Abstract
Objectives:
This study investigates (1) local tumor control and (2) normal tissue toxicity of pulsed low-dose rate radiotherapy (PLDR) for recurrent lung cancer.
Methods:
For study 1, nude mice were implanted with A549 tumors and divided into the following 3 groups: (1) control (n = 10), (2) conventional radiotherapy (RT; n = 10), and (3) PLDR (n = 10). Tumor-bearing mice received 2 Gy daily dose for 2 consecutive days. Weekly magnetic resonance imaging was used for tumor growth monitoring. For study 2, 20 mice received 8 Gy total body irradiation either continuously (n = 10) or 40 × 0.2 Gy pulses with 3-minute intervals (n = 10).
Results:
For study 1, both conventional RT and PLDR significantly inhibited the growth of A549 xenografts compared with the control group (>35% difference in the mean tumor volume; P < .05). The PLDR results were slightly better than conventional RT (8% difference in the mean tumor volume; P > .05). For study 2, the average weight was 20.94 ± 1.68 g and 25.69 ± 1.27 g and the survival time was 8 days and 12 days for mice treated with conventional RT and PLDR (P < .05), respectively.
Conclusion:
This study showed that PLDR could control A549 tumors as effectively as conventional RT, and PLDR induced much less normal tissue toxicity than conventional RT. Thus, PLDR would be a good modality for recurrent lung cancers.
Advances in Knowledge:
This article reports our results of an in vivo animal investigation of PLDR for the treatment of recurrent cancers, which may not be eligible for treatment because of the dose limitations on nearby healthy organs that have been irradiated in previous treatments. This was the first in vivo study to quantify the tumor control and normal tissue toxicities of PLDR using mice with implanted tumors, and our findings provided evidence to support the clinical trials that employ PLDR treatment techniques.
Keywords: pulsed low-dose rate radiotherapy (PLDR), total body irradiation (TBI), A549 xenograft model, normal tissue toxicity, tumor growth
Introduction
Locoregional cancer recurrence is one of the major causes of treatment failure for patients after primary radiation treatment. Locoregional recurrences are reported in about 41.7% of the patients at 3 years after concomitant radiochemotherapy for locally advanced head and neck squamous cell carcinoma,1 and 50% to 60% of patients died of locally recurrent tumors.2 The locoregional failure rate is about 85% after radiochemotherapy for locally advanced nonsmall cell lung cancer.3 For all stages (Ia-IVa) of cervical cancer, the locoregional recurrence rate was about 35% after being treated with radiochemotherapy.4 The treatment of locoregional recurrent tumors can be challenging, since many factors should be included such as histology type, stage of tumor, metastatic disease status, previous treatment, the interval time between primary treatment and locoregional recurrence, and performance status. There is also no consensus standard to treat patients with recurrent cancer who have previously been irradiated. Reirradiation of the tumor with or without chemotherapy is a major treatment option at some centers because therapeutic options are limited in many tumor sites after both primary surgical and radiotherapy (RT) treatment.5 The overall frequency of reirradiation for locoregional recurrences is unknown. For recurrent head and neck cancer, Dawson et al6 reported that a subset of patients are salvageable, and selected patients (78%) received high-dose reirradiation with concomitant chemotherapy. Creak et al7 concluded that reirradiation with concomitant chemotherapy should be considered for patients as it has the potential for cure. However, challenging problems exist for tumor reirradiation such as the risk of normal tissue toxicity in the reirradiation situation and the radioresistance of tumor clonogens which persist through the initial radiation course.8
In the recent years, the phenomenon of low-dose hyperradiosensitivity (HRS) has been reported in cells of more than 40×-irradiated human tumor cell lines.9,10 Low radiation dose <0.3 Gy induced more radio-sensitive tumor cells, and while the radiation dose was increased to 1 Gy, tumor cells became increasingly radioresistant per unit dose. Pulsed low-dose rate radiotherapy (PLDR) is used to take advantage of the different transition doses that can induce HRS in tumor cells at about 30 cGy, while generally greater in normal tissues, and induce more increasing normal tissue repair at low-dose rates.9,11 The PLDR divides the daily RT treatment into a number of subfractions (pulses) where each subfractional dose is less than the tumor transition dose but greater than the normal tissue transition dose so that the radiation repair can be triggered in normal tissues but not in tumor cells inducing HRS. The most commonly used PLDR treatment is 10 pulses of 0.2 Gy using a 3-minute interpulse to achieve an effective low dose rate of 6.7 cGy/min and maximize the normal tissue repair process.12,13 Several clinical studies have been reported using PLDR technique as reirradiation treatment for metastatic brain tumors and recurrence of breast cancer, glioblastoma, and nasopharyngeal carcinoma.14-18 However, most clinical reports using PLDR treatment are case reports. It is also controversial whether PLDR can provide a therapeutic advantage in RT in vivo in the animal. Krause et al19,20 reported that PLDR did not demonstrate a therapeutic benefit in subcutaneous gliomas using 3 fractions of 0.4 Gy/day, interval 4 hours, 7 days/week compared to conventional fractionation, and 1 fraction of 1.68 Gy/day, 5 days per week. Another experiment using ten 0.2-Gy pulses with 3-minute intervals between pulses compared with standard 2 Gy fractionation for 7 consecutive days in orthotopic glioma tumor models successfully demonstrated that PLDR was more effective than the standard fractionated treatment and was associated with less normal tissue damage.21 The main reason for different results in the animal experiments may be due to the difference in size of fraction dose of PLDR, where 0.4 Gy is sufficient to induce activation of the Ataxia Telangiectasia Mutated (ATM) gene, downstream repair, and checkpoint processes.22,23
Existing preclinical research on PLDR involves mostly gliomas, while other tumor models have rarely been used. To further investigate the in vivo effects of PLDR, we setup our animal studies using total body irradiation (TBI) to demonstrate the benefit of PLDR to normal tissues relative to tumor tissues and using an A549 subcutaneous tumor model to substantiate the treatment effect of PLDR.
