Abstract
Several studies on the effect of inhaled plutonium-dioxide particulates and the incidence of lung tumors in dogs reveal beneficial effects when the cumulative alpha-radiation dose is low. There is a threshold at an exposure level of about 100 cGy for excess tumor incidence and reduced lifespan. The observations conform to the expectations of the radiation hormesis dose-response model and contradict the predictions of the LNT hypothesis. These studies suggest investigating the possibility of employing low-dose alpha-radiation, such as from 239PuO2 inhalation, as a prophylaxis against lung cancer.
Keywords: plutonium-dioxide, inhalation, lung cancer, prophylaxis, radiation hormesis, adaptive protection
INTRODUCTION
The authors of a study on inhaled 239PuO2 particles in beagle dogs, Fisher and Weller (2010), identified the possibility of a beneficial health effect—lung tumor suppression at a low radiation dose. This was based on a statistical test they carried out “on the null hypothesis to determine the probability that the controls and the lowest dose groups have the same underlying probability of tumor incidence, and that tumor incidence is unrelated to dose (testing for a threshold effect).” Because of the infinitesimally small amount of plutonium in these studies, chemical toxicity does not need consideration.
The LNT hypothesis, recommended in 1956 (instead of the threshold dose-response model) for evaluating risks to the human genome, led to the adoption of the LNT model for cancer risk assessment. It states that the probability of the number of cancer deaths is proportional to the collective radiation dose of a population (Clarke and Valentin 2005). For the lung tumor data (spontaneously-occurring plus radiation-induced) in Fig. 4 of Fisher and Weller (2010), the LNT model would predict about 5 tumors in the 26 dogs that were exposed to a cumulative alpha-radiation dose in the range 8 to 48 cGy. The observed incidence of 0 tumors in the 16 dogs in the 8 to 22 cGy range and 1 tumor in the 10 dogs in the 27 to 48 cGy range suggests a beneficial effect, but the LNT statistical variations of these low tumor observations are too large to challenge the LNT prediction of 5 tumors.
However, the overall shape of the dose-response data shown in figures Fig. 1 to Fig. 4 is very non-linear. Furthermore, severe molecular damage, including many double-strand DNA breaks, occurs locally around each plutonium particle in tissue. These breaks are caused by the alpha radiation with its dense ionization, i.e., high LET, over a distance of several cell diameters. Since cancer is associated with DNA mutations, tumors are predicted at these locations. Considering the spontaneously-occurring lung tumors, it is quite surprising that only one tumor was observed among the 26 dogs that were exposed to cumulative lung absorbed doses in the range 8 to 48 cGy. At these doses all cells are hit at least once by an alpha-particle (Simmons and Richards 2010). The data and the biology conform to the lack of increased lung cancer in people living in houses with elevated radon concentration (Hall and Giaccia 2005) and contradict the LNT prediction that the probability of radiation-induced cancer is proportional to dose.
ADAPTIVE PROTECTION SYSTEMS
There is a large amount of data from a wide variety of medical treatments with radiation at doses below 100 cGy, including treatments for serious infections, and many radiobiological studies, all carried out over the past 120 years. These data show a remarkable effectiveness in healing. Moreover, even lower doses or lower level dose-rates, predominantly below 20 cGy, can up-regulate adaptive protection systems in terms of delayed and temporary detoxification, DNA-repair, and damage removal in various ways (Cuttler 2013, 2014). Such exposures have been shown to remove spontaneously-occurring cancer cells induced by endogenous effects and also remove radiation-induced damage. For example, oncologist K. Sakamoto observed the total removal of tumors in all regions of the body, probably by induction of protective mechanisms, of a patient with advanced ovarian cancer, after treatment with 15 whole-body doses of only 10 cGy x-rays, over a 5 week period (Sakamoto 2004).
