2000. Adenosine primes the opening of mitochondrial ATP-sensitive potassium channels: a key step in ischemic preconditioning? Circulation 102:800–5 [DOI] [PubMed] [Google Scholar]
- Schultz Je-J, Hsu AK, Nagase H, Gross GJ. 1998. TAN-67, a delta 1-opioid receptor agonist, reduces infarct size via activation of Gi/o proteins and KATP channels. Am J Physiol 274:H909–14 [DOI] [PubMed] [Google Scholar]
- Schwarz L, Kindermann W. 1992. Changes in beta-endorphin levels in response to aerobic and anaerobic exercise. Sports Med 13:25–36 [DOI] [PubMed] [Google Scholar]
- Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. 1994. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res 74:349–53 [DOI] [PubMed] [Google Scholar]
- Springhorn JP, Claycomb WC. 1989. Preproenkephalin mRNA expression in developing rat heart and in cultured ventricular cardiac muscle cells. Biochem J 258:73–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starnes JW, Taylor RP. 2007. Exercise-induced cardioprotection: endogenous mechanisms. Med Sci Sports Exerc 39:1537–43 [DOI] [PubMed] [Google Scholar]
- Steinberg SF. 1999. The molecular basis for distinct beta-adrenergic receptor subtype actions in cardiomyocytes. Circ Res 85:1101–11 [DOI] [PubMed] [Google Scholar]
- Sukhodub A, Jovanovic S, Du Q, Budas G, Clelland AK, Shen M, Sakamoto K, Tian R, Jovanovic A. 2007. AMP-activated protein kinase mediates preconditioning in cardiomyocytes by regulating activity and trafficking of sarcolemmal ATP-sensitive K(+) channels. J Cell Physiol 210:224–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernier G, Toumaniantz G, Erfanian M, Heymann MF, Laurent K, Langin D, Gauthier C. 2003. beta3-Adrenergic stimulation produces a decrease of cardiac contractility ex vivo in mice overexpressing the human beta3-adrenergic receptor. Cardiovasc Res 59:288–96 [DOI] [PubMed] [Google Scholar]
- Taylor RP, Harris MB, Starnes JW. 1999. Acute exercise can improve cardioprotection without increasing heat shock protein content. Am J Physiol 276:H1098–102 [DOI] [PubMed] [Google Scholar]
- Taylor RP, Olsen ME, Starnes JW. 2007. Improved postischemic function following acute exercise is not mediated by nitric oxide synthase in the rat heart. Am J Physiol Heart Circ Physiol 292:H601–7 [DOI] [PubMed] [Google Scholar]
- Taylor RP, Starnes JW. 2012. Reactive oxygen species are not a required trigger for exercise-induced late preconditioning in the rat heart. Am J Physiol Regul Integr Comp Physiol 303:R968–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H, Kinugawa S, Matsushima S. 2011. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol 301:H2181–90 [DOI] [PubMed] [Google Scholar]
- van den Brink OW, Delbridge LM, Rosenfeldt FL, Penny D, Esmore DS, Quick D, Kaye DM, Pepe S. 2003. Endogenous cardiac opioids: enkephalins in adaptation and protection of the heart. Heart Lung Circ 12:178–87 [DOI] [PubMed] [Google Scholar]
- Vegh A, Parratt JR. 2005. A common mechanism in the protective effects of preconditioning, cardiac pacing and physical exercise against ischemia and reperfusion-induced arrhythmias. Exp Clin Cardiol 10:200–5 [PMC free article] [PubMed] [Google Scholar]
- Venditti P, Di Meo S. 1996. Antioxidants, tissue damage, and endurance in trained and untrained young male rats. Arch Biochem Biophys 331:63–8 [DOI] [PubMed] [Google Scholar]
- Vidal M, Wieland T, Lohse MJ, Lorenz K. 2012. beta-Adrenergic receptor stimulation causes cardiac hypertrophy via a Gbetagamma/Erk-dependent pathway. Cardiovasc Res 96:255–64 [DOI] [PubMed] [Google Scholar]
- Wang J, Wolin MS, Hintze TH. 1993. Chronic exercise enhances endothelium-mediated dilation of epicardial coronary artery in conscious dogs. Circ Res 73:829–38 [DOI] [PubMed] [Google Scholar]
- Ward CW, Prosser BL, Lederer WJ. 2014. Mechanical stretch-induced activation of ROS/RNS signaling in striated muscle. Antioxid Redox Signal 20:929–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkinson WP, Foley DH, Rubio R, Berne RM. 1979. Myocardial adenosine formation with increased cardiac performance in the dog. Am J Physiol 236:H13–21 [DOI] [PubMed] [Google Scholar]
- Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. 2004. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A 101:13683–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil J, Zolk O, Griepentrog J, Wenzel U, Zimmermann WH, Eschenhagen T. 2006. Alterations of the preproenkephalin system in cardiac hypertrophy and its role in atrioventricular conduction. Cardiovasc Res 69:412–22 [DOI] [PubMed] [Google Scholar]
- Yamashita N, Hoshida S, Otsu K, Asahi M, Kuzuya T, Hori M. 1999. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J Exp Med 189:1699–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QJ, McMillin SL, Tanner JM, Palionyte M, Abel ED, Symons JD. 2009. Endothelial nitric oxide synthase phosphorylation in treadmill-running mice: role of vascular signalling kinases. J Physiol 587:3911–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Widmer RJ, Xie W, Jimmy Widmer A, Miller MW, Schroeder F, Parker JL, Heaps CL. 2010. Effects of exercise training on cellular mechanisms of endothelial nitric oxide synthase regulation in coronary arteries after chronic occlusion. Am J Physiol Heart Circ Physiol 298:H1857–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier JL, Wang P, Samouilov A, Kuppusamy P. 1995. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med 1:804–9 [DOI] [PubMed] [Google Scholar]