Materials and Methods
Animal Tumor Model
Male athymic Balb/c nude mice (6 weeks old) were purchased from Harlan (Indianapolis, Indiana). Animal studies were carried out in compliance with a protocol approved by the Institutional Animal Care and Use Committee of Fox Chase Cancer Center (FCCC). Human lung adenocarcinoma cells A549 were obtained from the American Type Culture Collection and cultured in Dulbecco modified Eagle medium-F12 medium, containing 10% fetal bovine serum, 1% l-glutamine, and 1% penicillin–streptomycin as described previously.24 A549 cells of 1 × 107 were injected subcutaneously into the left and right abdominal region. It took approximately 2 weeks for A549 tumors to reach 100 mm3 in diameter.
Experimental Design
Our study consisted of 2 experiments: (1) PLDR treatment of A549 tumor-bearing mice to investigate the local tumor control and (2) TBI to investigate the normal tissue toxicity of PLDR.
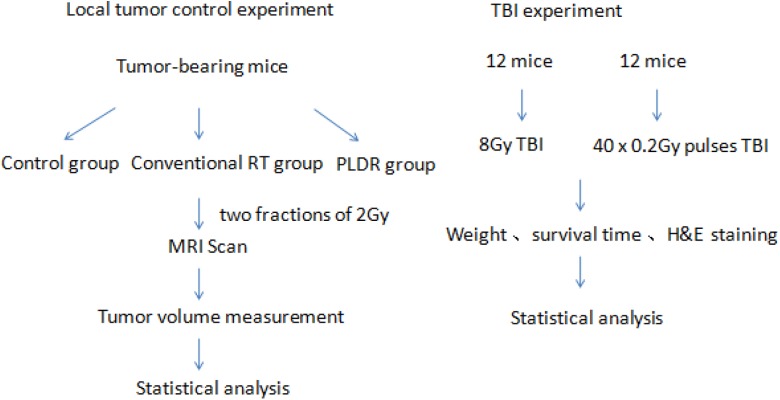
For the lung cancer tumor model, 1 × 107 A549 cells were injected subcutaneously into the left and right abdominal region. Tumor growth was monitored by daily measurements of tumor diameters with a caliper using the following formula: volume = 0.52 × (width2 × length). When tumors reached a mean volume of about 80 mm3, the tumor-bearing mice were randomly assigned into 3 groups: (1) control group (n = 10), (2) conventional RT group (n = 10), and (3) PLDR group (n = 10). All treatments were delivered in 2 fractions of 2 Gy each in 2 consecutive days. Following treatment, mice were scanned weekly using magnetic resonance (MR) imaging (MRI; resolution: 0.2 mm) for tumor growth monitoring. For the TBI experiment, 24 mice were divided into 2 groups randomly. One group was irradiated continuously at a typical RT dose rate of 300 MU/min. The other group was irradiated with 40 × 0.2 Gy pulses with a 3-minute interval. The total body radiation dose was 8 Gy in 1 treatment. The weight of each mouse was measured daily. Figure 1 shows the experimental procedures for the 2 experiments.
Figure 1.
The experiment setup and procedures for the tumor control experiment and total body irradiation experiment.
Magnetic Resonance Imaging
The A549 tumor growth was monitored weekly after tumor implantation using a 1.5-T GE MR scanner (GE Healthcare, Waukesha, Wisconsin). Animals were sedated and anesthetized with an intramuscular (IM) injection of a mixed solution of ketamine (60 mg/kg) and acepromazine (2.5 mg/kg) in 15 μL volume, resulting in a 15-minute immobilization of the animal during MR scanning. A ring-shaped surface coil (diameter: ˜8 cm) was used for the MR signal detection. T2-weighted MRIs were acquired using fast-recovery fast-spin-echo (FRFSE) sequence with the following parameters: repetition time (TR)/echo time (TE) = 2200/85 ms, number of excitations (NEX) = 3, matrix = 288 × 288, field of view (FOV) = 7 × 7 cm2 (resolution = 0.243 × 0.243 mm2), and slice thickness = 2 mm.