The biological phenomenon that results from adaptive protection is radiation hormesis (Feinendegen et al. 1987, 2011, 2013). This model predicts a reduction in the overall incidence of lung tumors, as a consequence of stimulation of protection by low level radiation. The Fisher and Weller (2010) data comply with these expectations. In the lowest range, from 8 to 22 cGy, no tumors were observed. Fig. 3 indicates a strong rise in the incidence of tumors, as the cumulative dose increases, which flattens starting at about 500 cGy. As shown in Fig.4, the NOAEL (no observed adverse effects level) is about 100 cGy. (Radiation pneumonitis is the cause of death following exposures above 3000 cGy.)
In challenging the LNT theory, Fisher and Weller (2010) also point to the measured lifespan dose-response relationship in Fig. 5 for dogs that inhaled 239PuO2. The lifespan appears to increase significantly from about 5000 to 6000 days, when the dose rate is raised by a factor of 3.6 from 0.0055 to 0.020 cGy per day. The threshold (NOAEL) for a return to 5000 days is ~0.030 cGy per day or 11 cGy per year (155 cGy lifetime). The data in Fig. 4 and 5 point to a beneficial effect after a low dose and contradict the LNT prediction of an excess risk of late health effects in proportion to the radiation dose.
BIOLOGICAL BASIS FOR RADIATION HORMESIS
Radiation hormesis theory (Feinendegen et al. 2013) is based on the following biological facts:
The DNA molecules in all organisms are being damaged at a relatively high rate by endogenous processes, which include temperature, reactive oxygen species and other agents. Damage to DNA, cells and tissues/organs also occurs due to external causes, such as thermal burns, physical injuries, infectious pathogens, ingestion of chemical substances, etc. The rate of damage to DNA due to average background radiation, including double strand breaks, is relatively negligible even if the quality of radiogenic DNA damage is on average more severe than endogenous DNA damage (Pollycove and Feinendegen 2003) .
Biological organisms have very powerful protection systems that prevent, repair and remove DNA damage, replace damaged cells and tissues, cure infections and endeavour to restore health. The overall ability of these systems to enable individuals to survive even severe stresses and the many daunting challenges of life is very impressive. There are immediately-acting protection systems and adaptive systems responding to internal signals under genetic control (more than 150 genes involved) with a delay of up to hours and lasting from days to months and even years. These responses depend on the sensitivity of the affected system with various thresholds. With increasing impact of stressors, adaptive protections tend to fail and give way to damage. The eventually resulting damage, such as obvious cancer, is then the difference between prevention of damage from different sources by adaptive protection, induced by low dose radiation, and damage caused by the primary radiation impact.
With increasing acute radiation doses or dose rates, different genes are activated at different thresholds (Mitchel 2015) and “turn on” appropriate adaptive protection systems, which act against the consequences of harmful agents, regardless of their sources, to restore health. When the radiation dose or dose rate increases beyond the ranges of response sensitivity of the impacted system, protection diminishes and eventually fails and the response of the organism to the exposure moves into the range of harmful effects. Still, when this occurs, other mechanisms are activated by other genes to mitigate the harm and improve the probability of survival, for instance, by removal of damaged cells and structural replacement with functional restoration.
RADIO-TOXICITY OF INHALED 239PUO2
A note by Simmons and Richards (2012) refers to the work by Muggenburg et al. (2008) on radio-toxicity of inhaled 239PuO2, plotting the incidence of lung tumors at low doses and observing an apparent threshold at approximately 100 cGy, which is consistent with the data of Fisher and Weller (2010). After assessing strong evidence on the basis of microdosimetric analysis and results of other studies, they concluded that there is no reduction in lifespan at lung doses below about 100 cGy and no significant increase in incidence of lung tumors at lung doses below 62 cGy. All of the studies “show that there is a threshold in the region of about 100 cGy in the lung dose required to induce cancer in that organ” (Simmons and Richards 2012).