External Beam Radiation
For the normal tissue toxicity study, the radiation dose was delivered using a Siemens Artiste linear accelerator (Siemens Medical Systems, Malvern, Pennsylvania). The mice were kept in a small box of ∼2 cm internal height during treatment. A 1-cm bolus was added to the top of the box for dose buildup. The source–surface distance (SSD) from the radiation source to the bottom of bolus was 98 cm. The photon energy was 6 MV, and the dose rate was 300 MU/min. An open beam with 20 × 20 cm field size and 760 MUs (output factor included) was used for the TBI (the prescription point was the midpoint of the mouse body).
For the local control study, the 2 Gy radiation dose prescribed to the A549 lung tumors in groups 2 and 3 was delivered with the same machine mentioned earlier. The photon energy was 6 MV and the dose rate was 300 MU/min. Mice were anesthetized with ketamine and acepromazine IM and placed on the treatment table in the head-first supine position. Since the tumor was very shallow (˜2 mm close to the skin of the mouse), a 1.5 cm bolus was added to the top of the mouse’s skin for radiation dose buildup. Mice were placed away from the radiation source with a SSD of 100 cm, that is, 100 cm SSD to the surface of the bolus. An open beam with a 3 × 3 cm field size and 210 MUs (output factor included) was used for treatment.
Hematoxylin and Eosin
The resected tissues were fixed with 10% neutral formalin, embedded with paraffin, and serially sectioned at 5 μm. The sections were stained with Hematoxylin and eosin (H&E) according to standard protocols and examined under a light microscope.
Tumor Volume Measurement
To measure the tumor volume precisely, a high-resolution T2-weighted MR scan protocol was developed, which used the FRFSE sequence with the same values of TR/TE, NEX, matrix size, and FOV, as described previousl. However, both coronal and axial scans were performed and a smaller slice thickness, 1.2 mm, was used for axial MR scanning. This resulted in a resolution of 0.243 × 0.243 × 1.2 mm3 per voxel for the axial scan. The tumor volume measurement was performed on axial MR images using the accustom software. In detail, the area of tumor on each image was calculated by summing the areas of all pixels in the tumor region. The total tumor volume was calculated by adding up the tumor area on each MR image and multiplying the slice thickness. The relative tumor volume was calculated by normalizing the tumor volume of each week to that on the treatment day.
Statistical Analysis
Statistical analysis was performed using the SPSS 13.0 software. The survival time of the mice in the TBI experiment was assessed using Kaplan-Meier curves. The mean and standard deviation of the mean (SEM) were calculated ,and the results were expressed as mean ± SEM. To determine whether there was a significant difference between various groups, Student t test was used and statistical significance was established at P < .05.
Results
Effect of PLDR on A549 Tumor Xenograft Growth
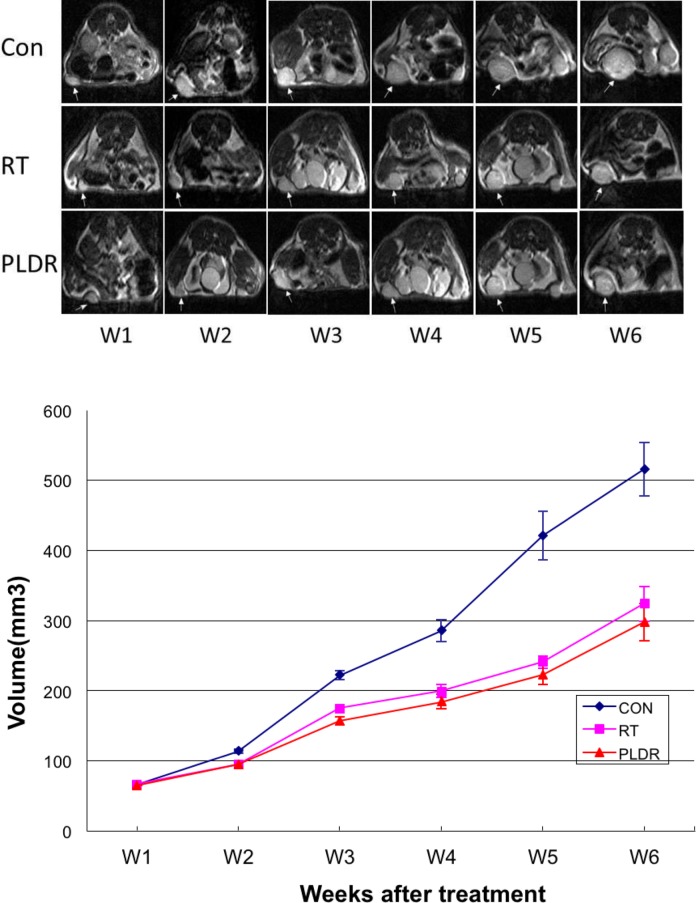
For the local tumor control experiment using the A549 xenograft model, we treated the mice with standard RT and PLDR separately. The total RT dose was 4 Gy (2 Gy daily doses for 2 consecutive days). Growth curves in nude mice were determined by measuring the tumor size using MRI (Figure 2) every week after A549 cells were implanted into the animals. The results of the tumor growth curve in Figure 1 showed that standard RT and PLDR significantly inhibited the growth of A549 xenografts compared with untreated control group (P < .05). The results of PLDR are better than the standard RT treatment, but there was no significant difference (P > .05) between the 2 groups.
Figure 2.
Top: Weekly magnetic resonance (MR) images of A549 tumors after the radiation treatment (W1 is the treatment week). Bottom: the average tumor volumes for the control mice (CON), and the mice received 4 Gy conventional radiotherapy (RT) and pulsed low-dose rate radiotherapy (PLDR).
Normal Tissue Toxicity of PLDR
To compare the difference in toxicity of normal tissue treated with standard RT and PLDR radiotherapy, the TBI method was an effective and definitive technique to use. We setup the proper radiation parameters to ascertain that the whole body of the mouse received the dose uniformly and that the experiment could be easily repeated. After 8 Gy TBI radiation, we found that the average weight of the mice in the standard RT group declined gradually until all mice died by the eighth day after radiation, while in the PLDR group, the animal weight did not decrease (Figure 3) until the 11th day when the mice began to die. There was a significant difference in the weight of the mice between the 2 groups (P < .05). There was also a significant difference in the survival time between the mice in the 2 groups (P < .05; Figure 4). The damage from 8 Gy TBI radiation was proven to be lethal from the experiment showing that all mice died 8 days after the treatment for the regular RT group, however, when using the PLDR treatment, the survival time of the mice was increased to 12 days, which is a more than a 50% improvement compared with the standard RT group. This difference verified the hypothesis that PLDR treatment could reduce the damage to normal tissues resulting in slower weight decline and longer survival.
Figure 3.
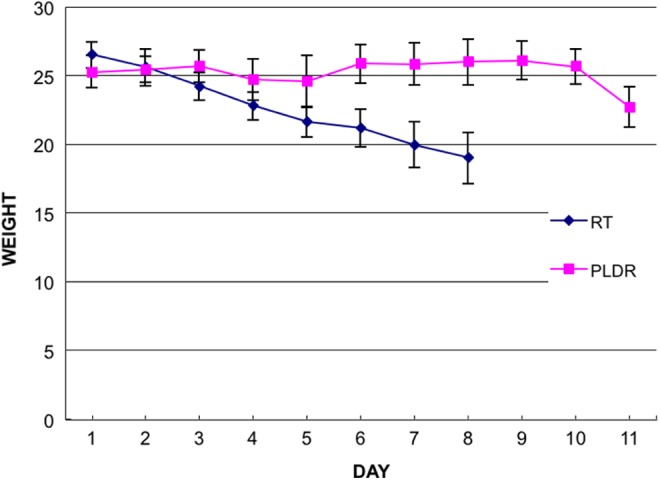
The average weight of mice in the conventional radiotherapy (RT)group and the pulsed low-dose rate radiotherapy (PLDR) group. The mice in the conventional RT group received continuous 8 Gy TBI treatment and the mice in the PLDR group received 8 Gy total body irradiation (TBI) in 40 × 0.2 Gy pulses with 3-minute intervals.
Figure 4.
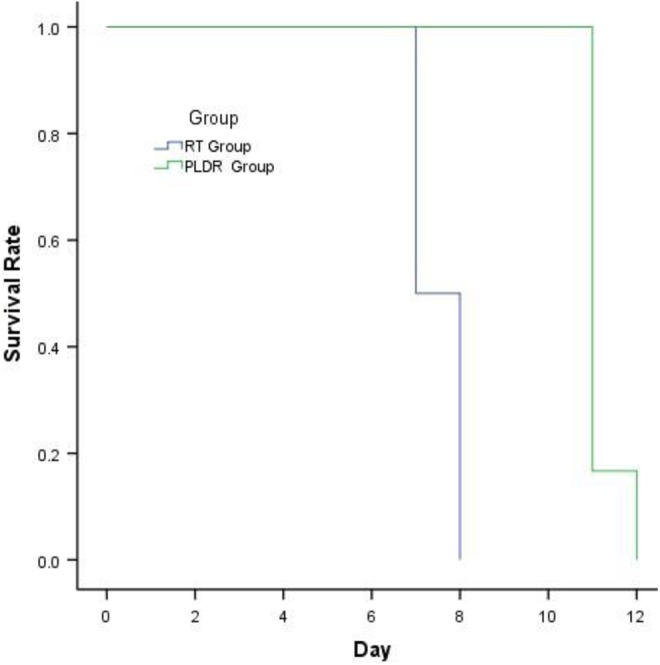
The survival time of mice in the conventional radiotherapy (RT) group and the pulsed low-dose rate radiotherapy (PLDR) group. The mice in the conventional RT group received continuous 8 Gy TBI treatment and the mice in the PLDR group received 8 Gy TBI in 40 × 0.2 Gy pulses with 3-minute intervals.