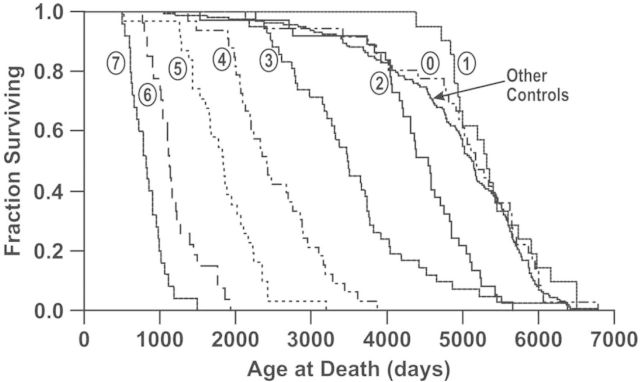
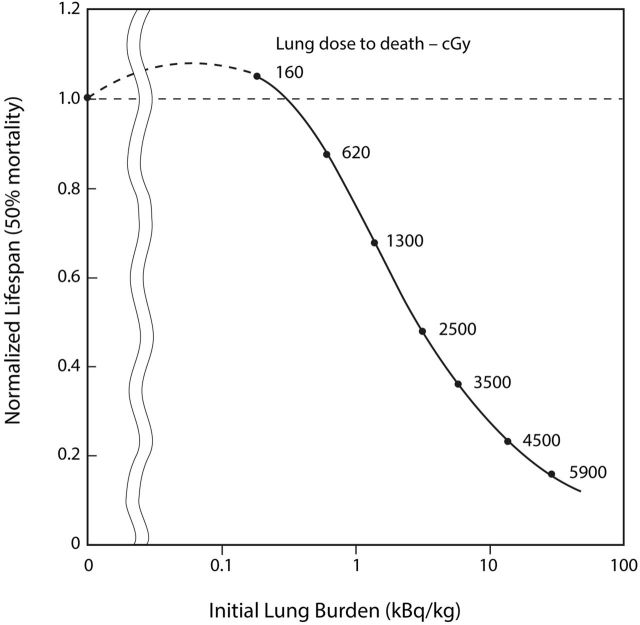
The study by Muggenburg et al. (2008) was designed to measure the lifespan health effects of different degrees of alpha-particle dose non-uniformity in the lung. The lowest exposure level was an initial lung burden of 0.16 kBq/kg, which corresponds to an accumulated 160 cGy lung dose to death. The dose range is well above the level at which lung tumor inhibition could be expected, based on the study by Fisher and Weller (2010). However, the data in Fig. 4 in the paper by Muggenberg et al. (2008) on the survival of dogs present evidence of a possible beneficial health effect for low-level inhalation (reproduced here as Figure 1). Table 1 and Figure 2, derived from this figure, reveal a clear threshold above which inhalation of plutonium-dioxide leads to reduced lifespan. It also suggests the possibility of an extended lifespan due to a lower incidence of spontaneously-occurring lung cancer.
Figure 1.
Survival curves for dogs that inhaled graded activity levels of 239PuO2(from Muggenburg et al. 2008, with permission from Radiation Research).
Table 1.
Normalized lifespan of beagle dogs following 239PuO2 inhalation
| Exposure Level | Initial Lung Burden kBq/kg | Lung Dose to Death cGy | Normalized Lifespan 50% mortality |
|---|---|---|---|
| Controls | 0 | 0 | 1.00 |
| 1 | 0.16 | 160 | 1.03 |
| 2 | 0.63 | 620 | 0.88 |
| 3 | 1.6 | 1300 | 0.68 |
| 4 | 3.7 | 2400 | 0.47 |
| 5 | 6.4 | 3500 | 0.36 |
| 6 | 14 | 4500 | 0.22 |
| 7 | 29 | 5900 | 0.16 |
Figure 2.
Normalized lifespan of beagle dogs following 239PuO2 inhalation (Adapted from Muggenburg et al. 2008).