Hematoxylin and Eosin Results of Normal Tissues After TBI
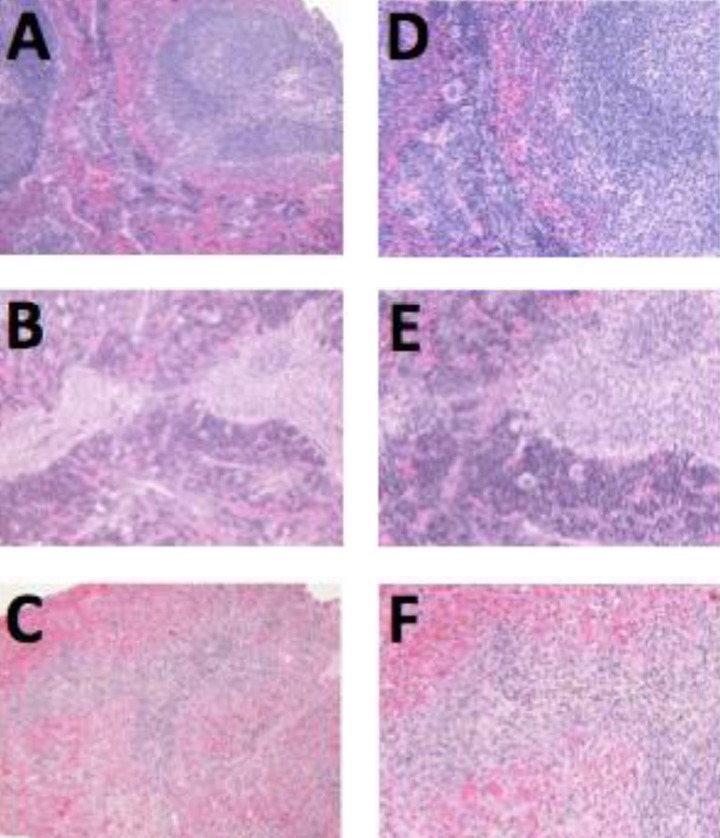
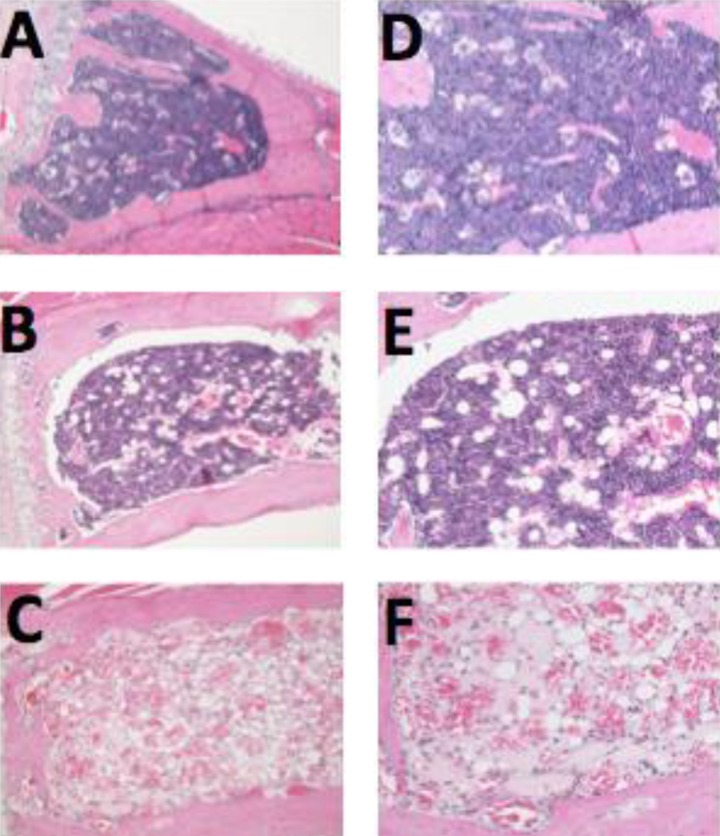
Histopathological examination revealed atrophy in several irradiated organs. The degree of atrophy was mild to moderate in the PLDR group but severe in the RT group. The most pronounced morphological abnormalities were in the immune and hematopoietic systems, such as spleen and bone marrow (Figures 5 and 6). Stomach atrophy was seen in the RT group (3/3) but was only seen in 1 mouse in the PLDR group (¼).
Figure 5.
The morphological abnormalities of spleen in 3 groups. Compared to the control group (A, ×100 and D, ×200), the degree of atrophy in the spleen was mild to moderate in the pulsed low-dose rate radiotherapy (PLDR) group (B, ×100 and E, ×200), but severe in the conventional radiotherapy (RT) group (C, ×100 and F, ×200).
Figure 6.
The morphological abnormalities of bone marrow in 3 groups. Compared to the control group (A, ×100 and D, × 200), the degree of atrophy in the bone marrow was mild to moderate in the pulsed low-dose rate radiotherapy (PLDR) group (B, ×100 and E, ×200), but severe in the conventional radiotherapy (RT) group (C, ×100 and F, ×200).
Discussion
A major concern of reirradiation of patients with locoregional recurrence after primary RT is normal tissue tolerance. Generally, minor to moderate damage to the acute responding tissues will recover a few months after radiation treatment.25 Severe acute toxicities should be avoided as an important consideration in reirradiation treatment planning. Toxicities to the late responding tissues are a concern as well, but they are of less significance considering the palliative nature of most reirradiation cases. The PLDR with 0.2 Gy/pulse and a 3-minute interval can protect the normal tissue effectively because of the differential repair capacities of normal tissues and tumor cells. The reduced dose per pulse and the small interval between pulses can improve the sublethal damage repair of normal tissues and, therefore, reduce toxicity. Since long-term follow-up is difficult in animal models to evaluate normal tissue tolerance in the reirradiation situation, and less severe or more subtle toxicities cannot be evaluated in animal studies especially with the 2 Gy daily PLDR treatment, we used the TBI method and from previous experiments we found 8 Gy to be the suitable dose to show the benefit of PLDR to normal tissues. Our previous experiments showed that when treated with 6 Gy or less, both groups of mice showed almost no obvious damage but when treated with 10 Gy or more both groups of mice died in 5 days (data not shown). When we used 8 Gy for the TBI of nude mice, we found a significant difference in the weight and survival time. Rapid weight loss and normal tissue damage occurred in mice that received 8 Gy in regular radiation dose rates (300 MU/min), whereas the nude mice that received PLDR TBI showed no decline in their body weight in the first 10 days after the TBI treatment, and the normal tissue damage was significantly reduced. This demonstrated that radiation damage could be effectively repaired when using 0.2 Gy pulsed radiation plus a 3-minute interval. The survival time was also an effective index. The significant difference in survival time between the 2 groups also indicated reduced radiation damage as a result of effective repair of sublethal damage to prolong the mice’s survival. The underlying mechanism contributing to the difference between the 2 groups is currently under investigation.
As we mentioned earlier, the locoregional recurrent tumor may be more radioresistant than the primary tumor. Tumor growth that escapes primary radiation-induced cell kill is mainly due to intrinsic radioresistance or secondary to hypoxia or proliferation of cells and results in accelerated repopulation and activation of prosurvival/poor prognosis oncogenes such as epidermal growth factor receptor or c-MET.26,27 Moreover, the recurrent tumor may be relatively hypoxic after original treatment such as surgery, RT, and chemotherapy which change the blood supply locally. Hence, increasing the tumor radiosensitivity using certain methods would increase the efficiency of reirradiation treatment of recurrent tumors. These methods may include multidisciplinary treatments such as a combination of chemotherapy including induction chemotherapy and concurrent chemoradiotherapy,28 antiangiogenic therapy,29 and radiation sensitizers30 which are more effective and have been widely used in the clinic.
The treatment scheme of PLDR may also play an important role in its efficacy. Such a treatment scheme may be designed based on the radioresistance characteristics of recurrent tumors. In recent years, PLDR has been shown as an effective method to resolve the radioresistance characteristics of tumor cells in vitro. In brief, when acute doses below 0.3 to 0.4 Gy were used, DNA damage was increased due to absent, ineffectual, or defective DNA repair processes determined by cell cycle-related events involving the ATM-dependent and Cdc25c phosphatase G2-phase cell cycle checkpoint.31-33 Our experimental results also showed that the 2 fractional treatments of ten 0.2-Gy pulses with a 3-minute interpulse interval delayed the average tumor growth slightly more than the standard fractional treatments, although there was no significant difference between the 2 groups. Nevertheless, our results showed that the PLDR treatment was at least as effective as regular RT for malignant tumors and therefore could be a better option for the treatment of recurrent tumors because of its reduced normal tissue toxicities. Regarding the different results in vitro and in vivo, it can be explained that there were different proportions of G2 cells and different tumor micromilieu inducing ATM phosphorylation status in vitro and in vivo, which are important to the HRS effect of PLDR.34 An alternative explanation might be the potential reduction in the HRS effect by the accumulation of pulsed doses and the finite interpulse break time that might have partially triggered the repair mechanisms.35
We have now developed treatment planning strategies for PLDR treatments of recurrent cancers utilizing advanced delivery techniques including intensity-modulated radiation therapy (IMRT) and volumetric-modulated radiation therapy (VMAT).36-40 In comparison with 3-dimensional conformal radiation therapy (3DCRT), IMRT and VMAT exhibited significantly improved target dose conformity and organ at risk dose sparing, and clinical trials using PLDR with IMRT or VMAT techniques are ongoing for the management of recurrent cancers at FCCC.
Conclusion
In this study, we performed in vivo experiments to investigate (1) local tumor control and (2) normal tissue toxicity of PLDR by comparing with regular RT with standard dose rates. For the local tumor control study, nude mice were implanted with A549 tumors and treated with PLDR or regular RT for 4 Gy. For the normal tissue toxicity study, nude mice were irradiated by 8 Gy of PLDR or regular RT. The tumor growth delay was measured accurately by MR imaging, and normal tissue toxicity was investigated by histopathology.