INHALED BETA-GAMMA EMITTING RADIONUCLIDES
A review was carried out recently on dogs that inhaled 90Sr, 144Ce, 91Y and 90Y, imbedded in an insoluble, fused-clay matrix (Brooks et al. 2009). The lungs, heart and nearby lymph nodes were the targets of the chronic low-LET radiation dose. Cumulative doses to the lungs ranged from zero up to 105 cGy, and the initial dose rates ranged from zero up to 7000 cGy per day. In the high-dose region, cancer increased with radiation dose to a very high frequency. However, there was no evidence of radiation-induced cancer when the doses were below 2500 cGy. This is apparent in the data in Fig. 6 in the paper by Brooks et al. (2009). “The 53 dogs in the control group had a total of 8 lung cancers, for a baseline incidence of 15%.” “In the 80 exposed dogs that received doses less than 25 Gy, there were a total of 6 lung tumors observed.” Total cancer frequency in the control dogs was about 50% (26 cancers in 53 dogs). In the group of 20 dogs in lowest dose group (50 cGy), there were only 3 total cancers observed—-significantly decreased relative to the controls.
CONCLUSION
The 50-year study of plutonium exposure to the Manhattan Project plutonium workers (Voelz et al. 1997) suggested beneficial health effects. There was no evidence that the incidence of lung cancer was elevated, as would be expected from the exposures received, based on the LNT hypothesis. Furthermore, the 30-year follow-up of a plutonium-americium inhalation exposure, with a committed effective dose of the order of 100 cSv, shows no evidence of lung cancer; the subject is in good health (Wernli et al. 2015).
All medical treatments with low radiation were discontinued following the 1956 recommendation of the National Academy of Science (NAS) Committee on the Biological Effects of Atomic Radiation (BEAR) (1956). It recommended the LNT instead of the threshold dose-response model to evaluate the risk of genetic mutations from ionizing radiation. This led to the enormous radiation scare that links any exposure to a risk of cancer. After Calabrese prepared several carefully researched papers on the history of the LNT model, he reviewed (Calabrese 2015a, 2015b) the archives of the BEAR committee and determined that the LNT concept for assessing genetic risk due to radiation did not comply with data available at that time and that the LNT model was a construct.
Lung cancer is the leading cancer killer in the United States. The studies on inhaled plutonium-dioxide suggest that the incidence of spontaneously-occurring lung tumors could be significantly reduced by low-dose alpha-radiation, such as from a single inhalation of 239PuO2, as a prophylaxis. Because the amount of plutonium is infinitesimally small, plutonium toxicity is of no concern in this context. Pollycove (2007) has summarized the rationale and radiobiological basis of low-dose irradiation for the prevention and therapy of cancer. This and many other medical applications of low-dose radiation treatment should be investigated.
Contributor Information
Jerry M. Cuttler, Cuttler & Associates Inc.