Our experiments showed that the PLDR treatment was effective for the A549 tumors compared with conventional RT with standard dose rates, and the tumor growth delay achieved by PLDR was even slightly better than conventional RT. Most importantly, the PLDR treatment induced significantly less toxicities to normal tissues,a nd the damage of 8 Gy TBI by PLDR to the spleen and bone marrow was only mild or moderate while the same 8 Gy TBI by conventional RT caused severe damage to these organs. These results indicated that the PLDR treatment could be a promising method for the reirradiation of recurrent lung tumors.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Bourhis J, Sire C, Graff P, et al. Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99-02): an open-label phase 3 randomised trial. Lancet Oncol. 2012;13(2):145–153. [DOI] [PubMed] [Google Scholar]
- 2. Jones A. The untreated patient with squamous carcinoma of the head and neck. Am J Clin Oncol. 1995;18(4):363. [DOI] [PubMed] [Google Scholar]
- 3. Blackstock AW, Govindan R. Definitive chemoradiation for the treatment of locally advanced non-small-cell lung cancer. J Clin Oncol. 2007;25(26):4146–4152. [DOI] [PubMed] [Google Scholar]
- 4. Vale C, Jakobsen A. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer:: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26(35):5802–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paleri V, Kelly C. Re-irradiation with concurrent chemotherapy in recurrent head and neck cancer: a decision analysis model based on a systematic review. Clin Otolaryngol. 2008;33(4):331–337. [DOI] [PubMed] [Google Scholar]
- 6. Dawson LA, Myers LL, Bradford CR, et al. Conformal re-irradiation of recurrent and new primary head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50(2):377–385. [DOI] [PubMed] [Google Scholar]
- 7. Creak A, Harrington K, Nutting C. Treatment of recurrent head and neck cancer: re-irradiation or chemotherapy? Clin Oncol. 2005;17(3):138–147. [DOI] [PubMed] [Google Scholar]
- 8. Weichselbaum RR, Beckett MA, Schwartz JL, Dritschilo A. Radioresistant tumor cells are present in head and neck carcinomas that recur after radiotherapy. Int J Radiat Oncol Biol Phys. 1988;15(3):575–579. [DOI] [PubMed] [Google Scholar]
- 9. Joiner MC, Marples B, Lambin P, Short SC, Turesson I. Low-dose hypersensitivity: current status and possible mechanisms. Int J Radiat Oncol Biol Phys. 2001;49(2):379–389. [DOI] [PubMed] [Google Scholar]
- 10. Marples B, Lambin P, Skov KA, Joiner MC. Low dose hyper-radiosensitivity and increased radioresistance in mammalian cells. Int J Radiat Biol. 1997;71(6):721–735. [DOI] [PubMed] [Google Scholar]
- 11. Hall EJ, Giaccia AJ. Radiobiology for the Radiologist Philadelphia: Wolters Kluwer Health; 2006. [Google Scholar]
- 12. Dilworth JT1, Krueger SA, Dabjan M, et al. Pulsed low-dose irradiation of orthotopic glioblastoma multiforme (GBM) in a pre-clinical model: effects on vascularization and tumor control. Radiother Oncol. 2013;108(1):149–154. [DOI] [PubMed] [Google Scholar]
- 13. Lee DY, Chunta J, Park SS, et al. Pulsed versus conventional radiation therapy in combination with temozolomide in a murine orthotopic model of glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2013;86(5):978–985. [DOI] [PubMed] [Google Scholar]
- 14. Cannon GM, Tomé WA, Robins HI, Howard SP. Pulsed reduced dose-rate radiotherapy: case report. J Neurooncol. 2007;83(3):307–311. [DOI] [PubMed] [Google Scholar]
- 15. Richards GM, Tomé WA, Robins HI, et al. Pulsed reduced dose-rate radiotherapy: a novel locoregional retreatment strategy for breast cancer recurrence in the previously irradiated chest wall, axilla, or supraclavicular region. Breast Cancer Res Treat. 2009;114(2):307–313. [DOI] [PubMed] [Google Scholar]
- 16. Adkison JB, Tomé W, Seo S, et al. Reirradiation of large-volume recurrent glioma with pulsed reduced-dose-rate radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79(3):835–841. [DOI] [PubMed] [Google Scholar]
- 17. Li G-H, Liu Y, Tang J-L, et al. Pulsed reduced dose-rate radiotherapy as re-irradiation for brain metastasis in a patient with lung squamous-celled carcinoma. Jpn J Clin Oncol. 2012;42(9):856–860. [DOI] [PubMed] [Google Scholar]
- 18. Li G-H, Zhu B, Yang F, Ma C-K, Yang D-Q. Use of cetuximab in combination with pulsed reduced dose-rate radiotherapy in a patient with recurrence of nasopharyngeal carcinoma in the neck. Exp Ther Med. 2012;3(5):869–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krause M, Hessel F, Wohlfarth J, et al. Ultrafractionation in A7 human malignant glioma in nude mice. Int J Radiat Biol. 2003;79(6):377–383. [DOI] [PubMed] [Google Scholar]