Ludwig E. Feinendegen, Brookhaven National Laboratory, Biological, Environmental & Climate Sciences
REFERENCES
- Brooks AL, Eberlein PE, Couch LA, Boecker BB. 2009. The role of dose-rate on risk from internally-deposited radionuclides and the potential need to separate dose-rate effectiveness factor (DREF) from the dose and dose-rate effectiveness factor (DDREF). Health Phys 97(5): 458–469. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. 2015a. Cancer risk assessment foundation unraveling: New historical evidence reveals that the US National Academy of Sciences (US NAS), Biological Effects of Atomic Radiation (BEAR) Committee Genetics Panel falsified the research record to promote acceptance of the LNT. Arch Toxicol. Available at: http://link.springer.com/article/10.1007/s00204-015-1455-3 [DOI] [PubMed]
- Calabrese EJ. 2015b. An abuse of risk assessment: how regulatory agencies improperly adopted LNT for cancer risk assessment. Arch Toxicol. Available at: http://link.springer.com/article/10.1007/s00204-015-1454-4 [DOI] [PubMed]
- Clarke R, Valentin J. 2005. A history of the International Commission on Radiological Protection. Health Phys 88(5): 407–422. [PubMed] [Google Scholar]
- Cuttler JM. 2013. Commentary on Fukushima and beneficial effects of low radiation. Dose-Response 11(4): 432–443. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3834738/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttler JM. 2014. Remedy for radiation fear—Discard politicized science. Dose-Response 12(2): 170–184. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4036393/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinendegen LE, Muehlensiepen H, Bond VP, Sondhaus CA. 1987. Intracellular stimulation of biochemical control mechanisms by low-dose low-LET irradiation. Health Phys 52(5): 663–669. [DOI] [PubMed] [Google Scholar]
- Feinendegen LE, Brooks AL, Morgan WF. 2011. Biological Consequences and Health Risks of Low-Level Exposure to Ionizing Radiation: Commentary on the Workshop. Health Phys 100(3): 247–259. [DOI] [PubMed] [Google Scholar]
- Feinendegen LE, Pollycove M, Neumann RD. 2013. Hormesis by low dose radiation effects: Low-dose cancer risk modeling must recognize up-regulation of protection. In: Baum RP. (ed.). Therapeutic Nuclear Medicine. Springer: ISBN 973-3-540-36718-5. Available at: http://radiationeffects.org/wp-content/uploads/2014/08/Feinendegen-2013-Hormesis-in-Therapeutic-Nuclear-MedicinePDFxR.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DR, Weller RE. 2010. Carcinogenesis from inhaled in beagles: Evidence for radiation homeostasis at low doses? Health Phys 99(3): 357–362. [DOI] [PubMed] [Google Scholar]
- Hall EJ, Giaccia AJ. 2005. Radiobiology for the Radiologist. 6th Edition, Williams LippincottWilkins; New York, USA. [Google Scholar]
- Mitchel REJ. 2015. Adaption by low dose radiation exposure: a look at scope and limitations for radiation protection. Dose-Response (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muggenburg BA, Guilmette RA, Hahn FF, Diel JH, Mauderly JL, Seilkop SK, Boecker BB. 2008. Radiotoxicity of inhaled 239PuO2 in dogs. Radiat Res 170: 736–757. [DOI] [PubMed] [Google Scholar]
- National Academy of Science (NAS)/National Research Council (NRC). 1956. The biological effects of atomic radiation. A report to the public. Washington. [Google Scholar]
- Pollycove M, Feinendegen LE. 2003. Radiation-induced versus endogenous DNA damage: possible effect of inducible protectice responses in mitigating endogenous damage. Hum Exp Toxicol 22: 290–306. [DOI] [PubMed] [Google Scholar]
- Pollycove M. 2007. Radiobiological basis of low-dose irradiation in prevention and therapy of cancer. Dose-Response 5(1): 26–38. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2477707/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K. 2004. Radiobiological Basis for Cancer Therapy by Total or Half-body Irradiation. Nonlinearity in Biology, Toxicology, and Medicine 2(4): 293–316. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2657505/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons JA, Richards SR. 2010. A microdosimetric reassessment of new data on the effects of plutonium dioxide inhalation by beagle dogs. Radiat Res 173: 818–828. [DOI] [PubMed] [Google Scholar]
- Simmons JA, Richards SR. 2012. Further comments on the induction of lung tumors by plutonium dioxide in beagles. Health Phys 102(3): 346–347. [DOI] [PubMed] [Google Scholar]
- Voelz GL, Lawrence JNP, Johnson ER. 1997. Fifty years of plutonium exposure to the Manhattan Project plutonium workers: an update. Health Phys 73(4): 611–619. [DOI] [PubMed] [Google Scholar]
- Wernli C, Eikenberg J, Marzocchi O, Breustedt B, Oestreicher U, Romm H, Gregoratto D, Marsh J. 2015. 30-Y follow-up of a Pu/Am inhalation case. Radiation Protection Dosimetry (in press). [DOI] [PubMed] [Google Scholar]