- 20. Krause M, Wohlfarth J, Georgi B, et al. Low-dose hyperradiosensitivity of human glioblastoma cell lines in vitro does not translate into improved outcome of ultrafractionated radiotherapy in vivo. Int J Radiat Biol. 2005;81(10):751–758. [DOI] [PubMed] [Google Scholar]
- 21. Park SS, Chunta JL, Robertson JM, et al. MicroPET/CT imaging of an orthotopic model of human glioblastoma multiforme and evaluation of pulsed low-dose irradiation. Int J Radiat Oncol Biol Phys. 2011;80(3):885–892. [DOI] [PubMed] [Google Scholar]
- 22. Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421(6922):499–506. [DOI] [PubMed] [Google Scholar]
- 23. Xu B, Kim S-T, Lim D-S, Kastan MB. Two molecularly distinct G2/M checkpoints are induced by ionizing irradiation. Mol Cell Biol. 2002;22(4):1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen X, Cvetkovic D, Ma C-M, Chen L. Quantitative study of focused ultrasound enhanced doxorubicin delivery to prostate tumor in vivo with MRI guidance. Med Phys. 2012;39(5):2780–2786. [DOI] [PubMed] [Google Scholar]
- 25. De Crevoisier R, Bourhis J, Domenge C, et al. Full-dose reirradiation for unresectable head and neck carcinoma: experience at the Gustave-Roussy Institute in a series of 169 patients. J Clin Oncol. 1998;16(11):3556–3562. [DOI] [PubMed] [Google Scholar]
- 26. Seiwert TY, Salama JK, Vokes EE. The concurrent chemoradiation paradigm—general principles. Nat Clin Pract Oncol. 2007;4(2):86–100. [DOI] [PubMed] [Google Scholar]
- 27. Uchida D, Kawamata H, Omotehara F, et al. Role of HGF/c-met system in invasion and metastasis of oral squamous cell carcinoma cells in vitro and its clinical significance. Int J Cancer. 2001;93(4):489–496. [DOI] [PubMed] [Google Scholar]
- 28. Salama JK, Vokes EE. Concurrent chemotherapy and re-irradiation for locoregionally recurrent head and neck cancer. Semin Oncol. 2008;35(3):251–261. [DOI] [PubMed] [Google Scholar]
- 29. Wachsberger P, Burd R, Dicker AP. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents exploring mechanisms of interaction. Clin Cancer Res. 2003;9(6):1957–1971. [PubMed] [Google Scholar]
- 30. Cohen EE, Rosine D, Haraf DJ, et al. Phase I trial of tirapazamine, cisplatin, and concurrent accelerated boost reirradiation in patients with recurrent head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;67(3):678–684. [DOI] [PubMed] [Google Scholar]
- 31. Marples B, Collis SJ. Low-dose hyper-radiosensitivity: past, present, and future. Int J Radiat Oncol Biol Phys. 2008;70(5):1310–1318. [DOI] [PubMed] [Google Scholar]
- 32. Krueger SA, Wilson GD, Piasentin E, Joiner MC, Marples B. The effects of G2-phase enrichment and checkpoint abrogation on low-dose hyper-radiosensitivity. Int J Radiat Oncol Biol Phys. 2010;77(5):1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao Y, Cui Y, Han J, Ren J, Wu G, Cheng J. Cell division cycle 25 homolog c effects on low-dose hyper-radiosensitivity and induced radioresistance at elevated dosage in A549 cells. J Radiat Res. 2012;53(5):686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eli Y, Przedecki F, Levin G, Kariv Na, Raz A. Comparative effects of indomethacin on cell proliferation and cell cycle progression in tumor cells grown in vitro and in vivo. Biochem pharmacol. 2001;61(5):565–571. [DOI] [PubMed] [Google Scholar]
- 35. Krause M, Wohlfarth J, Georgi B, et al. Low-dose hyperradiosensitivity of human glioblastoma cell lines in vitro does not translate into improved outcome of ultrafractionated radiotherapy in vivo. Int J Radiat Biol. 2005;81(10):751–758. [DOI] [PubMed] [Google Scholar]
- 36. Ma C-M, Luxton G, Orton C. Point-Counter-Point: Pulsed reduced dose rate radiation therapy is likely to become the treatment modality of choice for recurrent cancers. Med Phys. 2011;38:4909–4911. [DOI] [PubMed] [Google Scholar]
- 37. Ma C-M, Lin MH, Dai XF, et al. Investigation of pulsed low dose rate radiotherapy using dynamic arc delivery techniques. Phys Med Biol. 2012;57:4613–4626. [DOI] [PubMed] [Google Scholar]
- 38. Lin MH, Price RA, Li JS, Kang SW, Li J, Ma C-M. Investigation of pulsed IMRT and VMAT for re-irradiation treatments: dosimetric and delivery feasibilities. Phys Med Biol. 2013;58:8179–8196. [DOI] [PubMed] [Google Scholar]
- 39. Kang SW, Lang J, Wang P, et al. Optimization strategies for pulsed low-dose-rate IMRT of recurrent lung and head and neck cancers. J Appl Clin Med Phys. 2014;15(3):102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li J, Lang J, Wang P, et al. Intensity-modulated radiation therapy for pancreatic and prostate cancer using pulsed low-dose rate deliver techniques. Med Dosimetry. 2014;39:330–336. [DOI] [PubMed] [Google Scholar]